Abstract
A salient feature of the developing brain is that spontaneous oscillations (SOs) and waves may influence the emergence of synaptic connections. Whilst gamma-amino butyric acid (GABA) produces depolarization and may support SOs in the neurons of developing rodents, it elicits hyperpolarization and diminishes SOs in developing gerbil auditory cortex (ACx). Therefore, we asked whether SOs exist in developing gerbil ACx in vivo and if GABAergic involvement can be manipulated. In vivo extracellular recordings in P3-5 ACx revealed SOs with longer burst durations and shorter inter-event intervals compared to ACx SOs in slices. ACx was then validated by gross anatomical features and lesions created at the in vivo recording site that corresponded with the electrophysiological coordinates of thalamorecipient ACx in slices. Further, NeuroVue Red, a lipophilic dye loaded at the in vivo recording sites, stained anatomically identifiable fiber tracks between the ACx and the auditory thalamus, medial geniculate body (MG). Separately, to chronically perturb GABAergic role in SOs, P2-5 pups were administered daily with GABAA receptor blocker, bicuculline (BIC). We then recorded from P14-17 ACx neurons in slices generated after hearing onset. ACx neurons from BIC-administered pups exhibited spontaneous action potentials in contrast to subthreshold synaptic potentials in neurons from sham-injected animals. Finally, to elucidate whether the gap junction blocker mefloquine (MFQ) previously shown to dampen ACx SOs in slices affected GABAergic transmission, MFQ was acutely applied in P3-5 slices while spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded. Whereas MFQ increased the amplitude and frequency of sIPSCs in ACx neurons, the broad-spectrum gap junction blocker carbenoxolone decreased sIPSC amplitudes only. Together, we show that P2-5 gerbil ACx can endogenously generate SOs in vivo. Persistence of activity in ACx in P14-17 slices from pups administered with BIC at P2-5 implies that inhibitory GABAergic activity linked with gap-junction participates in the maturation of ACx.
Keywords: Spontaneous activity, auditory, inhibition, GABA, connexin
The rate of spontaneous activity during development is low; however, it is essential for cell survival, as well as the establishment of circuits and sensory maps (Constantine-Paton et al., 1990; Shatz, 1990; Wong, 1993, 1999; Pallas, 2001; Stellwagen and Shatz, 2002; Kanold et al., 2003; Butts et al., 2007; Kotak et al., 2007; Gonzalez-Islas and Wenner, 2010; Sanes and Kotak., 2011). For example, recordings from isolated retina of neonatal ferrets and embryonic cats show spontaneous oscillations (SOs) in the form of synchronous bursts of action potentials occurring every 1-2 minute (Shatz and Stryker, 1988). Numerous other studies have also described patterned spontaneous activity in sensory and motor circuits and explored their developmental significance. For instance, when sodium-dependent action potentials are blocked in utero, retinogeniculate axons fail to segregate properly to the visual cortex lamina or sprout out of the lamina normally and instead make aberrant arbors (Shatz and Stryker, 1988). In the hippocampus, blockade of SOs increases spontaneous excitatory postsynaptic currents and expression of key elements of the presynaptic release machinery, suggesting SOs influence the establishment of excitatory synapses in vivo (Lauri et al., 2003). However, there is a dearth of in vivo studies on the characteristics of SOs in the developing auditory cortex (ACx), transmitter and modulator mechanisms, and whether it is feasible to perturb them chronically. Such evaluations are important in understanding the mechanisms of SOs before exploring their developmental significance. As a first step to that end, we illustrate that it is feasible to record repetitive SOs in the gerbil ACx after birth and pharmacologically manipulate activity in vivo to reveal involvement of GABAergic transmission.
Although sensory epithelia show bursting patterns of ATP-driven correlated activity (Tritsch and Bergles, 2010; Tritsch et al., 2010), cortical SOs in neonates may be driven by intrinsic central pattern generators because: 1) thalamocortical inputs are not fully mature at these ages (Pallas, 2001); 2) SOs are recorded for many hours in acute slice preparations sans sub-thalamic relay structures, afferent activation, or pharmacological manipulations, and in cultured neurons and glia (Garaschuk et al., 2000; Kotak et al., 2007). Correlated calcium waves also occur in neurons and astrocytes in the superficial cortex in vivo (Hirase et al., 2004). In one study, it is proposed that developing brain waves are initiated one day prior to birth by pacemaker neurons in the septal nucleus and ventral cortex before they spread to dorsal cortical regions (Conhaim et al., 2011). In newborn brain slice preparations of the neocortex and hippocampus, spontaneous activity and calcium waves self-propagate across cell ensembles involving all layers, as assessed by paired recordings and two-photon fluorescent imaging. Thus, such SOs have been characterized as cortical early network oscillations (Garaschuk et al. 2000), sharp waves (Karlsson et al., 2006); oscillations and waves (Kotak et al., 2007), spontaneous synchronized activity (McCabe et al., 2007), spindle burst oscillations (Minlebaev et al., 2007) or spontaneous network activity (Gonzalez-Islas and Wenner, 2010). Comparable activity has been also recorded in the neuronal and glial ensembles of postnatal mouse and rat neocortex, and cerebellum in vivo under different physiological states (Hirase et al., 2004; Adelsberger et al., 2005; Watt et al., 2009). However, the attributes and manipulations of SOs in the developing ACx in vivo have not been identified, and these are major issues addressed in this study.
The developmental significance of depolarizing GABAergic activity after birth is well established in various brain regions including the hippocampus and neocortex. GABAergic synapses may synergize with functionally sub-optimal excitatory synapses after birth and support depolarization-induced calcium influx that in turn mediates a myriad of intracellular developmental processes (Ben-Ari et al., 2007; Root et al., 2008; Gonzalez-Islas and Wenner, 2010). In contrast, in P3-5 gerbil thalamocortical slices, GABA application triggers hyperpolarizing IPSPs that dampen SO spiking in ACx neurons whereas GABAA receptor blocker bicuculline (BIC) disrupts SOs leading to higher discharge (Kotak et al., 2007). Thus, the gerbil developmental time frames of synaptogenesis and refinement of GABAergic synapses may differ unlike the rat and mouse neocortex where a switch of depolarizing to hyperpolarizing GABAergic activity is believed to terminate SOs (Garaschuk et al., 2000; McCabe et al., 2007; Baltz et al., 2010; Conhaim et al. 2011). Another issue closely linked with GABAergic transmission is that inhibitory network after birth may be interconnected via dynamically evolving gap junction proteins that may facilitate sub- as well as suprathreshold synchrony among heterogeneous populations of neurons (review Connors and Long, 2004). In thalamocortical slices, the connexin-36 specific gap junction blocker MFQ disrupted SOs by decreasing neuronal discharge in the gerbil ACx when acutely applied in slices (Kotak et al., 2007). Moreover, gap junction populations not only transmit information across various neuronal and glial populations during development, but also interconnect inhibitory GABAergic interneurons (Yang and Ling, 2007; Tovar et al., 2009). Therefore, we were curious to know whether GABAergic activity can be manipulated in vivo and in separate brain slice experiments, whether gap junction manipulation can affect inhibitory synapse function when SOs are prominent.
Using a single tethered wire, we explored whether SOs can be detected in vivo in the gerbil auditory cortex (ACx) to reveal whether they displayed any periodicity. We also examined gross topographic, vasculature and fiber tracks between the cortex and MG to confirm these recording sites were performed within the ACx. Further, in an attempt evaluate whether GABAergic involvement can be chronically manipulated in vivo, we administered P2-5 pups with the GABAA receptor blocker and then recorded spontaneous activity in slices generated after hearing onset (P12). Finally, the role of gap junctions on inhibitory transmission and SOs was assessed by acute application of gap junction blockers and recording spontaneous inhibitory postsynaptic currents (sIPSCs) or SOs from the thalamorecipient ACx neurons in P3-5 brain slices.
EXPERIMENTAL PROCEDURES
Animals
Neonatal (P2-5) and juvenile (P9, and P14-17) gerbils (Meriones unguiculatus) born from breeding pairs (Charles River) were used. The age range includes the time during which cortical SOs are prominent, inner hair cells become spontaneously active, ear canals open, and synaptic connections and coding properties mature (Sanes et al., 2011). All protocols are approved by the Institutional Animal Care and Use Committees at New York University, and City College CUNY, New York.
