Abstract
Amphetamine withdrawal in both humans and rats is associated with increased anxiety states, which are thought to contribute to drug relapse. Serotonin in the ventral hippocampus mediates affective behaviors, and reduced serotonin levels in this region are observed in rat models of high anxiety, including during withdrawal from chronic amphetamine. This goal of this study was to understand the mechanisms by which reduced ventral hippocampus serotonergic neurotransmission occurs during amphetamine withdrawal. Serotonin synthesis (assessed by accumulation of serotonin precursor as a measure of the capacity of in vivo tryptophan hydroxylase activity), expression of serotonergic transporters, and in vivo serotonergic clearance using in vivo microdialysis, were assessed in the ventral hippocampus in adult male Sprague Dawley rats at 24 hours withdrawal from chronic amphetamine. Overall, results showed that diminished extracellular serotonin at 24 hours withdrawal from chronic amphetamine was not accompanied by a change in capacity for serotonin synthesis (in vivo tryptophan hydroxylase activity), nor serotonin transporter expression or function in the ventral hippocampus, but instead was associated with increased expression and function of organic cation transporters (low affinity, high capacity serotonin transporters). These findings suggest that 24 hours withdrawal from chronic amphetamine reduces the availability of extracellular serotonin in the ventral hippocampus by increasing organic cation transporter-mediated serotonin clearance, which may represent at future pharmacological target for reversing anxiety states during drug withdrawal.
Keywords: Serotonin Transporter, Organic Cation Transporter, Selective Serotonin Reuptake Inhibitor, Rat, Microdialysis
Introduction
Amphetamine abuse is associated with a withdrawal syndrome that includes anxiety symptoms which may contribute to drug relapse (Koob & Le Moal, 2001; Romanelli et al., 2006; Gossop, 2009; Shoptaw et al., 2009). In rats, 24 hours withdrawal from chronic amphetamine treatment increases anxiety-like behaviors which persist during long-term withdrawal (Barr et al., 2010; Vuong et al., 2010). Therefore, the rat represents a model for investigating neurobiological changes during amphetamine withdrawal to better understand mechanisms underlying withdrawal-induced increases in anxiety states.
Serotonin (5-HT) in the hippocampus is important for adaptive coping with anxiogenic environments, and dysfunction of 5-HT neurotransmission in this region is thought to underlie heightened anxiety states (Guimaraes et al., 1993; Graeff et al., 1996; Joca et al., 2003; Storey et al., 2006). For example, rats bred for high anxiety-like behavior have reduced stress-induced hippocampal 5-HT levels (Keck et al., 2005), while direct enhancement of hippocampal 5-HT reduces heightened anxiety-like behaviors of rats (Guimaraes et al., 1993; Graeff et al., 1996). Ex vivo tissue 5-HT levels in the ventral hippocampus are reduced in rats at 24 hours of amphetamine withdrawal, the same time point as increased anxiety behaviors emerge (Barr et al., 2010; Vuong et al., 2010; Barr and Forster, 2011). Furthermore, corticosterone-induced increases in extracellular 5-HT within the ventral hippocampus are attenuated in rats at 24 hours withdrawal from chronic amphetamine (Barr and Forster, 2011). Together, these findings suggest reduced 5-HT neurotransmission in the ventral hippocampus of rats undergoing amphetamine withdrawal may underlie increased anxiety states. The goal of this study was to investigate mechanisms by which reduced ventral hippocampus serotonergic neurotransmission occurs during amphetamine withdrawal, important for future work addressing reversing withdrawal-induced anxiety states.
A potential mechanism for reduced 5-HT function in the ventral hippocampus during amphetamine withdrawal includes enhancement of 5-HT transporters that clear extracellular 5-HT. Normally, the 5-HT transporter (SERT) is the primary mechanism for clearing extracellular 5-HT (Gainetdinov and Caron, 2003). In contrast, organic cation transporter 3 (OCT3), a low affinity, high capacity transporter, contributes to 5-HT clearance under conditions of elevated 5-HT release, such as in response to stress (Daws et al., 1998; Daws, 2009; Feng et al., 2009; Gasser et al., 2009). Amphetamine and its derivatives are substrates for SERT (Schuldiner et al., 1993; Tatsumi et al., 1997), and interact with SERT to increase 5-HT efflux (Hilber et al., 2005; Seidel et al., 2005). Acute amphetamine administration also increases SERT activity at the plasma membrane by suppressing protein kinase C-mediated SERT phosphorylation and internalization. (Ramamoorthy and Blakely, 1999; Qian et al. 1997). Amphetamine may also interact with OCT3s directly (Wu et al., 1998; Amphoux et al., 2006; although see Zhu et al., 2010) and indirectly by stimulating adrenal release of corticosterone (Kynch and Eisenburg, 1979; Swerdlow et al., 1993) which is a substrate for OCT3s (Gasser et al., 2006). Therefore, we tested the hypothesis that repeated amphetamine treatment enhances 5-HT transport mechanisms in the ventral hippocampus that are apparent in early (24 hours) withdrawal, thus reducing serotonergic neurotransmission during amphetamine withdrawal.
Methods
Animals
One hundred and forty-nine male Sprague Dawley rats (3 weeks old) were purchased from the University of South Dakota Animal Resource Center. Rats were housed in pairs for the entire experiment, and maintained at 22 °C on a reverse 12 h light 12 h dark cycle with free access to food and water. Pair-housing of rats from weaning onwards was used as a standard housing procedure since isolation rearing increases anxiety-like behaviors (Lukkes et al., 2009) and amphetamine differentially alters behavior of rats reared in isolation versus pair-housed controls (e.g. Zeeb et al., 2012). Rats were used in the following studies once they reached early adulthood (8 weeks of age). The procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011), and all efforts were made to reduce animal suffering and animal numbers.
Amphetamine Treatment
Rats were treated with amphetamine (2.5 mg/kg, ip.; n = 76) or saline (n = 73) daily for two weeks. Injections were always made during the dark phase of the light cycle, between 11am and 1pm. This treatment regime produces anxiety states that are observed at 24 hours to 4 weeks of withdrawal (Barr et al., 2010; Vuong et al., 2010). Rats used in the following experiments were at 24 hours of withdrawal at the time of testing or brain collection.
Experiment 1: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on Stimulated Levels of 5-HT in the Ventral Hippocampus
Rats exposed to chronic amphetamine treatment and 24 hours withdrawal exhibit attenuated corticosterone-induced increases in extracellular 5-HT within the ventral hippocampus, thought to be due to a reduction in glucocorticoid receptors in the ventral hippocampus (Barr and Forster, 2011). However, ex vivo 5-HT tissue content in the dentate gyrus of the ventral hippocampus is reduced at 24 hours withdrawal from chronic amphetamine treatment (Barr et al., 2010), suggesting a potential for extracellular serotonergic transmission per se to be dampened in this region, independent of direct glucocorticoid application. Therefore, the purpose of this experiment was to determine whether stimulated extracellular levels of 5-HT in the ventral hippocampus are dampened at 24 hours withdrawal from chronic amphetamine pretreatment. In the current experiment, reverse dialysis of 100 mM KCl was used to stimulate 5-HT release through local depolarization. Local application of a high K+-containing solution is frequently used to evoke changes in extracellular neurotransmitter levels (Trillat et al., 1997).
Microdialysis Procedures
The morning following the last injection of amphetamine, rats were anesthetized with urethane (1.8 g/kg, ip., Sigma-Aldrich, St. Louis, MO, USA) and placed within a small mammal stereotaxic frame (Kopf, Tujunga, CA, USA). Animals remained under anesthesia throughout the course of the experiment (Barr and Forster, 2011), with body temperature held at 37 °C by a temperature-controlled heating pad (Harvard Apparatus, Holliston, MA, USA). Urethane has minimal effects on neurotransmitter release and neuronal firing rates, with baseline and elicited 5-HT release similar between freely-moving and urethane-anesthetized rats (Maggi and Meli, 1986; Forster et al., 2006; 2008). A laboratory-made microdialysis probe (2.5–3.0 mm exposed membrane length, average recovery for 5-HT was 19.6%) was inserted into the ventral hippocampus (AP, −5.2 mm from bregma; ML, 4.5 mm from midline; DV, −8.6 mm from dura; Paxinos & Watson, 1996). Artificial cerebrospinal fluid (aCSF; composed of the following in mM: 147 NaCl, 2.7 KCl, 1 NaH2PO4, 1.4 Na2HPO4, 2.1 MgCl2, and 1.6 CaCl2) was continuously perfused through the probe at a rate of 0.4 μL/min. Dialysate (8 μL/sample) collection began 4 hrs following probe insertion (e.g. Forster et al., 2008; Barr and Forster, 2011), with dialysates collected at 20 min intervals and 5-HT levels measured using high-performance liquid chromatography (HPLC) with electrochemical detection (see below for details).
