Abstract
The oscillator of the circadian clock of cyanobacteria is composed of three proteins, KaiA, KaiB, and KaiC, which together generate a self-sustained ∼24-h rhythm of phosphorylation of KaiC. The mechanism propelling this oscillator has remained elusive, however. We show that stacking interactions between the CI and CII rings of KaiC drive the transition from the phosphorylation-specific KaiC–KaiA interaction to the dephosphorylation-specific KaiC–KaiB interaction. We have identified the KaiB-binding site, which is on the CI domain. This site is hidden when CI domains are associated as a hexameric ring. However, stacking of the CI and CII rings exposes the KaiB-binding site. Because the clock output protein SasA also binds to CI and competes with KaiB for binding, ring stacking likely regulates clock output. We demonstrate that ADP can expose the KaiB-binding site in the absence of ring stacking, providing an explanation for how it can reset the clock.
Keywords: dynamic allostery, NMR, protein structure, kinase, kinetics
Organisms exhibit circadian rhythms across multiple biological scales in anticipation of daily swings in ambient light and temperature (1). The oscillators of circadian clocks generate these rhythms. In cyanobacteria, the oscillator is composed of only three proteins, KaiA, KaiB, and KaiC (2). Through temporally separated interactions with KaiA and KaiB, KaiC executes ∼24-h cycles of (auto)phosphorylation and (auto)dephosphorylation (3, 4): ST → SpT → pSpT → pST → ST →..., where S/pS and T/pT are the unphosphorylated/phosphorylated states of residues S431 and T432, respectively (5, 6).
KaiC is a homohexamer composed of two rings, CI and CII, with S431 and T432 residues residing in the CII ring (7). During the subjective day, KaiA stimulates KaiC phosphorylation (8, 9) by binding to the A loops on the CII ring (10, 11). In the subjective evening, KaiB binds to KaiC upon phosphorylation of the S431 residues and inactivates KaiA (5, 6, 12, 13). Models show KaiB binding to the CII ring (13, 14). The ensuing dephosphorylation phase completes one ∼24-h cycle. The clock output protein SasA transduces this biochemical rhythm downstream (15–17), by binding to the CI domains of KaiC (18). The oscillator can be reset by ADP during the phosphorylation phase but not during the dephosphorylation phase (19). This observation explained why dark pulses could reset the circadian rhythms of entrained cyanobacteria under constant light during the subjective day, but not during the subjective night (19).
Despite these advances, many significant questions remain; for example: What determines the temporal pattern of phosphorylation and dephosphorylation? Does the CI ring play a role in generating the circadian rhythm of phosphorylation? Why does ADP have an effect only during the phosphorylation phase? There are also some inconsistencies in the literature. For instance, phosphorylation of the S431 residues and KaiC–KaiB binding have been proposed to expand the CII ring (20, 21); however, that notion is not supported by the observation that on S431 phosphorylation, breathing motions of the CII ring cease (18), suggesting ring contraction. Likewise, it is not clear how KaiB and SasA can compete for KaiC binding (13, 22) when their respective binding sites are supposedly on opposite sides of KaiC.
Here we provide answers to these questions and resolve the inconsistencies by investigating the clock proteins of the cyanobacterium Thermosynechococcus elongatus (23). Specifically, we demonstrate that the CI and CII rings of KaiC work together to drive the oscillator clockwise. We also show that KaiB, like SasA, binds to the CI domains of KaiC. Interestingly, either CI-CII ring stacking or ADP is necessary to expose the KaiB-binding site, presumably by partially separating the subunits of the CI ring.
Results
Ring Stacking in KaiC Governs KaiC–KaiB Binding.
