Abstract
The changes in red blood cells (RBC) as they age and the mechanisms for their eventual removal have been of interest for many years. Proposed age-related changes include dehydration with increased density and decreased size, increased membrane IgG, loss of membrane phospholipid asymmetry, and decreased activity of KCl cotransport. The biotin RBC label allows unambiguous identification of older cells and exploration of their properties as they age. Autologous normal human RBC were labeled ex vivo and, after reinfusion, compared with unlabeled RBC throughout their lifespan. RBC density increased with age, with most of the change in the first weeks. Near the end of their lifespan, RBC had increased surface IgG. However, there was no evidence for elevated external phosphatidylserine (PS) even though older RBC had significantly lower activity of aminophospholipid translocase (APLT). KCl cotransport activity persisted well past the reticulocyte stage, but eventually decreased as the RBC became older. These studies place limitations on the use of density fractionation for the study of older human RBC, and do not support loss of phospholipid asymmetry as a mechanism for human RBC senescence. However, increased levels of IgG were associated with older RBC, and may contribute to their removal from the circulation.
Introduction
Normal human red blood cells (RBC) all survive to about the same age. This implies that a molecular “alarm clock” keeps track of a cell’s age, and at the proper time triggers a change that leads to removal by the reticuloendothelial system. For many years there has been great interest in the nature of this process, and evidence has been presented for proposed mechanisms. Several lines of investigation have implicated naturally occurring antibodies as important, but a definitive model of RBC aging and senescence has remained elusive [1]. The proposed targets for the antibodies include proteolytically modified Band 3 [2,3], α-galactosyl carbohydrate [4,5], and clustered Band 3 [6,7]. The biotin label, introduced in 1987, has provided detailed and unequivocal information about age-dependent normal RBC changes in animals [8–20]. While much has been learned from these studies, the different patterns of red cell removal in various species complicate the application of these findings to human RBC. Dog RBC have been proposed [17] as an appropriate model for human RBC since they survive about the same length of time and are removed in an age-dependent manner. Senescent dog RBC, identified with a biotin label, were shown to have elevated levels of membrane immunoglobulin [15].
Studies in rodents [19,20] indicate that phosphatidylserine (PS), which is normally confined to the inner membrane leaflet, is externalized toward the end of the RBC lifespan. Since macrophages have PS receptors, the presence of external PS could contribute to the removal of senescent RBC. However, it remains in doubt whether the appearance of PS on older RBC is directly related to their removal. In mice, recent studies have shown that a loss of aminophospholipid translocase (APLT) activity in older RBC may contribute to loss of phospholipid asymmetry [21]. Most studies have shown that the removal of mouse RBC from the circulation is not strongly age-dependent, with random RBC dominating clearance kinetics [22]. However, a recent study that sampled very small volumes of blood to determine the number of labeled RBC found a linear survival curve, implying strictly age-dependent removal [23].
There is good evidence that RBC tend to become more dense as they age, and many studies have used density as a surrogate for age. Nevertheless, it has been a matter of some controversy whether the enrichment of older RBC in the dense fraction is adequate for this purpose. Biotin label studies in rabbits [24] showed minimal enrichment of older RBC in the dense fraction whereas analogous studies in dog [16] resulted in much better discrimination. Subsequent studies have suggested that the oldest human RBC may gain sodium and rehydrate prior to removal from the circulation [25]. If so, the cells most representative of the senescent state would not be in the dense fraction.
The mechanism for dehydration as RBC age is not well understood, and may not be the same for younger and older cells. Reticulocytes have relatively high activity of KCl cotransport (KCC), and this pathway is thought to mediate the decrease in hydration, and therefore size, as the cells progress to mature RBC. KCl cotransport activity is not confined to reticulocytes, however, and may be activated in mature RBC by urea [26] and by high hydrostatic pressure [27]. Another possibility for dehydration is that episodic increases in intracellular Ca++, perhaps related to passage through regions with high shear rate, cause activation of the Ca++-dependent Gardos K+ efflux pathway [28–30].
The study of RBC aging requires a method to label and follow RBC in the circulation as they age, with periodic analysis of the property of interest. Ideally, this would be accomplished by labeling an age cohort of cells as they came out of the bone marrow. However, there is no available cohort label for human subjects that allows separation of labeled cells and subsequent analysis of their properties. In the studies reported here a sample of circulating RBC of all ages was labeled with biotin. At the start of the experiment the mean ages of the labeled RBC and the entire RBC population were the same, about fifty days [31]. As the labeled RBC aged in the circulation, their mean age increased and their age distribution became narrower. Toward the end of the experiment all of the labeled cells approached senescence. Labeled RBC were detected and separated by flow cytometry, so it was possible to identify the aging RBC on a cell-by-cell basis rather than as an average activity as would be the case with a radioactive label. These labeled RBC could then be examined for potential age-related changes using a second fluorescent label in the flow cytometer or by other techniques. We examined some of the RBC properties that have been of interest in RBC aging, including morphology, density, size, surface IgG, external PS, APLT activity, and KCC activity. Our findings indicate that old cells are on average denser than the entire population, but the change takes place mostly within the first one or two weeks after labeling and is characterized by movement of cells from the lighter fractions. Although APLT activity decreased with age, we found no evidence for increased external PS in old human RBC. There was, however, evidence for increased membrane-associated IgG as the RBC aged. Maximum KCC activity decreased in older cells, but higher levels than expected remained past the reticulocyte stage.
Methods
RBC label
Autologous RBC from six normal subjects were labeled with biotin and reinfused after informed consent was given, but not all RBC properties were measured in every subject These subjects were the controls for a study of HbA1c generation in diabetes, and their RBC survival curves have been previously published [32]. Subject characteristics, cell labeling procedures, and methods for post-infusion identification of biotin labeled RBC (B-RBC) by flow cytometry have also been described [32]. During the labeling procedure care was taken to retain cells at the top of the red cell column to minimize the loss of reticulocytes and other light erythroid cells. At selected post-infusion time points, blood samples were collected in EDTA and either kept stored at 4°C until the next day for analysis in Cincinnati or shipped overnight on ice for analysis at Purdue. An advantage of the biotin label is the presence of two types of internal controls. The first is a comparison of labeled and unlabeled RBC immediately after reinfusion. If their properties are not identical, then the labeling itself has changed the cells. The second internal control is the stability of the properties of the unlabeled RBC during the four month experiment. If the unlabeled RBC change, then the subject is not at steady state. There was no evidence for a change due to labeling or for instability of unlabelled cells for the variables studied here. In addition, the labeled RBC must be older than the time since reinfusion since the labeling reagent is present only in vitro, and there is no recirculation of the label in vivo.
