Introduction
Cardiac arrhythmias are a major epidemiological and public health problem and significantly contribute to sudden cardiac death, heart failure, stroke, suffering, debilitation and heath care expense. In the United States alone sudden cardiac death is estimated to kill 250,000–400,000 people annually.1 Most sudden death is due to cardiac arrhythmias2 with ventricular tachycardia and fibrillation as the most commonly (~80%) recorded rhythms in out-of-hospital cardiac arrests.3 In patients with structural heart disease, mostly due to a history of myocardial infarction, arrhythmias are the main cause of death.4 Atrial fibrillation (AF) and sinus node dysfunction (SND) are the most common sustained arrhythmias. AF affects ~2.3 million patients in the United States5 and because the prevalence of AF increases with age, it is predicted to increase by a 2.5-fold by 2050.6 Patients with AF have approximately twice the mortality rate of patients in sinus rhythm6 and the incidence of stroke is increased by 2 to 7-fold.7 AF is a costly disease and causes a public health burden estimated from $6.0 to $26.0 billion annually in the United States.8 SND is associated with increased sudden cardiac death, particularly in patients with heart failure, and a large portion (~40%) of mortality in hospitalized patients with heart failure may be secondary to SND.9 SND is the indication for ~60% of the 180,000 pacemakers implanted in the United States each year, a procedure that in 2004 accounted for ~$2 billion in expense.10, 11 The negative impact of arrhythmias on human health and medical economics are major motivating factors for establishing new and effective therapeutic approaches.
Cardiac arrhythmias are the result of cell membrane hyperexcitability (the cause of automatic and triggered tachyarrhythmias), defective impulse formation (the cause of SND) or reduction in normal cell-to-cell electrical coupling (the cause of conduction system ‘block’ and a component of the zone of slow conduction in most arrhythmias supported by a reentrant circuit). Ion channels, macromolecular protein complexes with a cell membrane-spanning conductance pathway, are the fundamental units of membrane excitability and rare congenital defects in ion channels or proarrhythmic off-target actions of many drugs can be sufficient to promote arrhythmia risk. However, most arrhythmias are not attributable to monogenic defects or drugs and occur in the biological context of various proarrhythmic factors, such as advanced age, increased oxidant stress, ischemia, tissue injury, inflammation and systemic disease (e.g. hypertension, diabetes, heart failure). These proarrhythmic factors appear to favor structural remodeling of cardiac tissue and to predispose certain ion channels to initiate or sustain arrhythmias. Unfortunately, ion channel antagonist drugs have not proven to be broadly applicable, safe or effective antiarrhythmic agents.12, 13 Thus, a major goal for science and industry is to define molecular pathways and mechanisms that cause common and life-threatening arrhythmias, in order to develop new and improved therapies. The multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) has emerged as a highly validated molecular mechanism with the potential to connect ‘upstream’ proarrhythmic factors, such as oxidation, with ‘downstream’ responses, such as ion channel hyperactivity, defective intracellular Ca2+ homeostasis, tissue damage and scar formation that promote arrhythmias. Here we will review modern concepts of CaMKII molecular physiology in the context of fundamental arrhythmia mechanisms and consider evidence that CaMKII inhibition could be a broad-spectrum anti-arrhythmic strategy.
A Brief Overview of Arrhythmia Mechanisms
In order to move forward with this review, we have decided to pause and put forth a parsimonious but global concept for understanding arrhythmias. Although there will be exceptions that appear to violate this framework, they will be unusual and outside of the experience of most clinical practice. Most consequential arrhythmias cause heart rates that are too fast or too slow for optimal mechanical performance of the heart. Arrhythmias can be relatively regular (i.e. occur at a constant period) or irregular. Fast (tachy) rhythms arise from one or more of three basic mechanisms (Fig 1). Triggered arrhythmias are the result of enhanced cell membrane excitability due to an imbalance in currents that favor excessive net inward current. The inward current depolarizes the cell membrane causing a positive deflection in the membrane potential called an afterdepolarization. Early afterdepolarizations (EADs) occur during action potential depolarization and delayed afterdepolarizations (DADs) interrupt the diastolic interval after action potential repolarization is complete. EADs and DADs trigger arrhythmias when they depolarize the cell membrane to the threshold for action potential initiation. Current evidence suggests that some forms of atrial fibrillation, ventricular tachycardia and ventricular fibrillation may be initiated and sustained by EADs and DADs.14 Automatic arrhythmias typically arise in cardiomyocytes associated with the pacemaking or specialized conduction system, including cells in the pulmonary outflow track and in pulmonary vein myocytes that are linked to some forms of ventricular tachycardia, atrial tachycardia and atrial fibrillation Automatic and triggered arrhythmias are fundamentally similar, as both depend on cell membrane hyperexcitability that is primarily driven by Ca2+ and/or adrenergic receptor stimulation. Reentry refers to an arrhythmia substrate that permits a repetitively excitable circuit, which may be anatomically constituted, for example by fibrosis, or functional, for example by electrical refractoriness due to action potential prolongation, membrane depolarization or tissue gradients of activation and/or repolarization. Stable reentrant circuits require an excitable gap, a zone of slow conduction and a preferred direction for conduction, as may occur with unidirectional block. Reentry is the basis for common forms of supraventricular tachycardia (including typical atrial flutter, atrio-ventricular nodal reentrant tachycardia and accessory pathway mediated tachycardias) and ventricular tachycardia (particularly in the setting of structural heart disease). Triggered and reentrant mechanisms likely co-exist, particularly in structurally diseased tissue. Slow (brady) arrhythmias also may be due to conduction slowing or unidirectional conduction block, for example as occurs in atrio-ventricular conduction disturbances or 'block'. SND results from defective impulse formation, due to intrinsic or acquired defects in depolarizing current, loss of sinoatrial nodal pacemaker cells, or inherited or acquired impairment of conduction. Remarkably, excessive activation of CaMKII is implicated in tachy- and bradyarrhythmias by each of these mechanisms. The surprising breadth of CaMKII participation in arrhythmias appears to be rooted in the role of CaMKII signaling to ion channels and the involvement of excessively activated CaMKII in promoting cell death and fibrosis.
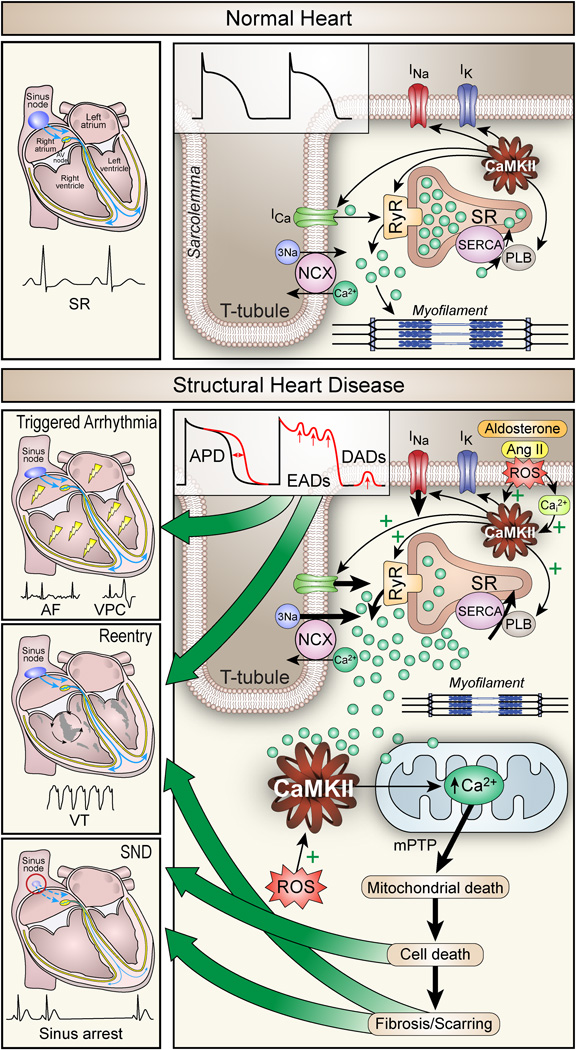
Figure 1.
The role of CaMKII in normal heart (top) and CaMKII-linked proarrhythmic implications in structural heart disease (bottom). Normal system for excitation-contraction coupling (ECC) and conduction in the heart leads to sinus rhythm as detected by the surface ECG (top left). On the single cardiomyocyte level (top right), excitation opens voltage-gated Na+ channels responsible for Na+ current (INa) leading to depolarization and triggering voltage-gated L-type Ca2+ current (ICa) to initiate myofilament cross bridge formation that supports contraction by stimulating ryanodine receptors (RyR) to release Ca2+ from the sarcoplasmic reticulum (SR). Relaxation mainly occurs by Ca2+ uptake to the SR by phospholamban (PLB) regulated sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA2a) and extrusion to the extracellular space by the Na+/Ca2+ exchanger (NCX). CaMKII is an integral part of ECC and orchestrates all its key components (arrows right panel). The sarcolemmal currents shape the action potential (inset middle). In structural heart disease (bottom panels), overexpressed and activated CaMKII is further activated by oxidation through an aldosterone and angiotensin II (Ang II) dependent increase of reactive oxygen species (ROS). In this setting, CaMKII disturbs Ca2+ homeostasis by hyperphosphorylating CaV1.2, NaV1.5 and RyR leading to increased intracellular Ca2+ (Ca2+i), early and late afterdepolarization (EADs, DADs, right panel and inset middle). Afterdepolarizations can initiate and sustain arrhythmias, including atrial and ventricular premature complexes and atrial fibrillation (AF, left upper panel). Increased Ca2+i causes mitochondrial death and apoptosis leading to scarring and reparative fibrosis (bottom right), which promote conduction slowing and reentry circuits (VT, left middle panel). Similarly, in the sinus node CaMKII-activation causes apoptosis of pacemaker cells leading to decreased impulse formation and sinus node dysfunction (SND, left bottom panel).
