Background: VahC toxin from Aeromonas hydrophila inactivates actin by transferring ADP-ribose from NAD+.
Results: The crystal structure of VahC reveals new insights into the interaction of the actin substrate with the actin-targeting toxins, and consequently, potent inhibitors have been developed.
Conclusion: Broad spectrum inhibitors have been developed against actin-targeting toxins from the ADP-ribosyltransferase family.
Significance: This new insight provides impetus for therapeutic development for treating bacterial diseases.
Keywords: Actin, Bacterial Toxins, Drug Development, Enzyme Inhibitors, Protein Structure, Bacterial Diseases, Enzyme Mechanisms, Protein-Protein Interactions
Abstract
The mono-ADP-ribosyltransferase (mART) toxins are contributing factors to a number of human diseases, including cholera, diphtheria, traveler's diarrhea, and whooping cough. VahC is a cytotoxic, actin-targeting mART from Aeromonas hydrophila PPD134/91. This bacterium is implicated primarily in diseases among freshwater fish species but also contributes to gastrointestinal and extraintestinal infections in humans. VahC was shown to ADP-ribosylate Arg-177 of actin, and the kinetic parameters were Km(NAD+) = 6 μm, Km(actin) = 24 μm, and kcat = 22 s−1. VahC activity caused depolymerization of actin filaments, which induced caspase-mediated apoptosis in HeLa Tet-Off cells. Alanine-scanning mutagenesis of predicted catalytic residues showed the predicted loss of in vitro mART activity and cytotoxicity. Bioinformatic and kinetic analysis also identified three residues in the active site loop that were critical for the catalytic mechanism. A 1.9 Å crystal structure supported the proposed roles of these residues and their conserved nature among toxin homologues. Several small molecules were characterized as inhibitors of in vitro VahC mART activity and suramin was the best inhibitor (IC50 = 20 μm). Inhibitor activity was also characterized against two other actin-targeting mART toxins. Notably, these inhibitors represent the first report of broad spectrum inhibition of actin-targeting mART toxins.
Introduction
Virulence factors produced by invading pathogenic microbes play an important role in diseases caused by bacterial infections. These virulence factors aid in various infection processes, including quorum sensing, subverting the host immune response, and killing of host cells (1, 2). Virulence factors have become the target of antimicrobial therapy focusing on pathogen inactivation, rendering them harmless or more susceptible to the host immune system (3). This new paradigm in antimicrobial treatment has promise for the production of antimicrobial agents with increased specificity and fewer bacterial resistance issues, specifically with respect to antibiotics (4, 5). Identification of new virulence factors and understanding of their role during infection is the first step in the advancement of new and innovative anti-virulence therapies (6).
Aeromonas hydrophila is a Gram-negative bacterium found ubiquitously in aquatic environments. It is known to cause motile Aeromonad septicemia in freshwater fish species (7), as well as gastrointestinal and extraintestinal infections in humans (8). Its ability to resist chlorination upon biofilm formation in drinking water distribution systems has made it a health concern (9), and A. hydrophila infections were prevalent among the survivors of the 2004 Indian Ocean tsunami (10). Infection by A. hydrophila is propagated by an array of virulence factors, including pili and adhesins (11, 12), O-antigens and capsules (13), exoenzymes (14), and a wide variety of exotoxins (aerolysin/cytotoxic enterotoxin and heat-labile and heat-stable cytotoxic enterotoxins) (15, 16).
There is low sequence homology across the mART5 family, but the structure of the catalytic core is highly conserved (48). Comprising this catalytic core are separate motifs, contained within three distinct regions, which are conserved across all but one mART toxin subgroup (Fig. 1).
FIGURE 1.
Sequence alignment of actin-binding mART toxins. The multiple-sequence alignment was performed with T-Coffee (58), and the figure was prepared with ESPript (59) and modified manually. The fully conserved residues are shaded. Those residues with global similarity scores over 0.7 are boxed and were calculated using the Risler matrix method. Structural elements are based on the reported crystal structure of VahC. Residue numbering is according to the VahC primary sequence (top) and iota primary sequence (bottom). The active site loop and motifs 1–3 for ADPRT activity are shown in boldface type. Regions 1–6 (R1 to R6) are shown as potential actin binding regions in the sequences. The gray asterisks show the actin binding residues (from the Ia actin structure, PDB entry 3BUZ).
In this study, we characterize a new actin-targeting toxin as the protein product of the virulence gene (vahC), from A. hydrophila PPD134/91 (17). The gene was originally annotated as vsdC for its resemblance to a Salmonella virulence gene but has been renamed to prevent confusion (18), and we identified it with a bioinformatics strategy. The virulence factor, called VahC, is similar to the recently reported virulence factor VgrG1 from A. hydrophila ATCC7966 but lacks the N-terminal VgrG domain that mediates secretion through the Type VI secretion system (19). VahC, classified as a Type IV mART toxin, ADP-ribosylates actin at Arg-177 and targets all actin isoforms. Elucidation of the catalytic signature of VahC by Ala-scanning mutagenesis and a fluorescence-based kinetic assay (20) was combined with a Saccharomyces cerevisiae-based cytotoxicity assay (21). We have shown that this toxin disrupts the actin cytoskeleton in mammalian cells and induces caspase-mediated apoptosis. Structural insight from a 1.9 Å crystal structure reveals the nature of conserved residues among actin-targeting homologues. Uniquely, we have observed effective in vitro inhibition of VahC and similar Type IV mART toxins by novel inhibitors identified from a virtual screen method (22).
EXPERIMENTAL PROCEDURES
Expression and Purification of VahC
The ORF for vahC was cloned into the NdeI-XhoI sites of a modified pET-28a(+) vector (called pET-TEV) for expression of the vahC gene encoding a 5′ polyhistidine tag. Escherichia coli Rosetta (DE3) (Novagen, Madison, WI) cells were transformed with the pET-TEV vector containing the vahC gene and grown to an A600 of 0.6 at 37 °C. Cells were incubated overnight at 15 °C after the addition of isopropyl-β-d-thiogalactoside to 1 mm. Cells were pelleted via centrifugation at 5000 × g for 10 min and resuspended in L buffer (50 mm Tris, pH 7.9, 50 mm NaCl). Cells were lysed via passage twice through a French press and clarified by centrifugation at 14,000 × g for 30 min. Supernatants were spiked to 5 mm imidazole and passed twice over a chelating Sepharose FF column (Amersham Biosciences, Baie d'Urfe, Canada) charged with ZnSO4. The column was washed with W buffer (50 mm Tris, pH 7.9, 50 mm NaCl, 40 mm imidazole), and protein was eluted using E buffer (50 mm Tris, pH 7.9, 50 mm NaCl, 100 mm imidazole) and S buffer (100 mm EDTA). Elution fractions were collected and pooled with S buffer fractions and dialyzed with 4 liters of L buffer for 24 h. Protein was concentrated using an Amicon Centriprep concentrator (Millipore, Billerica, MA) and quantified using a calculated extinction coefficient of 24,410 m−1 cm−1 at A280. Samples were assessed for purity via Coomassie staining of SDS-PAGE. Mutants were generated using the QuikChange site-directed mutagenesis method (Stratagene, Santa Clara, CA) and purified as above.
For crystallography, a truncated, active VahC construct, VahC-67T, lacking the first 67 N-terminal residues, was expressed and purified as described for the native protein. It was followed by the removal of the polyhistidine tag with TEV V8 protease. Subtractive nickel ion-Sepharose, and size exclusion chromatography on Superdex 200 (GE Healthcare) was carried out to improve the purity of the VahC-67C protein. The purified protein was concentrated to 20 mg/ml in 100 mm NaCl, 10 mm Tris-HCl (pH 7.6), and 2 mm DTT.
Folding Integrity by Intrinsic Trp Fluorescence
The VahC (4 μm) Trp emission spectra were obtained with excitation at 295 nm and the emission spectra collection from 305 to 450 nm (2.5-nm band pass for both) as described previously (23).
