Summary
Streptomyces scabies is a model organism for the investigation of plant–microbe interactions in Gram‐positive bacteria. Here, we investigate the type VII protein secretion system (T7SS) in S. scabies; the T7SS is required for the virulence of other Gram‐positive bacteria, including Mycobacterium tuberculosis and Staphylococcus aureus. The hallmarks of a functional T7SS are an EccC protein that forms an essential component of the secretion apparatus and two small, sequence‐related substrate proteins, EsxA and EsxB. A putative transmembrane protein, EccD, may also be associated with T7S in Actinobacteria. In this study, we constructed strains of the plant pathogen S. scabies carrying marked mutations in genes coding for EccC, EccD, EsxA and EsxB. Unexpectedly, we showed that all four mutant strains retain full virulence towards several plant hosts. However, disruption of the esxA or esxB, but not eccC or eccD, genes affects S. scabies development, including a delay in sporulation, abnormal spore chains and resistance to lysis by the Streptomyces‐specific phage ϕC31. We further showed that these phenotypes are specific to the loss of the T7SS substrate proteins EsxA and EsxB, and are not observed when components of the T7SS secretion machinery are lacking. Taken together, these results imply an unexpected intracellular role for EsxA and EsxB.
Introduction
Secreted proteins play a number of essential roles in bacteria, including the colonization of niches and host–pathogen interactions. Gram‐negative bacteria, which have double‐membrane cell envelopes, exhibit six major protein secretion pathways (types I–VI) that move proteins from the cytoplasm to the cell exterior by a one‐ or two‐step mechanism (reviewed in Desvaux et al., 2009). These secretion systems are absent from Gram‐positive bacteria, which generally (with the exception of mycobacteria and closely related genera) have a simpler cell envelope organization comprising a single membrane. The Sec and Tat pathways, which are located in the cytoplasmic membrane of Gram‐negative and Gram‐positive bacteria, directly secrete extracellular proteins in Gram‐positive bacteria (e.g. Sibbald et al., 2006; Widdick et al., 2006). In addition to these general export pathways, Gram‐positive bacteria possess a specialized protein export system, termed the type VII or ESX secretion system (Burts et al., 2005; Hsu et al., 2003; Pym et al., 2003; Stanley et al., 2003).
The type VII secretion system (T7SS) was initially described in mycobacteria, where it was demonstrated that a secretion system was encoded by the region of difference 1 (RD1) of Mycobacterium bovis that was required for the secretion of the small proteins ESAT‐6 and CFP‐10 (now renamed EsxA and EsxB, respectively; Hsu et al., 2003; Pym et al., 2003). These small proteins lack N‐terminal signal sequences and are structurally closely related, comprising a helical hairpin with a conserved W–x–G motif at the hairpin bend (Renshaw et al., 2005; Sundaramoorthy et al., 2008). The presence of one or more genes encoding an EsxA family protein is one of the genetic hallmarks for a T7SS, together with a gene coding for an ATPase of the FtsK‐SpoIIIE family, which is required for EsxA secretion (Brodin et al., 2006; Burts et al., 2005; Stanley et al., 2003). The T7SS is found throughout the Actinobacteria and also in the Firmicutes. However, the similarity between the secretion systems of the Firmicutes and Actinobacteria is very low, with the EsxA/B proteins and the FtsK‐SpoIIIE domain protein being the only conserved elements. In addition, there are a number of system‐specific components that are essential for T7S in the different phyla. The T7SS is required for full virulence of a number of Gram‐positive pathogens, including Mycobacterium tuberculosis, Mycobacterium bovis, Staphylcoccus aureus and Bacillus anthracis (Burts et al., 2005; Garufi et al., 2008; Hsu et al., 2003; Pym et al., 2003; Stanley et al., 2003).
Streptomyces forms the largest genus of Actinobacteria and contains mycelial organisms that undergo complex morphological differentiation involving the formation of aerial hyphae and spores (Flardh and Buttner, 2009). Streptomyces spp. are frequently soil dwelling, and are prolific protein secretors, particularly during their vegetative growth stage (Chater et al., 2010). At least one copy of a T7SS is encoded in the genomes of all streptomycetes sequenced to date. Streptomyces spp. are predominantly saprophytic, although a few members of the genus are able to cause disease. The best studied of the disease‐causing streptomycetes is Streptomyces scabies, which is the causal agent of common scab of potato. Thaxtomin, a nitrated dipeptide toxin, is one of the major pathogenicity factors secreted by S. scabies and is a potent inhibitor of cellulose synthesis (Bischoff et al., 2009; Scheible et al., 2003). Protein secretion is also essential for the pathogenesis of S. scabies. Nec1, which is probably secreted by the Sec pathway, is a protein that, to date, has only been found in pathogenic streptomycetes, and is required for root colonization (Bukhalid and Loria, 1997; Joshi et al., 2007a). The analysis of protein secretion by the S. scabies Tat pathway has shown that at least seven Tat substrates also contribute to virulence (Joshi et al., 2010), and there are likely to be additional proteinaceous virulence factors yet to be identified.
In this study, we have characterized the T7SS of S. scabies. Surprisingly, we show that, unlike most T7SSs previously characterized in pathogenic bacteria, the S. scabies T7S machinery is fully dispensable for virulence. However, as reported previously for one of the T7SSs of the nonpathogen Streptomyces coelicolor, disruption of the genes encoding the EsxA and EsxB proteins results in delayed sporulation and abnormal spore phenotypes (Akpe San Roman et al., 2010). We further show that strains lacking esxA or esxB are resistant to lysis by the Streptomyces‐specific phage ϕC31. Interestingly, the delayed sporulation and phage resistance phenotypes are unique to strains deleted for one or other of esxA or esxB, and are not observed when other essential components of the T7SS are lacking. Taken together, these results imply an unexpected intracellular role for EsxA and EsxB.
Results
Genes coding for conserved components of a T7SS are present in S. scabies
To determine whether a T7SS was encoded in the genome of S. scabies (accession number FN554889), blastp analysis was performed using the amino acid sequences of components of the previously analysed T7SS from S. coelicolor and M. tuberculosis (Akpe San Roman et al., 2010; Bitter et al., 2009). This led to the identification of a gene, scab58621, coding for the conserved EccC component (the FtsK‐SpoIIIE domain protein), which was found to be in close proximity to a second gene, scab58651, which encodes the conserved EccD component (Fig. 1A). EccD is a highly hydrophobic protein, and analysis of the coding sequence of S. scabies EccD suggests that it has 11 transmembrane domains.
Figure 1.

Genetic organization of the type VII secretion system (T7SS) coding region in Streptomyces scabies. (A) Organization of the genes coding for T7SS components on the chromosome of S. scabies. The numbers show the sizes of the intergenic regions between each gene, in base pairs (bp). (B) The esxA and esxB genes are co‐expressed. RNA was isolated from S. scabies cultured on tryptone soy broth (TSB) or thaxtomin defined growth medium (TDM) and cDNA was prepared as described in Experimental procedures. cDNA was used as a template in polymerase chain reactions (PCRs) using primers that amplify within esxA or esxB, or across the esxA–esxB or esxB–scab58661 junctions. The expected sizes of the amplified products are indicated to the right. The RNA sample without the reverse transcriptase step served as a template for a negative control and yielded no products (and is therefore not shown). Chromosomal DNA was also used as a template with the same primers as a positive control. Samples were analysed on the same agarose gel; intervening lanes have been excised for clarity.
