Abstract
Purpose
To determine the effect of the novel D3 dopamine receptor agonist, D-264, on activation of D3 and D2 dopamine receptor signal transduction pathways and cell proliferation.
Methods
AtT-20 neuroendocrine cells stably expressing Human D2S, D2L and D3 dopamine receptors were treated with D-264 and the coupling of the receptors to mitogen-activated protein kinase (MAPK) and G-protein coupled inward rectifier potassium (GIRK) channels determined using Western blotting and whole cell voltage clamp recording, respectively.
Results
D-264 potently activated MAPK signaling pathway coupled to D2S, D2L and D3 dopamine receptors. The activation of MAPK was more pronounced than the reference agonist quinpirole and was longer lasting. D-264 also activated GIRK channels coupled to D2S, D2L and D3 receptors. In addition, D-264 dose-dependently induced cell proliferation in AtT-D2L and AtT-D3 cells.
Conclusion
These results indicate that D-264 robustly activates GIRK channels and MAPK coupled to D2 and D3 dopamine receptors in AtT-20 cells. D-264 is also a potent inducer of cell proliferation.
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta (Wooten). PD affects approximately 1% of people older than 65 years of age. Principle symptoms associated with PD involve rigidity, bradykinesia, resting tremor and postural instability along with cognitive and psychiatric complications (Paulus and Jellinger, 1991; Sherer et al., 2001). Currently, the most effective therapy for PD involves restoration of the dopaminergic neurotransmission by administration of levodopa (Birkmayer and Hornykiewicz, 2001; Cotzias et al., 1969), the precursor of dopamine (Lopez et al., 2001). However, as the disease progresses, the levodopa therapy often results in the emergence of motor complications consisting of on–off phenomena and levodopa-induced dyskinesias (Marsden and Parkes, 1976). As an alternative to levodopa, dopamine receptor agonists are also used clinically to treat PD (Clarke and Guttman, 2002; Dutta, 2006; Foley et al., 2004).
Dopamine receptors are members of the G protein-coupled receptor (GPCR) family of proteins. They are classified into two main subfamilies based on their sequence homology and pharmacological properties. The D1-like dopamine receptors include D1 and D5 receptor subtypes whereas the D2-like dopamine receptors include D2, D3 and D4 receptor subtypes (Civelli et al., 1993; Giros et al., 1990; Kebabian and Calne, 1979; Sokoloff et al., 1990; Strange, 1993; Sunahara et al., 1991; Van Tol et al., 1991). The stimulation of D1-like receptors leads to activation of adenylate cyclase, which promotes synthesis of cAMP, whereas D2-like receptor activation leads to inhibition of adenylate cyclase activity. In the CNS, D1-like receptors are located post-synaptically, whereas D2-like receptors are located both pre- and post-synaptically and have high affinity for dopamine (Emilien et al., 1999). In addition to adenylate cyclase, the D2-like dopamine receptors also couple to several signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway and G-protein coupled inward rectifier potassium (GIRK) channels. In vitro and in vivo studies have also shown that D2-like receptors modulate cell proliferation.(Winner et al., 2009)
Currently, a number of dopamine receptor agonists are being used in the clinic to treat PD. Some of these agonists are ergot alkaloids and include pergolide, bromocriptine, and lisuride. Recently, newer, non-ergot classes of molecules pramipexole and ropinirole along with apomorphine have been clinically used (Rascol et al., 1998; Rinne et al., 1998). Most dopamine receptor agonists with antiparkinsonian effects have high affinity for D2 and D3 receptors. Interestingly, two dopamine receptor agonists that are used clinically, ropinirole and pramipexole, have a higher affinity for D3 receptor compared to D2 receptor. Results from in vitro and in vivo studies have indicated that D3 receptor activation can potentially afford neuroprotection (Du et al., 2005; Joyce, 2001).
Recently, we reported the development of novel D3 preferring agonists based on our hybrid template. One of our lead compounds, (−)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine (D-264) exhibited high affinity and good selectivity for the D3 receptor (Biswas et al., 2008a; Biswas et al., 2008b; Johnson et al., 2012), Table 1. This compound demonstrated potent antiparkinsonian activity in 6-OHDA lesioned animal models of PD (Biswas et al., 2008a). Furthermore in our in vivo neuroprotection study, using both acute MPTP and progressive lactacystin mouse models of PD, we demonstrated that D-264 significantly improved the behavioral symptoms of PD (Li et al., 2010). D-264 exhibited pronounced neuroprotective effect in both the MPTP- and lactacystin-lesioned models as the animals pretreated with D-264 maintained significant number of tyrosine hydroxylase-positive cells and had normal levels of dopamine and its metabolites (Li et al., 2010). The neuroprotective effect of D-264 was specifically mediated by D3 receptors as pretreatment with D3 receptor-preferring antagonist, U99194, blocked the D-264-induced neuroprotection (Li et al., 2010). While implicating D3 receptors, these in vivo studies with D-264 did not determine if the neuroprotection was mediated directly by D3 receptor signaling pathways or indirectly through production of trophic factors. In this paper, we directly evaluated the effect of D-264 on signaling mediated by D2 and D3 receptors heterologously expressed in AtT-20 neuroendocrine cells.