Morphology and RBC IgG
Fixation and permeabilization of RBC
RBC were washed three times in PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Ka2HPO4, 1.5 mM KH2PO4, pH 7.4) and 50 lL of pelleted RBC were fixed in eppendorf tubes at room temperature for exactly 5 min in 1 mL of 0.5% acrolein (Sigma-Aldrich) made fresh in PBS. After fixation the samples were centrifuged at 1,000g for 1 min, resuspend in PBS containing 0.1 M glycine to neutralize the unreacted aldehydes, and then rinsed three times in the same buffer. Fixed cells were permeabilized in PBS containing 0.1% Triton X-100 (Pierce) for 5 min at room temperature, followed by three washes in PBS/glycine containing 0.2% fish skin gelatin (Sigma). RBC were left 1 hr in this blocking solution before incubation with antibodies. For triple labeling experiments erythrocytes were incubated with mouse monoclonal anti band 3 (IVF12, a gift from Dr. M.L. Jennings) and streptavidin conjugated with AF488 (Invitrogen) for 40 min and washed three times in blocking buffer. A second incubation of 40 min with F(ab’)2 fragments of anti-mouse Ig conjugated with Cy5 and F(ab’)2 fragments of anti-human IgG conjugated with rhodamine Red-X (Jackson ImmunoResearch) was performed for 40 min, followed by washes as described above. Erythrocytes were attached to poly-l-lysine (Sigma) coated cover slips, mounted to slides using Aquamount (Fisher Scientific) and sealed using nail polish. Images were acquired using an MRC-1024 UV confocal system (Bio-Rad, Hemel Hempstead, England) on a Diaphot 300 inverted microscope (Nikon, Tokyo, Japan) using a 60× 1.4 NA oil-immersion lens. The acquisition software was Lasersharp 2000 version 5.0 (Bio-Rad, Hemel Hempstead, England).
Unfixed nonpermeabilized RBC
Whole blood was pelleted at 1,000g and the buffy coat removed. RBC were washed three times in PBS. A volume of 50 lL of washed RBC were resuspended in PBS containing 0.5% BSA for 60 min to block nonspecific binding. Cells were then incubated with streptavidin-AF488 (Invitrogen) and/or anti- F(ab’)2 fragments of anti-human IgG conjugated with rhodamine (Jackson ImmunoResearch) for 40 min, and washed three times in PBS/BSA before analysis.
Isolated RBC
RBC were washed and processed as described above for unfixed RBC and incubated with Streptavidin Microbeads (Miltenyi Biotech) following manufacturer guidelines. B-RBC were isolated using MS columns (Miltenyi Biotech) and labeled as described above for unfixed RBC. If the cells were not analyzed at once, they were left in PBS containing 2% paraformaldehyde to prevent any changes or lysis.
Density
RBC were fractionated using a discontinuous Optiprep® gradient that was prepared as previously described [33], except seven fractions were isolated with the following densities: <1085 (fx 1), 1.085–1.090 (fx 2), 1.090–1.095 (fx 3), 1.095–1.100 (fx 4), 1.100–1.105 (fx 5), 1.105–1.110 (fx 6), >1.100 (fx 7). For each fraction at each postinfusion time point, the percentage of total RBC was determined using an electronic cell counter (Coulter Z1) and the percentage of biotinylated RBC (B-RBC) was determined by flow cytometry. From these data the percentage of total B-RBC present in each fraction was calculated, yielding the density distribution of the B-RBC. A time-dependent shift towards higher fractions indicates an increase in density as the B-RBC become older.
Forward light scatter
The flow cytometric forward light scatter (FS) parameter gives a relative estimate of cell size. For each post-infusion time point, the mean FS was determined for fluorescent regions containing either unlabeled RBC or B-RBC. These values were plotted versus time and the slopes (ΔFS/day) were determined.
PS and APLT
Externalized PS (PS+) on labeled and unlabeled RBC at each post-infusion time point was determined using a two color (Annexin-FITC/Streptavidin-PE) flow cytometric protocol as previously described [34]. To normalize for small sample to sample variation in the percentage of PS(+) unlabeled RBC, the data were expressed as the difference between the percent PS(+) RBC in the labeled and unlabeled RBC populations.
Changes in APLT activity with cell age were determined using a two color flow cytometric assay (NBD-PS/Streptavidin-PE). At selected postinfusion time points, RBC were first labeled with streptavidin-PE and then an NBD-PS incorporation assay was performed as previously described [35]. Briefly, RBC were incubated with NBD-PS, which is transferred to the inner leaflet by APLT. After incubation, the remaining NBD-PS in the outer leaflet is removed by extraction with albumin. The NBD-PS fluorescence is determined before and after extraction for the unlabeled RBC and B-RBC, and APLT activity is expressed as the fraction of total fluorescence remaining after extraction.
KCl cotransport
We tested the hypothesis that KCC activity persists past the reticulocyte stage and for part of the lifespan of mature RBC, but is not present in older RBC. This was done by using a combination of two approaches. First, unlabeled RBC were swollen using the ionophore nystatin to MCHC 24 gm/dl [26]. KCC was then stimulated by incubating two hours in HEPES-buffered saline (HBS) with 600 mM urea [26]. The MCHC increase due to KCC-mediated loss of K+ from the cells was measured using an Advia 120 clinical hematology analyzer which measures each cell and displays an MCHC distribution histogram. If the percentage of the cells that shift to higher MCHC is greater than the percentage of reticulocytes, then mature RBC must also have KCC activity. However, this approach alone does not definitively show that it is the younger mature RBC with KCC activity. To explore this we used the biotin-labeled RBC. At selected post-reinfusion time points, RBC were treated with nystatin and then incubated in urea in a similar way. Before and after incubation the RBC were analyzed on a five fraction discontinuous Optiprep density gradient and density distributions of labeled and unlabeled RBC were determined as described above. Under these conditions a shift to higher density indicates KCC activity. Since all the biotin labeled RBC must be older than the time since reinfusion, loss of activity part way through the lifespan means that older RBC lack activity.
Results
The survival curves for the biotin-labeled normal human RBC used in the current experiments were previously published as a nondiabetic (NDM) control group [32]. The coding for individual subjects (NDMn, n = 1 thru 6) is retained from the previous publication.
Morphology and membrane IgG
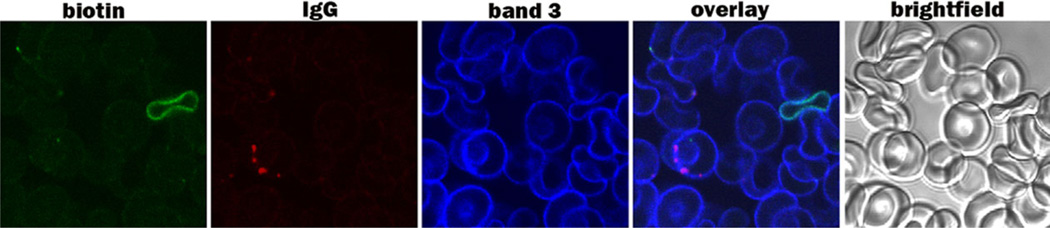
Normal RBC exhibit little change in morphology during the bulk of their lifespan, as shown by the remarkable uniformity observed in routine blood smears. There has been little previous effort, however, to specifically examine cells that are reaching the end of their lifespan for changes in their appearance. Figure 1 shows fixed RBC from a blood sample that was drawn 78 days after reinfusion of biotinylated RBC (B-RBC), establishing that the B-RBC are at least 78 days old. The field shows one B-RBC that is negative for IgG and one RBC that is not biotinylated but positive for IgG in the form of several distinct clusters. These data show that IgG binding is not necessarily located on the B-RBC, and is therefore not an artifact of labeling. In this sample the IgG positive cell could easily be older than the biotinylated cell, since only a small number of biotinylated RBC were reinfused into the patient. In general, band 3 stained more densely adjacent to the IgG clusters, but this clustering of band 3 was sometimes difficult to photograph in the same focal plane as the clumps of IgG. Quantitative analysis of erythrocytes collected and stained 78 days after re-infusion of the biotinylated cells revealed that ~25% of the biotinylated population displayed both clustered band 3 and cell surface IgG. By 92 days after re-infusion of biotinylated cells nearly 80% of the biotinylated fraction showed clustered band 3 and attached IgG, suggesting that band 3 clustering and IgG binding may constitute a marker for cell aging.