CaMKII Molecular Physiology
CaMKII structure determines CaMKII function, and the molecular physiology of CaMKII is ideal for connecting upstream signals encoded in intracellular Ca2+ and oxidation into downstream events that support core physiological functions in the cardiovascular system, such as fight or flight heart rate increases and excitation-contraction coupling. However, post-translational modifications can convert CaMKII from a Ca2+ and calmodulin-regulated enzyme to a Ca2+ and calmodulin-independent enzyme that plays a role in diverse forms of cardiovascular disease, including arrhythmias. CaMKII is a multifunctional serine-threonine protein kinase with widespread expression in muscle, nerve and immune tissues. The role of CaMKII, and other protein kinases, is to lower the free energy barrier and thus markedly hasten (i.e. catalyze) the reaction rate for transferring the terminal phosphate (γ phosphate) of ATP to a serine or threonine, which is part of a consensus sequence, on a target protein. Phosphorylation is a fundamental mechanism for biological systems to rapidly change the rate or function of many types of molecules (i.e. proteins and lipids). Ion channel proteins and Ca2+ homeostatic proteins involved in excitation-contraction coupling are CaMKII targets of immediate importance to arrhythmias. Protein phosphorylation is reversible by protein phosphatases, enzymes that catalyze the removal of phosphate adducts from proteins. Although important and functionally complementary to kinases, a discussion of phosphatases is beyond the scope of this review format. The 'rules' for the CaMKII consensus sequence are not inviolate. However, in general CaMKII prefers to catalyze phosphorylation within a RXXS/T motif, where R is arginine, X is any amino acid and S is a serine and T is a threonine.15 We can use the RXXS/T sequence motif as a guide to identifying candidate CaMKII 'sites.' There are four different CaMKII genes and each gene encodes a distinct CaMKII isoform (α, β, γ, δ). All CaMKII isoforms appear to share common regulatory mechanisms and protein targets, but differ in tissue distribution. CaMKIIδ is abundant in myocardium and recent studies using knock out mice lacking CaMKIIδ (CaMKIIδ−/−) have shown resistance to myocardial hypertrophy16 and heart failure after aortic banding surgery,17 exhibit reduced proarrhythmic intracellular Ca2+ release events18 and are resistant to vascular injury,19 validating the concept that CaMKIIδ can promote cardiovascular disease, including arrhythmias and sudden death.20 CaMKIIδ has multiple splice variants (i.e. specific molecular sequences are determined by exon skipping a process of making 'choices' about which particular exons to translate and which to leave untranslated), including two with the potential to guide subcellular localization of the CaMKII holoenzyme (see below).
CaMKII assembles into a dodecameric holoenzyme built from of a pair of stacked hexamers.21 Each CaMKII monomer consists of a core regulatory domain bound by an N terminus catalytic domain and a C terminus association domain (Fig 2A). Like other kinases, the catalytic domain has an ATP binding pocket that creates a microenvironment to lower the energy required to hydrolyze ATP, enhancing the rate of transfer for the γ phosphate from ATP to a target S/T and ejecting ADP. The association domain allows for auto-assembly of CaMKII monomers into a holoenzyme, which is critical to the molecular physiology of CaMKII. The junction between the association and regulatory domains includes a 'hypervariable' region with variable amino acid sequences across splice variants. One prominent example in CaMKIIδ are the splice variants CaMKIIδC (or CaMKIIδ2) and CaMKIIδB (or CaMKIIδ3). The hypervariable domain of CaMKIIδB contains a nuclear localization sequence that favors partitioning of CaMKIIδB to the nucleus.22 The CaMKIIδC splice variant lacks this sequence and preferentially resides in the cytoplasm. Although these distinctions are not straightforward,23 the presence of the nuclear localization sequence in a majority of constituent CaMKII monomers does favor residence of CaMKII holoenzymes in the nucleus, leading to important effects on transcriptional activity of genes involved in muscle hypertrophy, while CaMKIIδC splice variants in the cytoplasm have a prominent role in regulating membrane excitability and intracellular Ca2+ homeostasis.24, 25 Recently, a CaMKII holoenzyme crystal structure was resolved, revealing that variation in the length of the hypervariable region modulates access of Ca2+ bound calmodulin (Ca2+/CaM) to the regulatory domain.21 Thus, the length of the hypervariable linker appears to be a fundamental structural mechanism for tuning the Ca2+ sensitivity of CaMKII, an aspect of CaMKII molecular pathophysiology with potential but untested implications for the Ca2+ dependence of arrhythmias. Under resting conditions (i.e. low redox potential and low Ca2+i), CaMKII is enzymatically inactive, because the catalytic domain is bound to an autoinhibitory region embedded within the N terminus portion of the regulatory domain (Fig 2B). CaMKII is initially activated when an increase in Ca2+i favors Ca2+ binding to calmodulin (CaM), a ubiquitous intracellular Ca2+ binding protein. Ca2+/CaM binds to a CaM-binding region that resides in the C terminus of the CaMKII regulatory domain, leading to a conformational distortion that displaces the autoinhibitory region from the catalytic domain and causes CaMKII to become enzymatically active. Initially, Ca2+/CaM-unbinding reverses CaMKII activation. However, under conditions where elevated Ca2+i is persistent or where reactive oxygen species (ROS) are increased (see below), CaMKII can sustain activity even after Ca2+/CaM unbinding. Most cardiovascular diseases, including arrhythmias, appear to be associated with excessive Ca2+/CaM autonomous activity, so a brief discussion of mechanisms promoting Ca2+/CaM-independent activity follows.
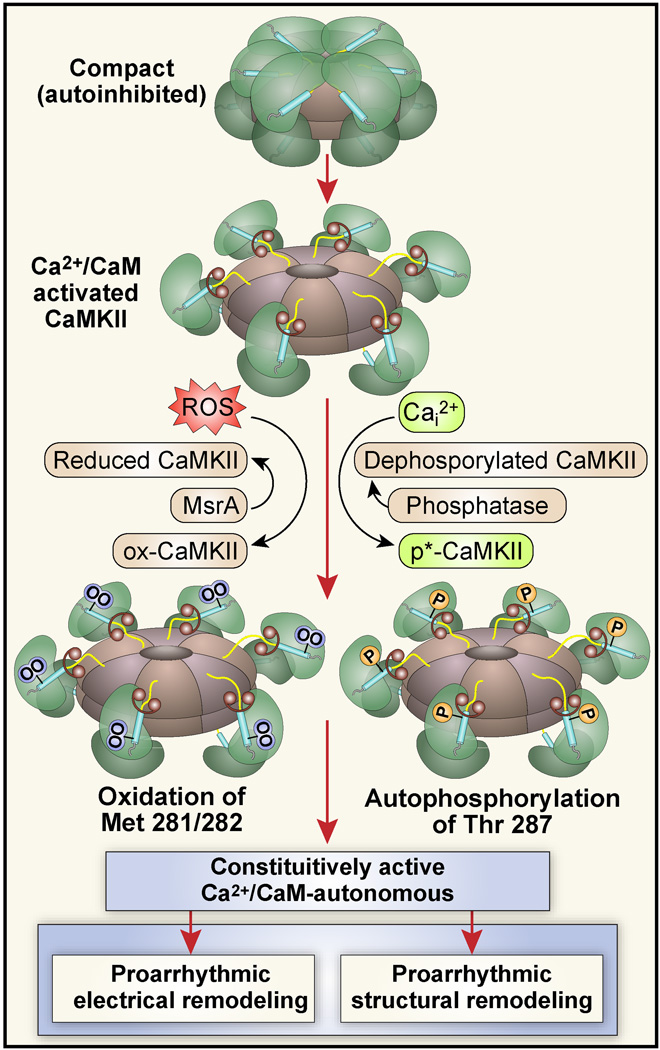
Figure 2.
The domain structure of a CaMKII. A. Each CaMKII monomer has a C terminus association domain (right side) and an N terminus catalytic domain (left side). The internal regulatory domain consists of a C terminus Ca2+/CaM binding region (CaM-B) and an N terminal side autoinhibitory region (AI, upper panel). When inactive, the catalytic domain is constrained by the AI sequence on the regulatory domain (lower panel, right side). Oxidation at Met 281/282 or autophosphorylation at Thr 287 prevent reassociation of the catalytic domain by disabling the AI sequence, leading to constitutive, Ca2+/CaM-independent CaMKII activity. B. The CaMKII holoenzyme is a dodecamer, assembled from CaMKII monomers as a stacked pair of hexameric rings.
When Ca2+/CaM is persistently bound to CaMKII, CaMKII undergoes inter-subunit autophosphorylation at threonine (Thr) 287 (the specific numbering varies slightly by isoform). Given the holoenzyme structure of CaMKII, it is intuitive that activated CaMKII monomers bound up in close proximity to a desirable CaMKII target site (i.e Thr 287) leads to autophosphorylation. Thr 287 autophosphorylation promotes CaMKII activity by two processes. First, autophosphorylation increases the affinity of Ca2+/CaM for CaMKII by 1,000-fold, so called “calmodulin trapping”. Second, even after Ca2+/CaM unbinding the Thr 287 autophosphorylated form of CaMKII has residual enzymatic activity, because phosphorylation of Thr 287 prevents effective reassociation and constraint of the catalytic domain by the autoinhibitory region. Thr 287 autophosphorylation is favored by high frequency Ca2+/CaM stimulation (i.e. as occurs in tachycardia) and prolonged (i.e. as occur in proarrhythmic electrical remodeling in heart failure or in the long QT syndromes) intracellular Ca2+ spikes,26 features that have led to the assertion that CaMKII is a 'memory' molecule. In contrast to the activating effects of autophosphorylation at Thr 287, autophosphorylation of Thr 306, in the CaM-binding region, can reduce Ca2+/CaM-dependent CaMKII activity by decreasing the affinity of CaMKII for Ca2+/CaM.27 Thus, CaMKII autophosphorylation represents a post-translational modification with the capacity to tune CaMKII activity and to transform CaMKII into a Ca2+/CaM-autonomous enzyme.