A hallmark of a T7SS is the presence of genes coding for EsxA and EsxB, small proteins of the WXG100 protein family that usually contain a conserved W–x–G motif. Simple blastp analysis using the S. coelicolor WXG100 protein sequences EsxA (Sco5725), EsxB (Sco5724) and Sco4509 (Akpe San Roman et al., 2010; Palmer and Hutchings, 2010) to query the S. scabies protein database failed to identify any obvious homologues. However, visual inspection of the genomic region around scab58621 revealed the presence of three open reading frames (ORFs) (scab58661, scab58671 and scab58681) encoding proteins of the expected size for a typical WXG100 protein (100–150 amino acids). The translated protein sequences were analysed using the secondary structure prediction program phyre (Kelley and Sternberg, 2009), and both SCAB58671 (henceforth EsxA) and SCAB58681 (EsxB) were predicted to be members of the EsxAB dimer‐like superfamily of proteins, with estimated precision values of 95% (E‐value 0.02) and 85% (E‐value 0.34), respectively. Neither EsxA nor EsxB contains the canonical WXG motif, with EsxB having an –FNG– motif at this position and EsxA having an –FQA– motif (Fig. S1, see Supporting Information).
Other conserved T7SS components found in M. tuberculosis and S. coelicolor are EccB (Sco5721 in S. coelicolor), a predicted monotopic membrane protein, and one or more subtilisin‐like serine proteases (MycP1–5 in M. tuberculosis, Sco5722 and Sco5731 in S. coelicolor). The S. scabies genome does not encode any protein with similarity to EccB, but does encode several predicted subtilisin‐type serine proteases, although none of them are encoded in the proximity of the T7SS. Of the other genes that are located close to SCAB58621, SCAB58631 is a proline/alanine‐rich protein that is homologous to M. tuberculosis EspI (blast E‐value of 7e−13 in 231 amino acid overlap) and SCAB58611 is a conserved hypothetical protein, the closest homologue of which in M. tuberculosis is the PPE family protein Rv2352c (blast E‐value of 0.019 in 109 amino acid overlap). SCAB58661 is a predicted monotopic membrane protein, whose closest orthologues are encoded in some Streptomyces and also appear to be linked to putative T7SS gene clusters. However, no closely related proteins to SCAB58661 are encoded in any other genus. The genetic organization of the S. scabies T7SS is shown in Fig. 1A.
The esxA and esxB genes form a transcription unit and are expressed under laboratory growth conditions
The genetic organization and spacing are consistent with the genes scab58681 (esxA) to scab58611 being organized as a probable transcription unit. To ascertain whether this probable transcription unit was expressed, we isolated mRNA and prepared complementary DNA (cDNA) from the S. scabies 87.22 wild‐type strain that had been cultured for 20 h in either tryptone soy broth medium (a standard laboratory growth medium for Streptomyces; Kieser et al., 2000) or thaxtomin defined medium (which contains cellobiose and is known to induce the expression of S. scabies virulence genes, e.g. Bignell et al., 2010; Johnson et al., 2007; Joshi et al., 2007a). The cDNA was used as a template in polymerase chain reactions (PCRs) to determine whether the individual esxA (scab58681) and esxB (scab58671) genes were expressed in either of these growth conditions, and whether they were co‐transcribed with each other, and with the neighbouring scab58661 gene.
As shown in Fig. 1B, PCR products of the expected sizes were obtained for the esxA and esxB genes under both of the growth conditions tested. No products were obtained if the reverse transcriptase step was omitted, indicating that the mRNA was not contaminated with chromosomal DNA (not shown). Furthermore, amplified DNA products of the expected sizes were also seen when oligonucleotide primers were used that span the esxA and esxB genes, and the esxB–scab58661 genes. This indicates that esxA and esxB are expressed under standard laboratory growth conditions and that the two genes form a transcription unit together with at least the adjacent downstream gene, scab58661.
Mutations in genes coding for core T7SS components have differential effects on S. scabies growth and development
In order to investigate the requirement of the S. scabies T7SS in its physiology, and to explore any role in virulence, we constructed gene deletions in the esxA, esxB, eccC and eccD genes, that were each marked with an apramycin resistance cassette, using the λ RED REDIRECT approach (Gust et al., 2003). Each mutant strain was verified by Southern blot analysis (Fig. S2, see Supporting Information), and the verified strains were designated JKF1 (ΔeccC::Apra), JKF2 (ΔeccD::Apra), JKF3 (ΔesxB::Apra) and JKF4 (ΔesxA::Apra).
We firstly investigated whether any of these mutations affected the growth of S. scabies by assessing the ability of strains JKF1–JKF4 to grow on nine different types of solid media typically used for the laboratory cultivation of Streptomyces strains (Fig. 2). Clear differences in strain behaviour were revealed by this analysis on some of the growth media. For example, the strain carrying an apramycin‐marked deletion of eccC (JKF1) appeared to form aerial hyphae (observed as a white appearance to the surface of the biomass) more readily than any of the other strains, including the wild‐type strain, on R2 medium or R2 medium containing yeast extract (Fig. 2D,E). Similarly, when grown on soyflour mannitol medium (Fig. 2A), the same strain also showed increased sporulation in comparison with the wild‐type (observed as darker grey pigmentation). These observations indicate that the loss of eccC, which encodes the FtsK‐SpoIIIE domain protein, results in a media‐dependent acceleration of the S. scabies developmental cycle.
Figure 2.

Growth analysis of Streptomyces scabies strains carrying mutations in the esxA, esxB, eccC and eccD genes. (A–I) Phenotypic analysis of growth on solid media. Equal numbers of spores of strain 87.22 (wild type, WT), JKF1 (eccC –), JKF2 (eccD –), JKF3 (esxB –) and JKF4 (esxA –) strains were streaked from glycerol stock and incubated at 30 °C for 5 days. The different growth media used were soyflour mannitol (A), oatbran (B), instant potato mash (C), R2 (D), R2 with yeast extract (E), minimal with 1% mannitol (F), minimal with 1% glucose (G), Difco nutrient agar (H) and yeast malt extract (I). The images shown are representative examples from five independent growth experiments. (J) Growth rate analysis of the same strains in tryptone soy broth. Aliquots of tryptone soy broth (100 mL) were inoculated with spores of the strain of interest to a density of 1 × 106 spores/mL of culture medium, and incubated at 30 °C with shaking. Samples (1 mL) were withdrawn from the cultures every 3 h and total cytosolic protein was prepared as described in Experimental procedures. Error bars represent the standard error of the mean, where n = 3.