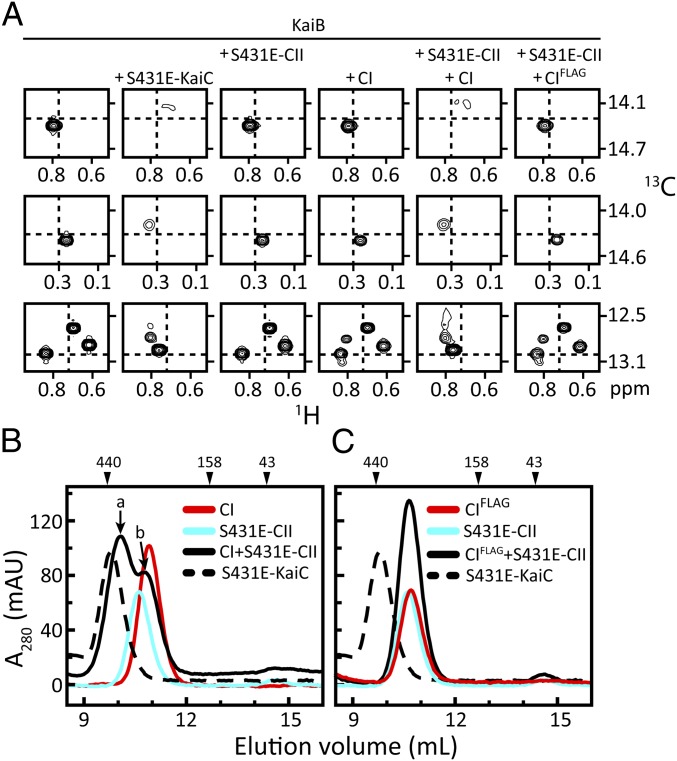
Formation of the KaiC–KaiB complex initiates the transition from KaiA-stimulated phosphorylation to KaiA-inactivated dephosphorylation of KaiC (24). KaiB binds KaiC upon tightening of the CII ring, which occurs on phosphorylation of the S431 residues (18). The molecular details of KaiC–KaiB binding remain incompletely understood, however. Formation of this supramolecular complex (20) can be monitored by NMR spectroscopy (25–27), because it induces significant perturbations in the chemical shifts of the methyl resonances of U-[15N, 2H]-Ile-δ1-[13C, 1H]–labeled KaiB (Fig. 1A, column 1) on the addition of S431E-KaiC, a phosphomimic of pST-KaiC (Fig. 1A, column 2). To gain further insight into the mechanism of the KaiC–KaiB interaction, we tested the isolated CI and CII domains for KaiB binding. There were no chemical shift perturbations in the presence of S431E-CII (Fig. 1A, column 3), and only minor perturbations in the presence of CI (Fig. 1A, column 4). Details of the different protein constructs are provided in SI Appendix, Table S1.
Fig. 1.
Stacking of CI and CII rings of KaiC is required for KaiC–KaiB binding. (A) Selected regions from methyl-TROSY spectra (26, 27) of U-[15N, 2H]-Ile-δ1-[13C, 1H]–labeled KaiB alone (column 1) or in the presence of S431E-KaiC (column 2), S431E-CII (column 3), CI (column 4), S431E-CII and CI (column 5), or S431E-CII and CIFLAG (column 6). Complete spectra are shown in SI Appendix, Fig. S1. (B and C) Gel-filtration profiles of mixtures of CI (B) and CIFLAG (C) with S431E-CII. Full-length S431E-KaiC was run as a control. Peaks denoted by a and b were checked by SDS/PAGE (SI Appendix, Fig. S2). The additional FLAG tag at the C terminus of CIFLAG was included to maximize potential repulsive interactions with the N-terminal FLAG tag on isolated S431E-CII domains. The same runs of S431E-CII and S431E-KaiC are shown in B and C. Details on the protein constructs are provided in SI Appendix, Table S1.
These observations were unexpected, given that isolated S431E-CII and CI domains, like the full-length protein, form stable hexameric rings (18). Gel-filtration experiments also showed no evidence of KaiB binding to the rings of either CI or S431E-CII (SI Appendix, Fig. S3); however, on addition of a 1:1 molar ratio of CI and S431E-CII (Fig. 1A, column 5), KaiB adopted the same chemical shifts as it did in the presence of S431E-KaiC. Gel-filtration chromatography indicated that the CI + S431E-CII mixture formed a complex of similar size as S431E-KaiC (Fig. 1B). The CI and S431E-CII rings likely stacked together, as was observed in S431E-KaiC and S431E-T432E-KaiC (18). KaiB binding was abolished (Fig. 1A, column 6) when the stacking interaction between CI and S431E-CII was weakened by adding an extra FLAG tag to CI (Fig. 1C). Likewise, we recently showed that KaiB did not bind to forms of KaiC with CI and CII domains that did not stack together (i.e., the unstacked state) (18). Collectively, these results support the notion that CI-CII ring stacking is necessary for KaiC–KaiB complex formation.
KaiB Binds to CI.
