Abstract
Huntington disease (HD) is a neurodegenerative disease caused by expansion of CAG repeats in the huntingtin (Htt) gene. The expression of hMTH1, the human hydrolase that degrades oxidized purine nucleoside triphosphates, grants protection in a chemical HD mouse model in which HD-like features are induced by the mitochondrial toxin 3-nitropropionic acid (3-NP). To further examine the relationship between oxidized dNTPs and HD-like neurodegeneration, we studied the effects of hMTH1 expression in a genetic cellular model for HD, such as striatal cells expressing mutant htt (HdhQ111). hMTH1 expression protected these cells from 3-NP and H2O2-induced killing, by counteracting the mutant htt-dependent increased vulnerability and accumulation of nuclear and mitochondrial DNA 8-hydroxyguanine levels. hMTH1 expression reverted the decreased mitochondrial membrane potential characteristic of HdhQ111 cells and delayed the increase in mitochondrial reactive oxygen species associated with 3-NP treatment. Further indications of hMTH1-mediated mitochondrial protection are the partial reversion of 3-NP-induced alterations in mitochondrial morphology and the modulation of DRP1 and MFN1 proteins, which control fusion/fission rates of mitochondria. Finally, in line with the in vitro findings, upon 3-NP in vivo treatment, 8-hydroxyguanine levels in mitochondrial DNA from heart, muscle and brain are significantly lower in transgenic hMTH1-expressing mice than in wild-type animals.
Abbreviations: HD, Huntington disease; Htt, huntingtin; polyQ, polyglutamine; 3-NP, 3-nitropropionic acid; ROS, Reactive oxygen species; 8-oxodG, 8-hydroxyguanine; DDR, DNA damage response; BER, Base excision repair; DSBs, Double strand breaks; DRP1, Dynamin related protein; MFN1, Mitofusin 1
Keywords: Oxidative stress, Mitochondria, hMTH1, 8-hydroxyguanine
Highlights
► hMTH1 provides defence against cell death in mutant htt-expressing striatal cells ► hMTH1 improves mitochondrial functionality in mutant htt-expressing striatal cells ► hMTH1 protects in vivo mitochondria from endogenous and 3-NP induced oxidation
Introduction
Huntington disease (HD) is a progressive neurodegenerative disorder with autosomal dominant inheritance (Bates, 2005). The cause of the disease is an abnormal expansion of CAG repeats in the first exon of the gene coding for huntingtin (Htt) (Group and disease, 1993). The normal and expanded allele sizes have been defined respectively as CAG6–37 and CAG35–121 repeats and expression of Htt with an extended polyglutamine (polyQ) stretch is harmful and results in a selective neuronal loss in the brain, particularly in the striatum.
3-nitropropionic acid (3-NP), an irreversible inhibitor of succinate dehydrogenase and a mitochondrial toxin, has been extensively used as a chemical model for HD. The metabolic impairment produced by 3-NP is associated with oxidative stress (Fontaine et al., 2000). Both in primates (Brouillet et al., 1995) and in mice (Brouillet et al., 1999) chronic exposure to 3-NP is capable to replicate most of the clinical and pathophysiological hallmarks of HD, including dystonic movements, cognitive deficits and progressive striatal degeneration. Mice carrying wild-type HdhQ7 and mutant HdhQ111 knockin Htt gene (Mangiarini et al., 1996; Wheeler et al., 1999), and striatal cell lines derived from them (Trettel et al., 2000), provide alternative experimental HD models. Because the mutant Htt affects a variety of cellular processes, the nature of the toxic insult is still not fully understood. Indeed mutant Htt expression in both neuronal and non-neuronal cells is highly pleiotropic. It is associated with major changes in transcription, the formation of intraneuronal aggregates/inclusion containing the abnormal protein, impaired intracellular trafficking and energy metabolism and increased oxidative DNA damage (Browne and beal, 2006; DiFiglia et al., 1997; Lin and Beal, 2006; Sorolla et al., 2008; Wyttenbach et al., 2002). Besides its direct effects, mutant Htt expression is also known to increase the susceptibility to a concomitant stressful challenge. Therefore, to fully depict the cell dysfunction caused by mutant Htt, 3NP is often used as a second challenge. It is generally recognized that formation of reactive oxygen species (ROS) and subsequent oxidative stress play a major role in the neurodegeneration associated with HD (Bertoni et al., 2011; Bogdanov et al., 2001; Browne et al., 1999; Giuliano et al., 2003; Polidori et al., 1999). Increased oxidative damage to DNA, proteins and lipids has been reported in HD both in humans and in mouse models (for review see ref. Lin and Beal, 2006). In particular, findings of increased levels of DNA 8-hydroxyguanine (8-oxodG) have been reported in post-mortem brains of HD patients (Polidori et al., 1999) and during the progression of the disease in R6/2 mice (Bogdanov et al., 2001). Htt-associated oxidative stress is also accompanied by DNA breaks and activation of a DNA damage response (DDR) identifiable in the accumulation of phosphorylated ATM/ATR proteins in Htt-expressing PC12 cells or in fibroblasts from HD patients (Bertoni et al., 2011; Giuliano et al., 2003).
Several DNA repair systems protect mammalian cells against the accumulation of 8-oxodG in the genome. The major one is the base excision repair (BER) pathway, which via the OGG1 glycosylase directly removes this oxidized base from DNA. Another significant level of protection is provided by a family of hydrolases which eliminates oxidized precursors from the dNTP/NTP pool (Ishibashi et al., 2003). hMTH1, the major human 8-oxodGTPase, degrades both 8-oxodGTP and 8-oxoGTP to the corresponding monophosphates, and prevents the incorporation of 8-oxoG into DNA and RNA (Hayakawa et al., 1999; Sakumi et al., 1993). Studies with mth1−/− mice exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrine identified a major protective role of MTH1 in dopaminergic neurons in a mouse model for Parkinson's disease (Yamaguchi et al., 2006) and in hippocampal microglia during kainate-induced excitotoxicity (Kajitani et al., 2006). Complementary to these observations, transgenic mice expressing the human MTH1 hydrolase are protected against 3-NP-induced HD-like striatal neurodegeneration and motor impairment (De Luca et al., 2008). In addition hMTH1 expression in HdhQ111 progenitor striatal cell lines containing Htt gene with expanded CAG repeats protected them against the toxicity associated with the mutant Htt (Ventura et al., 2010). hMTH1 is localized both in the cytosol and in the mitochondrial matrix and contributes to the sanitization of both nuclear and mitochondrial dNTP pools (Kang et al., 1995).In view of the effects of hMTH1 on these two targets, here we report an investigation of the mechanisms underlying the hMTH1-mediated defence against HD-associated neurodegeneration. We show that although hMTH1 protects both nuclear and mitochondrial cellular compartments against oxidative damage, the major factor in hMTH1-mediated neuroprotection is improved mitochondrial functionality.