By contrast, strains carrying marked deletions of esxA (JKF4) or esxB (JKF3) showed a decrease in aerial hyphae formation and sporulation in comparison with the wild‐type strain on several different growth media, including soyflour mannitol medium (Fig. 2A) oatbran medium (Fig. 2B), instant potato mash medium (Fig. 2C), minimal medium plus glucose (Fig. 2G) and, most prominently, yeast malt extract (Fig. 2I). In each case, it appeared that the growth defect was slightly more pronounced for JKF4 than for JKF3. Thus, it appears that the inactivation of genes coding for the EsxAB dimer‐like superfamily proteins results in a developmental delay. A similar developmental delay has been reported for a strain of S. coelicolor with a transposon insertion in esxB (Akpe San Roman et al., 2010). It should be noted that strain JKF2 (which carries a marked deletion of eccD) showed a similar growth behaviour to the wild‐type on all the solid growth media tested.
We next assessed the growth of the strains in tryptone soybroth liquid culture. Streptomyces scabies, like S. coelicolor, does not sporulate in liquid culture, and so these experiments primarily assess vegetative growth. As Streptomyces strains grow as mycelial clumps in liquid culture, we assessed growth by harvesting mycelia at different time points and by measuring total cytosolic protein (Joshi et al., 2010; Widdick et al., 2006; Fig. 2J). Strains JKF1 and JKF2 grew similarly to the wild‐type strain, entering the logarithmic growth phase between 21 and 24 h post‐inoculation and reaching a peak by 33 h post‐inoculation. By contrast, strains JKF3 and JKF4 entered the logarithmic growth phase earlier than the wild‐type strain, at 18 h post‐inoculation. The rate of growth remained similar to that of the other strains for the duration of the logarithmic growth period, as each slope had a similar gradient (Fig. 2J). Strain JKF4 entered the stationary phase at 27 h post‐inoculation, whereas all of the other strains, including the wild‐type, entered the stationary phase at 33 h post‐inoculation. However, the final levels of total cytosolic protein were similar for all of the strains, indicating that similar biomasses were achieved at the end of the growth cycle.
Taken together these results suggest that loss of esxA and esxB affects normal growth and development of S. scabies, resulting in growth acceleration during the early growth phase, but a clear delay in the onset of aerial hyphae production and sporulation. Interestingly, however, loss of the gene coding for a key component of the T7SS, the FtsK‐SpoIIIE domain protein EccC did not phenocopy the esxA and esxB strains and had noticeably different effects on S. scabies development than the loss of EsxA and EsxB.
Spore chains of strains lacking EsxA and EsxB show morphological defects
As we observed delayed sporulation of S. scabies strains carrying marked mutations in esxA or esxB, we next analysed the morphology of the spore chains of S. scabies that had been cultured on instant potato mash agar. This was chosen as it permits the sporulation of the esxA and esxB mutant strains and is the medium in which the development of these strains most closely resembles that of the wild‐type (Fig. 2C). The S. scabies wild‐type strain, together with JKF1–JKF4, were cultured on instant potato mash agar, and samples of each strain showing spore development were prepared following 10 days of growth for analysis by scanning electron microscopy.
As shown in Fig. 3, abundant spirally arranged spore chains were visible for the wild‐type strain and for the strains carrying marked deletions in eccC or eccD (Fig. 3A–C,F,G). However, for the strains carrying marked deletions in esxA or esxB, there was very little evidence of spiral spore chain formation (Fig. 3D,E,I,J). When examined under higher magnification, it could also be seen that the pre‐spores in the spore chains from strains JKF3 and JKF4 were not of uniform size, with many lysed aerial hyphae and irregularly sized pre‐spores (compare Fig. 3I,J with Fig. 3F–H). Thus, it seems that EsxA and EsxB (but not EccC or EccD) are not only required for the onset of aerial hyphae formation and sporulation (at least on some growth media), but also for correct spore formation.
Figure 3.

Scanning electron microscopy images of spore chains from the following Streptomyces scabies strains: (A, F) 87.22 (wild‐type); (B, G) JKF1 (eccC –); (C, H) JKF2 (eccD –); (D, I) JKF3 (esxB –); (E, J) JKF4 (esxA –). The strains were inoculated onto instant potato mash agar from glycerol stock and incubated at 30 °C for 10 days.
Introduction of a second copy of esxA–esxB into wild‐type S. scabies alters the timing of development
To attempt to complement the sporulation defect of strains JKF3 and JKF4, we constructed an integrating vector carrying esxA and esxB, together with 225 base pairs upstream of esxA, which should cover the natural promoter region (predicted to lie approximately 100 base pairs upstream of the esxA start codon according to the bprom prediction program; http://linux1.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb). We introduced this construct into the marked esxA and esxB deletion strains, and also into the wild‐type strain, and cultured these, alongside the nontransformed strains, on yeast malt extract agar. This was chosen as growth medium as it gave the most prominent developmental defect for the esxA and esxB deletion strains (Fig. 2I), and was therefore expected to most clearly indicate whether complementation of these strains was successful.
Following 2 days of growth on this medium, almost the entire surface of the biomass formed by the wild‐type strain was covered with white aerial hyphae (Fig. 4). By contrast, the development of aerial hyphae appeared to be delayed for the wild‐type strain harbouring an additional copy of esxAB, as it was only evident at the edges of the biomass. As expected, no aerial hyphae were visible for the esxA or esxB marked deletion strains; however, the same strains carrying an ectopic copy of esxAB had started to show some formation of aerial hyphae around the edges of the biomass.
Figure 4.

Partial complementation of the developmental defect of the Streptomyces scabies esxA and esxB strains is afforded by supplying an in trans copy of esxAB. Equal numbers of spores of strains 87.22 (wild‐type, WT), 87.22/pesxAB, JKF3 (esxB –), JKF3/pesxAB, JKF4 (esxA –) and JKF4/pesxAB were streaked from glycerol stock onto yeast malt extract medium and incubated at 30 °C for the indicated time periods.
After 6 days of growth, the wild‐type strain had sporulated (observed as a grey appearance on the surface of the biomass in Fig. 4). Sporulation was also observed for the wild‐type strain harbouring an extra copy of esxAB, but, in this case, the surface of the biomass appeared darker than that of the untransformed wild‐type strain, indicating that more spores were produced following the introduction of a second copy of esxA and esxB. Both of the esxA and esxB mutant strains showed more prolific formation of aerial hyphae when they carried an ectopic copy of esxAB, although development was still clearly retarded relative to the wild‐type strain. This indicates that partial complementation of the mutant strains was afforded by supplying an additional copy of esxA and esxB in trans.
Taken together, these results indicate that the cellular levels of EsxA and EsxB (and possibly the relative amounts of the two proteins) are critical for the timing of S. scabies development.
Loss of EsxA and EsxB confers resistance to infection by bacteriophage ϕC31
As T7SS components have been associated with bacteriophage infection, we carried out plaque assays using two different Streptomyces‐specific bacteriophages, ϕR4 and ϕC31, which are in different immune groups (Chater and Carter, 1979). Each of the bacteriophages tested was able to form plaques on the S. scabies wild‐type strain, with ϕR4 producing very small plaques and ϕC31 very large plaques (Fig. 5). Each of the T7S mutant strains was also susceptible to lysis by ϕR4, giving similar numbers and similar plaque sizes to those seen during infection of the wild‐type strain (Fig. 5, top panel). Interestingly, although the strains carrying marked mutations in eccC or eccD behaved similarly to the wild‐type strain for ϕC31 infection, the strains with marked deletions in esxA or esxB appeared to be fully resistant to infection, as there was a complete absence of plaque formation (Fig. 5, bottom panel). This implies that the EsxAB dimer‐like superfamily proteins are required for lytic infection by ϕC31, but not by ϕR4.