Protein Crystallization and Structure Determination
VahC-67C crystals were obtained by using hanging drop vapor diffusion against a reservoir solution consisting of 25% PEG 3350, 0.2 m NaCl, 0.1 m Hepes (pH 7.5), 5% glycerol, 2 mm DTT at room temperature with a protein concentration of 20 mg/ml. Needle-shaped crystals appeared within 24 h and grew to full size with dimensions of 0.7 × 0.2 × 0.05 mm in 4 days. Crystal cryoprotection was achieved by adding glycerol in the crystallization drop to a final concentration of 15% (v/v). Cryoprotected crystals were flash frozen in liquid nitrogen for data collection.
The diffraction data were collected to 1.93 Å at the Canadian Macromolecular Crystallography Facility (08ID-1) and processed in XDS (24). Crystals were of the hexagonal space group, P6522, with three molecules in the asymmetric unit. The structure was solved with PHASER (25) by molecular replacement using the related toxin SpvB (PDB entry 2GWL) as the search model. The structure was refined against 1.93 Å resolution data with PHENIX (26), applying TLS and 2-fold NCS restraints. Manual model building was performed with COOT (27). Table 3 lists data collection and final model refinement statistics. Structure figures were prepared using PyMOL (28).
TABLE 3.
Data collection and refinement statistics for VahC
| Data collection | |
| Sample | VahC (apo) |
| Beamline | CLSI 08-ID-1 |
| Wavelength (Å) | 0.979 |
| Space group | P6 (5)22 |
| Cell dimensions | |
| a, b, c (Å) | 91.0, 91.0, 303.1 |
| α, β, γ (degrees) | 90.0, 90.0, 120.0 |
| Resolution (Å) | 1.93 |
| Total reflections | 1,236,032 |
| Unique reflections | 57,036 |
| Completeness | 99.9% |
| Rsyma (%) | 8.5 |
| Multiplicity | 21.7 |
| I/σ(I) | 31.2 |
| Refinement statistics | |
| Overall B-factor (Å) | 61 |
| Rworkb (%) | 19.11 |
| Rfreec (%) | 21.72 |
| Root mean square deviations | |
| Bond lengths (Å) | 0.020 |
| Bond angles (degrees) | 1.495 |
| Ramachandran plot (%) | |
| Most favored | 97.43 |
| Additionally allowed | 2.39 |
a Rsym = Σ|(I − 〈I〉)|IΣ(I), where I is the observed intensity.
b Rwork = (Σh‖Fo| − k·|Fc‖/Σh|Fo|, where Fo and Fc are the observed and calculated structure factor, respectively, and k is a scaling factor.
c Rfree factor is identical to R-factor on a subset of test reflections not used in the refinement.
Detection of FITC-ADP-ribose Actin Isoforms
β- and γ-actin were purified using a baculovirus expression system as described previously (29). FITC-ADP-ribose labeling detection was performed as described previously (30).
mART Activity Assays and Inhibition
Chicken skeletal actin for kinetic assays was purified as described previously (31) and quantified using an extinction coefficient of 67,742 m−1 cm−1 at 290 nm. Activity assays were performed following a slightly modified protocol as described previously (30). In brief, appropriate concentrations of VahC (62 nm) or VahC mutants (0.22–3.3 μm) were mixed with varying concentrations of ϵ-NAD+ or chicken skeletal actin (50 and 35 μm for saturating concentrations, respectively), and fluorescence emission was monitored in a stopped-flow spectrometer, model SX20-MV (Applied Photophysics, Leatherhead, UK) as the reaction progressed at 25 °C (295-nm excitation, 395-nm emission cut-off filter). Inhibitor compounds were derived from a virtual screen method as described previously (22). Inhibition assays were conducted in a similar manner on a Cary Eclipse fluorescence spectrophotometer (Varian Instruments, Mississauga, Canada), with reaction volumes of 70 μl. For the inhibition of VahC and SpvB, 120 μm ϵ-NAD+ and 38 μm actin were combined with 12.5 nm toxin (final concentration). For Photox, 250 μm ϵ-NAD+ and 24 μm actin were combined with 40 nm toxin. In each case, inhibition was determined by monitoring the relative decrease in activity with increasing inhibitor concentrations. Activity was measured via excitation at 305 nm and emission at 405 nm over 5 min at 25 °C. IC50 values were determined after fitting the data using nonlinear regression in GraphPad Prism. Ki values were calculated from these IC50 measurements using the Cheng-Prusoff equation (32).
NAD+ and Inhibitor Binding
NAD+ and inhibitor binding constants (KD) were experimentally determined as described previously (33) with minor variations. In brief, increasing concentrations of NAD+ or inhibitor were titrated with 1 μm VahC, and Trp fluorescence quenching was monitored (295-nm excitation, 340-nm emission, 25 °C) using a Cary Eclipse fluorescence spectrophotometer.
Growth Inhibition Assays
S. cerevisiae haploid strains W303 (MATa leu2 trp1 can1 ura3 ade2 his) and ACT-RA (MATa his3 leu2 ura3 tub2 act1::LEU2 (pJD301(HIS3) - ACT1 R177A)) were cultured at 30 °C on yeast-peptone-dextrose or synthetic dextrose minimal medium missing the appropriate amino acid. The vahC gene constructs were individually cloned into the modified yeast shuttle vector pRS415 and pRS416 containing the yeast CUP1 promoter (21) for expression in yeast. The ACT-RA strain was prepared as described previously (30). All yeast growth inhibition assays were performed as described previously (21) with slight modification.
pBI-EGFP Cloning
The vahC ORF was cloned into the MluI and NheI restriction sites of the pBI-EGFP vector. Briefly, the sequences of vahC and vahC E/E were PCR-amplified from the pET-TEV plasmid carrying the genes of interest using a set of primers containing the MluI (forward 5′-TCATACGCGTATGGAGTCCATGGCGCCGCTGG-3′) or NheI (reverse 5′-GGTCGCTAGCTCATAATACGGAAACCTCAATCAG-3′) restriction sites. The PCR products and the pBI-EGFP vector were digested with the corresponding enzymes and ligated using T4 DNA ligase. E. coli DH5α cells were transformed with the recombinant plasmids and selected for ampicillin resistance. Several colonies were tested by restriction enzyme digestion to confirm the ligation. The pBI-EGFP-vip-2 plasmid was prepared as described recently (19).
Transfection of HeLa Tet-Off Cells
HeLa Tet-Off cells (Clontech) were grown in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% Tet approved fetal bovine serum (Clontech) under standard cell culture conditions at 37 °C and 5% CO2. Prior to electroporation, the cells were ∼70% confluent and were detached with a non-enzymatic cell dissociation solution (Sigma) and washed twice with DMEM without serum. The cells were resuspended at a concentration of ∼5 × 106 cells/ml, and 600 μl of cells were electroporated with 5 μg of plasmid in a Gene Pulser Xcell (Bio-Rad) using 0.4-mm cuvettes and 250-V/950-microfarad/∞-ohm exponential protocol. The electroporated cells were recovered in a complete medium and grown in 6-well plates. The percentage of transfected cells was measured by examining expression of the gene encoding EGFP by flow cytometry (19).
Western Blot Analysis
After 24 h of transfection, HeLa Tet-Off cells expressing and producing various proteins were lysed in SDS-Tris-glycine buffer and subjected to 15% SDS-PAGE. Proteins from the gels were transferred to nitrocellulose membranes, and then nonspecific sites were blocked with 5% skim milk, 1% BSA in TBS overnight. Specific mouse serum containing anti-VIP-2 antibodies (41) was used as the primary antibody at a 1:1000 dilution for 2 h. Subsequently, the membrane was incubated with HRP-conjugated goat anti-mouse antibody (Southern Biotech) at a 1:5000 dilution for 1 h. Between steps, three washes with 0.05% Tween 20 in TBS for 10 min were performed on the membranes. The blot was developed using Super Signal West Pico chemiluminescent substrate (Pierce) followed by x-ray film exposure.