Methods
Striatal cell cultures, DNA transfection and measurements of cell death
Cells derived from wild-type and mutant htt knockin mice (HdhQ7 and HdhQ111) (Coriell Cell Repositories, Camden, NJ, US) were routinely grown at 33 °C in high-glucose DMEM (Lonza, Basel, CH) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) (complete medium). Following transfection with Lipofectamine (Invitrogen Life Technologies, Carlsbad, CA, USA) of exponentially growing HdhQ111 cells with pcDEB∆ (De Luca et al., 2008), hygromycin-resistant clones were isolated after approximately 20 days growth in selective medium (200–300 mg/ml Hygromycin, Roche, Basel, CH). Survival was determined by clonogenic assay after a 24 hr treatment in serum-free DMEM with 3-NP or 15 min exposure to H2O2 in 20 mM Hepes containing complete medium. Cultures (100–200 cells/dish) were treated with the drug 18 hr after seeding, fed with complete medium and 1–2 weeks later surviving colonies were fixed, stained with Giemsa and counted. The number of colonies in treated cells was expressed as a percentage of that in untreated cells.
Analysis of 8-oxodG by HPLC/EC
8-oxodG was measured by high-performance liquid chromatography with electrochemical detection (HPLC/EC) following DNA extraction, RNase treatment, and enzymatic hydrolysis. DNA was extracted by a high-salt protein precipitation method that avoids possible artifacts. Briefly, cells were lysed in 1 ml of Tris–HCl 10 mM pH 8, EDTA 10 mM, NaCl 10 mM and SDS 0.5%, treated for 1 hr at 37 °C with RNAse (20 μg/ml) and digested overnight with 1 mg/ml proteinase K (Qiagen, Milan, Italy). Proteins were precipitated by adding NaCl to 1.5 M, and DNA in the supernatant was collected by addition of 2 volumes of ethanol. The DNA pellet was resuspended in 10 mM Tris-EDTA. Enzymatic digestion was then performed at 37 °C with nuclease P1 (Boehringer Mannheim, Monza, Italy) for 2 hr and alkaline phosphatase (Boehringer Mannheim) for 1 hr. Enzymes were precipitated by addition of CHCl3, and the upper layer was stored for analysis of 8-oxodGua at 80 °C under N2.
Mitochondria from striatal cell lines were prepared using the Pierce Mitochondrial isolation kit (Thermo Fisher Scientific, Rockford, IL, USA) and mtDNA was prepared using the protocol described above. The PromoKine Mitochondrial DNA isolation kit (PromoCell GmbH, Heidelberg, Germany) was used to isolate mtDNA from mouse tissues.
The DNA hydrolysate was analyzed by HPLC/EC (Coulochem; ESA Inc., Thermo Scientific) with a C18 5 μm Uptishere column (250 by 46 mm; Interchim) equipped with a C18 guard column. The eluent was 50 mM ammonium acetate, pH 5.5, containing 9% methanol, at a flow rate of 0.7 ml/min. The potentials applied were 150 and 400 mV for E1 and E2, respectively. The retention time of 8-oxodG was 23 min. Deoxyguanosine was measured in the same run of corresponding 8-oxodG with a UV detector (model SPD-2A; Shimadzu) at 256 nm; the retention time was 17 min.
Western blot analysis
Cell extracts were prepared from 106 cells lysed in RIPA Buffer (Tris–HCl 50 mM pH 7.4), NaCl 150 mM, EDTA 1 mM (pH 8), 1% NP40, NaF 1 mM, and a protease inhibitor cocktail tablets (Complete mini, Roche) for 1 hr on ice and then centrifuged at 14,000 rpm for 30 min. Protein concentration was evaluated using the Bradford method and 20–40 μg of total extract was separated on SDS polyacrilamide gels, transferred to nitrocellulose membranes (Whatman) with a TransBlot cell apparatus (Bio-Rad), and probed using the indicated primary antibodies: overnight at 4 °C with rabbit anti-hMTH1 (1:500, a kind gift of Y. Nakabeppu), rabbit anti-DRP1 (1:500, Abcam, Cambridge, UK) and mouse anti-MFN1 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies followed by the appropriate secondary antibody. ECL detection reagents (Invitrogen) were used to develop the blots. Anti-PCNA (1:5000, Santa Cruz Biotechnology) and anti-MnSOD (1:2000, Assay Designs/Stressgen, Enzo Life Sciences) antibodies were used as loading controls.
Measurement of mitochondrial membrane potential (∆ψm)
The potentiometric dye TMRE (tetramethylrhodamine ethyl ester perchlorate) was used to estimate mitochondrial inner membrane potential (∆ψm) by the “redistribution” method (Duchen et al., 2003), which is adequate for the comparison of ∆ψm between populations of cells. Loading of the dye was achieved by keeping the cells for 30 min in the presence of TMRE 30 nM before recording, in order to reach equilibrium between cell compartments and between cytoplasm and extracellular space (the external solution), where the loading concentration of the dye was maintained throughout the experiment. The saline solution used for loading and recording had the following composition: NaCl 140 mM, KCl5 mM, CaCl2 2.5 mM, MgCl2 1 mM, d-glucose 10 mM, HEPES/NaOH 10 mM (RT, pH 7.4, 290 mOsmol l− 1).
An oil immersion objective (Olympus: 40 ×, 1.35 NA) mounted on an inverted microscope (Axiovert 135, Zeiss; Germany) was utilized for fluorescence video imaging. An excitation wavelength of 535 nm was applied by means of a monochromator (Till Photonics, Polychrome II; Germany) and the emission light at 590 nM was collected by a CCD, cooled digital camera (PCO, Sensicam; Germany) and recorded on the hard disk of a PC computer. The Imaging Workbench 6.0 software package (Indec BioSystems; CA, USA) was used for recording and off-line analysis of the data. The software allowed the measurement of the emission values also along line profiles crossing mitochondria. A minimum of two peaks of amplitude was used to calculate the average amplitude of a given mitochondrion. Since the organelles were densely packed around a mitochondria-free area corresponding to the nucleus and single mitochondria were detectable only in the periphery of a cell, only mitochondria in this latter area were chosen for analysis. TMRE-loaded cultures were also used to evaluate mitochondrial morphology and to classify the cells accordingly.
ROS measurements
Proliferating striatal cell lines (2-4 × 105 cells in 60-mm dish) were treated for increasing time periods with 3-NP 10 mM, washed with PBS, incubated for 30 min in the dark at RT with CM-H2DCFDA (10 μM final concentration) (Invitrogen), trypsinized and resuspended in PBS/EDTA. Mitochondrial ROS levels were quantified as described by the manufacturer (Molecular Probes, Invitrogen). Before harvesting, cells were incubated with MitoSOX™ Red reagent 5 μM (Molecular Probes, Invitrogen) for 10 min. Cells were washed with PBS, collected, and kept on ice in the dark for immediate detection with the flow cytometer. Fluorescence was measured on a FACScan (Becton‐Dickinson, BD Biosciences) using excitation/emission wavelengths of 485/535 nm, and 510/580 nm for CM-H2DCFDA and MitoSox respectively. The values were expressed as mean fluorescence of the cell population.
Immunofluorescence analysis
Cultures were fixed with 4% PFA in PBS for 15 min. After fixation, cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min at RT and incubated, for the blocking step, for 60 min at RT with PBS containing 3% BSA/0.1% Triton X-100. Cells were incubated with the primary antibodies: anti-γ-H2AX (Millipore, Billerica, MA, USA) (1:1000 dilution, 1 hr at RT), anti-MnSOD (1:600 dilution, 2 hr at RT) (Assay Designs/Stressgen), and anti-DRP1 (1:500, Abcam). As secondary antibodies, Goat anti-rabbit IgG FITC-conjugated (1:200, Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) were used. Nuclei were stained using Hoechst-33258 (5 μg/ml for 20 min). Coverslips were mounted with Vectashield Mounting Medium and examined using an Eclipse 80i Nikon Fluorescence Microscope, equipped with a VideoConfocal (ViCo) system or examined using a Leica DM4000B fluorescence microscope equipped with a DFC420C digital camera and Leica Application Suite Software (260RI) for image acquisition (Leica, Wetzlar, Germany). For analysis of γ-H2AX foci, at least 200 nuclei were examined, and foci were scored at × 60 magnification. Only nuclei showing more than five bright foci were counted as positive.