Figure 1.
Confocal analysis of fixed and permeabilized RBC (subject NDM2) 78 days after reinfusion. A cell entirely coated with biotin (green) and a different cell with a small biotin cluster are shown in the first picture. A different cell in the field is seen to contain 4 clusters of IgG (second picture). The same cell that has IgG has a strong band 3 label (third picture). The fourth picture shows the overlay of all labels.
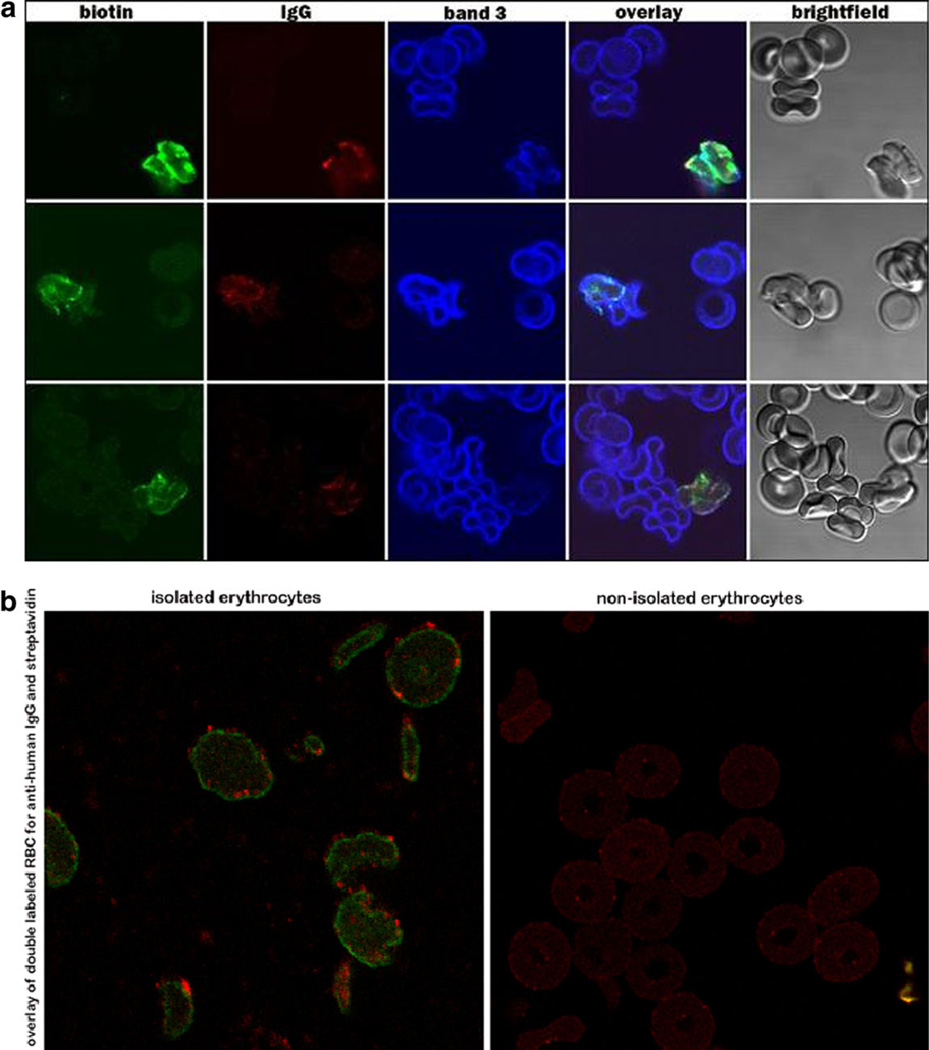
Figure 2 shows labeled RBC 126 days after reinfusion. These cells, which are close to the end of their lifespan, generally have an irregular morphology, and in some cases exhibit a very uneven and perhaps discontinuous distribution of band 3 in the membrane. These morphological abnormalities were not observed when biotinylated cells collected <100 days post-infusion were examined, suggesting that neither biotinylation nor fixation for immunofluorescence microscopy caused the structural changes. Interestingly, at this late stage most biotinylated cells were coated with IgG, and the band 3 often appeared clumped. Some of the very old RBC also displayed decreased overall staining for band 3 (Fig. 2A, 3rd row, panel 3).
Figure 2.
IgG on old RBC. A: (NDM1, day 126) At day 126 the biotinylated cells appear misshapen, and are all IgG labeled. B: IgG coated >100 day old biotinylated RBC isolated using magnetic beads derivatized with streptavidin (left panel), and the nonisolated aliquot from the same sample (right panel).
To corroborate that the attachment of IgG is not an artifact of processing, unfixed cells were also analyzed for the presence of IgG. As seen in Fig. 2B (left panel), the aforementioned association between old RBC and IgG was also present in unfixed, magnetically isolated RBC. The right panel of Fig. 2B shows cells of the same sample that were not captured by the streptavidin beads. They were collected as control cells during washing of the beads before elution of the bound biotinylated RBC. They were then labeled with streptavidin and anti-human IgG exactly as the cells in the left panel (Fig. 2B). As anticipated, the control cells contain no bound IgG.
Density and size
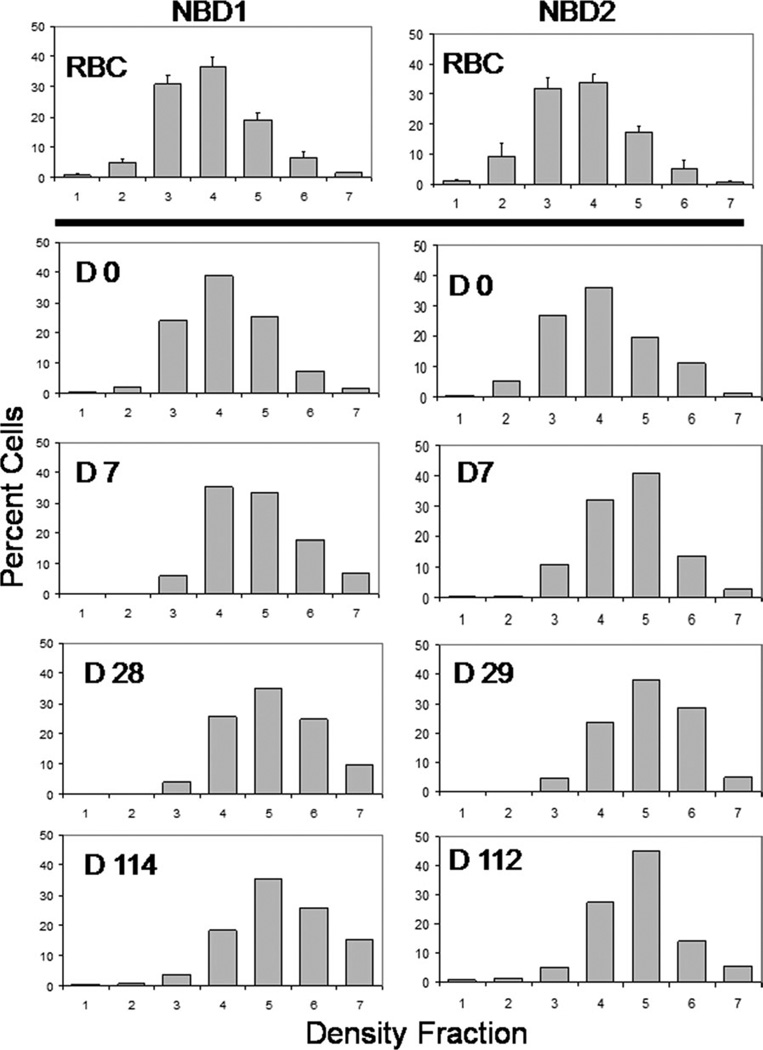
In two subjects density profiles were generated for labeled and unlabeled RBC at postinfusion time points. As shown by the small error bars in the top panel of Fig. 3, there was little or no time-dependent change in the steady-state density of unlabeled RBC. The density profile of biotin- labeled RBC immediately after reinfusion was essentially identical to the unlabeled RBC, indicating that no change in cellular hydration occurred during labeling. As the labeled cells aged, there was a shift to higher density that occurred mostly in the first 1 or 2 weeks (Fig. 3), followed by smaller changes in the following months. Most of this early shift was from the relatively light fraction 3 (1.090–1.095 g/cc) to more dense fractions. These data suggest that while there is a significant density change early in the RBC lifespan, there is little change during most of the time in the circulation. Thus isolation of a high density population of RBC for study would eliminate the youngest cells, but would not produce a highly enriched population of the oldest cells.