Figure 5.

The Streptomyces scabies esxA and esxB mutant strains are resistant to infection by bacteriophage ϕC31. Approximately 108 spores of S. scabies strains 87.22 (wild‐type, WT), JKF1 (eccC –), JKF2 (eccD –), JKF3 (esxB –) and JKF4 (esxA –) were mixed with a suspension of bacteriophage ϕR4 (top panel) or ϕC31 (bottom panel). Plates were incubated at 30 °C for 18 h to allow plaque formation prior to being photographed.
The T7SS is dispensable for S. scabies virulence
Streptomyces scabies is a broad host range pathogen that causes root rot on both model plant species and agricultural crops (reviewed in Loria et al., 2006). We used three different plant hosts and bioassays to assess virulence, namely the ability to cause necrosis in vitro on disc slices of potato tuber (Fig. 6A), and to cause necrosis on, and affect the growth of, radish (Fig. 6B) and tobacco (Fig. 7).
Figure 6.
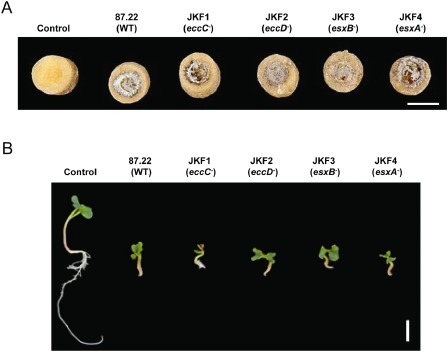
The Streptomyces scabies eccC –, eccD –, esxB – and esxA – strains show necrosis on potato tuber slices and are virulent for infection in the radish root assay. (A) Potato tuber slices in Petri dishes were inoculated spore side down with agar plugs from plates of each of the indicated strains showing confluent sporulation, as described in Experimental procedures. The Petri dishes were sealed with parafilm and incubated in the dark at room temperature for 7 days prior to being photographed. (B) Radish seeds, which had been grown as described in Experimental procedures, were transferred to water agar and inoculated with equal numbers of spores of each of the indicated S. scabies strains. Petri dishes were sealed with parafilm and incubated at 21 ± 2 °C with a 16‐h photoperiod for 6 days prior to the excision of roots from the agar. Seedlings were assessed for disease symptoms and photographed. Scale bars, 1 cm.
Figure 7.

The Streptomyces scabies eccC –, eccD –, esxB – and esxA – strains show virulence on Nicotiana tabacum. Tobacco seedlings, germinated in individual magenta boxes as described in Experimental procedures, were inoculated with approximately 5 × 107 spores of the indicated strains. Plants were incubated at 21 ± 2 °C with a 16‐h photoperiod for 9 weeks prior to being photographed from the side (A), above (B) and below (C). Scale bars, 2 cm.
The first virulence bioassay performed involved the inoculation of aseptically prepared potato tuber slices with plugs taken from agar plates showing confluent sporulation of each strain. Lesion size and severity were assessed following 7 days of incubation. In this potato disc assay (Fig. 6A), it was not possible to differentiate the size and severity of lesions among tuber discs inoculated with the wild‐type strain and the T7SS mutant strains (JKF1–JKF4). In contrast, the noninoculated tuber disc lacked the necrosis and pitting found in the inoculated tuber tissue.
The second virulence bioassay performed involved the inoculation of young radish seedlings with an equal number of spores of each strain. Disease symptoms were assessed following 6 days of incubation. The root growth of radish seedlings inoculated with the wild‐type strain (Fig. 6B) was severely stunted when compared with that of water‐inoculated seedlings (marked ‘control’ in Fig. 6B), and there was browning at both the root tip and at regions in which the formation of secondary roots would have occurred in noninoculated plants. However, similar symptoms were observed when radish seedlings were inoculated with each of the JKF1–JKF4 strains.
The third virulence bioassay performed involved the inoculation of 7‐day‐old Nicotiana tabacum seedlings growing in agar with an equal number of spores of each strain. Disease symptoms were assessed following 9 weeks of incubation. The growth of seedlings inoculated with the wild‐type strain was severely reduced compared with that of water‐inoculated control seedlings (Fig. 7). The plant height was greatly reduced (Fig. 7A), although the overall condition of the plants appeared healthy, with little etiolation of the stems and leaves (indeed, when viewed from above in Fig. 7B, it is difficult to distinguish control plants from S. scabies‐infected plants). This is consistent with reports that the foliage of potato plants infected with S. scabies usually shows no evidence of infection. Inspection of the roots revealed that they were underdeveloped, possessing much less root mass (estimated visually), and there was very little evidence of lateral roots (Fig. 7C). However, broadly similar effects were observed when Nicotiana seedlings were inoculated with each of the S. scabies mutant strains JKF1–JKF4 (Fig. 7).
Discussion
Protein secretion systems are intimately linked to virulence in all bacterial pathogens (e.g. Alvarez‐Martinez and Christie, 2009; Lindeberg et al., 2009), and even those protein export systems that play a housekeeping role in some organisms are frequently co‐opted for virulence functions during pathogenesis (e.g. Joshi et al., 2010; Rigel and Braunstein, 2008). In this study, we have addressed the role of the putative T7SS encoded in the genome of S. scabies in relation to its physiology and virulence. We constructed marked mutations in genes coding for two of the components, EccC and EccD, that have previously been reported to be essential for T7S in M. tuberculosis (Brodin et al., 2006; Raghavan et al., 2008; Stanley et al., 2003), as well as each of the two genes coding for the small secreted proteins, EsxA and EsxB.
Surprisingly, we found that there was no detectable role for any of the T7S components in S. scabies virulence in any of the plant infection models tested. This is in contrast with a clear role for this system in the virulence of the animal pathogens M. tuberculosis, M. bovis, S. aureus and B. anthracis (Burts et al., 2005; Garufi et al., 2008; Hsu et al., 2003; Pym et al., 2003; Stanley et al., 2003). Studies with Listeria monocytogenes showed that a deletion of the gene coding for the ESAT‐6 (EsxB) homologue did not affect virulence in a mouse model of infection, although any role for the secretion system itself in this study was not investigated (Way and Wilson, 2005). It remains possible that the T7SS of S. scabies plays a subtle role during infection that we cannot reliably detect in the qualitative growth tests used here to assess virulence.
