Abstract
Dysfunction of pulmonary surfactant in the lungs is associated with respiratory pathologies such as acute respiratory distress syndrome or meconium aspiration syndrome. Serum, cholesterol, and meconium have been described as inhibitory agents of surfactant’s interfacial activity once these substances appear in alveolar spaces during lung injury and inflammation. The deleterious action of these agents has been only partly evaluated under physiologically relevant conditions. We have optimized a protocol to assess surfactant inhibition by serum, cholesterol, or meconium in the captive bubble surfactometer. Specific measures of surface activity before and after native surfactant was exposed to inhibitors included i), film formation, ii), readsorption of material from surface-associated reservoirs, and iii), interfacial film dynamics during compression-expansion cycling. Results show that serum creates a steric barrier that impedes surfactant reaching the interface. A mechanical perturbation of this barrier allows native surfactant to compete efficiently with serum to form a highly surface-active film. Exposure of native surfactant to cholesterol or meconium, on the other hand, modifies the compressibility of surfactant films though optimal compressibility properties recover on repetitive compression-expansion cycling. Addition of polymers like dextran or hyaluronic acid to surfactant fully reverses inhibition by serum. These polymers also prevent surfactant inhibition by cholesterol or meconium, suggesting that the protective action of polymers goes beyond the mere enhancement of interfacial adsorption as described by depletion force theories.
Introduction
Pulmonary surfactant is a complex mixture of lipids and proteins lining the alveolar air-water interface (1,2). Inhibition of pulmonary surfactant action is associated with many respiratory diseases, rendering surfactant dysfunctional and affecting lung compliance and gas exchange. Acute respiratory distress syndrome (ARDS) develops after different lung injuries like pneumonia, aspiration of gastric contents, near-drowning, or edema. In ARDS, alveoli are filled with protein-enriched edema fluid, which impairs surfactant action. ARDS causes 60,000 deaths per year in the USA (3,4). Meconium aspiration syndrome also remains an important cause of morbidity and mortality affecting >20,000 neonates per year in the United States. Meconium aspiration syndrome is characterized by airway obstruction, pneumonitis, pulmonary hypertension, acidosis, and hypoxemia (5). Common to these diseases is surfactant inactivation. Studying and understanding the mechanisms of inhibition of surfactant is important not only for understanding the respiratory pathophysiology, but also for developing new strategies for treatment.
Three main functions determine the activity of surfactant preparations and the impact of inhibition. First, surfactant must adsorb quickly (in a few seconds) to reduce interfacial surface tension. Second, surfactant must be efficiently reabsorbed to the air-subphase interface (as occurs during inspiration in alveoli), and finally surfactant must form rigid films on compression of the surface (as occurs during expiration), which exhibit low surface tension. Inhibition of surfactant may impair adsorption (e.g., as with serum) (6–8), or cause perturbation of the composition and structure of surfactant films (e.g., by cholesterol or bile salts) (9–11), which prevents attainment of maximally compressed states.
The surface behavior of pulmonary surfactant and the mechanisms by which it is altered by different inhibitory substances have been analyzed using different approaches, including Langmuir surface balances (12), the pulsating bubble surfactometer (13–15), axisymmetric drop shape analysis in conjunction with constrained sessile drop surfactometry (16), or the captive bubble surfactometer (CBS) (8,17–19). Limitations of these methods prevent study of inhibition under (patho) physiologically relevant conditions that include temperature and humidity, high concentrations of both surfactant and inhibitory substances, and alveolar geometry.
For a variety of reasons, inhibition has been difficult to study in the CBS (9). In this study, we have modified the method of application of the sample to simulate competition between serum proteins and surfactant complexes for the interface (6–8,20). Traditionally, serum has been introduced into the subphase in which the bubble is formed. We have applied undiluted serum onto the surface of the bubble thus allowing serum proteins to form a concentrated film at the interface before the application of surfactant. Surface tension of serum at the interface falls to ≈50 mN/m (21). Although the actual concentration of serum leaked into an injured lung is difficult to establish, testing a layer at high concentrations of serum mimics the most potentially harmful condition. Surfactant is then applied near the surface of the bubble, instead of directly onto the surface of the bubble as it has been done in the past. Therefore, surfactant finds a steric barrier to adsorption, as would happen in vivo after surfactant is freshly secreted by pneumocytes, and must traverse a serum layer in an injured and edematous alveolus to adsorb at the interface. Similarly, exogenous surfactant introduced via the trachea as therapy, must also find its way to the alveolar interface through the leaky, inflamed interior of the lung.