Figure 3.
Age-dependent cellular density changes for subjects NDM1 and NDM2. RBC were separated by density using a discontinuous gradient. The top charts in each column show the average percent cells (± 1 sd) in each fraction for the unlabeled RBC at all time points (N = 16 for NDM1, N = 13 for NDM2). The bottom four charts in each column show the density distributions for labeled RBC at selected time points. Day 0 is immediately after reinfusion.
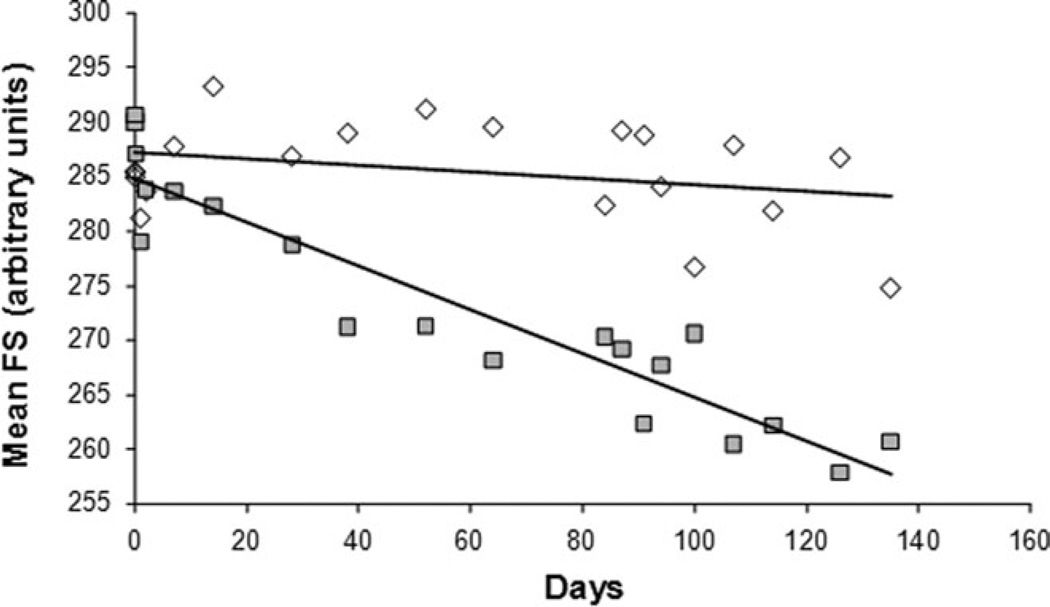
The forward scatter (FS) value one day after reinfusion was 271.1 ± 18.4 (1 SD, N = 6, arbitrary units) for unlabeled RBC and 277.2 ± 13.1 (1 SD, N = 6) for biotin labeled RBC. These values are not significantly different (paired t-test P = 0.37), indicating that there was no change in cell size during labeling and other in vitro manipulation. Figure 4 shows FS as a function of the time since reinfusion for subject H11-1. As expected, there was little or no change in FS for the steady state unlabeled RBC. However, there is a linear decrease in FS with age for the labeled RBC. For all subjects the slope for unlabeled RBC (FS/day) was −0.0015 ± 0.1034 (1 SD, N = 6) and for labeled RBC was −0.1677 ± 0.0699. These slopes were significantly different by paired t-test (P = 0.0018), and would indicate a total decrease in cell size of about 7% during the entire experiment as estimated by FS. Since on day one the average cell age is about one half of the maximum age, the total change in size from young to old mature RBC would be about twice this, or 14% (assuming linearity throughout the lifespan). This is less than the total MCV range that has been observed in blood [36], but since the slope derived from the labeled RBC is heavily weighted for mature RBC, the changes due to reticulocyte maturation are not fully taken into account.
Figure 4.
The mean forward scatter FS for unlabeled (◇) and labeled (■) RBC as a function of time since reinfusion.
External PS
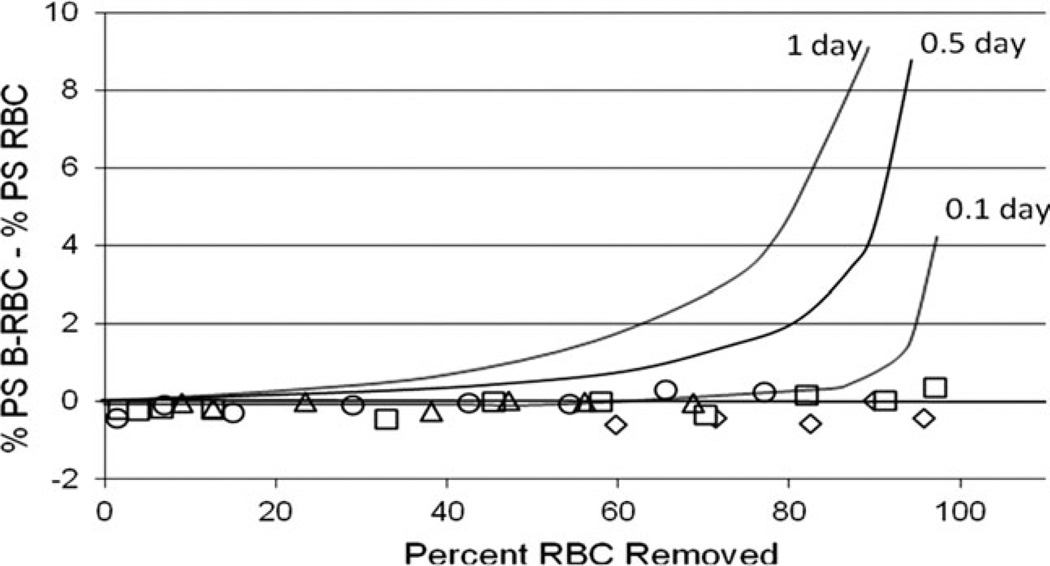
We determined whether the percentage of labeled RBC with external PS increased as the cells aged in the circulation (Fig. 5). To smooth small day-to-day variation in the assay, the data was expressed as a difference between the labeled and unlabeled RBC. To normalize the data for small differences in red cell survival in these normal subjects this difference was plotted as a function of the percentage of labeled RBC that have been removed from the circulation, taken from the RBC survival curve for each subject. Unlike previous results in rodents [19,20], the percentage of labeled cells with external PS remained constant for the entire lifespan. There was no gradual increase that would have indicated an age-related but probably not senescent change, and there was no abrupt increase at the end of the lifespan that would suggest a senescent change leading to removal. Of course, it is possible that a major, abrupt increase in external PS occurs shortly before removal, and therefore only a very small percentage of older cells in the circulation would be positive. To establish some temporal limits regarding the duration of PS positivity, Fig. 5 also shows three hypothetical curves with the calculated percent PS(+) cells for three assumed durations of external PS prior to removal. (e.g., for a one day marker and a lifespan 100 days, about 1% of the unlabeled cells are positive, and about 10% of the labeled RBC are positive after 90 percent have been removed. Therefore, the difference between labeled and unlabeled in Fig. 5 is 9%). These curves indicate that if this duration is greater than 0.1 day, it should have been possible to detect PS(+) cells in these experiments. This analysis suggests that PS externalization could be a signal for removal of senescent RBC only if externalization is very abrupt and removal occurs in less than 0.1 days after the signal appears.