In vivo treatment with 3-NP
Animal care, genotyping and all animal procedures were carried out according to EU Directive 86/609/EEC and to Italian legislation on animal experimentation. A previously described colony of hMTH1-Tg mice was generated at our Institute and hMTH1-Tg animals were genotyped as previously reported (De Luca et al., 2008). All animals were housed under standardized temperature, humidity and on a 12 hr light-12 hr dark cycle with free access to water and food. 3-NP (Sigma-Aldrich, Milan, Italy) was dissolved in PBS (pH adjusted with NaOH) and administered twice a day (9.00 am and 16.00 pm) at the dose of 60 mg/kg i.p. Wild-type and hMTH1-Tg mice were used. Baseline body weight was determined before the start of the experiment and weight was monitored daily, immediately before the 9.00 injection. Animals were killed by decapitation 16 hr after the last injection and muscle, heart and brain areas were removed and immediately frozen in liquid nitrogen.
Results
hMTH1 protects against mutant Htt-associated cell death during oxidative stress
We have previously shown that hMTH1 overexpression reverted the increased sensitivity of HdhQ111 cells towards 3-NP induced oxidative stress, without significantly affecting killing in HdhQ7 cells (De Luca et al., 2008). Here we investigated the molecular mechanisms underlying the protective role provided by hMTH1 against Htt-mediated changes, in an early-passage clone of HdhQ111 cells (passage 7) transfected with hMTH1 cDNA.
A pooled population of clones expressing hMTH1 was used for comparative studies with the parental cells as well as the wild-type HdhQ7 striatal cells. Levels of hMTH1 expression were similar to those previously published and obtained in an hMTH1-transfectant clone derived from late passage HdhQ111 cells (> 20 passages) (Fig. 1A) (De Luca et al., 2008). As expected, the mouse MTH1 protein was not detectable in western blots probed with an antibody raised against hMTH1. HdhQ111 cells are more sensitive than wild-type HdhQ7 cells to killing by 3-NP (Gines et al., 2003; Ruan et al., 2004), and this sensitivity is reversed by hMTH1 overexpression (De Luca et al., 2008). These findings were confirmed in the low-passage hMTH1-expressing HdhQ111 cells (Fig. 1B). hMTH1 expression also protected HdhQ111 cells against H2O2-induced death (Fig. 1C). Thus hMTH1 defence is not limited to cell death induced by a selective mitochondrial toxin, but it is effective also against a more generalized state of oxidative stress induced by an unspecific toxic agent such as H2O2.
Fig. 1.
Protection conferred by hMTH1 against cytotoxicity induced by 3-NP and H2O2. A. Early passage (passage 7) HdhQ111 striatal cells (lane 5) were transfected with hMTH1 cDNA (lane 4) and proteins were separated and probed with an antibody against hMTH1. hMTH1 expression in the neuroblastoma SH-SY5Y cell line, (lane 3), late passage HdhQ111 cells (lane 2) and their hMTH1 transfectant (lane 1) is shown for comparison. B. Survival measured by clonal assays in HdhQ7, HdhQ111 and HdhQ111–hMTH1 cells after a 24 hr exposure to 3-NP. C. Survival measured by clonal assays in HdhQ7, HdhQ111 and HdhQ111–hMTH1 cells after a 15 min exposure to H2O2. Data are the mean ± SE from 3 experiments. The asterisks indicate significant differences by Student's t-test (p < 0.05).
hMTH1 reduces 8-oxodG levels in mitochondrial and nuclear DNA
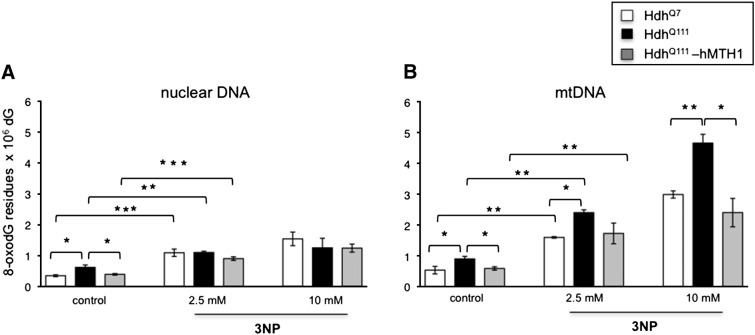
To examine whether hMTH1 expression influenced DNA 8-oxodG levels, we measured steady-state and oxidant-induced 8-oxodG in nuclear as well as in mitochondrial DNA (mtDNA). In agreement with several reports (Richter et al., 1988; Souza-Pinto et al., 2001) basal levels of 8-oxo-dG were higher in mitochondrial than in nuclear DNA — approximately 1.5-fold in each of the striatal cell lines (Figs. 2A and B, control bars). Furthermore, expression of the mutant Htt gene in HdhQ111 cells is associated with an additional 1.5-fold increase in both nuclear and mtDNA 8-oxodG oxidation. hMTH1 expression in HdhQ111 transfectants decreased DNA 8-oxo-dG to wild-type levels in both cellular compartments (Figs. 2A and B).
Fig. 2.
Levels of 8-oxodG measured by HPLC-EC in striatal cell lines exposed to 3-NP. 8-oxodG was measured in nuclear (A) and mtDNA (B) extracted from HdhQ7 (open bar), HdhQ111 (black bar) and HdhQ111–hMTH1 (gray bar) cells either untreated or following a 24 hr exposure to 2.5 and 10 mM 3-NP. Data are the mean ± SE from 3 experiments. The asterisks indicate significant differences by Student's t-test (*p < 0.05; **p < 0.01; ***p < 0.001).
A 24 hr exposure to 3-NP (2.5 and 10 mM) induced a clear dose-dependent increase in mtDNA 8-oxodG (increases ranging from 3 to > 5-fold) (Figs. 2A and B). Expression of mutant Htt resulted in higher levels of mtDNA oxidation in comparison to wild-type cells (1.5-fold) and this increase was reverted by hMTH1 expression (Fig. 2B). In contrast, 3-NP-induced oxidation was less pronounced in nuclear DNA, with no differential oxidation in cells expressing mutant Htt and/or hMTH1 expression (Fig. 2A).
Following exposure to H2O2 (range 0.25–1 mM) levels of 8-oxodG increased in both nuclear and mitochondrial DNA and expression of a mutant Htt was generally associated with enhanced DNA 8-oxo-dG levels (Figs. 3A and B). hMTH1 conferred significant protection against nuclear DNA oxidation over a range of H2O2 concentrations (Fig. 3A). Statistically significant differences in mtDNA 8-oxo-G were observed only in cells exposed to the lowest H2O2 dose (0.25 mM) (Fig. 3B), although a similar trend was evident following exposure to highly toxic H2O2 doses (0.5 and 1 mM). It is possible that the protection provided by hMTH1 is masked by the high variability in the cellular response to highly toxic H2O2 doses (Fig. 3B).