Tethered wire recordings in un-anesthetized neonatal pups
To gain a first approximation whether in vivo spontaneous cortical activity can be recorded, we initially designed a relatively simple recording preparation in a few neonatal animals. To circumvent an incision over the cranium and protracted anesthetic exposure that can affect SOs, pups were anesthetized by hypothermia for ~10 minute during which the following was done. The pups were wrapped in surgical gauze, placed on ice, and the skin above the presumptive ACx was resected. The bones above the right left temporal cortex caudal to the bregma suture (temporal lobe area 1; ACx), and above the cerebellum were exposed. Two fine perforations were made through the cranium with a 301/2-gauge syringe needle, through which two Teflon-coated silver wires shaved off their tips and bent vertically down (tip diameter, 0.008″, AM-systems) were inserted into the cortex and cerebellum area, the latter as a ground. A small drop of cyanoacrylate glue was applied at the insertion sites to stabilize the electrodes. The cortex wire penetrated about 200-400 μm deep into the cortical laminae. Thus, the neonatal pups were subjected to hypothermia only during the preparation phase, and not during recordings. The pups were then immediately taken off the ice and transferred to a small (~5″ diameter) Petri dish. For restraint, lukewarm agar (1%; ~30°C) was poured over a pup while its mouth and nostrils remained exposed. The pup, with these 2 tethered wires, was taken to a recording chamber and the preparation was placed over a heating system (32 ± 1°C). The semi-liquid agar envelope restrained the animal and did not hinder breathing or heartbeats. The breathing and heartbeats were measured after 5 minutes, at 32 ± 1°C and compared with known rates in age-matched pups (respiratory rate 80-100/minute; heart beats 260-300/minute measured from cardiac artifacts during the recordings). The connectors at the other ends of the two wires were inserted into a head stage and a differential amplifier (Warner PC-501). Spontaneous multiunit activity (MUA) was continuously acquired for 2 minutes at a time, for up to a maximum of 15 minutes at 1 kHz filter setting on the amplifier and digitized at 1 kHz during acquisition.
After the recording sessions, the pup was euthanized by chloral hydrate (100 mg/kg body weight), the brains fixed in 4% paraformaldehyde (PF), and standard 500 μm thalamocortical slices generated after 2 days of fixation to identify lesion marks created by the recording wire.
Postmortem confirmation of ACx
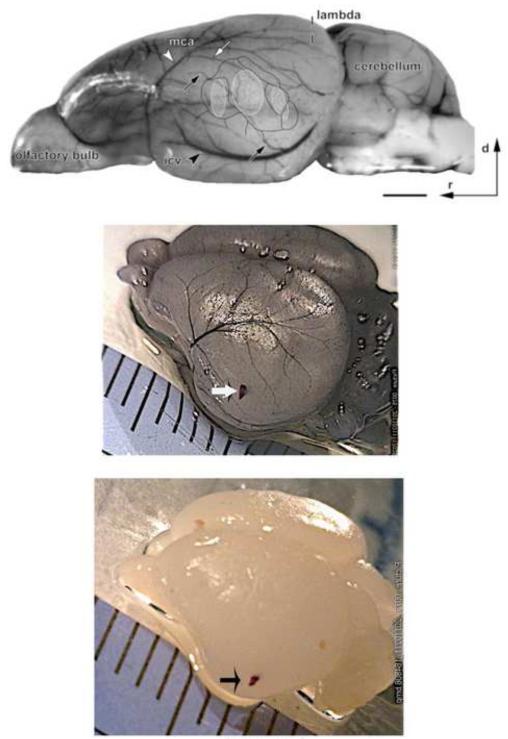
Subsequently, we wanted to validate whether the lesion marks corresponded with anatomically identifiable ACx in neonates. To this end, we first performed several additional anatomical and physiological assays. To test whether gross anatomical features could be identified in P3-5 brains, we evaluated the anatomical landmarks described by Büdinger et al. (2000, 2006). Using geometric derivatives to locate the center of a cortex, single lines were drawn between the opposite sides of the area limited by 2 major vasculature landmarks; the mid-cerebral artery (MCA) and the inferior cerebral vein (ICV) that surround the ACx (Fig 3A). We employed the logic that the intersection of those lines (center of the area within the 2 blood vessels) corresponds to the putative location of ACx in the adult gerbils (Büdinger et al., 2000, 2006; Fig. 3A, B).
Fig. 3.
Identification of auditory cortex (ACx) in postnatal pups. Top panel. View of an adult gerbil brain showing key regions and vasculature landmarks around the ACx (Büdinger et al., 2000, 2006). The 2 major vasculature landmarks, the mid-cerebral artery (MCA) and inferior cerebral vein (ICV) are labeled respectively by white and black-arrowheads while the arrows point at their major branches. The outlined ACx is shown to contain primary auditory cortex (A1) as a gray shaded oblong while the adjacent smaller anterior and posterior association auditory cortices are similarly shaded. Scale bar = 2 mm; d: dorsal, r: rostral. Middle panel. Top view of a P14 gerbil brain that was transcardially perfused with ink, showing similar vascular landmarks as above (2A). The white arrow indicates the NeuroVue Red filter. Bottom panel. A P4 gerbil brain without any ink perfusion, showing the NeuroVue loaded filter (arrow). The rulers in the middle and bottom panels display millimeter scales.
To accentuate the vasculature in developing gerbils, 2 P9 and 1 P14 gerbils were transcardially perfused with PB solution mixed with regular ink (~5%). The ink perfusion left a ubiquitous black color filled vasculature over the gray cortical tissue. The brains were then photographed in order to compare known topographic characteristics (Fig. 3A,B; Büdinger et al., 2000, 2006).
We recorded MG-evoked responses from slices generated from a normal P3 and P4 pups, and measured the dorsoventral and rostrocaudal axes coordinates in slices from which electrical activation of MG generated reliable field responses (Kotak et al., 2007). Thus, this strategy allowed us to directly compare the location of thalamorecipient ACx with the lesion marks observed in slices from fixed P3-5 brains following in vivo recordings (Fig. 2A-D). Separately, in a P4 and a P3 pup, immediately following in vivo recordings (≤ 3 min), we obtained fresh brain slices, stimulated the MG and recorded in areas adjacent to the lesion created by the in vivo recording wire (Fig. 2G,H).
We performed additional anatomical assays in 9 pups (P2-5) to determine whether loading lipophilic tracer dye at previously defined coordinate landmark (ACx) could label the fiber tracks between the ACx, the site of dye loading, and the MG. For this, pups were anesthetized with ketamine (20mg/kg body weight), and transcardially perfused with the fixative; 4% paraformaldehyde in 0.1M PB with 30% sucrose, and then post-fixed for 3 days. Filter paper squares coated with NeuroVue Red (0.1mm wide, 0.5mm long, MTTI) were placed in the putative location of A1, ~ 0.5 mm deep in the cortex. Dye was loaded by visual identification of the loops formed by the inferior cerebral vein and the middle cerebral artery. The precise placement of the tracer (filter paper loaded with the dye) was at the center of the area limited by that loop, located respectively 1.5 mm away from the ventral surface (Figs. 3B,C).
Fig. 2.