CaMKII activity is promoted by conditions of increased ROS, as occur in myocardium prone to tachycardia28, 29 and bradycardia, due to SND.30 High ROS conditions can enhance CaMKII activity by direct and indirect actions. Oxidation may increase the abundance of Thr 287 autophosphorylated CaMKII by inactivating phosphatases.31 Our group identified a mechanism where oxidation of methionines 281 and 281 (Met281/282) in CaMKIIδ leads to Ca2+/CaM autonomous activity by a post-translational modification that is analogous to Thr 287 autophosphorylation (Fig 3).32 Like Thr 287 autophosphorylation, Met 281/282 oxidation prevents reassociation of the catalytic and autoinhibitory domains even in the absence of Ca2+/CaM binding, causing constitutive, Ca2+/CaM autonomous CaMKII activity. Importantly, oxidation may increase the sensitivity of CaMKII to activation by Ca2+/CaM,33 suggesting that CaMKII could become proarrhythmic in the setting of increased ROS, even in the absence of increased Ca2+i. The first oxidation step (i.e. methionine sulfoxide) for Met 281/282 oxidization is enzymatically reversible by methionine sulfoxide reductase A (MsrA). MsrA is an interesting reductase, in part because it is implicated as a determinant of lifespan; mice lacking MsrA have shorter lives,34 while non-mammalian model organisms (i.e. worms and flies) exhibit increased lifespan by MsrA over-expression. Our findings suggest CaMKII is an important target of MsrA and that MsrA knock out increases susceptibility to myocardial oxidant injury,32, 35 while myocardial MsrA over-expression is protective against oxidant injury.35 At this point, proof-of-concept studies are lacking to test if increasing MsrA activity is antiarrhythmic. Oxidized CaMKII (ox-CaMKII) does not show CaM trapping, because oxidation of Met 308 reduces the affinity of Ca2+/CaM binding.32 Thus, the CaMKII holoenzyme is able to detect ROS and the frequency and duration of intracellular Ca2+ signals. The ability of CaMKII to transition into a Ca2+/CaM autonomous enzyme appears to be critical to the pathological roles of CaMKII in cardiovascular diseases, including arrhythmias.
Figure 3.
CaMKII is activated by autophosphorylation and/or oxidation, and constitutively active CaMKII promotes proarrhythmic electrical and structural remodeling. Under resting conditions the catalytic subunit is conformationally constrained so that CaMKII is inactive (top). CaMKII is initially activated when Ca2+ bound calmodulin (Ca2+/CaM, dumbbell shapes) binds to the regulatory domain (blue segments) causing a more extended conformation where the catalytic domain (green) becomes accessible to substrate proteins and ATP. Sustained increases in Ca2+i lead to autophosphorylation at Thr 287, enhancing the avidity of CaM binding but also inducing a Ca2+/CaM-independent form of CaMKII after CaM unbinding. Oxidation of Met 281/282 induces a Ca2+/CaM-independent form of CaMKII without CaM trapping, and increased levels of constitutively active CaMKII promote defective intracellular Ca2+ homeostasis leading to proarrhythmic electrical and structural remodeling.
Ion Channels are Fundamental
The development of the ECG by Einthoven36 marked the beginning of cardiac electrophysiology and initiated an explosion of mechanistically-informed research that contributed to a growing understanding of arrhythmias. A key event in this discovery process was the development of a voltage clamp technique applicable to single cells (so called "patch clamp”) in 1981 (Fig 4).37 Patch clamp allowed for very high resistance seals between a glass micropipette, containing an electrode, and the cell membrane that was required to resolve tiny currents (10−10 – 10−12 Amperes) associated with individual ion channels. Scientists armed with new patch clamp technology rapidly identified discrete ionic currents in heart, leading to biophysical understanding and naming of many ionic currents that were plausibly linked to cardiac electrophysiology and arrhythmias. The identification of patients with heritable arrhythmias, with eventual linkage to genetic mutations encoding defective ion channel proteins,38, 39 made it clear that ion channel defects alone, even in the absence of structural heart disease, could be sufficient to induce arrhythmias. The rise of molecular biology allowed investigators to begin to understand how genetic mutations could lead to defective ion channel protein structure, function and arrhythmias. The use of genetically modified mouse models provided important insights into the effects of ion channel encoding gene deletion in vivo.40, 41 Improved high resolution crystal structures42 and tools for dynamic imaging of ion channels provide hope that integrated application of complementary experimental approaches will improve our understanding of ion channel biology and the mechanisms by which ion channels contribute to arrhythmias.
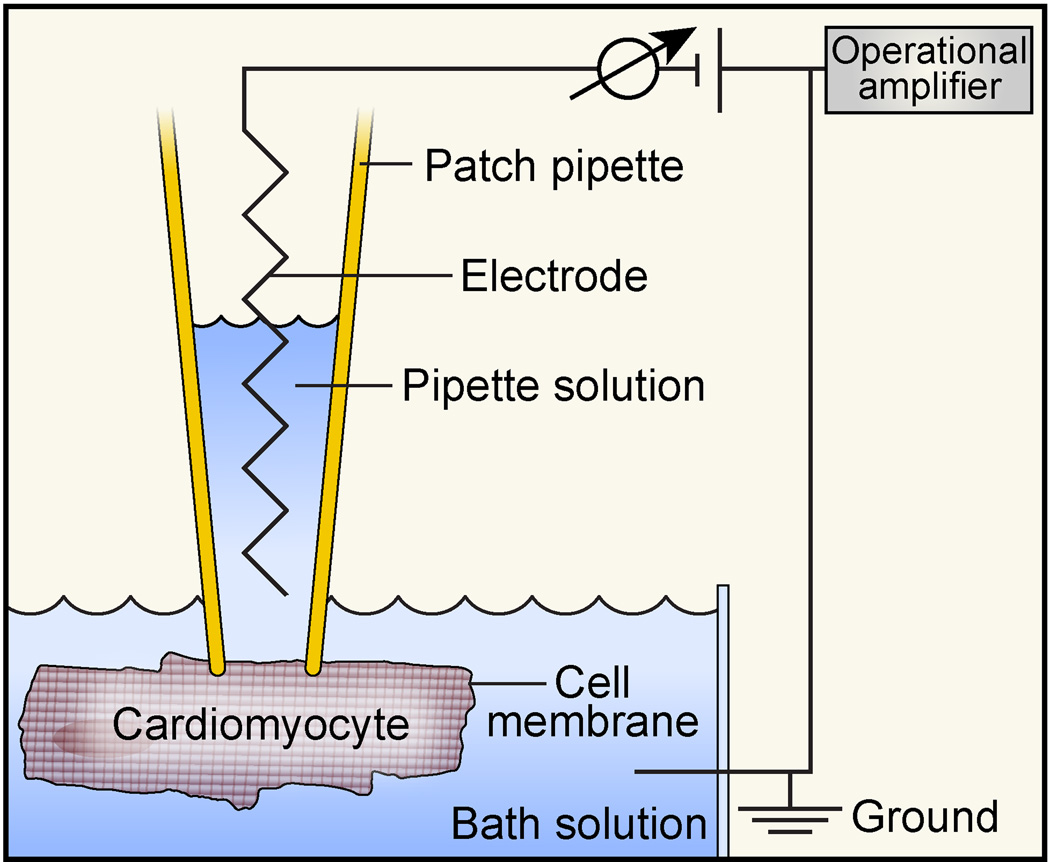
Figure 4.
Simplified schematic of a patch-clamp setup. A thin glass patch pipette (open tip ~1μm in diameter) is tightly attached (‘sealed’) to an area of the cardiomyocyte cell membrane (‘patch’). In this ‘cell on’ configuration it is possible to record ionic currents from single ion channels. By applying suction, it is possible to break the patch while maintaining the high resistance seal (‘whole cell’ configuration), so that ionic current from all ion channels or a subset of pharmacologically or biophysically-selected ion channels in the cell membrane can be measured. The patch pipette includes a pipette solution and a silver wire electrode that is connected to an operational amplifier. The amplifier measures the voltage (or current) in the micropipette in relation to a reference electrode in the bath solution connected to the ground, and injects or withdraws current to maintain a command voltage (‘voltage clamp’).
Action potentials are built from ionic currents
Cardiac action potentials initiate each heart beat and are the result of highly coordinated activity of ion channels. The ventricular myocyte action potential has a long duration (~200–400 ms) compared to action potentials in neurons (~10 ms) and is divided into numbered phases (Fig 5). In the diastolic interval (phase 4), between action potentials, the inner resting cell membrane potential is negatively charged (~−90 mV in contracting myocardium and ~−60 mV in sinoatrial nodal pacemaker cells) compared to the extracellular space. In sinoatrial nodal pacemaker cells phase 4 is dynamic, exhibiting a pattern of late diastolic depolarization that culminates in action potential initiation. Pacemaker cell membrane diastolic depolarization rate (DDR) is under autonomic nerve control and catecholamines increase heart rate by augmenting the inward ‘funny’ current (If) through cyclic AMP responsive (gated) channels43 and by enhancing the electrogenic Na+/Ca2+ exchanger current (INCX) that is activated by release of Ca2+ from intracellular sarcoplasmic reticulum (SR) ryanodine receptor Ca2+ channels.44 Action potentials in atrial and ventricular myocardial cells are initiated (phase 0) by an inward (positive) Na+ current (INa), but in pacemaker cells, with less negatively charged cell membranes, Na+ channels are either absent or inactivated, and action potentials are initiated by inward Ca2+ current (ICa) and or inward Na+/Ca2+ exchanger current. In ventricular myocytes, at the conclusion of phase 0 the vast majority of Na+ channels are inactivated, while rapidly activating outward K+ currents open and sculpt a repolarizing notch in the AP prior to the plateau (phase 1).
Figure 5.