Table 1.
Stimulation of [35S]GTPγS Binding to Cloned Human D2 Receptor and D3 receptor expressed in CHO cells.
| Compound | hCHO-D2 | hCHO-D3 | |||
|---|---|---|---|---|---|
| EC50 (nM) [35S]GTPγS | % Emax | EC50 (nM) [35S]GTPγS | % Emax | D2/D3 | |
| DA | 227 ± 11 | 100 | 8.57 | 100 | 26.5 |
| D-264a | 33.1 ± 6.6 | 104 ± 5 | 1.51 ± 0.22 | 90.0 ± 4.3 | 22.1 |
EC50 is the concentration producing half-maximal stimulation. For each compound, maximal stimulation (Emax) is expressed as a percent of the Emax observed with 1 mM (D2) or 100 μM (D3) of the full agonist DA (% Emax). Results are means ± SEM for 3–6 independent experiments, each performed in triplicate.
The D3 receptor has been previously shown to couple to MAPK signaling and GIRK channels in AtT-20 neuroendocrine cells stably expressing human D3 receptors (Kuzhikandathil et al., 2004; Westrich and Kuzhikandathil, 2007). In this manuscript, we report the effect of D-264 on MAPK-mediated ERK phosphorylation as well as activation of GIRK channels coupled to human D2S, D2L or D3 dopamine receptors. In addition, we determined the effect of various concentrations of D-264 on the proliferation of AtT-20 cells. Our goal was to understand D2 and D3 dopamine receptor signal transduction pathways activated by D-264 and determine if D-264 modulated proliferation of cell lines heterologously expressing D2 and D3 receptors.
METHODS
Cell culture
AtT-20 mouse pituitary cells were grown in Ham's F10 medium with 5% FBS, 10% heat-inactivated horse serum, 2 mM glutamine and 50 μg/ml gentamicin (Invitrogen, Carlsbad, CA, USA). AtT-20 cells stabling expressing the human D2S, D2L, and D3 receptor were maintained in the above F10 culture media supplemented with 500 μg/ml G418 (Invitrogen). For electrophysiological characterization, cells were plated onto glass coverslips coated with 40 μg/ml poly L-lysine (Sigma, St. Louis, MO, USA). The generation and characterization of the AtT-20 cells stably expressing various human dopamine receptors have been previously reported.(Kuzhikandathil and Bartoszyk, 2006; Kuzhikandathil and Oxford, 1999)
Electrophysiology
Agonist-activated G-protein coupled inward rectifier potassium (GIRK) currents were measured by the whole-cell patch clamp technique in voltage clamp mode as described previously. (Kuzhikandathil and Bartoszyk, 2006; Kuzhikandathil and Oxford, 1999; Kuzhikandathil et al., 1998) Briefly, cells were held at −65 mV and inward K+ currents induced by drug solutions were measured. The external solution (SES) used for K+ current measurements was: 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes [pH7.4], and 10mM glucose and the pipette solution contained: 130 mM K-Aspartate, 20 mM NaCl, 1 mM MgCl2, 10 mM Hepes [pH7.4], 10 mM glucose, 0.1 mM GTP, 5 mM Mg-ATP, and 1 mM EGTA. To enhance inwardly rectifying K+ currents, controls and drug exposures were performed in solutions with elevated extracellular [K+] (30 mM) by substitution for Na+. (−) Quinpirole (Sigma, St. Louis, MO, USA) was dissolved in water and used at indicated concentrations. A 10 mM stock of D-264 was freshly dissolved in DMSO, diluted in SES and used at indicated concentrations. Drug solutions were delivered to cells via a multi-barreled micropipette array. The current responses were normalized to the cell capacitance, to account for variation in cell size.