Phalloidin Staining
HeLa cells expressing and producing various proteins were fixed in situ with Cytofix/Cytoperm solution (BD Biosciences) for 20 min. Then the cells were scraped, washed with cytoperm wash buffer, and stained with Alexa Fluor-568 conjugated to phalloidin (Invitrogen) for 1 h. Subsequently, the cells were washed and kept in 2% paraformaldehyde. For phalloidin quantification, the suspension of cells was acquired in a FACScan flow cytometer (BD Biosciences) by gating on EGFP in order to exclude untransfected cells. For fluorescence microscopy, the cells in suspension were attached to a slide by cytospin at 1500 rpm for 5 min, and the coverslips were mounted with a solution that contained DAPI (Vector, Burlingame, CA). The image visualization was performed using fluorescence microscopy (Olympus BX51/DPManager version 1.2.1.107/DP Controller version 1.2.1.108, Olympus Optical Co. Ltd.).
Assay for Apoptosis
After 24 h of transfection, cytoplasmic nucleosomes were detected by the cell death ELISA kit by following the manufacturer's instructions (Roche Applied Science). Briefly, the supernatant from HeLa Tet-Off cells was removed, and the cells were lysed in 500 μl of lysis buffer for 20 min by continuous shaking. Cells in suspension were recovered by centrifugation and returned to the well to be lysed. After lysis, the solution was centrifuged at 200 × g for 10 min, and the supernatant was used for the ELISA. Antibodies to histone conjugated with biotin and anti-DNA conjugated with peroxidase were incubated with the 20 μl of supernatant in a streptavidin-coated plate for 2 h. The plate was washed with assay buffer three times, and the reaction was developed with 2,2″-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate. The absorbance was read at 405 nm in a VersaMax microplate reader. The colorimetric caspase-3 activity assay (Biovision Inc., Mountain View, CA) was performed as follows. 200 μg of protein (whole-cell lysate from HeLa Tet-Off cells expressing and producing different proteins), 50 μl of 2× reaction buffer, and 5 μl of peptide substrate (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-p-nitroanilide) were incubated at 37 °C between 2 and 24 h. The color reaction was measured every hour at a 405-nm wavelength in a VersaMax microplate reader.
RESULTS
VahC ADP-ribosylates Actin at Arg-177 and Targets All Actin Isoforms
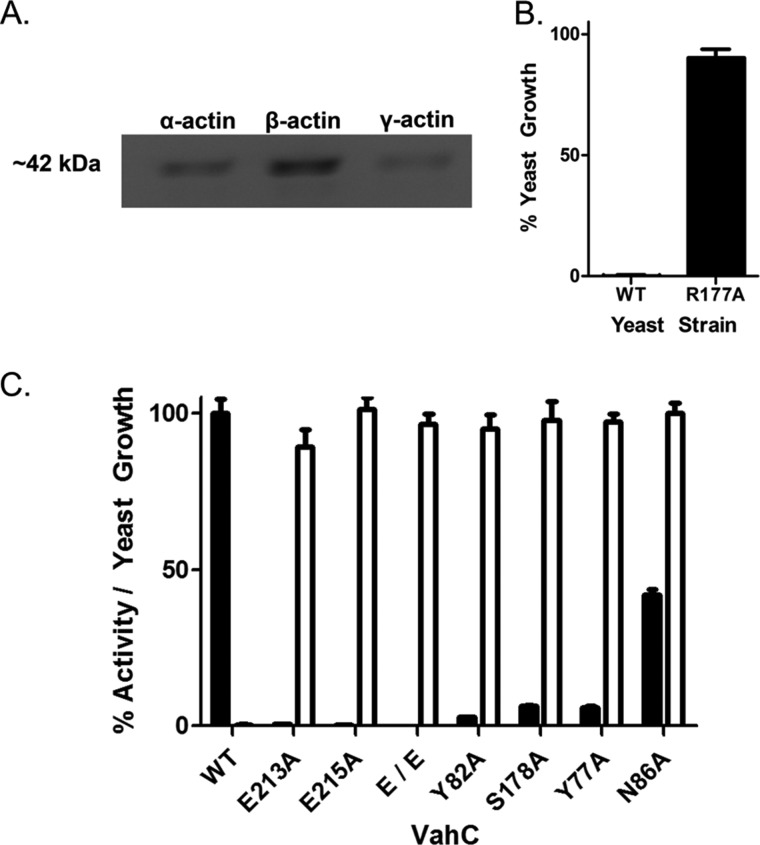
The ability of VahC to ADP-ribosylate actin isoforms was assessed using an FITC-NAD+ conjugate. Purified α-, β-, or γ-actin was incubated with VahC, and the fluorescently modified actin was visualized under UV light. VahC is capable of attaching ADP-ribose to all three major actin isoforms, indicating that it is a nonspecific actin-targeting toxin (Fig. 2A). To confirm that VahC transfers the ADP-ribose group to Arg-177 of actin, we employed a yeast-based growth inhibition assay. In this method, we control the expression of the vahC gene with the CUP1 (copper-inducible) promoter, and an active toxin will cause a growth defect phenotype in yeast (21). Expression of the vahC gene in the W303 WT S. cerevisiae strain inhibited growth to 0.02% (Fig. 2B). However, when expressed in a mutant strain of yeast called ACT-RA, containing a plasmid-encoded actin gene with an R177A substitution, the viability of yeast cells expressing the vahC gene was largely restored (90.5% growth) (Fig. 2B).
FIGURE 2.
VahC targets all actin isoforms and inhibits growth by ADP-ribosylation of Arg-177. A, the mART activity was assessed by incubating active VahC with α, β, and γ isoforms of actin and FITC-labeled NAD+. The reaction mixtures were separated on a 12.5% SDS-polyacrylamide gel, and FITC-ADP-ribosylated actin was visualized with UV light. B, VahC produced in S. cerevisiae causes a reduction in yeast growth, which is restored when expressed in the ACT-RA yeast strain, which ubiquitously expresses a mutant actin, R177A. C, Ala-scanning mutagenesis of the predicted catalytic signature was conducted, and the catalytic activity was characterized in vitro using an established fluorescence-based assay and expressed as a percentage of native VahC activity (black bars). The WT mART activity was 22.3 ± 2.0 s−1 and is shown as 100% (black bars). Cell-based toxicity is expressed as a percentage of yeast growth (white bars). All mutants showed a marked decrease in enzyme activity, which corresponded to the restoration of yeast growth. Statistical analysis by one-way analysis of variance and Tukey's test revealed that all mutants were significantly different (p < 0.05) from controls in both catalytic activity and yeast cytotoxicity. Error bars, S.D.
Alanine-scanning Mutagenesis Reveals VahC Catalytic Signature Is Necessary for in Vitro Activity and Growth Inhibition in Yeast
Wild-type VahC showed Michaelis-Menten mART activity with both NAD+ and actin as substrates (Km = 6 ± 0.4 and 24 ± 2 μm, respectively) with a kcat value of 22 s−1 (Table 1). To conclusively verify the mART catalytic signature of VahC, alanine-scanning mutagenesis of the active site residues was performed. All catalytic mutant proteins were purified in the same manner as wild-type VahC. The folded integrity of each variant was assessed using Trp emission spectroscopy, and all mutants were similar to the wild-type fluorescence emission maximum value (332 nm). In vitro activity was monitored via a fluorescence-based kinetic assay (20), and the cell-based activity was assessed using a high throughput S. cerevisiae cytotoxicity assay (Fig. 2C). Mutagenesis of the predicted key catalytic residues of VahC showed a marked decrease in enzyme activity from as low as 0.04% (E213A/E215A) to 41.9% (N86A) of native enzyme activity (Fig. 2C, black bars). Cytotoxicity of VahC was also abrogated by substitution of residues within the catalytic signature, restoring yeast growth to 86.8% (Y82A) and to >95% (E215A, Tyr-77, Asn-86, Ser-178, and the double mutant, E213A/E215A), compared with the wild-type VahC (Fig. 2C, white bars). VahC mutants T179A and R148A could not be characterized in vitro due to expression and purification difficulties, but they restored yeast growth to 81.8 and 95%, respectively (data not shown). The cell-based data reflected the in vitro loss of activity in nearly a binary manner due to a lack of sensitivity from the yeast-based assay. The loss in catalytic activity of these mutant enzymes was sufficient to restore yeast growth and verified that the catalytic activity was the sole cause of toxicity and also revealed the seminal role of the catalytic signature residues.