Ventricular action potential (AP) and ionic currents. The morphology of a ventricular AP is shaped by 4 phases (top, left). Phase 0 – Rapid upstroke, generated by the voltage-gated Na+ current; Phase 1 – Early repolarization, inactivation of Na+ current and activation of repolarizing transient outward K+ current Ito; Phase 2 – Plateau, orchestrated by Ca2+ currents and repolarizing K+ currents; Phase 3 – Late repolarization, inactivation of Ca2+ currents and sustained activation of K+ currents. Phase 4 – Diastole, the resting membrane potential is mainly governed by the K+ current IK1. A schematic overview of transsarcolemmal ionic movements is shown at bottom left. Typical ionic current flows that orchestrate a ventricular AP are shown in the right panel along with the pore-forming proteins and encoding genes.
The cardiac action potential plateau (phase 2) is marked by a relatively low amount of ion movement and high cell membrane resistivity, so that small currents have relatively large effects on membrane potential. These features cause the action potential plateau to be particularly vulnerable to arrhythmia-triggering EADs.45 Action potential repolarization (late phase 2 and phase 3) occurs when inward current wanes and outward currents (mainly due to K+) increase. Action potentials spread across the myocardium as a wave front of depolarization to trigger mechanical systole. The coupling between electrical and mechanical events is called excitation-contraction coupling and is accomplished by a Ca2+-induced Ca2+ release process.46 Action potentials resolve as a wave back, leaving behind electrically excitable myocardium, poised to generate another action potential.
Ion channel antagonist drugs are suboptimal agents
The improved understanding of ion channels and the recognition of their role as determinants of cardiac arrhythmia occurred at a time when an increasingly sophisticated pharmaceutical industry became intent on designing molecularly focused drug therapies. Flecainide and encainide, potent and selective INa antagonists, were famously evaluated in the Cardiac Arrhythmia Suppression Trial (CAST).13 CAST was motivated by the fact that patients with structural heart disease (left ventricular ejection fraction ≤30% after myocardial infarction) have a high rate of sudden death due to arrhythmias. Earlier work had shown that patients with depressed ejection fractions and/or frequent premature ventricular contractions, a potential consequence of afterdepolarizations, were at highest risk for sudden death. The CAST pilot study first established the efficacy of flecainide and encainide in suppressing premature ventricular contractions, by ≥80%, using Holter monitoring. Nonetheless, CAST showed that patients treated with encainide or flecainide were significantly more likely to die suddenly compared to placebo-treated patients, presumably due to the proarrhythmic effects of Na+ channel inhibition. The Survival with Oral d-Sotalol (SWORD) was a second critical study that definitively demonstrated increased mortality in post-myocardial infarction patients with reduced ejection fractions treated with a relatively selective K+ channel antagonist.12 Like many drugs, sotalol is a racemic modification where the l-enantiomer has K+ channel antagonist and β adrenergic receptor antagonist actions. In contrast, the d-enantiomer lacks activity at β adrenergic receptors. Patients treated with d-sotalol had increased mortality compared to controls. Taken together, these seminal studies suggested that ion channel antagonist drugs were suboptimal for treating arrhythmias, and were potentially dangerous in patients at high risk for life threatening arrhythmias due to structural heart disease.
The emergence of anti-arrhythmic device therapies
The failure of ion channel-targeted antiarrhythmic drugs in CAST and SWORD occured during a period of discovery for the long QT syndromes. In many cases, the defective ion channel encoding genes in long QT syndrome patients led to proarrhythmic defects that mimicked the effects of antiarrhythmic drugs, suggesting to many that ion channel targeted therapy alone was unlikely to be a broadly applicable or successful antiarrhythmic strategy, because of the risk of proarrhythmia. The surprising results of CAST marked the beginning of a shift away from ion channel antagonist therapies and toward the use of surgically implanted cardiac defibrillators (ICDs).47 Although ICD therapy does not affect biological factors driving progression of structural heart disease, cardiac resynchronization therapy by biventricular pacing can reverse adverse structural remodeling of failing left ventricles.48 Studies in dogs show increased myocardial CaMKII activity and apoptosis in the lateral left ventricle in tachy-pacing induced heart failure with left bundle branch ablation that is reversed by cardiac resynchronization therapy.49 These findings suggest that CaMKII activity may be favorably modulated by biventricular pacing and that normalization of CaMKII activity could account for some of the clinical benefits of cardiac resynchronization therapy.
Understanding proarrhythmic actions of CaMKII at ion channels
CaMKII is now known to orchestrate connections between intracellular Ca2+ and membrane excitability by actions at virtually all known voltage-gated ion channels in heart. It is also likely that CaMKII connects changes in redox potential to cell membrane potential through actions at ion channels.50 Given the central role of CaMKII in modulating electrical activity in heart, it is perhaps not surprising that excessive CaMKII activation favors arrhythmias (see below), in part, by actions at ion channels. A comprehensive discussion of CaMKII actions on ion channels is beyond the scope of this review and has been published elsewhere.51 Instead, we will focus on two cell membrane (sarcolemmal) ion channels that are prominently represented in ventricular myocardium, voltage-gated Ca2+ (CaV1.2) and Na+ (NaV1.5) channels, where the role of CaMKII in arrhythmogenesis is relatively well-established.28, 52 In a later section we will also discuss the effects of CaMKII on an intracellular Ca2+ releasing ion channel, the ryanodine receptor, in order to illustrate important molecular and cellular concepts relevant to clinical arrhythmias.
CaMKII catalyzes phosphorylation of the pore forming α-subunit of the predominant ventricular myocardial L-type Ca channel, CaV1.2, at Ser1512 and Ser1570 and the accessory β2a-subnit, atThr498.53–55 The effect of CaMKII-dependent phosphorylation on whole cell (i.e. macroscopic) ICa is to promote a dynamic process called facilitation,56 which results in enhanced peak and slowed ICa inactivation as an initial response to repetitive cell membrane depolarizations (Fig 6). At a single channel (i.e. microscopic) level CaMKII promotes frequent, prolonged openings,57 so-called mode 2 openings,58 a biophysical response shared with β adrenergic receptor, catecholamine, agonists.59 CaMKII effects on CaV1.2 may contribute to prolongation of the action potential duration,54, 60 as a consequence of increasing inward current that opposes action potential repolarization (Fig 6). However, CaMKII activity appears to be essential for the proarrhythmic effects of pathological action potential and QT interval prolongation, because in cell and animal models CaMKII inhibition effectively suppresses afterdepolarizations and arrhythmias without significantly shortening repolarization.54, 61–63 Thus, action potential prolongation is part of a proarrhythmic, feed forward, circuit that favors arrhythmias by increasing cellular Ca2+ entry through ICa and activating CaMKII (Fig 7).62, 64, 65
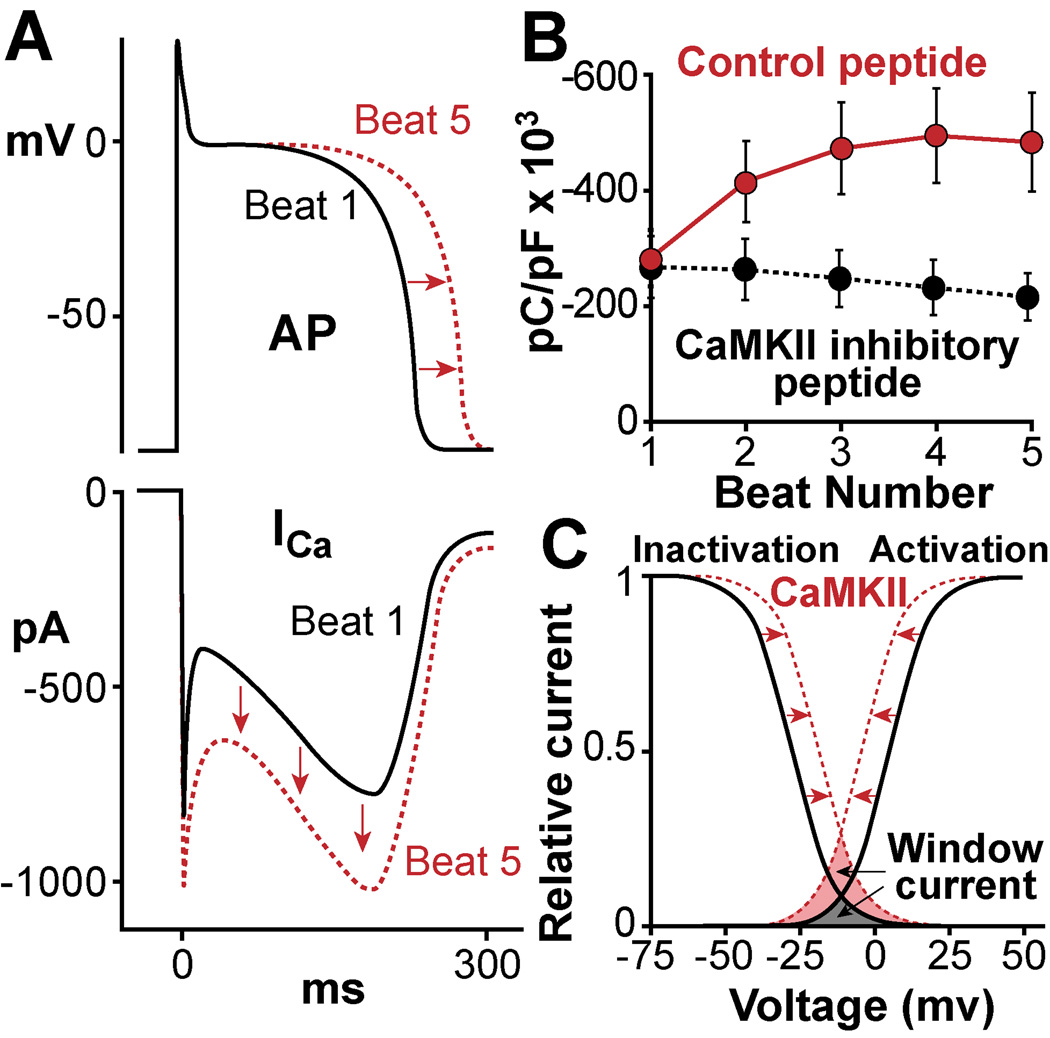
Figure 6.