In contrast with the apparent lack of any role for the T7SS in plant infection, we clearly demonstrated that components encoded by the T7SS gene cluster are required for the normal growth and development of S. scabies. This is in close agreement with the studies of Akpe San Roman et al. (2010) in the related streptomycete S. coelicolor. We showed that the loss of the genes coding for EsxA and EsxB leads to a failure of S. scabies to undergo normal aerial development and sporulation during the growth cycle on several types of solid growth media. Moreover, any spore chains that were formed by these mutant strains were clearly aberrant with irregularly sized pre‐spores. This is very similar to the findings of Akpe San Roman et al. (2010), who showed that the disruption of esxBA resulted in a sporulation delay, and that the spore morphology and DNA content were substantially affected in the knockout strain.
It is intriguing to note that phenotypic differences have been observed previously between strains lacking EccC, an essential component of the T7S secretion apparatus, and strains lacking EsxA and EsxB, the substrate proteins of the T7SS. Wherever examined, including M. tuberculosis, M. smegmatis, S. aureus and S. coelicolor, loss of EccC abolishes the export of EsxA and EsxB (Akpe San Roman et al., 2010; Burts et al., 2005; Coros et al., 2008; Stanley et al., 2003). In both S. scabies and S. coelicolor, the eccC mutant strain does not phenocopy the esxA/esxB strains. This indicates that it is not simply the case that failure to secrete EsxA and EsxB gives rise to the aberrant development of Streptomyces. Instead, it points to an intracellular role for these two small proteins. This is also supported by our findings that an ectopic copy of esxAB expressed in an otherwise wild‐type S. scabies strain also interferes with the developmental cycle, as this might be expected to shift the balance between intracellular and extracellular EsxA and EsxB in this situation.
It has been proposed that the EsxAB heterodimer controls the co‐ordination of cell division with the segregation of nucleoids, most probably through interaction with other factors (for example, the morphogenic regulator BldB) that regulate nucleoid condensation (Akpe San Roman et al., 2010). Although BldB is directly linked to the T7S‐encoding loci in some streptomycetes, the S. scabies BldB homologue, encoded by scab25271, does not cluster with this locus. It is interesting to note that the T7SS of the saprophyte M. smegmatis is essential for conjugal DNA transfer, with the implication that either co‐secretion of EsxAB with other T7S substrate proteins, and/or EsxAB interaction with other proteins in the cell envelopes of the donor and recipient, mediates DNA transfer (Coros et al., 2008; Flint et al., 2004).
One of the components encoded by the putative T7SS of Bacillus subtilis, YueB, is a membrane protein which is essential for irreversible binding of the bacteriophage SPP1 to the bacterial surface and ejection of phage DNA into the host cell (Sao‐Jose et al., 2004, 2006). Furthermore, it has been shown that, although yukA and yukE (coding for an EccC homologue and an EsxA homologue, respectively) are not essential for phage infection, strains with mutations in these genes give smaller plaques (Sao‐Jose et al., 2004).
In this study, we have shown that the loss of EsxA and EsxB prevents lytic infection by the bacteriophage ϕC31, another process which is also closely linked with DNA mobility. However, a strain lacking the FtsK‐SpoIIIE domain protein, EccC, which is essential for the export of EsxA and EsxB to the cell envelope in all organisms examined to date (Akpe San Roman et al., 2010; Burts et al., 2005; Champion et al., 2006), behaves very differently to the esxA/esxB deletion strains by showing sensitivity to ϕC31 infection. Like the sporulation defect, this phenotype appears to be linked to intracellular levels of EsxA/B.
It is possible that intracellular EsxA and EsxB interact with proteins that regulate phage DNA integration and/or excision or, alternatively, it may be that EsxA/B themselves interact with DNA. Regardless of the precise intracellular roles for EsxA and EsxB, one of the major functions of the T7S machinery of Streptomyces, identified by Akpe San Roman et al. (2010) and confirmed here, is the regulated secretion of EsxA and EsxB to control the progression of the developmental growth cycle. Other roles for the T7SS or extracellular EsxA and EsxB cannot be ruled out; however, they are likely to be subtle in comparison with the intracellular role identified here.
In conclusion, we have shown that two small proteins encoded at the T7SS locus of S. scabies are required for normal growth of the organism, and that the probable role of the T7SS is in the regulated secretion of these proteins. Although we observed no overt virulence phenotype associated with the loss of T7SS components or substrates in S. scabies, it should be noted that, for the most part, the virulence assays used here are associated with the growth of vegetative hyphae (e.g. Joshi et al., 2010). However, dispersal of the pathogen through the environment is mediated by spores, and therefore any mutation that leads to a defect in sporulation would be expected to have a major effect on the dissemination of S. scabies during infection.
Experimental Procedures
Bacterial strains, plasmids, media and culture conditions
Streptomyces scabies strains used in this study were routinely cultured on instant potato mash agar (Joshi et al., 2010) and incubated at 30 °C. Where required, the medium was supplemented with apramycin (100 μg/mL), hygromycin B (100 μg/mL), kanamycin (50 μg/mL) or nalidixic acid (25 μg/mL). Spore stocks were prepared as described previously (Kieser et al., 2000).
Targeted disruption of the S. scabies eccC, eccD, esxB and esxA genes employed the REDIRECT PCR targeting system (Gust et al., 2003), with Escherichia coli strain BW25113 containing pIJ790 (Gust et al., 2003) and S. scabies cosmid 212 (which harbours all of the genes of the T7SS‐encoding cluster) as the initial host. Plasmid pIJ773 served as the source of the apramycin resistance cassette, which was amplified using the oligonucleotide primer pairs listed in Table S1 (see Supporting Information) designed to disrupt the individual genes of interest. After individual disruption of each gene, mutant cosmids were conjugated into S. scabies from the E. coli methylation‐deficient host strain ET12567/pUZ8002 (Bignell et al., 2010; MacNeil et al., 1992). Double crossovers were selected by resistance to apramycin and sensitivity to kanamycin. Gene deletions were verified by Southern blot analysis using the method of Bignell et al. (2010). For this, S. scabies strains were cultured in tryptone soy broth (Kieser et al., 2000) and incubated at 30 °C with shaking at 200 rpm for 2 days prior to extraction of chromosomal DNA using the MasterPure Gram‐positive DNA purification kit (Epicentre Biotechnologies, Madison, WI, USA). The oligonucleotides used for probe preparation are given in Table S1. The verified strains were designated JKF1 (ΔeccC::Apra), JKF2 (ΔeccD::Apra), JKF3 (ΔesxB::Apra) and JKF4 (ΔesxA::Apra).
For the ectopic expression of esxAB, DNA covering the esxA and esxB region, together with 225 bp of upstream DNA and 93 bp of downstream DNA, was amplified from cosmid 212 using oligonucleotide primers esxAB_comp_fwd and esxAB_comp_rev (Table S1). Following digestion with EcoRI and EcoRV, it was ligated into similarly digested pBluescript, verified by DNA sequencing and subcloned into pSET‐Hyg (Muller et al., 2007) as an EcoRI–EcoRV fragment, yielding pesxAB. This plasmid was then transferred to S. scabies by intergeneric conjugation, after which integration into the chromosome at the ϕC31 attachment site was selected by hygromycin resistance.