Validation of in vivo recording site. Following in vivo recordings, 500 μm thalamocortical slices were generated from fixed or fresh brains to validate recording sites (white arrows). Postnatal ages are indicated on top right of each panel. (A) The lesion site ~ 3.4 mm from the rostral end, corresponding to the region in fresh slices that respond to the stimulation of the auditory thalamus, MG, and thus ACx. This slice was derived from the animal recordings shown in Fig. 1. For anatomical landmarks, see B. (B) The lesion site indicates the lesion is ~ 1.4 mm from the rostral end, and therefore possibly somatosensory. MG: medial geniculate body, ACx: auditory cortex, H: hippocampus, Peri: perirhinal cortex. (C) The lesion site is 3.3 mm from the rostral end indicates a more temporal and deeper location and correspond to coordinates of thalamorecipient ACx as follows. (D). The lesion site 5.5 mm indicates a caudal location for the recording site, corresponding to the perirhinal cortex. This lesion had opened up appearing as a wedge during vibratome sectioning, and thus appears deeply cut. (E, F) Photomicrographs of thalamocortical slices from which coordinates of thalamorecipient ACx were measured. When the auditory thalamus (MG) was stimulated extracellularly (bipolar stimulating electrode immediately rostral to the MG at the emerging afferent fibers, blue arrows in the recordings indicate stimulus artifact, 5 mA/500 μS), brief field potentials are recorded, validating that the site of recordings was thalamorecipient ACx. Such responses are shown at the bottom panels of E and F where MG-evoked field responses are shown (blue traces). Note the several milliseconds time delay between the stimulus artifact and the onset of inward response. Recording sites were chosen 1 mm apart to illustrate the approximate caudorostral dimension of ACx. The gray traces represent failed synaptic transmission when subthreshold stimulation at lower intensities (100- 200 μA/100-200 μS duration, blue arrows indicate stimulus artifacts) did not elicit any detectable response. G and H are images of brain slices from 2 pups in which in which < 3 min in vivo recordings were first performed (Fig. 1B and C). Pups were then anesthetized and brain slices made immediately and taken to the recording chamber. When the auditory thalamus (MG) was stimulated field responses could be recorded in areas (asterisks) adjacent to the lesion (< 500 μm) created during the in vivo recordings (white arrows), confirming the in vivo recordings were from thalamorecipient auditory cortex (ACx). The bottom 2 traces show thalamically-evoked responses. Blue arrows indicate stimulus artifacts.
The brains were subsequently immersed in the fixative with sucrose and stored at room temperature for one month. The brains were then embedded in 10% gelatin, 50 μm coronal slices generated within 2 mm of the anatomical coordinates and then examined under a fluorescence microscope (wave length, 565 nm). The images obtained were processed using Nikon AIS software for acquisition and Photoshop CS5 for acquiring high-resolution images. Adjacent brain slices from the same brains were additionally stained by DAPI (4′, 6-diamidino-2-phenylindole), a fluorescent stain that binds specifically to adenine-thiamine rich portions of the DNA and images captured at wavelength of 358 nm (ultraviolet) and its emission maximum is at 461 nm (blue). DAPI staining allowed us to re-define the corticothalamic anatomy in relation to the key brain regions adjacent to the ACx and MG.
We obtained coordinates of medial geniculate body (MG) from the NeuroVue track tracing data as follows. 10 images obtained from 50 μm coronal sections from a P3, P4 and P5 brain each believed to pass through the cortex and MG as judged by the vasculature and gross topography were analyzed. Two measurements were made: the distance from coronal end to the center of MG and distance from temporal end to the center of MG.
Recordings in fresh thalamocortical brain slices
For brain slice physiology (Kotak et al., 2007, 2008), animals (P2-5 and P14-17) were anesthetized by injecting chloral hydrate. The animal’s response to nociceptive (toe pinch) stimulus was tested to verify anesthesia level. The animal was then transcardially perfused with chilled (0-4°C) artificial cerebrospinal fluid (ACSF, ~ 15 ml) and the brain rapidly dissected out in chilled oxygenated ACSF containing in mM: 123 NaCl, 4 KCl, 1.2 KH2PO4, 1.3 MgSO4, 24 NaHCO3, 15 glucose, 2.4 CaCl2, 0.2 ascorbic acid; pH=7.35 after bubbling with 95% O2/5% CO2.) In P14-17 animals, the brain was vibratome sectioned at 500 μm at a 15° angle to preserve thalamocortical connections. For younger pups (P2-5), the slicing angle was reduced to ~7° (Kotak et al., 2007). All slices were treated for 30 min at 33°C, and then left for an hour at room temperature before taking one to a recording chamber with continuously oxygenated ACSF superfused at ~ 3 ml/min at 32 ± 1°C. In two each P3 and P4 slices, we stimulated MG and recorded field responses at 3 different locations in the cortex ~300 μm apart to assess the extent of the spatial spread of thalamorecipient ACx along the caudorostral axis in young pups.
For testing the characteristics of SOs in slices from normal, acutely treated or manipulated animals, single cell-attached recordings were obtained (1.5 mm OD glass electrodes, 4-10 MΩ, Sutter) from the supra-granular neurons (L2-4) of the thalamorecipient ACx. To additionally explore subthreshold membrane fluctuations and suprathreshold discharge, whole-cell recordings were obtained in current-clamp condition. The electrodes (5-10 MΩ) were made using 1.5 mm OD glass capillaries and backfilled with the internal patch solution containing: (mM) 130 K+-gluconate, 5 KCl, 2 MgCl2, 2 ATP, 0.3 GTP, 0.6 EGTA, and 10 HEPES (pH 7.2 with KOH). Supragranular pyramidal neurons with resting membrane potential of less than -40 mV (for P3-5 neurons) or less than -60 mV (for P14-17 neurons) and responding to brief stimulation (500 μS, 5 mA) of the MG with a field potential or an EPSP/EPSC were included. In whole-cell recording conditions, RSERIES was monitored by injection of a -10pA/1500 ms step hyperpolarizing current in the soma. Experiments were discontinued if RSERIES exceeded 40 MΩ.
We performed 3 additional in vivo tethered wire recordings (described before) that were followed up by validation of thalamorecipient ACx in brain slices generated immediately after in vivo recordings. Briefly, we restricted our recording time to 3 minutes or less, then immediately anesthetized the pup, generated 500 μm thalamocortical slices, transferred slices in the recording chamber, stimulated the MG, and recorded from the cortical region adjacent to the lesion area (within < 0.5 mm) created during in vivo recording. In slices from 2 of the 3 pups we could record MG-evoked responses confirming these regions to be thalamorecipient ACx (in vivo data from 1 pup is not included). This approach is somewhat similar to the one we used following calcium imaging (Kotak et al. 2007). In that study, following calcium imaging, we had validated thalamorecipient ACx in the same slice by stimulating the MG and recording field responses in the cortical areas corresponding to the regions of interest from which calcium imaging was done.
Manipulation to perturb spontaneous activity in vivo: effect of GABAA receptor antagonist
One key objective in this study was an attempt to disrupt spontaneous cortical activity during P2-5 by administering pups everyday for 4 days with BIC (20 mg/kg), the GABAA receptor blocker, and then assessing the effects in thalamocortical slices generated after hearing onset (P12) when no spontaneous discharge is present in the supragranular ACx neurons.
One caveat of this approach is the systemic drug exposure can affect the developing central and peripheral nervous system. We opted for the sub-cutaneous (s.c.) administration route because of the following advantages (Kotak et al., 2011): i) Dug delivery is non-invasive, ii) BIC (or sham, vehicle alone) was delivered quickly (< 10 sec/pup), iii) Drug administration avoided an additional injection of an anesthetic that could affect SOs on a daily basis, iv) The drug with its vehicle remained un-intrusively beneath the dorsal skin for some time as identified visually as a small ‘bubble’ of liquid, that did not hinder locomotory or feeding behavior, viii) The drug crossed the blood brain barrier on a sustained basis. This latter factor is advantageous in that SOs may be affected daily at a sustained levels rather than rapidly at an acute level. The latter strategy (acute ‘in vivo’ effects) would require a different set of experiments that we did not perform in this study.
Pharmacological manipulations to perturb GABAergic activity in slices: acute application of GABA
Previous work demonstrated that focal delivery of GABA (10 ms puff, 10 mM) in close proximity of the recorded neuron elicited IPSPs and thus disrupted OWs. However, to explore whether much stronger and longer duration of GABA exposure affected OWs without causing the collapse of chloride gradient, GABA was directly bath-applied at the inflow of ACSF (10-30 μl, 10 mM; final bath concentration, ~1 mM). It took about 1 sec to carefully deliver the drug. It took several seconds to minutes before we observed the reversal of the inhibitory effects, depending upon the amount delivered.
Pharmacological manipulations to perturb gap junctions in slices: effect on spontaneous inhibitory currents
MFQ (25 μM) was bath applied and sIPSCs were recorded as inward currents in voltage clamp condition at -60 mV holding potential after blocking ionotropic glutamate receptors with DNQX (20 μM) and AP-5 (50 μM) in P2-5 neurons. sIPSC amplitudes and frequency are good measures respectively of postsynaptic GABAA receptor function and presynaptic release properties. One caveat of this approach is that it is not possible to know which subpopulation of presynaptic inputs is active. In this study however, our aim was to determine whether the 2 major classes of gap junction blockers affected the amplitude and frequency of sIPSCs and thus GABAergic transmission. Five 30-second traces were acquired for each recorded neuron. To determine whether another gap junction blocker similarly affected sIPSCs, a broad-spectrum connexin protein blocker carbenoxolone (CBN), a steroid, was also employed (200 μm). These data were filtered at 5 kHz on the amplifier and digitized at 1 kHz bandwidth using the custom software SLICE. The program detected each inwardly deflecting IPSC larger than -8 pA.