Regulation of L-type Ca channels (LTCC) by CaMKII. CaMKII phosphorylation of LTCC alters channel gating by increasing open probability. A. In myocardium this mechanism leads to a frequency-dependent increase of Ca2+ current (ICa, lower panel), a process termed facilitation, which may also contribute to action potential (AP) prolongation (upper panel). B. Facilitation is a positive CaMKII-dependent staircase of ICa amplitudes over a series of several APs. C. CaMKII phosphorylation alters inactivation and activation properties of LTCC current, moving the availability of Ca2+ channels to more positive membrane potentials and the activation to more negative potentials. In this way CaMKII increases the ‘window current’ (shaded field between the curves) of the ICa increasing the probability of Ca2+ channels to (re)open during phase 2 of the AP and cause EADs.
Figure 7.
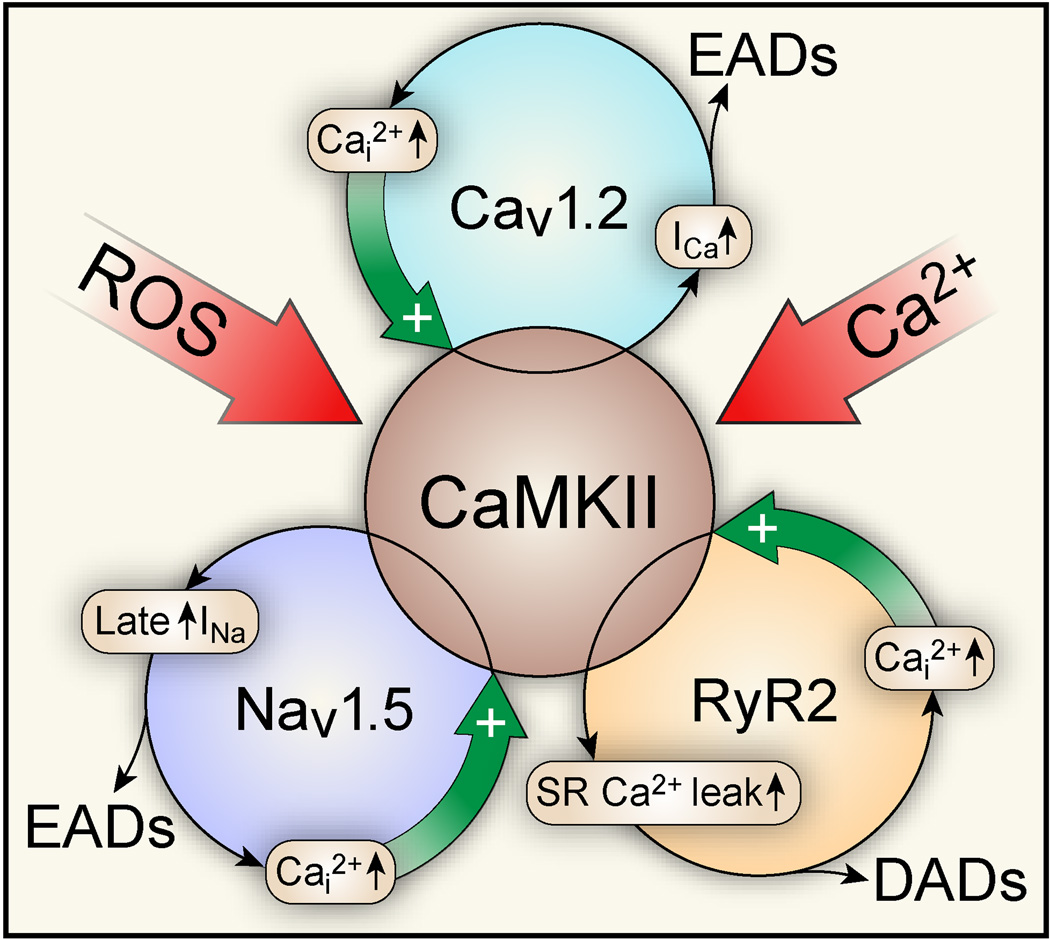
Feed forward nature of CaMKII-activation in structural heart disease and arrhythmias. CaMKII is activated by elevated intracellular reactive oxygen species (ROS) and Ca2+ (Ca2+i). Activated CaMKII phosphorylates L-type Ca channels (CaV1.2), which increases channel open probability (mode 2 gating), increases Ca2+i and causes EADs. Phosphorylation of Na+ channels (NaV1.5) moves their availability to more negative membrane potentials (loss-of-function), but also enhances the long-lasting late INa (gain-of-function) favoring EADs. CaMKII phosphorylation of ryanodine receptors (RyR) increases sarcoplasmic (SR) Ca2+ leak and Ca2+i that in turn fuels NCX forward mode causing DADs. Importantly, CaMKII integrates several proarrhythmic mechanisms that may work to further augment CaMKII activity by increasing Ca2+i and ROS.
The proarrhythmic actions of CaMKII on CaV1.2 occur preferentially during the plateau phase of the cardiac action potential,61, 65, 66 at least in part because the plateau potential is an electrical substrate that favors opening of CaV1.2, a 'voltage-gated' ion channel where plateau membrane potentials favor structural rearrangements of the channel protein that increase the probability of opening. Phosphorylation by CaMKII or by protein kinase A, the principal kinase activated by β adrenergic receptor agonists, synergizes with cell membrane potential to enhance the probability of CaV1.2 opening. The relatively long cardiac action potential plateau in ventricular myocytes is necessary for myocardial physiology. The action potential plateau grades CaV1.2 openings that increase ICa and trigger SR Ca2+ release, which ultimately induces myofilament crossbridge formation and cardiac systole, by a Ca2+-induced Ca2+ release process (Fig 1).46 Unfortunately, the physiological advantages of the prolonged action potential plateau appear to go hand in glove with proarrhythmic vulnerability. Pathological action potential prolongation is a prominent outcome of a proarrhythmic electrical remodeling process in heart failure,67 in response to drugs68 and in the much rarer long QT genetic arrhythmia syndromes (discussed below).38, 39 The Timothy Syndrome is a very rare form of the long QT syndrome due to a mutation in CaV1.2 that prevents normal inactivation, leading to increased ICa, action potential and QT interval prolongation, life-threatening arrhythmias and multisystem defects.69 We found that CaMKII activation was essential for amplifying the genetic CaV1.2 defect in adult rat ventricular myocytes and that CaMKII inhibition normalized the action potential duration and eliminated afterdepolarizations,70 suggesting that CaMKII activation is part of a proarrhythmic feed forward mechanism in at least one type of long QT syndrome. Excessive action potential prolongation can promote arrhythmias by multiple mechanisms. CaV1.2 channels close and reopen within the action potential plateau, operating within a 'window' between membrane potential-driven channel opening and inactivation (Fig 6). Ca2+ channel agonist drugs71 and CaMKII72 favor increased ICa window current during the action potential plateau. The membrane resistance is relatively high during the action potential plateau73 so, according to Ohm's law (voltage = current × resistance), small increases in inward current cause large depolarizations in membrane potential (voltage), which are the basis for EADs. The efficacy of Ca2+ channel 'blockers' as antiarrhythmic agents is limited, likely because concentrations adequate for significantly inhibiting ICa prevent physiological cardiac and smooth muscle function leading to inadequate inotropy and hypotension. Dihydropyridine CaV1.2 antagonists work by preventing mode 2 gating58 and are effective at suppressing EADs in experimental animal models.62, 65 In contrast, CaMKII54, 57 and the CaV1.2 channel agonist BayK 8644 increase CaV1.2 mode 2 activity, ICa window current and EADs.58, 71 CaMKII inhibition using a small molecule CaMKII inhibitor (KN-93)61, 74 or a calmodulin antagonist drug, W-7,63, 75 is effective in preventing ventricular tachycardia. BayK 8644 induces mode 2 CaV1.2 openings by direct actions on the pore forming α-subunit that overrides the effects of CaMKII inhibition to reduce CaV1.2 opening probability. Addition of BayK 8644 induces ventricular tachycardia even after treatment with the calmodulin antagonist W-7,63 a condition with reduced CaMKII activity, suggesting that ICa is a critical site for proarrhythmic actions of CaMKII, a hypothesis supported by modeling studies.54, 76 In our view, the best available evidence is that the proarrhythmic actions of CaMKII at CaV1.2 occur by enhanced phosphorylation of a specific site (Thr 498) on the CaV1.2 βsubunit protein, because elimination of this site prevents mode 2 gating and significantly reduces EADs.54 It is interesting that the Thr 498 site also contributes to intracellular Ca2+ overload that contributes to cell death, suggesting the possibility that CaMKII proarrhythmic effects on CaV1.2 may extend beyond arrhythmia triggering afterdepolarizations and include cell death, a feature of pathological tissue remodeling that contributes to sinus node dysfunction30 and reentrant circuits (Fig 1).29
CaMKII reduces the cell membrane voltage-dependence of Na+ current (INa) availability and at the same time increases a non-inactivating component of INa implicated in action potential prolongation and EADs by effects on the predominant cardiac voltage-gated Na+ channel, Nav1.5.77 The reduction in NaV1.5 channels available to open resembles the Brugada syndrome,78 and the increase in the non-inactivating component of INa mirrors changes to INa seen in heart failure,79 long QT syndrome 3 and in the presence of increased oxidant stress,80 suggesting the hypothesis that CaMKII is a common proarrhythmic signal for NaV1.5. In cardiomyocytes and neurons CaMKII is brought into close proximity to the NaV1.5 protein complex by βIV spectrin, a cytoskeletal protein. Mutant mice lacking a CaMKII binding motif on βIV spectrin are resistant to proarrhythmic effects of CaMKII on INa and EADs.81 NaV1.5 and CaV1.2 share a general structure with four homologous ‘repeats’ that are comprised of six transmembrane helices (Fig 8). The four homologous repeat domains are connected by intracellular linkers and the linker domain between repeat I and II appears to be a 'hot spot' for CaMKII-mediated phosphorylation and binding.81, 82 Serine (Ser) 571 appears to be a key residue for phosphorylation in cardiomyocytes,81 but not in HEK293 cells82 where Ser 516 and Thr 594 appear to be better CaMKII targets. The complete effects of CaMKII on INa, reduced availability and increased non-inactivating late current, are evident in cardiomyocytes but not in HEK293 cells, suggesting that multiple components contribute to proarrhythmic actions of CaMKII on NaV1.5. CaMKII inhibition or ranolazine,83 a drug that preferentially inhibits the non-inactivating late component of INa, can both reduce EADs, suggesting that NaV1.5 late current is an important ion channel target for proarrhythmic effects of CaMKII.