Fig. 3.
Levels of 8-oxodG measured by HPLC-EC in striatal cell lines exposed to H2O2. 8-oxodG was measured in nuclear (A) and mtDNA (B) extracted from HdhQ7 (open bar), HdhQ111 (black bar) and HdhQ111–hMTH1 (grey bar) cells either untreated or following a 15 min exposure to 0.25, 0.5 and 1 mM H2O2. Data are the mean ± SE from 3 experiments. The asterisks indicate significant differences by Student's t-test (*p < 0.05; **p < 0.01).
We conclude from these data that sanitization of the dNTP pool by hMTH1 protects both nuclear and mitochondrial DNA from the increased susceptibility to oxidation due to Htt-expression.
Modulation of γH2AX foci by Htt and hMTH1
To examine whether the Htt-dependent increase in steady-state DNA oxidation resulted in the formation of DNA double strand breaks (DSBs), γH2AX foci were measured in striatal cells. In the absence of exogenous oxidant, a significantly higher percentage of HdhQ111 cells than HdhQ7 cells were γH2AX-positive. hMTH1 expression in HdhQ111 cells reverted this type of DNA damage to the levels observed in HdhQ7 cells (Fig. 4).
Fig. 4.
Induction of γH2AX foci by 3-NP and H2O2 in HdhQ7, HdhQ111 and HdhQ111–hMTH1 cells. A. The number of cells positive for γH2AX foci was determined in the striatal cell lines exposed for 24 hr to 0 (control), 2.5 and 10 mM 3-NP in serum-free media. B. The number of cells positive for γH2AX foci was determined in the striatal cell lines either untreated or exposed for 15 min to increasing concentrations of H2O2. Data are the mean ± SE from 3 experiments. The asterisks indicate significant differences by Student's t-test (p < 0.01). HdhQ7 (open bar), HdhQ111 (black bar) and HdhQ111–hMTH1 (gray bar).
3-NP treatment increased the fraction of γH2AX foci-positive HdhQ7 and HdhQ111 cells. hMTH1 expression in HdhQ111 cells was associated with a significant reduction in the frequency of positive cells (Fig. 4A). In contrast these effects were not appreciated in H2O2-treated cells and both the Htt-dependent increase and the hMTH1-dependent reduction in the number of γH2AX-positive cells were not statistically significant in the three cell populations (Fig. 4B). Thus it is possible that a large fraction of these breaks derives from direct DNA strand breakage and is not affected by the level of oxidized dNTPs.
As before hMTH1 expression counteracts the Htt-dependent increase in DSBs generated by oxidative stress. Furthermore we confirm the specific susceptibility of HdhQ111 cells to mitochondrial damage induced by 3NP.
hMTH1 expression alters mitochondrial membrane potential
The mitochondrial dysfunction in striatal neurons of HD is associated with a lower membrane potential and depolarization at lower calcium loads than mitochondria from controls (Lim et al., 2008; Panov et al., 2002). Similarly mutant Htt expression in HdhQ111 cells has been shown to be associated with a lower mitochondrial inner membrane potential (∆ψm) compared to HdhQ7 cells (Lim et al., 2008) and this observation was confirmed in our experimental conditions (data not shown). Here we investigated whether hMTH1 expression could influence the affected mitochondrial membrane potential in HdhQ111 cells. For the analysis, performed by the TMRE “redistribution” method, single mitochondria located at the cell periphery were chosen (Fig. 5A). hMTH1 expressing cells showed a significantly higher TMRE fluorescence signal (p < 0.01) in comparison to the parental HdhQ111 cells (Fig. 5B), thus suggesting that the respiratory chain dysfunction caused by mutant Htt expression is reversed by hMTH1 overexpression.
Fig. 5.
Measurement of mitochondrial membrane potential in HdhQ111 and HdhQ111–hMTH1 cells. A. The panel shows a TMRE-loaded HdhQ111 cell with single peripheral mitochondria (arrows) chosen for membrane potential measurement. B. TMRE-loaded cells were excited with 535 λ and fluorescence intensity was recorded. The amplitudes of the fluorescence signals in single mitochondria were averaged and shown in the graph as indication of ∆ψm in the three cell lines. Data are the mean ± SE from 4 experiments. Asterisks indicate significant differences by Student's t-test (p < 0.01). HdhQ111 (black bar) and HdhQ111–hMTH1 (gray bar).
Reactive oxygen species measurements
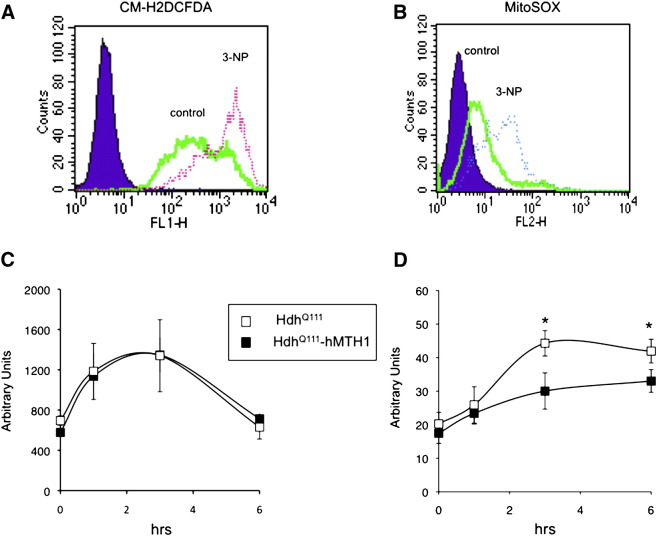
Expression of a mutant Htt protein in neuronal cells is associated with increased ROS levels (Bogdanov et al., 2001; Hands et al., 2011; Polidori et al., 1999; Tabrizi et al., 2000; Firdaus et al., 2006). To investigate whether hMTH1 expression influenced ROS production, intracellular ROS were measured by two fluorogenic dyes, the chloromethyl derivative of dihydrofluorescein diacetate (CM-H2DCFDA) and MitoSOX staining. The first one is a nonfluorescent dye that is oxidized into highly green fluorescence when reacting with peroxides or peroxide-derived reactive species, while the second one detects superoxides in the mitochondria of live cells. The intensity of fluorescence generated by these dyes is proportional to the amount of ROS and can be quantified by FACS analysis (Mukhopadhyay et al., 2007). Examples are shown in Figs. 6A and B. HdhQ111 and hMTH1- HdhQ111 cells showed similar basal ROS levels as measured by CM-H2DCFDA and MitoSOX (Figs. 6C and D, 0 hr). Exposure to 10 mM 3-NP for 1, 3 or 6 hr resulted in a significant increase in ROS production in both cell lines. This increase was unaffected by hMTH1 expression when total cellular ROS were evaluated (Fig. 6C), while a significant decrease was observed in the levels of mitochondrial ROS detected by MitoSOX staining (Fig. 6D).
Fig. 6.
Measurements by flow cytometry of total and mitochondrial ROS in HdhQ111 and HdhQ111–hMTH1 cells. Analysis by flow cytometry of CM-H2DCFDA (A) and MitoSOX (B) fluorescence in an untreated and 3-NP-treated HdhQ111 cell population. Cytofluorimetric determinations of total ROS by CM-H2DCFDA (C) and mitochondrial ROS by MitoSOX (D) in HdhQ111 and HdhQ111–hMTH1 cells either untreated or exposed for 1, 3 and 6 hr to 10 mM 3-NP. Data are the mean ± SE from 3 experiments. The asterisks indicate significant differences by Student's t-test (p < 0.05). HdhQ111 (open square) and HdhQ111–hMTH1 (closed square).