Following collection of at least three comparable baseline samples, perfusion with aCSF was changed to perfusion of aCSF containing high KCl levels (100 mM; Trillat et al., 1997) through the dialysis probe for 20 min after which perfusion with normal aCSF was re-established (n = 6 for amphetamine and n = 5 for saline pretreated rats). When KC1 was brought from 2.7 to 100 mM, NaC1 concentration was reduced concurrently to maintain physiological osmolarity. Dialysates were collected until 5-HT returned to baseline levels following reintroduction of normal aCSF.
HPLC Measurement of 5-HT from Dialysates
High performance liquid chromatography with electrochemical detection (HPLC-ED) was used to detect 5-HT in dialysates (Forster et al., 2008; Barr and Forster, 2011). The mobile phase (containing per liter: 680 mg EDTA, 500 mg sodium octanesulfonate, 4.8 g NaH2PO4, 500 μL triethylamine and 170 mL methanol, pH 5.2; all obtained from Sigma-Aldrich) was pumped through a UniJet 3 μm C18 microbore column (Bioanalytical Systems; West Lafayette, IN, USA) under nitrogen gas pressure (2000 psi). Dialysates were injected onto the chromatographic system using a rheodyne injector via a 5 μL loop (Bioanalytical Systems). The perfusion rate of 0.4μL/min resulted in the collection of approximately 8μL of dialysate/20 min to ensure that the loop was overfilled with each sample. Following separation by the column, 5-HT was detected by a glassy carbon electrode (Bioanalytical Systems), which was maintained at +0.6 V with respect to an Ag/AgCl2 reference electrode using an LC-4C potentiostat (Bioanalytical Systems). The voltage output was recorded by Clarity v2.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic) and 5-HT peaks were identified by comparison to a 5-HT standard (7.9 pg/5 μL 5-HT). The 2:1 signal to noise detection limit for 5-HT using this system was 0.06 +/− 0.01 pg.
Histology
At the conclusion of each experiment, rats were killed by an overdose of sodium pentobarbital (0.5 mL Fatal Plus, i.p.; Vortech, Dearborn, MI, USA). The brains were removed and fixed in 10% buffered formalin (Fisher Scientific, Fair Lawn, NJ, USA). Brains were sectioned (60 μm) frozen on a sliding microtome and examined under a light microscope by two experimenters, one blind to treatment and results, to determine probe placement. Only data from rats with correct placements in the ventral hippocampus were included in the analyses.
Experiment 2: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on in vivo Tryptophan Hydroxylase Activity in the Ventral Hippocampus
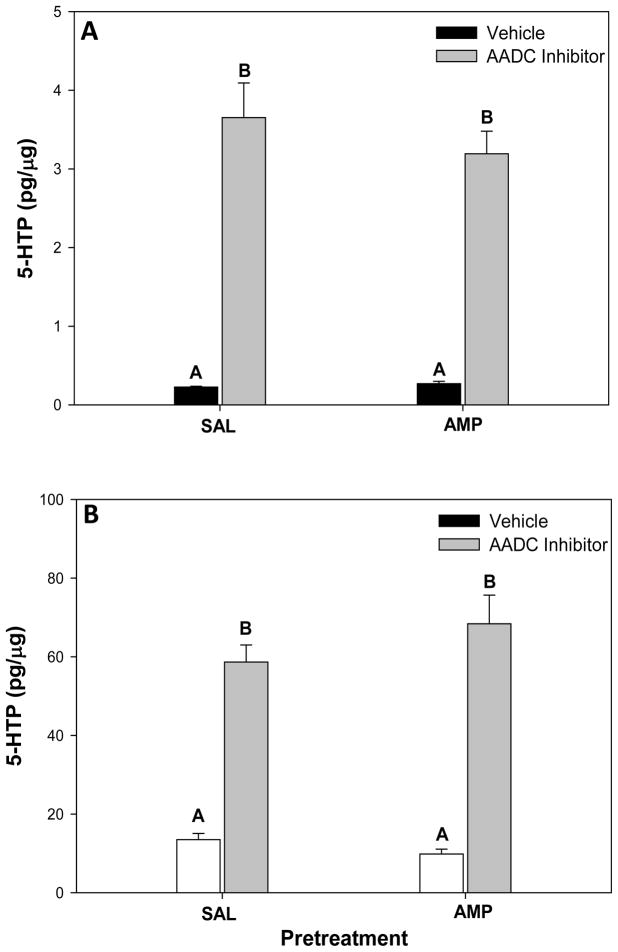
This experiment tested whether chronic amphetamine treatment and withdrawal altered the activity of the rate-limiting enzyme in serotonin synthesis, tryptophan hydroxylase (TpH), to account for reduced K+-stimulated increases in extracellular 5-HT concentrations that were observed in the ventral hippocampus of amphetamine pretreated rats at 24 hours withdrawal (see Results). Tryptophan is converted to 5-hydroxytryptophan (5-HTP) by TpH, and in turn, 5-HTP is converted to 5-HT by aromatic amino acid decarboxylase (AADC). The inhibition of AADC prevents 5-HTP conversion to 5-HT, and the accumulation of 5-HTP over a fixed time period serves as a measure of in vivo TpH activity (Johnston and Moore, 1983). The median raphe nucleus (mRN) was also examined, as functional and anatomical evidence suggests that the majority of 5-HT innervation of the ventral hippocampus is from the mRN (Azmitia and Segal, 1978; Miguez et al., 2002).
AADC Inhibition and Collection of Brain Tissue
Twenty-four hours after the last amphetamine or saline injection, rats received an injection of either the AADC inhibitor NSD-1015 (100 mg/kg, ip.; Sigma-Aldrich; Evans et al., 2009) or vehicle (equivalent volume of 0.9% saline) and returned to their home cages (n = 11 per treatment group; 44 rats total). Thirty minutes following AADC or vehicle injection (Evans et al., 2009), rats were decapitated, and brains were rapidly removed and frozen on dry ice. Brains were stored at −80 °C until sectioning. Frozen brains were serially sliced at 300 μm in the coronal plane, at −10°C within a cryostat (Leica Jung CM 1800; North Central Instruments, Plymouth, MN). The sections were thaw-mounted on glass slides and stored at −80°C until microdissection. The ventral hippocampus and the mRN were identified using Paxinos and Watson (1996), and bilaterally microdissected with a 23 gauge cannula using a dissecting microscope and freezing stage (Physiotemp; North Central Instruments). The microdissected tissue was expelled into 60 μl of sodium acetate buffer (pH 5.0) containing 0.1 μM of internal standard (α-methyl-dopamine), and the cells were lysed by freeze-thawing samples (e.g., Scholl et al., 2009; 2010)
Brain regions were analyzed for tissue 5-HTP concentrations using HPLC-ED. Details of this assay have been published elsewhere (e.g. Renner et al, 1987; Scholl et al., 2009; 2010). Briefly, 2 μL of a 1 mg/mL ascorbate oxidase solution (Sigma-Aldrich) was added to each sample prior to centrifuging at 17,000 × g for 3 minutes. The supernatant was removed and 45 μL injected into a chromatographic system (Waters Associates, Inc., Milford, MA, USA) and analyzed electrochemically with a LC-4B potentiostat (BioAnalytical Systems). The electrode potential was set at +0.6 V with respect to an Ag/AgCl reference electrode. The mobile phase consisted of 14 g citric acid, 8.6 g sodium acetate, 110 mg sodium octanesulfonate, 150 mg EDTA disodium salt, and 100 ml of methanol in 1 L of deionized water (all obtained from Sigma-Aldrich). Flow rate was maintained at 1.4 mL/min.
The tissue pellet remaining from each sample was dissolved in 45 μL (mRN) or 110 μL (ventral hippocampus) 0.4 M NaOH. Protein concentrations were determined within 5 μL sample duplicates using a Bradford Kit (BioRad Laboratories, Hercules, CA) and a microplate reader (Bio-Tek Instruments, Winooski, VA, USA). Concentrations of 5-HTP were obtained and corrected for recovery using CSW32 v1.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic), and expressed as pg amine/μg protein.