For growth rate analysis, 100 mL of tryptone soy broth (Kieser et al., 2000) was inoculated with S. scabies spores to a final spore concentration of approximately 1 × 106 spores/mL; 1‐mL samples, in triplicate, were removed every 3 h over the course of 45 h and mycelia were pelleted by centrifugation. Following resuspension in 1 mL of 1 m NaOH, samples were boiled for 10 min and the supernatant obtained following centrifugation was used to estimate the total cytosolic protein concentration in the sample, employing the Bio‐Rad DC protein assay kit (Hemel Hempstead, Hertfordshire, UK).
For phenotypic analysis, S. scabies strains were cultured alongside each other on soy flour mannitol agar (Hobbs et al., 1989), oat bran agar (4% oat bran and 1.2% agar in tap water), instant potato mash agar, R2 (Okanishi et al., 1974), R2YE (as R2 with 0.5% yeast extract), minimal media (Hopwood, 1967) supplemented with either 1% mannitol or 1% glucose, Difco nutrient agar (Kieser et al., 2000) or yeast malt extract agar (YME; 0.4% glucose, 0.4% yeast extract, 1% malt extract and 1.5% agar in tap water).
For the detection of esxA and esxB transcripts, S. scabies was cultured in either tryptone soy broth or thaxtomin defined medium (Johnson et al., 2007) at 30 °C with shaking for 20 h, after which time RNA was isolated as described previously (Joshi et al., 2007b). An additional incubation step with DNase (Qiagen, Valencia, CA, USA) at 37 °C for 30 min was included to ensure the destruction of genomic DNA, which was subsequently removed using StrataClean Resin (Stratagene, San Diego, CA, USA). Synthesis of cDNA from RNA (2 ng) was performed with random hexamer primers using a SuperScript III First‐Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Control reactions were carried out that excluded reverse transcriptase from the reaction mix (NRT sample). Transcript analysis using cDNA as PCR template used oligonucleotide primer pairs esxA_transcript_fwd and esxA_transcript_rev (to detect esxA expression), esxB_transccript_fwd and esxB_transcript_rev (to detect esxB expression), esxA_transcript_fwd and esxA_transcript_rev (to detect esxA–esxB co‐expression) or esxB‐ssc58661_fwd and esxB‐ssc58661_rev (to detect esxB–scab58661 co‐expression) (sequences listed in Table S1). The absence of genomic DNA was confirmed as no PCR products were obtained when NRT samples were used as the template.
Scanning electron microscopy of S. scabies spore chains
Spores of S. scabies strains 87‐22, JKF1, JKF2, JKF3 or JKF4 were inoculated onto instant potato mash agar and incubated at 30 °C for 10 days to allow confluent sporulation. A square section was excised from the agar surface of each plate and mounted on the sample holder using Tissue‐Tech OCT Compound (BDH Laboratory Supplies, Poole, UK). This was rapidly frozen in liquid N2 slush and then transferred under vacuum to the cryo‐preparation stage and warmed to −95 °C for 5 min to remove surface water. After sublimation, the samples were then cooled to –115 °C and coated with approximately 5 nm of Au/Pd. Samples were examined using a Philips XL 30 emission scanning electron microscope (ESEM) operating at an accelerating voltage of 15 kV.
Phage plaque assays
Phage plaque assays were performed as described previously (Dowding, 1973). Briefly, serial dilutions of phage were prepared in Difco nutrient broth and 100‐μL aliquots were transferred to the surface of Difco nutrient agar supplemented with 0.5% glucose, 10 mm MgSO4 and an appropriate concentration of calcium ions (8 mm for ϕC31 and 25 mm for ϕR4). Spores of S. scabies (to a final concentration of approximately 107–108/mL) were added to molten soft nutrient agar; 1‐mL aliquots were then transferred to the surface of the media containing the phage, allowed to set for 15 min, inverted and incubated at 30 °C for 18–20 h, after which time plaque formation was assessed.
Virulence bioassays
Potato tuber slice assay
Potato tuber discs (cv. Chippewa) were prepared, surface sterilized as described previously (Loria et al., 1995) and transferred to a Petri dish lined with moist filter paper. Agar plugs excised from instant potato mash agar plates of S. scabies strains showing confluent sporulation were placed spore side down onto the potato tissue, and each Petri dish was sealed with parafilm. As a control, an agar plug was also excised from a sterile plate and placed on the potato tissue. The discs were incubated in the dark at room temperature for 7 days.
Radish seedling assay
Radish seeds were surface sterilized [5 min in 70% (v/v) ethanol, followed by 10 min in 15% (v/v) bleach (Chlorox) and then several washes with sterile water) and then transferred to a moist lined Petri dish, sealed with parafilm and incubated in the dark at room temperature for 40 h to germinate. Seedlings were selected that displayed a similar level of germination, and were transferred to wells in 1.5% water agar plates. Freshly prepared S. scabies spore suspensions were diluted in sterile water to give an optical density at 600 nm (OD600) of 0.5, and 200 μL of this suspension was used to inoculate the radish seedlings. Petri dishes were sealed with parafilm and incubated at 21 ± 2 °C with a 16‐h photoperiod for 6 days.
Tobacco assay
Nicotiana tabacum seeds were surface sterilized by exposure to chlorine gas inside a sealed chamber for 3 h; 1 mL of 0.1% phytagar (Sigma‐Aldrich, St Louis, MO, USA) was then added to the seeds, which were stored at 4 °C in the dark for 2–5 days prior to sowing onto Murashige and Skoog medium supplemented with 1% sucrose and 0.8% agar in sterile magenta boxes. These were incubated at 21 ± 2 °C with a 16‐h photoperiod for 7 days; the surface of the agar in each box was then flooded with approximately 1 mL of sterile water containing 5 × 107 spores from a pre‐enumerated spore stock of S. scabies. Plants were incubated at 21 ± 2 °C with a 16‐h photoperiod, and disease progression was monitored over a period of 9 weeks.
Supporting information
Fig. S1 Alignment of EsxA and EsxB proteins from Gram‐positive bacteria. EsxA and EsxB proteins from Mycobacterium tuberculosis, Staphylococcus aureus and Streptomyces coelicolor were aligned with SCAB58671 (Streptomyces scabies EsxA) and SCAB58681 (S. scabies EsxB) using clustalw2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Fig. S2 Southern blot analysis of Streptomyces scabies strains JKF1, JKF2, JKF3 and JKF4 to confirm the chromosomal deletion of genes at the type VII secretion system (T7SS) gene cluster. Chromosomal DNA and DNA probe preparation and Southern blotting were carried out as described in Experimental procedures. (A) SalI‐digested DNA from chromosomal DNA from S. scabies 87.22 (wild‐type, WT) and JKF1 (ΔeccC::Apra) was probed with a DNA probe that hybridizes upstream of eccC; (B) SalI‐digested chromosomal DNA from S. scabies 87.22 (WT) and JKF2 (ΔeccD::Apra) was probed with a DNA probe that hybridizes upstream of eccD; (C) SalI‐digested chromosomal DNA from S. scabies 87.22 (WT) and JKF3 (ΔesxB::Apra) was probed with a DNA probe that hybridizes upstream of esxB; (D) MluI‐digested chromosomal DNA from S. scabies 87.22 (WT) and JKF4 (ΔesxA::Apra) was probed with a DNA probe that hybridizes upstream of esxA. The expected sizes of the hybridizing DNA bands for the wild‐type and mutant strains are indicated in (E).