To study meconium/cholesterol inhibition we premixed and incubated each with surfactant to allow these inhibitors to incorporate into surfactant complexes, because the main inhibitory mechanism for both agents is disruption of surfactant structure (8,9). In a lung full of serum or meconium, surfactant may suffer dilution. Therefore, we also studied various surfactant concentrations. For inhibition experiments, we use critical conditions, that is to say, low concentrations of surfactant, which are still functional but susceptible to inhibition. The concentration of surfactant in the subphase of healthy alveoli is estimated at ∼30–100 mg/mL. However, during inflammation, reduced production by damaged epithelia, an accelerated catabolism by lipases and proteases, and dilution by edema, all contribute to a substantial decrease of surfactant concentrations.
By optimizing the CBS technique, we have been able to study and compare inhibition by serum, meconium, and cholesterol, under more restrictive conditions than assessed previously. We have confirmed that serum imposes a steric barrier to surfactant adsorption, which effectively competes with native surfactant to reach the interface. Exposure to cholesterol or meconium alters the compressibility of surfactant films. Surprisingly, addition of polymers such as dextran or hyaluronic acid (HA) prevents surfactant inhibition both by serum, and by meconium or cholesterol despite differing mechanism of inhibition.
Materials and Methods
Materials
Native porcine lung surfactant (NS) was purified from bronchoalveolar lavage by NaBr density gradient centrifugation as previously described (6). Isolated surfactant was used in aqueous suspensions without organic extraction. Native surfactant contains a full complement of surfactant proteins SP-A, SP-B, and SP-C. Its concentration was measured by analysis of lipid phosphorous (23). Surfactant amounts and concentrations are expressed as phospholipid mass, taking 750 Da as the average phospholipid molecular mass. Three different batches of native surfactant were used in different experiments. Dilutions of material were made with 5 mM Tris pH 7 buffer, containing 150 mM NaCl. First passed meconium from term infants was collected, pooled, and lyophilized. Dry meconium was diluted in Tris buffer and mixed with surfactant at a final concentration of 10 mg/ml for both. Water-soluble cholesterol (complexed with methyl-β-cyclodextrine (MβCD)) was obtained from Sigma (C4951, St. Louis, MO) and mixed (at final proportion of 4% cholesterol w/w) with surfactant. We determined that exposure of NS to cholesterol-loaded MβCD increases the proportion of cholesterol from 3.1 ± 0.2% to 5.8 ± 0.8% (w/w), (average of three experiments (10)). Porcine serum was obtained from blood and used at a protein concentration of 100 mg/ml. Hyaluronic acid (HA, 120 k) and dextran (Dex, 148 k) were obtained from Sigma and mixed at final concentrations of 0.25% for HA and 5% dextran (w/v) with surfactant.
Captive bubble surfactometry
Surfactant at 37°C was evaluated as described previously (19,24). The chamber contained 5 mM Tris-HCl pH7, 150 mM NaCl, and 10% sucrose. After a small air bubble (0.035–0.040 cm3) was formed, ∼150 nL of surfactant (10 mg/ml) was deposited below the bubble surface with a transparent capillary. Following the introduction of surfactant, the change in surface tension (γ) was monitored over 5 min from changes in shape of the bubble (17). The chamber was then sealed and the bubble was rapidly (1 s) expanded to 0.15 cm3, to record postexpansion adsorption. 5 min after expansion, quasistatic cycles started, where the bubble size was first reduced (by 20% of its previous volume) and then enlarged in a stepwise fashion. There was 1 min intercycle delay between each of four quasistatic cycles and a further 1 min delay before starting dynamic cycles, in which the bubble was continuously varied at 20 cycles/min. Data from initial and postexpansion adsorption are presented as averages from three experiments, whereas graphs plotting quasistatic (Q-static) and dynamic cycles correspond to single representative experiments. Data from replicates are shown in the tables (see Supporting Material).
To study serum inhibition we first injected serum to form a layer at the air-liquid interface, and then injected surfactant in the subphase. To study meconium or cholesterol inhibition, inhibitor and surfactant were premixed, and then the mixtures were injected into the subphase. We used a concentration of surfactant of 10 mg/ml. In additional experiments, polymers were premixed with surfactant and immediately injected into the subphase as described previously.
Data reproducibility and statistics
Figures represent the mean ± SD after averaging data from three experiments. CBS compression-expansion experiments are shown as representative isotherms. Significance was determined by t-Student test. The Holm-Sidak method was applied for multiple comparisons with a significance level < or = 0.05.