Experiment 3: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on Expression of 5-HT Transporters in the Ventral Hippocampus
Extracellular 5-HT levels (as measured by microdialysis) are contributed to by both 5-HT release and clearance. Thus, changes in 5-HT clearance may account for reduced extracellular concentrations of 5-HT in the ventral hippocampus following chronic amphetamine treatment. This experiment was conducted to examine whether chronic amphetamine treatment alters the expression of the 5-HT transporters SERT or OCT3 in the ventral hippocampus. While other cation transporters exist, 5-HT uptake by OCT1 is minimal, and expression of OCT2 (which can transport 5-HT with a similar affinity than OCT3) in brain is for the most part confined to the subventricular region (Gorboulev et al., 1997; Gründemann et al., 1997 Amphoux et al., 2006; Schomig et al., 2006; Koepsell et al., 2007).
Collection of Brain Tissue
Rats (n = 8 per amphetamine or saline pretreatment) were decapitated 24 hrs following last treatment and the brains were rapidly removed. Brains were frozen, sectioned, the ventral hippocampus microdissected as described for Experiment 2, and homogenized in 40 μL of HEPES buffer. Protein concentrations were determined from 5 μL sample duplicates as described for Experiment 2.
Western Immunoblotting
Samples (50 μg/lane) were loaded on a 7.5% SDS polyacrylamide gel (PAGE) and electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories) from the gel by a semi-dry blotting apparatus (Bio-Rad Laboratories). The blotted membrane was then blocked with 5% skim milk containing 0.1% BSA at 4°C overnight and then incubated with goat polyclonal antibody against SERT (SC-1458; Santa Cruz, Santa Cruz, CA; 1:400; O’Reilly et al., 2007; which recognized a single band at 75 kDa) or rabbit polyclonal antibody against rat OCT3 (OCT31-A; Alpha Diagnostics International, Cambridge, United Kingdom; 1:1000; Gasser et al., 2009; which recognized a single band at 53 kDa) in Tris-buffered saline containing 0.1% Tween-20 (TBST) at 4 °C for 24 hr. Membranes were washed three times with TBST, and then incubated with IRDye 800-conjugated donkey anti-goat IgG secondary antibody (605-732-125; Rockland Inc., Gilbertsville, PA 1:5,000) or IRDye 800-conjugated goat anti-rabbit IgG secondary antibody (611-132-122; Rockland Inc.; 1:5,000) for 2 hr at room temperature. After the incubation, membranes were washed three times with TBST before visualization. Control for protein loading was achieved by using primary antibodies to actin (1:2000; Chemicon International; MAB1501R) and IRDye 800-conjugated affinity purified anti-mouse IgG as secondary antibodies (1:5000; Rockland Inc.; 610-132-121). Proteins were detected using the Odyssey infrared imaging system (excitation/emission filters at 780 nm/820 nm range, LI-COR Biosciences, Lincoln, NE). Optical densities from each individual sample were corrected against actin levels and expressed as a percentage of the saline-injected control group (Burke et al., 2011).
Experiment 4: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on the Functional Expression of 5-HT Transporters in the Ventral Hippocampus
In vivo microdialysis was used to test if SERT or OCT3-mediated accumulation of 5-HT was altered by chronic amphetamine treatment and withdrawal by measuring extracellular 5-HT concentrations after pharmacological blockade of the respective transporters (Miguez et al., 2002; Feng et al., 2005). This method effectively tests functional expression of the 5-HT transporters, since the level of 5-HT accumulation produced by a given concentration of a 5-HT transporter inhibitor reflects the level of functional protein expressed at the cell surface available for pharmacological blockade.
Microdialysis Procedures
Rats underwent microdialysis experiments 24 hrs after the last amphetamine or saline injection using the general procedures outlined for Experiment 1. Experiments were initiated following the collection of at least 3 stable baseline samples of 5-HT. To assess the effects of acute amphetamine withdrawal on extracellular concentrations of 5-HT in the ventral hippocampus following blockade of SERT-mediated 5-HT, paroxetine (0.1 μM (n = 5/treatment group), 0.5 μM (n = 6/treatment group) or 3 μM (n = 5/treatment group; Miguez et al., 2002) was perfused into the ventral hippocampus via the microdialysis probe for 20 min. Paroxetine has a higher affinity for SERT than other selective serotonin reuptake inhibitors (Hirano et al., 2005). The vehicle for paroxetine was aCSF. Rats perfused with aCSF in the absence of paroxetine (n = 4/treatment group) had perfusion lines containing aCSF disconnected and reconnected to simulate start and end of drug perfusion and control for changes in line pressure.
To assess the effects of acute amphetamine withdrawal on extracellular concentrations of 5-HT in the ventral hippocampus following blockade of OCT3-mediated 5-HT clearance, 30 or 100 μM concentrations of the OCT3 inhibitor decynium 22 (D-22) were perfused into the ventral hippocampus via the microdialysis probe for 20 min (30 μM, n = 7 for saline and 8 for amphetamine pretreated rats; 100 μM, n = 6/treatment group; Feng et al. 2005) and the dialysate was analyzed for extracellular 5-HT. D-22 inhibits up to 48% of monoamine clearance thought to be dependent on OCT3, as estimated by histamine clearance (Gasser et al., 2006). It should be noted that D-22 also inhibits OCT1-, OCT2-, and plasma membrane monoamine transporter (PMAT)-mediated monoamine clearance with similar affinity, but due to the distribution and affinity differences discussed above (Schomig et al., 2006; Amphoux et al., 2006; Koepsell et al., 2007; Duan and Wang, 2010), the major contributor to 5-HT accumulation within the ventral hippocampus following D-22 perfusion is OCT3, and possibly PMAT. Stock D-22 (1,11-diethyl-2,22-cyanine iodide, Sigma-Aldrich) was dissolved in 100% ethanol and diluted in aCSF with a final ethanol concentration of 4.5%. Rats perfused with aCSF in the absence of D-22 (n = 5 for saline and 6 for amphetamine pretreated rats) were perfused with aCSF containing 4.5% ethanol. Once 5-HT returned to baseline levels, rats were euthanized and the brains were histologically evaluated for probe placement.
Data analysis
For microdialysis experiments, the height of the 5-HT peaks in three baseline dialysis samples were averaged and post-drug 5-HT peak heights were calculated as a percentage change from mean baseline levels for each animal (Forster et al., 2008; Barr and Forster, 2011). For each experiment, 5-HT levels were analyzed with respect to time (within-subject factor) and treatment group (between-subject factor) using two-way ANOVA with one repeated measure. Significant effects of treatment at a given time-point were further analyzed by Student–Newman-Keul’s (SNK) multiple comparison procedure. When a significant effect of time was noted, a one-way ANOVA with one repeated measure was performed across time for each given treatment. Resulting significant time-points were identified by Holm-Sidak post hoc tests for multiple comparisons with a single control, where the sample collected immediately before the drug infusion served as the control sample. Separate two-way ANOVA were used to analyze the effect of amphetamine on tissue concentrations of 5-HTP in AADC-inhibition and vehicle groups, with significant main effects followed by SNK multiple comparison tests. Separate one-way ANOVA were used to measure the effect of drug treatment on hippocampal SERT and OCT3 optical density (corrected for actin). All analyses were performed using SigmaStat v.3.5, with α set at 0.05.
Results
Probe Placements and Baseline 5-HT Levels for Microdialysis Experiments
The placement of microdialysis probes ensured that the 2.5–3.0 mm length of dialysis membrane sampled from the ventral hippocampus ( 5.2 to 5.8 mm posterior from bregma; Paxinos and Watson, 1996; Fig. 1), and were similarly distributed in saline and amphetamine treated animals across the three different pharmacological experiments. A total of five rats were excluded from further analysis due to probe placements outside the ventral hippocampus. Baseline levels of 5-HT for amphetamine pretreated rats were 1.12 ± 0.15 pg/5 μL, and for saline pretreated rats were 1.16 ± 0.16 pg/5 μL (uncorrected for recovery). There was no statistical difference in baseline levels between groups (F1,57 = 0.0209, P>0.05).
Figure 1.
Representative coronal diagrams of microdialysis probe membrane placements in saline (SAL) and amphetamine (AMP) pretreatment groups. Black bars represent the membrane of one or more probes. Figure adapted from Paxinos & Watson (1996, bregma −5.20 mm).
