Abstract
Expressed ubiquitously, PrBP/δ functions as chaperone/co-factor in the transport of a subset of prenylated proteins. PrBP/δ features an immunoglobulin-like β-sandwich fold for lipid binding, and interacts with diverse partners. PrBP/δ binds both C-terminal C15 and C20 prenyl side chains of phototransduction polypeptides and small GTP-binding (G) proteins of the Ras superfamily. PrBP/δ also interacts with the small GTPases, ARL2 and ARL3, which act as release factors (GDFs) for prenylated cargo. Targeted deletion of the mouse Pde6d gene encoding PrBP/δ resulted in impeded trafficking to the outer segments of GRK1 and cone PDE6 which are predicted to be farnesylated and geranylgeranylated, respectively. Rod and cone transducin trafficking was largely unaffected. These trafficking defects produce progressive cone-rod dystrophy in the Pde6d−/− mouse.
Keywords: cGMP phosphodiesterase 6 (PDE6), Prenyl binding protein PrBP/δ, prenylation, PrBP/δ crystal structure, small GTPases, rod photoreceptors, G-protein coupled receptor kinase 1 (GRK1)
1. Introduction
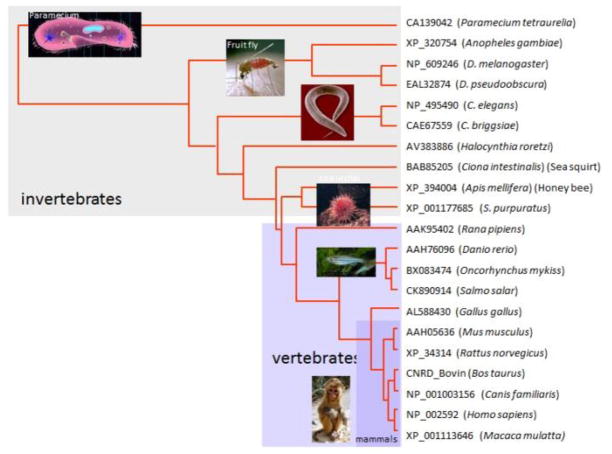
The mouse Pde6d gene encodes PrBP/δ, a 17 kDa protein that functions as a prenyl binding protein (Ismail et al., 2011; Gillespie et al., 1989; Cook et al., 2000; Zhang et al., 2004; Norton et al., 2005). By NCBI homology search, PrBP/δ orthologs were identified in essentially all animals (Fig. 1), e.g., fruit fly, the eyeless C. elegans (Li and Baehr, 1998), and the unicellular protozoan Paramecium (Zhang et al., 2007). PrBP/δ protein sequence is highly conserved throughout the animal kingdom, with at least 70% sequence identity within vertebrates, and ~50% sequence identity among invertebrates. The closest relatives of PrBP/δ are the two UNC119 paralogs, UNC119A and UNC119B (see accompanying paper, (Constantine et al., 2012)). PDE6D and UNC119 paralogs constitute a new class of neural genes whose common function as lipid-binding proteins has been maintained through metazoan evolution. This review focuses on structure/function relationships of PrBP/δ with some of its interaction proteins.
Figure 1.
Dendrogram of 21 PrBP/δ orthologs. Amino acid sequences were retrieved using the accession numbers shown, and aligned using ClustalW. The dendrogram was generated from the alignment. Sequences among vertebrates are highly conserved. PrBP/δ sequences of C. elegans and human are 65% similar suggesting conserved function through evolution.
2. Protein prenylation
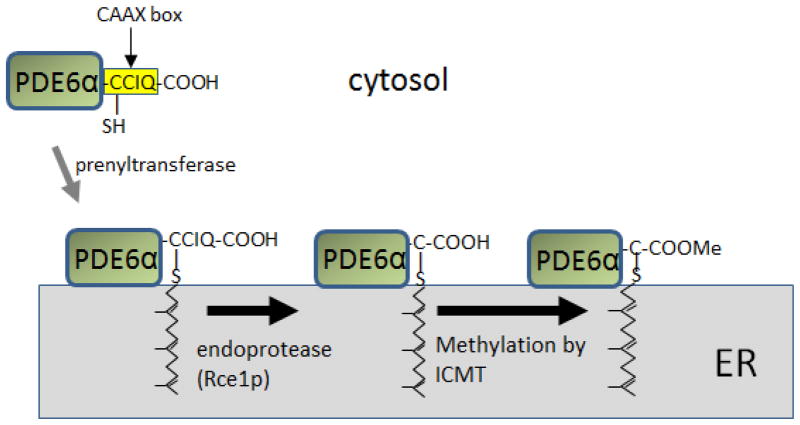
Protein prenylation is a common posttranslational modification of eukaryotic cells affecting up to 2% of all proteins expressed in mammalian cells (referred to as the “prenylome”) (Nguyen et al., 2010b). Prenyl side chains are synthesized in all living organisms via the mevalonate pathway and attached to newly synthesized cytosolic proteins carrying a C-terminal CAAX box motif (C = cysteine, A = aliphatic amino acid, X = any amino acid) (Magee and Seabra, 2005; McTaggart, 2006). The C-terminal X determines the nature of the lipid chain as leucine specifies geranylgeranylation and all other residues result in farnesylation. The prenyl chain is attached to the CAAX box cysteine via a thioether bond by cytosolic prenyl transferases (Zhang and Casey, 1996). Prenylated proteins dock to the endoplasmic reticulum (ER) and are further processed by the ER-associated enzymes RCE1 protease (ras-converting enzyme 1), which cleaves AAX of the CAAX box and an isoprenyl cysteine carboxymethyl transferase (ICMT), which carboxymethylates the cysteine COOH (Winter-Vann and Casey, 2005) (Fig. 2). Both enzymes are essential for mouse development as deletion of either RCE1 or ICMT are embryonic lethal (Bergo et al., 2002; Bergo et al., 2001). Deletion of RCE1 in retina prevented transport of rod PDE6 to the outer segments, but had no effect on GRK1 (Christiansen et al., 2011). The number of CAAX box-containing proteins in the human and mouse genome, as defined by ORFs followed by CAAX box and a stop codon, has been estimated as ~280 candidates (Winter-Vann and Casey, 2005). Known prenylated CAAX proteins include visual cascade components, members of the Ras superfamily and G protein γ-subunits, among others (Nguyen et al., 2010a; Winter-Vann and Casey, 2005) (Table 1).