Results
To study changes in biophysical properties when inhibitory agents are added to NS, we modified the usual CBS protocol in three ways: First we tested relatively low concentrations of surfactant, 10 mg/ml phospholipid, applying the sample without contacting with the surface bubble (see images in Fig. 1 a). Second, we injected undiluted serum near the surface of the bubble. As a consequence of the high density of the sucrose-containing subphase, the undiluted serum forms a layer of concentrated material surrounding the bubble. This can be observed when serum is doped with a trace of fluorescently labeled albumin (see image in Fig. 1 b).
Figure 1.

CBS model to test surfactant inhibition by serum. (a) Sequential images of a surfactant sample immediately before (t = 0) and at different times after injection underneath an air bubble at the CBS. (b) Fluorescence image of a serum sample doped with a trace of rhodamine-labeled albumin, injected underneath the bubble at the CBS.
Fig. 2 shows the surface activity of a low concentration of uninhibited NS. Surface tension decreased to 22.1 ± 1.2 mN/m after 5 min of initial adsorption, and remained at 22.9 ± 1.1 mN/m after expanding the bubble for 5 min. Initial adsorption occurs within a few seconds (as described by Schürch et al.(19)). Fig. 2 also shows how serum (2 μl) applied near the surface of the bubble decreases surface tension to 45.4 ± 2.4 mN/m after 5 min of initial adsorption and to 49.5 ± 2.3 mN/m after 5 min the expansion of the bubble (7).
Figure 2.

Interfacial adsorption of native surfactant in the absence or presence of serum. Initial (left panel) and post-expansion (right panel) adsorption kinetics of surfactant (NS) (black dots), serum alone (white dots), NS 10 mg/ml applied underneath the bubble surface coated with a preformed serum layer (triangles), and NS 20 mg/ml applied underneath the bubble surface coated with serum (squares), all at 37°C. Data are mean ± SD after averaging data from three experiments.
When we applied NS (10 mg/ml) underneath the preformed serum film, without touching the bubble surface, no adsorption of NS occurred (surface tension remained 39.8 ± 3.3 mN/m, significantly higher than that reached by NS in the absence of serum). After expansion, the minimal surface tension after 5 min also remained unchanged 43.9 ± 1.5 mN/m (Fig. 2). Fig. 2 also shows that a concentration of surfactant of 20 mg/ml is not inhibited by serum. In other experiments, surfactant at 10 mg/ml was applied directly into the bubble surface, thus breaking through the serum protein layer, resulting in equilibrium surface tensions similar to noninhibited surfactant (not shown).
Fig. 3 summarizes compression-expansion isotherms of surfactant films formed in the absence or presence of serum. We have compared isotherms obtained at both slow (Q-static) and fast (Dynamic, physiological-like), cycling regimes. Q-static cycling provides details on the intrinsic compressibility properties of the films as they are formed at the interface, including their potential to undergo compression-driven reorganizations or to reach very low surface tension under conditions permitting local or long-range relaxation. Dynamic cycling mimics physiological-like compression-expansion rates and allow testing surface properties of films far from equilibrium but close to breathing conditions. In the absence of serum, NS exhibits well-characterized behavior, with the first quasistatic cycle showing greater hysteresis than the subsequent cycles. Thus, at the end of the fourth quasistatic cycle surface tension is 1.6 ± 0.6 mN/m after area compression of ∼20% (see Table S1 in the Supporting Material). Reorganization of material during Q-static compression is something we were not able to study at high surfactant concentrations, 25 or 72 mg/ml (17,19). Dynamic cycles show normal function of surfactant in the absence of serum, with very low surface tension (1.8 ± 0.7 mN/m) with less than a 20% area reduction. In Fig. 3, the surface tension of a pure serum layer under Q-static and dynamic conditions is never lower than 37.8 ± 2.4 mN/m. NS (10 mg/mL) applied just below the surface of the bubble, underneath a preformed serum layer, did not lower surface tension below 21.7 ± 1.6 mN/m (Q-static) or 17.1 ± 6.0 mN/m (dynamic cycles), even after as much as a 50% reduction of surface area. These values were always significantly higher than the surface tension reached by NS applied in the same conditions in the absence of serum. When we doubled the concentration of surfactant, the film was still not able to reduce surface tension below 20 mN/m under slow quasi-static compression-expansion cycling. However, if cycled quickly (20 cycles/min), films made by concentrated surfactant reduced tension below 10 mN/m during the first compression and reached minimal tensions below 5 mN/m with very little compression in all the subsequent cycles. This means that surfactant at concentrations ≥20 mg/mL is only partly inhibited by undiluted serum when rapid compression-expansion is imposed. We therefore used 10 mg/ml as an appropriate critical surfactant concentration to study inhibition in the following experiments.