We conclude that although hMTH1 expression does not affect overall steady-state ROS levels, it does decrease the production of ROS in response to a mitochondrial toxin such 3-NP. Increased ROS levels can be considered an early sign of mitochondrial dysfunction (Liot et al., 2009), and our data indicate that hMTH1 confers significant protection against the effects of this mitochondrial toxin that dramatically affects mitochondria bioenergetics.
hMTH1 affects mitochondrial morphology
3-NP-induced mitochondrial dysfunction can be accompanied by changes in mitochondrial morphology (Liot et al., 2009). Confocal imaging was used to determine whether hMTH1 induced protection was paralleled by changes in mitochondrial morphology.
Immunofluorescence examination indicated that untreated HdhQ111 and HdhQ111–hMTH1 striatal cells all contained mitochondria with a clear reticular/ramified (rod-like) shape, and very few spherical mitochondria, which are a typical sign of mitochondrial dysfunction, were observed (Figs. 7A and B).
Fig. 7.
Morphological changes in mitochondria induced by exposure of HdhQ111 and HdhQ111–hMTH1 cells to 3-NP. Mitochondrial morphology was visualized by confocal immunofluorescence in cells labelled with the superoxide dismutase type 2 (Mn-SOD) antibody. Untreated HdhQ111 (A) and HdhQ111–hMTH1 (B) cells are shown above the same cell lines (C and D, respectively) treated for 24 hr with 10 mM 3-NP. Examples of spheric, reticular/spheric and reticular mitochondria visualized in TMRE-loaded cells are shown in E. The percentage of cells showing the different mitochondrial morphologies (F) are indicated in HdhQ111 and HdhQ111–hMTH1 cells after 24 hr exposure to 10 mM 3-NP, depicting an increase of cells with reticular and a decrease of cells with spherical mitochondria induced by hMTH1 (*p < 0.05; ***p < 0.001).
A completely different picture emerged when cells were challenged for 24 hr with 10 mM 3-NP (Figs. 7C and D). This treatment caused a striking effect on mitochondrial morphology, with a major shift from the reticular/ramified shape to the dysfunctional spherical one in both cell lines. Single cells were then analyzed by thorough visual inspection for the different shapes of their mitochondria and classified accordingly. Three shapes were identified: the reticular/ramified shape (normal), a spherical point-like (diameter up to 1 μm) and a spherical vesicular-like shape. The last two are typical of stressed mitochondria (examples of the three shapes are shown in Fig. 7E). After 3-NP treatment, cells containing only reticular-shaped mitochondria were rare in the HdhQ111 and HdhQ111–hMTH1 cell lines (Fig. 7F). hMTH1 expression was associated with a significantly higher proportion of cells with mixed reticular/spherical mitochondria and a lower proportion of spherical mitochondria compared to the parental HdhQ111 cell line (Fig. 7F).
These changes in mitochondrial morphology indicate that hMTH1 protects HdhQ111 against mitochondrial stress and consequent dysfunction.
hMTH1 expression affects mitochondrial dynamics
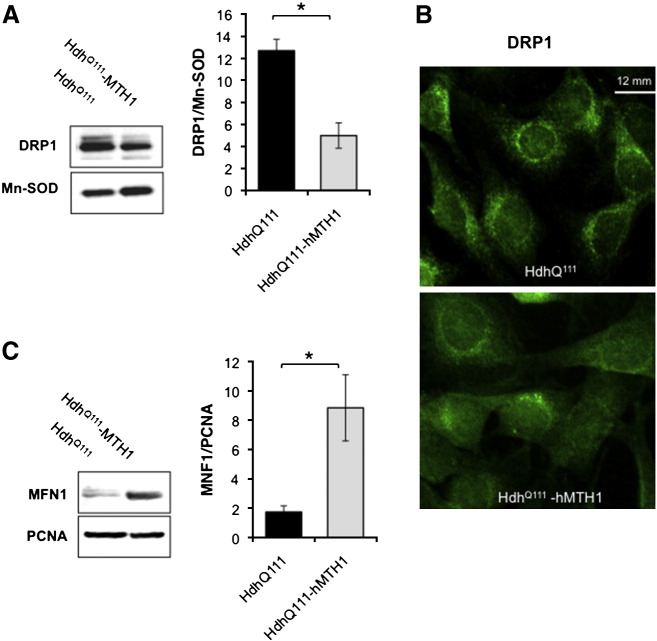
Mitochondrial structural abnormalities in HD are accompanied by alterations in the equilibrium between the two opposing events of organelle fission and fusion (Johri and Beal, 2012; Song et al., 2011). To verify whether the observed hMTH1-associated changes in mitochondrial shape and size were accompanied by alterations of mitochondrial dynamics, we examined the expression of the dynamin-related protein (DRP1) and mitofusin 1 (MFN1), two of the primary proteins controlling respectively mitochondrial fission and fusion, in the striatal cell lines. Comparison by western blotting indicated that mitochondria from HdhQ111 cells contained more DRP1 than those of hMTH1-expressing cells (Fig. 8A). The effect of hMTH1 expression on DRP1 mitochondrial localization in HdhQ111 cells was confirmed by immunofluorescence analysis (Fig. 8B) showing a more diffuse DRP1 labelling in hMTH1 expressing cells. hMTH1 expression had the reverse effect on the level of the pro-fusion MFN1 protein and MNF1 levels were increased in HdhQ111–hMTH1 cells when compared to the parental cells (Fig. 8C).
Fig. 8.
Analysis of mitochondrial fission and fusion. A. Western blot analysis of the DRP1 fission protein in mitochondrial extracts prepared from HdhQ111 and HdhQ111–hMTH1 cells. DRP1/ Mn-SOD ratios are shown in the graph. Error bars indicate mean ± SE from three independent determinations. Asterisks indicate significant differences by Student's t-test (*p < 0.05). HdhQ111 (black bar) and HdhQ111–hMTH1 (grey bar). B. Immunofluorescence analysis of the DRP1 protein, indicating a different expression and distribution of the protein in HdhQ111 and HdhQ111–hMTH1 cells. C. Western blot analysis of the MNF1 fusion protein in cell-free extracts prepared from HdhQ111 and HdhQ111–hMTH1 cells. MNF1/PCNA ratios are shown in the graph. Error bars indicate mean ± SE from four independent determinations. The asterisks indicate significant differences by Student's t-test (*p < 0.05). HdhQ111 (black bar) and HdhQ111–hMTH1 (gray bar).
The changes we observed in DRP1 and MFN1 levels indicate that hMTH1 expression shifts the equilibrium of mitochondrial fission and fusion proteins towards a more favourable dynamic.
In vivo protection of mitochondria by hMTH1
Since the protective role of hMTH1 towards mitochondrial dysfunction of HdhQ111 cells was investigated in proliferating cells in vitro, we examined whether hMTH1 expression protected mtDNA also in vivo by using a transgenic mouse expressing hMTH1 in several organs. These mice are resistant to neurodegeneration induced by a 5-day chronic exposure to 3-NP (De Luca et al., 2008). Since no quantitative information is available on this protection at DNA level, here we isolated mitochondria from different brain areas as well as from heart and skeletal muscles and measured 8-oxodG levels in mtDNA in untreated animals or following a 2-day exposure to 3-NP (Figs. 9A–E). Compared to wild-type animals a significantly lower basal level of mtDNA 8-oxodG was observed in the striatum, hippocampus, heart and muscles of hMTH1-Tg animals. The single exception was the cortex where no difference was observed between the two genotypes.