Data acquisition and analysis
Data were acquired using a Macintosh G5 PPC running on an Igor-based custom software (WaveMetrics, v4.04) called SLICE, and analyzed off-line using a package, SLICE ANALYSIS Traces were sampled at 10 kHz during acquisition of I/V curves and MG-evoked responses and 1 kHz during acquisition of SOs. The thalamorecipient ACx was validated from the surrounding non-auditory cortical areas, by a brief single electrical stimulation of the MG (500 μS/5 mA pulse) that triggered a robust field potential/current. Statistical tests (ANOVA, students’ t-test or Wilcoxon) were performed using the SAS-based JMP 5.0.1 package, depending upon whether the data were normally distributed or not, respectively.
To characterize SOs recorded initially by the tethered wire offline by using the SLICE analysis (intra-burst discharge and inter-burst intervals), a spike threshold was set, which was the absolute voltage that a spike must reach to be unequivocally discriminated from the much smaller, regular amplitude and rhythmic cardiac artifacts (≥ 250/minute). A minimum burst period of 500 ms, and minimum inter-burst interval of 2 seconds were considered as separate SOs. Sporadic tonic spikes amongst SOs were counted manually and not included in SOs bursts (Fig. 1A-C). Sub-threshold synaptic events in sham-treated P14-17 controls were measured manually offline by making 100 μV and 5 ms grids over the 30-second acquisition traces. EPSPs and IPSPs larger than 400 μV amplitude were counted and measured by overlying 100 μV grids along the Y-axis. Generally, in the recorded age-range, the amplitudes of these spontaneous synaptic events varied between 0.4 and 7 mV. Spontaneous synaptic potentials smaller than 400 μV may exist but were not considered owing to a poor signal to noise ratio at < 400 μV.
Fig. 1.
SOs exist in the P3-5 developing gerbil cortex. (A-C) Six consecutive sweeps (five in C) show recurring neuronal bursts recorded by a tethered wire electrode. Sporadic tonic spikes are also observed interspersed among the bursts. (D) Scatter plots of SO characteristics recorded in the ACx slice preparation (N=6) vs. those recorded in P2-5 pups using tethered wire preparations (N=7). Horizontal bars are means. Note that the in vivo intra-burst firing frequency is higher (upper panel) and the inter-burst interval is significantly longer (lower panel). In this and all subsequent figures, asterisks indicate that the differences are statistically significant. Details on the levels of significance for each figure panel marked by asterisks appear in the results section.
RESULTS
The data in this paper were collected from a total of 71 gerbils that include: tethered wire recordings in vivo, N = 7 (P2-5); age-matched recordings of SO characteristics in brain slices, N = 6; coordinates of ACx characterized by thalamic responses in slices, N = 6 (P2-5); gross anatomy and vasculature landmarks in intact fixed brains, NeuroVue Red dye labeling in fixed brains, N = 9 (P2-5, P9, P14); acute MFQ effects on inhibitory currents, N = 8 (P2-5); acute CBN effects on SOs in slices, N = 7 (P2-5); acute CBN effects on inhibitory currents, N = 8 (P2-5); acute GABA effects on SOs in slices N = 5 (P2-5); acute BIC effects on SOs in slices, N = 3 (P2-5); administration of BIC in vivo, N = 3 (in vivo injections at P2-5, recordings in slices, P14-17); spontaneous PSPs in slices after sham administration in vivo, N = 9 (sham injection in vivo at P2-5, recordings in slices at P14-17).
Synchronized SOs exist in the neonate gerbil ACx
A major objective in this study was to determine the in vivo existence and characteristics of SOs within the ACx layers during the first postnatal week and assess how they compare with those recorded in vitro. Initially, a tethered silver wire preparation was developed for in vivo recordings in unanesthetized restrained P2-5 pups (N = 7 pups; methods). Fig. 1A shows several continuously acquired traces of SOs (black) occurring repetitively as bursts of spikes recorded in a P3 animal. The repetitive multiunit-activity (MUA) was interspersed with single units. Such low levels of interspersed single action potentials are also observed in slices of the thalamorecipient ACx (Kotak et al., 2007). For comparison, recordings were additionally obtained in thalamorecipient ACx slices from seven age-matched gerbils by single cell-attached or whole cell recording. Comparison between all in vivo vs. slice data showed that both the mean MUA frequency during SOs and the inter-burst interval were significantly higher in vivo (Fig. 1D; mean intra-burst firing, Hz ± SEM, in vivo, 17.9 ± 2.3 vs. slice, 11 ± 1.7, t = 2.4; P = 0.04; mean inter-burst interval, sec ± SEM, in vivo, 49 ± 6.8 vs. slice, 26 ± 6, t = 2.3, P = 0.03; mean burst duration, sec ± SEM, in vivo, 2.4 ± 0.4 vs. slice, 1.1 ± 0.3, X2 = 5.1, P = 0.02; for all comparisons, in vivo: N=7, slice: N=6).
Postmortem histological analysis showed that recordings were located in temporal cortex Te3 and at the caudal end within the perirhinal cortex (Fig. 2A-D), consistent with previous observations that SOs and calcium waves sweep across these regions in slices of age-matched gerbils (Kotak et al., 2007) and rats (Garaschuk et al., 2000). Specifically, two of the lesion- marked neocortical areas corresponded with the coordinates of the thalamorecipient ACx obtained in fresh slices (Compare Fig. 2A & C, with 2E & F). However, the lesion marks seen in Fig. 2B, as a result of test lesion, were more rostral to ACx likely to be putative somatosensory area, while the lesion in Fig. 2D was caudal, within the perirhinal cortex, though SOs also exist at that location (Kotak et al., 2007). Fig. 2E and F are images of fresh thalamocortical slices generated respectively from normal P3 and P4 normal gerbils in which consistent field responses could be evoked by stimulation of the auditory thalamus, the MG (bottom 2 traces, the top gray traces are failures). We performed 3 additional in vivo tethered wire recordings in P3-5 pups and validated the recording site as being thalamorecipient ACx. In brain slices generated immediately after in vivo recordings, consistent field responses were recorded from the cortical region adjacent to the lesion area (within ~ 0.5 mm) created by the tethered wire insertion and withdrawal in 2 of these pups (Figure 2 G, H). We further characterized the site of in vivo recordings in greater detail by anatomical criteria in fresh and fixed slices. For this, we carried out additional experiments described below.
ACx can be further characterized in neonate gerbils
ACx was first characterized by gross topographical and vasculature landmarks in whole fresh postmortem brains that were then compared with published topography of adult gerbil cortex (Fig. 3A; Büdinger et al., 2006). Second, anatomical coordinates of the neocortical regions from which MG stimulation consistently evoked field responses were used as the benchmarks for loading the dyes at the putative ACx sites (Fig. 3B,C). In a 500μm thalamocortical brain slice, thalamically (MG)-evoked field and synaptic responses were consistently recorded approximately 1 to 1.5 mm from the ventral surface, 4 to 4.5 mm from the dorsal surface, 4 to 4.5 mm from the rostral end about 1.5 mm from the caudal end of the cortex (Figs. 3,4; also Kotak et al., 2007). The precise tracer placement was the center of the area limited by that loop, located respectively 1.5 and 4 mm away from the ventral surface and rostral end (± 0.5 mm, Figs. 3A,B, 4), and the coordinates obtained from fresh brain slices of thalamorecipient ACx.
Fig. 4.