Figure 3.

Compression-expansion isotherms of native surfactant films in the absence or presence of serum. Q-static (upper panels) and dynamic (lower panels) compression/expansion isotherms from surfactant films formed upon injection of NS at 10 or 20 mg/mL phospholipid, in the absence or in the presence of a preformed serum layer. A representative experiment is shown here after repeating three independent experiments with each sample.
Figs. 4–6 show a summary of the inhibitory effect of serum, meconium, or cholesterol on the surface behavior of surfactant and the prevention of this inhibition by preexposure of surfactant to polymers such as HA (negatively charged) or dextran (noncharged). Panel a of Fig. 4 illustrates how the presence of a preformed serum layer at the bubble surface does not allow surfactant to reach the interface, because the surface tension reached upon surfactant injection is not lower than 40 mN/m. Application of surfactant premixed with the polymers (HA 0.25% w/v or dextran 0.5% w/v) restores the ability of surfactant to reach the interface and lower surface tension to 22–23 mN/m. Addition of the polymers by themselves does not affect interfacial adsorption of NS, as surfactant premixed with polymers had surface tensions that were not significantly different from the tensions reached by NS alone. The polymers tested do not possess surface activity by themselves, because the introduction of the polymers alone in the subphase does not produce changes in the surface tension of the saline solution, either in the CBS or as tested in Langmuir troughs (not shown). To test inhibition of adsorption by meconium or cholesterol, we premixed each with NS, before applying the mixture near the surface of the bubble. Fig. 4 (panel b) also illustrates that the equilibrium surface tension reached upon adsorption, either initially or after bubble expansion of meconium- or cholesterol-pretreated surfactant, was not different from that of NS alone. It can be noticed that the surface tension reached upon 5 min of expansion of the bubble coated with NS premixed with meconium is apparently higher, but with a rather large error as a consequence of high heterogeneity in the behavior of replicas, likely associated with the intrinsic complexity and heterogeneity of meconium. Fig. 5 summarizes quasistatic cycling of NS in the absence or in the presence of serum, meconium, or cholesterol, with or without premixing with dextran or HA. Q-static cycling of NS in the presence of a serum film (2 μl, 100 mg/ml total protein) was highly defective, with surface tension decaying only to 37.9 ± 2.4 mN/m after ∼50% of area compression (see also Table S2). Addition of 4% (w/w) cholesterol to NS, either as taking part of meconium or solubilized by MβCD, produced a similar deleterious effect on the behavior of NS complexes, which required an area compression of 50% to reach surface tension of only around 20 mN/m (19.6 ± 0.8 mN/m for meconium- and 20.2 ± 0.6 mN/m for cholesterol-MβCD-treated surfactant; see Table S3). The inhibitory effect of the exposure of surfactant to cholesterol-loaded MβCD is clearly due to the increase in the proportion of cholesterol produced in surfactant membranes, from 3% to around 6% with respect to phospholipid (see above), because exposure of surfactant to cholesterol-free cyclodextrine does not produce any effect on Q-static or dynamic compression-expansion isotherms (see Fig. S1). Addition of dextran or HA totally restored the behavior of NS films during Q-static compression-expansion cycling: these reached very low tensions with only ∼20% area compression even in the presence of serum, meconium, or cholesterol.
Figure 4.

Interfacial adsorption of native surfactant in the absence or presence of inhibitors and polymers. (a) Minimal surface tension upon 5 min of initial (black bars) or post-expansion (gray bars) adsorption of a native surfactant sample combined or not with the indicated polymers, injected under a clean or a serum-coated bubble. (b) Minimal surface tension upon initial or post-expansion adsorption of surfactant in the absence or presence of meconium, cholesterol, and/or polymers. Data are mean ± SD after averaging three independent experiments from each sample.
Figure 5.

Quasi-static compression-expansion cycling isotherms for surfactant injected under the bubble at the CBS, in the absence or in the presence of serum, meconium, or cholesterol, combined or not with HA or dextran. A representative experiment is shown in each panel, after three repetitions from each sample.
Figure 6.