Figure 2.
CAAX-Protein prenylation and processing (example: PDE6α). After prenylation in the cytosol, PDE6α protein docks at the ER and undergoes enzymatic processing by RCE1P protease that removes AAX, and an S-adenosylmethionine-dependent methyltransferase (ICMT) that carboxymethylates the C-terminal cysteine.
Table 1.
Polypeptides interacting with PrBP/δ. Column 1, proteins involved in phototransduction (red); small G proteins (green); other interacting proteins (black). Column 2, CAAX box sequences. Column 3, prenyl side chains at the C-terminal Cys, f = farnesyl; gg = geranylgeranyl. Column 4, acyl side chains at G2. Column 5, References.
| Target | CAAX | prenyl | acyl | References. |
|---|---|---|---|---|
| PDEα | cciq | f | - | (Cook et al., 2001) |
| PDEβ | ccil | gg | - | (Cook et al., 2001) |
| GRK1 | clis | f | - | (Zhang et al., 2004) |
| GRK7 | clll | gg | - | (Zhang et al., 2004) |
| cTγ | cvls | f | - | (Zhang et al., 2007) |
| Tγ | cvis | f | - | (Zhang et al., 2007) |
| DmPDE5/6 | call | gg | - | (Day et al., 2008) |
| Rab13 | cllg | f | - | (Marzesco et al., 1998) |
| Rheb | csvm | f | (Hanzal-Bayer et al., 2002) (Ismail et al., 2011; Gelb et al., 2006) |
|
| Rho6 (Rnd1) | csim | f | 16:0? | (Nancy et al., 2002) |
| Rap1a | clll | gg | - | (Nancy et al., 2002) |
| Rap1b | cqll | gg | - | (Nancy et al., 2002) |
| Rap2a | cniq | f | (Nancy et al., 2002) | |
| Rap2b | cvil | gg | 16:0? | (Nancy et al., 2002) |
| H-Ras | cvls | f | (Nancy et al., 2002) | |
| N-Ras | cvvm | f | 16:0 | (Nancy et al., 2002) |
| K-Ras | cvim | f | (Nancy et al., 2002) | |
| RhoA | clvl | gg | - | (Nancy et al., 2002) |
| RhoB | ckvl | gg | 16:0? | (Nancy et al., 2002; Hanzal-Bayer et al., 2002) |
| Prostacyclin-R | cslc | f | (Wilson and Smyth, 2006) | |
| RPGR-RCC1 | - | - | (Li et al., 1998; Linari et al., 1999b) | |
 -GTP -GTP |
- | - | (Renault et al., 2001) | |
 -GTP -GTP |
- | - | (Linari et al., 1999a) |
3. Photoreceptor PDE6 and PrBP/δ
cGMP-specific phosphodiesterase 6 (PDE6) belongs to a large PDE superfamily (PDE1–11) whose members regulate cellular concentrations of cAMP and cGMP (Conti and Beavo, 2007). PDE6 is expressed in rods and cones and consists of two catalytic subunits (rod Pde6αβ and cone Pde6α′, respectively) and two inhibitory PDE6γ subunits (Miki et al., 1975; Baehr et al., 1979). PDE6α (~99 kDa), PDE6β (~99 kDa) and two PDE6γ (~10 kDa) form a heterotetramer PDE6αβγγ (Fung et al., 1990). Each catalytic subunit carries a C-terminal cysteine, part of a CAAX box motif for post-translational prenylation (Anant et al., 1992). Mammalian PDE6α is farnesylated while PDE6β is geranylgeranylated, resulting in modifications that facilitate membrane attachment. PrBP/δ was originally identified as a protein co-purifying with PDE6αβγ2 and named PDE6δ (Gillespie et al., 1989). Under isotonic conditions, most PDE6 is peripherally membrane-associated (Baehr et al., 1979), but a fraction (20–30%) remains soluble (Gillespie et al., 1989). Affinity purification using a monoclonal antibody column to purify soluble PDE6, yielded a novel 15 kDa polypeptide (Gillespie et al., 1989). Cloning this peptide’s cDNA and northern blotting revealed that PrBP/δ was present in several different bovine tissue mRNA preparations, the strongest of which was present in the retina (Florio et al., 1996).
Addition of a GST-PrBP/δ fusion protein to permeabilized rod outer segment preparations resulted in a reduction of the maximal rate of cGMP hydrolysis in response to light (Cook et al., 2001) suggesting that GST-PrBP/δ may modify the activity of the phototransduction cascade by uncoupling transducin’s normal activation of PDE6. However, it was later demonstrated that very little PrBP/δ is present in the rod outer segment (ROS) rendering this in-vitro uncoupling mechanism physiologically insignificant (Norton et al., 2005) has purified PrBP/δ has no effect on PDE activity in vitro.