Detection of ERK phosphorylation
For the mitogen-activated protein kinase (MAPK) studies, cell lines were grown in 6-well plates until 80–90% confluent. The cells were incubated in Opti-MEM serum-free medium (Invitrogen) for 3 hours prior to treatment. The cells were then pretreated with either 100 nM D-264, 200 nM quinpirole or SES for 1 min, followed by several thorough SES washes for 30 min. Both control and pretreated cells were thoroughly washed several times in SES for a total of 30 minutes. The wash procedure included three quick rinses with 3 ml of SES, followed by six rinses with 3 ml SES. Each of the six rinses was for five minutes at room temperature (25°C). Subsequently, the cells were treated with SES, 200 nM quinpirole or 100 nM D-264 for 5 min and lysed using a lysis reagent (62.5 mM Tris-Cl [pH 6.8], 10 mM sodium fluoride, 1 mM phenylmethylsulphonyl fluoride (PMSF), 1 mM sodium orthovanadate, 1 mM EDTA, 1 mM EGTA, 200 mM DTT, 1 μg/ml phosphatase inhibitor (Sigma), 1 μg/ml protease inhibitor cocktail (Sigma), 2% SDS, 0.5% TritonX-100, and 10% glycerol). Following centrifugal clarification, the supernatants were boiled for 3 minutes at 95°C and 30 μl of cell lysate loaded on a 10% SDS polyacrylamide gel. The separated proteins were electroblotted onto nitrocellulose membrane and blocked in 5% nonfat milk. Activated (phosphorylated) MAPK were first detected using monoclonal phospho-p44/p42 MAPK antibody (1:1000 dilution; Cell Signaling Technology, Danvers, MA). Following the detection of phosphorylated proteins, the membrane was stripped and reprobed to detect total MAPK proteins. Total MAPK proteins were detected using a polyclonal antibody (1:1000 dilution; Cell Signaling Technology) overnight at 4°C. Horseradish peroxidase conjugated anti-mouse or anti-rabbit secondary antibodies (1:2,000, Pierce Biochemicals, Rockford, IL) and SuperSignal® West Dura extended duration substrate chemiluminescence detection kit (Pierce Biochemicals, Rockford, IL) were used to detect the signals. Immunoblots shown are from representative experiments that were repeated at least three independent times with comparable results.
Cell proliferation experiment
AtT-D2L or AtT-D3 cells (50,000 cells/well) were seeded in 48 well plates in F-10 media (with phenol red, 10% heat-inactivated horse serum, 5 % fetal bovine serum, 1% geneticin, 0.1% gentamicin and 1% L-glutamine). The plates were incubated for 48 hours (37°C, 5% CO2). Media was replaced with 200 μL fresh media containing control DMSO or increasing concentrations of D-264 (0.01 nM to 100 nM) and incubated for an additional 48 hours. The media was replaced with 200 μL, 10% MTT (stock solution in DMEM, high glucose without phenol red) in DMEM (high glucose, 5% heat-inactivated horse serum, 2 % fetal bovine serum along with other components as mentioned before except no DMSO). After a 4-hour incubation (37°C, 5% CO2), 200 μL MTT solubilizing solution was added to each well. The plates were gently shaken on an Eppendorf Themomix R to dissolve the formazan salt at a mixing rate of 350–500 rpm for 1–2 hours. The absorbance was recorded at 570 nM and 690 nM on a BioTek Epoch plate reader and analyzed using Gen 5 software.