Figure 5.
Age-dependent cellular external PS. The x-axis shows the percentage of the labeled RBC that have been removed from the circulation. The y-axis shows the percentage of labeled RBC with external PS normalized by subtracting the concurrent percentage of unlabeled RBC with external PS. Data on 4 subjects (open symbols) are compared to theoretical curves (lines) calculated assuming RBC with external PS remain in the circulation for 1, 0.5, or 0.1 days prior to removal.
APLT activity
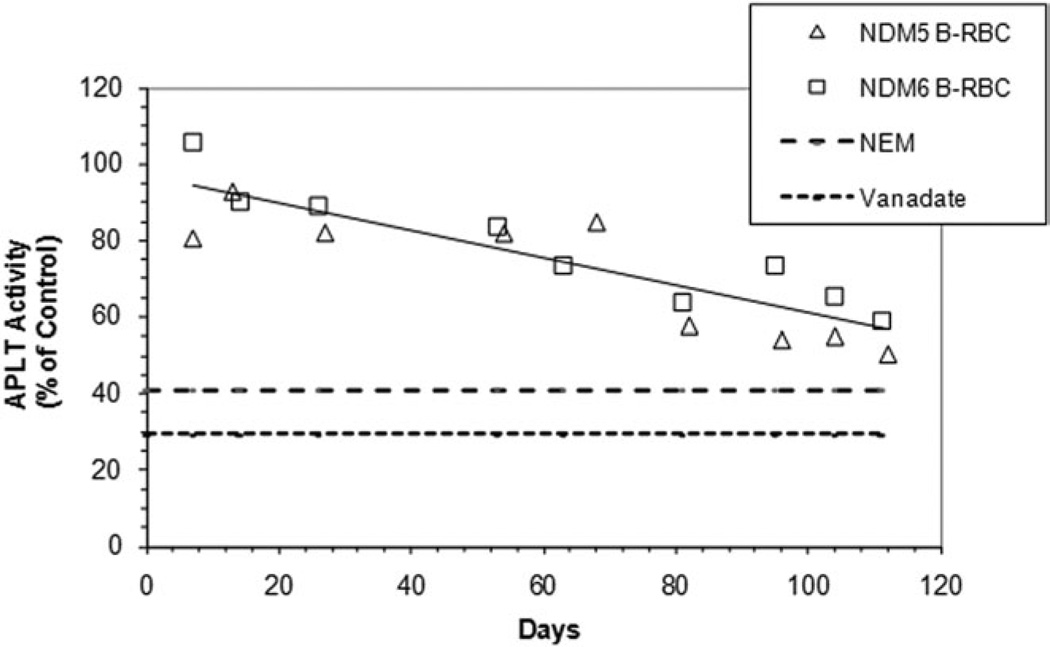
De Yong et al. showed that in mice aminophospholipid translocase (APLT) activity decreases with RBC age [21]. We measured this activity for biotin-labeled RBC in two human subjects using internalization of NBD-labeled PS, normalized as a percentage of internalization in the unlabeled RBC present in the same sample. As shown in Fig. 6, APLT activity decreases with time after reinfusion with the oldest cells having about 60% of the activity of the steady state population. As shown in Fig. 5, this does not result in the externalization of measurable amounts of PS, presumably because there is little or no activation of scramblase in these normal cells.
Figure 6.
Age-dependent cellular change in APLT activity. The fraction of NBD-PS transferred from the outer to the inner leaflet in biotin-labeled RBC was determined and expressed relative to the fraction in unlabeled RBC in the same sample. The effects of compounds that inhibit APLT activity (2 µM NEM and 10 mM vanadate) are also shown as controls.
KCC activity
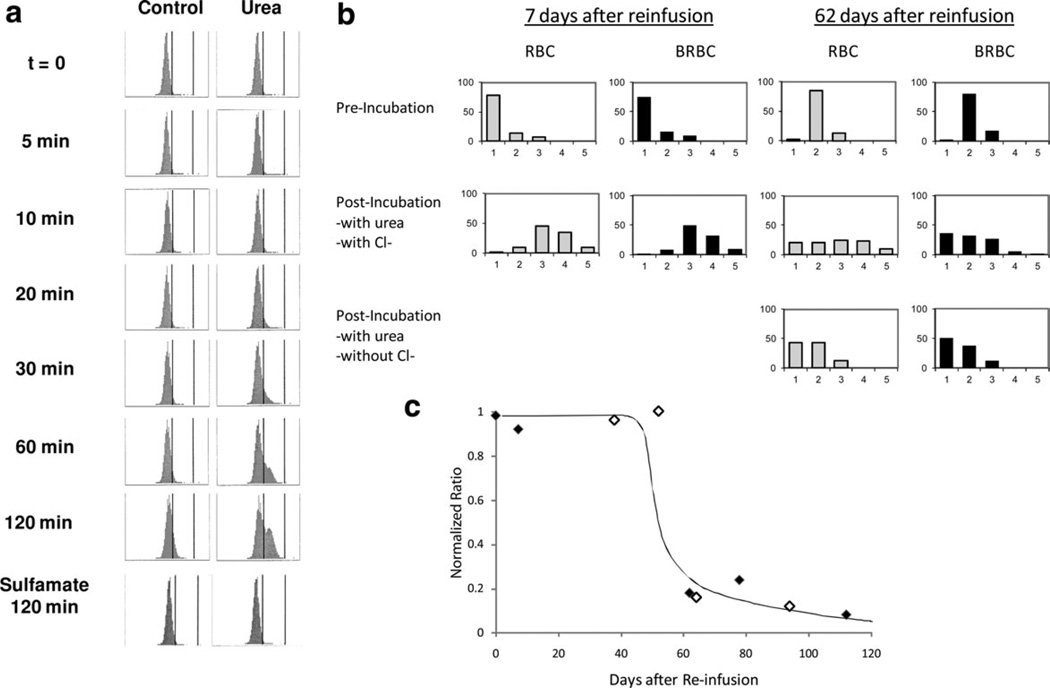
Changes in cellular hemoglobin concentration (CHC) were measured in a separate group of 7 normal subjects using the Advia 120 [26] and used to monitor KCC activity in mature RBC. Figure 7A shows CHC frequency distributions in mature normal RBC swollen by nystatin treatment and then incubated in HBS without (control) and with 600 mM urea, which activates KCC [26]. After 30 min with urea, a population of cells with higher CHC is present and by 120 min a distinct population of nonreticulocytes that has shifted to higher CHC is apparent. After 120 min without urea (control) there is a slight rightward shift and a shoulder on the CHC distribution. These density shifts were blocked in Cl-free sulfamate media, indicating they were mediated by KCC. With urea, 37% of the RBC demonstrated volume regulation by peak area analysis. Assuming a linear distribution of cell ages among the entire RBC population, this would be consistent with KCC activity persisting for 30–50 days. Similar results were obtained in each of seven independent experiments.