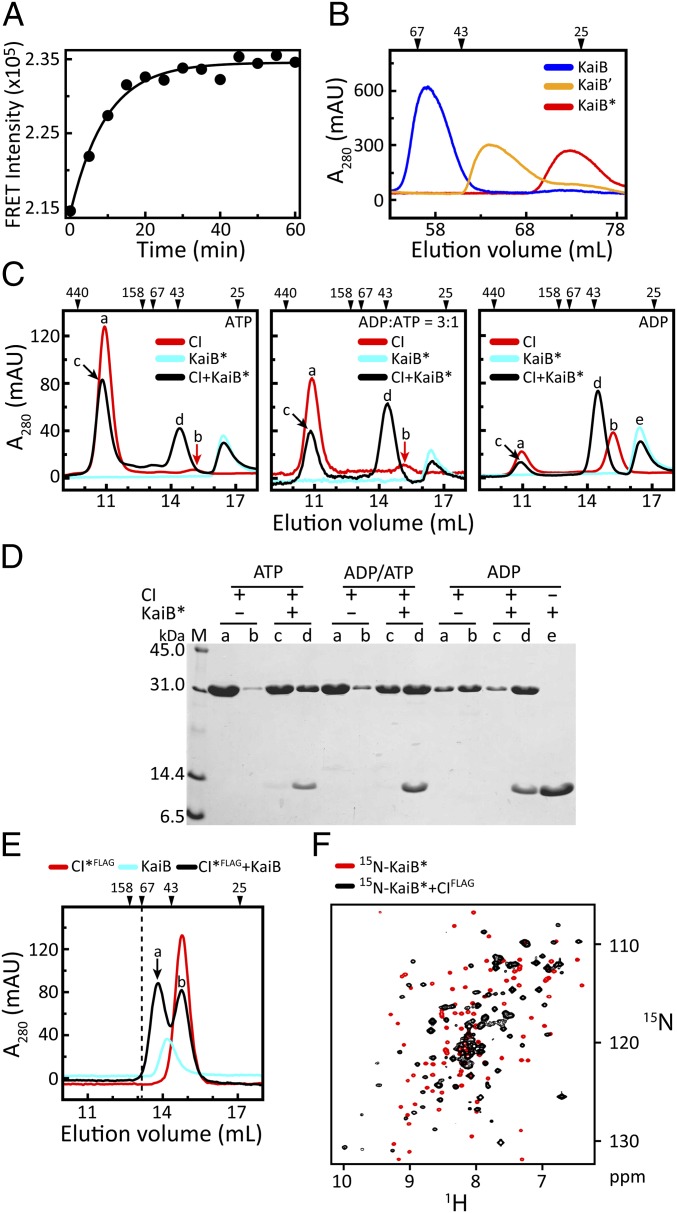
The presence of small changes in the NMR spectra of KaiB in the presence of CI, but not of CII, was unanticipated, given recent reports suggesting that for homologous proteins from Synechococcus elongatus, KaiB (88% identical) bound to the CII side (82% identical) of KaiC (13, 14). This discrepancy prompted us to reinvestigate the KaiB-binding site on KaiC. We wished to test KaiB binding to isolated CI and CII domains, so that our results would be unambiguous; however, because KaiC–KaiB binding requires the presence of both CI and CII (Fig. 1), we needed to engineer a variant of KaiB that would bind tightly, even in the absence of ring stacking. Because models suggest that KaiB binds as a dimer to KaiC (20) but by itself exists as a dimer of dimers (28), we reasoned that binding could be enhanced by destabilizing the tetramer to favor the dimer. Fluorescence experiments revealed that KaiB exists in a dynamic equilibrium (Fig. 2A), most likely between the dominant tetrameric form and a sparsely populated dimeric form; thus, we designed mutants of KaiB that progressively favored the dimeric form (Fig. 2B). Truncating the protein after residue Y94 (22) and introducing two amino acyl substitutions, Y8A and Y94A, produced the dimeric mutant KaiB*.
Fig. 2.
KaiB binds to monomeric CI. (A) FRET measurements of KaiB subunit exchange after mixing two samples of KaiB separately labeled with the donor and acceptor fluorophores 1,5-IAEDANS and 6-IAF. Excitation and emission wavelengths were set to 347 nm and 530 nm, respectively. (B) Gel-filtration profiles of KaiB and KaiB variants KaiB′ and KaiB* (SI Appendix, Table S1). KaiB′ differs from KaiB by two amino acyl substitutions, Y8A and Y94A. KaiB* differs from KaiB′ by being truncated after residue Y94A. (C) Gel-filtration profiles of mixtures of CI and KaiB* in the presence of [ATP] = 0.25 mM (Left), [ADP]/[ATP] = 0.75 mM/0.25 mM (Middle), and [ADP] = 0.25 mM (Right). Peaks denoted by a, b, c, d, and e were checked by SDS/PAGE. For clarity, a red arrow indicates peak b. (D) SDS/PAGE gel of peaks a, b, c, d, and e in C. (E) Gel-filtration profile of a mixture of a monomeric variant CI*FLAG (SI Appendix, Table S1) with KaiB. Peaks denoted by a and b were checked by SDS/PAGE (SI Appendix, Fig. S6). The dashed line indicates the position of a 67-kDa marker. The size of a complex of CI* and a KaiB tetramer would exceed 67 kDa. (F) 15N, 1H-HSQC spectra of 15N-labeled KaiB* free (red contours) and bound (black contours) to CIFLAG. The NMR spectra were recorded at 37 °C (SI Appendix, Table S2). Note that CIFLAG exists as a monomer in an ATP-free buffer (SI Appendix, Fig. S5). Details on the purification of these proteins are provided in SI Appendix, Table S3.