Micromolar concentrations of prenylated and carboxymethylated PrBP/δ C-terminal peptides block the Pde6- PrBP/δ interaction. Soluble PDE6 from ROS was five-fold more highly methylated than membrane-bound PDE6 suggesting that PrBP/δ preferentially binds to carboxymethylated PDE6 (Cook et al., 2000). The PDE6-PrBP/δ complex is relatively stable with a half-life of about 3.5 h. Exploiting the intrinsic tryptophan fluorescence of PrBP/δ and using dansylated prenyl cysteines as fluorescent ligands in a fluorescence resonance energy transfer (FRET) experiment, recombinant PrBP/δ was shown to specifically bind geranylgeranyl and farnesyl moieties lacking bound amino acids with Kds of ~20 and ~1 μM, respectively, establishing unambiguously that PrBP/δ functions as a prenyl-binding protein (Zhang et al., 2004). In photoreceptors, PrBP/δ was shown to interact with PDE6 subunits, farnesylated rhodopsin kinase (GRK1) and geranylgeranylated GRK7 (Zhang et al., 2004). A cryo-EM reconstruction of the PDE6/PrBP/δ complex at 18 Å (Goc et al., 2010) is shown in Fig. 3. The structure roughly resembles a skull showing two pronounced cavities, the larger of which is formed by the catalytic domains at its top (behind the PDE6γ binding sites) and the GAF-B domains at its bottom. GAF domains are important as they function as non-catalytic cGMP binding sites, sequestering most of the cGMP present in photoreceptors (Martinez et al., 2008; Ho et al., 2000).
Figure 3.
PDE6 may be membrane-bound or soluble. A, Schematic of PDE6αβγγ bound to ROS disc membranes via prenyl side chains. B, Association of PDE6αβγγ with PrBP/δ (delta) forms a soluble and diffusible complex. C, Structure of soluble PDE6αβγγδδ by Cryo-EM at 18 Å resolution in two orientations (Goc et al., 2010).
4. Interaction of PrBP/δ with prenylated Ras and Rho GTPases
Doubts concerning the identity of PrBP/δ as a PDE6 subunit arose when it was shown that the eyeless nematode C. elegans expressed a PrBP/δ ortholog (C27H5) whose functional properties were identical to human PrBP/δ (Li and Baehr, 1998). Both proteins eluted native PDE6 from ROS membranes with nearly identical efficiencies (Fig. 4). Further, human PrBP/δ interacts with several prenylated small GTPases of the Ras and Rho subfamilies present in essentially all cells (Table 1). Almost all members of the Ras family carry the CAAX motif and are prenylated, a modification which is essential for function.
Figure 4.
Function and sequence comparison of human and C. elegans PrBP/δ. A, Extraction of PDE6 as a function of human PrBP/δ versus its ortholog expressed in C. elegans. B, Sequence alignment of human PrBP/δ (1) and C. elegans PrBP/δ (2) (Li and Baehr, 1998).
Recombinant PrBP/δ extracted Rab13 from cellular membranes as it dissociates PDE6 from photoreceptor disk membranes (Marzesco et al., 1998). This PrBP/δ function was determined to be specific as RhoGDI, another known prenyl binding protein specific for Rho GTPases, was unable to substitute for PrBP/δ. PrBP/δ also interacts with many other small GTPases e.g., Ras, Rac, Rap, Rho, Rheb, RhoA, RhoB and Rho6 (Table 1), each of which is prenylated (Nancy et al., 2002; Hanzal-Bayer et al., 2002). These experiments established PrBP/δ as a promiscuous prenyl-binding protein capable of interacting with multiple prenylated proteins. The physiological significance of most GTPase interaction with PrBP/δ remains unknown. Yeast two-hybrid screens indicate that several prenylated proteins do not interact with PrBP/δ, suggesting that specificity is mediated in part by protein-protein interactions. Examples of non-interacting prenylated GTPases include Rala, Ralb, and Rab6 (Nancy et al., 2002), as well as Arf1, Arf6, Arl6, Rac1, Rab1, Rab2, Rab7, and Ran (Hanzal-Bayer et al., 2002).
PrBP/δ-interacting Ras GTPases are oncogenes which, in normal cells, act as a switch to signal cell growth when cell surface receptors are stimulated by hormones or other agents. When HRAS is “switched on,” it signals the cell to grow, but when “switched off,” the cell is dormant. HRAS is an important oncogene as it is mutated in approximately one third of human cancers causing unchecked cell growth. Such a mutation may not, in and of itself, be enough to cause a cell to become cancerous as mutations in other genes may also be required. Rheb (Ras homolog enriched in brain), a novel, highly conserved member of the Ras superfamily of G-proteins, is vital in regulation of growth and cell cycle progression and was recently co-crystallized with PrBP/δ (Ismail et al., 2011) (see below). Rho6 (Rho-related GTP-binding protein 6) lacks intrinsic GTPase activity and constitutively binds GTP. Rho6 also controls rearrangements of the actin cytoskeleton. In general, the Rho family of small GTPases regulate the actin cytoskeleton in various cell types (Etienne-Manneville and Hall, 2002). Like other GTPases of the Ras superfamily, RhoGTPases serve as molecular switches by cycling between GDP- and GTP-bound states. In the GTP-bound state, interaction with effectors leads to a variety of biological functions. The physiological role of PrBP/δ interacting with Rho and Ras GTPases remains unknown; most likely PrBP/δ acts as a GDI-like solubilizing factor contributing to Ras and Rho signaling in cells (Chandra et al., 2012).
5. Interaction of Pde6d with non-prenylated proteins
Yeast two-hybrid screens have shown that PrBP/δ interacts with the RCC1-like domain of RPGR (Becker et al., 1998; Linari et al., 1999b). RCC1 is a GEF for the small GTP-binding protein Ran, which helps to control transport between the nucleus and cytoplasm (Clarke and Zhang, 2008). The interaction between RPGR and PrBP/δ was confirmed by both pull-down assays (Li and Baehr, 1998) and surface plasmon resonance experiments (Linari et al., 1999b). Oddly, the C-terminal region of RPGR which carries a CAAX box motif did not interact with PrBP/δ suggesting that RPGR may lack posttranslational prenylation (Linari et al., 1999b). Missense mutations in RPGR linked to X-linked Retinitis pigmentosa 3 (RP3) showed reduced interaction with PrBP/δ, suggesting that RPGR mutations may give rise to retinal degeneration via the dysregulation of intracellular protein localization and transport. PrBP/δ also interacts with the Arf-like proteins, Arl2 and Arl3, in GTP-specific manner (Linari et al., 1999a) (Renault et al., 2001). Closely-related, Arl2 and Arl3 are neither myristoylated nor prenylated (Hanzal-Bayer et al., 2002). This interaction was verified by co-crystallization (Hanzal-Bayer et al., 2002; Renault et al., 2001), fluorescence spectroscopy and co-immunoprecipitation (Hanzal-Bayer et al., 2005).