Figure 7.
A: KCC mediated volume reduction in mature RBC. Advia 120 histograms of CHC of nonreticulocyte AA RBC are shown for a single experiment representative of six others. Cells were swollen with nystatin to CHCM = 24 g/dl (t = 0) then incubated at 37°C without (control) or with 600 mM urea (to activate KCC) for the times indicated. Cells were washed in cold HBS and kept on ice until Advia analysis. Vertical markers in each histogram represent CHC of 27 and 41 g/dl. B: Blood samples were taken 7 days or 62 days after reinfusion of BRBC, treated with nystatin, and incubated with urea to activate KCC as above. Density distributions pre- and posturea incubation are shown for unlabeled RBC (gray bars) and labeled BRBC (black bars). At 7 days, when the BRBC are only slightly older than the RBC, a similar shift in density is apparent in both RBC and BRBC. After 62 days a density shift upon KCC activation is again apparent in the steady state RBC, but does not occur in the BRBC, which are at least 62 days old. Lower panes (62 days only) indicate that removal of Cl eliminates the density shift as would be expected for KCC. Data represents a single experiment, replicated independently in another subject. Fx1 = 1.080 g/cc; Fx2 = 1.085; Fx3 = 1.090; Fx4 = 1.095; Fx5 = 1.100 (C) Since hydration changes are subject to experimental variability as discussed in the text, the changes in BRBC were normalized to the changes in steady state RBC at each timepoint by calculating the ratio of the percentage BRBC exhibiting urea-stimulated, KCC-mediated density shift to that of RBC (see text for details). Data from two subjects indicate that for approximately the first half of the red cell lifespan KCC can be activated under these conditions. Data from two subjects are shown.
We also examined cell age dependent, KCC-mediated density shifts using the biotin labeling technique. Figure 7B shows discontinuous density distributions before and after incubation with urea for RBC collected 7 days and 62 days after reinfusion of biotin-labeled cells. Cells were first swollen with nystatin, so preincubation density profiles show mostly low density cells (<1.080). Unlabeled RBC (solid bars) and labeled RBC (stippled) had nearly identical preincubation density distributions for each day, but due to unavoidable experimental variations in nystatin treatment and gradient separation there were appreciable day to day differences. Nevertheless, on both Days 7 and 62 all of the RBC, both labeled and unlabeled, were in the low density fractions (1–3) prior to incubation. On Day 7 (left panel) incubation in the presence of urea resulted in a shift to higher density fractions 4 and 5 (>1.095 g/ml) for 41% of the labeled RBC and 44% of the unlabeled RBC. These data for unlabeled RBC are consistent with the Advia data in Fig. 7A, which showed a density shift for 35% of the cells. On Day 62 (right panel), the percentage of unlabeled cells shifting to higher density after incubation with urea is quite similar to that seen at 7 days (36%). However, only 6% of the biotin-labeled older RBC (age = 62 days) exhibited a density shift after urea incubation, indicating less KCC activity in these RBC. Incubation without Cl− (sulfamate media, lowest panel) yielded no dense cells, indicating the Cl− dependence of the density shift.
Since the fraction of unlabeled RBC demonstrating a urea-stimulated, KCC-mediated density shift varied from day to day, we normalized the data for each experiment by dividing the percentage of biotin-labeled cells shifting density by the percentage of unlabeled cells becoming dense. For example, in Fig. 7B at 7 days, 44% of the unlabeled cells and 41% of the biotin-labeled cells shifted density, so the normalized ratio was 0.93 (41/44); at 62 days the ratio was 0.17 (6/36). Figure 7C shows these normalized ratios for two experiments (NDM1 and NDM2) measured at nine time points. Up until the midpoint of RBC lifespan (about 60 days), labeled RBC showed density shifts similar to the unlabeled RBC population (ratio ~1). In RBC older than 60 days, KCC-mediated density shift was considerably less than the whole RBC population. Since the density shift assay is highly sensitive to experimental conditions (e.g., urea concentration, incubation time, choice of discontinuous density gradients), the time at which RVD is lost is somewhat arbitrary. The Advia data demonstrating that about one third of mature RBC exhibit KCC mediated volume reduction supports the idea that KCC activity persists well into the lifespan of the red cell and is not restricted to reticulocytes.
Discussion
One of the early uses of RBC biotinylation was the study of senescence [9–11], first in rabbits, and then in other species [16,18]. The characteristics of RBC survival vary widely among species, implying that senescent mechanisms also vary, and making a species-specific approach necessary. The studies reported here are the first to apply this versatile technique to examine senescence in human RBC.
The biotin label is well-suited to this purpose due to its ability to unequivocally identify the oldest RBC and use multicolor techniques and magnetic isolation [32,34,37] to study a variety of cell variables. The number of subjects was limited in this human study for which each experiment required four months of data collection. Furthermore, due to limited resources it was not possible to perform every assay in each subject, and as a result statistical analyses were limited.
The major disadvantage of the biotin label in the study of senescence is that it is not an age cohort label. RBC of all ages are labeled, and their properties at the time of labeling are therefore average values. Furthermore, if there is heterogeneity in cell survival, short surviving cells will have less representation in the labeled population compared to their production in the bone marrow. A mathematical model originally developed for the analysis of tagged wild animal populations has been useful to address this limitation [31]. As the labeled cells become older their age range narrows until only very old RBC remain. Therefore, using this method it is possible to compare the properties of old cells with an average value but not to directly compare very young and very old RBC. This limitation can be partly overcome by using a reticulocyte marker, transferrin receptor, to identify very young RBC [32].