Anatomical connections between cortex and MG. The lipophilic dye NeuroVue Red was loaded at the putative ACx area in intact brains and they were left for a month in a sucrose paraformaldehyde fixative. Fig 4A-D are fluorescent micrographs of 50 μm coronal slices passing through the thalomorecipient auditory cortex from several brains that show track-traced dye that had traveled to the MG several millimeters medial (MG, arrow heads). Arrows in each panel indicate the lesion mark that was created in the intact brains during insertion of the filter paper with the dye. Asterisks indicate en passant thalamocortical fibers. The first 3 images are from different P3 pups, while panel 4D micrograph is from a P4 pup. E is a de-saturated, enhanced fluorescent micrograph of a DAPI stained coronal section to identify some major landmarks around the ACx and MG. DG, dentate gyrus (labeled on contra side), 1, 2, and 3 are respectively CA1, CA2 and CA3 of the hippocampus, ACx, auditory cortex. Scale bar = 1.2 mm. The MG is seen as a bulbous structure between the ipsilateral dentate gyrus and CA3 (MG, arrowhead); its location corresponds with the NeuroVue-filled MG (A-D). F, G, and H are fluorescent micrographs from another P3 brain section respectively showing DAPI stained, NeuroVue stain and a merged image of the two. Arrow in H indicates the site of dye loading. Scale bar = 1 mm in F also for G and H.
When NeuroVue Red was loaded in the proximity (± 500 μm) of ACx based on these coordinates, it stained anatomically identifiable fiber tracks between the ACx and auditory thalamus, as well as the MG in several P3-P5 brains in which we had allowed one month of transport (Fig. 4A-D). In several DAPI-stained sections, we could further identify MG and the key regions of the hippocampus including the dentate gyrus in corresponding areas of the NeuroVue labeled sections (Fig. 4E). Furthermore, the connectivity between the ACx and MG was also visible in merged images of NeuroVue and DAPI (Fig. 4F,G). The coordinates derived from coronal end to the center of MG (CM) and temporal end to the center of MG (TM) from these 50μm coronal slices (mean mm ± SEM) of MG were: for P5 CM: 3.58 ± 0.04, TM: 3.42 ± 0.04, n=10 sections; for P4 CM: 3.48 ± 0.03, TM: 3.15 ± 0.02, n=10 sections; for P3 CM: 3.4 ± 0.03, TM: 2.73 ± 0.05, n=10 sections.
Acute effect of GABA on SOs in slices
We previously showed that SOs are disrupted by brief (10 ms) focal delivery of GABA (Kotak et al. 2007). Here, we wanted to determine whether much longer duration GABA exposure affects SOs without causing a collapse of the intracellular chloride gradient that may lead to depolarization and excitation common in developing neurons. A higher and longer duration GABA dose was directly bath applied (10-30 μl, 10 mM; final bath concentration, 1 mM or less). Under this condition too, GABA completely blocked action potentials and caused long-lasting hyperpolarization (up to 9 mV hyperpolarization, lasting from 1-5 minutes, N = 3 Fig. 5C,D). This reiterates the fact that protracted GABAergic activity may not lead to excitation.
Fig. 5.
GABA application blocks SOs. (A) Acute bath application of GABA (30 μl, ~1 mM effective concentration, arrowhead) dampens of SOs (top trace) leading to single action potentials followed by prolonged hyperpolarization in a P3 ACx neuron in brain slice. B. In the middle panel, a similar damping effect of GABA is shown for a P2 neuron. Action potentials within an SO are slowed and this is followed by prolonged hyperpolarization. C. In another slice (P3) a much lesser volume of GABA (10μm, effective concentration ~400 μM) leads to disruption of an SO (see second burst, arrow); action potentials are stunted, followed by hyperpolarization and a rebound SO. In a P5 neuron, 20 μl application of GABA (effective concentration ~ 700 μM) leads to similar block of spikes in an SO (arrow) followed by hyperpolarization. After recovery, SOs are irregular with some IPSPs. D. Application of 30 μl GABA (effective concentration ~ 1 mM) led to SO blockade (arrowhead) and prolonged hyperpolarization, in this case, ~ 10 mV. The cell resumed SOs about 4 minutes thereafter.
In vivo manipulation of GABAergic activity at P2-5 can be evaluated in slices after hearing onset
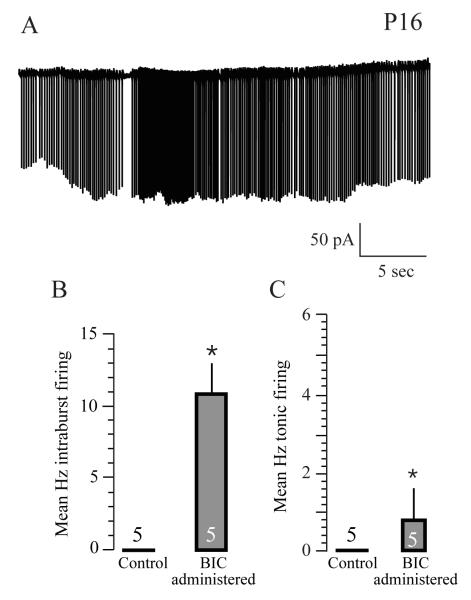
Our other key objective was to attempt manipulating activity in vivo daily at a chronic level when SOs are robust by injecting the GABAA receptor blocker BIC. This is because in P2-5 ACx neurons in slices, focal micro-delivery of GABA perturbs SOs by causing the membrane potential to hyperpolarize while acute BIC exposure produced a greater discharge rate (Kotak et al., 2007, and Fig. 5). Thus, BIC administration could block the predicted hyperpolarizing GABAergic activity in the ACx in vivo. Our prediction was also that by perturbing SOs, we might observe persistent or aberrant changes in spontaneous activity that may differ than that recorded in age-matched control or slices from sham-injected animals. After in vivo administration of BIC for 4 days (P2-5), we assayed spontaneous activity in slices following hearing onset (P12) because SOs or single spontaneous action potentials are not detected in P14-17 pyramidal neurons (Kotak et al., 2007).
Recording from ACx neurons in slices from BIC-treated pups showed high level of spontaneous discharge rate (Fig. 6A). All 5 pups administered with BIC showed spontaneous firing with some SO-like features (Intraburst firing in slices from BIC administered pups; Mean Hz ± SEM; 10.8 ± 2.2, Fig. 6B; tonic firing in slices from BIC treated pups; Mean Hz ± SEM, 0.8 ± 0.4; N = 5; Fig. 6C). In contrast, sham-injected pups did not show any spontaneous action potentials. The functional properties of these neurons, such as their firing characteristics in response to step depolarizing current injection, input resistance, and resting membrane potential, were comparable to those in age-matched L2/3 ACx (not shown). The characteristics of the sub-threshold synaptic activity in sham-injected pups were as follows: Mean EPSP size; 0.3-6 mV; mean EPSP frequency, 6.1 ± 0.35 Hz, n = 9. Mean IPSP size; 0.3-3 mV; mean IPSP frequency, 1.53 ± 0.1 Hz, N = 9 (calculated from five continuously acquired 30 second sweeps, Figure not shown, but for similarity of sham-injected PSP data with un-injected controls, see Kotak et al., 2007).
Fig. 6.
GABAergic transmission may influence the maturation of spontaneous activity in vivo. (A) A representative cell-attached recordings from a P16 ACx neuron previously (P2-5) administered with BIC displays some SO-like and tonic discharge. (B) Bar graphs representing mean intra-burst discharge rate (left panel) and tonic firing (right panel) from all BIC treated and age-matched control neurons. Asterisks indicate that the means are significantly different. The numbers above or within bars graphs represent N values.