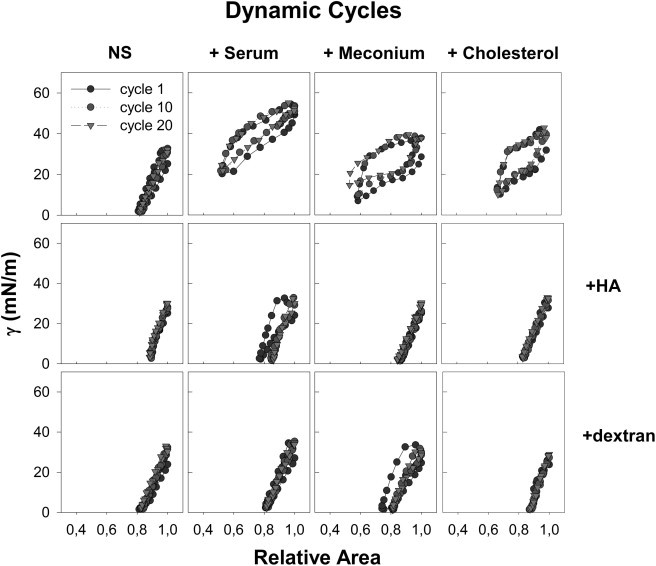
Dynamic compression-expansion cycling isotherms for surfactant injected under the bubble at the CBS, in the absence or in the presence of serum, meconium, or cholesterol, combined or not with HA or dextran. A representative experiment is shown in each panel, after three repetitions from each sample.
Fig. 6 confirms the differences in dynamic cycling between inhibited and polymer/surfactant samples. Presence of a serum film resulted in NS reaching minimal surface tension under dynamic cycling of 17.0 ± 6.0 mN/m, with an area reduction of 50%. Addition of 4% cholesterol (w/w) contained in 10 mg/ml of meconium resulted in minimal surface tension of 18.3 ± 3.7 mN/m (area compression 50%) and 4% cholesterol solubilized by MβCD allowed NS to reach 7.2 ± 4.0 mN/m (area compression 30%). Despite the presence of the inhibitory agents, the exposure of NS to either HA or dextran prevented the inhibitory effects observed under dynamic cycling, resulting in NS films that were completely functional, with parameters that were similar to those exhibited in the absence of inhibitors. The films were able to reach the lowest surface tensions, statistically indistinguishable than those reached in the absence of inhibitors, with practically no hysteresis, needing only ∼20% of area compression.
Discussion
In this study, we modified CBS protocols to study inhibition of surfactant by several substances relevant to human lung injury. Furthermore, we have documented the remarkable ability of ionic (HA) and nonionic (dextran) polymers to reverse not only serum inhibition of surfactant, but also inhibition by meconium or cholesterol, two substances that inhibit surface activity by mechanisms that differ from serum.
The ability of surfactant to reach the interface in the presence of inhibitors depends on surfactant concentration. NS is highly surface active in very low concentrations (10 mg/mL) and is resistant to inhibition at phospholipid concentrations ≥20 mg/mL. In vivo, after lung injury, relative concentrations of serum and other inflammatory inhibitors and surfactant are difficult to ascertain and vary over the course of the disease. Lung injury and concomitant edema is associated not only with leakage of serum, lipoproteins, cholesterol, and other inhibitory agents into alveoli but also produces an effective dilution of the surfactant, which could be further reduced by impairment in synthesis/secretion of new surfactant due to epithelium damage.
Our data suggest that a decrease in concentrations of surfactant below a certain threshold permits inhibition. Therefore, one therapeutic strategy is to overcome inhibition associated with lung injury by the introduction of exogenous surfactant. However, previous work has shown that current clinical surfactants are substantially more susceptible to inhibition than NS (6).
Our model confirms that serum can form an interfacial barrier composed of serum proteins that impedes proper adsorption of surfactant. This explains why minimal tensions achieved by diluted surfactant are not much lower than surface tensions produced by pure serum layers. Surfactant fails to reach an interface that is previously occupied by a concentrated layer of surface-active serum proteins. Our results show that a brief disruption of the serum layer caused by injecting surfactant at the bubble surface can be enough to give surfactant the opportunity to compete with serum components for adsorption into a newly opened interface, thereby displacing serum proteins to reach an (uninhibited) low tension. The concept of competitive adsorption of serum and surfactant has been developed in the inhibition models proposed by the Zasadzinski group (7,25–27). We show here that the mere mechanical disruption of the inhibitory layer can also be a crucial contribution to overcome surfactant inactivation provided that surfactant is active enough to compete efficiently with the less surface active but much more concentrated serum components.