The changes shown in Fig. 3 confirm that normal RBC become more dense as they age, but also demonstrate the limitations on using density alone as a surrogate for RBC age. Most of the change occurs in the first few weeks after labeling and involves movement from the lighter to the intermediate density fractions. This timing suggests that most of the density change occurs early in the RBC lifespan. In addition, the oldest RBC still show a range of density, and many are still in the intermediate density fractions. This implies that increased density is not uniformly associated with age, and that the dense fraction contains a selected population of old cells that may not be representative of the majority. It now appears that age-dependent RBC density changes—predominantly a function of hemoglobin concentration— occur in three phases: an initial phase in reticulocytes and young mature RBC, a long phase in mature RBC that results in dehydration of some but not all older RBC, and a proposed third phase in the oldest RBC that causes rehydration prior to removal [38]. This complex temporal sequence invalidates the use of density alone to study RBC aging. A multidimensional approach to cell fractionation, such as the use of both density and size (elutriation) may yield better age discrimination [39]. We also used the forward scatter (FS) parameter in the flow cytometer as an approximate measure of cell size as the labeled RBC age, and it also showed changes consistent with time-dependent volume reduction (Fig. 4). However, the FS parameter continued to decline with time and thus indicated a continuing decrease in size for older RBC. The loss of volume in older RBC without increase in density is consistent with the known loss of approximately 15 percent of cellular hemoglobin and a similar percentage of membrane surface area from normal RBC as they age [40], and suggests a mechanism that involves budding of hemoglobin-containing vesicles [41].
Cell volume changes constitute an important maturational event in reticulocytes and are mediated by KCC. Our studies indicate that KCC activity persists for a substantial portion of the lifespan of the RBC and is not restricted to reticulocytes. This raises the possibility that the density changes observed in younger mature RBC are also mediated by KCC activation, perhaps by transient exposure to acidic regions in the circulation or to high urea concentrations in the kidney.
It is often asserted that external PS is present in senescent RBC and leads to their removal from the circulation. In rodents, there is support for the former [19,20], but no proof that it is the mechanism for removal. In humans, the current studies provide no evidence for involvement of PS in RBC senescence. Nevertheless, it is possible that the lack of external PS in aged human RBC is due to the immediate removal of these cells. Our studies suggest that such removal of PS(+) cells, if it occurred, would be within 0.1 days of PS externalization. This seems unlikely from other evidence, including the presence of external PS on normal reticulocytes [42–44] and the presence of many PS(+) RBC in patients with sickle cell disease [42,44,45]. Furthermore, the persistence of biotin-labeled PS(+) sickle RBC, was not consistent with rapid removal [34,37,44]. The decreased level of APLT activity in older RBC does suggest that older human RBC would have a greater tendency to externalize PS in the presence of active scramblase, and the current studies do not rule out the possibility that this occurs as a terminal event and is followed by very rapid removal of the PS(+) cells.
The presence of increased IgG binding on the oldest normal RBC is consistent with the appearance of a neoantigen during aging, but increased nonspecific binding is not ruled out. Whether the increased IgG binding causes removal of the old RBC cannot be determined from these studies, but earlier studies have demonstrated that several different stimuli that trigger band 3 clustering also trigger autologous IgG binding and RBC clearance [46]. In contrast, a recent study in normal blood donors with positive antiglobulin test [47] suggests that RBC IgG may play a protective role.
The morphology of the oldest RBC, with an irregular outline and a somewhat ragged look, implies that it may be possible to recognize them on a routine blood smear, and that the small number of cells with this appearance that are currently passed over as artifact or nonspecific changes are in reality senescent RBC.
The methods used here introduce a useful and unambiguous approach for the study of senescence in human RBC. The findings provide support for the importance of IgG binding but not for externalization of PS as a mechanism for RBC removal. However, the only true test of a proposed senescence mechanism is the demonstration of prolonged RBC lifespan in its absence.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: DK63088 [RC], 5UL1RR026314, GM24417-33 [PL], VA (1 IO1 CX000121-01 [RC].
Footnotes
Author Contributions
Robert S. Franco: designed research, analyzed data, wrote the paper. M. Estela Puchulu-Campanella: designed research, performed research, analyzed data, reviewed the paper. Latorya A. Barber: performed research, reviewed the paper. Mary B. Palascak: performed research, reviewed the paper. Clinton H. Joiner: designed research, analyzed data, reviewed the paper. Philip S. Low: designed research, analyzed data, reviewed the paper. Robert. M. Cohen: designed research, analyzed data, reviewed the article.
Conflict of interest: Nothing to report
References
- 1.Clark MR. Senescence of red blood cells: Progress and problems. Physiol Rev. 1988;68:503–554. doi: 10.1152/physrev.1988.68.2.503. [DOI] [PubMed] [Google Scholar]
- 2.Kay MM. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci USA. 1984;81:5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kay MM, Marchalonis JJ, Hughes J, Watanabe K, Schluter SF. Definition of a physiologic aging autoantigen by using synthetic peptides of membrane protein band 3: Localization of the active antigenic sites. Proc Natl Acad Sci USA. 1990;87:5734–5738. doi: 10.1073/pnas.87.15.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorette MP, Galili U, Clark MR. Comparison of serum anti-band 3 and anti-gal antibody binding to density-separated human red blood cells. Blood. 1991;77:628–636. [PubMed] [Google Scholar]
- 5.Galili U, Flechner I, Knyszynski A, Danon D, Rachmilewitz EA. The natural anti-alpha-galactosyl IgG on human normal senescent red blood cells. Br J Haematol. 1986;62:317–324. doi: 10.1111/j.1365-2141.1986.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 6.Low PS, Waugh SM, Zinke K, Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985;227:531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 7.Waugh SM, Willardson BM, Kannan R, et al. Heinz bodies induce clustering of band 3, glycophorin, and ankyrin in sickle cell erythrocytes. J Clin Invest. 1986;78:1155–1160. doi: 10.1172/JCI112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T, Dale GL. Biotinylated erythrocytes: In vivo survival and in vitro recovery. Blood. 1987:791–795. [PubMed] [Google Scholar]
- 9.Suzuki T, Dale GL. Senescent erythrocytes: Isolation of in vivo aged cells and their biochemical characteristics. Proc Natl Acad Sci USA. 1988;85:1647–1651. doi: 10.1073/pnas.85.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T, Dale GL. Membrane proteins in senescent erythrocytes. Biochem J. 1989;257:37–41. doi: 10.1042/bj2570037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimran A, Forman L, Suzuki T, et al. In vivo aging of red cell enzymes: Study of biotinylated red blood cells in rabbits. Am J Hemat. 1990;33:249–254. doi: 10.1002/ajh.2830330407. [DOI] [PubMed] [Google Scholar]