TABLE 1.
Binding and kinetic constants for WT VahC and various active site mutants
Binding affinities and Michaelis constants were determined as described under “Experimental Procedures” using the fluorescence-quenching assay and the mART enzyme assay, respectively. The values represent the mean ± S.D. for at least triplicate independent measurements. Statistical analysis was performed using a one-way analysis of variance followed by Tukey's test. Differences in KD values among mutant and WT enzymes were not statistically significant (p > 0.05), and actin Km values were also not significantly different (p > 0.05). NAD+ Km values for the E215A, Y82A, and S178A mutants were statistically significant (p < 0.05).
| Mutant | KD | KM actin | KM NAD+ | kcat |
|---|---|---|---|---|
| μm | μm | μm | s−1 | |
| WT | 9 ± 0.4 | 24 ± 2 | 6 ± 0.4 | 22.3 ± 2.0 |
| E213A | 9 ± 0.7 | 20 ± 1 | 5 ± 0.4 | 0.11 ± 0.01 |
| E215A | 18 ± 3 | 23 ± 3 | 14 ± 2 | 0.05 ± 0.006 |
| Y77A | 11 ± 1 | 37 ± 5 | 8 ± 1 | 1.3 ± 0.09 |
| Y82A | 15 ± 2 | 35 ± 5 | 12 ± 0.5 | 0.65 ± 0.07 |
| N86A | 21 ± 2 | 11 ± 1 | 4 ± 0.1 | 9.35 ± 1.1 |
| S178A | 6 ± 0.3 | 33 ± 2 | 18 ± 1 | 1.38 ± 0.11 |
Binding constants were determined for the NAD+ substrate for the wild-type and mutant enzymes as well as Michaelis kinetic constants for both substrates (Table 1), showing that reduced mART activity could be attributed to a loss in ADP-ribose transferase ability and was not due to a defect in substrate binding. No KD values for mutant enzymes were found to be significantly different (p > 0.05) from the WT enzyme. The Km for actin was determined at saturating NAD+ conditions (50 μm) and was fit to a standard Michaelis-Menten function, whereas the Km for NAD+ was determined at the highest possible actin concentration (38 μm)6 and fit to a substrate inhibition model, as previously reported for certain mART toxins (kcat = 22.3 s−1). Importantly, VahC showed no detectable glycohydrolase activity in the absence of the actin substrate. There were no mutants with an affinity for actin markedly different from that of WT VahC (p > 0.05). Michaelis constants for NAD+ were significantly increased (p < 0.05) for the E215A, Y82A, and S178A mutants by 2.3-, 2-, and 3-fold, respectively (Table 1).
Expression of the vahC Gene in HeLa Tet-Off Cells Induces Rounded Cell Morphology and Disruption of the Actin Cytoskeleton
To determine whether VahC was cytotoxic in mammalian cells, the native and mutant E/E (E213A/E215A) vahC genes from A. hydrophila PPD134/91 were cloned into the pBI-EGFP vector and expressed in HeLa Tet-Off cells. Successfully transfected cells were readily visualized through co-expression of the gene encoding EGFP in this vector. Expression and production of VahC were confirmed via Western blot analysis of cell lysates following transfection using a VIP-2-specific antibody (Fig. 3A, lanes 2 and 3). The VIP-2 domain of VgrG1 served as a positive control (Fig. 3A, lane 4); cells transfected only with the pBI-EGFP vector acted as a negative control (Fig. 3A, lane 1).
FIGURE 3.
Intracellular expression and production of VahC cause actin cytoskeleton disruption and induced caspase-mediated apoptosis in mammalian cells. A, episomal expression of the vahC gene in HeLa Tet-Off cells. Shown is a Western blot on HeLa Tet-Off cells producing VahC and VahC E/E, vector alone, and the VIP-2 domain of VgrG1 using sera from mice immunized with VIP-2 from A. hydrophila ATCC 7966. B, disruption of actin cytoskeleton by expression of the vahC gene in the HeLa Tet-Off cell system. HeLa Tet-Off cells were transfected with the pBI-EGFP vector containing the vahC gene or the mutated vahC E/E gene. The vector alone and the vector carrying the VIP-2 domain of VgrG1 from A. hydrophila ATCC 7966 were stained with Alexa 568-conjugated phalloidin and mounted with a mounting solution containing DAPI. Images were acquired by fluorescence microscopy (×40 magnification). C, quantification of phalloidin staining by flow cytometry. HeLa Tet-Off cells expressing and producing VahC, VahC E/E, VIP-2 domain from VgrG1, and vector alone were stained with Alexa Fluor-568-conjugated phalloidin. Cells were acquired in a FACScan based on EGFP expression. The quadrants show the differences in percentage of cells positive for phalloidin in different groups. D, induction of apoptosis in HeLa Tet-Off cells by VahC. Cells expressing and producing VahC and VahC E/E, VIP-2 domain of VgrG1, and the vector alone were tested for rates of apoptosis using cytoplasmic nucleosome ELISA (top) and a colorimetric caspase-3 assay (bottom). Treatment with camptothecin was used as a positive control. Statistical differences were measured by one-way analysis of variance followed by Tukey's multiple comparison test. Error bars, S.D.
HeLa cells expressing the vahC gene were observed 24 h post-transfection and displayed a rounded morphology (Fig. 3B, panel III) similar to that observed in cells expressing the VIP-2 domain of VgrG1 (Fig. 3B, panel II). The morphology of cells transfected with only pBI-EGFP or vahC E/E was consistent with that of healthy cells (Fig. 3B, panels I and IV). Integrity of the actin cytoskeleton in these cells was assessed using Alexa 568-conjugated phalloidin to stain actin followed by fluorescence microscopy. Healthy cells had an intact cytoskeleton (Fig. 3B, panels I and IV), whereas active toxin-treated cells displayed a rounded phenotype with a severely disrupted cytoskeleton (Fig. 3B, panels II and III).
Precise quantification of phalloidin-stained intact cytoskeletal actin filaments was determined using flow cytometry (Fig. 3C). HeLa Tet-Off cells expressing and producing either the VIP-2 domain of VgrG1 or VahC were found to be only 7.4 and 6.8% phalloidin-positive, respectively. Conversely, the majority of cells transfected with only pBI-EGFP or vahC E/E were phalloidin-positive (95.5 and 94.2%, respectively).
Expression of the vahC Gene in HeLa Tet-Off Cells Induces Apoptosis
Induction of apoptosis was assessed via the presence of cytoplasmic nucleosomes and caspase-3. The presence of nucleosomes, a hallmark of apoptotic cells (34), was detected by ELISA and found to be present in a larger fraction of cells producing VahC but drastically reduced in cells expressing and producing VahC E/E (Fig. 3D, top, columns 2 and 3). Detection of caspase-3 activity, an executor of apoptosis, supported caspase-mediated induction of apoptosis. Reflecting the presence of nucleosomes, caspase-3 activity was detected in a larger fraction of cells producing VahC rather than the VahC E/E mutant (Fig. 3D, bottom, lanes 2 and 3). Camptothecin, a potent inducer of apoptosis through inhibition of DNA topoisomerase I, was used as a positive control (Fig. 3D, column 5). Cells transfected with pBI-EGFP only or the VIP-2 domain of VgrG1 served as negative and positive controls, respectively (Fig. 3C, columns 1 and 4).