Fig. 9.
Levels of 8-oxodG in mtDNA from several organs of wild-type and hMTH1-Tg mice. Groups of wild-type (n = 15) and hMTH1-Tg mice (n = 15) that had received saline injections or 3-NP (60 mg/kg) twice daily for 2 days were sacrificed and levels of 8-oxo-dG were determined by HPLC-EC in mtDNA prepared from striatum (A), hippocampus (B), cortex (C), heart (D) and muscles (E) heart. Values are indicated as mean ± S E. The asterisks indicate significant differences by Student's t-test (*p < 0.05; **p < 0.01). Wild-type (black bar) and hMTH1-Tg mice (open bar).
Following treatment with 3-NP (2 daily injections of 60 mg/kg) the differential mtDNA oxidation between the two genotypes was maintained. The ratios between 8-oxodG levels in wild-type and hMTH1-Tg mice were 1.4-fold in striatum and hippocampus (p < 0.04 and p < 0.05, Student's t-test), 1.5-fold in muscle (p < 0.02, Student's t-test) and 1.7-fold in the heart (p < 0.004, Student's t-test). A similar trend, but with no statistical significance was observed in the cortex (Figs. 9A–E).
These results indicate that hMTH1 protects mtDNA from both endogenous and 3-NP-induced oxidation in the intact animal.
Discussion
Transcriptional dysregulation, protein aggregation, oxidative stress and mitochondrial abnormalities have all been proposed as contributors to neurodegeneration in HD. The results we present here indicate that the hMTH1 hydrolase represents a major protective element against oxidative damage and mitochondrial dysfunctions associated with expression of mutant Htt in striatal cells. Mutant Htt expression causes oxidative stress (for a review see ref. Browne et al., 1999) and oxidized DNA bases, lipids (e.g. F2-isoprostanes) and proteins accumulate in HD patients and in mouse models for HD (Bogdanov et al., 2001; Giuliano et al., 2003; Sorolla et al., 2010). Our data show that Htt expression is associated with increased DNA 8-oxodG in both nuclei and mitochondria. Both these targets are protected by hMTH1. Although mtDNA synthesis occurs independently from nuclear DNA replication, the deoxynucleotide pools for nuclear and mtDNA synthesis are in a dynamic equilibrium (Leanza et al., 2008). The mitochondrial dNTP pool may however be more at risk of oxidation because of its proximity to superoxide anions leaking from the respiratory chain, particularly during anomalous mitochondrial activity. The double localization of an 8-oxodGTPase in the mitochondrial matrix as well as in the cytosol indicates its likely involvement in protecting the genetic integrity of mtDNA.
Oxidative stress associated with mitochondrial impairment has been identified as an early event in HD ethiopathogenesis (Bossy-Wetzel et al., 2008; Browne and Beal, 2004). The phenotype of HD cells might reflect a direct interaction of polyQ-expanded Htt protein with mitochondria. The mitochondria related changes include dysfunctional Ca2 + homeostasis, with abnormal permeability transition pore opening (Milakovic et al., 2006), increased ROS production exacerbated by complex II inhibitors such as 3-NP, and a failure to maintain a balance between mitochondrial fission and fusion (Cui et al., 2006; Song et al., 2011). These features of mutant Htt expressing cells were all retained in HdhQ111 striatal cells.
Consistent with a role in maintaining healthy mitochondria, hMTH1 expression resulted in a higher membrane potential at steady state level and a decreased production of mitochondrial ROS following 3-NP treatment. These features were associated with a larger proportion of mitochondria in a filamentous mitochondrial morphology, which correlates with a shifted equilibrium towards fusion in the balance between fusion/fission of mitochondria and a higher bioenergetic efficiency of mitochondria.
Moreover, mutant htt has been shown to enhance the expression of NMDA-receptor 2B subtype in medium sized spiny neurons, rendering this particular neuronal population the most susceptible to excitotoxicity among striatal neurons (Fan and Raymond, 2007). In consideration of the involvement of mitochondria in the excitotoxic pathway, mitochondrial protection provided by hMTH1 would contribute to counteract excitotoxic neuronal degeneration in HD.
hMTH1 sanitizes the dNTP pool by degrading oxidized DNA precursors. This role is entirely consistent with the decreased mtDNA oxidation we observed. It is well known that Htt acts as a transcriptional regulator of nuclear genes (Sugars and Rubinsztein, 2003), including the major transcriptional regulator of mitochondrial biogenesis peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α (Cui et al., 2006)). Thus the protection provided by hMTH1 towards mitochondria might benefit from the double protective role of this repair enzyme on the stability of both nuclear and mtDNA.
3-NP induces a relatively modest increase in nuclear DNA 8-oxodG, which is unaffected by either mutant Htt or hMTH1 expression. This result is intriguing when we consider the parallel accumulation of DSBs identified by γH2AX foci and modulated by expression of polyQ Htt and hMTH1. Then the question comes up on the mechanism underlying these DNA breaks. The occurrence of DNA fragmentation associated with expression of polyQ-expanded Htt has been reported both in vitro (Bertoni et al., 2011) and in vivo (Butterworth et al., 1998; Kim and Chan, 2001). In addition it has been shown that polyQ-expanded proteins rapidly induce ROS and a significant ATM/ATR mediated DDR (Bertoni et al., 2011; Giuliano et al., 2003). Intriguingly, in the absence of 3-NP exposure, hMTH1 overexpression fully reverts accumulation of γH2AX foci in HdhQ111 cells, suggesting that the DDR triggered by polyQ-expanded Htt specifically derives from intermediates arising during repair of incorporated 8-oxoG from the dNTP pool. In contrast following 3-NP exposure suppression of DNA breaks by hMTH1 is only partial, possibly because treatment with this toxin combined with polyQ Htt expression generates additional oxidative DNA lesions whose repair intermediates may equally well trigger a more sustained DDR.
We (De Luca et al., 2008) and others (Kajitani et al., 2006) have shown that hMTH1 expression reduces the level of mRNA oxidation in vivo. Since transcriptional errors caused by oxidative damage are counteracted by hMTH1 (Hori et al., 2010; Ishibashi et al., 2005), elimination of 8-oxoGTP from the precursors pool may also ensure more accurate protein synthesis. An excess of carbonylated proteins, particularly among mitochondrial proteins, is a feature of cells expressing the polyQ Htt protein (Browne et al., 1999; Perluigi et al., 2005; Tunez et al., 2011) and synthesis of aberrant proteins because of reduced transcriptional fidelity might also favour protein oxidation (Dukan et al., 2000). By analogy to the function of the E. coli MutT protein (Dukan et al., 2000), it is possible that the protective role of hMTH1 towards mutant Htt-associated dysfunction of mitochondria might derive from the pleiotropic role of the repair enzyme.
Finally it is widely accepted that oxidative stress associated with expression of mutant Htt ultimately leads to neuronal cell death. The increased survival of hMTH1-expressing HdhQ111 striatal cells following exogenous oxidative stress (3-NP or H2O2) is consistent with the pool of oxidized dNTP/NTPs being a major contributor to the neurodegenerative process occurring in HD.