- 12.Dale GL, Daniels RB, Beckman J, Norenberg SL. Characterization of senescent red cells from the rabbit. In: Magnani M, DeFlora A, editors. Red Blood Cell Aging. New York: Plenum Press; 1991. pp. 93–103. [DOI] [PubMed] [Google Scholar]
- 13.Dale GL, Daniels RB. Quantitation of immunoglobulin associated with senescent erythrocytes from the rabbit. Blood. 1991;77:1096–1099. [PubMed] [Google Scholar]
- 14.Waugh RE, Narla M, Jackson CW, et al. Rheologic properties of senescent erythrocytes: Loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 15.Christian JA, Rebar AH, Boon GD, Low PS. Senescence of canine biotinylated erythrocytes: Increased autologous immunoglobulin binding occurs on erythrocytes aged in vivo for 104 to 110 days. Blood. 1993;82:3469–3473. [PubMed] [Google Scholar]
- 16.Christian JA, Wang J, Kiyatkina N, et al. How old are dense red blood Cells? The dog’s tale. Blood. 1998;92:2590–2591. [PubMed] [Google Scholar]
- 17.Christian JA, Rebar AH, Boon GD, Low PS. Methodologic considerations for the use of canine in vivo aged biotinylated erythrocytes to study RBC senescence. Exp Hematol. 1996;24:82–88. [PubMed] [Google Scholar]
- 18.Rettig MP, Low PS, Gimm JA, et al. Evaluation of biochemical changes during in vivo erythrocyte senescence in the dog. Blood. 1999;93:376–384. [PubMed] [Google Scholar]
- 19.Gifford SC, Yoshida T, Shevkoplyas SS, Bitensky MW. A high-resolution, double- labeling method for the study of in vivo red blood cell aging. Transfusion. 2006;46:578–588. doi: 10.1111/j.1537-2995.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- 20.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA. 1998;95:3077–3081. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeJong K, Bhagat A, Emerson RK, Kuypers FA. Flippase activity decreases throughout erythrocyte life-span in normal and sickle mice [abstract] Blood. 2006;108 [Google Scholar]
- 22.Coupland LA, Cromer D, Davenport MP, Parish CR. A novel fluorescent-based assay reveals that thrombopoietin signaling and Bcl-X(L) influence, respectively, platelet and erythrocyte lifespans. Exp Hematol. 2010;38:453–461. doi: 10.1016/j.exphem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, von Loehneysen K, Noack D, Vu A, et al. A novel approach for in vivo measurement of red cell redox status [abstract] Blood. 2010;116:2036. doi: 10.1182/blood-2011-03-342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dale GL, Norenberg SL. Density fractionation of erythrocytes by percoll/hypaque results in only a slight enrichment for aged cells. Biochim Biophys Acta. 1990;1036:183–187. doi: 10.1016/0304-4165(90)90032-r. [DOI] [PubMed] [Google Scholar]
- 25.Bookchin RM, Etzion Z, Sorette M, et al. Identification and characterization of a novel population of high-Na+, low-K+, low density sickle and normal red cells. Proc Natl Acad Sci USA. 2000;97:8045–8050. doi: 10.1073/pnas.130198797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joiner CH, Rettig RK, Jiang M, et al. Urea stimulation of KCl cotransport induces abnormal volume reduction in sickle reticulocytes. Blood. 2007;109:1728–1735. doi: 10.1182/blood-2006-04-018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall AC, Ellory JC. Effects of high hydrostatic pressure on ‘passive’ monovalent cation transport in human red cells. J Membr Biol. 1986;94:1–17. doi: 10.1007/BF01901009. [DOI] [PubMed] [Google Scholar]
- 28.Larsen FL, Katz S, Roufogalis BD, Brooks DE. Physiological shear stresses enhance the Ca2+ permeability of human erythrocytes. Nature. 1981;294:667–668. doi: 10.1038/294667a0. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RM, Tang K. Induction of a Ca(2+)-activated K+ channel in human erythrocytes by mechanical stress. Biochim Biophys Acta. 1992;1107:314–318. doi: 10.1016/0005-2736(92)90418-l. [DOI] [PubMed] [Google Scholar]
- 30.Dyrda A, Cytlak U, Ciuraszkiewicz A, et al. Local membrane deformations activate Ca2+-dependent K+ and anionic currents in intact human red blood cells. PLoS One. 2010;5:e9447. doi: 10.1371/journal.pone.0009447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsell CJ, Franco RS, Smith EP, Joiner CH, Cohen RM. A method for the continuous calculation of the age of labeled red blood cells. Am J Hematol. 2008;83:454–457. doi: 10.1002/ajh.21148. [DOI] [PubMed] [Google Scholar]
- 32.Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–4291. doi: 10.1182/blood-2008-04-154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holtzclaw JD, Jiang M, Yasin Z, et al. Rehydration of high-density sickle erythrocytes in vitro. Blood. 2002;100:3017–3025. doi: 10.1182/blood-2002-02-0631. [DOI] [PubMed] [Google Scholar]
- 34.Franco RS, Yasin Z, Lohmann JM, et al. The survival characteristics of dense sickle cells. Blood. 2000;96:3610–3617. [PubMed] [Google Scholar]
- 35.Barber LA, Palascak MB, Joiner CH, Franco RS. Aminophospholipid translocase and phospholipid scramblase activities in sickle erythrocyte subpopulations. Br J Haematol. 2009;46:447–455. doi: 10.1111/j.1365-2141.2009.07760.x. [DOI] [PubMed] [Google Scholar]
- 36.Bosch FH, Werre JM, Roerdinkholder-Stoelwinder B, et al. Characteristics of red blood cell populations fractionated with a combination of counterflow centrifugation and Percoll separation. Blood. 1992;79:254–260. [PubMed] [Google Scholar]
- 37.Franco RS, Lohmann J, Silberstein EB, et al. Time-dependent changes in the density and hemoglobin F content of biotin-labeled sickle cells. J Clin Invest. 1998;101:2730–2740. doi: 10.1172/JCI2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lew VL, Daw N, Etzion Z, et al. Effects of age-dependent membrane transport changes on the homeostasis of senescent human red blood cells. Blood. 2007;110:1334–1342. doi: 10.1182/blood-2006-11-057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasch J, Kullertz G, Opalka JR. Separation of erythrocytes into age-related fractions by density or size? Counterflow centrifugation. Clin Chem Lab Med. 2000;38:629–632. doi: 10.1515/CCLM.2000.092. [DOI] [PubMed] [Google Scholar]
- 40.Gifford SC, Derganc J, Shevkoplyas SS, Yoshida T, Bitensky MW. A detailed study of time-dependent changes in human red blood cells: From reticulocyte maturation to erythrocyte senescence. Br J Haematol. 2006;135:395–404. doi: 10.1111/j.1365-2141.2006.06279.x. [DOI] [PubMed] [Google Scholar]
- 41.Willekens FL, Werre JM, Groenen-Dopp YA, et al. Erythrocyte vesiculation: A self-protective mechanism? Br J Haematol. 2008;141:549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 42.Wood BL, Gibson DF, Tait JF. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: Flow-cytometric measurement and clinical associations. Blood. 1996;88:1873–1880. [PubMed] [Google Scholar]
- 43.de Jong K, Larkin SK, Styles LA, Bookchin RM, et al. Characterization of the phosphatidylserine-exposing subpopulation of sickle cells. Blood. 2001;98:860–867. doi: 10.1182/blood.v98.3.860. [DOI] [PubMed] [Google Scholar]
- 44.Yasin Z, Witting S, Palascak MB, et al. Phosphatidylserine externalization in sickle red blood cells: Associations with cell age, density, and hemoglobin F. Blood. 2003;102:365–370. doi: 10.1182/blood-2002-11-3416. [DOI] [PubMed] [Google Scholar]
- 45.de Jong K, van Rossum L, Lubin BH, et al. Phosphatidyserine externalization in subpopulations of sickle cells [abstract] Blood. 1997;90:125a. [Google Scholar]
- 46.Turrini F, Arese P, Yuan J, Low PS. Clustering of integral membrane proteins of the human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J Biol Chem. 1991;266:23611–23617. [PubMed] [Google Scholar]
- 47.Alaia V, Frey BM, Siderow A, et al. A pair of naturally occurring antibodies may dampen complement-dependent phagocytosis of red cells with a positive antiglobulin test in healthy blood donors. Vox Sang. 2009;97:338–347. doi: 10.1111/j.1423-0410.2009.001214.x. [DOI] [PubMed] [Google Scholar]