Experiment 1: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on Stimulated Levels of 5-HT in the Ventral Hippocampus
This experiment aimed to establish whether chronic amphetamine administration and withdrawal altered extracellular 5-HT concentrations in the rat ventral hippocampus as a response to 100 mM KCl perfused into the ventral hippocampus for 20 min through a microdialysis probe (Fig. 2). There was no significant effect of drug treatment (F1,9 = 1.34; P>0.05), but a significant effect of time (F11,115 = 13.642; P< 0.001), and an interaction between treatment and time (F11,115 = 3.488; P< 0.001) were observed. Perfusion of 100 mM KCl solution into the ventral hippocampus significantly increased extracellular 5-HT concentrations over time for both saline (F11,52 = 14.554; P < 0.001) and amphetamine (F11,62 = 3.039; P < 0.01) pretreatment groups. The effect of KCl was apparent 20 and 40 min post-infusion when compared with pre-perfusion levels (Holm-Sidak, P < 0.001, Fig. 2). However, amphetamine pretreated rats showed significantly less KCl-evoked 5-HT levels at 20 and 40 min post perfusion (SNK, P<0.001) than saline pretreated rats (Fig. 2).
Figure 2.
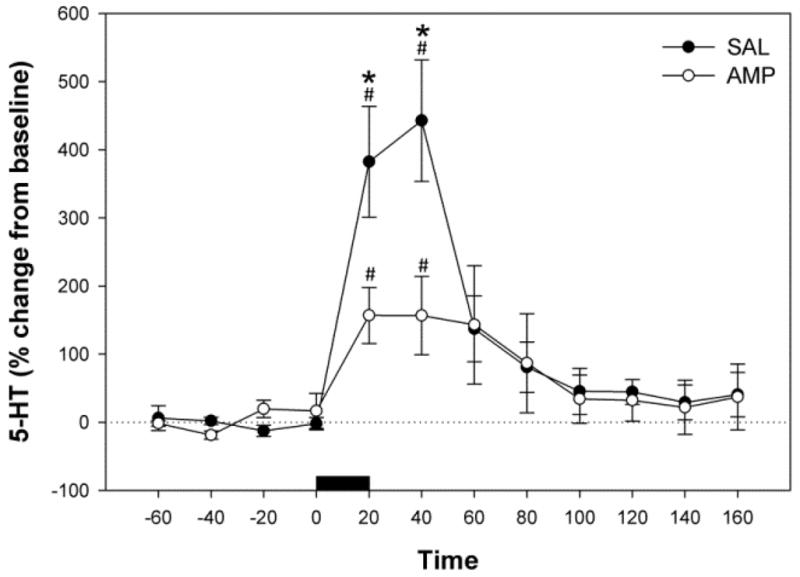
Effects of KCl-induced depolarization on extracellular 5-HT concentrations in the ventral hippocampus of saline (SAL) and amphetamine (AMP) pretreated rats. KCl was added to the perfusate at a concentration of 100 mM during a sample period (20 min) marked by the horizontal black bar. Data are mean ±SEM. #Significantly different from pre-infusion levels (P < 0.05). *Significant differences between saline and amphetamine pretreatment groups (P < 0.05).
Experiment 2: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on in vivo Tryptophan Hydroxylase Activity in the Ventral Hippocampus
The goal of this experiment was to test whether the decrease in KCl-stimulated extracellular 5-HT concentrations in the ventral hippocampus of chronic amphetamine pretreated rats at 24 hours withdrawal might be due to reduced 5-HT synthesis. We assessed this possibility by measuring the accumulation of 5-HTP in the ventral hippocampus following AADC inhibition as an index of in vivo TpH activity and thus potential for 5-HT synthesis. In the ventral hippocampus (Fig. 3A), there was no significant effect of pretreatment (F1,39 = 0.593, P>0.05) and no significant interaction between pretreatment and inhibitor treatment (F1,39 = 0.681, P>0.05), but there was a significant main effect of inhibitor treatment (F1,39 = 138.328, P < 0.001). Post-hoc tests revealed that the AADC inhibitor significantly increased 5-HTP levels over vehicle-treated groups in both saline pretreated rats (SNK, P < 0.001) and amphetamine pretreated rats (SNK, P < 0.001). Likewise in the mRN (Fig. 3B), there was no significant effect of pretreatment (F1,38 = 0.467, P>0.05) and no significant interaction between pretreatment and inhibitor treatment (F1,38 = 0.2.263, P>0.05), but there was a significant main effect of inhibitor treatment (F1, 38 = 134.819, P< 0.001). The AADC inhibitor significantly increased 5-HTP levels over vehicle-treated groups in both saline pretreated rats (SNK, P < 0.001) and amphetamine pretreated rats (SNK, P < 0.001) within the mRN.
Figure 3.
Tissue concentrations of 5-hydroxytryptophan (5-HTP) in the (A) ventral hippocampus and (B) median raphe nucleus within saline (SAL) or amphetamine (AMP) pretreated rats 30 minutes after rats were treated with either vehicle or the amino acid decarboxylase (AADC) inhibitor NSD-1015 (100 mg/kg, i.p.). Data represent mean +/− SEM. Bars with different superscript letters are significantly different from one another (P < 0.001).
Experiment 3: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on Expression of 5-HT Transporters in the Ventral Hippocampus
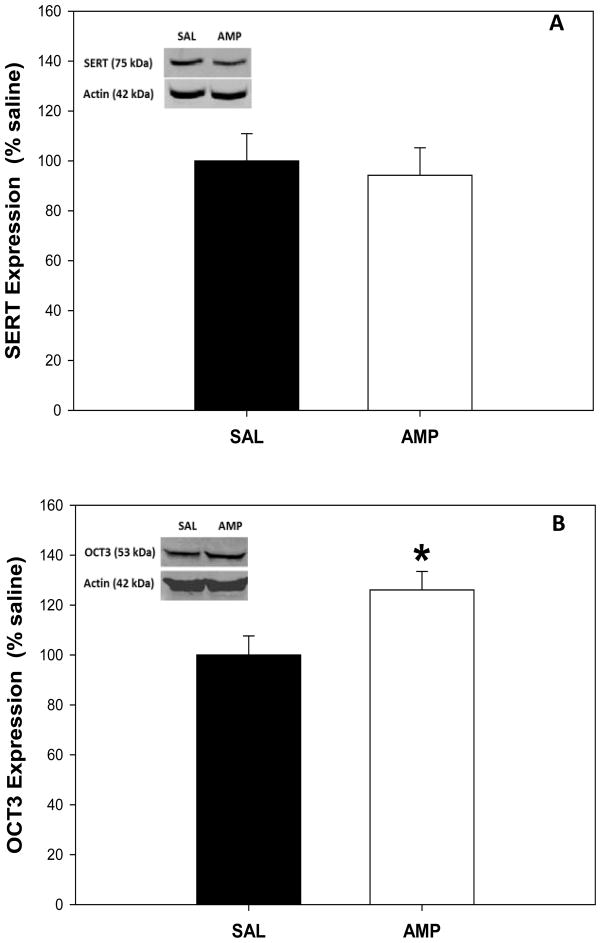
This experiment examined whether either SERT or OCT3 protein expression in the ventral hippocampus was altered following pretreatment with amphetamine for 2 weeks and acute withdrawal for 24 h, using western immunoblotting. There was no significant effect of amphetamine pretreatment on SERT expression in the ventral hippocampus when compared to saline pretreated controls (Figure 4A, F1,15 = 0.160, P>0.05). However, OCT-3 protein expression in amphetamine pretreated rats was significantly higher than saline pretreated rats in the ventral hippocampus (Figure 4B; F1,15 = 5.986, P< 0.05).
Figure 4.
(A) Serotonin transporter (SERT) and (B) organic cation transporter 3 (OCT3) protein levels in ventral hippocampal tissue of saline (SAL) and amphetamine (AMP) pretreated rats. Levels of SERT and OCT3 protein were determined by western immunoblot with antibodies recognizing a SERT band at 75 kDa and an OCT3 band at 53 kDa (Insert: examples from a single animal). Means ± S.E.M. are shown for all groups. * Significant difference between saline and amphetamine pretreatment groups (P < 0.05).
Experiment 4: Effects of Chronic Amphetamine Treatment and Acute Withdrawal on the Functional Expression of 5-HT Transporters in the Ventral Hippocampus
This experiment aimed to establish whether chronic amphetamine administration and withdrawal altered 5-HT accumulation in the rat ventral hippocampus in response to local perfusion with SERT or OCT3 inhibitors, as a measure of functional expression of each transporter.