6. Structure of the PrBP/δ-Arl2-GTP complex
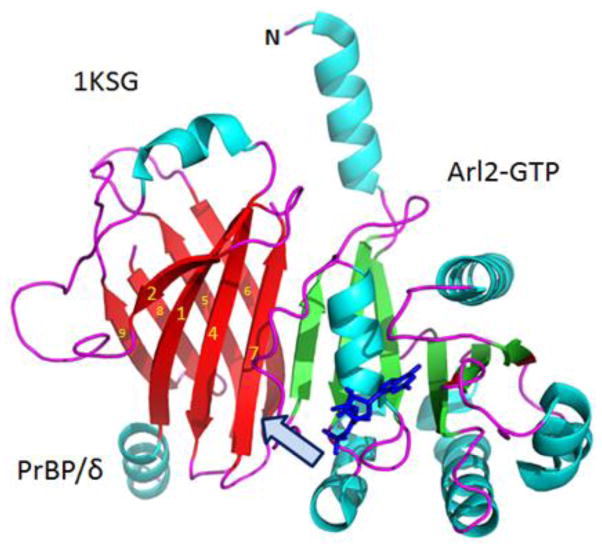
The 2.3 Å co-crystal structure of PrBP/δ and Arl2-GTP was a significant breakthrough in resolving how PrBP/δ interacts with both prenylated and nonprenylated proteins (Fig. 5) (Renault et al., 2001; Hanzal-Bayer et al., 2002). The complex crystallized in two crystal forms (Renault et al., 2001) (Protein Data Bank codes 1KSG, 1KSH and 1KSJ): form-1 grew within days and form-2 crystallized over the course of several months to 1 year (Renault et al., 2001; Hanzal-Bayer et al., 2002). Form-1 contained Arl2-GTP, whereas form-2 contained partially hydrolyzed GTP (GDP and phosphate). The Arl2 structure in both forms is very similar exhibiting no significant structural differences. The PrBP/δ structure (Figs. 5, 6) contains an immunoglobulin-like β-sandwich fold comprised of two β-sheets forming a hydrophobic pocket. One sheet is formed by strands β1, β2, β4 and β7, while the other is formed by β3, β5, β6, β8 and β9. The N-terminal region forms an α-helix (α1) and the loop connecting β7 and β8 is disordered. The interface between Arl2-GTP and PrBP/δ is formed by β-sheet interactions involving β2 from Arl2 and β7 from PrBP/δ. The immunoglobulin-like β-sandwich fold of PrBP/δ is closely related to RhoGDI (Hoffman et al., 2000) and UNC119A (Zhang et al., 2011) despite the sequence similarity between these polypeptides being relatively low. The major structural differences between these three proteins consist of the length and structure of the loops connecting β-sheets and the N-terminal regions (Hanzal-Bayer et al., 2002). In contrast to RhoGDI and PrBP/δ, UNC119A is an acyl-binding protein with specificity for lauroylated and myristoylated N-termini of G-protein α-subunits (Zhang et al., 2011).
Figure 5.
Ribbon representation of the PrBP/δ-Arl2/GTP complex (PDB 1KSH). GTP (shown as sticks in dark blue) is bound to Arl2. Arl2 blocks the entrance (arrow) through which the lipid side chain of prenylated proteins may insert. Figure was created with PyMOL (www.pymol.org).
Figure 6.
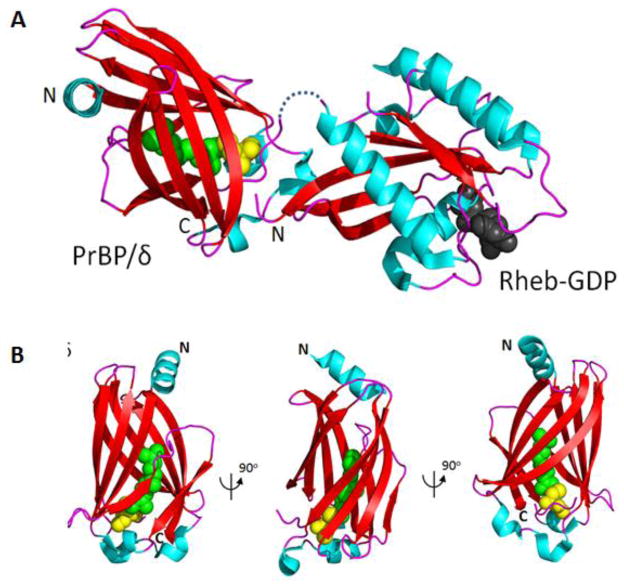
Structure of the Rheb(GDP)-PrBP/δ complex. Top, structure modified from Ismail, et al. (PDB 3T5I). The C-terminal farnesyl chain of Rheb (green) is inserted into the β-sandwich structure of PrBP/δ. GDP (dark gray) of Rheb is shown. Bottom, the structure of PrBP/δ with inserted farnesyl (green). Cys (yellow) of Rheb is shown. The middle and right structures were generated by 90° counterclockwise rotation.