VahC, Photox, and SpvB Are Inhibited by Several Small Molecules in Vitro
Previously, our group conducted a virtual screen of a chemical library of compounds against the mART toxin, cholix toxin (which shares high homology with ExoA), and a small library of compounds were identified that showed efficacy against both cholix and ExoA (22). One of our research goals is to develop “broad spectrum” antivirulence compounds against the family of mART toxins. Accordingly, we tested our mART toxin inhibitor library against VahC enzyme activity, and we identified four compounds that showed good inhibition of VahC ADP-ribosyltransferase activity. The structure of each compound is shown in Fig. 4A. Compounds V7, V8, and V31 are commercially available compounds, and suramin is a well characterized anti-trypanosomal drug. V7, V8, and V31 share some chemical similarity with the structure of the mART substrate, NAD+, whereas suramin looks remarkably like two NAD+ molecules fused end-to-end. The inhibition efficiency of each inhibitor was measured using an in vitro VahC enzyme activity assay, and the mechanism of inhibition is competitive with the NAD+ substrate (Fig. 4B) as shown previously for ExoA and cholix toxins (22). Suramin showed the strongest inhibition with an inhibition constant (Ki) of 1.0 ± 0.1 μm. The weakest inhibitor of these four compounds was V8 with a Ki of 7.5 ± 0.9 μm (Table 2). Suramin demonstrated the weakest binding to VahC of the inhibitors tested, with a dissociation constant (KD) of 482.7 ± 22.4 nm, but each of the inhibitors tested showed relatively similar binding affinity. Compound V8 bound with the highest affinity to VahC (KD = 185.8 ± 15.3 nm), and this binding was ∼3-fold tighter than with suramin. These inhibitors were also tested against other actin-targeting mARTs, namely Photox and SpvB, to assess their broad spectrum efficacy among some representative Type IV mART toxins. As with VahC, each compound was able to inhibit the mART activity of both Photox and SpvB to a comparable degree, as seen in Fig. 4C.
FIGURE 4.
Inhibition of VahC, Photox, and SpvB mART activity. A, structures of inhibitor compounds effective in reducing mART activity of VahC, Photox, and SpvB. B, IC50 determinations of VahC by suramin (white circles), V31 (black circles), V7 (white squares), and V8 (black squares). Activity loss was assessed as described under “Experimental Procedures.” C, relative inhibition of Photox (gray), VahC (black), and SpvB (white). Activities were compared in the presence and absence of each inhibitor at its respective IC50 concentration against VahC using reaction conditions described under “Experimental Procedures.” Error bars, S.D.
TABLE 2.
Characterization of small molecule inhibitors of VahC
IC50 values and inhibitory and dissociation constants were obtained as described under “Experimental Procedures” using the mART enzyme assay, the Cheng-Prusoff equation, and the fluorescence-quenching assay, respectively. These values represent means ± S.E. of the mean for at least three independent measurements.
| Inhibitor | IC50 | Ki | Kd |
|---|---|---|---|
| μm | μm | μm | |
| V7 | 85.8 ± 12.4 | 4.1 ± 0.6 | 259.0 ± 24.4 |
| V8 | 157.4 ± 17.9 | 7.5 ± 0.9 | 251.7 ± 11.9 |
| V31 | 86.3 ± 4.2 | 4.1 ± 0.2 | 185.8 ± 15.3 |
| Suramin | 20.3 ± 0.7 | 1.0 ± 0.1 | 482.7 ± 22.4 |
VahC Crystal Structure Shows Similarity to Homologous Actin-targeting mARTs
Although three monomers were detected in the asymmetric unit of the VahC crystal, the monomer represents the biologically relevant form (Fig. 5A and Table 3). VahC forms a typical mART active site with most of the relevant binding and catalytic residues (Tyr-77, Tyr-82, Asn-86, Arg-148, Ser-178, and Glu-215), showing no significant structural deviation from other members of the Type IV toxins (Fig. 5A, enlargement).
FIGURE 5.
High resolution (1.9 Å) crystal structure of VahC. A, crystal structure of VahC (PDB entry 4FML) shown as a schematic diagram. Secondary structural elements (α-helices; β, β-strands) are shown and numbered in succession. Adjacent to the VahC structure is an expanded view of the active site with the important binding/catalytic residues shown in stick format. Motif 1 residues are in violet, motif 2 residues are in orange, motif 3 residues are in magenta, and the proposed critical residues of the active site loop are in yellow. B, structural comparison of VahC (green) with SpvB (PDB entry 2GWM; yellow) based on an iterative three-dimensional alignment of protein backbone Cα atoms. C, structural comparison of VahC (green) with ι-toxin catalytic domain, Ia (PDB entry 1GIQ; magenta) based on an iterative three-dimensional alignment of protein backbone Cα atoms. The structural differences are highlighted by orange arrows.
Overall, the VahC structural topology is similar to that of the SpvB toxin from Salmonella enterica (alignment in Fig. 5B). However, it differs from the catalytic domains of other binary toxins (iota, VIP2, and C2I) in four areas (Fig. 5C). These include (i) the presence of a helix-loop insertion between helix α2 and α3, (ii) a shorter loop-helix region between β1 and β2, (iii) a shorter PN-loop region between β3 and β4, and (iv) the presence of new insertion helix-loop at the C terminus between β7 and β8.
The VahC crystal structure aligns best with that of SpvB (1.12 Å root mean square deviation for Cα atoms for 160 residues; PDB entry 2GWM). However, even with some structural differences visible in the VahC catalytic domain, the root mean square deviation values in key regions of the catalytic domains of iota (1.87 Å for 93 residues, PDB entry 1GIQ), VIP2 (1.31 Å for 92 residues, PDB entry 1QS1), and C2I (1.75 Å for 97 residues, PDB entry 2J3Z) highlight the strongly conserved active site structure of these Type IV mART toxins.
Significance of Surface Potential in Actin Recognition
The crystal structures of VahC and other actin binding toxins suggest the existence of at least two different substrate recognition models among the Type IV bacterial toxins/effectors that inhibit actin polymerization by modifying actin at Arg-177. One subtype, Ia/C2I, is formed by the binary actin-ADP-ribosylating toxin members, such as C2 toxin (Clostridium botulinum), ι-toxin (Clostridium perfringens), VIP2 (Bacillus cereus), CDT (Clostridium difficile), and CST (Clostridium spiriforme). These binary toxins contain a binding/translocation component and an enzymatic component. The enzymatic component consists of an N-terminal adaptor domain (that interacts with the binding domain) and a C-terminal ADP-ribosyltransferase domain. The second subtype, SpvB/VahC, includes toxins that are not binary in structure and include members such as SpvB (S. enterica), VahC (A. hydrophila), and Photox (Photorhabdus luminescens). In addition, AexT (A. hydrophila) is a bifunctional Exo-S/Exo-T-like enzyme, which exhibits both GTPase-activating protein and ADP-ribosyltransferase activity, resulting in depolymerization of the host cell actin cytoskeleton (35). SpyA (Streptococcus pyrogenes) is a C3-like ADP-ribosyltransferase, which directly modifies actin filaments (36). Although AexT and SpyA structures are not currently available, their homologue enzyme structures (e.g. C3-like mARTs for SpyA) suggest that their enzyme-substrate interface might differ from the aforementioned subtypes. VgrG1 (A. hydrophila) is another actin-targeting toxin and has sequence identity similar to VahC (19).
Iota (Ia) and SpvB are the best-characterized members of their respective subtypes (37, 38). A recent crystal structure of ι-toxin (Ia) with actin has shown that ι-toxin binds actin through five loops present in the catalytic and N-terminal adaptor domains (37). Sequence alignment of Type IV toxins that bind actin, however, showed that the binding residues in the loop regions are poorly conserved (Fig. 1). Further analysis of these regions based upon crystal structures (iota, C2I, VIP2, and SpvB) and also from the current high resolution crystal structure of VahC shows that the enzyme-substrate interface for these Type IV mARTs in general possesses a similar pattern for the electrostatic surface potential. However, the interface surface potential signature does show some unique differences for each of the two actin-targeting subtypes (Ia/C2I and SpvB/VahC) and can potentially be used as an identifier for each subtype (Fig. 6, A and B).
FIGURE 6.
