Background: TLRs are key sensors of viral and bacterial components and lead to the production of proinflammatory cytokines.
Results: 14-3-3ϵ and 14-3-3σ proteins curtail TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated proinflammatory cytokine induction.
Conclusion: 14-3-3ϵ and 14-3-3σ play a critical, hitherto underappreciated role in modulating TLR functionality.
Significance: Learning how TLRs are modulated is crucial for understanding innate immunity.
Keywords: TLR Signaling, 14–3-3 Protein, Cytokine, Negative Regulation
Abstract
Toll-like receptors (TLRs) are a group of pattern recognition receptors that play a crucial role in the induction of the innate immune response against bacterial and viral infections. TLR3 has emerged as a key sensor of viral double-stranded RNA. Thus, a clearer understanding of the biological processes that modulate TLR3 signaling is essential. Limited studies have applied proteomics toward understanding the dynamics of TLR signaling. Herein, a proteomics approach identified 14-3-3ϵ and 14-3-3σ proteins as new members of the TLR signaling complex. Toward the functional characterization of 14-3-3ϵ and 14-3-3σ in TLR signaling, we have shown that both of these proteins impair TLR2, TLR3, TLR4, TLR7/8, and TLR9 ligand-induced IL-6, TNFα, and IFN-β production. We also show that 14-3-3ϵ and 14-3-3σ impair TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated NF-κB and IFN-β reporter gene activity. Interestingly, although the 14-3-3 proteins inhibit poly(I:C)-mediated RANTES production, 14-3-3 proteins augment Pam3CSK4, LPS, R848, and CpG-mediated production of RANTES (regulated on activation normal T cell expressed and secreted) in a Mal (MyD88 adaptor-like)/MyD88-dependent manner. 14-3-3ϵ and 14-3-3σ also bind to the TLR adaptors and to both TRAF3 and TRAF6. Our study conclusively shows that 14-3-3ϵ and 14-3-3σ play a major regulatory role in balancing the host inflammatory response to viral and bacterial infections through modulation of the TLR signaling pathway. Thus, manipulation of 14-3-3 proteins may represent novel therapeutic targets for inflammatory conditions and infections.
Introduction
The human innate immune system provides the first line of defense against various infectious agents, such as bacteria, viruses, parasites, and helminths. Toward the effective functioning of innate immunity, various classes of protein-based receptors serve to sense various danger-associated molecular patterns and pathogen-associated molecular patterns (PAMPs)2 (1). These different classes of pathogen recognition receptors include membrane-bound pathogen recognition receptors, such as Toll-like receptors (TLRs); receptor kinases; mannose receptors; cytoplasmic pathogen recognition receptors, such as nucleotide oligomerization domain receptors, the RIG-I (retinoic acid-inducible gene I)-like receptor (RLR) family, and the recently described AIM2 and DAI cytosolic DNA receptors, the activation of which leads to the production of proinflammatory cytokines and type I interferon (IFN) (2).
To date, 10 human TLRs have been identified, and each member of the TLR family recognizes different kinds of PAMPs. For example, TLR1 in combination with TLR2 or TLR6 recognizes triacyl lipopeptides and diacyl lipopeptides, respectively (1). TLR4 recognizes lipopolysaccharide (LPS) from Gram-negative bacteria, and TLR5 recognizes flagellin from bacterial flagellum. In contrast to the above mentioned plasma membrane-localized TLRs, the so-called antiviral TLRs, TLR3, TLR7/8, and TLR9 are located on endocytic vesicles and recognize double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), and DNA, respectively (3). Upon recognition of PAMPs and danger-associated molecular patterns, TLRs are activated, which initiates the recruitment of proximal cytoplasmic Toll/IL-1 receptor (TIR) domain-containing adaptor proteins. Four activationary TLR adaptor proteins have been identified, namely MyD88 (myeloid differentiation factor 88), Mal (MyD88 adaptor-like) (also known as Toll/IL-1 adaptor protein (TIRAP)), TIR domain-containing adaptor-inducing interferon (IFN)-β (TRIF) (also known as TICAM-1), and TRIF-related adaptor molecule (TRAM) (1). Additionally, an inhibitory TLR adaptor protein called SARM (sterile α and TIR motif-containing protein) has also been identified (4, 5). MyD88 is the common downstream adaptor that is recruited by all TLRs, except TLR3, and leads to activation of nuclear factor (NF)-κB. Mal is required for signaling by TLR4 and, to a lesser extent, TLR2 (1, 6). TRIF mediates TLR3 and TLR4 signaling, and TRAM serves as a bridging adaptor between TRIF and TLR4 and leads to activation of interferon regulatory factor 3 (IRF3) (3, 7). Upon recruitment of the activationary adaptor proteins to the TLR via homotypic TIR domain interactions, a series of TLR signaling cascades are elicited, which culminates in the production of proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-1β, and IL-12. In addition, activation of TLR3, -7, -8, and -9 induces the production of anti-viral IFN-β and IFN-α (2).
TLR3 plays a critical role in innate immunity because it serves to recognize viral dsRNA originating from dsRNA viruses, such as reovirus (4). TLR3 also recognizes dsRNA produced during the replication of many viruses, including ssRNA viruses, such as West Nile virus, respiratory syncytial virus, and encephalomyocarditis virus. In addition, TLR3 also recognizes a synthetic analog of dsRNA known as polyriboinosinic:polyribocytidylic acid (poly(I:C)) (3). It has been shown that activation of TLR3 using poly(I:C) inhibits HIV infection by increasing the expression of type I IFN antiviral factors, thus restricting HIV expression and replication (8). Also, poly(I:C) has proven beneficial as a mucosal adjuvant for an influenza virus vaccine in a murine infection model (4). Activation of TLR3 signaling has also been linked to the inhibition of tumor cell growth (9) and to amplification of inflammation following the sensing of necrosis in viral dsRNA-independent settings, such as experimental polymicrobial septic peritonitis and ischemic gut injury (10). Given the pleiotropic role exerted by TLR3 in innate immunity, it is essential that the molecular mechanisms that serve to modulate its functionality be fully deciphered. To this end, we have shown that Mal and MyD88 have the ability to inhibit TLR3-mediated IFN-β production (7, 11). Further, we have shown that the transcription factor YinYang1 (YY1), induced by poly(I:C), translocates to the nucleus, where it interacts with the IFN-β promoter and inhibits the binding of IRF-7 to the latter (12).
Here, we identify and functionally characterize 14-3-3 proteins as key modulators of TLR3 signaling. Using two-dimensional gel electrophoresis and mass spectrometry, we found that 14-3-3 expression was initially suppressed and then induced in bone marrow-derived macrophages (BMDMs) following their stimulation with poly(I:C). The 14-3-3 proteins are a family of ubiquitously expressed, acidic proteins, consisting of seven known mammalian isoforms (β, γ, ϵ, σ, ζ, τ, and η), which are expressed in all mammalian cells (13–15). 14-3-3 proteins have been shown to bind to a multitude of functionally diverse signaling proteins, including kinases, phosphatases, and transmembrane receptors (16). The 14-3-3 proteins play a vital role in cell survival, proliferation, growth, differentiation, and intracellular signaling (16) and have been implicated in many diseases, such as cancer and neurological diseases (17). Recently, 14-3-3 proteins have been implicated in the modulation of TLR signaling, whereby stimulation of RAW cells with LPS induced PKCϵ phosphorylation and its association with 14-3-3β in a MyD88-dependent manner (18). Also, 14-3-3ζ was shown to exert differential effects on TLR2 and TLR4 signaling, whereby 14-3-3ζ inhibited TLR2-mediated NF-κB activation but enhanced TLR4-mediated NF-κB activation (19). Herein, we focused on 14-3-3ϵ (also called tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, ϵ polypeptide, or YWHAE) and 14-3-3σ (also called stratifin or SFN) toward characterizing their functional role in TLR signaling. We opted to investigate the ability of 14-3-3 proteins to modulate TLR2, TLR3, TLR4, TLR7/8, and TLR9 signaling following stimulation of cells with the corresponding ligands, Pam3CSK4, poly(I:C), LPS, R848, and CpG, respectively. We show that 14-3-3ϵ and 14-3-3σ inhibit TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated signaling. In contrast, 14-3-3ϵ and 14-3-3σ enhance TLR2-, TLR4-, TLR7/8-, and TLR9-mediated RANTES production and inhibit TLR3-mediated RANTES production.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Wild-type (WT), MAVS−/−, TRIF−/−, and MyD88−/− immortalized BMDMs (iBMDMs), HEK293, HEK293-TLR2, HEK293-TLR3, HEK293-TLR4, HEK293-TLR7/8, and HEK293-TLR9 were gifts from Professor Katherine Fitzgerald (University of Massachusetts Medical School). HEK293-Blue IFN-α/β were purchased from InvivoGen. Cells were cultured in DMEM with GlutaMAX (Invitrogen) and supplemented with 10% FBS, 1% penicillin-streptomycin, 1% Fungizone and were maintained at 37 °C in a humidified atmosphere of 5% CO2. The HEK293-TLR4 culture medium was also supplemented with blasticidin (100 μg/ml) and hygrogold (100 μg/ml). The HEK293-TLR2, HEK293-TLR3, and HEK293-TLR7/8 culture medium was supplemented with blasticidin (100 μg/ml). The HEK293-TLR9 cell culture medium was supplemented with gentamycin (250 μg/ml; G418, Sigma). The HEK293-Blue IFN-α/β culture medium was supplemented with zeocin (100 μg/ml) and blasticidin (10 μg/ml). U373-CD14 cells were cultured in RPMI 1640 with GlutaMAX (Invitrogen) supplemented with 10% FBS, 1% penicillin-streptomycin, 1% Fungizone, and 250 μg/ml G418. Human fibroblast-like synoviocytes were purchased from Cell Applications and were cultured in synoviocyte growth medium (Cell Applications) maintained at 37 °C in a humidified atmosphere of 5% CO2. Pam3CSK4 (InvivoGen), naked polyinosinic:polycytidylic acid (poly(I:C)) (InvivoGen), highly purified protein-free LPS derived from Escherichia coli strain 011:B4 (Alexis), R848 (InvivoGen), CLO97 (InvivoGen), and CpG (InvivoGen) were used for all treatments. 14-3-3ϵ and 14-3-3σ endoribonuclease-prepared siRNAs (esiRNAs) were purchased from Sigma-Aldrich. Lamin A/C-negative control was from Ambion.