Gel-filtration chromatography and NMR experiments showed that KaiB* did not form detectable complexes with S431E-CII, but did form stable complexes with CI (SI Appendix, Figs. S4 and S5). The finding that KaiB* bound to CI was surprising, given that current models show KaiB binding to the CII side of KaiC (13, 14). We think that the incorrect assignment resulted from the difficulty in distinguishing the rings of KaiC by EM and small-angle X-ray scattering.
Isolated CI domains form hexameric rings with ATP molecules bound at the interfaces between the subunits (7, 29). We anticipated that KaiB* would bind to these rings, but instead, smaller complexes formed, consistent with one KaiB* and one CI subunit (Figs. 2 C and D). Binding of KaiB* to CI could be promoted by increasing the ADP/ATP ratio, which shifts CI from a hexameric ring to disassociated monomers (29). In contrast to KaiB*, KaiB did not form a 1:1 complex with a CI subunit when CIs were associated as rings (SI Appendix, Fig. S3A); however, KaiB did bind to a monomeric variant, CI*FLAG (Fig. 2E). An NMR spectrum of a mixture of 15N-labeled KaiB* and CIFLAG (Fig. 2F) suggests that the interaction is specific. These results support the notion that the ring structure hides, at least partially, the KaiB-binding site, and that the site can be exposed by disassociating the CI subunits.
Phosphomimics of pSpT-KaiC experience significantly higher rates of subunit exchange compared with unphosphorylated KaiC (30, 31). Our results suggest that the higher rates of exchange are promoted by stacking-induced disjoining of the ring of CI, as in pSpT-KaiC. Recall that stacking occurs when the CII ring is tight, as in the pST and pSpT phosphoforms of KaiC, but not when the CII ring is undergoing breathing motions, as in the ST and SpT phosphoforms (18). Because the CII ring is tightest for pST-KaiC (18), we expect that the rate of subunit exchange for pST-KaiC is slower than that for pSpT-KaiC.
The finding that KaiB binds to CI when CI is monomeric or the rings of KaiC are stacked supports the idea that stacking exposes the KaiB-binding site. We think that when the CII ring stacks on the CI ring, it wedges the CI domains apart to some extent, thereby exposing the KaiB-binding site. Now that we have established that both KaiB and SasA (18) bind to CI, we think that they compete (13, 22) for partially overlapping binding sites. We also expect that the competition is regulated by ring stacking.
KaiB Sequesters KaiA on CI.
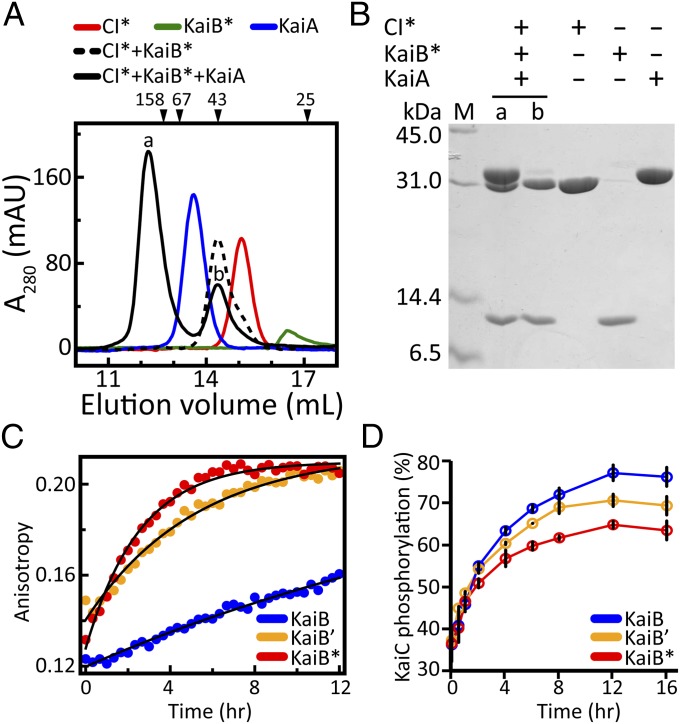
On binding to KaiC, KaiB sequesters KaiA, thereby terminating the phosphorylation phase and inducing the dephosphorylation phase (6, 12, 32). Therefore, a critical test of the functional importance of the complex between KaiB and CI is whether it can bind KaiA. Gel-filtration chromatography indicated that adding KaiA to a solution of CI*–KaiB* complexes produced a larger complex (Fig. 3A) in which all three proteins were present (Fig. 3B). In the absence of KaiB*, KaiA did not form a complex with CI* (SI Appendix, Fig. S7 A and B). Likewise, KaiA and KaiB* in the absence of CI* did not form a stable complex (SI Appendix, Fig. S7 C and D), which is consistent with other reports (33, 34).