SERT Inhibition with Paroxetine
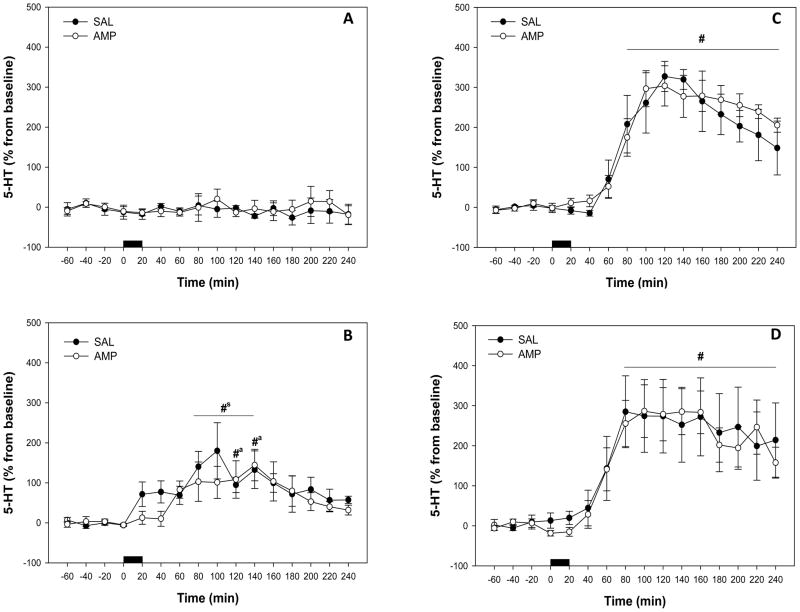
Extracellular 5-HT concentrations in the ventral hippocampus were unchanged by perfusion with vehicle (Fig. 5A). There were no significant effects of pretreatment (F1,6 = 0.273, P>0.05), or time (F15,124 = 0.816, P>0.05), and no significant interaction between treatment and time (F15,124 = 0.704, P>0.05).
Figure 5.
Effect of local paroxetine perfusions on extracellular 5-HT concentrations in the ventral hippocampus in saline (SAL) and amphetamine (AMP) pretreated rats, expressed as percentage of baseline. Horizontal black bar = perfusion of (A) vehicle, (B) 0.1 μM paroxetine (C) 0.5 μM paroxetine, and (D) 3μM paroxetine. Data represent mean ± SEM. #sSignificantly different from pre-perfusion levels in saline pretreated rats, #aSignificantly different from pre-perfusion levels in amphetamine pretreated rats, #Significantly different from pre-perfusion levels in both saline and amphetamine pretreated rats, (P < 0.05).
Perfusion of 0.1μM paroxetine into the ventral hippocampus increased extracellular 5-HT concentrations to approximately 100–150% above baseline levels (Fig. 5B). There was no significant effect of pretreatment (F1,8 = 0.111, P>0.05) and no significant interaction between treatment and time (F15,133 = 0.980, P>0.05). However, there was a significant effect of time (F15,133 = 5.504, P< 0.001). Animals that received saline pretreatment (F15,59 = 3.065; P<0.01) and animals that received amphetamine pretreatment (F15,73 = 3.395; P< 0.001) both had a significant increase in hippocampal 5-HT over time. Serotonin levels were significantly different from pre-perfusion levels at 80–140 min post-perfusion (Holm-Sidak, P < 0.05) for saline pretreated rats and at 120–140 min post-perfusion (Holm-Sidak, P < 0.05) for amphetamine pretreated rats (Fig. 5B).
Local application of 0.5μM paroxetine increased extracellular 5-HT concentrations in the ventral hippocampus to approximately 300% of baseline levels (Fig. 5C) This response appeared to be a maximal effect of SERT blockade since further increases in 5-HT were not evident with the perfusion of 3 μM paroxetine (see Fig. 5C–D). Like the 0.1 μM paroxetine infusion, there was no significant effect of pretreatment (F1,10 = 0.0884, P>0.05) and no significant interaction between treatment and time (F15,142 = 0.684, P>0.05), but a significant effect of time (F15,142 = 36.636, P< 0.001) was observed. Animals that received saline pretreatment (F15,59 = 21.783; P< 0.001) and animals that received amphetamine pretreatment (F15,82 = 20.278; P< 0.001) both had a significant increase in hippocampal 5-HT over time. Serotonin levels were significantly different from pre-infusion levels at 80–240 min post-perfusion (Holm-Sidak, P < 0.05) for both saline and amphetamine pretreated rats (Fig. 5C).
Perfusion of 3μM paroxetine into the ventral hippocampus also increased extracellular 5-HT approximately 300 % above baseline values (Fig. 5D). There was no significant effect of pretreatment (F1,8 = 0.0203, P>0.05) and no significant interaction between treatment and time (F15,156 = 0.164, P>0.05), although a significant effect of time was apparent (F15,156 = 16.115, P< 0.001). Animals that received saline pretreatment (F15,87 = 6.528; P< 0.001) and animals that received amphetamine pretreatment (F15,68 = 15.072; P< 0.001) both exhibited a significant increase in hippocampal 5-HT over time. Similar to the results obtained from the 0.5μM paroxetine perfusion, 5-HT levels were significantly different from pre-perfusion levels at 80–240 min post-perfusion (Holm-Sidak, P<0.05) for saline pretreated rats and at 60–240 min post-perfusion (Holm-Sidak, P< 0.05) for amphetamine pretreated rats (Fig. 5D) following 3 μM perfusion of paroxetine.
OCT3 Inhibition with D-22
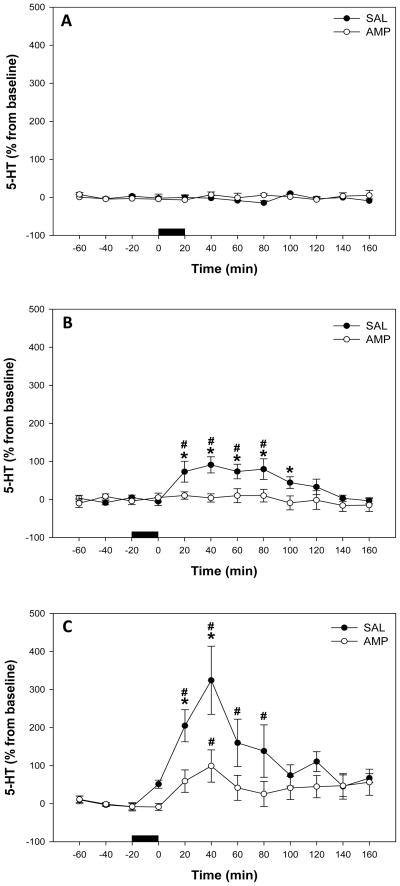
Local perfusion of vehicle for D-22 did not alter extracellular 5-HT concentrations in the ventral hippocampus (Fig. 6A). There was no significant effect of pretreatment (F1,9 = 0.753, P>0.05), of time (F11,82 = 0.662, P>0.05), nor a significant interaction between treatment and time (F11,82 = 0.728, P>0.05).
Figure 6.
Effect of local D-22 perfusion on extracellular 5-HT levels in the ventral hippocampus in saline (SAL) and amphetamine (AMP) pretreated rats, expressed as percentage of baseline. Horizontal black bar = perfusion of (A) vehicle, (B) 30 μM D-22 and (C) 100μM D-22. Data represent mean ± SEM. #Significantly different from pre-perfusion levels in both saline and amphetamine pretreated rats, (P < 0.05). *Significantly different from amphetamine pretreated rats, (P < 0.05).
Local application of 30μM D-22 produced significantly higher extracellular levels of 5-HT in the ventral hippocampus of saline pretreated rats (Fig. 6B). There was a significant effect of pretreatment (F1,13 = 6.983, P<0.05), a significant effect of time (F11,172 = 4.882, P<0.001), and a significant interaction between treatment and time (F11,172 = 3.891, P<0.001). Animals receiving saline pretreatment had a significant increase in hippocampal 5-HT over time (F11,79 = 7.683; P<0.001), with 5-HT levels significantly different from pre-perfusion levels at 20–80 min post-perfusion (Holm-Sidak, P<0.05; Fig. 6B). Conversely, 5-HT levels were not significantly altered over time following 30μM D-22 perfusion in rats pretreated with amphetamine (F11,92 = 0.300, P>0.05). When saline and amphetamine groups were compared at each time-point, saline pretreated rats exhibited significantly higher 5-HT levels at 20–100 min post-D-22 perfusion (SNK, P< 0.05; Fig 6B).
In the 100μM D-22 groups (Fig. 6C), there was no significant effect of pretreatment (F1,17 = 0.449, P>0.05), although there was a significant effect of time (F11,102 = 8.448, P< 0.001), and a significant interaction between treatment and time (F11,102 = 3.735, P< 0.001). Animals receiving saline pretreatment had a significant increase in hippocampal 5-HT over time (F9,51 = 7.376; P< 0.001), which was significantly higher than pre-perfusion levels at 20–80 min post-perfusion (Holm-Sidak, P<0.05; Fig. 6C). Serotonin levels were also significantly increased over time in amphetamine pretreated rats following 100 μM D-22 perfusion (F8,51 = 2.137; P< 0.05), with only 40 min post-perfusion higher than pre-perfusion levels (Holm-Sidak, P<0.05; Fig. 6C). When saline and amphetamine groups were compared at each time-point, saline pretreated rats exhibited significantly higher 5-HT levels at 20 and 40 min post-D-22 perfusion (SNK, P < 0.05; Fig 6C).