Figure 8.
Structure of the L-type Ca channel CaV1.2, the Na channel NaV1.5 and the SR Ca release channel RyR with indication of CaMKII phosphorylation sites. The pore-forming α1-subunit of CaV1.2 and NaV1.5 have four domains (I–IV) with each domain having six homologous transmembrane regions (1–6). CaMKII phosphorylates CaV1.2 at both the α1-subunit (Ser 1512 and Ser 1570) and the accessory the β2a-subunit (Thr498). CaMKII phosphorylates NaV1.5 at the linker loop 1 between domain I and II, at multiple possible candidate sites (indicated as “hot spot” which includes e. g. Ser 571, Ser 516, Thr 594). The RyR contains four transmembrane spanning domains (1–4), and CaMKII phosphorylates Ser 2809 and Ser2814 on the cytosolic and N-terminal side.
The best antiarrhythmic drugs are not ion channel antagonists
In contrast to the lack of success with ion channel antagonist drugs, antagonist drugs targeting neurohumoral pathways activated during conditions favoring common arrhythmias, such as hypertension and myocardial infarction, were found to be effective in reducing arrhythmias and sudden death. The β adrenergic receptor antagonist drugs84 and drugs that inhibit the renin angiotensin II85 aldosterone86 system (RAAS) are effective for reducing heart failure and sudden death in patients with reduced left ventricular ejection fractions after myocardial infarction. While these drugs engage specific receptors they have widespread biological actions by virtue of the multivalent nature of signaling pathways activated by β adrenergic, angiotensin and mineralocorticoid receptors. Agonist stimulation at each of these signaling pathways can produce broad-based proarrhythmic and cardiomyopathic effects, which are associated with increased reactive oxygen species and disturbed intracellular Ca2+ homeostasis. The lack of efficacy of selectively-targeted ion channel antagonist drugs combined with the relative efficacy of neurohumoral antagonist drugs suggests that successful antiarrhythmic therapies will need to address complex relationships between signaling pathways, myocardial hypertrophy, survival and ion channels.
The purpose of ion channels is to ‘trigger’ automaticity and contraction
The purpose of the elaborate cell membrane (sarcolemmal) ultrastructure of cardiomyocytes and the rich variety of ion channel complexes is to initiate coordinated contraction that is essential for physiologically tunable cardiac output. Phase 0 of the cardiac action potential is the ‘excitation’ in excitation-contraction coupling. Cell membrane excitation (by INa in the vast majority of cardiomyocytes purposed to mechanical duties and by ICa in the small number of pacemaker and atrioventricular nodal cardiomyocytes) couples to contraction by a Ca2+-induced Ca2+ release process. In sinoatrial nodal pacemaker cells, which lack a clear mechanical purpose, excitation-contraction coupling is a misnomer. An arguably better descriptor for these pacemaker cells is excitation-excitation coupling, because DDR is a consequence of cell membrane excitation from If and spontaneous SR Ca2+ release that promotes INCX. In contracting cardiomyocytes phase 0 depolarizes (i.e. makes the inner cell membrane relatively more positive) the cell membrane, which enhances the opening probability of voltage-gated Ca2+ channels (Fig 5). ICa triggers a coherent release of relatively massive amounts of SR Ca2+ from RyR (Fig 1). The SR is the primary source of activator Ca2+ for driving myofilament cross bridge formation, which is the molecular basis for myocardial contraction. The peak ICa represents the maximum number of open Ca2+ channels in response to membrane depolarization. Soon after voltage-gated Ca2+ channels open they begin to inactivate and become reluctant to reopen until completion of action potential repolarization (phase 3) and attainment of the physiological negative resting cell membrane potential (phase 4). Diastole is an active process that requires reduction of myofilament bound and free cytoplasmic Ca2+ by active reuptake into the SR by the sarcoplasmic-endoplasmic reticulum ATPase (SERCA2a) and to a lesser extent by mitochondrial uptake. Cytoplasmic Ca2+ removal also occurs by extrusion from the cell, mostly by the Na+/Ca2+ exchanger and to a lesser extent by the sarcolemmal Ca2+ ATPase. Thus, the ion channel components that orchestrate myocardial cell excitability are intimately involved with cellular proteins required for intracellular Ca2+ homeostasis and electrical automaticity that underlies physiological pacing and mechanical control of myocardium. This interdependence of electrical and Ca2+ homeostatic systems occurs on an ultrastructure of cellular and organelle membranes that is best understood in ventricular myocytes (Fig 1). More than half of the ventricular myocyte membrane is involved in repetitively spaced invaginations called T-tubules that reach deep into the myocyte interior. T tubular membranes are richly decorated with voltage-gated Ca2+ channels that face off across a narrow span of cytoplasm (~10 nm) to engage dyadically arrayed ryanodine receptors. These dyads provide a near idealized spatial environment for Ca2+-induced Ca2+ release. However, when these relationships are disturbed at an ultrastructural and molecular level, as occurs in myocardial injury, they become prone to arrhythmias and sudden death.
Managing intracellular Ca2+ constitutes a major ATP cost for cardiomyocytes. The Ca2+ concentration gradient between the extracellular space (~1 mM) and cytoplasm (~100 nM) is massive (~10,000× more extracellular than bulk cytoplasmic Ca2+). There is broad variation in the concentration of free versus protein bound intracellular Ca2+, spatially and over time (e.g. systole versus diastole), so that intracellular Ca2+ in the SR and in the dyadic space between voltage-gated Ca2+ channels, integral to T-tubular membranes, and SR bound ryanodine receptors during systole may approach extracellular values. CaMKII catalyzes the phosphorylation of important Ca2+ homeostatic proteins, including voltage-gated Ca2+ channels,54, 56, 57 ryanodine receptors87–89 and the SERCA2a regulatory protein phospholamban.90 Thus, CaMKII is positioned to enhance cellular Ca2+ fluxes and to coordinate physiological goals of excitation-contraction coupling, lusitropy and heart rate. It is therefore not necessarily surprising that CaMKII when excessively activated may also contribute to pathological derangement of membrane excitability and mechanical function, promoting arrhythmias and heart failure.
Understanding non-ion channel proarrhythmic actions of CaMKII
Although ion channels are the final effectors of cell membrane excitability, multiple cellular and tissue events contribute to arrhythmia initiation and perpetuation. The proarrhythmic effects of excessive CaMKII activity are due to actions at multiple protein targets that affect intracellular Ca2+ homeostasis, myocardial survival, matrix and inflammation. Intracellular Ca2+ homeostasis - CaMKII has been shown to regulate both SR Ca2+ uptake and release. For relaxation to occur, the largest fraction of the cytosolic Ca2+ is removed to the SR by SERCA2a, which is inhibited by phospholamban. CaMKII phosphorylates phospholamban at Thr 17,90 which reduces the inhibitory effect of phospholamban on SERCA2a, thereby increasing Ca2+ reuptake by the SR and myocardial relaxation. CaMKII catalyzes phosphorylation of several known sites on the cardiac ryanodine receptor.87, 88 One highly investigated site is Ser 2814.89 CaMKII-dependent phosphorylation of Ser 2814 increases proarrhythmic diastolic SR Ca leak.24 Diastolic SR Ca2+ leak (measured as Ca2+ sparks91 and waves) increases cytosolic Ca2+ and reduces SR Ca2+ content, which in turn increases forward-mode INCX leading to (late phase 3) EADs and DADs. There is a growing body of evidence that this concept of CaMKII- and afterdepolarization-dependent arrhythmogenesis is valid for both ventricular and atrial tachyarrhythmias, including atrial fibrillation (discussed below). Increased diastolic SR Ca2+ leak and hyperphosphorylation of ryanodine receptors are also features of heart failure,92 which may contribute to impaired contractility and arrhythmias.93 Cell death - Excessive CaMKII activity induces cell death, which can contribute to reparative fibrosis and adverse remodeling.20, 30, 32, 94 In non-cardiac tissues CaMKIIγ promotes apoptosis by Fas death receptor and mitochondrial pathways.95 In cardiac tissue, excessive and sustained β1AR stimulation results in increased apoptosis, adverse remodeling and heart failure. CaMKII is critically involved in β1AR mediated apoptosis, and mice with transgenic expression of the CaMKII-inhibitory protein AC3-I are in part protected from apoptosis upon βAR stimulation and after myocardial infarction.94, 96 Inflammation - Inflammation is a feature of arrhythmia-prone myocardium, after myocardial infarction, in various cardiomyopathies and in atrial fibrillation.97–99 CaMKII is involved in the induction of the sarcolemmal injury by activating a local inflammatory response through the NF-κB pathway.99 CaMKII is also oxidatively activated during myocardial infarction or by endotoxin as part of a toll-like receptor/MyD88 pathway.100 The potential for CaMKII to activate and to be activated by inflammatory signaling suggests CaMKII may be an important molecular connection between inflammation and arrhythmias. Extracellular matrix and scar formation - We recently found that aldosterone leads to oxidative activation of CaMKII and myocardial matrix metalloproteinase 9 (MMP9) synthesis by activation of a myocyte enhancer factor 2 transcriptional pathway.35 In the setting of myocardial infarction and hyperaldosteronism, myocardial MMP9 production was sufficient to increase the likelihood of death due to myocardial rupture. The untoward effects of myocardial CaMKII on extracellular matrix are potentially consistent with the anti-fibrotic effects of myocardial CaMKII inhibition,30 a contributing factor in SND and reenterant arrhythmias. Oxidized CaMKII may contribute to peri-infarct scar properties that promote reenterant arrhythmias.29 CaMKII can also increase fibrosis as a consequence of promoting cell death. CaMKII actions at Ca2+ homeostatic proteins54 and mitochondria95 appear to activate myocyte death programs under conditions of disease stress. Thus, myocardial CaMKII has the potential to modify tissue substrates that can promote arrhythmias by contributing to structural heart disease.