All the experiments demonstrating the protective role of hMTH1 in the genetic model for HD were performed in active proliferating cells. We were however able to demonstrate that hMTH1 expression significantly diminishes also DNA damage at mitochondrial level in differentiated tissues in vivo (brain, heart and muscles) both at steady state and following 3-NP exposure. Indeed heart, similarly to the brain, has a tremendous dependence on mitochondrial function and oxidative metabolism and mitochondria in both these organs have been shown to be major targets for 3-NP toxicity (Gabrielson et al., 2001). The efficiency of hMTH1 in protecting mtDNA in a condition of strong oxidative stress such as 3-NP treatment is noteworthy and suggests that the protective role of hMTH1 we previously identified against 3-NP-induced neurodegeneration (De Luca et al., 2008) can be mostly ascribed to mitochondria protection. It is worthwhile to stress that in the experimental conditions used in this study the length of exposure to the mitochondrial toxin was relatively short when compared to the time needed to observe the neurological symptoms of HD (2 versus 5 days). In the absence of early signs of toxicity (including weight loss), a significant increase of 8-oxodG levels was observed in all the investigated tissues. All these observations on DNA damage are consistent with the hypothesis of oxidative stress as one of the early events in 3-NP toxicity, which precedes clinical symptoms.
We conclude that the hMTH1-mediated maintenance of mtDNA stability protects cells from the exacerbated susceptibility to oxidant injury associated with expression of a polyQ-expanded Htt protein, but also defends the organism against the in vivo process of 3-NP-induced neurodegeneration in the mouse model for HD.
Funding
Grant support: Telethon (Project GGP06100) and Fondo per gli Investimenti della Ricerca di Base from Ministry of Education to MB and PS-NEURO (ex 56/05/20) from Ministry of Health to MB and LM.
Acknowledgments
We thank P. Karran and P. Pichierri for comments and fruitful discussions.
Contributor Information
Luisa Minghetti, Email: luisa.minghetti@iss.it.
Margherita Bignami, Email: margherita.bignami@iss.it.
References
- Bates G.P. History of genetic disease: the molecular genetics of Huntington disease — a history. Nat. Rev. Genet. 2005;6:766–773. doi: 10.1038/nrg1686. [DOI] [PubMed] [Google Scholar]
- Bertoni A., Giuliano P., Galgani M., Rotoli D., Ulianich L., Adornetto A., Santillo M.R., Porcellini A., Avvedimento V.E. Early and late events induced by polyQ-expanded proteins: identification of a common pathogenic property of polYQ-expanded proteins. J. Biol. Chem. 2011;286:4727–4741. doi: 10.1074/jbc.M110.156521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M.B., Andreassen O.A., Dedeoglu A., Ferrante R.J., Beal M.F. Increased oxidative damage to DNA in a transgenic mouse model of Huntington's disease. J. Neurochem. 2001;79:246–1249. doi: 10.1046/j.1471-4159.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Petrilli A., Knott A.B. Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci. 2008;31:609–616. doi: 10.1016/j.tins.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet E., Hantraye P., Ferrante R.J., Dolan R., Leroy-Willig A., Kowall N.W., Beal M.F. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet E., Conde F., Beal M., Hantraye P. Replicating Huntington's disease phenotype in experimental animals. Prog. Neurobiol. 1999;59:427–468. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Browne S., Beal M.F. The energetics of Huntington's disease. Neurochem. Res. 2004;29:531–546. doi: 10.1023/b:nere.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- Browne S., Beal M.F. Oxidative damage in Huntington's disease pathogenesis. Antioxid. Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- Browne S., Ferrante R., Beal M.F. Oxidative stress in Huntington's disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth N., Williams L., Bullock J., Love D., Faull R.L.M., Dragunow M. Trinucleotide [CAG] repeat length is positively correlated with the degree of DNA fragmentation in Hungtinton's disease striatum. Science. 1998;87:49–53. doi: 10.1016/s0306-4522(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Cui L., Jeong H., Borovecki F., Parkhurst C.N., Tanese N., Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- De Luca G., Russo M.T., Degan P., Tiveron C., Zijno A., Meccia E., Ventura I., Mattei E., Nakabeppu Y., Crescenzi M. A role for oxidized DNA precursors in Huntington's disease-like striatal neurodegeneration. PLoS Genet. 2008;4:e1000266. doi: 10.1371/journal.pgen.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M., Sapp E., Chase K., Davies S., Bates G., Vonsattel J., Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Duchen M., Surin A., Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–389. doi: 10.1016/s0076-6879(03)61019-0. [DOI] [PubMed] [Google Scholar]
- Dukan S., Farewell A., Ballesteros M., Taddei F., Radman M., Nyström T. Protein oxidation in response to increased transcriptional or translational errors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5746–5749. doi: 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M.M., Raymond L.A. N-methyl-d-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog. Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Firdaus W.J.J., Wyttenbach A., Giuliano P., Kretz-Remy C., Currie R.W., Arrigo A.-P. Huntingtin inclusion bodies are iron-dependent centers of oxidative events. FEBS J. 2006;273:5428–5441. doi: 10.1111/j.1742-4658.2006.05537.x. [DOI] [PubMed] [Google Scholar]
- Fontaine M.A.L., Geddes J.W., Banks A., Butterfield D.A. Effect of exogenous and endogenous antioxidants on 3-nitropropionic acid-induced in vivo oxidative stress and striatal lesions: insights into Huntington's disease. J. Neurochem. 2000;75:1709–1715. doi: 10.1046/j.1471-4159.2000.0751709.x. [DOI] [PubMed] [Google Scholar]
- Gabrielson K.L., Hogue B., Bohr V., Cardounel J., Nakajima W., Kofler J., Zweier J.L., Rodriguez E.R., Martin L.J., de Souza-Pinto N.C. Mitochondrial toxin 3-nitropropionic acid induces cardiac and neurotoxicity differentially in mice. Am. J. Pathol. 2001;159:1507–1520. doi: 10.1016/S0002-9440(10)62536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gines S., Seong I.S., Fossale E., Ivanova E., Trettel F., Gusella J.F., Wheeler V.C., Persichetti F., Macdonald M.E. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington's disease knock-in mice. Hum. Mol. Genet. 2003;12:497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- Giuliano P., De Cristofaro T., Affaitati A., Pizzulo G.M., Feliciello A., Criscuolo C., De Michele G., Filla A., Avvedimento E.V., Varrone S. DNA damage induced by polyglutamine-expanded proteins. Hum. Mol. Genet. 2003;12:2301–2309. doi: 10.1093/hmg/ddg242. [DOI] [PubMed] [Google Scholar]
- Group T.H., disease C.R. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Hands S., Sajjad M.U., Newton M.J., Wyttenbach A. In vitro and in vivo aggregation of a fragment of huntingtin protein directly causes free radical production. J. Biol. Chem. 2011;286:44512–44520. doi: 10.1074/jbc.M111.307587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa H., Hofer A., Thelander L., Kitajima S., Cai Y., Oshiro S., Yakushiji H., Nakabeppu Y., Kuwano M., Sekiguchi M. Metabolic fate of oxidized guanine ribonucleotides in mammalian cells. Biochemistry. 1999;38:3610–3614. doi: 10.1021/bi982361l. [DOI] [PubMed] [Google Scholar]