Expression Vectors/Recombinant Plasmids
HA-tagged pcDNA3–14-3-3ϵ and 14-3-3σ were kind gifts from Dr. Christian Ottmann (Chemical Genomics Center of the Max Planck Society, Dortmund, Germany). The plasmids pcDNA3:MyD88-cmyc and pcDNA3:p38-FLAG were kind gifts from Professor Luke O'Neill (Trinity College Dublin). The plasmids pcDNA3:TRAM-FLAG, pcDNA3:TRIF-FLAG, pcDNA3:TRAF3-FLAG, pcDNA3:TRAF6-FLAG, pcDNA3:IRF7-FLAG, and pcDNA3:MAL-FLAG were kind gifts from Professor Paul Moynagh (National University of Ireland Maynooth). The IFN-β and CCL5 reporter gene construct, FLAG-IKKϵ, FLAG-IRF3, and plasmids were as described (20). The NF-κB luciferase reporter construct was as described (21).
Two-dimensional Gel Electrophoresis
Whole cell lysates were extracted from WT and MAVS−/− iBMDMs following stimulation with poly(I:C) for various times (0, 1.5, 8, and 24 h). The proteins were precipitated using the acetone precipitation method followed by incubation in lysis buffer (7 m urea, 4% CHAPS, 2 m thiourea, 100 mm DTT, 5% ampholytes, and one protease inhibitor mixture tablet (PICS)/50 ml of lysis buffer). Protein separation by two-dimensional gel electrophoresis in the first dimension was performed by isoelectric focusing using 24-cm pH 4–7 IPG strips (GE Healthcare) and in the second dimension by SDS-PAGE. Rehydration of IPG strips, isoelectric focusing, equilibration of focused strips, and SDS-PAGE second dimensional separation was carried out as described previously (22). The separated proteins were visualized by silver staining (23), and high resolution gel images where scanned using a Typhoon Trio variable mode imager from GE Healthcare. Comparative and statistical analysis of two-dimensional gels was performed with the Progenesis software program from Non-Linear Dynamics (Newcastle, Tyne, UK).
Mass Spectrometry Analysis (Progenesis, MS, Mascot)
Differentially expressed proteins were subjected to in-gel trypsin digestion, and the resulting peptides were analyzed by peptide mass fingerprinting using an Ion Trap LC/MS apparatus from Agilent Technologies (model 6430). Excision, washing, destaining, trypsin digestion, and peptide recovery was performed as described previously (22). Peptides were separated using a nanoflow Agilent 1200 series system, equipped with a Zorbax 300SB C18 5-μm, 4-mm, 40-nl precolumn and a Zorbax 300SB C18 5-μm, 43 mm × 75-μm analytical reversed phase column using HPLC-Chip technology, and 0.1% formic acid was used as mobile phase A, and 50% acetonitrile, 0.1% formic acid was used as mobile phase B. Samples were loaded at a flow rate of 4 μl/min onto the enrichment column, and the peptide fragments were eluted with a constant nanopump flow rate of 0.6 ml/min. The capillary voltage was set to 1900 V, and the flow and the temperature of the drying gas were 4 liters/min and 300 °C, respectively. Database searches were performed using Mascot MS/MS ion search (Matrix Science, London, UK). All pI values and molecular masses of the identified proteins were compared with the relative position of their corresponding two-dimensional spots on analytical slab gels.
First Strand cDNA Synthesis
Total cellular RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Thereafter, 1 μg of total RNA was mixed with 1 μl of random hexamers (500 μg/ml), and water was added to a final volume of 17 μl and incubated for 5 min at 70 °C. The mixture was then briefly centrifuged and chilled on ice for 2 min. Thereafter, the other reaction components were added in the following order: 5 μl of 5× RT buffer, 1.3 μl of 10 mm dNTP, 0.7 μl of RNasin (Promega), 1 μl of Moloney murine leukemia virus reverse transcriptase (Promega), and nuclease-free water to a total volume of 25 μl. Reactions were incubated at 37 °C for 40 min followed by 42 °C for 40 min and heating to 80 °C for 5 min. The first strand cDNA was stored at −20 °C for up to 1 month.
Quantitative Real-time PCR
First strand cDNA was used as starting material for real-time RT-PCR quantitation with 2× SensiMixTM SYBR No-ROX Mastermix (Bioline) on a real-time PCR system (DNA Engine Opticon system, MJ Research). For amplification of specific genes, the following primers were used: hIFNβ, forward (5′-AACTGCAACCTTTCGAAGCC-3′) and reverse (5′-TGTCGCCTACTACCTGTTGTGC-3′); hTNFα, forward (5′-CACCACTTCGAAACCTGGGA-3′) and reverse (5′-CACTTCACTGTGCAGGCCAC-3′); hCCL5, forward (5′-TGCCTGTTTCTGCTTGCTCTTGTC-3′) and reverse (5′-TGTGGTAGAATCTGGGCCCTTCAA-3′); hIP-10, forward (5′-ATTATTCCTGCAAGCCAATTTTG-3′) and reverse (5′-TCACCCTTCTTTTTCATTGTAGCA-3′); hIL-6, forward (5′-AAATTCGGTACATCCTCGACGGCA-3′) and reverse (5′-GTGCCTCTTTGCTGCTTTCACACA-3′); h14-3-3ϵ, forward (5′-TGCAGAACTGGATACGCTGAGTGA-3′) and reverse (5′-TCACCCTGCATGTCTGAAGTCCAT-3′); h14-3-3σ, forward (5′-AAGAAGCGCATCATTGACTCAGCC-3′) and reverse (5′-TGTTGGCGATCTCGTAGTGGAAGA-3′); m14-3-3ϵ, forward (5′-TGCAGAACTGGATACGCTGAGTGA-3′) and reverse (5′-TCACCCTGCATGTCTGAAGTCCAT-3′); m14-3-3σ, forward (5′-AAGAAGCGCATCATTGACTCAGCC-3′) and reverse (5′-TGTTGGCGATCTCGTAGTGGAAGA-3′); mMAVS, forward (5′-AAGCACCTGCCAACACAATACCAC-3′) and reverse (5′-AGCTCAGTGGACCTCGCCATATTT-3′). For each mRNA quantification, the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference point using the following primers: mGAPDH, forward (5′-GCACAGTCAAGGCCGAGAAT-3′) and reverse (5′-GCCTTCTCCATGGTGGTGAA-3′); hGAPDH, forward (5′-TCGACAGTCAGCCGCATCTTCTTT-3′) and reverse (5′-ACCAAATCCGTTGACTCCGACCTT-3′). Real-time PCR data were analyzed using the 2−ΔΔCT method.
Reporter Gene Assays
HEK293 and HEK293-TLR2, -3, -4, -7/8, and -9 cells (5 × 104 cells/well; 96-well plate) were transfected with 80 ng/well luciferase reporter gene plasmid for NF-κB, IFN-β, and CCL5 and cotransfected with the expression vectors encoding 14-3-3ϵ or 14-3-3σ and MyD88, Mal, TRIF, or TRAM using Lipofectamine as described previously (7). A total of 40 ng/well phRL-TK (TK-Renilla-luciferase) reporter gene was co-transfected simultaneously to normalize data for transfection efficiency. After 24 h, cells were stimulated with ligands as indicated for a further 24 h. Thereafter, cell lysates were prepared and reporter gene activity was measured using the Dual-Luciferase assay system (Promega). Data are expressed as the mean -fold induction relative to control levels, for a representative experiment from a minimum of three separate experiments, each performed in triplicate.
esiRNA Transfection
Human U373-CD14 cells were transfected with 14-3-3ϵ, 14-3-3σ, and control esiRNA to target the suppression of 14-3-3ϵ, 14-3-3σ, and negative control, respectively. In a 6-well plate, 20 nm esiRNA was transfected into cells using Dreamfect Gold (OZ Biosciences) as described by the manufacturer. After 24 and 48 h, efficiency of 14-3-3ϵ and 14-3-3σ knockdown was assessed by RT-PCR using 14-3-3ϵ and 14-3-3σ forward and reverse primers.
Cytokine Analysis
14-3-3ϵ-, 14-3-3σ-, and control esiRNA-transfected U373-CD14 cells were stimulated with Pam3CSK4, poly(I:C), LPS, R848, and CpG as indicated. After 24 h, cell-free supernatants were removed and analyzed for IL-6, TNFα, and CCL5 cytokine release as described by the manufacturer (Peprotech). Regarding IFN measurements, Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-induced human type I IFNs were measured using HEK293-Blue IFN-α/β cells (InvivoGen), essentially as described by the manufacturer.
Extraction of Cellular Nuclear Fraction
14-3-3ϵ, 14-3-3σ, and control esiRNA transfected U373-CD14 cells were stimulated with Pam3CSK4, poly(I:C), LPS, R848, and CpG for the indicated times. After ligand stimulations, the cells were collected, and nuclear extracts were prepared using the nuclear extraction kit as described by the manufacturer (Cayman Chemical).
p65, p38, and ERK1/2 Immunoblot Analysis
14-3-3ϵ-, 14-3-3σ-, and control esiRNA-transfected U373-CD14 cells were stimulated with Pam3CSK4, poly(I:C), LPS, R848, and CpG as described, and whole cell lysates were subjected to SDS-PAGE followed by immunoblot analysis with an anti-phospho-p65 antibody (Cell Signaling Technology), anti-phospho-p38 antibody (Cell Signaling Technology), and anti-phospho-ERK1/2 antibody (Cell Signaling Technology).
Transfection and Co-immunoprecipitation
HEK293, HEK293-TLR3, and U373-CD14 cells (1 × 106 cells/well; 6-well plate) were transfected with the indicated plasmids, whereby the total amount of DNA (3 μg/well) was kept constant using Lipofectamine 2000 (Invitrogen). After 24 h, cells were stimulated with the indicated ligands followed by cell lysis in 600 μl of lysis buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 2 mm EDTA, pH 8.0, 1% Nonidet P-40, 0.5% sodium deoxycholate supplemented with 1 mm PMSF, 1 mm, DDT, 1 mm NaVO3, 5 mm EGTA, and 1 tablet/10 ml of PICS) and left on ice for 20 min. The lysates were subjected to centrifugation for 10 min at 14,000 rpm to remove cell debris. Next, 2 μg of the indicated antibody was incubated with 70 μl of A/G Plus-agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4 °C, washed, and incubated with the cell lysates for 2 h at 4 °C. The immune complexes were precipitated, washed, and eluted by the addition of Laemmli loading buffer followed by SDS-PAGE and immunoblotting using the indicated antibodies.