Table S1 Oligonucleotides used in this study. Restriction sites are underlined.
Acknowledgements
JKF was funded by a joint University of Dundee/James Hutton Institute PhD studentship. Drs David Widdick, Martin Zoltner, Beata Ruban‐Osmialowska, Madhumita Joshi and Isolde Francis are thanked for helpful discussions and advice, Martin Kierans for assistance with scanning electron microscopy, Kent Loeffler for the photography of plants and Drs Paul Herron and Emma Bell for providing bacteriophages ϕC31 and ϕR4. The UK Society for General Microbiology and the British Society for Plant Pathology are thanked for supporting this research through funding visits of JKF to RL's laboratory.
References
- Akpe San Roman, S. , Facey, P.D. , Fernandez‐Martinez, L. , Rodriguez, C. , Vallin, C. , Del Sol, R. and Dyson, P. (2010) A heterodimer of EsxA and EsxB is involved in sporulation and is secreted by a type VII secretion system in Streptomyces coelicolor . Microbiology, 156, 1719–1729. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Martinez, C.E. and Christie, P.J. (2009) Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73, 775–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell, D.R. , Seipke, R.F. , Huguet‐Tapia, J.C. , Chambers, A.H. , Parry, R.J. and Loria, R. (2010) Streptomyces scabies 87‐22 contains a coronafacic acid‐like biosynthetic cluster that contributes to plant–microbe interactions. Mol. Plant–Microbe Interact. 23, 161–175. [DOI] [PubMed] [Google Scholar]
- Bischoff, V. , Cookson, S.J. , Wu, S. and Scheible, W.R. (2009) Thaxtomin A affects CESA‐complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J. Exp. Bot. 60, 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter, W. , Houben, E.N. , Bottai, D. , Brodin, P. , Brown, E.J. , Cox, J.S. , Derbyshire, K. , Fortune, S.M. , Gao, L.Y. , Liu, J. , Gey van Pittius, N.C. , Pym, A.S. , Rubin, E.J. , Sherman, D.R. , Cole, S.T. and Brosch, R. (2009) Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 5, e1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin, P. , Majlessi, L. , de Marsollier, L., Jonge, M.I. , Bottai, D. , Demangel, C. , Hinds, J. , Neyrolles, O. , Butcher, P.D. , Leclerc, C. , Cole, S.T. and Brosch, R. (2006) Dissection of ESAT‐6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 74, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhalid, R.A. and Loria, R. (1997) Cloning and expression of a gene from Streptomyces scabies encoding a putative pathogenicity factor. J. Bacteriol. 179, 7776–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burts, M.L. , Williams, W.A. , DeBord, K. and Missiakas, D.M. (2005) EsxA and EsxB are secreted by an ESAT‐6‐like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc. Natl. Acad. Sci. USA, 102, 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion, P.A. , Stanley, S.A. , Champion, M.M. , Brown, E.J. and Cox, J.S. (2006) C‐terminal signal sequence promotes virulence factor secretion in. Mycobacterium tuberculosis. Science, 313, 1632–1636. [DOI] [PubMed] [Google Scholar]
- Chater, K.F. and Carter, A.T. (1979) A new, wide host‐range, temperate bacteriophage (R4) of Streptomyces and its interaction with some restriction‐modification systems. J. Gen. Microbiol. 115, 431–442. [Google Scholar]
- Chater, K.F. , Biro, S. , Lee, K.J. , Palmer, T. and Schrempf, H. (2010) The complex extracellular biology of Streptomyces . FEMS Microbiol. Rev. 34, 171–198. [DOI] [PubMed] [Google Scholar]
- Coros, A. , Callahan, B. , Battaglioli, E. and Derbyshire, K.M. (2008) The specialized secretory apparatus ESX‐1 is essential for DNA transfer in Mycobacterium smegmatis . Mol. Microbiol. 69, 794–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvaux, M. , Hebraud, M. , Talon, R. and Henderson, I.R. (2009) Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 17, 139–145. [DOI] [PubMed] [Google Scholar]
- Dowding, J.E. (1973) Characterization of a bacteriophage virulent for Streptomyces coelicolor A3(2). J. Gen. Microbiol. 76, 163–176. [DOI] [PubMed] [Google Scholar]
- Flardh, K. and Buttner, M.J. (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 7, 36–49. [DOI] [PubMed] [Google Scholar]
- Flint, J.L. , Kowalski, J.C. , Karnati, P.K. and Derbyshire, K.M. (2004) The RD1 virulence locus of Mycobacterium tuberculosis regulates DNA transfer in Mycobacterium smegmatis . Proc. Natl. Acad. Sci. USA, 101, 12 598–12 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garufi, G. , Butler, E. and Missiakas, D. (2008) ESAT‐6‐like protein secretion in Bacillus anthracis . J. Bacteriol. 190, 7004–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust, B. , Challis, G.L. , Fowler, K. , Kieser, T. and Chater, K.F. (2003) PCR‐targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA, 100, 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, G. , Frazer, C.M. , Gardner, D.C.J. , Cullum, J.A. and Oliver, S.G. (1989) Dispersed growth of Streptomyces in liquid culture. Appl. Microbiol. Biotechnol. 31, 272–277. [Google Scholar]
- Hopwood, D.A. (1967) Genetic analysis and genome structure in Streptomyces coelicolor . Bacteriol. Rev. 31, 373–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, T. , Hingley‐Wilson, S.M. , Chen, B. , Chen, M. , Dai, A.Z. , Morin, P.M. , Marks, C.B. , Padiyar, J. , Goulding, C. , Gingery, M. , Eisenberg, D. , Russell, R.G. , Derrick, S.C. , Collins, F.M. , Morris, S.L. , King, C.H. and Jacobs, W.R., Jr (2003) The primary mechanism of attenuation of bacillus Calmette–Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA, 100, 12 420–12 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, E.G. , Joshi, M.V. , Gibson, D.M. and Loria, R. (2007) Cello‐oligosaccharides released from host plants induce pathogenicity in scab‐causing Streptomyces species. Physiol. Mol. Plant Pathol. 71, 18–25. [Google Scholar]
- Joshi, M. , Rong, X. , Moll, S. , Kers, J. , Franco, C. and Loria, R. (2007a) Streptomyces turgidiscabies secretes a novel virulence protein, Nec1, which facilitates infection. Mol. Plant–Microbe Interact. 20, 599–608. [DOI] [PubMed] [Google Scholar]
- Joshi, M.V. , Bignell, D.R. , Johnson, E.G. , Sparks, J.P. , Gibson, D.M. and Loria, R. (2007b) The AraC/XylS regulator TxtR modulates thaxtomin biosynthesis and virulence in Streptomyces scabies . Mol. Microbiol. 66, 633–642. [DOI] [PubMed] [Google Scholar]