The addition of nonionic or anionic polymers such as dextran or HA can improve substantially the resistance of surfactant to inactivation. This activity of polymers had been extensively documented (6,15,28–30), but not under the restrictive conditions imposed in our assay. The action of polymers has been explained by models that invoke polymer-created depletion forces that push the large surfactant complexes against the interface, thereby overcoming the steric and electrostatic barrier imposed by serum layers (6–8). Still, it is difficult to envision how depletion forces can propel surfactant, injected far—in molecular terms—below the interface, to cross the concentrated layer of serum to reach the interface where surface-active species are finally transferred. The polymers in our experiments are restricted to the small volume of injected surfactant. After injection of surfactant/polymer combinations, the polymers are likely homogenously distributed and diluted into the thin layer of fluid constituting the subphase, without a particular accumulation at the interface, because injection of equivalent amounts of polymer alone does not produce apparent effects on surface tension. These conditions are different from previously published work. In our experiments the polymers are not included in the subphase, where in principle they are diluted to concentrations much lower than those required to promote depletion forces shortly after injection of the surfactant/polymer mixtures. The ability of the polymers to allow surfactant to successfully cross the serum layer and adsorb at the interface is remarkable. Preliminary experiments using a quartz-microbalance model (31) discarded binding of HA to surfactant complexes (not shown), indicating that the effect of HA to promote interfacial adsorption in the presence of the serum layer is not due to the formation of polymer/surfactant complexes with particularly favorable surface properties. It remains to be determined whether the establishment of interactions between negatively charged polymers, such as HA, and cationic protein components in serum, could play a role in counteracting serum-promoted surfactant inhibition. However, this possibility does not seem likely because i), a nonionic polymer like dextran seems to produce qualitatively similar effects, and ii), the total amount of polymer introduced into the surfactant/polymer combinations of our experiments is orders of magnitude smaller than the amount of serum proteins in the surface layer.
One would not have predicted that the addition of polymers to surfactant mixtures would have a similar beneficial effect after inhibition by meconium or cholesterol, which acts by mechanisms that differ from true competitive adsorption by serum proteins (9,10,32). These agents, meconium and cholesterol, do not cause strong inhibition of interfacial adsorption of surfactant, but rather cause a substantial modification of its compression-expansion properties. This can be explained by insertion of the excess of cholesterol into surfactant membranes and films, and a concomitant reduction of their compressibility. Cholesterol, solubilized either by bile salts (as in meconium) or by MβCD, is transferred into surfactant films rendering them dysfunctional during Q-static and dynamic cycles. It seems that altered films experiment early collapse and do not lower surface tension as a result of the incorporation of 4% w/w cholesterol. Even though this is a relatively small amount of cholesterol (33), it seems that in a soluble state (in complexes with bile salts or MβCD) cholesterol has a particularly notable deleterious effect.
Exposure to the polymers counteracts the perturbation of rheological properties of surfactant caused by incorporation of excess cholesterol. It is conceivable that a potential depletion force-originated compaction of membrane structures, with creation of extensive membrane-membrane interactions, promotes a highly cohesive multilayered structure that compensates for a loss of rigidity caused by an excess of cholesterol-promoted fluidification. We have shown that exposure to polymers such as dextran or HA do in fact create compaction of surfactant structures and a generation of membrane-based networks by different surfactant preparations (34). We cannot discard the possibility that HA would also interact with surfactant membranes and/or films providing a surplus of mechanical stability compensating the likely higher deformability of cholesterol-enriched phases. The reversal or prevention of surfactant inhibition by polymers has been extensively documented both in vitro (25,30,35–37) and in vivo (35,38–40). The new, to our knowledge, approach described here demonstrates that certain polymers help NS overcome not only the inactivating effect of serum, but also that of meconium and cholesterol. Thus, the reversion mechanism is general for NS and not specific for inhibitory agents that we tested. We are not aware of previous data showing that polymers not only promote interfacial adsorption but also restore compressibility of surfactant films during compression-expansion cycling in the presence of inhibitors. Further studies are required to analyze in more detail the ways in which polymers may structure and organize surfactant membranes and films, particularly when surfactant has been pathologically altered. We speculate that exposure to polymers like dextran or HA may induce an activation of surfactant by modifying its structure so that it is much more resistant to different types of inhibition and has better surface properties in terms of both better interfacial adsorption and improved rheological properties to sustain high pressures without collapsing. If this is the case, addition or treatment with polymers may produce new clinical surfactants for lung injuries for which there is as yet not good therapy.
In summary, to our knowledge, the novel conditions of our experiments may mirror the introduction of therapeutic surfactant into edematous alveoli that are replete with inflammatory inhibitors after acute lung injuries. Our results affirm the potential use of certain polymers as useful additives to therapeutic surfactants.
Acknowledgments
This research was supported by grants from the Spanish Ministry of Science (BIO2009-09694, BIO2012-30733, CSD2007-00010), Madrid Regional Government (S0505/MAT/0283), and National Institutes of Health (NIH) (HLBI RO1 HL 66410).