Data Analyses
Unpaired Student's t test and two-way ANOVA tests were used to carry out statistical analysis of data. The p values ≤0.05 were considered to indicate a statistically significant difference (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
RESULTS
14-3-3 Proteins Are Novel Components of the TLR Signaling Pathway
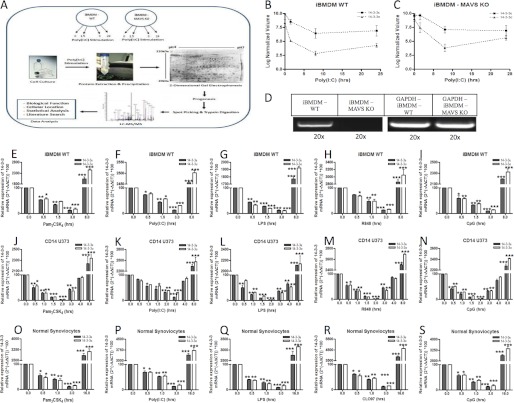
In order to explore the molecular mechanisms that modulate TLR3 functionality following viral sensing, iBMDMs were stimulated with the synthetic TLR3 ligand, poly(I:C), followed by an assay of changes in protein expression using two-dimensional gel electrophoresis. Although poly(I:C) is sensed by TLR3, it may also be sensed by the RLRs RIG-1 and Mda-5, which utilize the signaling adaptor MAVS (24); thus, MAVS−/− iBMDMs were included in the study as a control to preclude the signaling proteins induced through the cytosolic RLRs. Following stimulation of WT and MAVS−/− iBMDMs with poly(I:C) for 0–24 h and two-dimensional gel electrophoresis, differentially expressed proteins were selected using Progenesis software and were then subjected to in-gel trypsin digestion followed by nano-LC-MS/MS (Fig. 1A). A number of proteins were found to be differentially expressed (up-/down-regulated) at all time points following poly(I:C) stimulation of WT iBMDMs when compared with control (0 h) iBMDMs (Table 1). Importantly, we found that several members of the 14-3-3 protein family (14-3-3 protein ζ, δ, σ, β, α, ϵ, and θ) were suppressed upon stimulation with poly(I:C) for 1.5 and 8 h. Similarly, suppression of 14-3-3 expression was also observed in MAVS−/− iBMDMs following poly(I:C) stimulation, indicating that poly(I:C)-mediated suppression of 14-3-3 proteins occurs through TLR3 rather than through the RLRs (Table 2). Although a limited number of studies have described a role for 14-3-3 proteins in TLR signaling (18, 25), their functional characterization and role in TLR signaling require further clarification. Given this, we opted to validate and functionally characterize the role of two 14-3-3 proteins, 14-3-3ϵ and 14-3-3σ, in TLR signaling. Statistical comparison of the two-dimensional gels using Progenesis software showed comparable suppression of 14-3-3ϵ and 14-3-3σ protein expression in both WT and MAVS−/− BMDMs following stimulation with poly(I:C) for 1.5 and 8 h (Fig. 1, B and C). Thus, the absence of MAVS expression does not significantly affect the modulatory effects of poly(I:C) on 14-3-3ϵ and 14-3-3σ mRNA expression and confirms that poly(I:C) mediates its effects primarily through TLR3 in iBMDMs. An absence of MAVS adaptor expression in MAVS−/− BMDMs was confirmed using RT-PCR (Fig. 1D). In WT BMDMs, using quantitative RT-PCR, we show that suppression of 14-3-3ϵ and 14-3-3σ mRNA was evident between 0.5 and 3 h of stimulation with Pam3CSK4, poly(I:C), LPS, R848, and CpG (Fig. 1, E–I). However, significant induction of 14-3-3ϵ and 14-3-3σ mRNA was observed in WT BMDMs at 8 h (Fig. 1, E–I). To negate the possibility of species and ligand-dependent differences, the expression levels of 14-3-3ϵ and 14-3-3σ were also investigated in human U373-CD14 cells, whereupon comparable suppression (0.5–3 h) and enhanced mRNA expression (3–8 h) profiles were observed upon stimulation with Pam3CSK4, poly(I:C), LPS, R848, and CpG (Fig. 1, J–N). Similar to iBMDMs (Fig. 1, E–I) and U373-CD14 cells (Fig. 1, J–N), it was found that 14-3-3ϵ and 14-3-3σ mRNA expression was suppressed upon stimulation of synoviocytes with Pam3CSK4, poly(I:C), LPS, CLO97, and CpG for 0.5–3 h (Fig. 1, O–S). Thereafter, significant induction of 14-3-3ϵ and 14-3-3σ mRNA was observed following stimulation of the synoviocytes with TLR ligands for 16 h (Fig. 1, O–S). Thus, 14-3-3ϵ and 14-3-3σ expression is initially suppressed and then induced following stimulation of cells with TLR2, -3, -4, -7/8, and -9 ligands.
FIGURE 1.
14-3-3 proteins are novel components of TLR signaling pathway. A, experimental workflow. Wild-type and MAVS−/− iBMDMs were stimulated with poly(I:C) (20 μg/ml) for a range of times (0, 1.5, 8, and 24 h). The proteins from the cells were extracted using acetone precipitation, and samples were subjected to two-dimensional gel electrophoresis as described under “Experimental Procedures.” Gel cross-comparison was performed using Progenesis software, and differentially expressed protein spots were picked, digested with trypsin, and identified using nano-LC-MS/MS as described under “Experimental Procedures.” The data analysis was performed using the MASCOT database, and biological and cellular characteristics of the proteins were recorded. B and C, average -fold change of the differentially expressed protein spots shown as log normalized volumes comparing the protein spot volume in gels stimulated for 1.5, 8, and 24 h with poly(I:C) as compared with the control gels (0 h). D, RT-PCR was performed to verify the absence of MAVS adaptor in MAVS−/− iBMDMs using forward and reverse primers. The samples were diluted 20× and were subjected to DNA gel electrophoresis. GAPDH was used to normalize all samples, and -fold changes were determined relative to control. Wild-type iBMDMs (E–I), U373-CD14 (J–N), and synoviocyte cells (O–S) were stimulated with Pam3CSK4 (1 μg/ml), poly(I:C) (20 μg/ml), LPS (1 μg/ml), R848 (1 μg/ml), and CpG (5 μg/ml) for a range of times (0, 0.5, 1.5, 3, 4, and 8 h), and total RNA was isolated, converted to first strand DNA, and used as a template for quantitative real-time RT-PCR as described under “Experimental Procedures” to assay the expression levels of 14-3-3ϵ and 14-3-3σ. The data presented are representative of at least three independent experiments performed in triplicate (mean ± S.E. (error bars)). Statistical analysis was performed using unpaired Student's t test and two-way ANOVA tests comparing the test samples with their respective controls.
TABLE 1.
Mass spectrometry identification of differentially expressed protein spots in wild-type BMDMs stimulated with poly(I:C) (20 μg/ml) over a range of times (0, 1.5, 8, and 24 h)
The -fold change represents an average differential change of the protein spot over time compared with the control (0 h). The experiment and two-dimensional gel electrophoresis for each stimulation time point were performed in triplicate as independent experiments (mean ± S.E.).
| Accession no. | Protein name | No. of peptide matches | Mascot Score | Coverage | pI | Mr | Subcellular localization | Gene ontology | Average -fold change upon poly(I:C) stimulation | ANOVA (p) |
|---|---|---|---|---|---|---|---|---|---|---|
| % | -fold | |||||||||
| LMNA/162287370 | Prelamin-A/C | 2 | 87 | 3 | 6.54 | 74,483 | Nucleus | Intermediate filament | 2 | 0.01 |
| PEBP1/84794552 | Phosphatidylethanolamine-binding protein 1 | 6 | 220 | 56 | 5.19 | 20,991 | Cytoplasm | ATP binding | −2.6 | 0.01 |
| PRDX2/148747558 | Peroxiredoxin-2 | 2 | 42 | 14 | 5.2 | 21,939 | Cytoplasm | Cell redox homeostasis, anti-apoptosis | −2.6 | 0.01 |
| HSP7C/13242237 | Heat shock cognate 71-kDa protein | 3 | 75 | 7 | 5.37 | 71,059 | Cytoplasm | Stress response, transcription regulation | −2.3 | 0.011 |
| TCPA/110625624 | T-complex protein 1 subunit α | 4 | 143 | 16 | 5.82 | 60,875 | Cytoplasm | Protein folding | −5.4 | 0.012 |
| 1405933 | M2-type pyruvate kinase | 6 | 191 | 19 | 7.18 | 58,458 | Cytoplasm, nucleus | Glycolysis, programmed cell death | −3.1 | 0.014 |
| 1433Z/6756041 | 14-3-3 protein ζ/δ | 3 | 222 | 15 | 4.73 | 27,928 | Cytoplasm | Numerous biological processes and pathways | −5.3 | 0.016 |
| 1433S/134023662 | 14-3-3 protein σ | 4 | 163 | 12 | 4.72 | 27,803 | Cytoplasm | Numerous biological processes and pathways | −5.3 | 0.016 |
| 1433B/31543974 | 14-3-3 protein β/α | 7 | 314 | 24 | 4.77 | 28,183 | Cytoplasm | Numerous biological processes and pathways | −5.3 | 0.016 |
| 1433E/5803225 | 14-3-3 protein ϵ | 3 | 149 | 11 | 4.63 | 29,329 | Cytoplasm | Numerous biological processes and pathways | −5.3 | 0.016 |
| 1433T/6756039 | 14-3-3 protein θ | 3 | 95 | 5 | 4.69 | 28,051 | Cytoplasm | Numerous biological processes and pathways | −5.3 | 0.016 |
| ACTB/4501885 | Actin, cytoplasmic 1 | 2 | 47 | 7 | 5.29 | 42,052 | Cytoplasm | De novo post-translational protein folding | 10.7 | 0.019 |
| PLSL/31542113 | Plastin-2 | 11 | 239 | 25 | 5.2 | 70,743 | Cytoplasm, cytoskeleton | T cell activation involved in immune response | −8.3 | 0.025 |
| EWS/2500583 | RNA-binding protein EWS | 4 | 71 | 8 | 9.37 | 68,666 | Cytoplasm, nucleus | Transcription regulation | −8.3 | 0.025 |
| PLSI/85986577 | Plastin-1 | 2 | 53 | 4 | 5.23 | 70,769 | Cytoplasm | T cell costimulation | −8.3 | 0.025 |
| 6PGL/13384778 | 6-Phosphogluconolactonase | 8 | 187 | 45 | 5.55 | 27,469 | Cytoplasm | 6-Phosphogluconolactonase activity | −3.7 | 0.043 |
| TCPE/6671702 | T-complex protein 1 subunit ϵ | 4 | 159 | 10 | 5.72 | 60,050 | Cytoplasm | De novo post-translational protein folding, response to viruses | −4.8 | 0.05 |
TABLE 2.