- Joshi, M.V. , Mann, S.G. , Antelmann, H. , Widdick, D.A. , Fyans, J.K. , Chandra, G. , Hutchings, M.I. , Toth, I. , Hecker, M. , Loria, R. and Palmer, T. (2010) The twin arginine protein transport pathway exports multiple virulence proteins in the plant pathogen Streptomyces scabies . Mol. Microbiol. 77, 252–271. [DOI] [PubMed] [Google Scholar]
- Kelley, L.A. and Sternberg, M.J. (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371. [DOI] [PubMed] [Google Scholar]
- Kieser, T. , Bibb, M.J. , Buttner, M.J. , Chater, K.F. and Hopwood, D.A. (2000) Practical Streptomyces Genetics. Norwich, Norfolk: The John Innes Foundation. [Google Scholar]
- Lindeberg, M. , Cunnac, S. and Collmer, A. (2009) The evolution of Pseudomonas syringae host specificity and type III effector repertoires. Mol. Plant Pathol. 10, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria, R. , Bukhalid, R.A. , Creath, R.A. , Leiner, R.H. , Olivier, M. and Steffens, J.C. (1995) Differential production of thaxtomins by pathogenic Streptomyces species in vitro. Phytopathology, 85, 537–541. [Google Scholar]
- Loria, R. , Kers, J. and Joshi, M. (2006) Evolution of plant pathogenicity in Streptomyces . Annu. Rev. Phytopathol. 44, 469–487. [DOI] [PubMed] [Google Scholar]
- MacNeil, D.J. , Gewain, K.M. , Ruby, C.L. , Dezeny, G. , Gibbons, P.H. and MacNeil, T. (1992) Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene, 111, 61–68. [DOI] [PubMed] [Google Scholar]
- Muller, C. , Nolden, S. , Gebhardt, P. , Heinzelmann, E. , Lange, C. , Puk, O. , Welzel, K. , Wohlleben, W. and Schwartz, D. (2007) Sequencing and analysis of the biosynthetic gene cluster of the lipopeptide antibiotic Friulimicin in Actinoplanes friuliensis . Antimicrob. Agents Chemother. 51, 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi, M. , Suzuki, K. and Umezawa, H. (1974) Formation and reversion of Streptomycete protoplasts: cultural condition and morphological study. J. Gen. Microbiol. 80, 389–400. [DOI] [PubMed] [Google Scholar]
- Palmer, T. and Hutchings, M.I. (2010) Protein secretion in Streptomyces In: Streptomyces Molecular Biology and Biotechnology (Dyson P.J., ed.), pp. 87–104. Wymondham: Caister Academic Press. [Google Scholar]
- Pym, A.S. , Brodin, P. , Majlessi, L. , Brosch, R. , Demangel, C. , Williams, A. , Griffiths, K.E. , Marchal, G. , Leclerc, C. and Cole, S.T. (2003) Recombinant BCG exporting ESAT‐6 confers enhanced protection against tuberculosis. Nat. Med. 9, 533–539. [DOI] [PubMed] [Google Scholar]
- Raghavan, S. , Manzanillo, P. , Chan, K. , Dovey, C. and Cox, J.S. (2008) Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature, 454, 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw, P.S. , Lightbody, K.L. , Veverka, V. , Muskett, F.W. , Kelly, G. , Frenkiel, T.A. , Gordon, S.V. , Hewinson, R.G. , Burke, B. , Norman, J. , Williamson, R.A. and Carr, M.D. (2005) Structure and function of the complex formed by the tuberculosis virulence factors CFP‐10 and ESAT‐6. EMBO J. 24, 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigel, N.W. and Braunstein, M. (2008) A new twist on an old pathway—accessory Sec [corrected] systems. Mol. Microbiol. 69, 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sao‐Jose, C. , Baptista, C. and Santos, M.A. (2004) Bacillus subtilis operon encoding a membrane receptor for bacteriophage SPP1. J. Bacteriol. 186, 8337–8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sao‐Jose, C. , Lhuillier, S. , Lurz, R. , Melki, R. , Lepault, J. , Santos, M.A. and Tavares, P. (2006) The ectodomain of the viral receptor YueB forms a fiber that triggers ejection of bacteriophage SPP1 DNA. J. Biol. Chem. 281, 11464–11470. [DOI] [PubMed] [Google Scholar]
- Scheible, W.R. , Fry, B. , Kochevenko, A. , Schindelasch, D. , Zimmerli, L. , Somerville, S. , Loria, R. and Somerville, C.R. (2003) An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell, 15, 1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbald, M.J. , Ziebandt, A.K. , Engelmann, S. , de Hecker, M., Jong, A. , Harmsen, H.J. , Raangs, G.C. , Stokroos, I. , Arends, J.P. , van Dubois, J.Y. and Dijl, J.M. (2006) Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol. Mol. Biol. Rev. 70, 755–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, S.A. , Raghavan, S. , Hwang, W.W. and Cox, J.S. (2003) Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. USA, 100, 13 001–13 006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy, R. , Fyfe, P.K. and Hunter, W.N. (2008) Structure of Staphylococcus aureus EsxA suggests a contribution to virulence by action as a transport chaperone and/or adaptor protein. J. Mol. Biol. 383, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way, S.S. and Wilson, C.B. (2005) The Mycobacterium tuberculosis ESAT‐6 homologue in Listeria monocytogenes is dispensable for growth in vitro and in vivo. Infect. Immun. 73, 6151–6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdick, D.A. , Dilks, K. , Chandra, G. , Bottrill, A. , Naldrett, M. , Pohlschroder, M. and Palmer, T. (2006) The twin‐arginine translocation pathway is a major route of protein export in Streptomyces coelicolor . Proc. Natl. Acad. Sci. USA, 103, 17 927–17 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Alignment of EsxA and EsxB proteins from Gram‐positive bacteria. EsxA and EsxB proteins from Mycobacterium tuberculosis, Staphylococcus aureus and Streptomyces coelicolor were aligned with SCAB58671 (Streptomyces scabies EsxA) and SCAB58681 (S. scabies EsxB) using clustalw2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Fig. S2 Southern blot analysis of Streptomyces scabies strains JKF1, JKF2, JKF3 and JKF4 to confirm the chromosomal deletion of genes at the type VII secretion system (T7SS) gene cluster. Chromosomal DNA and DNA probe preparation and Southern blotting were carried out as described in Experimental procedures. (A) SalI‐digested DNA from chromosomal DNA from S. scabies 87.22 (wild‐type, WT) and JKF1 (ΔeccC::Apra) was probed with a DNA probe that hybridizes upstream of eccC; (B) SalI‐digested chromosomal DNA from S. scabies 87.22 (WT) and JKF2 (ΔeccD::Apra) was probed with a DNA probe that hybridizes upstream of eccD; (C) SalI‐digested chromosomal DNA from S. scabies 87.22 (WT) and JKF3 (ΔesxB::Apra) was probed with a DNA probe that hybridizes upstream of esxB; (D) MluI‐digested chromosomal DNA from S. scabies 87.22 (WT) and JKF4 (ΔesxA::Apra) was probed with a DNA probe that hybridizes upstream of esxA. The expected sizes of the hybridizing DNA bands for the wild‐type and mutant strains are indicated in (E).
Table S1 Oligonucleotides used in this study. Restriction sites are underlined.