Fig. 3.
Formation of the CI*–KaiB*–KaiA complex. (A) Gel-filtration profiles of CI* alone (red line), KaiB* alone (green line), KaiA alone (blue line), mixtures of CI* + KaiB* (dashed black line), and CI* + KaiB* + KaiA (solid black line). Elution profiles of mixtures of CI* + KaiA and KaiB* + KaiA are shown in SI Appendix, Fig. S7. (B) SDS/PAGE of peaks denoted by a and b in A. (C) Fluorescence anisotropy of the binding kinetics of 6-IAF–labeled KaiB and KaiB variants (KaiB′ or KaiB*) to S431E-KaiC (blue, KaiB; orange, KaiB′; red, KaiB*). (D) Phosphorylation kinetics profiles of KaiC in the presence of KaiA and KaiB or KaiB variants (KaiB′ or KaiB*). Color-coding is the same as in C. Each data point represents the average of two experiments. Error bars represent SEM. Values were determined from densitometric analysis of SDS/PAGE gels stained with Coomassie blue (SI Appendix, Fig. S8).
Based on these observations, we conclude that KaiB induces the transition from phosphorylation to dephosphorylation by recruiting KaiA to the CI side of KaiC. The unusually large changes in the chemical shifts of KaiB* on binding to the CI monomer (Fig. 2F) indicates that it adopted a significantly different conformation. It has been suggested that KaiB binds to KaiC as a dimer (20); if so, then the NMR signals of KaiB* would be expected to double, because its twofold symmetry would be broken on binding to the CI monomer. Peak doubling was not observed, however (Fig. 2F). Further experiments are needed to determine whether KaiB binds to KaiC as a dimer or perhaps as a monomer.
The rate of formation of the KaiC–KaiB complex is very slow (Fig. 3C). To gain insight into the significance of this slow formation, we measured the kinetics of binding and phosphorylation using KaiB variants. Destabilization of the KaiB tetramer to favor the dimer (Fig. 2B) enhanced the rate of formation of the KaiC–KaiB complex (Fig. 3C). More rapid binding was correlated with an attenuated amplitude of phosphorylation (Fig. 3D), likely owing to overly rapid sequestration of KaiA. Based on the faster binding observed for dimeric forms of KaiB, the tetramer ↔ dimer equilibrium might act to tune binding rates such that robust amplitudes can be generated. It should be noted that despite the relatively fast binding kinetics of KaiB* to a phosphomimic of KaiC (Fig. 3C), KaiB cannot bind to KaiC until the CI and CII rings stack.
Discussion
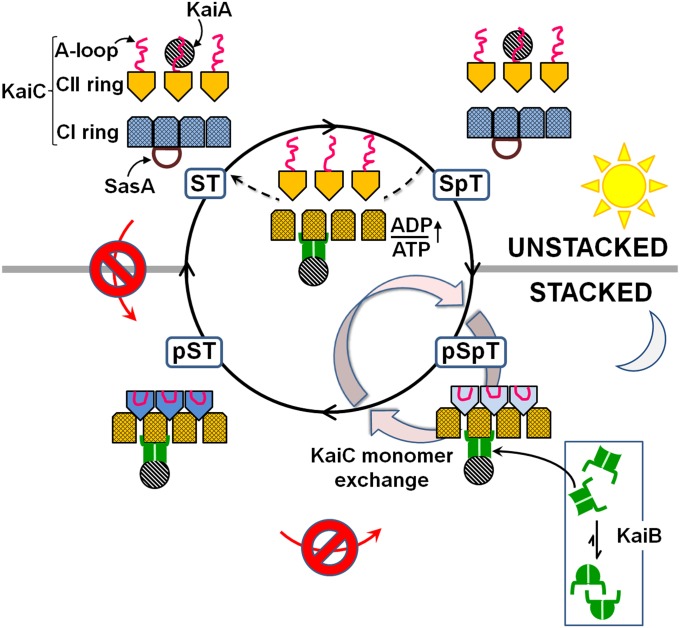
Fig. 4 illustrates our allosteric model. Here we discuss three predictions of this model, that rhythmic ring stacking ↔ unstacking in KaiC (i) drives the oscillator clockwise, (ii) dictates the phase dependence of ADP-induced clock resetting, and (iii) regulates clock output signals.
Fig. 4.