Electrostatic potential representation of the Type IV mART toxins that ADP-ribosylate Arg-177 in α-actin. A, surface potential of the Ia/C2I subtype members that include ι-toxin (Ia) (magenta ribbon and surface potential models) and VIP2 and C2I (PDB entries 1QS1 and 2J3Z, respectively; surface potential models only). The five regions (1–5) of iota believed to be involved in actin substrate recognition are shown as numbered circles (green). B, surface potentials of the SpvB/VahC subtype toxins are shown, and the iota C-terminal catalytic domain is also shown for comparison. Potential regions that may be involved in actin substrate recognition are also highlighted with green circles. These regions (Regions 2 and 5) correspond to those regions highlighted for Ia/C2I (above) except that Region 6 is unique to the SpvB/VahC subtype. The C/ denotes the catalytic subunit of the toxin only. Molecular surfaces are colored by the relative electrostatic potential (red, negative or acidic; blue, basic or positive). Surface potentials were calculated using PyMOL APBS software (60).
DISCUSSION
Growth inhibition in yeast (Fig. 2C) and cytotoxicity in HeLa Tet-Off cells (Fig. 3) via ADP-ribosylation of actin at Arg-177 (Fig. 2B) support the classification of VahC as another member of the Type IV mART toxins. ADP-ribosylation of all actin isoforms is consistent with some Type IV mART toxins (Photox, SpvB, iota, Sa, and CDTa), but it is not observed in all cases (30, 39–41). This in turn leads to the disruption of the actin cytoskeleton and caspase-induced apoptosis, as observed in this study and others (Fig. 3) (42–45). The use of a catalytically inactive VahC construct (E213A/E215A; E/E in Fig. 2C) further demonstrated that yeast cytotoxicity was caused by the mART activity of VahC and not another unknown function associated with the protein/toxin. Furthermore, the sole target of VahC is actin because the R177A actin mutant conferred yeast resistance to the toxin (Fig. 2B). These results provide support for VahC as a virulence factor of A. hydrophila, which acts as a Type IV mART toxin capable of disrupting the actin cytoskeleton of mammalian cells, inducing caspase-mediated apoptosis.
Previous structural studies have shown that there are conserved residues in mART toxins that play key roles in actin ADP-ribosylation (37). Herein, we add credence to the importance of conserved residues within the three catalytic signature/motif regions of mART enzymes. In vitro and cell-based data from Ala-scanning mutagenesis provided an excellent perspective and coordinated view of the role of a number of key catalytic residues (Fig. 2C). Each critical catalytic residue resulted in a loss of activity and a restoration of yeast growth upon substitution with Ala. As expected, Ala substitutions within the classical (E/Q)XE motif caused the greatest loss in mART activity. Results showing loss of function from mutations to the conserved STS motif (S178A and T179A) further corroborate a conserved catalytic mechanism across the Type IV mART family. Additionally, we demonstrated the importance of Tyr-82 (equivalent to Tyr-251 in ι-toxin (Ia)) in the active site loop, supporting the previous results of Tsuge et al. (37) as a critical residue in actin ADP-ribosylation. Additionally, our in silico analysis identified two conserved residues also within the active site loop, Tyr-77 and Asn-86, that were shown to be critical for enzyme activity. The loss of mART activity may result from the lost ability of Asn-86 to hydrogen-bond with Tyr-77 (Fig. 5) and may point to a role for Tyr-77 in repositioning the N-ribose ring upon cleavage. The loss of function resulting from the Y77A and N86A substitutions fit with data obtained from mutagenesis of another actin-targeting mART, iota (Ia) (5). Residues Leu-89 and Leu-217 (Fig. 1) were also identified in silico as conserved residues, but we did not perform Ala-scanning mutagenesis at these sites because of their likely role in maintaining fold integrity as nonpolar, non-ionizable side chains.
Most mARTs possess “NAD+-ase” activity in the absence of a target substrate, but the physiological relevance of this activity is not yet understood (46, 47). Intriguingly, VahC, in line with some other actin-targeting toxins (Photox and SpvB), does not possess detectable NAD+-ase activity, and therefore the effect of point mutations could not be assessed on that basis (30, 40). Our in vitro results demonstrate that the observed mART activity loss in mutant enzymes is not due to (p > 0.05) a defect in NAD+-binding capabilities (Table 1, column 2) or structural perturbation (Trp emission λem(max) analysis). Additionally, comparison of Km values allowed us to indirectly confirm that loss of activity was in fact primarily due to a defect in transferase activity and not substrate(s) interaction. No mutant enzyme had Km values for either substrate that differed by more than an order of magnitude from the wild type, which suggests that loss of function is from a direct involvement of that residue(s) in the catalytic transferase mechanism (Table 1, columns 3 and 4). The statistically significant (p < 0.05) 2–3-fold reductions observed suggest only minor roles for Glu-215, Tyr-82, and Ser-178 in maintaining NAD+ substrate catalytic efficiency. Furthermore, no significant differences (p > 0.05) were observed for actin Km values. However, the kcat values for E213A, E215A, Y82A, S178A, and Y77A were all less than 7% of the WT value (Table 1, column 5). The only active site mutant that showed significant ADPRT activity was N86A (42% of WT activity; Table 1), and it may be explained because of its proposed role as a secondary catalytic residue (Fig. 1, active site loop) in stabilizing Tyr-77. The lack of any physiologically significant changes in KD and Km values supports the proposal that the observed losses in actin-ADP-ribosylation and yeast cytotoxicity among VahC mutants are from a loss of catalytic transferase ability.
The strong correlation between our in silico analysis, our cell-based and in vitro mART activity assays, and the crystal structure of the VahC catalytic domain bodes strongly for the precision and power of the bioinformatic method for the identification of new members of the mART toxin family (48, 49). Importantly, the mutagenesis and structural data support the proposal of a new motif in the Type IV mART family; a YX4YX3N motif in the active site loop that aids in catalysis. Furthermore, in silico examination revealed that this motif is also conserved across the Type III mART toxin family (target small GTPases), and we predict it also plays a critical role in this mART type.
The high resolution crystal structure of VahC shows the structural conservation of the active site motifs and important mART active site residues (Tyr-77, Tyr-82, Arg-148, Ser-178, and Glu-215) among the Type IV mART toxin family (Fig. 5). The overall structural topology and surface potential pattern at the proposed enzyme-(actin) substrate interface points to the existence of at least two subtypes of mART enzymes that show structural differences in substrate (actin) recognition (Fig. 6). Iota, VIP2, and C2 binary toxins belong to the first subtype, designated Ia/C2I, and show NAD+-glycohydrolase activity in addition to transferase activity. VahC, Spvb, and Photox belong to the second subtype, Spvb/VahC, and they lack NAD+-glycohydrolase activity due to the absence of essential residues (corresponding to Phe-349 in Ia) and a shorter PN loop (Region 4). The N-terminal adaptor portion of the enzymatic component of binary toxins (Region 1; Fig. 6A) is critical for actin recognition in the Ia/C2I subtype (40), whereas it is absent in the Spvb/VahC subtype toxins (Fig. 6B). The latter subtype contains a special 30-residue insertion (helix-loop segment) at the C terminus, which could probably substitute for the loss of the N-terminal adaptor element in these toxins, which are still able to recognize and modify actin. This subtype also contains a 20-residue helix-loop insertion following Region 2; however, the significance of this insertion is not yet understood.
In summary, the surface of binary toxins in Regions 3 and 4 is more basic, as can be seen in the iota catalytic domain (Fig. 6, A and B). Thus, binary toxins need aid from neighboring N-terminal domain hydrophobic side chains for sufficiently strong interaction with the actin substrate. If the N-terminal domain is removed/absent, then the actin-binding activity is lost, as has been demonstrated in the iota/C2I subtype (50). In the VahC/SpvB subtype, the 30-residue insertion helix-loop is made up of acidic and hydrophobic residues (Figs. 1 and 6B) and can probably compensate for the loss of Region 1 (absence of the N-terminal domain) and Region 4 (shorter PN loop) and can effectively aid in actin substrate binding.