RESULTS
D-264 activates D2 and D3 dopamine receptor-MAPK signaling
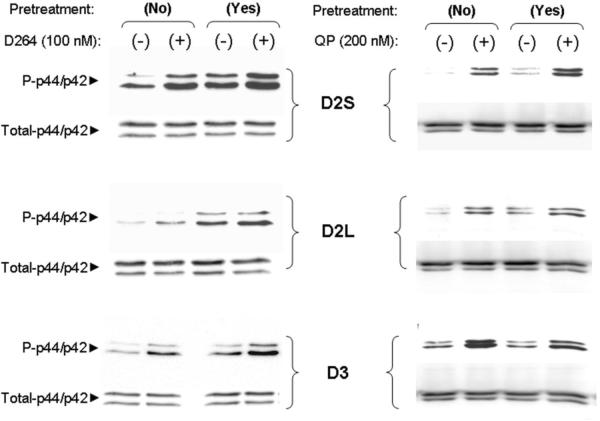
D2 and D3 dopamine receptors couple robustly to MAPK signaling pathways.(Cussac et al., 1999; Westrich and Kuzhikandathil, 2007) Figure 1 shows that an acute 5 minute, 100 nM D-264 treatment significantly increased the level of phosphorylated MAPK in AtT-20 cells stably expressing either human D2S, D2L or D3 dopamine receptors. In our previous study, we showed that the D3 receptor exhibits a tolerance property following treatment with D2/D3 receptor agonist, quinpirole (Westrich and Kuzhikandathil, 2007). To determine if D-264 exhibited this tolerance property we used our previously described protocol in which cells are pretreated with agonist for one minute and then washed for at least 5 minutes before a second treatment (Westrich and Kuzhikandathil, 2007). We compared the effect of this treatment paradigm using D-264 and quinpirole on AtT-20 cells stably expressing D2S, D2L and D3 receptors. In initial experiments, in all three stable cell lines, when cells were pretreated with D-264 and the agonist washed out for 5 minutes, we observed persistent D-264-induced MAPK activation (data not shown); therefore, we extended the duration of the washout to 30-minutes. Figure 2 and Figure 3 show that 100 nM D-264 pretreatment for one minute results in a persistent activation of MAPK signaling even after a 30 minute washout. Consequently, additional stimulation following pretreatment results in a smaller increase in phosphorylated MAPK levels (Fig. 2 and 3). Unlike D-264, the classical D2/D3 receptor agonist, quinpirole, does not show the persistent activation of the MAPK signaling after a 30 minute washout (Fig. 2 and 3). In addition, as reported previously, (Westrich and Kuzhikandathil, 2007), the D3 receptor exhibits tolerance wherein the cells pretreated with agonist elicits a significantly reduced second response (Fig. 3). The quinpirole-induced tolerance is not observed with D2S and D2L activation. Taken together this result suggests that, in contrast to quinpirole, D-264 stimulation robustly and persistently activates D2 and D3 receptor coupled MAPK signaling.
Figure 1.

D-264 activates D2/D3-MAPK signaling pathway. (A) Representative Western blots showing 100 nM D-264-induced phosphorylation of p44/p42 MAPK proteins in AtT20 cells stably expressing human D2S, D2L or D3 dopamine receptors. Cells were treated with vehicle (−) or 100 nM D-264 (+) for 5 minutes. (B) Cumulative data showing the relative increase of phospho MAPK in D2S, D2L and D3 expressing AtT-20 cells following a 5 minute, 100 nM D-264 treatment. The levels of D-264-induced phosphorylated MAPK proteins were normalized to the levels of total MAPK proteins and were compared to vehicle treated cells (which was set to 1 for each cell line). *, P<0.05, statistically significant, two-tailed Student's t-test. Statistical comparison was between MAPK activation with and without agonist treatment in each cell line separately. The experiments were repeated three independent times.
Figure 2.
D-264 induces persistent activation of the D2/D3-MAPK signaling pathway. Representative Western blots showing 100 nM D-264 (left panel) and 200 nM quinpirole (QP) (right panel) induced phosphorylation of p44/p42 MAPK proteins in AtT20 cells stably expressing human D2S, D2L or D3 dopamine receptors. Cells were pretreated for 1 minute with control SES (No pretreatment) and either 100 nM D-264 or 200 nM quinpirole (Yes pretreatment), washed for 30 minutes and subsequently treated with control SES (−) and 100 nM D-264 or 200 nM quinpirole (+) for 5 minutes.
Figure 3.

D-264, but not quinpirole, induces persistent activation of the D2/D3-MAPK signaling pathway. Cumulative data showing the ratio of phosphorylated to total MAPK induced by 100 nM D-264 or 200 nM quinpirole (QP) without (−) and with (+) pretreatment in AtT20 cells stably expressing human D2S, D2L or D3 dopamine receptors. Cells were pretreated for 1 minute with control SES (No pretreatment)and either 100 nM D-264 or 200 nM quinpirole (Yes pretreatment), washed for 30 minutes and subsequently treated with control (−) and 100 nM D-264 or 200 nM quinpirole (+) for 5 minutes. The levels of D-264-induced P-p44/p42 MAPK proteins were normalized to the levels of total-p44/p42 MAPK proteins. The experiments were repeated three independent times. *, #, ψ, P<0.05, statistically significant, ANOVA, post-hoc Holms test. *, comparing control (−/−) to samples that received 2nd treatment only (−/+). #, comparing control (−/−) to samples that were only pretreated (+/−). ψ, comparing samples that received pretreatment only (+/−) to those that received both treatments (+/+).