7. The structure of the Rheb(GDP)-PrBP/δ complex
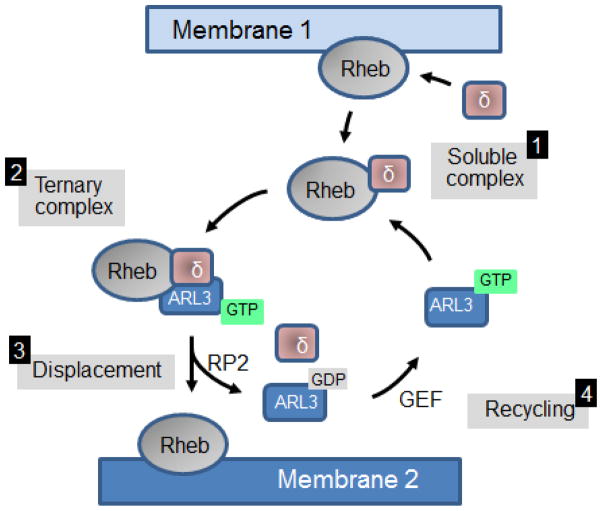
Rheb (Ras homolog enriched in brain), a novel, highly conserved member of the Ras superfamily of G-proteins, regulates mTORC1 (mammalian Target Of Rapamycin complex 1), which in turn regulates cell growth and proliferation (Avruch et al., 2006; Aspuria and Tamanoi, 2004; Ismail et al., 2011). The 1.7-Å structure of PrBP/δ in complex with farnesylated Rheb was solved by model building with a non-prenylated truncated Rheb and the crystallization of PrBP/δ with a C-terminal prenylated octopeptide (Ismail et al., 2011). The structure shows the farnesyl group of Rheb buried deeply in the hydrophobic pocket of PrBP/δ, an interaction that is independent of the nucleotide status of Rheb (Ismail et al., 2011). PrBP/δ and the switch regions of Rheb do not interact. Importantly, the interaction of Rheb with PrBP/δ is regulated allosterically by Arl2 and Arl3 in a GTP-dependent manner, establishing that GTP-bound Arl proteins act as GDI displacement factors (GDFs). In general, GDFs function by helping to displace small prenylated G proteins (or GRK1, PDEα′) from their lipid binding proteins, preventing further interaction by stabilizing the “closed” form of the GDI (or PrBP/δ, or UNC119A) (Fig. 7).
Figure 7.
ARL3-GTP functions as a GDF (GDI-displacement factor). Top, Step1: Rheb is extracted from membrane 1 by PrBP/δ, Step 2: an intermediate ternary complex is formed with ARL3-GTP. Step 3: Rheb is displaced to membrane 2 by ARL3-GTP acting as a GDF. The cycle is completed by the ARL3 GAP, RP2, which produces ARL3-GDP and free PrBP/δ.
8. Deletion of PrBP/δ in mouse produces retinal degeneration
At least six prenylated proteins are involved in mammalian phototransduction (Table 1): three catalytic subunits of PDE6 (α, β in rods and α′ in cones), two G protein-coupled receptor kinases (GRK1 and GRK7), and the rod and cone γ subunits of transducin. PDE6α, GRK1, and both Tγ subunits are farnesylated, while PDE6β and GRK7 are geranylgeranylated. These polypeptides are synthesized in the cytosol, and posttranslationally prenylated by soluble prenyl transferases. Prenylated proteins dock to the ER surface, where further processing occurs (i.e., proteolytic cleavage of -AAX of the CAAX box and carboxymethylation of Cys) ) (Hannoush and Sun, 2010; Christiansen et al., 2011). Following ER processing, GRK1 and PDE6 must be targeted to outer segment disk membranes to participate in phototransduction.
Pde6d gene deletion resulted in a viable adult mouse that developed normally and was fertile, but exhibited a significantly reduced body size early in life (Zhang et al., 2007). Phenotypically the Pde6d−/− mouse primarily exhibits transport deficiencies of a subset of membrane-associated proteins (Figs. 8, 9). In particular, defects are seen in transporting GRK1 to rod and cone outer segments (COS) and in cone PDE6 to COS, which resulted in anomalous photoreceptor physiology. In Pde6d−/− rod single-cell recordings, sensitivity to single photons was increased (Zhang et al., 2007). Further, double-flash electroretinograms indicated a delay of more than 20 minutes in recovery to the dark state in Pde6d−/− rods, which is likely due to severely reduced levels of GRK1 in rod outer segments (Zhang et al., 2007).
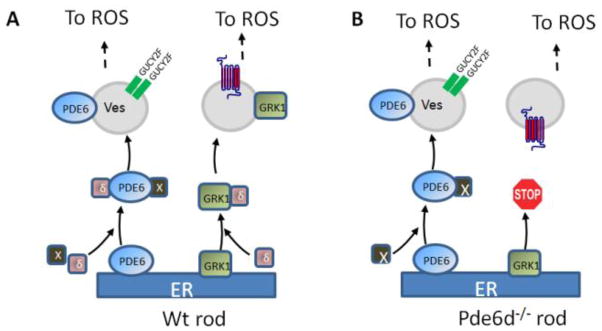
Figure 8.
PrBP/δ-dependent export of PDE6 and GRK1 from the ER in rods. A, GRK1 and PDE6αβ dock to the ER after prenylation and processing. Prenylated proteins may be extracted from the ER by PrBP/δ (δ, purple) forming a diffusible complex. Additional prenyl binding proteins likely exist (X, in black). Transfer from ER (membrane 1) to the transport vesicle (membrane 2) is likely mediated by ARL2/3 functioning as a GDF, as outlined in Fig. 7. B, Deletion of PrBP/δ prevents GRK1 exit from the ER. With the help of X, PDE6 still travels to the OS although some PDE6 is retained and mislocalized in the inner segment (Zhang et al., 2007). This model is based on (Zhang et al., 2007; Baehr et al., 2007; Karan et al., 2008).
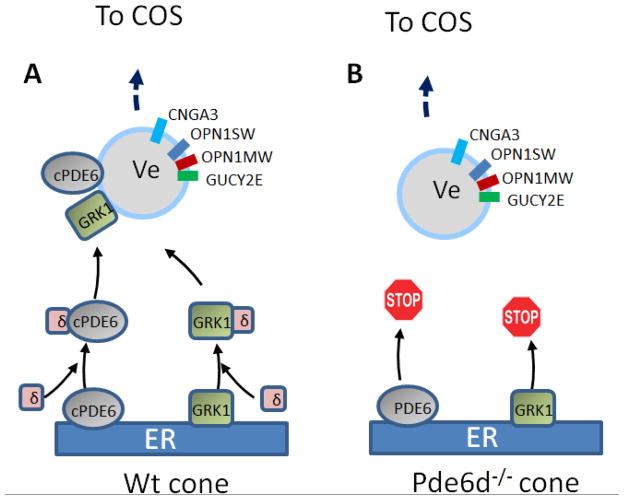
Figure 9.
