Abstract
We report the complex case of a 12-month-old female with stage IV hepatoblastoma accompanied by thrombosis and cavernous transformation of the portal vein. Following neoadjuvant chemotherapy, she underwent right hepatectomy, which was complicated by iatrogenic injury of her left hepatic duct, and subsequently developed a postoperative biloma and chronic biliocutaneous fistula. Concomitant with multiple percutaneous interventions to manage the biloma nonoperatively while the child completed her adjuvant chemotherapy, she progressed to develop chronic malnutrition, jaundice, and failure to thrive. Once therapy was completed and the child was deemed free of disease she underwent exploratory laparotomy with roux-en-Y biliary cyst-enterostomy for definitive management, resulting in resolution of her biliary fistula, jaundice, and marked improvement in her nutritional status. Roux-en-Y biliary cyst-enterostomy is a unique and efficacious management option in the highly selected patient population with chronic biliary leak refractory to minimally invasive management.
Keywords: hepatoblastoma, biloma, roux-en Y biliary cyst enterostomy
1.0 Introduction
Hepatoblastoma is the most common pediatric liver malignancy, yet only 100–150 new cases are diagnosed annually in North America.[1, 2] It is most often diagnosed before 3 years of age. Current cisplatin-based treatment protocols have contributed to an overall survival rate between 75–80%, but complete surgical resection remains the cornerstone to cure this otherwise highly lethal malignancy.[3, 4] The timing and extent of partial hepatectomy or orthotopic liver transplant is directed by the radiographic pretreatment extent of disease (PRETEXT) system, which guides the surgical management of hepatoblastoma based on the number of adjacent liver sections involved with tumor.[3] Surgical complications occur after 15–23% of partial liver resections for hepatoblastoma, and pose unique management challenges due to the young age of these patients and the potential to delay adjuvant chemotherapy and recovery.[5–8] Here, we present the complex management of a 12-month-old girl with stage IV hepatoblastoma and portal vein tumor thrombus resulting in cavernous transformation, who underwent right hepatectomy that was complicated by a chronic biliocutaneous fistula causing failure to thrive and jaundice.
2.0 Case Report
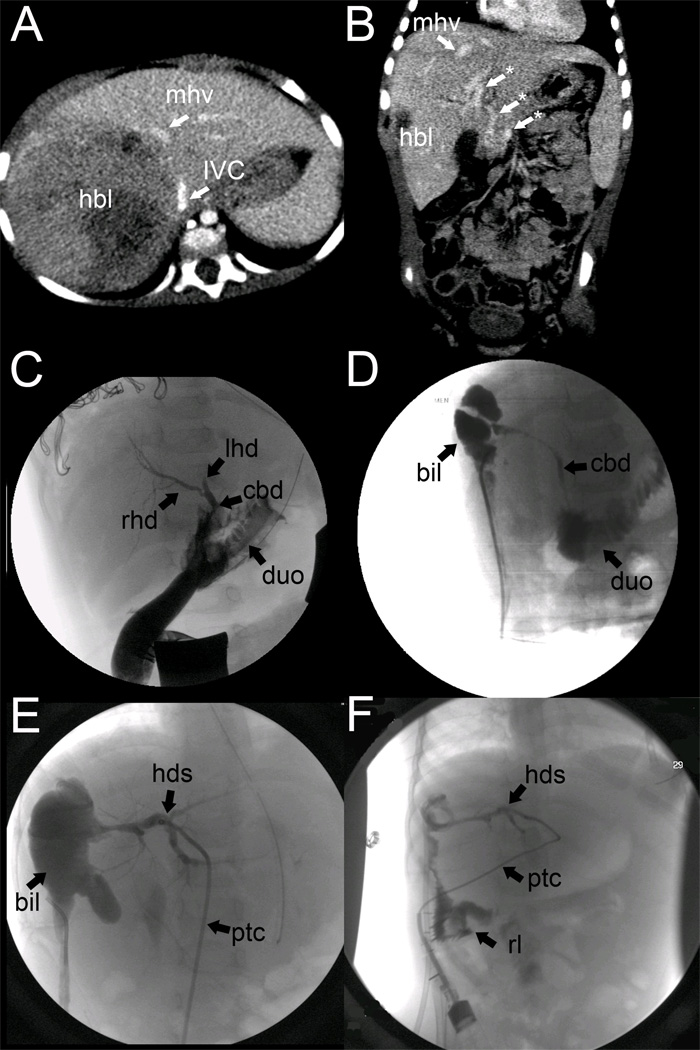
A 12-month-old previously healthy girl was admitted with an abdominal mass and failure to thrive (7 kg; <3rd percentile for age). Complete blood count showed a profound leukocytosis (27.7) without left shift, a hematocrit of 30%, and platelets of 808. Serum alpha-fetoprotein was 220,531 ng/mL. A diagnostic abdominal CT scan revealed a large, heterogeneous, hypodense mass in the right lobe of the liver measuring 7.3×7.8×10 cm and displacing the duodenum, pancreas, and upper pole of the right kidney, concerning for a PRETEXT II (V+P+) hepatoblastoma with concern for effacement of the portal vein (which was not well-visualized) and inferior vena cava (Figure 1A). Liver biopsy confirmed the diagnosis of mixed epithelial-type hepatoblastoma with less favorable histology consisting of embryonal and fetal elements. A staging chest CT showed bilateral basilar pulmonary nodules consistent with Stage IV disease. The patient was enrolled in COG-P9645 and underwent 4 neoadjuvant cycles of cisplatin, 5- fluorouracil, and vincristine (CFV).