Supporting Material
References
- 1.Pérez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim. Biophys. Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Serrano A.G., Pérez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem. Phys. Lipids. 2006;141:105–118. doi: 10.1016/j.chemphyslip.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Taeusch H.W. Treatment of acute (Adult) respiratory distress syndrome. The holy grail of surfactant therapy. Biol. Neonate. 2000;77(Suppl 1):2–8. doi: 10.1159/000047050. [DOI] [PubMed] [Google Scholar]
- 4.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Nkadi P.O., Merritt T.A., Pillers D.A. An overview of pulmonary surfactant in the neonate: genetics, metabolism, and the role of surfactant in health and disease. Mol. Genet. Metab. 2009;97:95–101. doi: 10.1016/j.ymgme.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taeusch H.W., Bernardino de la Serna J., Zasadzinski J.A. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys. J. 2005;89:1769–1779. doi: 10.1529/biophysj.105.062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zasadzinski J.A., Alig T.F., Taeusch H.W. Inhibition of pulmonary surfactant adsorption by serum and the mechanisms of reversal by hydrophilic polymers: theory. Biophys. J. 2005;89:1621–1629. doi: 10.1529/biophysj.105.062646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo Y.Y., Veldhuizen R.A., Possmayer F. Current perspectives in pulmonary surfactant—inhibition, enhancement and evaluation. Biochim. Biophys. Acta. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Gunasekara L., Schoel W.M., Amrein M.W. A comparative study of mechanisms of surfactant inhibition. Biochim. Biophys. Acta. 2008;1778:433–444. doi: 10.1016/j.bbamem.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Rodriguez E., Echaide M., Perez-Gil J. Meconium impairs pulmonary surfactant by a combined action of cholesterol and bile acids. Biophys. J. 2011;100:646–655. doi: 10.1016/j.bpj.2010.12.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vockeroth D., Gunasekara L., Veldhuizen R.A. Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L117–L125. doi: 10.1152/ajplung.00218.2009. [DOI] [PubMed] [Google Scholar]
- 12.Wüstneck R., Perez-Gil J., Pison U. Interfacial properties of pulmonary surfactant layers. Adv. Colloid Interface Sci. 2005;117:33–58. doi: 10.1016/j.cis.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Hall S.B., Lu R.Z., Notter R.H. Inhibition of pulmonary surfactant by oleic acid: mechanisms and characteristics. J. Appl. Physiol. 1992;72:1708–1716. doi: 10.1152/jappl.1992.72.5.1708. [DOI] [PubMed] [Google Scholar]
- 14.Holm B.A., Wang Z., Notter R.H. Multiple mechanisms of lung surfactant inhibition. Pediatr. Res. 1999;46:85–93. doi: 10.1203/00006450-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Lu K.W., Goerke J., Taeusch H.W. Hyaluronan reduces surfactant inhibition and improves rat lung function after meconium injury. Pediatr. Res. 2005;58:206–210. doi: 10.1203/01.PDR.0000169981.06266.3E. [DOI] [PubMed] [Google Scholar]
- 16.Saad S.M., Policova Z., Neumann A.W. A double injection ADSA-CSD methodology for lung surfactant inhibition and reversal studies. Colloids Surf. B Biointerfaces. 2009;73:365–375. doi: 10.1016/j.colsurfb.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Schoel W.M., Schürch S., Goerke J. The captive bubble method for the evaluation of pulmonary surfactant: surface tension, area, and volume calculations. Biochim. Biophys. Acta. 1994;1200:281–290. doi: 10.1016/0304-4165(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 18.Schürch S., Bachofen H., Possmayer F. A captive bubble method reproduces the in situ behavior of lung surfactant monolayers. J. Appl. Physiol. 1989;67:2389–2396. doi: 10.1152/jappl.1989.67.6.2389. [DOI] [PubMed] [Google Scholar]
- 19.Schürch S., Green F.H.Y., Bachofen H. Formation and structure of surface films: captive bubble surfactometry. Biochim. Biophys. Acta. 1998;1408:180–202. doi: 10.1016/s0925-4439(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 20.Nag K., Vidyashankar S., Panda A.K. Physicochemical studies on the interaction of serum albumin with pulmonary surfactant extract in films and bulk bilayer phase. J. Colloid Interface Sci. 2010;352:456–464. doi: 10.1016/j.jcis.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Stenger P.C., Isbell S.G., Zasadzinski J.A. Rediscovering the Schulze-Hardy rule in competitive adsorption to an air-water interface. Langmuir. 2009;25:10045–10050. doi: 10.1021/la9009724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reference deleted in proof.