Discussion
Rats undergoing 24 hours withdrawal from chronic amphetamine treatment exhibited decreased stimulated (KCl-induced) increases of extracellular 5-HT in the ventral hippocampus. This effect could not be explained by reduced TpH activity in either the ventral hippocampus or in the mRN, which contains serotonergic cell bodies that project to the ventral hippocampus (Azmitia and Segal, 1978; Miguez et al., 2002). Reduced KCl-induced ventral hippocampus 5-HT was also not explained by an apparent change in SERT protein expression or functional expression (as measured by SERT blockade) following chronic amphetamine pretreatment and 24 hours withdrawal. Instead, chronic amphetamine and withdrawal increased OCT3 protein expression in the ventral hippocampus. Consistent with this effect, there was a reduction in the ability for the OCT3 inhibitor D-22 to increase extracellular 5-HT accumulation in the ventral hippocampus in amphetamine pretreated rats at 24 hours withdrawal.
High K+ increases extracellular neurotransmitter by depolarizing synaptic terminals and initiating vesicular exocytosis. The increase in extracellular 5-HT concentrations induced by K+ and measured by microdialysis reflects a balance between release and reuptake (Badoer et al., 1989). Previous studies have shown that transgenic mice overexpressing SERT or rats with enhanced 5-HT reuptake via SERT exhibit significantly reduced KCl-elicited increases in extracellular 5-HT (Jennings et al., 2006; Benmansour et al., 2008) which is similar to the response to KCl we observed in amphetamine pretreated rats during withdrawal. Deficits in KCl-elicited 5-HT in SERT overexpressing mice are reversed by local or systemically applied paroxetine to inhibit SERT function (Jennings et al., 2006). Therefore, it is likely that reduced KCl-elicited extracellular 5-HT levels in the ventral hippocampus of amphetamine pretreated rats during withdrawal is a result of decreased accumulation of extracellular 5-HT due to increased 5-HT clearance in this region, although changes to the mechanisms underlying KCl-related 5-HT release might also play a role. Alternative possibilities include amphetamine-induced changes to the sensitivity or expression of 5-HT autoreceptors that might limit 5-HT neurotransmission in the ventral hippocampus. For example, 5-HT1B terminal autoreceptors in the ventral hippocampus inhibit 5-HT release in this region (Trillat and Malagie, 1997), and it remains to be seen if chronic amphetamine treatment and acute withdrawal increase 5-HT1B receptor sensitivity/expression. However, chronic treatment and 24 hours withdrawal from a related compound (3,4-methylenedioxymethamphetamine) does not affect expression of 5-HT1B receptors in the hippocampus (Sexton et al., 1999), and 5-HT1B knockout mice do not show altered basal or KCl- elicited 5-HT levels in the ventral hippocampus (Trillat et al., 1997), suggesting that reduced KCl-elicited 5-HT responses in amphetamine pretreated rats from the current study may not be due to changes in 5-HT1B autoreceptor function. It is also possible that increased sensitivity of 5-HT1A auto receptors in the raphe serotonergic cell body regions may serve to dampen 5-HT neurotransmission in the ventral hippocampus during amphetamine withdrawal. Increased binding sites for 5-HT1A receptors have been observed in the dorsal (but not median) raphe nucleus 24 hours following chronic amphetamine treatment (Bonhomme et al., 1995). However, spontaneous firing of these neurons appears to be either unaffected or increased by chronic amphetamine treatment and withdrawal, and decreased sensitivity of raphe neurons to a 5-HT1A receptor agonist is observed 24 hours following chronic amphetamine treatment (Heidenreich et al., 1987; Heidenreich and Rebec, 1989). These findings suggest that dampened KCl-elicited 5-HT neurotransmission in the ventral hippocampus of amphetamine pretreated rats at 24 hours withdrawal is unlikely to be due to increased autoinhibition at the cell body level.
Surprisingly, our results did not implicate SERT in increased clearance of 5-HT in the ventral hippocampus of amphetamine pretreated rats. The expression of SERT in the ventral hippocampus was not affected by chronic amphetamine pretreatment and withdrawal. Although the western immunoblotting procedure used in the current studies does not discriminate between intracellular and surface expressed SERT, the magnitude of the increase in 5-HT following local perfusion of paroxetine into the ventral hippocampus was similar between amphetamine and saline pretreatment groups for all drug concentrations tested. This suggests that the functional expression or density of SERT available for blockade was similar between saline and amphetamine pretreated rats. Since 5-HT clearance is related to the density of functional SERT (Montanez et al., 2002; Ramsey and DeFelice, 2002), the combined pharmacological and protein results suggests that chronic amphetamine treatment may not alter SERT clearance in the ventral hippocampus as measured at 24 hours withdrawal. While acute amphetamine has been shown to increase SERT activity at the membrane through the suppression of protein kinase C -mediated phosphorylation of SERT (Ramamoorthy and Blakely, 1999; Qian et al., 1997), a lack of change in SERT expression during withdrawal following chronic amphetamine treatment in the current study is consistent with previous work measuring SERT expression during withdrawal from chronic treatment with amphetamine derivatives such as D-fenfluramine, parachloroamphetamine or 3,4-methylenedioxymethamphetamine (Wang et al., 2004; 2005; Rothman et al., 2003; 2004; Williams et al., 2005).
Since dampened levels of hippocampal 5-HT in amphetamine pretreated rats were observed in response to KCl stimulation, it is likely that chronic amphetamine pretreatment and withdrawal affects alternate mechanisms of 5-HT clearance. Of importance, amphetamine and saline pretreated rats differ in KCl-stimulated and glucocorticoid-stimulated (Barr and Forster, 2011), but not SERT blockade-induced levels of 5-HT in the ventral hippocampus. Thus it is possible that KCl or glucocorticoid stimulation, which produce high extracellular concentrations of 5-HT, engage alternative clearance mechanisms that are not evident with the more modest increases in 5-HT concentrations induced by SERT blockade. A likely candidate is OCT3, a low affinity, high capacity transporter of 5-HT (Koepsell et al., 2007), which is believed to function in monoamine clearance, particularly under conditions when extracellular monoamine concentrations are high (Daws, 2009). Perfusion of sub-maximal concentrations of D-22(30 μM; Feng et al., 2005) resulted in an accumulation of 5-HT in the ventral hippocampus of saline pretreated rats. However, this effect of low D-22 concentration on extracellular 5-HT accumulation was absent in amphetamine pretreated rats at 24 hours withdrawal. A higher concentration of D-22 did enhance 5-HT concentrations in amphetamine pretreated rats. However, this effect was significantly attenuated when compared to the high concentration D-22 induced 5-HT increase measured in the ventral hippocampus of saline pre-treated controls. In addition, chronic amphetamine pretreatment and withdrawal resulted in an increase in OCT3 protein expression in the ventral hippocampus. Combined, these findings suggest that the greater concentration of D-22 required to block OCT3 sufficiently in the ventral hippocampus of amphetamine pretreated rats at 24 hours withdrawal reflects increased OCT3 functional expression, and hence greater 5-HT clearance by OCT3. Increased 5-HT clearance by OCT3 may explain, in part, the reduced extracellular 5-HT in the ventral hippocampus as a response to KCl or corticosterone (Barr and Forster, 2011) stimulation 24 hours after withdrawal from chronic amphetamine treatment.