- Hori M., Satou K., Harashima H., Kamiya H. Suppression of mutagenesis by 8-hydroxy-2′-deoxyguanosine 5′-triphosphate [7,8-dihydro-8-oxo-2'-deoxyguanosine 5'-triphosphate] by human MTH1, MTH2, and NUDT5. Free Radic. Biol. Med. 2010;48:1197–1201. doi: 10.1016/j.freeradbiomed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Hayakawa H., Sekiguchi M. A novel mechanism for preventing mutations caused by oxidation of guanine nucleotides. EMBO Rep. 2003;4:479–483. doi: 10.1038/sj.embor.embor838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Hayakawa H., Ito R., Miyazawa M., Yamagata Y., Sekiguchi M. Mammalian enzymes for preventing transcriptional errors caused by oxidative damage. Nucleic Acids Res. 2005;33:3779–3784. doi: 10.1093/nar/gki682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A., Beal M.F. Antioxidants in Huntington's disease. Biochim. Biophys. Acta. 2012;1822:664–674. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani K., Yamaguchi H., Dan Y., Furuichi M., Kang D., Nakabeppu Y. MTH1, an oxidized purine nucleoside triphosphatase, suppresses the accumulation of oxidative damage of nucleic acids in the hippocampal microglia during kainate-induced excitotoxicity. J. Neurosci. 2006;26:1688–1698. doi: 10.1523/JNEUROSCI.4948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D., Nishida J., Iyama A., Nakabeppu Y., Furuichi M., Fujiwara T., Sekiguchi M., Takeshige K. Intracellular localization of 8-oxo-dGTPase in human cells, with special reference to the role of the enzyme in mitochondria. J. Biol. Chem. 1995;14:659–665. doi: 10.1074/jbc.270.24.14659. [DOI] [PubMed] [Google Scholar]
- Kim G., Chan P. Oxidative stress and neuronal DNA fragmentation mediate age-dependent vulnerability to the mitochondrial toxin, 3-nitropropionic acid, in the mouse striatum. Neurobiol. Dis. 2001;8:114–126. doi: 10.1006/nbdi.2000.0327. [DOI] [PubMed] [Google Scholar]
- Leanza L., Ferraro P., Reichard P., Bianchi V. Metabolic interrelations within guanine deoxynucleotide pools for mitochondrial and nuclear DNA maintenance. J. Biol. Chem. 2008;283:16437–16445. doi: 10.1074/jbc.M801572200. [DOI] [PubMed] [Google Scholar]
- Lim D., Fedrizzi L., Tartari M., Zuccato C., Cattaneo E., Brini M., Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liot G., Bossy B., Lubitz S., Kushnareva Y., Sejbuk N., Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S.W. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Milakovic T., Quintanilla R.A., Johnson G.V.W. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J. Biol. Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P., Rajesh M., Haskó G., Hawkins B.J., Madesh M.V., Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panov A.V., Gutekunst C.-A., Leavitt B.R., Hayden M.R., Burke J.R., Strittmatter W.J., Greenamyre J.T. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Perluigi M., Poon H.F., Maragos W., Pierce W.M., Klein J.B., Calabrese V., Cini C., De Marco C., Butterfield D.A. Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: a model of Huntington disease. Mol. Cell. Proteomics. 2005;4:1849–1861. doi: 10.1074/mcp.M500090-MCP200. [DOI] [PubMed] [Google Scholar]
- Polidori M.C., Mecocci P., Browne S.E., Senin U., Beal M.F. Oxidative damage to mitochondrial DNA in Huntington's disease parietal cortex. Neurosci. Lett. 1999;272:53–56. doi: 10.1016/s0304-3940(99)00578-9. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J.W., Ames B.N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Lesort M., MacDonald M.E., Johnson G.V.W. Striatal cells from mutant huntingtin knock-in mice are selectively vulnerable to mitochondrial complex II inhibitor-induced cell death through a non-apoptotic pathway. Hum. Mol. Genet. 2004;13:669–681. doi: 10.1093/hmg/ddh082. [DOI] [PubMed] [Google Scholar]
- Sakumi K., Furuichi M., Tsuzuki T., Kakuma T., Kawabata S., Maki H., Sekiguchi M. Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J. Biol. Chem. 1993;268:23524–23530. [PubMed] [Google Scholar]
- Song W., Chen J., Petrilli A., Liot G., Klinglmayr E., Zhou Y., Poquiz P., Tjong J., Pouladi M.A., Hayden M.R. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorolla M.A., Reverter-Branchat G., Tamarit J., Ferrer I., Ros J., Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic. Biol. Med. 2008;45:667–678. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Sorolla M.A., Rodríguez-Colman M.J., Tamarit J., Ortega Z., Lucas J.J., Ferrer I., Ros J., Cabiscol E. Protein oxidation in Huntington disease affects energy production and vitamin B6 metabolism. Free Radic. Biol. Med. 2010;49:612–621. doi: 10.1016/j.freeradbiomed.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Souza-pinto N.C.D., Eide L., Hogue B.A., Thybo T., Stevnsner T., Seeberg E. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine DNA glycosylase [OGG1] gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1-defective mice. Cancer Res. 2001;61:5378–5381. [PubMed] [Google Scholar]
- Sugars K.L., Rubinsztein D.C. Transcriptional abnormalities in Huntington disease. Trends Genet. 2003;19:233–238. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- Tabrizi S., Workman J., Hart P., Mangiarini L., Mahal A., Bates G., Cooper J.M., Chapira A. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann. Neurol. 2000;47:80–86. doi: 10.1002/1531-8249(200001)47:1<80::aid-ana13>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- Trettel F., Rigamonti D., Hilditch-Maguire P., Wheeler V.C., Sharp A.H., Persichetti F., Cattaneo E., MacDonald M.E. Dominant phenotypes produced by the HD mutation in STHdh[Q111] striatal cells. Hum. Mol. Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- Tunez I., Sanchez-Lopez F., Aguera E., Fernandez-Bolanos R., Sanchez F.M., Tasset-cuevas I. Important role of oxidative stress biomarkers in Huntington's disease. J. Med. Chem. 2011;54:5602–5606. doi: 10.1021/jm200605a. [DOI] [PubMed] [Google Scholar]
- Ventura I., Russo M.T., De Luca G., Bignami M. Oxidized purine nucleotides, genome instability and neurodegeneration. Mutat. Res. 2010;703:59–65. doi: 10.1016/j.mrgentox.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Wheeler V.C., Auerbach W., White J.K., Srinidhi J., Auerbach A., Ryan A., Duyao M.P., Vrbanac V., Weaver M., Gusella J.F. Length-dependent gametic CAG repeat instability in the Huntington's disease knock-in mouse. Hum. Mol. Genet. 1999;8:31–35. doi: 10.1093/hmg/8.1.115. [DOI] [PubMed] [Google Scholar]
- Wyttenbach A., Sauvageot O., Carmichael J., Diaz-Latoud C., Arrigo A.-P., Rubinsztein D.C. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kajitani K., Dan Y., Furuichi M., Ohno M., Sakumi K., Kang D., Nakabeppu Y. MTH1, an oxidized purine nucleoside triphosphatase, protects the dopamine neurons from oxidative damage in nucleic acids caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Cell Death Differ. 2006;13:551–563. doi: 10.1038/sj.cdd.4401788. [DOI] [PubMed] [Google Scholar]