Allosteric model of how ring stacking in KaiC orchestrates the moving parts of the circadian oscillator of cyanobacteria. The model predicts that rhythmic ring stacking drives phosphorylation clockwise, reorganizes protein–protein interactions, provides a temporal window for ADP-induced resetting, and regulates clock output. Only three subunits of the CII ring and four subunits of the CI ring are shown for clarity. Likewise, the linkers connecting the CI and CII domains are not shown.
Ring Stacking Drives the Oscillator Clockwise.
KaiA can stimulate KaiC phosphorylation as long as KaiB does not bind KaiC. Because KaiC–KaiB binding depends on ring stacking, the unstacked clockwise direction ST → SpT → pSpT is uninhibited by KaiB, in contrast to the stacked counterclockwise direction, ST → pST → pSpT. Dephosphorylation depends on the ability of KaiB to sequester KaiA from the A loops of KaiC. Thus, the preferred direction of dephosphorylation is that in which the rings of KaiC remain stacked, pSpT → pST → ST (clockwise), rather than unstacked, pSpT → SpT → ST (counterclockwise). The overall clockwise directionality is further promoted by burial of the A loops in the stacked states of KaiC (18). The stacking-dependent affinities between KaiA and KaiC predicted by our model suggest a mechanism for maintaining phase coherence across the ensemble, as was presaged mathematically (32).
Ring Stacking Explains ADP-Induced Clock Resetting.
The circadian rhythms of entrained cyanobacteria under constant light could be reset by a pulse of darkness in the middle of the subjective day when KaiC was phosphorylating, because of the dark-induced increase in intracellular ADP/ATP ratios (19). In contrast, the oscillator was insensitive to ADP during the subjective night, when KaiC was dephosphorylating. Our model proposes that this phase dependence arises from the stacking-dependent effect of ADP on the ring structure of CI. When the rings are unstacked, as they are during the subjective day, the KaiB-binding sites are hidden by the associated subunits of the CI ring. Our results suggest that ADP resets the oscillator during this phase by prematurely disassociating these subunits to expose the KaiB-binding sites. During the subjective night, the CI and CII rings are stacked together. We think that when they stack, the CII ring wedges the CI subunits apart, exposing the KaiB-binding sites. Thus, according to our model, ADP would have no effect during this phase of the rhythm, as was observed experimentally (19).
One of the rings of KaiC expands on S431 phosphorylation (21) or KaiB binding (20). Our model predicts that this expanded ring is that of CI (Fig. 4).
Ring Stacking Regulates Clock Output.
The clock output protein SasA (15, 17, 35, 36) binds to the CI ring (18). Now that we have established that KaiB also binds to CI, the basis for their competitive binding to KaiC (13, 22) becomes clearer. Because the KaiC–KaiB interaction depends on ring stacking, the competition is also expected to depend on ring stacking. Consequently, SasA activity, and thus clock output, are likely regulated by ring stacking. The ATPase activity of KaiC appears to play an important role in clock function (37, 38) and cell division (16). The lower activity in KaiC proteins with stacked rings and higher activity in KaiC proteins with unstacked rings suggest that ring stacking regulates the ATPase.
It is now clear why KaiC has two rings. The added dimension from having a second ring (i.e., the CI ring) empowers KaiC to oscillate clockwise and also recalibrate itself to environmental cues.
Materials and Methods
Cloning, Protein Expression, and Purification.
T. elongatus kaiA, kaiB, and kaiC genes were cloned into pET-28b by PCR using the Nde I/Hind III sites as described previously (18). SI Appendix, Table S1 presents the abbreviations and complete names of the proteins and corresponding constructs. Proteins were expressed in BL21(DE3) Escherichia coli (Novagen) and purified by Ni-NTA affinity chromatography and size-exclusion chromatography. Detailed information on protein expression and purification is provided in SI Appendix.
Analytical Gel-Filtration Chromatography.
The gel-filtration profiles of KaiB and its variants were obtained using a HiLoad 16/60 Superdex 75 column (GE Healthcare). All other gel-filtration assays were performed with a Superdex 200 10/300 GL column (GE Healthcare). Detailed descriptions of sample preparation and running conditions are provided in SI Appendix.
In Vitro KaiC Phosphorylation Reactions.
KaiC was incubated with KaiA and KaiB or KaiB variants at 30 °C. Aliquots were obtained at indicated time points for SDS/PAGE analysis. Gels were stained with Coomassie brilliant blue R250 (EMD Chemicals), and the percentage of KaiC phosphorylation was determined by densitometry using ImageJ (National Institutes of Health) and PeakFit (SeaSolve Software).
Fluorescence Spectroscopy.