Overview of arrhythmias linked to CaMKII
Atrial Fibrillation
A growing body of evidence suggests an important causative role for CaMKII in AF. The hallmarks of abnormal cell membrane excitability in AF are enhanced triggered excitability and automaticity, and reduced refractoriness due to shortened action potential duration (APD). Profound fibrosis and atrial dilatation of the fibrillating atria provide the tissue substrate for conduction slowing, reentrant circuits and maintenance of AF. About a decade ago Tessier et al. discovered that CaMKIIδ expression is increased in chronic human fibrillating atria.101 They provided evidence, corroborated by others,102 that 1. CaMKII enhances the repolarizing transient outward K+ current (Ito) in fibrillating human atrial cardiomyocytes, thus shortening refractoriness and action potential duration, and 2. CaMKII leads to a disturbed frequency-dependent reactivation of Ito. Both mechanisms are proarrhythmogenic, per se, favoring functional reentry circuits and dispersion of atrial excitability. Indeed, direct CaMKII inhibition (by KN-93 and autocamtide-2-related inhibitory peptide) and indirect (by the calmodulin inhibitor calmidazolium) significantly reduced these effects. Enhanced CaMKII activity in fibrillating atria in animal models103 and patients104 causes hyperphosphorylation of the ryanodine receptor at Ser2814 leading to increased diastolic SR Ca2+ leak,104, 105 elevated cytosolic Ca2+ and increased susceptibility to AF.103, 104 This mechanism has been recently validated in human fibrillating atrial myocytes, tying CaMKII-dependent diastolic SR Ca2+ leak to the increased frequency of DADs as a trigger for AF.106 Consistently, CaMKII inhibition blocked this AF-triggering pathway at all levels by reducing SR Ca2+ leak,103, 104 DADs and ectopic beats,103, 106 and inducible AF.103
Sinus Node Dysfunction
SND results from defective impulse formation or propagation in the sinoatrial node or adjacent atrial myocardium. Patients with SND present with a variety of rhythm abnormalities including sinus bradycardia, sinus pauses and arrest, intermittent exit block and deficient heart rate response to exertion or chronotropic incompetence. In contrast to extrinsic and reversible SND (e. g. due to hypoxia, metabolic disturbance or drugs), intrinsic SND is not readily reversed because of pacemaker cell death and replacement fibrosis.107 The loss of sinoatrial node cells leads to physiologically inadequate summation and formation of the propagating pacemaker impulse, a phenomenon that is also referred to as electrical ‘source-sink mismatch’. Typically, intrinsic SND occurs in conditions of increased oxidative stress and high amounts of circulating angiotensin II, as occurs in elderly patients with hypertension, structural heart disease and heart failure. We recently reported a model of SND in mice induced by chronic angiotensin II treatment.30 These mice had sinus-pauses and chronotropic incompetence and their sinoatrial node regions showed cell loss due to increased cell death with extensive fibrosis. In our model, Angiotensin II induced ROS through a NADPH oxidase dependent pathway, and ROS activated CaMKII (ox-CaMKII),32 and we found ox-CaMKII was elevated in right atria from patients who required a pacemaker for SND. Sinoatrial node gene painting with focal pacemaker expression of a synthetic CaMKII inhibitory peptide protected angiotensin II-infused mice from SND and pacemaker cell death by preventing a source-sink mismatch between sinus node and surrounding atrium. These data suggested that SND could be prevented in high-risk patients by CaMKII inhibition targeted to sinoatrial nodal pacemaker cells.
Ventricular Tachyarrhythmias in Structural Heart Disease
Ventricular tachycardia and ventricular fibrillation are common causes of sudden death, as discussed earlier. Patients with structural heart disease are at highest risk, and myocardium from patients and from animal models of structural heart disease shows a consistent increase in the expression and/or activity of CaMKII.108, 109 There is now abundant evidence that CaMKII over-activity can promote potentially lethal ventricular arrhythmias by initiating afterdepolarizations18, 54, 61, 75, 77, 110 and causing proarrhythmic tissue remodeling that favors reentry.16, 20, 111, 112 Furthermore, CaMKII inhibition by small molecules and genetic approaches is effective in preventing or reducing ventricular arrhythmias in animal models in vivo, 18, 61, 74, 75, 77, 110 in isolated tissues and ventricular myocytes from animals18, 61, 77, 110, 113, 114 and in reducing proarrhythmic SR Ca2+ leak and improving contractility in failing human ventricles.115 These data, from diverse sources support a view that CaMKII can contribute to initiation and perpetuation of ventricular arrhythmias and that CaMKII inhibition is an effective antiarrhythmic therapy.
Inherited Tachyarrhythmias
CaMKII has been implicated in a number of genetic arrhythmia syndromes, including the Brugada Syndrome,77, 78, 82 long QT syndromes - particularly long QT syndrome 377 and the Timothy Syndrome,70 and catecholaminergic polymorphic ventricular tachycardia (CPVT).116, 117 CaMKII may be part of a feed forward proarrhythmic circuit in each of these examples by virtue of the tendency of CaMKII to become constitutively active under conditions of increased Ca2+i (e.g. CPVT where ryanodine receptors are 'leaky'),116, 117 prolonged intracellular Ca2+ transients (e.g. action potential prolongation in the long QT syndromes)62, 64, 65 or rapidly repetitive Ca2+i, as occurs in all tachyarrhythmias. CaMKII inhibition has been effective in reducing arrhythmias in animal models of genetic arrhythmias,110, 116 suggesting that CaMKII inhibitory therapy could be useful in reducing the arrhythmia risk in patients.
Conclusions
A modern understanding of arrhythmia mechanisms acknowledges that proarrhythmic behaviors of ion channels depend on a cellular and tissue context, which together contribute to common clinical arrhythmias. It is increasingly clear that CaMKII regulates physiological connections between ion channels and intracellular Ca2+ homeostasis in myocardium. Under pathological acquired or genetic stress excessive CaMKII activity promotes brady- and tachyarrhythmias. Proof of concept studies in animal models consistently show CaMKII inhibition provides anti-arrhythmic benefits. Thus, our field awaits development of clinically applicable CaMKII inhibitory drugs to determine if the experimentally observed benefits of CaMKII inhibition will also improve the lives of patients. Many questions remain, including whether systemic CaMKII will be tolerated, and multiple steps will be necessary, including development of small molecules with drug-like properties and adequate specificity - features that are lacking in currently available experimental inhibitors, before the clinical efficacy of CaMKII inhibition for treating arrhythmias can be tested.
Acknowledgements
We are grateful to Shawn Roach for the graphic design.
Funding Sources: This work was funded in part by US National Institutes of Health (R01HL70250, R01HL079031, R01HL113001 and R01HL096652) and a grant (08CVD01) from the Fondation Leducq as part of the ‘Alliance for CaMKII Signaling in Heart’. A.G.R. was supported by a Career Development Award from the Fondation Leducq.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: M.E.A. is a named inventor on intellectual property claiming to treat arrhythmias by CaMKII inhibition and is a co-founder of Allosteros Therapeutics, a biotech company aiming to develop enzyme-based therapies.