- 23.Rouser G., Siakotos A.N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966;1:85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Gil L., Schürch D., Pérez-Gil J. Pulmonary surfactant protein SP-C counteracts the deleterious effects of cholesterol on the activity of surfactant films under physiologically relevant compression-expansion dynamics. Biophys. J. 2009;97:2736–2745. doi: 10.1016/j.bpj.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenger P.C., Zasadzinski J.A. Enhanced surfactant adsorption via polymer depletion forces: a simple model for reversing surfactant inhibition in acute respiratory distress syndrome. Biophys. J. 2007;92:3–9. doi: 10.1529/biophysj.106.091157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernsler J.G., Zasadzinski J.A. Competitive adsorption: a physical model for lung surfactant inactivation. Langmuir. 2009;25:8131–8143. doi: 10.1021/la8039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zasadzinski J.A., Stenger P.C., Dhar P. Overcoming rapid inactivation of lung surfactant: analogies between competitive adsorption and colloid stability. Biochim. Biophys. Acta. 2010;1798:801–828. doi: 10.1016/j.bbamem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J.R., Su T.J., Penfold J. Adsorption of serum albumins at the air/water interface. Langmuir. 1999;15:6975–6983. [Google Scholar]
- 29.Lu K.W., Pérez-Gil J., Taeusch H.W. Pulmonary surfactant proteins and polymer combinations reduce surfactant inhibition by serum. Biochim. Biophys. Acta. 2011;1808:2366–2373. doi: 10.1016/j.bbamem.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taeusch H.W., Dybbro E., Lu K.W. Pulmonary surfactant adsorption is increased by hyaluronan or polyethylene glycol. Colloids Surf. B Biointerfaces. 2008;62:243–249. doi: 10.1016/j.colsurfb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Wolny P.M., Banerji S., Richter R.P. Analysis of CD44-hyaluronan interactions in an artificial membrane system: insights into the distinct binding properties of high and low molecular weight hyaluronan. J. Biol. Chem. 2010;285:30170–30180. doi: 10.1074/jbc.M110.137562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonenko Z., Gill S., Amrein M. An elevated level of cholesterol impairs self-assembly of pulmonary surfactant into a functional film. Biophys. J. 2007;93:674–683. doi: 10.1529/biophysj.107.106310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunasekara L.C., Pratt R.M., Amrein M.W. Methyl-beta-cyclodextrin restores the structure and function of pulmonary surfactant films impaired by cholesterol. Biochim. Biophys. Acta. 2010;1798:986–994. doi: 10.1016/j.bbamem.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Lu K.W., Pérez-Gil J., Taeusch H.W. Kinematic viscosity of therapeutic pulmonary surfactants with added polymers. Biochim. Biophys. Acta. 2009;1788:632–637. doi: 10.1016/j.bbamem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu K.W., Goerke J., Taeusch H.W. Hyaluronan decreases surfactant inactivation in vitro. Pediatr. Res. 2005;57:237–241. doi: 10.1203/01.PDR.0000150726.75308.22. [DOI] [PubMed] [Google Scholar]
- 36.Tashiro K., Kobayashi T., Robertson B. Dextran reduces surfactant inhibition by meconium. Acta Paediatr. 2000;89:1439–1445. doi: 10.1080/080352500456615. [DOI] [PubMed] [Google Scholar]
- 37.Taeusch H.W., Lu K.W., Clements J.A. Nonionic polymers reverse inactivation of surfactant by meconium and other substances. Am. J. Respir. Crit. Care Med. 1999;159:1391–1395. doi: 10.1164/ajrccm.159.5.9808047. [DOI] [PubMed] [Google Scholar]
- 38.Calkovska A., Mokra D., Javorka K. Bronchoalveolar lavage with pulmonary surfactant/dextran mixture improves meconium clearance and lung functions in experimental meconium aspiration syndrome. Eur. J. Pediatr. 2008;167:851–857. doi: 10.1007/s00431-007-0596-7. [DOI] [PubMed] [Google Scholar]
- 39.Campbell H., Bosma K., Lewis J. Polyethylene glycol (PEG) attenuates exogenous surfactant in lung-injured adult rabbits. Am. J. Respir. Crit. Care Med. 2002;165:475–480. doi: 10.1164/ajrccm.165.4.2106109. [DOI] [PubMed] [Google Scholar]
- 40.Dehority W., Lu K.W., Taeusch H.W. Polyethylene glycol-surfactant for lavage lung injury in rats. Pediatr. Res. 2005;58:913–918. doi: 10.1203/01.PDR.0000182581.39561.01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