D-264 activates D2/D3 dopamine receptor-GIRK channel signaling
D2 and D3 dopamine receptors couple robustly to GIRK channels.(Kuzhikandathil and Bartoszyk, 2006; Kuzhikandathil et al., 2004; Werner et al., 1996) Figure 4 shows that D-264 activates endogenous GIRK channels in AtT-20 cells stably expressing human D2S, D2L or D3 dopamine receptors; no activation is observed in control parental AtT-20 cells (Fig. 4A). In D2S- and D2L-expressing cells, the GIRK currents elicited by D-264 showed different kinetics of inactivation compared to GIRK currents elicited by quinpirole (Fig. 4B–D). The GIRK currents activated by D-264 terminated very slowly following agonist washout. This persistent activation is consistent with that observed with the MAPK activation in Figures 2 and 3. Consistent with our previous observations with other D3 receptor agonists (Kuzhikandathil et al., 2004), D-264 induced the D3 receptor tolerance and slow response termination properties (Fig. 4F).
Figure 4.

D-264 activates D2/D3-GIRK channel signaling pathway. Representative voltage clamp recordings from parental AtT20 cells (A) or AtT-20 cells stably expressing the human D2S (B), D2L (C and D) or D3 (E and F) dopamine receptors. All drugs were applied in 30 mM K+ external solution (open rectangle). Parental AtT-20 cells do not respond to 300 nM D-264 (black rectangle) or 300 nM quinpirole (cross hatch). D-264 only activates the GIRK currents in AtT-20 clones expressing the various D2-like dopamine receptor subtypes (B–F). Current traces were obtained in whole cell voltage clamp recording mode with the cells held at −65 mV. The cells were sequentially treated with SES (5 mM K+), 30 mM K+ external solution (open rectangle) and various doses of agonists in 30 mM K+ external solution. The time and duration of agonist application (typically- 60s) are indicated by the rectangles. Following each agonist application, the cells were treated with the 30 mM K+ external solution during washout (open rectangle). To test the integrity of the seal during the washout in 30 mM K+ external solution, following the D-264 treatment, the external solution was switched to SES (5 mM K+); the reduction in external K+ causes a rapid decrease in the inward current (for example, see panel D), suggesting that slow deactivation of GIRK currents induced by D-264 was not due to a loss of seal integrity.
D-264 dose-dependently activated GIRK currents in AtT-20 cells stably expressing human D2S, D2L or D3 dopamine receptors. The magnitude of normalized currents elicited by a high concentration (300 nM) of D-264 were not significantly different from those elicited by a high concentration (300 nM) of the full agonist quinpirole (Figure 5). These results suggest that D-264 is a full agonist and that the efficacy of D-264 at D2S, D2L and D3 dopamine receptors are similar.
Figure 5.

Dose-dependent activation of D2/D3 induced GIRK response by D-264. Cumulative data showing GIRK responses induced by 10, 100 and 300 nM D-264 in AtT-20 cells stably expressing the human D2S (A), D2L (B) or D3 (C) dopamine receptors. Current traces were obtained in whole cell voltage clamp recording mode with the cells held at −65 mV. The peak current value (pA) at −65 mV for each drug concentration was normalized to whole cell membrane capacitance (pF) to normalize differences due to cell size. The dashed line in each graph represents the mean GIRK response (in pA/pF) induced by 300 nM quinpirole in each cell line. In each cell line, the magnitude of GIRK response elicited by the three different doses of D-264 are significantly different (P<0.05, ANOVA, post-hoc SNK test).
D-264 induces cell proliferation in AtT-20 cells expressing D2 and D3 receptors
MAPK activation plays an important role in different neuronal functions (Hetman et al., 1999). The activation of MAPK is involved in cell survival, growth and differentiation (Xia et al., 1995). Stress-induced activation of MAPK pathways has been shown to be a mechanism for neuroprotection in the CNS (Impey et al., 1998). Similarly, studies in cancer cell lines have shown that activation of GIRK channels promotes cell proliferation (Plummer et al., 2005). Given our results with D-264-induced MAPK and GIRK channel activation, we next determined the effect of D-264 on AtT-20 cell proliferation. D-264 dose-dependently induced cell proliferation in both AtT-D2L and AtT-D3 cells with corresponding EC50 values of 0.225 nM and 0.113 pM, respectively. In the case of AtT-D2L, the concentration range of D-264 varied from 100 nM to 0.01 nM. The maximum effect on cell proliferation (121%) compared to untreated control (100%) was observed at 1 nM (Fig. 6A). AtT-D3 cells were treated with concentrations of D-264 that ranged from10 nM to 0.00001 nM and a dose-dependent effect was observed, with the maximum effect on proliferation (128%) observed at 0.001 nM (Fig. 6B). Comparing D2 and D3 receptors, it was found that the concentration of D-264 that induced the highest cell proliferation in D3 receptor-expressing cells was 1000-fold lower compared to D2L receptor-expressing cells (Figs. 6A and 6B). The reference agonist quinpirole induced the highest proliferation (147%) of D3 receptor-expressing AtT-20 cells at 0.0001 nM (Fig. 6C). Interestingly, we also observed that high concentrations of D-264 did not induce proliferation of AtT-20 cells stably expressing the D3 receptor but did induce proliferation of AtT-20 cells stably expressing the D2L receptor (Fig. 6).