GAP junctions may influence SOs via altering GABAergic currents
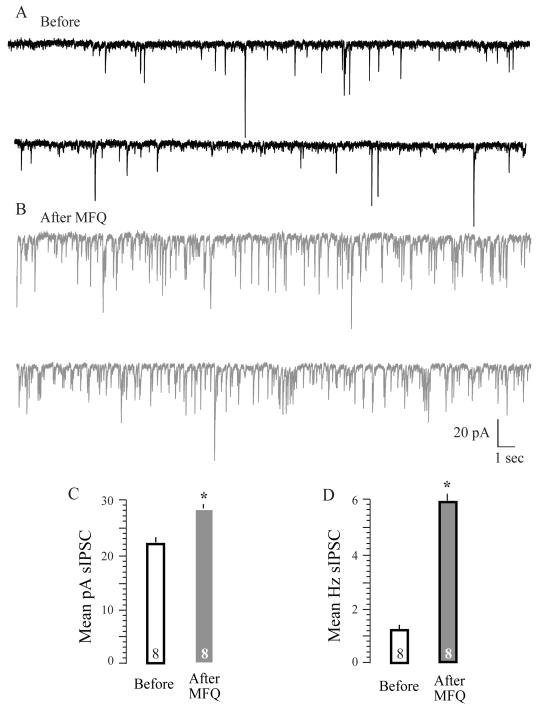
In developing brains, a variety of gap junctions are up and down-regulated among neurons, glia and interneurons. For instance, mefloquine (MFQ)-sensitive Cx36 gap junctions are expressed in neurons and upregulated during the first two postnatal weeks. We have previously shown that an acute application of MFQ dampens SOs in brain slices (Kotak et al., 2007). Because cortical inhibitory interneurons are also interconnected via gap junctions, we sought to determine whether MFQ affected inhibitory synapse function, which in turn could influence SOs. Thus, we examined the acute effects of MFQ on sIPSCs at doses that dampened SOs. It is known that an assay of spontaneous synaptic currents is a reliable index of both pre- as well as postsynaptic change in developing ACx neurons (Kotak et al., 2008). Bath superfusion of MFQ (25 μM) significantly increased sIPSC amplitude and frequency: sIPSC amplitude, mean pA ± SEM, before MFQ, 24 ± 1.73 vs. after MFQ, 28.16 ± 1.23, X2 = 7.4, P = 0.006, N = 8; sIPSC frequency, mean Hz ± SEM, before MFQ, 1.24 ± vs. after MFQ, 5.89 ± 0. X2 = 7.4 P = 0.001, N = 8; Fig. 7).
Fig. 7.
Gap junctions affect spontaneous inhibitory currents in vitro: Facilitation of inhibitory function by MFQ. (A) The top panel shows two representative sweeps of sIPSCs recorded at VH=−60 mV in the presence of DNQX and AP-5 for 30 seconds each in a P4 neuron. (B) Note that 15′ after bath application of MFQ (25 μM), the sIPSC amplitude and frequency have increased. (C) Bar graphs summarizing the mean amplitude and frequency of sIPSCs all recorded neurons. Note that the amplitude of mean sIPSCs (left panel) and frequency (filled bar graphs) are greater after the application of MFQ. The numbers within bars graphs represent N values.
With an aim to test the efficacy of a chemically unrelated gap junction blocker, carbenoxolone (CBN) was chosen because unlike MFQ, which is a quinine-based antimalarial drug, CBN is a water-soluble steroid-like compound and a broad-spectrum gap junction antagonist. The efficacy of CBN (200 μM) was first tested acutely on sIPSCs. Unlike MFQ, CBN significantly reduced inhibitory current amplitudes without affecting their frequency. (sIPSC amplitude, mean pA ± SEM, before CBN, 31.12 ± 1.86 vs. after CBN, 15.2 ± 2.31, X2 = 11.9, P = 0.001, N = 8; sIPSC frequency, mean Hz ± SEM, before CBN, 0.98 ± 0.22 vs. after CBN, 1.11 ± 0.21, X2 = 0.1, P = 0.9, N = 8).
If MFQ dampened SOs and facilitated inhibitory synapse function, then one would expect the damping effect of CBN on inhibitory synaptic activity might result in greater SO discharge and our results were consistent with this idea. Single cell-attached and whole-cell recordings from ACx neurons showed that bath application of CBN eliminated the SO bursts while the tonic firing pattern increased (before CBN: interburst interval, mean seconds ± SEM, 26 ± 1.7; intraburst frequency, mean Hz ± SEM, 11.1 ± 1.7; after CBN: no bursts were observed N = 7. Tonic firing, mean Hz ± SEM, before CBN, 0.79 ± 0.2 vs. after CBN, 5.3 ± 1, X2 = 9.8, P = 0.003, N = 7; Fig. 8).
Fig. 8.
Gap junctions affect spontaneous inhibitory currents in vitro: Disinhibition by CBN may disrupt SOs. (A) A representative cell-attached recording from a control P5 ACx neuron shows 2 inward deflecting SO bursts and a few spikes between inter-burst interval (top trace). Bath application of CBN (200 μM) disrupts SO leading to greater discharge. (B) Bar graph representation of total discharge rate before and 15 minutes after the application of 200 μM CBN from all recorded L2/3 ACx neurons (mean Hz ± SEM).
DISCUSSION
The key findings of this study are that endogenously generated SOs exist in vivo in the ACx of P3-5 gerbils (Fig. 1). Although this is consistent with their occurrence in age-matched ACx in slices shown by paired recordings and calcium imaging (Kotak et al., 2007); to our knowledge, this is the first report of SOs specifically within mammalian ACx in vivo, as confirmed by various anatomical criteria corresponding with thalamorececipient ACx by electrophysiology (Figs. 2-4). The persistence of tonic and SO-like activity in ACx from pups administered with GABAA receptor blocker implies that inhibitory GABAergic transmission may influence the termination of SOs (Figs. 5,6). Further, the ability of MFQ to potentiate inhibitory currents (Fig. 7) and dampen SOs (Kotak et al., 2007) supports the notion that inhibitory GABAergic activity may regulate SOs via membrane hyperpolarization. By contrast, the suppression of inhibitory currents by CBN may de-synchronize SOs leading to higher discharge (Fig. 8). Thus, SOs may use gap junction-mediated communication among different populations of developing neurons and SO characteristics may be modulated by action of ligands upon gap junctions. For example, activation of specific intracellular biochemical cascades can reduce gap junction communication in P4 hippocampus slices (Konietzko and Müller, 1994). Recently, by employing molecular manipulations in rodent brain, Park et al. (2011) have shown that activation of metabotropic glutamate receptors enhances and inactivation diminishes the developmental increase in neuronal gap junction coupling. In contrast, changes in GABAA receptor activity have the reverse effects. Therefore, a complex array of transmitter-gated mechanisms themselves may mediate signaling via electrical gap junction proteins associated with SOs (Conhaim et al., 2011).
SOs are heterogeneous among different regions of the developing nervous system
EEG studies, electrophysiological recordings, confocal imaging with voltage and calcium-sensitive markers, and modeling show that developing and adult SOs are associated with synaptic remodeling, circadian cycles, cognitive performances, consciousness, execution of motor programs, and pathologies. Their incidence ranges from very slow to ultra-fast (< 0.01 Hz to >1 kHz) and reflect properties of neuronal populations interconnected by electrical and chemical synapses (Shatz, 1990; Wong, 1999; Garaschuk et al., 2000; Hirase et al., 2004; Adelsberger et al., 2005; Buzsaki, 2006; Karlsson et al., 2006; Watt et al., 2009; Tritsch and Bergles, 2010; Gonzalez-Islas and Wenner, 2010; Conhaim et al. 2011; Sanes et al., 2011). In this study, repetitive occurrence of SO within the ACx in vivo during the first postnatal week (Fig. 1) is comparable with the network ensemble SOs and self-propagating calcium waves reported in the CNS of various mammals and traveling oscillations and waves across the developing thalamorecipient ACx in slices (Kotak et al., 2007). SOs and correlated neuronal bursts have been recorded in vivo in the urethane-anesthetized rat barrel cortex during the first postnatal week (Minlebeav et al., 2009), as well as in the retina and visual cortex of urethane anesthetized P12-20 pups (Hanganu et al., 2007), and un-anesthetized mouse neonatal cortex (Adelsberger et al., 2005). In the developing cerebellum, such correlated patterns of activity travel along interconnected Purkinje cells during the first postnatal week (Watt et al., 2009). In our study, greater spike discharge in individual SO bursts and slower inter-burst intervals in vivo compared to those in slices (Fig. 1D) may imply better regulation of SOs by modulators in vivo (Hanganu et al., 2007; Stacy et al., 2005). The agar restraint during the tethered-wire recordings could induce sleep, which is reminiscent of such activity during resting phases (Adelsberger et al., 2005). Because pups spend most of their time after birth sleeping rather than exploring, it is likely that SOs could provide instructional cues for the experience independent, self-determining synapse restructuring leading to greater specificity, eventually leading to the foundations of tonotopic maps in the ACx. Various studies for instance have demonstrated that deprivation or acceleration of neural activity can alter the biophysical and synaptic properties in the auditory system during early life (Kotak et al., 2008; Sanes et al. 2011; Sanes and Kotak, 2011; Oliver et al., 2011). Because SOs may be initiated prior to birth during late embryonic life (Conhaim et al. 2011) and maintained during early postnatal days and end before air-borne sound activity is initiated, synergism between the maturation of voltage and ligand-gated channels and gap junctions on one hand, and SOs on the other may provide cues for the normal development of ACx circuits. Such a model would involve a dynamically evolving and positive feedback between SOs and neuronal properties. Numerous in vivo and vitro investigations will be needed for a better appreciation of the significance of SOs in cortical development and sound-evoked performance in adults.