All data were collected with an ISS PC1 spectrofluorometer. For measuring the kinetics of KaiB subunit exchange, FRET experiments were performed using KaiB proteins labeled separately with donor and acceptor fluorophores 5-((((2-iodoacetyl)amino)ethyl)amino)naphthalene-1-sulfonic acid (1,5-IAEDANS) and 6-iodoacetamidofluorescein (6-IAF) (Invitrogen) and using excitation and emission wavelengths of 347 nm and 530 nm, respectively. For KaiC–KaiB binding, fluorescence anisotropies of 6-IAF–labeled KaiB and its variants were monitored on mixing with S431E-KaiC. Sample details and the experimental setup are provided in SI Appendix.
NMR Spectroscopy.
All NMR experiments were run on a Bruker 600 MHz AVANCE III spectrometer equipped with a TCI cryoprobe. Details of sample preparation and experimental parameters are provided in SI Appendix. Data were processed using NMRPipe and visualized using NMRDraw (39).
Supplementary Material
Acknowledgments
We thank Susan S. Golden for valuable discussions, Steve Grimaldi for NMR support, and Martin van de Ven for spectrofluorometry support. This research was supported by US Army Research Office Grant W911NF-10-1-0090. R.T. was supported by a National Science Foundation Graduate Research Fellowship.
Footnotes
The authors declare no conflict of interest.
†This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211508109/-/DCSupplemental.
See Commentary on page 16760.
References
- 1.Hogenesch JB, Ueda HR. Understanding systems-level properties: Timely stories from the study of clocks. Nat Rev Genet. 2011;12:407–416. doi: 10.1038/nrg2972. [DOI] [PubMed] [Google Scholar]
- 2.Ishiura M, et al. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science. 1998;281:1519–1523. doi: 10.1126/science.281.5382.1519. [DOI] [PubMed] [Google Scholar]
- 3.Tomita J, Nakajima M, Kondo T, Iwasaki H. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science. 2005;307:251–254. doi: 10.1126/science.1102540. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima M, et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- 5.Rust MJ, Markson JS, Lane WS, Fisher DS, O’Shea EK. Ordered phosphorylation governs oscillation of a three-protein circadian clock. Science. 2007;318:809–812. doi: 10.1126/science.1148596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiwaki T, et al. A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. EMBO J. 2007;26:4029–4037. doi: 10.1038/sj.emboj.7601832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pattanayek R, et al. Visualizing a circadian clock protein: Crystal structure of KaiC and functional insights. Mol Cell. 2004;15:375–388. doi: 10.1016/j.molcel.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki H, Nishiwaki T, Kitayama Y, Nakajima M, Kondo T. KaiA-stimulated KaiC phosphorylation in circadian timing loops in cyanobacteria. Proc Natl Acad Sci USA. 2002;99:15788–15793. doi: 10.1073/pnas.222467299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams SB, Vakonakis I, Golden SS, LiWang AC. Structure and function from the circadian clock protein KaiA of Synechococcus elongatus: A potential clock input mechanism. Proc Natl Acad Sci USA. 2002;99:15357–15362. doi: 10.1073/pnas.232517099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YI, Dong G, Carruthers CW, Jr, Golden SS, LiWang A. The day/night switch in KaiC, a central oscillator component of the circadian clock of cyanobacteria. Proc Natl Acad Sci USA. 2008;105:12825–12830. doi: 10.1073/pnas.0800526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vakonakis I, LiWang AC. Structure of the C-terminal domain of the clock protein KaiA in complex with a KaiC-derived peptide: Implications for KaiC regulation. Proc Natl Acad Sci USA. 2004;101:10925–10930. doi: 10.1073/pnas.0403037101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin X, et al. Intermolecular associations determine the dynamics of the circadian KaiABC oscillator. Proc Natl Acad Sci USA. 2010;107:14805–14810. doi: 10.1073/pnas.1002119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pattanayek R, et al. Combined SAXS/EM based models of the S. elongatus post-translational circadian oscillator and its interactions with the output His-kinase SasA. PLoS ONE. 2011;6:e23697. doi: 10.1371/journal.pone.0023697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akiyama S, Nohara A, Ito K, Maeda Y. Assembly and disassembly dynamics of the cyanobacterial periodosome. Mol Cell. 2008;29:1–14. doi: 10.1016/j.molcel.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Takai N, et al. A KaiC-associating SasA-RpaA two-component regulatory system as a major circadian timing mediator in cyanobacteria. Proc Natl Acad Sci USA. 2006;103:12109–12114. doi: 10.1073/pnas.0602955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong G, et al. Elevated ATPase activity of KaiC applies a circadian checkpoint on cell division in Synechococcus elongatus. Cell. 2010;140:529–539. doi: 10.1016/j.cell.2009.