Mass spectrometry identification of differentially expressed protein spots in MAVS−/− BMDMs stimulated with poly(I:C) (20 μg/ml) for a range of times (0, 1.5, 8, and 24 h)
The -fold change represents an average differential change of the protein spot over time compared with the control (0 h). The experiment and two-dimensional gel electrophoresis for each stimulation time point were performed in triplicate as independent experiments (mean ± S.E.).
| Accession no. | Protein name | No. of peptide matches | Mascot score | Coverage | pI | Mr | Subcellular localization | Gene ontology | Average -fold change upon poly(I:C) stimulation | ANOVA (p) |
|---|---|---|---|---|---|---|---|---|---|---|
| % | -fold | |||||||||
| HS90B/123681 | Heat shock protein HSP90-β | 10 | 464 | 11 | 4.97 | 83,615 | Cytoplasm | Molecular chaperone | −2.5 | 0.01 |
| PRDX3/6680690 | Thioredoxin-dependent peroxidase reductase | 6 | 274 | 22 | 7.15 | 28,337 | Mitochondrion | Positive regulation of NF-κB transcription factor activity | −4.4 | 0.01 |
| FABP5/6754450 | Fatty acid-binding protein, epidermal | 5 | 205 | 29 | 6.14 | 15,470 | Cytoplasm | High specificity for fatty acids. Highest affinity for C18 chain length | 5.2 | 0.011 |
| TBCA/6678225 | Tubulin-specific chaperone A | 6 | 259 | 35 | 5.24 | 12,807 | Cytoplasm | De novo post-translational protein folding | −3.5 | 0.014 |
| CH60/183396771 | 60-kDa heat shock protein, mitochindrial | 3 | 117 | 4 | 5.91 | 61,088 | Mitochondrion | De novo protein folding, T cell activation | −3.6 | 0.015 |
| 1433F/6756037 | 14-3-3 protein η | 13 | 597 | 41 | 4.81 | 28,365 | Cytoplasm | Numerous biological processes and pathways | −3 | 0.022 |
| 1433B/31543974 | 14-3-3 protein β/α | 7 | 314 | 24 | 4.77 | 28,183 | Cytoplasm | Numerous biological processes and pathways | −3 | 0.022 |
| 1433E/5803225 | 14-3-3 protein ϵ | 3 | 149 | 11 | 4.63 | 29,329 | Cytoplasm | Numerous biological processes and pathways | −3 | 0.022 |
| 1433G/9507245 | 14-3-3 protein γ | 6 | 239 | 20 | 4.8 | 28,456 | Cytoplasm | Numerous biological processes and pathways | −3 | 0.022 |
| 1433S/134023662 | 14-3-3 protein σ | 4 | 163 | 12 | 4.72 | 27,803 | Cytoplasm | Numerous biological processes and pathways | −3 | 0.022 |
| UBP1/31981898 | Ubiquitin C-terminal hydrolase 1 | 2 | 50 | 2 | 5.33 | 88,314 | Nucleus | Cell cycle, Wnt signaling pathway | −5.4 | 0.026 |
| PFD3/124248572 | Prefildin subunit | 2 | 84 | 10 | 6 | 22,592 | Cytoplasm | De novo post-translational protein folding | −3.9 | 0.032 |
| VIME/31982755 | Vimentin | 2 | 58 | 4 | 5.06 | 53,712 | Cell membrane | Cellular component movement | −6.7 | 0.05 |
| PSB6/238231384 | Proteasome subunit β type-6 | 3 | 203 | 13 | 4.97 | 25,591 | Cytoplasm | DNA damage response, cell cycle | −5.7 | 0.051 |
14-3-3ϵ and 14-3-3σ Negatively Regulate TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated Proinflammatory Cytokine Release
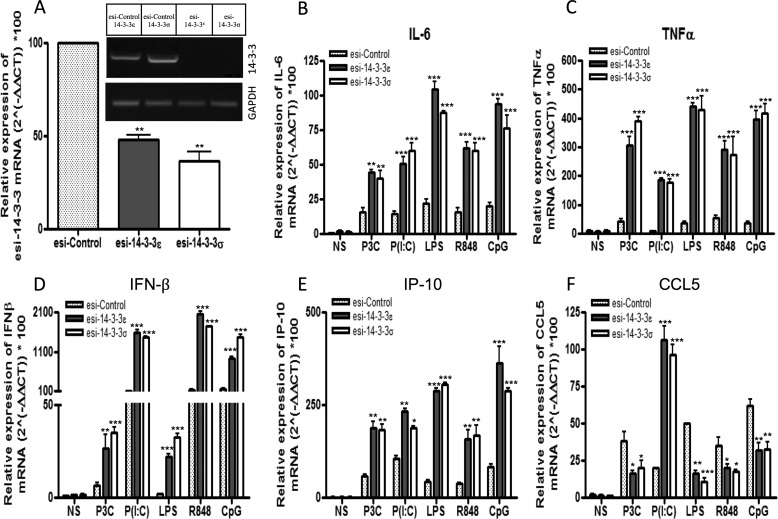
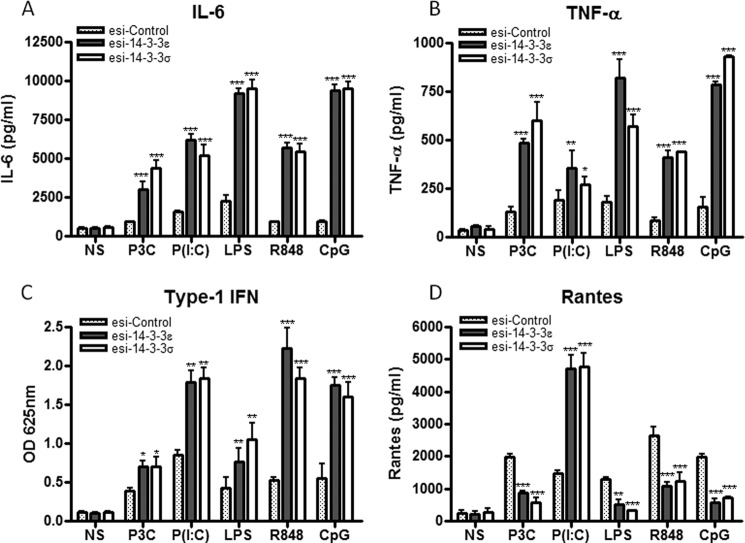
Given that suppression of 14-3-3ζ has previously been shown to modulate TLR2- and TLR4-mediated cytokine production (19), we sought to further define the role of 14-3-3ϵ and 14-3-3σ in TLR signaling. To do this, 14-3-3ϵ and 14-3-3σ expression was suppressed in U373-CD14 cells, a cell line of physiological relevance to TLR signaling (11), by transfecting them with esiRNA directed against 14-3-3ϵ and 14-3-3σ or control lamin A/C. Selective and specific suppression of endogenous expression of 14-3-3ϵ and 14-3-3σ was measured by semiquantitative RT-PCR (Fig. 2A, inset). Silencing of 14-3-3ϵ and 14-3-3σ significantly enhanced Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-induced IL-6, TNFα, IFN-β, and IP-10 gene induction in human cells (Fig. 2, B–E). However, CCL5 gene induction was differentially affected upon silencing of 14-3-3ϵ and 14-3-3σ. Specifically, upon silencing of 14-3-3ϵ and 14-3-3σ, CCL5 gene induction was enhanced following poly(I:C) stimulation but suppressed following Pam3CSK4, LPS, R848, and CpG stimulation (Fig. 2F). Next, we sought to investigate the role of 14-3-3 proteins in the translational regulation of IL-6, TNFα, IFN-β, and RANTES. To this end, 14-3-3ϵ and 14-3-3σ expression was suppressed in U373-CD14 cells, and cytokine release was measured by ELISA following stimulation of cells with Pam3CSK4, poly(I:C), LPS, R848, or CpG. Correlating with the mRNA expression data, suppression of 14-3-3ϵ and 14-3-3σ expression enhanced Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-mediated IL-6, TNFα, and IFN-β production when compared with control cells (Fig. 3, A–C). Notably, correlating with the mRNA data, suppression of 14-3-3ϵ and 14-3-3σ expression enhanced poly(I:C)-mediated RANTES production (Fig. 3D) but decreased Pam3CSK4-, LPS-, R848-, and CpG-mediated RANTES production when compared with control cells (Fig. 3D). Taken together, these data support the hypothesis that 14-3-3ϵ and 14-3-3σ are negative regulators of TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated IL-6, TNFα, and IFN-β induction at the transcriptional level. Notably, differences exist in terms of the role played by 14-3-3 proteins in TLR-mediated RANTES induction.
FIGURE 2.
Suppression of 14-3-3ϵ and 14-3-3σ affects TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated IL-6, TNF-α, IFNβ, IP-10, and CCL5 expression. A–F, U373-CD14 cells were pretreated with either control lamin A/C or 14-3-3ϵ and 14-3-3σ esiRNA to target their suppression. After 24 h, cells were stimulated with Pam3CSK4 (1 μg/ml; P3C), poly(I:C) (20 μg/ml; P(I:C)), LPS (1 μg/ml), R848 (1 μg/ml), and CpG (5 μg/ml) for 3 h, and total RNA was isolated, converted to first strand DNA, and used as a template for quantitative real-time RT-PCR as described under “Experimental Procedures” to assay the expression levels of IL-6 (B), TNF-α (C), IFN-β (D), IP-10 (E), and CCL5 (F) or basal 14-3-3ϵ and 14-3-3σ in unstimulated cells (A). GAPDH was used to normalize all samples, and -fold changes were determined relative to unstimulated control. The data presented are representative of at least three independent experiments performed in triplicate (mean ± S.E. (error bars)). Statistical analysis was performed using unpaired Student's t test and two-way ANOVA tests comparing the test samples with their respective controls. NS, non-stimulated.
FIGURE 3.
Suppression of 14-3-3ϵ and 14-3-3σ augments TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-induced proinflammatory cytokine release. A–D, U373-CD14 cells were pretreated with either control lamin or 14-3-3ϵ and 14-3-3σ esiRNA to target their suppression. After 24 h, cells were stimulated with Pam3CSK4 (1 μg/ml; P3C), poly(I:C) (20 μg/ml; P(I:C)), LPS (1 μg/ml), R848 (1 μg/ml), and CpG (5 μg/ml) for 24 h as indicated. Thereafter, IL-6 (A), TNFα (B), type I IFN (C), and RANTES (D) were measured by ELISA as described under “Experimental Procedures.” The data presented are representative of at least three independent experiments performed in triplicate (mean ± S.E. (error bars)). Statistical analysis was performed using unpaired Student's t test and two-way ANOVA tests comparing the test samples with their respective controls. NS, non-stimulated.