References
- 1.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association task force and the European Society of Cardiology committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 3.Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80:1675–1680. doi: 10.1161/01.cir.80.6.1675. [DOI] [PubMed] [Google Scholar]
- 4.Lehnart SE, Ackerman MJ, Benson DW, Jr, Brugada R, Clancy CE, Donahue JK, George AL, Jr, Grant AO, Groft SC, January CT, Lathrop DA, Lederer WJ, Makielski JC, Mohler PJ, Moss A, Nerbonne JM, Olson TM, Przywara DA, Towbin JA, Wang LH, Marks AR. Inherited arrhythmias: A National Heart, Lung, and Blood Institute and office of rare diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation. 2007;116:2325–2345. doi: 10.1161/CIRCULATIONAHA.107.711689. [DOI] [PubMed] [Google Scholar]
- 5.Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–672. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the Framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 8.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson WG, Stevenson LW, Middlekauff HR, Saxon LA. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation. 1993;88:2953–2961. doi: 10.1161/01.cir.88.6.2953. [DOI] [PubMed] [Google Scholar]
- 10.Lamas GA, Lee K, Sweeney M, Leon A, Yee R, Ellenbogen K, Greer S, Wilber D, Silverman R, Marinchak R, Bernstein R, Mittleman RS, Lieberman EH, Sullivan C, Zorn L, Flaker G, Schron E, Orav EJ, Goldman L. The mode selection trial (MOST) in sinus node dysfunction: Design, rationale, and baseline characteristics of the first 1000 patients. Am Heart J. 2000;140:541–551. doi: 10.1067/mhj.2000.109652. [DOI] [PubMed] [Google Scholar]
- 11.Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: A population-based analysis. J Gen Intern Med. 2008;23(Suppl 1):13–19. doi: 10.1007/s11606-007-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD investigators. Survival with orald-sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 13.Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The cardiac arrhythmia suppression trial (CAST) investigators. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 14.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 15.White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM. Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem. 1998;273:3166–3172. doi: 10.1074/jbc.273.6.3166. [DOI] [PubMed] [Google Scholar]
- 16.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Brown JH. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J Biol Chem. 2011;286:7990–7999. doi: 10.1074/jbc.M110.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The delta c isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 21.Chao LH, Stratton MM, Lee IH, Rosenberg OS, Levitz J, Mandell DJ, Kortemme T, Groves JT, Schulman H, Kuriyan J. A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin-dependent kinase II holoenzyme. Cell. 2011;146:732–745. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan M, Edman CF, Schulman H. Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J Cell Biol. 1994;126:839–852. doi: 10.1083/jcb.126.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282:35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 24.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltacoverexpression uniquely alters cardiac myocyte Ca2+handling: Reduced SR Ca2+load and activated SR Ca2+release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 25.Mishra S, Gray CB, Miyamoto S, Bers DM, Brown JH. Location matters: Clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ Res. 2011;109:1354–1362. doi: 10.1161/CIRCRESAHA.111.248401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 27.Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of Threonine 305/306 and Serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- 28.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: A computational analysis. PLoS Comput Biol. 2009;5:e1000583. doi: 10.1371/journal.pcbi.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem. 2004;279:44573–44581. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- 32.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 34.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Einthoven W. Ueber die Form des menschlichen Electrocardiogramms. Arch Gesamte Physiol. 1895:101–123. [Google Scholar]
- 37.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 38.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: hERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5a mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 40.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. HCN4 provides a 'depolarization reserve' and is not required for heart rate acceleration in mice. EMBO J. 2007;26:4423–4432. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 43.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 44.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004;94:802–809. doi: 10.1161/01.RES.0000122045.55331.0F. [DOI] [PubMed] [Google Scholar]
- 45.January CT, Riddle JM. Early afterdepolarizations: Mechanism of induction and block. A role for L-type Ca2+current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 46.Fabiato A, Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The antiarrhythmics versus implantable defibrillators (AVID) investigators. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 48.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 49.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, Abraham TP, Tomaselli GF, Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–1377. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 50.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: Sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and INaremodeling after myocardial infarction: Insights from mathematical modeling. J Mol Cell Cardiol. 2008;45:420–428. doi: 10.1016/j.yjmcc.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME. Cav1.2 beta-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci U S A. 2010;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blaich A, Welling A, Fischer S, Wegener JW, Kostner K, Hofmann F, Moosmang S. Facilitation of murine cardiac L-type Cav1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc Natl Acad Sci U S A. 2010;107:10285–10289. doi: 10.1073/pnas.0914287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca2+-induced enhancement of the L-type Ca2+current in rabbit ventricular myocytes. Circ Res. 1994;75:854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 57.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 58.Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 59.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 62.Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca2+/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 63.Mazur A, Roden DM, Anderson ME. Systemic administration of calmodulin antagonist W-7 or protein kinase a inhibitor h-8 prevents torsade de pointes in rabbits. Circulation. 1999;100:2437–2442. doi: 10.1161/01.cir.100.24.2437. [DOI] [PubMed] [Google Scholar]
- 64.Wu Y, Roden DM, Anderson ME. Calmodulin kinase inhibition prevents development of the arrhythmogenic transient inward current. Circ Res. 1999;84:906–912. doi: 10.1161/01.res.84.8.906. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+current: A cellular mechanism for long QT arrhythmias. Am J Physiol. 1999;276:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- 66.Wu Y, Kimbrough JT, Colbran RJ, Anderson ME. Calmodulin kinase is functionally targeted to the action potential plateau for regulation of L-type Ca2+current in rabbit cardiomyocytes. J Physiol. 2004;554:145–155. doi: 10.1113/jphysiol.2003.053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: A decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 68.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 69.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Cav1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 70.Thiel WH, Chen B, Hund TJ, Koval OM, Purohit A, Song LS, Mohler PJ, Anderson ME. Proarrhythmic defects in Timothy Syndrome require calmodulin kinase II. Circulation. 2008;118:2225–2234. doi: 10.1161/CIRCULATIONAHA.108.788067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.January CT, Riddle JM, Salata JJ. A model for early afterdepolarizations: Induction with the Ca2+channel agonist Bay K 8644. Circ Res. 1988;62:563–571. doi: 10.1161/01.res.62.3.563. [DOI] [PubMed] [Google Scholar]
- 72.Qi X, Yeh YH, Chartier D, Xiao L, Tsuji Y, Brundel BJ, Kodama I, Nattel S. The calcium/calmodulin/kinase system and arrhythmogenic afterdepolarizations in bradycardia-related acquired long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2:295–304. doi: 10.1161/CIRCEP.108.815654. [DOI] [PubMed] [Google Scholar]
- 73.Weidmann S. Effect of current flow on the membrane potential of cardiac muscle. J Physiol. 1951;115:227–236. doi: 10.1113/jphysiol.1951.sp004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khoo MS, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, Grueter CE, Guan X, Oddis CV, Zhang R, Mendes L, Ni G, Madu EC, Yang J, Bass M, Gomez RJ, Wadzinski BE, Olson EN, Colbran RJ, Anderson ME. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114:1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 75.Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, Dobrev D, Nattel S, Kodama I, Kamiya K. Ca2+-related signaling and protein phosphorylation abnormalities play central roles in a new experimental model of electrical storm. Circulation. 2011;123:2192–2203. doi: 10.1161/CIRCULATIONAHA.110.016683. [DOI] [PubMed] [Google Scholar]
- 76.Tanskanen AJ, Greenstein JL, O'Rourke B, Winslow RL. The role of stochastic and modal gating of cardiac L-type Ca2+channels on early after-depolarizations. Biophys J. 2005;88:85–95. doi: 10.1529/biophysj.104.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+calmodulin, and CaMKII in normal and failing dog cardiomyocytes: Similarities and differences. Am J Physiol Heart Circ Physiol. 2008;294:H1597–H1608. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated ca/calmodulin kinase II delta is required for late INaaugmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A betaIV-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, Bers DM, Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel Nav1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, Hirakawa R, Budas GR, Rajamani S, Shryock JC, Belardinelli L. Nav1.5-dependent persistent Na+influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–C586. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 84.Effect of metoprolol cr/xl in chronic heart failure: Metoprolol cr/xl randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 85.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 86.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 87.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 88.Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501(Pt 1):17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 90.Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261:13333–13341. [PubMed] [Google Scholar]
- 91.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 92.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 93.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 94.Yang Y, Zhu WZ, Joiner ML, Zhang R, Oddis CV, Hou Y, Yang J, Price EE, Gleaves L, Eren M, Ni G, Vaughan DE, Xiao RP, Anderson ME. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am J Physiol Heart Circ Physiol. 2006;291:H3065–H3075. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 95.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase a-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toischer K, Rokita AG, Unsold B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuss L, Gupta SN, Schmidt K, Lehnart SE, Kruger M, Linke WA, Backs J, Regitz-Zagrosek V, Schafer K, Field LJ, Maier LS, Hasenfuss G. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rudolph V, Andrie RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, Schwoerer AP, Lau D, Fu X, Klingel K, Sydow K, Didie M, Seniuk A, von Leitner EC, Szoecs K, Schrickel JW, Treede H, Wenzel U, Lewalter T, Nickenig G, Zimmermann WH, Meinertz T, Boger RH, Reichenspurner H, Freeman BA, Eschenhagen T, Ehmke H, Hazen SL, Willems S, Baldus S. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;16:470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, Singh M, Yang J, Guan X, Lowe JS, Weiss RM, Zimmermann K, Yull FE, Blackwell TS, Mohler PJ, Anderson ME. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor b gene expression in the mouse heart. J Clin Invest. 2009;119:986–996. doi: 10.1172/JCI35814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol. 2012 doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K+current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999;85:810–819. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- 102.Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, Allen BG, Nattel S. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res. 2008;103:733–742. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- 103.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+leak and elevated diastolic Ca2+levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 105.Greiser M, Neuberger HR, Harks E, El-Armouche A, Boknik P, de Haan S, Verheyen F, Verheule S, Schmitz W, Ravens U, Nattel S, Allessie MA, Dobrev D, Schotten U. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–394. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 106.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+leak and increased Na+-Ca2+exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evans R, Shaw DB. Pathological studies in sinoatrial disorder (sick sinus syndrome) Br Heart J. 1977;39:778–786. doi: 10.1136/hrt.39.7.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 109.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of camp-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 110.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 112.Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, Belke DD, Dillmann WH, Rogers TB, Schulman H, Ross J, Jr, Brown JH. The cardiac-specific nuclear deltabisoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2a activity. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 113.Belevych AE, Terentyev D, Terentyeva R, Ho HT, Gyorke I, Bonilla IM, Carnes CA, Billman GE, Gyorke S. Shortened Ca2+signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–577. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Z, Fefelova N, Shanmugam M, Bishara P, Babu GJ, Xie LH. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol. 2011;50:128–136. doi: 10.1016/j.yjmcc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittkopper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 116.Liu N, Ruan Y, Denegri M, Bachetti T, Li Y, Colombi B, Napolitano C, Coetzee WA, Priori SG. Calmodulin kinase II inhibition prevents arrhythmias in RyR2R4496c+/−mice with catecholaminergic polymorphic ventricular tachycardia. J Mol Cell Cardiol. 2011;50:214–222. doi: 10.1016/j.yjmcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 117.Dybkova N, Sedej S, Napolitano C, Neef S, Rokita AG, Hunlich M, Brown JH, Kockskamper J, Priori SG, Pieske B, Maier LS. Overexpression of CaMKIIdeltacin RyR2R4496c+/−knock-in mice leads to altered intracellular Ca2+handling and increased mortality. J Am Coll Cardiol. 2011;57:469–479. doi: 10.1016/j.jacc.2010.08.639. [DOI] [PubMed] [Google Scholar]