Inhibitory GABA may be involved in the regulation of SOs in vivo
Persistence of spontaneous activity in P14-17 ACx neurons in slices generated from P2-5 BIC treated pups implies a possible homeostatic rebound or delay in the maturation of SOs that may not have terminated by P7 (Kotak et al., 2007) owing to the disruption in hyperpolarizing GABAergic signaling. This is supported by the fact that prolonged hyperpolarization is triggered by increasing GABAergic activation (Fig. 5); this differs from the depolarizing GABAeregic actions in neonatal hippocampus, cortex and cultured neurons (Owens et al., 1996; Garaschuk et al., 2000; Ben-Ari et al., 2007; Gonzalez-Islaz and Wenner, 2010; Baltz et al., 2011). A recent report however demonstrates that activation of extrasynaptic GABA receptor subunits by very low levels of GABA exposure can induce tonic inhibition in newborn cortex that is sufficient to dampen discharge of pyramidal cells (Sebe et al., 2010). Our observations also imply that the chloride transporters and pumps in developing ACx are adult-like early on i.e. a higher activity of KCC2 and lower activity of NKCC1 may exist to bias lower intracellular chloride concentration contrary to that in the developing hippocampus (Ben Ari, 2002; Farrant and Kaila, 2007). Thus, we rule out the possibility that excitatory GABAergic ACx network may support SO discharge after birth at least in gerbils (Garaschuk et al., 2000; Ben-Ari et al. 2007; Baltz et al. 2010). Rather, the persistence of action potentials in ACx neurons after hearing onset in BIC-administered pups may result from a classic blockade of inhibitory synaptic activity during P2-5 (Fig. 6). In the mouse cortex just prior to and after birth, SOs are dependent on depolarizing GABAergic activity and predominantly restricted to the septum and ventral cortical pacemakers. As development proceeds and GABAergic transmission switches gradually to hyperpolarizing phenotype, wave initiation largely shifts to AMPAergic glutamate receptors that is associated with wave travel to the dorsal cortex (Conhaim et al., 2011). Other investigators have demonstrated persistence of activity if it is blocked during early neuronal maturation. For example, if embryonic slice cultures are treated with TTX, an established blocker of sodium channel-dependent action potentials, spontaneous activity does not terminate normally by P3; instead, it continues until P10 implying a compensatory extension of activity (McCabe et al., 2007). It is possible that an increasing strength or participation of inhibitory synaptic activity or and proliferation of such synapses may underlie SO termination much before hearing onset.
Gap junctions among inhibitory cells may be associated with SOs
Various gap junction connexin proteins such as Cx-26, 36, 43, 45 and 52 are functional among pyramidal neurons, glia and inhibitory interneurons in the nervous system during development, adulthood and disorders. For instance, a 3-D simulation and multiple whole-cell recordings have shown gap junctions among pyramidal cells in the frontal cortex of juvenile ferrets and rats that facilitate direct transfer of spike discharge or small signaling molecules leading to synchronous firing that may regulate cortical SOs (Kandler and Katz, 1998; Roerig and Feller, 2000; Connors and Long, 2004; Sutor and Hagerty, 2005; Vervaeke et al., 2010). In another study, connexin-36 gap junction coupling between Golgi cells in the cerebellum was shown to be located on the apical dendrites. Experimental and modeling studies show sparse synaptic excitation produces local and transient network de-synchronization via gap junction-mediated surround inhibition that can account for variance in their firing patterns in vivo (Vervaeke et al., 2010). As in the retina, an upregulated Cx-36 reaching its peak during the first postnatal week may mediate repetitive SOs in the ACx (Figs. 1). The ability of the Cx36 blocker MFQ to diminish SOs in P3-5 ACx neurons (Kotak et al., 2007) may be correlated with its ability to indirectly enhance GABA release on one hand and potentiate postsynaptic inhibitory strength on the other (increased frequency and amplitude of sIPSCs; Fig. 7). By contrast, the broad-spectrum connexin antagonist CBN can alter SO properties in vitro (Fig. 8) is consistent with its effect in selectively desynchronizing SOs in the somatosensory cortex slices in P0-3 pups (Sun and Luhmann, 2007). CBN is also known to block hemi-channel currents in the retina, uncouple brainstem neurons, dephosphorylate various other connexins (Ross et al., 2000; Xia et al., 2003; Minlebaev et al., 2007) and ACx SOs in slices during the first postnatal week (Kotak et al., 2007). In mouse hippocampal neurons cultured upon glial micro-plates, CBN can alter synaptic and intrinsic membrane properties (Tovar et al., 2009). Specifically, CBN significantly reduces GABAergic IPSC amplitudes in a dose-dependent manner and our results are consistent with these. Thus, the reduced magnitude of inhibitory currents following a CBN exposure may underlie enhanced SO firing (Fig. 8). The fact that SOs can be desynchronized via inhibitory interneuronal network whose strength is co-regulated by connexin proteins indicates that developing SOs properties may change by transmitters and modulators that may target these developing proteins (Figs. 6,7). For example, both glutamatergic and GABAergic transmission have opposite effects in the developmental regulation of Cx36 proteins; group II mGluRs-mediated enhancement of Cx36 expression occurs via cyclic adenosine mono phosphate/protein kinase A (cAMP/PKA)-dependent signaling, while its decreased expression occurs by GABAA receptors mediated calcium/protein kinase C (Ca2+/PKC)-dependent signaling (Park et al., 2011). Here, we did not design any in vivo experiment on the developmental significance of SOs.
Pre and postsynaptic elements involved in spontaneous synaptic activity may respond in opposing fashion under sensory deprivation (Kotak et al. 2005). Our observation that MFQ increase both the sIPSC frequency as well as amplitude may be linked with its greater specificity targeting connexin-36 protein gap junction proteins known to interconnect inhibitory interneuronal communication. By contrast, CBN is a broad-spectrum gap junction antagonist that may not affect the release probability (Gibson et al. 1999; Pangratz-Fuehrer and Hestrin 2011). A synapse-specific assay would entail paired recording among fast-spiking, non-fast spiking GABAergic interneurons and pyramidal neurons with pharmacological manipulations to further explore the association between gap-junction mediated communication, GABAergic transmission and SOs
CONCLUSION
To our knowledge, this is the first report of SOs in the ACx of neonatal gerbils in vivo, and this is consistent with their presence in the ACx in age-matched thalamocortical slices, implying SOs are associated with the development of cortical circuitry before hearing onset. A persisting discharge in ACx from BIC-administered animals suggests that regulation of SOs during the first postnatal week is associated with inhibitory GABAergic transmission. The disruption of SOs and inhibitory currents by gap junction blockers suggests that developing connexin proteins are integral in the normal expression of SOs and the maturation of ACx.
Neonatal gerbil ACx is endowed with spontaneous oscillations in vivo.
Thalamorecipient ACx is anatomically identifiable in neonatal brains.
Regulation of SOs in vivo may involve inhibitory GABAergic transmission.
Gap junctions may mediate inhibitory regulation of SOs.
Acknowledgements
VCK thanks Dan Sanes for candid discussions and Claudia Farb for slice photomicrographs. MP wishes to thank ARC for mentorship, V. Khatri for help, and the Loreto family (Cecilia, Emilia, and Matilde) for their support. We thank Jonathan Levitt (CUNY), and Tara Chowdhury and Jason M. Hunter (NYU) for proofing this manuscript. Supported by NIH DC011284 (DHS and VCK), G12-RR03060-25 and SC1HD068129-01 (ARC).