A pathogenic organism found ubiquitously in aquatic environments such as A. hydrophila could readily become a serious human health concern, in addition to its known reputation as a problem in the fish industry (51). Information gleaned from the aftermath of hurricane Katrina (52) and studies of drinking water distribution systems (53) further emphasizes that the health threats of A. hydrophila are real. Previous efforts to identify virulence genes have laid an excellent foundation to understand and treat A. hydrophila as a widespread human pathogen (17). Indeed, strong evidence of direct transmission of Aeromonas species from water to humans leading to the disease state has recently been reported (54). In the present study, we have extensively characterized the effects both in vitro and in cell-based systems of a new virulence factor, VahC, from A. hydrophila. It is the newest member of the Type IV mART toxin family, differing from VgrG1 by its lack of a VgrG domain to mediate secretion through the Type VI secretion system. Future studies will elucidate the secretion mechanism of VahC in A. hydrophila PPD134/91, which we speculate may be the Type III secretion system. Secretion could be mediated by an interaction with the Type III secretion system chaperone genetically associated with the vahC gene identified by Yu et al. (17). This would not be unprecedented because the toxicity of a similar Type IV mART, SpvB, was shown to be directly linked to the Type III secretion system of Salmonella (55).
Remarkably, this is the first report of inhibition of a Type IV mART toxin family member. Given the high structural homology among Type IV mARTs at the catalytic core, it is probable that the inhibitors studied here (V7, V8, V31, and suramin) could be effective lead compounds for the development of inhibitory agents for this entire group of actin-targeting mART toxins. This hypothesis has been proven in principle, given that similar inhibition of VahC, Photox, and SpvB was also demonstrated for V7, V8, and V31 compounds. Therefore, the task of developing antivirulence therapeutics to actin-targeting mARTs may hinge on the discovery of a single high affinity, inhibitory agent. Inhibitors of virulence factors have an advantage over standard antibiotic treatment of infections due to a higher specificity and a reduced chance of the target pathogen developing resistance (56). Unfortunately, many bacterial pathogens are not powered by a single virulence factor (57), heightening the need to identify other candidate virulence factors as targets for anti-virulence therapy in a “mixture” approach.
Acknowledgments
We sincerely thank Dawn White and Robert Gale for cloning and mutagenesis work that contributed greatly to the project. We thank Dr. Robert Fieldhouse for helpful discussions about the VahC toxin. We thank Ryan Demers for the generous donation of β- and γ-actin.
Due to the actin purification process and the tendency of actin to polymerize at high concentrations, kinetic parameters were determined with actin concentrations no higher than 34 μm, which was near saturation for this substrate.
- mART
- mono-ADP-ribosyltransferase
- TEV
- tobacco etch virus
- EGFP
- enhanced GFP
- PDB
- Protein Data Bank.
REFERENCES
- 1. Miller M. B., Bassler B. L. (2001) Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199 [DOI] [PubMed] [Google Scholar]
- 2. Nizet V. (2007) Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J. Allergy Clin. Immunol. 120, 13–22 [DOI] [PubMed] [Google Scholar]
- 3. Clatworthy A. E., Pierson E., Hung D. T. (2007) Targeting virulence. A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3, 541–548 [DOI] [PubMed] [Google Scholar]
- 4. Cegelski L., Marshall G. R., Eldridge G. R., Hultgren S. J. (2008) The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Escaich S. (2008) Antivirulence as a new antibacterial approach for chemotherapy. Curr. Opin. Chem. Biol. 12, 400–408 [DOI] [PubMed] [Google Scholar]
- 6. Escaich S. (2010) Novel agents to inhibit microbial virulence and pathogenicity. Expert. Opin. Ther. Pat. 20, 1401–1418 [DOI] [PubMed] [Google Scholar]
- 7. Kozińska A., Pȩkala A. (2012) Characteristics of disease spectrum in relation to species, serogroups, and adhesion ability of motile aeromonads in fish. ScientificWorldJournal 2012, 949358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janda J. M., Abbott S. L. (2010) The genus Aeromonas. taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 23, 35–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edberg S. C., Browne F. A., Allen M. J. (2007) Issues for microbial regulation. Aeromonas as a model. Crit. Rev. Microbiol. 33, 89–100 [DOI] [PubMed] [Google Scholar]
- 10. Uçkay I., Sax H., Harbarth S., Bernard L., Pittet D. (2008) Multiresistant infections in repatriated patients after natural disasters. Lessons learned from the 2004 tsunami for hospital infection control. J. Hosp. Infect. 68, 1–8 [DOI] [PubMed] [Google Scholar]
- 11. Pepe C. M., Eklund M. W., Strom M. S. (1996) Cloning of an Aeromonas hydrophila type IV pilus biogenesis gene cluster. Complementation of pilus assembly functions and characterization of a type IV leader peptidase/N-methyltransferase required for extracellular protein secretion. Mol. Microbiol. 19, 857–869 [DOI] [PubMed] [Google Scholar]
- 12. Quinn D. M., Wong C. Y., Atkinson H. M., Flower R. L. (1993) Isolation of carbohydrate-reactive outer membrane proteins of Aeromonas hydrophila. Infect. Immun. 61, 371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y. L., Arakawa E., Leung K. Y. (2002) Novel Aeromonas hydrophila PPD134/91 genes involved in O-antigen and capsule biosynthesis. Infect. Immun. 70, 2326–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pemberton J. M., Kidd S. P., Schmidt R. (1997) Secreted enzymes of Aeromonas. FEMS Microbiol. Lett. 152, 1–10 [DOI] [PubMed] [Google Scholar]
- 15. Laohachai K. N., Bahadi R., Hardo M. B., Hardo P. G., Kourie J. I. (2003) The role of bacterial and non-bacterial toxins in the induction of changes in membrane transport. Implications for diarrhea. Toxicon 42, 687–707 [DOI] [PubMed] [Google Scholar]
- 16. Sha J., Wang S. F., Suarez G., Sierra J. C., Fadl A. A., Erova T. E., Foltz S. M., Khajanchi B. K., Silver A., Graf J., Schein C. H., Chopra A. K. (2007) Further characterization of a type III secretion system (T3SS) and of a new effector protein from a clinical isolate of Aeromonas hydrophila. Part I. Microb. Pathog. 43, 127–146 [DOI] [PubMed] [Google Scholar]
- 17. Yu H. B., Zhang Y. L., Lau Y. L., Yao F., Vilches S., Merino S., Tomas J. M., Howard S. P., Leung K. Y. (2005) Identification and characterization of putative virulence genes and gene clusters in Aeromonas hydrophila PPD134/91. Appl. Environ. Microbiol. 71, 4469–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fang F. C., Krause M., Roudier C., Fierer J., Guiney D. G. (1991) Growth regulation of a Salmonella plasmid gene essential for virulence. J. Bacteriol. 173, 6783–6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suarez G., Sierra J. C., Erova T. E., Sha J., Horneman A. J., Chopra A. K. (2010) A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J. Bacteriol. 192, 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armstrong S., Merrill A. R. (2001) Application of a fluorometric assay for characterization of the catalytic competency of a domain III fragment of Pseudomonas aeruginosa exotoxin A. Anal. Biochem. 292, 26–33 [DOI] [PubMed] [Google Scholar]
- 21. Turgeon Z., White D., Jørgensen R., Visschedyk D., Fieldhouse R. J., Mangroo D., Merrill A. R. (2009) Yeast as a tool for characterizing mono-ADP-ribosyltransferase toxins. FEMS Microbiol. Lett. 300, 97–106 [DOI] [PubMed] [Google Scholar]