Plasma membrane transporters are subject to multiple levels of regulation that can rapidly alter 5-HT uptake and clearance rates (Blakely and Bauman 2000; Blakely et al. 2005; Steiner et al., 2008). Repeated amphetamine and withdrawal may have an impact on one or more regulatory pathways altering expression and function of OCT3 in the ventral hippocampus. For instance, repeated binding of OCT3 by amphetamine (Wu et al., 1998; Amphoux et al., 2006) may produce increased surface expression of the transporter or alter OCT3 transport activity to generate enhanced clearance of 5-HT. However, more recent reports suggest that amphetamine may not functionally interact with OCT3s (Zhu et al., 2010). Since acute amphetamine increases extracellular 5-HT by acting as a false substrate for SERT (Hilber et al., 2005; Seidel et al., 2005), it may be more likely that repeated increases in extracellular 5-HT levels during the chronic amphetamine treatment results in a compensatory increase in high capacity OCT3 transporter expression and a concomitant increase in 5-HT clearance in the ventral hippocampus over the course of the amphetamine treatment and acute withdrawal period. A complementary mechanism may be related to the ability of acute amphetamine to rapidly increase corticosterone (Kynch and Eisenburg, 1979; Swerdlow et al., 1993). Corticosterone inhibits OCT3 mediated uptake of 5-HT (Gasser et al., 2006) and repeated corticosterone-mediated inhibition of 5-HT clearance by OCT during chronic amphetamine treatment may also contribute to compensatory increases in OCT3 expression and function in the ventral hippocampus. It is clear that future studies should examine these potential mechanisms by which chronic amphetamine may increase OCT3 expression and function as revealed at 24 hours withdrawal. It also remains to be seen whether the increase in OCT3 function, and diminished ventral hippocampus 5-HT function, persists beyond the 24 hour withdrawal period measured here.
Rats that have undergone chronic amphetamine administration, identical to the treatment used in these studies, display heightened anxiety states apparent during early (24 hour) and late (4 weeks) withdrawal (Barr et al., 2010; Vuong et al., 2010). The current study shows dampened 5-HT neurotransmission in the ventral hippocampus at 24 hours withdrawal from chronic amphetamine. While a direct relationship between reduced ventral hippocampal 5-HT and increased anxiety-like behaviors can not be ascertained from the current study, previous work shows that 5-HT in the hippocampus is important for adaptive coping with anxiogenic environments, with dysfunction of 5-HT neurotransmission in this region thought to underlie symptoms of anxiety (Guimaraes et al., 1993; Graeff et al., 1996; Joca et al., 2003; Keck et al., 2005; Storey et al., 2006). It is likely that increases in hippocampal 5-HT that contribute to coping behaviors is induced by corticotropin-releasing factor (CRF) stimulation of serotonergic inputs into the hippocampus, as intracerebroventricular (icv.) administration of CRF increases 5-HT levels in the hippocampus, and stressor-induced increases in hippocampal 5-HT, which occur during active coping behaviors, are blocked by icv. infusion of a CRF receptor antagonist (Linthorst et al., 2002; Kagamiishi et al., 2003). Changes to CRF-mediation of ventral hippocampal 5-HT in amphetamine treated rats during withdrawal have not been investigated. However, CRF2 receptor levels are increased in the dorsal raphe nucleus at 20 hours and 6 weeks following withdrawal from chronic amphetamine (Pringle et al., 2008), suggesting that CRF would augment rather than attenuate 5-HT neurotransmission in the ventral hippocampus of rats exposed to chronic amphetamine treatment and withdrawal, as has been observed for the amygdala (Scholl et al., 2010). Therefore, the attenuated 5-HT neurotransmission in the ventral hippocampus (and any associated behaviors) of amphetamine-treated rats at 24 hours withdrawal appear to more readily relate to local changes in OCT3-mediated clearance.
Related, reduced OCT3 function is associated with reduced anxiety-like behaviors whereas indirect evidence suggests increased OCT3 expression or function increases anxiety states. For example, OCT3 knockout mice display an anxiolytic phenotype (Wultsch et al., 2009), and inhibition of OCT3 in adult rats has anxiolytic-like effects (Kitaichi et al., 2005; Baganz et al., 2008). Similarly, multiple studies have shown that SERT knockout mice, which have heightened OCT3 activity (Schmitt et al., 2003; Daws et al., 2006; Baganz et al., 2008), display increased anxiety-like behavior (Holmes et al., 2003; Kalueff et al., 2007). The increase of OCT3 mRNA in SERT knockout mice was detected in the hippocampus, but not other brain regions (Schmitt et al., 2003; Wultsch et al., 2009), suggesting OCT3 may have a region specific role for 5-HT reuptake in the hippocampus, and alterations to 5-HT reuptake specifically in the hippocampus might relate to anxiety states. Therefore, enhanced functional expression of OCT3 in the hippocampus of amphetamine pretreated rats may play an important role in the heightened anxiety states observed in these animals. Further studies are needed to determine whether alterations to ventral hippocampus OCT3 activity directly relate to increased anxiety states during amphetamine withdrawal.
In summary, the present study indicates that chronic amphetamine administration and withdrawal decreases serotonergic neurotransmission in the ventral hippocampus and alters 5-HT functional expression of OCT3 independent of any apparent effects on SERT. These results suggest that via changes in mechanisms of 5-HT reuptake, chronic amphetamine treatment and acute withdrawal attenuates elevated extracellular levels of 5-HT in the ventral hippocampus that may be necessary to generate adaptive responses to anxiogenic environments.
Acknowledgments
This work was supported by grants NIH R01 DA019921 (GLF), NSF grants IOS 0921874 (KJR) and NSF IOS 0921969 (CAL) & NIH P20 RR015567 which is a designated Center of Biomedical Research Excellence (COBRE).
Abbreviations
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HTP
5-hydroxytryptophan
- AADC
aromatic amino acid decarboxylase
- aCSF
artificial cerebrospinal fluid
- AP
anterioposterior
- CRF
corticotropin-releasing factor
- D-22
decynium 22
- DV
dorsoventral
- ML
mediolateral
- mRN
median raphe nucleus
- OCT
organic cation transporter
- PMAT
plasma membrane monoamine transporter
- SERT
serotonin transporter
- SNK
Student-Newman-Keuls
- TpH
tryptophan hydroxylase
Footnotes
All authors report no conflict of interest.
References
- Amphoux A, Vialou V, Drescher E, Bruss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bonisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Badoer E, Wurth H, Turck D, Qadri F, Itoi K, Dominiak P, Unger T. The K+-induced increases in noradrenaline and dopamine release are accompanied by reductions in the release of their intraneuronal metabolites from the rat anterior hypothalamus. An in vivo brain microdialysis study. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:54–59. doi: 10.1007/BF00165126. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JL, Forster GL. Serotonergic neurotransmission in the ventral hippocampus is enhanced by corticosterone and altered by chronic amphetamine treatment. Neuroscience. 2011;182:105–114. doi: 10.1016/j.neuroscience.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JL, Renner KJ, Forster GL. Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology. 2010;59:395–405. doi: 10.1016/j.neuropharm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Deltheil T, Piotrowski J, Nicolas L, Reperant C, Gardier AM, Frazer A, David DJ. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587:90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 2005;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- Bonhomme N, Cador M, Stinus L, Le Moal M, Spampinato U. Short and long-term changes in dopamine and serotonin receptor binding sites in amphetamine-sensitized rats: a quantitative autoradiographic study. Brain Research. 1995;675:215–223. doi: 10.1016/0006-8993(95)00067-z. [DOI] [PubMed] [Google Scholar]
- Burke AR, Watt MJ, Forster GL. Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience. 2011;197:269–279. doi: 10.1016/j.neuroscience.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Toney GM, Gerhardt GA, Frazer A. In vivo chronoamperometric measures of extracellular serotonin clearance in rat dorsal hippocampus: contribution of serotonin and norepinephrine transporters. J Pharmacol Exp Ther. 1998;286:967–976. [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–53. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AK, Heerkens JL, Lowry CA. Acoustic stimulation in vivo and corticotropin-releasing factor in vitro increase tryptophan hydroxylase activity in the rat caudal dorsal raphe nucleus. Neurosci Lett. 2009;455:36–41. doi: 10.1016/j.neulet.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Feng N, Lowry CA, Lukkes JL, Orchinik M, Forster GL, Renner KJ. Organic cation transporter inhibition increases medial hypothalamic serotonin under basal conditions and during mild restraint. Brain Res. 2010;1326:105–113. doi: 10.1016/j.brainres.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063:69–76. doi: 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Feng N, Telefont M, Kelly KJ, Orchinik M, Forster GL, Renner KJ, Lowry CA. Local perfusion of corticosterone in the rat medial hypothalamus potentiates D-fenfluramine-induced elevations of extracellular 5-HT concentrations. Horm Behav. 2009;56:149–157. doi: 10.1016/j.yhbeh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–81. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- Guimaraes F, Del Bel EA, Padovan CM, Netto SM, de Almeida RT. Hippocampal 5-HT receptors and consolidation of stressful memories. Behav Brain Res. 1993;58:133–139. doi: 10.1016/0166-4328(93)90098-b. [DOI] [PubMed] [Google Scholar]