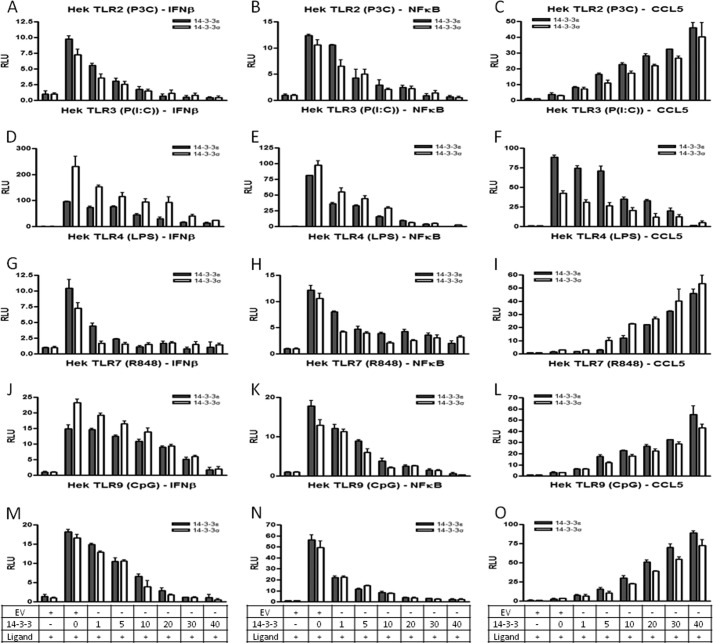
14-3-3 Proteins Inhibit TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated NF-κB and IFN-β Reporter Gene Activity but Differentially Affect CCL5 Reporter Gene Activity
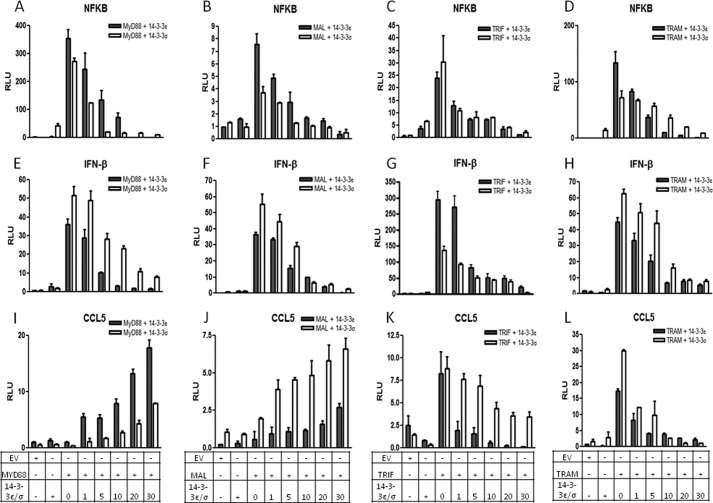
Given that Pam3CSK4, poly(I:C), LPS, R848, and CpG modulate 14-3-3ϵ and 14-3-3σ expression at the transcriptional level, we sought to examine and dissect the role of these proteins in TLR2, -3, -4, -7/8, and -9 signaling, respectively. Initially, we examined the ability of 14-3-3ϵ and 14-3-3σ to modulate TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-dependent transcription factor activation. To do this, HEK293 cells stably transfected with either TLR2 (HEK293-TLR2), TLR3 (HEK293-TLR3), TLR4 (HEK293-TLR4), TLR7 (HEK293-TLR7), or TLR9 (HEK293-TLR9) to render them Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-responsive, respectively, were transiently transfected with the IFN-β, NF-κB, and CCL5 reporter gene constructs and increasing amounts of 14-3-3ϵ and 14-3-3σ. We found that transfection of HEK293-TLR3 cells with 14-3-3ϵ and 14-3-3σ dose-dependently inhibited poly(I:C)-induced activation of the IFN-β, NF-κB, and CCL5 reporter genes (Fig. 4, D–F). Regarding HEK-TLR2, HEK-TLR4, HEK-TLR7, and HEK-TLR9 cells, although 14-3-3ϵ and 14-3-3σ dose-dependently inhibited IFN-β and NF-κB reporter gene activity, 14-3-3ϵ and 14-3-3σ dose-dependently augmented CCL5 reporter gene activity (Fig. 4, A–C and G–O). To further define the role of 14-3-3 proteins in TLR signaling and to understand and clarify the differences in the production of RANTES via different TLRs, we examined the ability of 14-3-3ϵ and 14-3-3σ to modulate signaling through the TLR adaptors, MyD88, Mal, TRIF, and TRAM. To do this, HEK293 cells were transiently transfected with the NF-κB, IFN-β, and CCL5 reporter gene constructs and increasing amounts of the 14-3-3ϵ and 14-3-3σ in the presence of a constant concentration of one of the four TLR adaptors (MyD88/Mal/TRIF/TRAM). We found that 14-3-3ϵ and 14-3-3σ inhibited MyD88-, Mal-, TRIF-, and TRAM-mediated activation of NF-κB and IFN-β reporter genes (Fig. 5, A–H). Interestingly, we found that whereas 14-3-3ϵ and 14-3-3σ inhibited TRIF- and TRAM-mediated activation of the CCL5 reporter gene, 14-3-3ϵ and 14-3-3σ augmented MyD88- and Mal-mediated activation of the CCL5 reporter gene (Fig. 5, I–L). Taken together, these data suggest that both 14-3-3ϵ and 14-3-3σ inhibit TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated IFN-β and NF-κB reporter gene activity. Interestingly, 14-3-3ϵ and 14-3-3σ drive TLR2-, TLR4-, TLR7/8-, and TLR9-mediated CCL5 reporter gene activity, but not TLR3-mediated CCL5 reporter gene activity, through a mechanism that involves MyD88 and Mal but not TRIF and TRAM. Together, these data provide evidence that 14-3-3ϵ and 14-3-3σ differentially modulate TLR-mediated RANTES production.
FIGURE 4.
14-3-3ϵ and 14-3-3σ inhibit TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated NF-κB and IFN-β reporter gene activity but enhance TLR2-, TLR4-, TLR7/8-, and TLR9-mediated CCL5 reporter gene activity. HEK293-TLR2 (A–C), HEK293-TLR3 (D–F), HEK293-TLR4 (G–I), HEK293-TLR7 (J–L), and HEK293-TLR9 cells (M–O) were co-transfected with vectors encoding a reporter gene for the IFN-β, NF-κB, or CCL5 promoter (80 ng) and either empty vector (40 ng) or increasing amounts of an expression vector encoding 14-3-3ϵ or 14-3-3σ (0, 1, 5, 10, 20, 30, or 40 ng), as indicated. A total of 40 ng/well phRL-TK (TK-Renilla-luciferase) reporter gene was co-transfected simultaneously to normalize data for transfection efficiency After 24 h, cells were stimulated with Pam3CSK4 (1 μg/ml; P3C) (A–C), poly(I:C) (20 μg/ml; P(I:C)) (D–F), LPS (1 μg/ml) (G–I), R848 (1 μg/ml) (J–L), and CpG (5 μg/ml) (M–O) for 24 h followed by harvesting of cell lysates and assessment of luciferase reporter gene activity. The data presented are representative of at least three independent experiments performed in triplicate (mean ± S.E. (error bars)). Statistical analysis was performed using unpaired Student's t test and two-way ANOVA tests comparing the test samples with their respective controls.
FIGURE 5.
14-3-3ϵ and 14-3-3σ inhibit TLR adaptor-dependent NF-κB and IFN-β reporter gene activity but differentially affect CCL5 reporter gene activity. A–L, HEK293 cells were co-transfected with vectors encoding a reporter gene for either the IFN-β, NF-κB, or CCL5 promoter (80 ng) and either empty vector (40 ng), MyD88, MAL, TRIF, or TRAM plasmids (10 ng) and increasing amounts of an expression vector encoding 14-3-3ϵ or 14-3-3σ (0, 1, 5, 10, 20, or 30 ng), as indicated. A total of 40 ng/well phRL-TK (TK-Renilla-luciferase) reporter gene was co-transfected simultaneously to normalize data for transfection efficiency After 24 h, cell lysates were harvested and assessed for luciferase reporter gene activity. The data presented are representative of at least three independent experiments performed in triplicate (mean ± S.E. (error bars)). Statistical analysis was performed using unpaired Student's t test and two-way ANOVA tests comparing the test samples with their respective controls.
Pam3CSK4-, LPS-, R848-, and CpG-mediated 14-3-3 Expression Is Controlled by MyD88; however, Poly(I:C)-mediated 14-3-3 Expression Is Controlled by TRIF
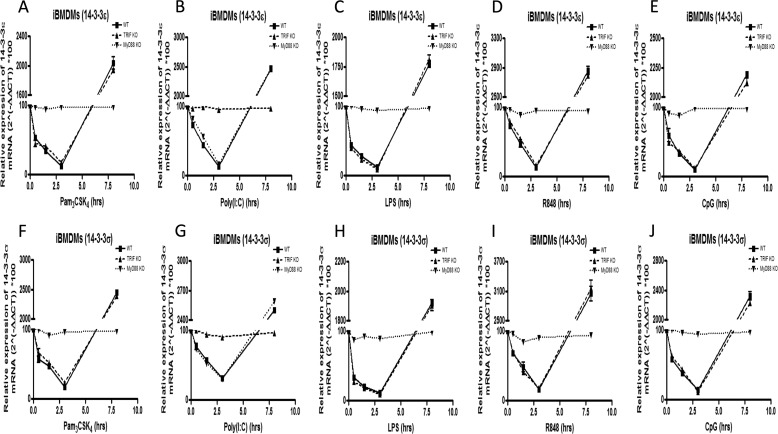
Upon observing the differential role of TRIF and MyD88 in TLR-mediated RANTES production, we sought to further explore the critical role of the adaptors TRIF and MyD88 in 14-3-3-dependent TLR signaling. To do this, WT, TRIF−/−, and MyD88−/− iBMDMs were stimulated with Pam3CSK4, poly(I:C), LPS, R848, and CpG for the indicated times, and 14-3-3ϵ and 14-3-3σ mRNA expression levels were quantified using quantitative RT-PCR. It was observed that TRIF−/− iBMDMs showed comparable modulation of 14-3-3ϵ and 14-3-3σ mRNAs relative to WT iBMDMs upon stimulation with Pam3CSK4, LPS, R848, and CpG (Fig. 6, A–J). As expected, the expression of 14-3-3 mRNA remained unaltered upon stimulation of TRIF−/− iBMDMs with poly(I:C) (Fig. 6, B and G). In contrast to TRIF−/− iBMDMs, it was observed that stimulation of MyD88−/− iBMDMs with poly(I:C) induced comparable modulation of 14-3-3ϵ and 14-3-3σ mRNAs relative to WT iBMDMs (Fig. 6, B and G). As expected, the expression of 14-3-3ϵ and 14-3-3σ mRNAs remained unaltered upon stimulation of MyD88−/− iBMDMs with Pam3CSK4, LPS, R848, and CpG (Fig. 6, A–J). Taken together, these data suggest that whereas TLR3-mediated 14-3-3ϵ and 14-3-3σ expression is controlled by TRIF, TLR2-, TLR4-, TLR7/8-, and TLR9-mediated 14-3-3ϵ and 14-3-3σ expression is controlled by MyD88.