- 22. Turgeon Z., Jørgensen R., Visschedyk D., Edwards P. R., Legree S., McGregor C., Fieldhouse R. J., Mangroo D., Schapira M., Merrill A. R. (2011) Newly discovered and characterized antivirulence compounds inhibit bacterial mono-ADP-ribosyltransferase toxins. Antimicrob. Agents Chemother. 55, 983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jørgensen R., Wang Y., Visschedyk D., Merrill A. R. (2008) The nature and character of the transition state for the ADP-ribosyltransferase reaction. EMBO Rep. 9, 802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., Terwilliger T. C. (2002) PHENIX. Building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 [DOI] [PubMed] [Google Scholar]
- 27. Emsley P., Cowtan K. (2004) Coot. Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 28. Delano W. L. (2002) The PyMOL User's Manual, DeLano Scientific, San Carlos, CA [Google Scholar]
- 29. Yates S. P., Otley M. D., Dawson J. F. (2007) Overexpression of cardiac actin with baculovirus is promoter-dependent. Arch. Biochem. Biophys. 466, 58–65 [DOI] [PubMed] [Google Scholar]
- 30. Visschedyk D. D., Perieteanu A. A., Turgeon Z. J., Fieldhouse R. J., Dawson J. F., Merrill A. R. (2010) Photox, a novel actin-targeting mono-ADP-ribosyltransferase from Photorhabdus luminescens. J. Biol. Chem. 285, 13525–13534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spudich J. A., Watt S. (1971) The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 246, 4866–4871 [PubMed] [Google Scholar]
- 32. Cheng Y., Prusoff W. H. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 [DOI] [PubMed] [Google Scholar]
- 33. Beattie B. K., Merrill A. R. (1999) A fluorescence investigation of the active site of Pseudomonas aeruginosa exotoxin A. J. Biol. Chem. 274, 15646–15654 [DOI] [PubMed] [Google Scholar]
- 34. Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. (1992) Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 10, 267–293 [DOI] [PubMed] [Google Scholar]
- 35. Fehr D., Burr S. E., Gibert M., d'Alayer J., Frey J., Popoff M. R. (2007) Aeromonas exoenzyme T of Aeromonas salmonicida is a bifunctional protein that targets the host cytoskeleton. J. Biol. Chem. 282, 28843–28852 [DOI] [PubMed] [Google Scholar]
- 36. Coye L. H., Collins C. M. (2004) Identification of SpyA, a novel ADP-ribosyltransferase of Streptococcus pyogenes. Mol. Microbiol. 54, 89–98 [DOI] [PubMed] [Google Scholar]
- 37. Tsuge H., Nagahama M., Oda M., Iwamoto S., Utsunomiya H., Marquez V. E., Katunuma N., Nishizawa M., Sakurai J. (2008) Structural basis of actin recognition and arginine ADP-ribosylation by Clostridium perfringens ι-toxin. Proc. Natl. Acad. Sci. U.S.A. 105, 7399–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pust S., Hochmann H., Kaiser E., von Figura G., Heine K., Aktories K., Barth H. (2007) A cell-permeable fusion toxin as a tool to study the consequences of actin-ADP-ribosylation caused by the Salmonella enterica virulence factor SpvB in intact cells. J. Biol. Chem. 282, 10272–10282 [DOI] [PubMed] [Google Scholar]
- 39. Gill D. M., Meren R. (1978) ADP-ribosylation of membrane proteins catalyzed by cholera toxin. Basis of the activation of adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 75, 3050–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakurai J., Nagahama M., Hisatsune J., Katunuma N., Tsuge H. (2003) Clostridium perfringens ι-toxin, ADP-ribosyltransferase. Structure and mechanism of action. Adv. Enzyme Regul. 43, 361–377 [DOI] [PubMed] [Google Scholar]
- 41. Aktories K., Lang A. E., Schwan C., Mannherz H. G. (2011) Actin as target for modification by bacterial protein toxins. FEBS J. 278, 4526–4543 [DOI] [PubMed] [Google Scholar]
- 42. Heine K., Pust S., Enzenmüller S., Barth H. (2008) ADP-ribosylation of actin by the Clostridium botulinum C2 toxin in mammalian cells results in delayed caspase-dependent apoptotic cell death. Infect. Immun. 76, 4600–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lesnick M. L., Reiner N. E., Fierer J., Guiney D. G. (2001) The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol. Microbiol. 39, 1464–1470 [DOI] [PubMed] [Google Scholar]
- 44. Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. (1986) Botulinum C2 toxin ADP-ribosylates actin. Nature 322, 390–392 [DOI] [PubMed] [Google Scholar]
- 45. Weigt C., Just I., Wegner A., Aktories K. (1989) Nonmuscle actin ADP-ribosylated by botulinum C2 toxin caps actin filaments. FEBS Lett. 246, 181–184 [DOI] [PubMed] [Google Scholar]
- 46. Beattie B. K., Prentice G. A., Merrill A. R. (1996) Investigation into the catalytic role for the tryptophan residues within domain III of Pseudomonas aeruginosa exotoxin A. Biochemistry 35, 15134–15142 [DOI] [PubMed] [Google Scholar]
- 47. Collier R. J. (2001) Understanding the mode of action of diphtheria toxin. A perspective on progress during the 20th century. Toxicon 39, 1793–1803 [DOI] [PubMed] [Google Scholar]
- 48. Fieldhouse R. J., Merrill A. R. (2008) Needle in the haystack. Structure-based toxin discovery. Trends Biochem. Sci. 33, 546–556 [DOI] [PubMed] [Google Scholar]
- 49. Fieldhouse R. J., Turgeon Z., White D., Merrill A. R. (2010) Cholera- and anthrax-like toxins are among several new ADP-ribosyltransferases. PLoS Comput. Biol. 6, e1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsuge H., Nagahama M., Nishimura H., Hisatsune J., Sakaguchi Y., Itogawa Y., Katunuma N., Sakurai J. (2003) Crystal structure and site-directed mutagenesis of enzymatic components from Clostridium perfringens ι-toxin. J. Mol. Biol. 325, 471–483 [DOI] [PubMed] [Google Scholar]
- 51. Monette S., Dallaire A. D., Mingelbier M., Groman D., Uhland C., Richard J. P., Paillard G., Johannson L. M., Chivers D. P., Ferguson H. W., Leighton F. A., Simko E. (2006) Massive mortality of common carp (Cyprinus carpio carpio) in the St. Lawrence River in 2001. Diagnostic investigation and experimental induction of lymphocytic encephalitis. Vet. Pathol. 43, 302–310 [DOI] [PubMed] [Google Scholar]
- 52. Presley S. M., Rainwater T. R., Austin G. P., Platt S. G., Zak J. C., Cobb G. P., Marsland E. J., Tian K., Zhang B., Anderson T. A., Cox S. B., Abel M. T., Leftwich B. D., Huddleston J. R., Jeter R. M., Kendall R. J. (2006) Assessment of pathogens and toxicants in New Orleans, LA following Hurricane Katrina. Environ. Sci. Technol. 40, 468–474 [DOI] [PubMed] [Google Scholar]
- 53. Jeppesen M. G., Ortiz P., Shepard W., Kinzy T. G., Nyborg J., Andersen G. R. (2003) The crystal structure of the glutathione S-transferase-like domain of elongation factor 1Bγ from Saccharomyces cerevisiae. J. Biol. Chem. 278, 47190–47198 [DOI] [PubMed] [Google Scholar]
- 54. Khajanchi B. K., Fadl A. A., Borchardt M. A., Berg R. L., Horneman A. J., Stemper M. E., Joseph S. W., Moyer N. P., Sha J., Chopra A. K. (2010) Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples. Suggestive evidence of water-to-human transmission. Appl. Environ. Microbiol. 76, 2313–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Browne S. H., Hasegawa P., Okamoto S., Fierer J., Guiney D. G. (2008) Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol. Med. Microbiol. 52, 194–201 [DOI] [PubMed] [Google Scholar]
- 56. Schmitt C. K., Meysick K. C., O'Brien A. D. (1999) Bacterial toxins. Friends or foes? Emerg. Infect. Dis. 5, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu H. J., Wang A. H., Jennings M. P. (2008) Discovery of virulence factors of pathogenic bacteria. Curr. Opin. Chem. Biol. 12, 93–101 [DOI] [PubMed] [Google Scholar]
- 58. Taly J. F., Magis C., Bussotti G., Chang J. M., Di Tommaso P., Erb I., Espinosa-Carrasco J., Kemena C., Notredame C. (2011) Using the T-Coffee package to build multiple sequence alignments of protein, RNA, DNA sequences, and three-dimensional structures. Nat. Protoc. 6, 1669–1682 [DOI] [PubMed] [Google Scholar]
- 59. Gouet P., Courcelle E., Stuart D. I., Métoz F. (1999) ESPript. Analysis of multiple sequence alignments in PostScript. Bioinformatics. 15, 305–308 [DOI] [PubMed] [Google Scholar]
- 60. Baker N. A., Sept D., Joseph S., Holst M. J., McCammon J. A. (2001) Electrostatics of nanosystems. Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]