PrBP/δ-dependent transport in cones. A, Processing of cone PDE6 and GRK1 in WT cones. Cone PDE6 consists of two identical geranylgeranylated subunits (PDEα′). Both are thought to interact with PrBP/δ. B, Deletion of PrBP/δ prevents trafficking of cone PDE6 subunits and GRK1 to the outer segments. Prenylated subunits, unable to exit the ER, are presumably degraded.
We hypothesized that PrBP/δ may be involved in extracting prenylated proteins from the ER surface (Figs. 8,9) and either delivering them to a post-TGN vesicular transport carrier in rods and cones (Zhang et al., 2007; Karan et al., 2008), or directly to ROS discs (Zhang et al., 2011). This process is likely regulated by GTP-bound Arl proteins, which presumably act in a GDF-like fashion. Curiously, rod PDE6 subunits were affected weakly and mislocalized only partially in the inner segments (Zhang et al., 2007), despite PrBP/δ being known to interact strongly with PDE6. One possible explanation for this phenotype is that PDE6 may use alternate trafficking pathways independent of PrBP/δ.
In contrast to Pde6d−/− rod outer segments, Pde6α′, predicted to be geranylgeranylated, was undetectable by immunofluorescence in Pde6d−/− cone outer segments (COS) (Zhang et al., 2007). Under photopic (bright light) ERG conditions, the Pde6d−/− cone response was diminished, which is consistent with reduced PDE6α′ levels in COS. Taken together, PrBP/δ deletion in photoreceptors results in defective transport of some prenylated proteins (PDE6 subunits and GRK1) to the outer segment. Transport defects varied, suggesting that additional unidentified prenyl binding proteins may substitute for PrBP/δ loss in photoreceptors. In Pde6−/− rods and cones, both the visual pigments and transducin trafficked, but rod Tγ mislocalized as some was retained in the inner segment (Zhang et al., 2007)
9. PrBP/δ expression in non-retina mouse tissues
According to the EST database, PrBP/δ is expressed in most mouse tissues, including RPE/choroid, brain, visual cortex, organ of Corti, placenta, lung, testicles, spermatids, bones, bone marrow, and tumor biopsy samples. Lung tissue expresses PDE1-5 at high levels, and while they were detected, PDE6 subunits including PrBP/δ were found at low levels in human alveolar epithelial cells (Nikolova et al., 2010). PDE6D mRNA, PrBP/δ protein levels, and PDE6γ protein levels were reduced in lungs affected by idiopathic pulmonary fibrosis (IPF), when compared with donor lungs, suggesting that these PDE6 subunits may contribute to IPF pathogenesis (Nikolova et al., 2010). Interestingly, both PDE5 and PDE6δ were pulled down from mouse lung using beads coated with the known PDE5 inhibitor, (N-(6-aminohexyl)-3-(1-ethyl-3-methyl-7-oxo-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-propoxybenzenesulfonamide) (PF-4540124), with very high specificity (Dadvar et al., 2009). PDE5 is closely related in sequence to photoreceptor PDE6α or PDE6β, but lacks the CAAX motif and is therefore not prenylated (thus excluding binding through prenyl side chains). Using recombinant PrBP/δ, it was demonstrated that PrBP/δ directly interacts with PF-4540124 with a Ka in the micromolar range, whereas the Ka of PDE5 to PF-4540124 is in the nanomolar range (Dadvar et al., 2009). The PrBP/δ domain that interacts with PF-4540124 is unknown, but the molecule is largely hydrophobic and may fit into the hydrophobic binding pocket. Despite these data, the precise function of PrBP/δ in mouse lung remains undefined.
10. PrBP/δ Expression in invertebrates
Drosophila melanogaster
PrBP/δ homologs are capable of interacting with non-retina PDEs through prenyl moieties. In Drosophila melanogaster, a homologue of both PDE5 and PDE6, termed DmPDE5/6, contains a C-terminal CAAX-box motif (CALL) (Day et al., 2008). DmPDE5/6 is expressed in the renal tubules of the fruit fly, but not in ommatidia. DmPDE5/6 is predicted to be geranylgeranylated with expression documented in the head and body. DmPDE5/6 associates with DmPrBP/δ, a 151 amino acid homolog with high sequence similarity (78%) to mammalian PrBP/δ (Zhang et al., 2007; Day et al., 2008). Association of DmPDE5/6 with DmPrBP/δ results in its translocation from the plasma membrane to the cytoplasm, a function identical to that of mammalian PrBP/δ.
C. elegans
A gene with an exon/intron arrangement very similar to PrBP/δ was identified in the eyeless nematode C. elegans as part of the genome sequencing project and was given the systematic identification tag C27H5.1 (Wilson et al., 1994). The gene product, termed PDL-1 (PDEdelta-like 1) (Wormbase at www.wormbase.org), is similar in sequence to PrBP/δ (Fig. 4) and also the C-terminal domain of neuron-specific unc-119 and mammalian UNC119A. Expression and characterization of PDL-1 demonstrated that it can elute PDE6 from ROS membranes (Fig. 4), a biological activity identical to PrBP/δ (Li and Baehr, 1998). Pull-downs with GST- PDL-1 showed that PDL-1 binds PDE6 subunits and the N-terminal of domain RPGR indistinguishably from PrBP/δ. Further, recent work has shown that mutations in PDL-1 affect gustatory plasticity in C. elegans (Hukema et al., 2006).
Highlights.