Figure 6.

Dose dependent effect of D-264 on proliferation of AtT-D2L and AtT-D3 cells. Cell viability was measured by MTT assay. Cell viability increased dose dependently with peak cell viability was observed at 1 nM for D2L and 0.001 nM. Reference quinpirole exhibited the highest effect for D3 receptor at 0.0001 nM. Bars represent means ± S.E.M. of cell viability values against control expressed as percentages of control for n = 3–4. ANOVA with Tukey's multiple comparison test was used to detect the differences among the treatments and control *P < 0.001.
Discussion
Our multifunctional drug development effort is directed toward developing antiparkinsonian drugs with neuroprotective properties. In this endeavor, we designed and synthesized a unique series of D3 receptor-preferring molecules with hybrid molecular structure. In our previous study, one of our lead molecules, D-264, with potent agonist activity at both D2 and D3 receptors, was shown to be neuroprotective in two well established animal models(Li et al., 2010). The D-264-mediated neuroprotection is partly attributable to its interaction with the D3 receptors (Li et al., 2010). The D3 receptor has been previously implicated in neuroprotection as D3 receptor-preferring agonists, such as pramipexole and ropinirole, protected DA neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) neurotoxicity more robustly than less selective D3 receptor-preferring agonists (Iida et al., 1999; Ramirez et al., 2003). It has been suggested that the neuroprotective role of D3 receptor is mediated indirectly via production of trophic factors such as BDNF and GDNF. Indeed, D3 receptor stimulation induces expression of BDNF as demonstrated in studies wherein D3 receptor-preferring agonists induced production of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and glial cell line-derived factor (GDNF) in cultured mesencephalic DA neurons and also in differentiated SH-SY5Y cells (Du et al., 2005; Joyce and Millan, 2007). An alternate hypothesis is that the neuroprotective effect of D3 receptor is directly mediated by its robust activation of MAPK signaling and GIRK channels. Both signaling effectors have been previously shown to mediate cell proliferation (Xia et al., 1995; (Plummer et al., 2005). MAPK signaling plays an important role in different neuronal functions (Hetman et al., 1999) and the activation of MAPK has been shown to contribute to cell survival, growth and differentiation (Xia et al., 1995). Stress induced activation of MAPK pathways has been shown to be one of the mechanisms for neuroprotection in the CNS (Impey et al., 1998). Studies in cancer cell lines have shown that specific inhibition of GIRK channels suppresses cancer cell proliferation (Plummer et al., 2005). This would suggest that activation of GIRK channels by D2-like dopamine receptors might also promote cell proliferation. The objective of our current study was to evaluate the effect of novel D2/D3 receptor agonist, D-264, on MAPK and GIRK channel activation and determine its ability to modulate cell proliferation. Our results show that D-264 potently activated the MAPK signaling pathway coupled to D2S, D2L and D3 receptors (Figs 1–3). D-264 also robustly induced GIRK current in AtT-20 cells stably expressing D2S, D2L and D3 receptors as evidenced in Figures 4B–D. D-264 was equally efficacious at activating GIRK channels coupled to D2 or D3 receptors (Fig. 5); this observation when taken in the context of the data in Table 1 suggests that D-264 might exhibit functional selectivity wherein it exhibits selectivity in some signal transduction pathways (MAPK, cell proliferation) but not others (GIRK).
While both D-264 and quinpirole activated MAPK signaling and GIRK channels, they differed in duration of activation. Unlike quinpirole, D-264 mediated activation of D2/D3 receptor signaling exhibited persistent activation (Fig. 2–4). D-264-induced MAPK and GIRK channel activation persisted in AtT-20 cells stably expressing D2S, D2L and D3 receptors, even 30 minutes after D-264 application was terminated. Such persistent activation could be due to slow off-rate of D-264 from the receptor or, alternatively, D-264 could induce an active receptor conformation that returns to the original conformation state very slowly. It is also possible that D-264 could partition into the membrane compartment and become resistant to washout. Regardless of the mechanism underlying persistent signaling activation by D-264, this property of D-264 is observed with both D2 and D3 receptors and might contribute to its duration of action. Potent D-264-induced activation of MAPK and GIRK channels via D2/D3 dopamine receptors could lead to increased cell survival. While the current experiments were performed in a heterologous expression system, the ability of D-264 to directly modulate two signaling pathways involved in cell proliferation leads us to suggest a hypothesis that direct modulation of D3 receptor signaling and might be partially responsible for the previously reported neuroprotective property of D-264.