FIGURE 6.
Pam3CSK4-, LPS-, R848-, and CpG-mediated 14-3-3 expression is MyD88-dependent, whereas poly(I:C)-mediated 14-3-3 expression is TRIF-dependent. A–J, WT, TRIF−/−, and MyD88−/− iBMDMs were stimulated with Pam3CSK4 (1 μg/ml) (A and F), poly(I:C) (20 μg/ml) (B and G), LPS (1 μg/ml) (C and H), R848 (1 μg/ml) (D and I), and CpG (5 μg/ml) (E and J) for the indicated times (0, 0.5, 1.5, 3, and 8 h). Then total RNA was isolated, converted to first strand DNA, and used as a template for quantitative real-time RT-PCR as described under “Experimental Procedures” to assay the expression levels of 14-3-3ϵ (A–E) and 14-3-3σ (F–J). The data presented are representative of at least three independent experiments performed in triplicate (mean ± S.E. (error bars)). Statistical analysis was performed using unpaired Student's t test and two-way ANOVA tests comparing the test samples with their respective controls.
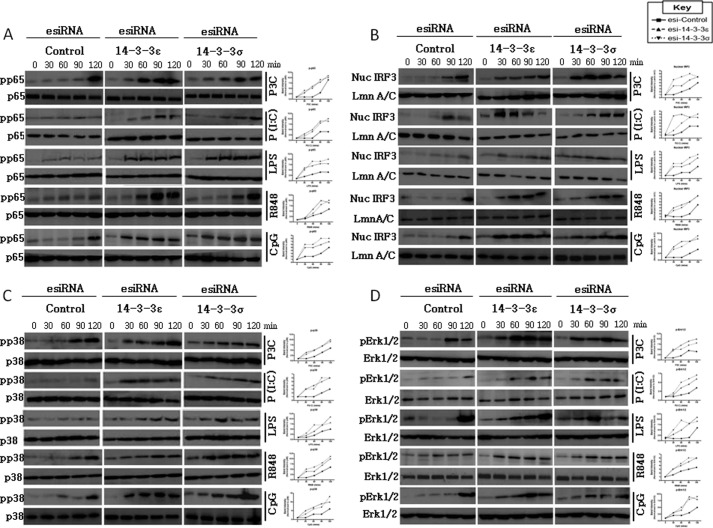
Suppression of 14-3-3ϵ and 14-3-3σ Enhances TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated Phosphorylation of p65, IRF3, p38, and ERK1/2
We have demonstrated that 14-3-3ϵ and 14-3-3σ impairs Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-induced activation of the NF-κB and IFN-β reporter genes and differentially affects CCL5 reporter gene activity. To support our data, we opted to investigate the ability of 14-3-3ϵ and 14-3-3σ to modulate the activity of the transcription factors, NF-κB and IRF3, and MAPK signaling. We found that suppression of 14-3-3ϵ and 14-3-3σ in U373-CD14 cells augmented Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-mediated phosphorylation of NF-κB (Fig. 7A). Similarly, suppression of 14-3-3ϵ and 14-3-3σ also augmented Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-mediated nuclear translocation of IRF3 (Fig. 7B). In addition, suppression of 14-3-3ϵ and 14-3-3σ also augmented Pam3CSK4-, poly(I:C)-, LPS-, R848-, and CpG-mediated phosphorylation of p38 and ERK1/2 (Fig. 7, C and D). These data were confirmed following normalization of active protein levels relative to total protein expression or lamin A/C using densitometry (Fig. 7 and supplemental Fig. S1). Together, these data support the hypothesis that 14-3-3ϵ and 14-3-3σ serve to inhibit TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated proinflammatory cytokine production through a mechanism involving curtailment of TLR adaptor-mediated activation of the transcription factors, NF-κB and IRF-3. More globally, 14-3-3ϵ and 14-3-3σ also serve to curtail TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated p38 activation and ERK signaling. Together, these data suggest that 14-3-3ϵ and 14-3-3σ may mediate their inhibitory effects upstream within the TLR signaling pathway via modulation of TLR adaptor functionality rather than through modulation of transcription factor activity.
FIGURE 7.
Suppression of 14-3-3ϵ and 14-3-3σ enhances TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated activation of NF-κB, IRF3, p38, and ERK1/2. U373-CD14 cells were pretreated with either control lamin A/C or 14-3-3ϵ or 14-3-3σ esiRNA to target their suppression. After 24 h, cells were stimulated with Pam3CSK4 (1 μg/ml; P3C), poly(I:C) (20 μg/ml; P(I:C)), LPS (1 μg/ml), R848 (1 μg/ml), and CpG (5 μg/ml) for 0–120 min as indicated. Next, the cell lysates were harvested, and NF-κB (phospho-p65 (A)), p38 (phospho-p38 (C)), and ERK1/2 (phospho-ERK1/2 (D)) signaling was assessed by immunoblot analysis with total protein serving as a loading control. Alternatively, the nuclear protein fraction was isolated, and immunoblot analysis was performed using an anti-IRF3 antibody (B). Equal protein loading was confirmed using an anti-lamin A/C antibody. Next, densitometry was performed to determine the relative activation of the indicated protein, whereby the band intensity of the activated protein was normalized relative to the respective total protein level. The data presented are representative of three independent experiments.
14-3-3ϵ and 14-3-3σ Bind to the TLR Adaptors, TRAF3, and TRAF6
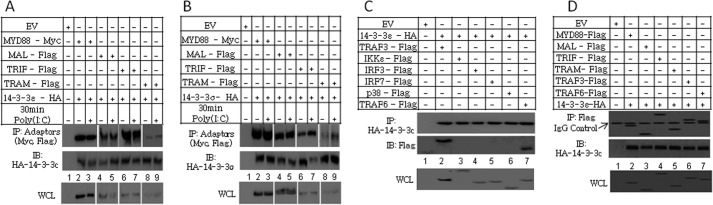
Given our results and the fact that MyD88 has previously been shown to interact with 14-3-3ζ following IL-1β and Pam3CSK4 stimulation (19), we sought to further explore the molecular mechanisms utilized by 14-3-3 proteins to impair TLR signaling. To do this, co-immunoprecipitation studies were performed in HEK-293 cells. It was found that MyD88, Mal, TRIF, and TRAM co-immunoprecipitated with 14-3-3ϵ and 14-3-3σ (Fig. 8, A and B) in a ligand-independent manner. Moreover, 14-3-3ϵ co-immunoprecipitated with TRAF3 (Fig. 8C, lane 2) and TRAF6 (lane 7) but not with IKKϵ (lane 3), IRF3 (lane 4), IRF7 (lane 5), or p38 (lane 6). Comparable co-immunoprecipitation results were obtained using 14-3-3σ (data not shown). To preclude the possibility of cell type-dependent differences in the protein interactions, co-immunoprecipitation of 14-3-3ϵ and 14-3-3σ with MyD88, MAL, TRIF, TRAM, TRAF3, and TRAF6 was also investigated in human astrocytoma U373-CD14 cells. Correlating with our HEK293 data, we found that MyD88, Mal, TRIF, TRAM, TRAF3, and TRAF6 co-immunoprecipitated with 14-3-3ϵ and 14-3-3σ (Fig. 8D and data not shown, respectively). Together, these data support the hypothesis that both 14-3-3ϵ and 14-3-3σ exert their inhibitory effects upstream in the TLR signaling pathway by binding to the TLR adaptors and to TRAF3 and TRAF6.
FIGURE 8.
14-3-3ϵ and 14-3-3σ bind to the TLR adaptors, TRAF3 and TRAF6. A and B, HEK293-TLR3 cells were co-transfected with vectors encoding MyD88-c-myc, Mal-FLAG, TRIF-FLAG, TRAM-FLAG, or empty vector (EV) and with either 14-3-3ϵ-HA (A) or 14-3-3σ-HA (B). After 24 h, cells were stimulated with poly(I:C) (20 μg/ml) for 30 min as indicated. Thereafter, immunoprecipitation (IP) of 14-3-3ϵ (A) and 14-3-3σ (B) was performed using an anti-HA antibody as described under “Experimental Procedures.” C, HEK293 cells were cotransfected with vectors encoding TRAF3-FLAG, IKKϵ-FLAG, IRF3-FLAG, IRF7-FLAG, p38-FLAG, TRAF-6, 14-3-3ϵ-HA, or empty vector as indicated. After 24 h, immunoprecipitation was performed using an anti-HA antibody as described under “Experimental Procedures.” D, U373-CD14 cells were co-transfected with vectors encoding MyD88-FLAG, Mal-FLAG, TRIF-FLAG, TRAM-FLAG, or empty vector and with 14-3-3ϵ-HA. After 24 h, immunoprecipitation was performed using an anti-FLAG antibody as described under “Experimental Procedures.” The data presented are representative of two independent experiments. IB, immunoblot; WCL, whole cell lysate.
Common Biological Processes Modulated by 14-3-3ϵ and 14-3-3σ in TLR Signaling Networks
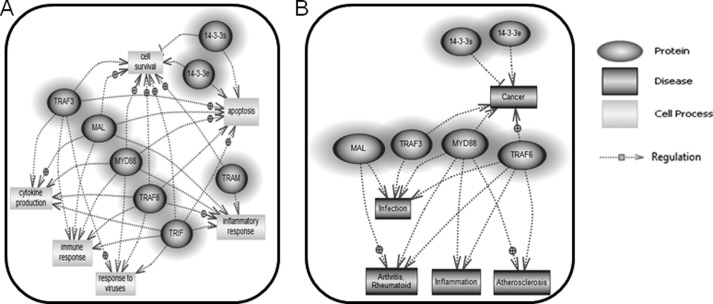
Upon identification and validation of MyD88, Mal, TRIF, TRAM, TRAF3, and TRAF6 as interacting partners of 14-3-3ϵ and 14-3-3σ, we opted to investigate whether 14-3-3ϵ and 14-3-3σ and their interacting proteins, MyD88, Mal, TRIF, TRAM, TRAF3, and TRAF6, are involved in the co-regulation of similar biological processes and diseases. To do this, Pathway Studio Software (Ariadne Genomics) was used to investigate and integrate the search for biological processes and diseases co-associated with 14-3-3 proteins and their interacting partners. Assimilated data demonstrated that both 14-3-3ϵ and 14-3-3σ and their interacting partners play a regulatory role in biological processes, such as cytokine production and the inflammatory response (Fig. 9A). Also, the 14-3-3 proteins and their interacting partners co-modulate pathological processes, such as atherosclerosis and rheumatoid arthritis (Fig. 9B). Given our data showing that 14-3-3ϵ and 14-3-3σ interact with TLR signaling molecules, including MyD88, Mal, TRIF, TRAM, TRAF3, and TRAF6, and concomitantly modulate TLR signaling, we can postulate that 14-3-3 proteins may play a role in TLR-mediated cellular processes, such as immune and inflammatory responses, cytokine production, and responses to viral and bacterial infections (Fig. 9).