- Gossop M. Review: limited evidence to support pharmacological therapy for amphetamine withdrawal. Evid Based Ment Health. 2009;12:122. doi: 10.1136/ebmh.12.4.122. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–41. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Gründemann D, Babin-Ebell J, Martel F, Ording N, Schmidt A, Schömig E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. J Biol Chem. 1997;272:10408–13. doi: 10.1074/jbc.272.16.10408. [DOI] [PubMed] [Google Scholar]
- Heidenreich BA, Basse-Tomusk AE, Rebec GV. Serotonergic dorsal raphe neurons: subsensitivity to amphetamine with long-term treatment. Neuropharmacology. 1987;26:719–724. doi: 10.1016/0028-3908(87)90233-4. [DOI] [PubMed] [Google Scholar]
- Heidenreich BA, Rebec GV. Serotonergic dorsal raphe neurons: Changes in spontaneous neuronal activity and responsiveness to 5-MeODMT following long-term amphetamine administration. Neuroscience Letters. 1989;103:81–86. doi: 10.1016/0304-3940(89)90489-8. [DOI] [PubMed] [Google Scholar]
- Hilber B, Scholze P, Dorostkar MM, Sandtner W, Holy M, Boehm S, Singer EA, Sitte HH. Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines and non-amphetamines. Neuropharmacology. 2005;49:811–819. doi: 10.1016/j.neuropharm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, Hashimoto H, Yamada S. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144:695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, Olverman HJ, Hastie ND, Harmar AJ, Shen S, Sharp T. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joca SR, Padovan CM, Guimaraes FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res. 2003;978:177–184. doi: 10.1016/s0006-8993(03)02943-3. [DOI] [PubMed] [Google Scholar]
- Johnston CA, Moore KE. Measurement of 5-hydroxytryptamine synthesis and metabolism in selected discrete regions of the rat brain using high performance liquid chromatography and electrochemical detection: pharmacological manipulations. J Neural Transm. 1983;57:49–63. doi: 10.1007/BF01250047. [DOI] [PubMed] [Google Scholar]
- Kagamiishi Y, Yamamoto T, Watanabe S. Hippocampal serotonergic system is involved in anxiety-like behavior induced by corticotropin-releasing factor. Brain Research. 2003;991:212–221. doi: 10.1016/j.brainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Keck ME, Sartori SB, Welt T, Muller MB, Ohl F, Holsboer F, Landgraf R, Singewald N. Differences in serotonergic neurotransmission between rats displaying high or low anxiety/depression-like behaviour: effects of chronic paroxetine treatment. J Neurochem. 2005;92:1170–1179. doi: 10.1111/j.1471-4159.2004.02953.x. [DOI] [PubMed] [Google Scholar]
- Knych ET, Eisenberg RM. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology. 1979;29:110–118. doi: 10.1159/000122912. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Linthorst ACE, Peñalva RG, Flachskamm C, Holsboer F, Reul JMHM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. European Journal of Neuroscience. 2002;16:2441–2452. doi: 10.1046/j.1460-9568.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Frontiers in Behavioral Neuroscience. 2009;3:1–12. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Miguez JM, Paz-Valinas L, Miguez I, Aldegunde M. Somatodendritic action of pindolol to attenuate the paroxetine-induced decrease in serotonin release from the rat ventral hippocampus: a microdialysis study. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:378–387. doi: 10.1007/s00210-002-0530-5. [DOI] [PubMed] [Google Scholar]
- Montanez S, Daws LC, Gould GG, Gerhardt GA, Frazer A. Differential in vivo clearance of serotonin in rat dorsal raphe nucleus and CA3 region. Brain Res. 2002;955:236–244. doi: 10.1016/s0006-8993(02)03470-4. [DOI] [PubMed] [Google Scholar]
- O’Reilly KC, Trent S, Bailey SJ, Lane MA. 13-cis-Retinoic acid alters intracellular serotonin, increases 5-HT1A receptor, and serotonin reuptake transporter levels in vitro. Exp Biol Med. 2007;232:1195–1203. doi: 10.3181/0703-RM-83. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. New York: Academic Press; 1996. [Google Scholar]
- Pringle RB, Mouw NJ, Lukkes JL, Forster GL. Amphetamine treatment increases corticotropin-releasing factor receptors in the dorsal raphe nucleus. Neuroscience Research. 2008;62:62–65. doi: 10.1016/j.neures.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, DeFelice LJ. Serotonin transporter function and pharmacology are sensitive to expression level: evidence for an endogenous regulatory factor. J Biol Chem. 2002;277:14475–14482. doi: 10.1074/jbc.M110783200. [DOI] [PubMed] [Google Scholar]
- Romanelli F, Smith KM. Clinical effects and management of methamphetamine abuse. Pharmacotherapy. 2006;26:1148–1156. doi: 10.1592/phco.26.8.1148. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Jayanthi S, Wang X, Dersch CM, Cadet JL, Prisinzano T, Rice KC, Baumann MH. High-dose fenfluramine administration decreases serotonin transporter binding, but not serotonin transporter protein levels, in rat forebrain. Synapse. 2003;50:233–239. doi: 10.1002/syn.10266. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Jayanthi S, Cadet JL, Wang X, Dersch CM, Baumann MH. Substituted amphetamines that produce long-term serotonin depletion in rat brain (“neurotoxicity”) do not decrease serotonin transporter protein expression. Ann N Y Acad Sci. 2004;1025:151–161. doi: 10.1196/annals.1316.020. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71:701–709. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Renner KJ, Forster GL, Tejani-Butt S. Central monoamine levels differ between rat strains used in studies of depressive behavior. Brain Res. 1355:41–51. doi: 10.1016/j.brainres.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL. Individual differences in amphetamine sensitization, behavior and central monoamines. Physiol Behav. 2009;96:493–504. doi: 10.1016/j.physbeh.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl JL, Vuong SM, Forster GL. Chronic amphetamine treatment enhances corticotropin-releasing factor-induced serotonin release in the amygdala. European Journal of Pharmacology. 2010;644:80–87. doi: 10.1016/j.ejphar.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schömig E, Lazar A, Gründemann D. Extraneuronal monoamine transporter and organic cation transporters 1 and 2: a review of transport efficiency. Handb Exp Pharmacol. 2006;175:151–80. doi: 10.1007/3-540-29784-7_8. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Steiner-Mordoch S, Yelin R, Wall SC, Rudnick G. Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol Pharmacol. 1993;44:1227–1231. [PubMed] [Google Scholar]
- Seidel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, Holy M, Koppatz K, Krivanek P, Freissmuth M, Sitte HH. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- Sexton TJ, McEvoy C, Neumaier JF. (+) 3,4-Methylenedioxymethamphetamine (‘Ecstasy’) transiently increases striatal 5-HT1B binding sites without altering 5-HT1B mRNA in rat brain. Molecular Psychiatry. 1999;4:572. doi: 10.1038/sj.mp.4000574. [DOI] [PubMed] [Google Scholar]
- Shoptaw SJ, Kao U, Heinzerling K, Ling W. Treatment for amphetamine withdrawal. Cochrane Database Syst Rev. 2009:CD003021. doi: 10.1002/14651858.CD003021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Robertson DA, Beattie JE, Reid IC, Mitchell SN, Balfour DJK. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res. 2006;166:220–229. doi: 10.1016/j.bbr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav. 1993;45:629–637. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Trillat AC, Malagie I, Scearce K, Pons D, Anmella MC, Jacquot C, Hen R, Gardier AM. Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: in vivo microdialysis studies. J Neurochem. 1997;69:2019–2025. doi: 10.1046/j.1471-4159.1997.69052019.x. [DOI] [PubMed] [Google Scholar]
- Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav Brain Res. 208:278–281. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53:240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Morales M, Rothman RB. (+/−)-3,4-Methylenedioxymethamphetamine administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J Pharmacol Exp Ther. 2005;314:1002–1012. doi: 10.1124/jpet.105.088476. [DOI] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- Wultsch T, Grimberg G, Schmitt A, Painsipp E, Wetzstein H, Breitenkamp AF, Gründemann D, Schömig E, Lesch KP, Gerlach M, Reif A. Decreased anxiety in mice lacking the organic cation transporter 3. J Neural Transm. 2009;116:689–97. doi: 10.1007/s00702-009-0205-1. [DOI] [PubMed] [Google Scholar]
- Zeeb F, Wong A, Winstanley C. Differential effects of environmental enrichment, social-housing, and isolation-rearing on a rat gambling task: Dissociations between impulsive action and risky decision-making. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2822-x. in press. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Appel DI, Grundemann D, Markowitz JS. Interaction of organic cation transporter 3 (SLC22A3) and amphetamine. J Neurochem. 114:142–149. doi: 10.1111/j.1471-4159.2010.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]