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valencia S J, et al. Phase-dependent generation and transmission of time information by the KaiABC circadian clock oscillator through SasA-KaiC interaction in cyanobacteria. Genes Cells. 2012;17:398–419. doi: 10.1111/j.1365-2443.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang YG, Kuo NW, Tseng R, LiWang A. Flexibility of the C-terminal, or CII, ring of KaiC governs the rhythm of the circadian clock of cyanobacteria. Proc Natl Acad Sci USA. 2011;108:14431–14436. doi: 10.1073/pnas.1104221108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rust MJ, Golden SS, O’Shea EK. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science. 2011;331:220–223. doi: 10.1126/science.1197243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattanayek R, et al. Structural model of the circadian clock KaiB-KaiC complex and mechanism for modulation of KaiC phosphorylation. EMBO J. 2008;27:1767–1778. doi: 10.1038/emboj.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murayama Y, et al. Tracking and visualizing the circadian ticking of the cyanobacterial clock protein KaiC in solution. EMBO J. 2011;30:68–78. doi: 10.1038/emboj.2010.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami R, et al. The roles of the dimeric and tetrameric structures of the clock protein KaiB in the generation of circadian oscillations in cyanobacteria. J Biol Chem. 2012;287:29506–29515. doi: 10.1074/jbc.M112.349092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura Y, et al. Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 2002;9:123–130. doi: 10.1093/dnares/9.4.123. [DOI] [PubMed] [Google Scholar]
- 24.Kitayama Y, Iwasaki H, Nishiwaki T, Kondo T. KaiB functions as an attenuator of KaiC phosphorylation in the cyanobacterial circadian clock system. EMBO J. 2003;22:2127–2134. doi: 10.1093/emboj/cdg212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kay LE. Solution NMR spectroscopy of supra-molecular systems, why bother? A methyl-TROSY view. J Magn Reson. 2011;210:159–170. doi: 10.1016/j.jmr.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE. A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR. 1999;13:369–374. doi: 10.1023/a:1008393201236. [DOI] [PubMed] [Google Scholar]
- 27.Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc. 2003;125:10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- 28.Iwase R, et al. Functionally important substructures of circadian clock protein KaiB in a unique tetramer complex. J Biol Chem. 2005;280:43141–43149. doi: 10.1074/jbc.M503360200. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi F, Iwase R, Uzumaki T, Ishiura M. Hexamerization by the N-terminal domain and intersubunit phosphorylation by the C-terminal domain of cyanobacterial circadian clock protein KaiC. Biochem Biophys Res Commun. 2006;348:864–872. doi: 10.1016/j.bbrc.2006.07.143. [DOI] [PubMed] [Google Scholar]
- 30.Ito H, et al. Autonomous synchronization of the circadian KaiC phosphorylation rhythm. Nat Struct Mol Biol. 2007;14:1084–1088. doi: 10.1038/nsmb1312. [DOI] [PubMed] [Google Scholar]
- 31.Kageyama H, et al. Cyanobacterial circadian pacemaker: Kai protein complex dynamics in the KaiC phosphorylation cycle in vitro. Mol Cell. 2006;23:161–171. doi: 10.1016/j.molcel.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 32.van Zon JS, Lubensky DK, Altena PRH, ten Wolde PR. An allosteric model of circadian KaiC phosphorylation. Proc Natl Acad Sci USA. 2007;104:7420–7425. doi: 10.1073/pnas.0608665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutoh R, et al. Direct interaction between KaiA and KaiB revealed by a site-directed spin labeling electron spin resonance analysis. Genes Cells. 2010;15:269–280. doi: 10.1111/j.1365-2443.2009.01377.x. [DOI] [PubMed] [Google Scholar]
- 34.Iwasaki H, Taniguchi Y, Ishiura M, Kondo T. Physical interactions among circadian clock proteins KaiA, KaiB and KaiC in cyanobacteria. EMBO J. 1999;18:1137–1145. doi: 10.1093/emboj/18.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniguchi Y, Takai N, Katayama M, Kondo T, Oyama T. Three major output pathways from the KaiABC-based oscillator cooperate to generate robust circadian kaiBC expression in cyanobacteria. Proc Natl Acad Sci USA. 2010;107:3263–3268. doi: 10.1073/pnas.0909924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwasaki H, et al. A KaiC-interacting sensory histidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell. 2000;101:223–233. doi: 10.1016/S0092-8674(00)80832-6. [DOI] [PubMed] [Google Scholar]
- 37.Terauchi K, et al. ATPase activity of KaiC determines the basic timing for circadian clock of cyanobacteria. Proc Natl Acad Sci USA. 2007;104:16377–16381. doi: 10.1073/pnas.0706292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami R, et al. ATPase activity and its temperature compensation of the cyanobacterial clock protein KaiC. Genes Cells. 2008;13:387–395. doi: 10.1111/j.1365-2443.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- 39.Delaglio F, et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.