FIGURE 9.
Cellular processes and diseases associated with 14-3-3ϵ and 14-3-3σ and interacting partners. The newly identified and verified 14-3-3ϵ and 14-3-3σ protein interacting partners, including MyD88, MAL, TRIF, TRAM, TRAF3, TRAF6 were uploaded onto Pathway Studio software (Ariadne Genomics). A and B, 14-3-3ϵ and 14-3-3σ and interacting partners were analyzed using Pathway Studio software for the cellular process network (A) and common diseases (B) shared between them. The dark highlighted circular/oval-shaped entities represent the 14-3-3 proteins and interacting partners. The rectangular entities indicate the cellular processes (A) and diseases (B) that are co-regulated by 14-3-3ϵ and 14-3-3σ and interacting partners.
DISCUSSION
TLRs are critically involved in mediating a normal physiological response to invading pathogens toward their elimination, and it is becoming increasingly appreciated that TLRs are involved in inflammatory pathologies (26–28). The innate immune system has evolved in such a way to provide many strategies to limit TLR functionality with a view to curtailing unwanted inflammatory responses (7, 11). Given the central role played by TLRs toward the sensing of PAMPs and danger-associated molecular patterns and emanation of the inflammatory response, albeit inappropriate in certain cases, we sought to investigate novel mechanisms that may have evolved toward the modulation of TLR functionality. Given that dsRNA is one of the most important viral PAMPs, we opted to investigate the immune responses that are instigated upon exposure of iBMDMs to synthetic dsRNA, namely poly(I:C). Using an integrated quantitative proteomics approach, we have identified 14-3-3ϵ and 14-3-3σ proteins as critical new regulators of the TLR2, TLR3, TLR4, TLR7/8, and TLR9 signal transduction pathways. Briefly, upon stimulation of murine iBMDMs with Pam3CSK4, poly(I:C), LPS, R848, and CpG, ligand-dependent degradation of 14-3-3ϵ and 14-3-3σ at 0.5–3 h was observed, followed by induction at later time points. Similarly, degradation followed by induction of 14-3-3 mRNA was also demonstrated in human U373-CD14 astrocytoma cells and human synoviocytes upon Pam3CSK4, poly(I:C), LPS, R848, and CpG stimulation. These findings correlate with another study (29), which showed that the increased expression of multiple 14-3-3 proteins was evident upon stimulation of keratinocytes with dsRNA for 18 h.
Toward the functional characterization of 14-3-3ϵ and 14-3-3σ in TLR signaling, we have shown that both of these proteins impair TLR2, TLR3, TLR4, TLR7/8, and TLR9 ligand-induced IL-6, TNFα, and IFN-β production. This disagrees, at least in part, with a previous study showing that 14-3-3ζ enhanced TLR2- and TLR4-mediated TNFα and IL-8 production in RAW264.7 cells (19). However, the same study also showed that 14-3-3ζ suppressed TLR2-, TLR4-, and TLR9-mediated IL-6 production and did not affect IP-10 production (19). We also show that 14-3-3ϵ and 14-3-3σ impair TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-mediated NF-κB and IFN-β reporter gene activity, which is in contrast to a previous study showing that 14-3-3ζ enhanced TLR4-mediated NF-κB reporter gene activity (19). Interestingly, the same study showed that 14-3-3ζ suppressed TLR2-mediated NF-κB reporter gene activity (19). However, it must be noted that differences in functionality exist between the various 14-3-3 isoforms and may account for the observed experimental discrepancies (25). Regarding the modulation of TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-driven RANTES production, we provide evidence that, similar to NF-κB and IFN-β induction, 14-3-3 proteins serve to inhibit poly(I:C)-mediated CCL5 mRNA and protein production. Interestingly, 14-3-3 proteins are required for Pam3CSK4-, LPS-, R848-, and CpG-mediated production of RANTES in a Mal/MyD88-dependent manner. Given these findings, we propose that the differential effects of Pam3CSK4, poly(I:C), LPS, R848, and CpG on RANTES production may be due to alternative adaptor utilization. More specifically, poly(I:C)-mediated CCL5 induction, facilitated by TRIF, is impaired by 14-3-3. In contrast, Pam3CSK4-, LPS-, R848-, and CpG-mediated 14-3-3 expression, facilitated by MyD88, is enhanced by 14-3-3. Because studies have shown that the CCL5 promoter contains numerous response elements for the transcriptional activators NF-κB, AP-1, SP-1, IRF3, IRF-1-RE, and STAT1 (31–33), we propose that transcriptional induction of CCL5 mRNA may be differentially regulated by MyD88 and TRIF through the induction/suppression of various transcriptional activators. Herein, we preclude NF-κB and IRF3 because they are comparably modulated following stimulation with the various PAMPs. Thus, we propose that the differential effects of TRIF and MyD88 on RANTES production may be facilitated through the differential activation of the other key CCL5 transcriptional activators, such as AP-1, SP-1, IRF-1-RE, and STAT1. Thus, it is plausible to speculate that PAMPs that drive MyD88-dependent TLR signaling may, in conjunction with 14-3-3, induce the expression of specific transcriptional activators to drive CCL5 transcription. In contrast, PAMPs that drive MyD88-independent TRIF signaling, in conjunction with 14-3-3, may induce the expression of inhibitory elements or suppress the expression of transcriptional activators toward the inhibition of CCL5 transcription. Interestingly, we have previously shown that differences exist in terms of the regulation of RANTES production by poly(I:C). Specifically, we have shown that although MyD88 inhibits poly(I:C)-mediated RANTES production, Mal does not (7, 11). Therefore, our work correlates with other studies showing that CCL5 induction is a complex cell type- and ligand-dependant process in vivo (32, 33).
Recently, the role of 14-3-3β in TLR signaling has been investigated (18). Following LPS stimulation of cells, PKCϵ is recruited to TLR4, followed by phosphorylation of PKCϵ and subsequent association with 14-3-3β in a MyD88-dependent manner (18). Binding of 14-3-3β to PKCϵ has been shown to lock PKCϵ in an open conformation, thus regulating its lipid binding activity (34). Another study has shown that dsRNA induces 14-3-3-mediated signaling pathways in human keratinocytes (29). However, the exact role played by 14-3-3 proteins in TLR signaling requires further clarification. Herein, we employed co-immunoprecipitation experiments and have shown that 14-3-3ϵ and 14-3-3σ bind to MyD88, Mal, TRIF, and TRAM. Further, 14-3-3ϵ and 14-3-3σ also bind TRAF3 and TRAF6 but not IKKϵ, IRF3, IRF7, or p38. Notably, although the interaction between the 14-3-3 proteins and the TLR signaling molecules is ligand-independent, our data clearly show that stimulation of cells with PAMPs is required to activate TLR signaling and to facilitate the immunomodulatory capacity of the 14-3-3 proteins toward the curtailment of TLR-mediated proinflammatory cytokine production. In support of these findings is the fact that 14-3-3 isoforms have previously been shown to interact with another member of the TRAF family, TRAF7, and components of the TLR signaling pathway, namely TAB2, TAK1, and TBK1 (TRAF family member-associated NF-κB activator-binding kinase 1) (1, 25, 35). Together, these data suggest that 14-3-3ϵ and 14-3-3σ mediate their cellular effects through the TLR adaptors and TRAF3 and TRAF6.
The 14-3-3 proteins are a family of conserved, ubiquitously expressed, acidic proteins, consisting of seven known mammalian isoforms (β, γ, ϵ, σ, ζ, τ, and η), which are differentially expressed in mammalian cells and are subject to phosphorylation (13–15). 14-3-3 proteins have at least 200 interacting partners, and in most cases, binding is dependent on the phosphorylation of the target protein at either serine or threonine motifs (15). Binding partners include a multitude of functionally diverse signaling proteins, including kinases, phosphatases, and transmembrane receptors, which allows 14-3-3 proteins to play a vital role in a variety of regulatory processes, such as metabolism, apoptosis, cell proliferation, growth, differentiation, and intracellular signaling (16). It is becoming increasingly appreciated that abnormal 14-3-3 expression or dysregulation of 14-3-3/target protein interactions contribute to many diseases (e.g. cancer, joint inflammation, and bacterial/viral infections) (17). More specifically, 14-3-3σ is purported to be a tumor suppressor and is silenced in breast cancers (17). In contrast, 14-3-3ϵ expression is enhanced in lung cancers (36). Because TLR activation and dysregulation have also been linked with such pathologies (1, 25, 30, 37), it is tempting to speculate that coordinated dysregulation of TLRs and 14-3-3 proteins may occur in these diseases. Herein, we propose that upon stimulation of TLR2, TLR3, TLR4, TLR7/8, and TLR9 with their respective ligands, 14-3-3ϵ and 14-3-3σ expression is reduced, thus permitting early proinflammatory cytokine production. Later, 14-3-3ϵ and 14-3-3σ expression is induced, thereby curtailing proinflammatory cytokine production. Whether 14-3-3 proteins serve to curtail the unwanted inflammatory milieu that exists during chronic inflammatory diseases is certainly worthy of further investigation.
Regarding the role played by 14-3-3 proteins during viral and bacterial infections, we propose that initial sensing of the pathogen by the TLR leads to suppression of 14-3-3ϵ and 14-3-3σ expression, thereby permitting the induction of TLR-driven early proinflammatory cytokine and type I IFN production toward the elimination of the infection. Later, following curtailment of the infection, 14-3-3ϵ and 14-3-3σ expression is induced, thus leading to the inhibition of proinflammatory cytokines and type I IFN production. Thus, we propose that TLR-mediated cytokine and chemokine production is a self-regulating process that is facilitated, at least in part, by 14-3-3ϵ and 14-3-3σ.