Finally, we observed that the D3 receptor-mediated cell proliferation exhibited a bell-shaped dose response curve wherein no proliferation was observed at higher concentrations (> 0.1 – 0.01 nM) of the agonists (Fig. 6B and 6C). This was different from the results obtained with D2L wherein, significant proliferation was observed at relatively higher concentrations (100 nM) of D-264 (Fig. 6A). The most parsimonious explanation is that higher concentrations of agonists induce desensitization or tolerance at D3 receptors but not at D2 receptors; this would be consistent with our previous studies where we have reported and characterized the D3 receptor tolerance properties (Kuzhikandathil et al., 2004). Alternatively, the higher concentrations of the agonists might induce specific D3 receptor-linked negative feedback mechanisms that inhibits signaling pathways involved in cell proliferation.
Conclusion
In this report we demonstrated that D-264 is a potent agonist at D2S, D2L and D3 dopamine receptors, leading to robust activation of MAPK and GIRK channels. Compared to the reference D2/D3 agonist quinpirole, D-264 exhibited a longer lasting activation of the signaling pathways. Finally, D-264 induced significant cell proliferation in AtT-20 cells expressing D2/D3 receptors. The results from these experiments suggest that novel D2/ D3 agonist, D-264, exhibits unique functional properties that might contribute to its in vivo neuroprotective efficacy in rodent models of PD (Li et al., 2010).
Acknowledgements
This work is supported by National Institute of Neurological Disorders and Stroke/ National Institute of Health (NS047198, AKD) and the F.M. Kirby Foundation (EVK).
References
- Birkmayer W, Hornykiewicz O. The effect of l-3,4-dihydroxyphenylalanine (= DOPA) on akinesia in parkinsonism. Wien Klin Wochenschr. 2001;113:851–854. 1961. [PubMed] [Google Scholar]
- Biswas S, Hazeldine S, Ghosh B, Parrington I, Kuzhikandathil E, Reith ME, Dutta AK. Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphth alen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity. J Med Chem. 2008a;51:3005–3019. doi: 10.1021/jm701524h. [DOI] [PubMed] [Google Scholar]
- Biswas S, Zhang S, Fernandez F, Ghosh B, Zhen J, Kuzhikandathil E, Reith ME, Dutta AK. Further structure activity relationships study of hybrid 7-{[2-(4-Phenyl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-2-ol analogues : Identification of a high affinity D3-preferring agonist with potent in vivo activity with long duration of action. J Med Chem. 2008b;51:101–117. doi: 10.1021/jm070860r. [DOI] [PubMed] [Google Scholar]
- Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969;280:337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Pasteau V, Millan MJ. Human dopamine D(3) receptors mediate mitogen-activated protein kinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol Pharmacol. 1999;56:1025–1030. doi: 10.1124/mol.56.5.1025. [DOI] [PubMed] [Google Scholar]
- Du F, Li R, Huang Y, Li X, Le W. Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. The European journal of neuroscience. 2005;22:2422–2430. doi: 10.1111/j.1460-9568.2005.04438.x. [DOI] [PubMed] [Google Scholar]
- Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors--physiological understanding to therapeutic intervention potential. Pharmacol Ther. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Giros B, Martres MP, Sokoloff P, Schwartz JC. [Gene cloning of human dopaminergic D3 receptor and identification of its chromosome] C R Acad Sci III. 1990;311:501–508. [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Iida M, Miyazaki I, Tanaka K, Kabuto H, Iwata-Ichikawa E, Ogawa N. Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain research. 1999;838:51–59. doi: 10.1016/s0006-8993(99)01688-1. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Johnson M, Antonio T, Reith ME, Dutta AK. Structure-Activity Relationship Study of N(6)-(2-(4-(1H-Indol-5-yl)piperazin-1-yl)ethyl)-N(6)-propyl-4,5,6,7-tetrahydroben zo[d]thiazole-2,6-diamine Analogues: Development of Highly Selective D3 Dopamine Receptor Agonists along with a Highly Potent D2/D3 Agonist and Their Pharmacological Characterization. J Med Chem. 2012 doi: 10.1021/jm300268s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN. Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacol Ther. 2001;90:231–259. doi: 10.1016/s0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor agonists for protection and repair in Parkinson's disease. Curr Opin Pharmacol. 2007;7:100–105. doi: 10.1016/j.coph.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Bartoszyk GD. The novel antidyskinetic drug sarizotan elicits different functional responses at human D2-like dopamine receptors. Neuropharmacology. 2006;51:873–884. doi: 10.1016/j.neuropharm.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Oxford GS. Activation of human D3 dopamine receptor inhibits P/Q-type calcium channels and secretory activity in AtT-20 cells. J Neurosci. 1999;19:1698–1707. doi: 10.1523/JNEUROSCI.19-05-01698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Westrich L, Bakhos S, Pasuit J. Identification and characterization of novel properties of the human D3 dopamine receptor. Mol Cell Neurosci. 2004;26:144–155. doi: 10.1016/j.mcn.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Yu W, Oxford GS. Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines. Mol Cell Neurosci. 1998;12:390–402. doi: 10.1006/mcne.1998.0722. [DOI] [PubMed] [Google Scholar]
- Li C, Biswas S, Li X, Dutta AK, Le W. Novel D3 dopamine receptor-preferring agonist D-264: Evidence of neuroprotective property in Parkinson's disease animal models induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and lactacystin. J Neurosci Res. 2010;88:2513–2523. doi: 10.1002/jnr.22405. [DOI] [PubMed] [Google Scholar]
- Lopez A, Munoz A, Guerra MJ, Labandeira-Garcia JL. Mechanisms of the effects of exogenous levodopa on the dopamine-denervated striatum. Neuroscience. 2001;103:639–651. doi: 10.1016/s0306-4522(00)00588-1. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Parkes JD. “On-off” effects in patients with Parkinson's disease on chronic levodopa therapy. Lancet. 1976;1:292–296. doi: 10.1016/s0140-6736(76)91416-1. [DOI] [PubMed] [Google Scholar]
- Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Plummer HK, 3rd, Dhar MS, Cekanova M, Schuller HM. Expression of G-protein inwardly rectifying potassium channels (GIRKs) in lung cancer cell lines. BMC Cancer. 2005;5:104. doi: 10.1186/1471-2407-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AD, Wong SK, Menniti FS. Pramipexole inhibits MPTP toxicity in mice by dopamine D3 receptor dependent and independent mechanisms. Eur J Pharmacol. 2003;475:29–35. doi: 10.1016/s0014-2999(03)02087-9. [DOI] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Brunt ER, Korczyn AD, Poewe WH, Stocchi F. Ropinirole in the treatment of early Parkinson's disease: a 6-month interim report of a 5-year levodopa-controlled study. 056 Study Group. Mov Disord. 1998;13:39–45. doi: 10.1002/mds.870130111. [DOI] [PubMed] [Google Scholar]
- Rinne UK, Bracco F, Chouza C, Dupont E, Gershanik O, Marti Masso JF, Montastruc JL, Marsden CD. Early treatment of Parkinson's disease with cabergoline delays the onset of motor complications. Results of a double-blind levodopa controlled trial. The PKDS009 Study Group. Drugs. 1998;55(Suppl 1):23–30. doi: 10.2165/00003495-199855001-00004. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Greenamyre JT. Pathogenesis of Parkinson's disease. Curr Opin Investig Drugs. 2001;2:657–662. [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Strange PG. New insights into dopamine receptors in the central nervous system. Neurochem Int. 1993;22:223–236. doi: 10.1016/0197-0186(93)90050-f. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Werner P, Hussy N, Buell G, Jones KA, North RA. D2, D3, and D4 dopamine receptors couple to G protein-regulated potassium channels in Xenopus oocytes. Mol Pharmacol. 1996;49:656–661. [PubMed] [Google Scholar]
- Westrich L, Kuzhikandathil EV. The tolerance property of human D3 dopamine receptor is determined by specific amino acid residues in the second cytoplasmic loop. Biochim Biophys Acta. 2007;1773:1747–1758. doi: 10.1016/j.bbamcr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Winner B, Desplats P, Hagl C, Klucken J, Aigner R, Ploetz S, Laemke J, Karl A, Aigner L, Masliah E, Buerger E, Winkler J. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp Neurol. 2009;219:543–552. doi: 10.1016/j.expneurol.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten GF. Neurologic Principles and Practice. McGraw-Hill; New York, NY, USA: 1997. Movement Disorders. [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]