Ubiquitously-expressed PrBP/δ belongs to the UNC119 gene family
PrBP/δ forms an immunoglobulin-like β-sandwich fold into which prenyl chains may insert
PrBP/δ interacts with a subset of prenylated G proteins of the ras gene family
PrBP/δ controls trafficking of GRK1 and PDE6 to photoreceptor outer segments
PrBP/δ deletion in mouse causes recessive cone/rod dystrophy
Acknowledgments
This work was supported by National Institute of Health grants EY08123, EY019298 (WB), EY014800-039003 (NEI core grant), by a Center Grant of the Foundation Fighting Blindness, Inc., to the University of Utah, and unrestricted grants to the Departments of Ophthalmology at the University of Utah from Research to Prevent Blindness (RPB; New York). WB is a recipient of a Research to Prevent Blindness Senior Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Anant JS, Ong OC, Xie H, Clarke S, O’Brien PJ, Fung BKK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. [PubMed] [Google Scholar]
- Aspuria PJ, Tamanoi F. The Rheb family of GTP-binding proteins. Cell Signal. 2004;16:1105–1112. doi: 10.1016/j.cellsig.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- Baehr W, Devlin MJ, Applebury ML. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979;254:11669–11677. [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Linari M, Manson F, Wright A, Meindl A, Meitinger T. The delta subunit of rod phosphodiesterase interacts with the RCC1 homologpus domain of RPGR. Invest Ophthalmol & Vis Sci. 1998;39:S953. [Google Scholar]
- Bergo MO, Ambroziak P, Gregory C, George A, Otto JC, Kim E, Nagase H, Casey PJ, Balmain A, Young SG. Absence of the CAAX endoprotease Rce1: effects on cell growth and transformation. Mol Cell Biol. 2002;22:171–181. doi: 10.1128/MCB.22.1.171-181.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem. 2001;276:5841–5845. doi: 10.1074/jbc.C000831200. [DOI] [PubMed] [Google Scholar]
- Chandra A, Grecco HE, Pisupati V, Perera D, Cassidy L, Skoulidis F, Ismail SA, Hedberg C, Hanzal-Bayer M, Venkitaraman AR, et al. The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nat Cell Biol. 2012;14:148–158. doi: 10.1038/ncb2394. [DOI] [PubMed] [Google Scholar]
- Christiansen JR, Kolandaivelu S, Bergo MO, Ramamurthy V. RAS-converting enzyme 1-mediated endoproteolysis is required for trafficking of rod phosphodiesterase 6 to photoreceptor outer segments. Proc Natl Acad Sci U S A. 2011;108:8862–8866. doi: 10.1073/pnas.1103627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol. 2008;9:464–477. doi: 10.1038/nrm2410. [DOI] [PubMed] [Google Scholar]
- Constantine R, Zhang H, Gerstner CD, Frederickson RM, Baehr W. UNC119A and UNC119B: Novel lipid binding proteinsinvolved in trafficking of myristoylated proteins. Vision REs. 2012 doi: 10.1016/j.visres.2012.08.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Cook TA, Ghomashchi F, Gelb MH, Florio SK, Beavo JA. Binding of the delta subunit to rod phosphodiesterase catalytic subunits requires methylated, prenylated C-termini of the catalytic subunits. Biochemistry. 2000;39:13516–13523. doi: 10.1021/bi001070l. [DOI] [PubMed] [Google Scholar]
- Cook TA, Ghomashchi F, Gelb MH, Florio SK, Beavo JA. The delta subunit of type 6 phosphodiesterase reduces light-induced cGMP hydrolysis in rod outer segments. J Biol Chem. 2001;276:5248–5255. doi: 10.1074/jbc.M004690200. [DOI] [PubMed] [Google Scholar]
- Dadvar P, O’Flaherty M, Scholten A, Rumpel K, Heck AJ. A chemical proteomics based enrichment technique targeting the interactome of the PDE5 inhibitor PF-4540124. Mol Biosyst. 2009;5:472–482. doi: 10.1039/b815709j. [DOI] [PubMed] [Google Scholar]
- Day JP, Cleghon V, Houslay MD, Davies SA. Regulation of a Drosophila melanogaster cGMP-specific phosphodiesterase by prenylation and interaction with a prenyl-binding protein. Biochem J. 2008;414:363–374. doi: 10.1042/BJ20080560. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Florio SK, Prusti RK, Beavo JA. Solubilization of membrane-bound rod phosphodiesterase by the rod phosphodiesterase delta subunit. J Biol Chem. 1996;271:24036–24047. doi: 10.1074/jbc.271.39.24036. [DOI] [PubMed] [Google Scholar]
- Fung BKK, Young JH, Yamane HK, Griswold-Prenner I. Subunit stoichiometry of retinal rod cGMP phosphodiesterase. Biochemistry. 1990;29:2657–2664. doi: 10.1021/bi00463a006. [DOI] [PubMed] [Google Scholar]
- Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H. Therapeutic intervention based on protein prenylation and associated modifications. Nat Chem Biol. 2006;2:518–528. doi: 10.1038/nchembio818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Prusti RK, Apel ED, Beavo JA. A soluble form of bovine rod photoreceptor phosphodiesterase has a novel 15-kDa subunit. J Biol Chem. 1989;264:12187–12193. [PubMed] [Google Scholar]
- Goc A, Chami M, Lodowski DT, Bosshart P, Moiseenkova-Bell V, Baehr W, Engel A, Palczewski K. Structural characterization of the rod cGMP phosphodiesterase 6. J Mol Biol. 2010;401:363–373. doi: 10.1016/j.jmb.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannoush RN, Sun J. The chemical toolbox for monitoring protein fatty acylation and prenylation. Nat Chem Biol. 2010;6:498–506. doi: 10.1038/nchembio.388. [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer M, Linari M, Wittinghofer A. Properties of the interaction of Arf-like protein 2 with PDEdelta. J Mol Biol. 2005;350:1074–1082. doi: 10.1016/j.jmb.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer M, Renault L, Roversi P, Wittinghofer A, Hillig RC. The complex of Arl2-GTP and PDE delta: from structure to function. EMBO J. 2002;21:2095–2106. doi: 10.1093/emboj/21.9.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YS, Burden LM, Hurley JH. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor. EMBO J. 2000;19:5288–5299. doi: 10.1093/emboj/19.20.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- Hukema RK, Rademakers S, Dekkers MP, Burghoorn J, Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 2006;25:312–322. doi: 10.1038/sj.emboj.7600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail SA, Chen YX, Rusinova A, Chandra A, Bierbaum M, Gremer L, Triola G, Waldmann H, Bastiaens PI, Wittinghofer A. Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat Chem Biol. 2011;7:942–949. doi: 10.1038/nchembio.686. [DOI] [PubMed] [Google Scholar]