Thus, by identifying 14-3-3ϵ and 14-3-3σ as critical negative regulators of TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-dependent proinflammatory and type I IFN production, the present study provides insight into the mechanisms that serve to modulate TLR-dependent signaling. More specifically, 14-3-3ϵ and 14-3-3σ inhibit TLR2-, TLR3-, TLR4-, TLR7/8-, and TLR9-dependent IL-6, TNFα, and IFN-β induction. Interestingly, 14-3-3ϵ and 14-3-3σ augment, rather than impair, TLR2-, TLR4-, TLR7/8-, and TLR9-mediated RANTES production but not TLR3-mediated RANTES production. These effects are mediated through interaction of the 14-3-3 proteins with the TLR adaptors and both TRAF3 and TRAF6. This interaction may serve to recruit auxiliary immunomodulatory molecules to the complex. Alternatively, the interaction may curtail the ability of the TLR adaptors and/or TRAF3 and TRAF6 to engage with downstream signaling proteins, thus impeding cytokine production. Although the precise molecular role for 14-3-3 in TLR signaling requires further investigation, it is clear that the 14-3-3 proteins respond to changes in the phosphorylation status of target proteins within the cell, so they respond to the dynamic changes that occur within a cell upon sensing of invading pathogens.
In conclusion, 14-3-3ϵ and 14-3-3σ play a major regulatory role in balancing the host inflammatory response to viral and bacterial infections. Thus, monitoring and manipulation of 14-3-3 proteins may represent novel diagnostic and therapeutic targets for inflammatory conditions and infections.
This work was supported by a Science Foundation Ireland award (to S. M.).

This article contains supplemental Fig. S1.
- PAMP
- pathogen-associated molecular pattern
- BMDM
- bone marrow-derived macrophage
- iBMDM
- immortalized BMDM
- TIR
- Toll/interleukin-1 receptor
- TLR
- Toll-like receptor
- TRAM
- TRIF-related adaptor molecule
- TRIF
- TIR domain-containing adaptor-inducing IFN-β
- RLR
- RIG-I-like receptor
- esiRNA
- endoribonuclease-prepared siRNA
- ANOVA
- analysis of variance
- IPG
- immobilized pH gradient.
REFERENCES
- 1. Miggin S. M., O'Neill L. A. (2006) New insights into the regulation of TLR signaling. J. Leukoc. Biol. 80, 220–226 [DOI] [PubMed] [Google Scholar]
- 2. Keating S. E., Baran M., Bowie A. G. (2011) Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol. 32, 574–581 [DOI] [PubMed] [Google Scholar]
- 3. Kumar H., Kawai T., Akira S. (2009) Pathogen recognition in the innate immune response. Biochem. J. 420, 1–16 [DOI] [PubMed] [Google Scholar]
- 4. Vercammen E., Staal J., Beyaert R. (2008) Sensing of viral infection and activation of innate immunity by Toll-like receptor 3. Clin. Microbiol. Rev. 21, 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Neill L. A., Bowie A. G. (2007) The family of five. TIR domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. 7, 353–364 [DOI] [PubMed] [Google Scholar]
- 6. Kenny E. F., Talbot S., Gong M., Golenbock D. T., Bryant C. E., O'Neill L. A. (2009) MyD88 adaptor-like is not essential for TLR2 signaling and inhibits signaling by TLR3. J. Immunol. 183, 3642–3651 [DOI] [PubMed] [Google Scholar]
- 7. Siednienko J., Gajanayake T., Fitzgerald K. A., Moynagh P., Miggin S. M. (2011) Absence of MyD88 results in enhanced TLR3-dependent phosphorylation of IRF3 and increased IFN-β and RANTES production. J. Immunol. 186, 2514–2522 [DOI] [PubMed] [Google Scholar]
- 8. Zhou Y., Wang X., Liu M., Hu Q., Song L., Ye L., Zhou D., Ho W. (2010) A critical function of Toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology 131, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu M., Levine S. J. (2011) Toll-like receptor, RIG-I-like receptors and the NLRP3 inflammasome. Key modulators of innate immune responses to double-stranded RNA viruses. Cytokine Growth Factor Rev. 22, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavassani K. A., Ishii M., Wen H., Schaller M. A., Lincoln P. M., Lukacs N. W., Hogaboam C. M., Kunkel S. L. (2008) TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 205, 2609–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siednienko J., Halle A., Nagpal K., Golenbock D. T., Miggin S. M. (2010) TLR3-mediated IFN-β gene induction is negatively regulated by the TLR adaptor MyD88 adaptor-like. Eur. J. Immunol. 40, 3150–3160 [DOI] [PubMed] [Google Scholar]
- 12. Siednienko J., Maratha A., Yang S., Mitkiewicz M., Miggin S. M., Moynagh P. N. (2011) Nuclear factor κB subunits RelB and cRel negatively regulate Toll-like receptor 3-mediated β-interferon production via induction of transcriptional repressor protein YY1. J. Biol. Chem. 286, 44750–44763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu H., Subramanian R. R., Masters S. C. (2000) 14-3-3 proteins. Structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40, 617–647 [DOI] [PubMed] [Google Scholar]
- 14. Muslin A. J., Tanner J. W., Allen P. M., Shaw A. S. (1996) Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84, 889–897 [DOI] [PubMed] [Google Scholar]
- 15. Yaffe M. B., Rittinger K., Volinia S., Caron P. R., Aitken A., Leffers H., Gamblin S. J., Smerdon S. J., Cantley L. C. (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91, 961–971 [DOI] [PubMed] [Google Scholar]
- 16. Obsilová V., Silhan J., Boura E., Teisinger J., Obsil T. (2008) 14-3-3 proteins. A family of versatile molecular regulators. Physiol. Res. 57, S11–S21 [DOI] [PubMed] [Google Scholar]
- 17. Zhao J., Meyerkord C. L., Du Y., Khuri F. R., Fu H. (2011) 14-3-3 proteins as potential therapeutic targets. Semin. Cell Dev. Biol. 22, 705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faisal A., Saurin A., Gregory B., Foxwell B., Parker P. J. (2008) The scaffold MyD88 acts to couple protein kinase Cϵ to Toll-like receptors. J. Biol. Chem. 283, 18591–18600 [DOI] [PubMed] [Google Scholar]
- 19. Schuster T. B., Costina V., Findeisen P., Neumaier M., Ahmad-Nejad P. (2011) Identification and functional characterization of 14-3-3 in TLR2 signaling. J. Proteome Res. 10, 4661–4670 [DOI] [PubMed] [Google Scholar]
- 20. Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. (2003) IKKϵ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 [DOI] [PubMed] [Google Scholar]
- 21. Fitzgerald K. A., Rowe D. C., Barnes B. J., Caffrey D. R., Visintin A., Latz E., Monks B., Pitha P. M., Golenbock D. T. (2003) LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the Toll adapters TRAM and TRIF. J. Exp. Med. 198, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mullen E., Ohlendieck K. (2010) Proteomic profiling of non-obese type 2 diabetic skeletal muscle. Int. J. Mol. Med. 25, 445–458 [DOI] [PubMed] [Google Scholar]
- 23. Heukeshoven J., Dernick R. (1985) Characterization of a solvent system for separation of water-insoluble poliovirus proteins by reversed-phase high performance liquid chromatography. J. Chromatogr. 326, 91–101 [DOI] [PubMed] [Google Scholar]
- 24. Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K. J., Yamaguchi O., Otsu K., Tsujimura T., Koh C. S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 [DOI] [PubMed] [Google Scholar]
- 25. Zuo S., Xue Y., Tang S., Yao J., Du R., Yang P., Chen X. (2010) 14-3-3 ϵ dynamically interacts with key components of mitogen-activated protein kinase signal module for selective modulation of the TNF-α-induced time course-dependent NF-κB activity. J. Proteome Res. 9, 3465–3478 [DOI] [PubMed] [Google Scholar]
- 26. Sellam J., Berenbaum F. (2010) The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 [DOI] [PubMed] [Google Scholar]
- 27. Sacre S. M., Andreakos E., Kiriakidis S., Amjadi P., Lundberg A., Giddins G., Feldmann M., Brennan F., Foxwell B. M. (2007) The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am. J. Pathol. 170, 518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scanzello C. R., Plaas A., Crow M. K. (2008) Innate immune system activation in osteoarthritis. Is osteoarthritis a chronic wound? Curr. Opin. Rheumatol. 20, 565–572 [DOI] [PubMed] [Google Scholar]
- 29. Ohman T., Lietzén N., Välimäki E., Melchjorsen J., Matikainen S., Nyman T. A. (2010) Cytosolic RNA recognition pathway activates 14-3-3 protein-mediated signaling and caspase-dependent disruption of cytokeratin network in human keratinocytes. J. Proteome Res. 9, 1549–1564 [DOI] [PubMed] [Google Scholar]
- 30. McCormack W. J., Parker A. E., O'Neill L. A. (2009) Toll-like receptors and NOD-like receptors in rheumatic diseases. Arthritis Res. Ther. 11, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wickremasinghe M. I., Thomas L. H., O'Kane C. M., Uddin J., Friedland J. S. (2004) Transcriptional mechanisms regulating alveolar epithelial cell-specific CCL5 secretion in pulmonary tuberculosis. J. Biol. Chem. 279, 27199–27210 [DOI] [PubMed] [Google Scholar]
- 32. Liu H., Ning H., Men H., Hou R., Fu M., Zhang H., Liu J. (2012) Regulation of CCL5 expression in smooth muscle cells following arterial injury. PloS One 7, e30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar D., Hosse J., von Toerne C., Noessner E., Nelson P. J. (2009) JNK MAPK pathway regulates constitutive transcription of CCL5 by human NK cells through SP1. J. Immunol. 182, 1011–1020 [DOI] [PubMed] [Google Scholar]
- 34. Saurin A. T., Durgan J., Cameron A. J., Faisal A., Marber M. S., Parker P. J. (2008) The regulated assembly of a PKCϵ complex controls the completion of cytokinesis. Nat. Cell Biol. 10, 891–901 [DOI] [PubMed] [Google Scholar]
- 35. Bouwmeester T., Bauch A., Ruffner H., Angrand P. O., Bergamini G., Croughton K., Cruciat C., Eberhard D., Gagneur J., Ghidelli S., Hopf C., Huhse B., Mangano R., Michon A. M., Schirle M., Schlegl J., Schwab M., Stein M. A., Bauer A., Casari G., Drewes G., Gavin A. C., Jackson D. B., Joberty G., Neubauer G., Rick J., Kuster B., Superti-Furga G. (2004) A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat. Cell Biol. 6, 97–105 [DOI] [PubMed] [Google Scholar]
- 36. Qi W., Liu X., Qiao D., Martinez J. D. (2005) Isoform-specific expression of 14-3-3 proteins in human lung cancer tissues. Int. J. Cancer 113, 359–363 [DOI] [PubMed] [Google Scholar]
- 37. Matijevic T., Pavelic J. (2010) Toll-like receptors. Cost or benefit for cancer? Curr. Pharm. Des. 16, 1081–1090 [DOI] [PubMed] [Google Scholar]