ABBREVIATIONS
- A1
primary auditory cortex
- ACSF
artificial cerebrospinal fluid
- ACx
thalamorecipient auditory cortex
- AP-5
2-amino-5 phosphonopentanoate
- ANOVA
one-way analysis of variance
- AV
anterior ventral cortex
- BIC
bicuculline
- Ca2+/PKC
calcium/protein kinase C
- CB
cerebellum
- CBN
carbenoxolone
- cAMP/PKA
cyclic adenosine mono phosphate-protein kinase A
- Cx
Connexin
- DNQX
6,7-Dinitroquinoxaline-2,3-dione
- DP
dorsal posterior cortex
- EGTA
ethylene glycol-bis(beta-amino ethyl ether)-N,N,N’,N’-tetra acetic acid
- EPSP
excitatory postsynaptic potential
- Hepes
4-2-hydroxyethyl-1-piperazineethanesulfonic acid; H: hippocampus
- L2/3
cortical layers 2 and 3
- FS
fast spiking
- ICV
inferior cerebral vein
- i.p.
intraperitoneal
- IPSP
inhibitory postsynaptic potential
- siRNA
small interfering ribonucleic acid
- sIPSC
spontaneous inhibitory postsynaptic current
- MCA
mid cerebral artery
- MFQ
mefloquine
- MG
ventral division of the medial geniculate nucleus
- MTTI
molecular targeting tools Inc
- MUA
multiunit activity
- NMDA
N-methyl-D-aspartate
- OD
outer diameter
- P
postnatal day
- PB
phosphate buffer
- Peri
perirhinal cortex
- PF
paraformaldehyde
- S.C.
subcutaneous
- SO
spontaneous oscillation
- RINPUT
input resistance
- RSERIES
series resistance
- VHOLD
holding membrane potential
- VREST
resting membrane potential
- Vm
membrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8:988–990. doi: 10.1038/nn1502. [DOI] [PubMed] [Google Scholar]
- Baltz T, de Lima AD, Voigt T. Contribution of GABAergic interneurons to the development of spontaneous activity patterns in cultured neocortical networks. Front Cell Neurosci. 2010;4:1–17. doi: 10.3389/fncel.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neursoci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Büdinger Heil P., Scheich H. Functional organization of auditory cortex in the Mongolian gerbil (Meriones unguiculatus). Anatomical subdivisions and corticocortical connections. Eur J Neurosci. 2000;12:2425–2451. doi: 10.1046/j.1460-9568.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- Büdinger E, Heil P, Hess A, Scheich H. Multisensory processing via early cortical stages: Connections of the primary auditory cortical field with other sensory system. Neuroscience. 2006;143:1065–1083. doi: 10.1016/j.neuroscience.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based "Hebbian" learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. Oxford University Press; New York: 2006. [Google Scholar]
- Conhaim J, Easton CR, Becker MI, Barahimi M, Emily R, Cedarbaum ER, Moore JG, Mather LF, Dabagh S, Daniel J, Minter DJ, Moen SP, Moody WJ. Developmental changes in propagation patterns and transmitter dependence of waves of spontaneous activity in the mouse cerebral cortex. J Physiol. 2011;589:2529–2541. doi: 10.1113/jphysiol.2010.202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Ann Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptors in developing visual pathways. Ann Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Progr Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas CE, Wenner P. Role of spontaneous activity in the maturation of GABAergic synapses in embryonic spinal circuits. In: Pallas S, editor. Developmental plasticity of inhibitory circuitry. Springer; NY: 2010. pp. 27–39. [Google Scholar]
- Hanganu IL, Staiger JF, Ben-Ari Y, Khazipov R. Cholinergic modulation of spindle bursts in the neonatal rat visual cortex in vivo. J Neurosci. 2007;27:5694–5705. doi: 10.1523/JNEUROSCI.5233-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Quian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:e96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Mohns EJ, di Prisco GV, Blumberg MS. On the co-occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus. 2006;16:959–965. doi: 10.1002/hipo.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki A, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neuroscience. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sadahiro M, Fall CP. Developmental expression of endogenous oscillations and waves in the auditory cortex involves calcium, gap junctions, and GABA. Neuroscience. 2007;146:1629–1639. doi: 10.1016/j.neuroscience.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DS. GABAA agonist rescues cortical inhibitory synaptic function following developmental hearing loss. Asso Res Otolaryngol. 2011 Abstr # 396. [Google Scholar]
- Konietzko U, Müller CM. Astrocytic dye coupling in rat hippocampus: topography, developmental onset, and modulation by protein kinase C. Hippocampus. 1994;3:297–306. doi: 10.1002/hipo.450040313. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Lamsa K, Pavlov I, Riekki R, Johnson BE, Molnar E, Rauvala H, Taira T. Activity blockade increases the number of functional synapses in the hippocampus of newborn rats. Mol Cell Neurosci. 2003;22:107–117. doi: 10.1016/s1044-7431(02)00012-x. [DOI] [PubMed] [Google Scholar]
- McCabe AK, Easton CR, Lischalk JW, Moody WJ. Roles of glutamate and GABA receptors in setting the developmental timing of spontaneous synchronized activity in the developing mouse cortex. Dev Neurobiol. 2007;67:1574–1588. doi: 10.1002/dneu.20533. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Izquierdo MA, Malmierca MS. Persistent effects of early augmented acoustic environment on the auditory brainstem. Neuroscience. 2011;184:75–87. doi: 10.1016/j.neuroscience.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Marion B, Davis E, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas SL. Intrinsic and extrinsic factors that shape neocortical specification. Trends Neurosci. 2001;24:417–423. doi: 10.1016/s0166-2236(00)01853-1. [DOI] [PubMed] [Google Scholar]
- Park WM, Wang Y, Park S, Denisova JV, Fontes JD, Belousov AB. Interplay of chemical neurotransmitters regulates developmental increase in electrical synapses. J Neurosci. 2011;3116:5909–5920. doi: 10.1523/JNEUROSCI.6787-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross FM, Gwyn P, Spanswick D, Davies SN. Carbenoxolone depresses spontaneous epileptiform activity in the CA1 region of rat hippocampal slices. Neuroscience. 2000;100:789–796. doi: 10.1016/s0306-4522(00)00346-8. [DOI] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Root CM, Velázquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Kotak VC. Developmental plasticity of auditory cortical inhibitory synapses. Hear Res. 2011;279:140–148. doi: 10.1016/j.heares.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Reh TA, Harris WA. Development of the Nervous System. III Edition Academic Press; New York: 2011. [Google Scholar]
- Sebe JY, Looke-Stewart EC, Estrada RC, Baraban SC. Robust tonic GABA currents can inhibit cell firing in mouse newborn neocortical pyramidal cells. J Neurosci. 2010;32:1310–1318. doi: 10.1111/j.1460-9568.2010.07373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Stacy RC, Demas J, Burgess RW, Sanes JR, Wong RO. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci. 2005;25:9347–9357. doi: 10.1523/JNEUROSCI.1800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta. 2005;1719:59–68. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Sun JJ, Luhmann HJ. Spatio-temporal dynamics of oscillatory network activity in the neonatal mouse cerebral cortex. Eur J Neurosci. 2007;26:1995–2004. doi: 10.1111/j.1460-9568.2007.05819.x. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission neuronal membrane properties. J Neurophysiol. 2009;102:974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the mammalian cochlea. J Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Rodríguez-Contreras A, Crins TTH, Wang HC, Borst JGG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci. 2010;13:1050–1052. doi: 10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Lorincz A, Gleeson P, Farinella M, Nusser Z, Silver RA. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron. 2010;67:435–451. doi: 10.1016/j.neuron.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Cuntz H, Mori M, Nusser Z, Sjöström J, Haüsser M. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci. 2009;4:463–473. doi: 10.1038/nn.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ROL. The role of spatiotemporal firing patterns in neuronal development of sensory systems. Curr Biol. 1993;3:595–601. doi: 10.1016/0959-4388(93)90061-3. [DOI] [PubMed] [Google Scholar]
- Wong ROL. Retinal waves: stirring up a storm. Neuron. 1999;24:493–495. doi: 10.1016/s0896-6273(00)81102-2. [DOI] [PubMed] [Google Scholar]
- Yang L, Ling DSF. Carbenoxolone modifies spontaneous inhibitory and excitatory synaptic transmission in rat somatosensory cortex. Neurosci Lett. 2007;18:221–226. doi: 10.1016/j.neulet.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Nawy S. The gap junction blockers carbenoxolone and 18beta-glycyrrhetinic acid antagonize cone-driven light responses in the mouse retina. Vis Neurosci. 2003;20:429–435. doi: 10.1017/s0952523803204089. [DOI] [PubMed] [Google Scholar]