- Karan S, Zhang H, Li S, Frederick JM, Baehr W. A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 2008;48:442–452. doi: 10.1016/j.visres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Baehr W. Expression and characterization of human PDEδ and its Caenorhabditis elegans ortholog CEδ. FEBS Lett. 1998;440:454–457. doi: 10.1016/s0014-5793(98)01501-4. [DOI] [PubMed] [Google Scholar]
- Li N, Florio SK, Pettenati MJ, Rao PN, Beavo JA, Baehr W. Characterization of human and mouse rod cGMP phosphodiesterase delta subunit (PDE6D) and chromosomal localization of the human gene. Genomics. 1998;49:76–82. doi: 10.1006/geno.1998.5210. [DOI] [PubMed] [Google Scholar]
- Linari M, Hanzal-Bayer M, Becker J. The delta subunit of rod specific cyclic GMP phosphodiesterase, PDE delta, interacts with the Arf-like protein Arl3 in a GTP specific manner. FEBS Lett. 1999a;458:55–59. doi: 10.1016/s0014-5793(99)01117-5. [DOI] [PubMed] [Google Scholar]
- Linari M, Ueffing M, Manson F, Wright A, Meitinger T, Becker J. The retinitis pigmentosa GTPase regulator, RPGR, interacts with the delta subunit of rod cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1999b;96:1315–1320. doi: 10.1073/pnas.96.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T, Seabra MC. Fatty acylation and prenylation of proteins: what’s hot in fat. Curr Opin Cell Biol. 2005;17:190–196. doi: 10.1016/j.ceb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Martinez SE, Heikaus CC, Klevit RE, Beavo JA. The structure of the GAF A domain from phosphodiesterase 6C reveals determinants of cGMP binding, a conserved binding surface, and a large cGMP-dependent conformational change. J Biol Chem. 2008;283:25913–25919. doi: 10.1074/jbc.M802891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzesco AM, Galli T, Louvard D, Zahraoui A. The rod cGMP phosphodiesterase delta subunit dissociates the small GTPase Rab13 from membranes. J Biol Chem. 1998;273:22340–22345. doi: 10.1074/jbc.273.35.22340. [DOI] [PubMed] [Google Scholar]
- McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki N, Baraban JM, Keirns JJ, Boyce JJ, Bitensky MW. Purification and Properties of the Light-activated Cyclic Nucleotide Phosphodiesterase of Rod Outer Segments. J Biol Chem. 1975;250:6320–6327. [PubMed] [Google Scholar]
- Nancy V, Callebaut I, El Marjou A, de Gunzburg J. The delta subunit of retinal rod cGMP phosphodiesterase regulates the membrane association of Ras and Rap GTPases. J Biol Chem. 2002;277:15076–15084. doi: 10.1074/jbc.M109983200. [DOI] [PubMed] [Google Scholar]
- Nguyen UT, Goody RS, Alexandrov K. Understanding and exploiting protein prenyltransferases. Chembiochem. 2010a;11:1194–1201. doi: 10.1002/cbic.200900727. [DOI] [PubMed] [Google Scholar]
- Nguyen UT, Wu Y, Goodall A, Alexandrov K. Analysis of protein prenylation in vitro and in vivo using functionalized phosphoisoprenoids. Curr Protoc Protein Sci. 2010b;Chapter 14(Unit14) doi: 10.1002/0471140864.ps1403s62. [DOI] [PubMed] [Google Scholar]
- Nikolova S, Guenther A, Savai R, Weissmann N, Ghofrani HA, Konigshoff M, Eickelberg O, Klepetko W, Voswinckel R, Seeger W, et al. Phosphodiesterase 6 subunits are expressed and altered in idiopathic pulmonary fibrosis. Respir Res. 2010;11:146. doi: 10.1186/1465-9921-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton AW, Hosier S, Terew JM, Li N, Dhingra A, Vardi N, Baehr W, Cote RH. Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors. J Biol Chem. 2005;280:1248–1256. doi: 10.1074/jbc.M410475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault L, Hanzal-Bayer M, Hillig RC. Coexpression, copurification, crystallization and preliminary X-ray analysis of a complex of ARL2-GTP and PDE delta. Acta Crystallogr D Biol Crystallogr. 2001;57:1167–1170. doi: 10.1107/s0907444901009556. [DOI] [PubMed] [Google Scholar]
- Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, et al. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans [see comments] Nature. 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Smyth EM. Internalization and Recycling of the Human Prostacyclin Receptor Is Modulated through Its Isoprenylation-dependent Interaction with the {delta} Subunit of cGMP Phosphodiesterase 6. J Biol Chem. 2006;281:11780–11786. doi: 10.1074/jbc.M513110200. [DOI] [PubMed] [Google Scholar]
- Winter-Vann AM, Casey PJ. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer. 2005;5:405–412. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, et al. UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci. 2011;14:874–880. doi: 10.1038/nn.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li S, Doan T, Rieke F, Detwiler PB, Frederick JM, Baehr W. Deletion of PrBP/{delta} impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci U S A. 2007;104:8857–8862. doi: 10.1073/pnas.0701681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu XH, Zhang K, Chen CK, Frederick JM, Prestwich GD, Baehr W. Photoreceptor cGMP phosphodiesterase delta subunit (PDEdelta) functions as a prenyl-binding protein. J Biol Chem. 2004;279:407–413. doi: 10.1074/jbc.M306559200. [DOI] [PubMed] [Google Scholar]