Figure 1.
(A) PRETEXT II hepatoblastoma (hbl) involving the right anterior and right posterior liver sections with impingement on the middle hepatic vein (mhv) and inferior vena cava (ivc). The portal vein was not well visualized on this study. (B) Coronal view of CT scan prior to resection, but after neoadjuvant chemotherapy, demonstrates regression of hepatoblastoma (hbl) from the middle hepatic vein (mhv) and the presence of numerous portal varices (*) consistent with cavernous transformation of the portal vein. (C) Intraoperative cholangiogram prior to right hepatectomy illustrates the right hepatic duct (rhd) an abrupt cutoff in the left hepatic duct (lhd) due to suture ligation injury and patency of the common bile duct (duct) with free flow of contrast into the duodenum (duo). (D) First cholangiogram documenting a postoperative biloma (bil) in communication with the common bile duct (cbd). (E) Postoperative percutaneous transhepatic cholangiogram (catheter = ptc) demonstrating communication between the biloma (bil) and hepatic ductal system (hds), but not the common bile duct, suggesting stricture. (F) PTC after roux-en-Y biliary cyst enterostomy demonstrating flow of contrast from the hepatic ductal system (hds) into the roux limb (rl).
Following 4 cycles of neoadjuvant chemotherapy, the patient underwent repeat preoperative imaging. At that time, abdominal ultrasound and CT scan suggested areas of branching attenuation within the main portal vein consistent with thrombosis without clear evidence of direct tumor invasion. Additionally, multiple varices were identified at the hepatic hilum concerning for cavernous transformation of the portal vein (Figure 1B). The tumor was noted to be free from the middle and left hepatic veins, and thus right hepatectomy was planned (Figure 1B). Intraoperatively, thrombosis of the right portal vein with resultant cavernous transformation and tremendous varices investing the common, right and left bile ducts were noted. The operation was complicated by inadvertent suture-ligation injury of the extrahepatic portion of the left hepatic duct in an attempt to ligate heavily bleeding periportal varices. The injury was first noted on intraoperative cholangiogram, which also identified free flow of contrast through the common bile duct and into the duodenum (Figure 1C). The ligature occluding the left hepatic duct was removed, and a small needle injury in this duct was repaired primarily. A Jackson-Pratt drain was left in the subhepatic space. Surgical margins of the tumor were negative on histology, which identified microscopic lymphovascular invasion, but no tumor at the portal vein margin.
Her early postoperative course was complicated by an asymptomatic bile leak, noted in her Jackson-Pratt drain, which was managed expectantly and by antibiotic suppression, until she completed adjuvant chemotherapy. The child’s drain was removed inadvertently 2.5 months after surgery, and she promptly experienced bilious drainage from the tract. An abdominal ultrasound demonstrated a 5.3×2.0×2.9 cm biloma with associated biliocutaneous fistula. Initially, by cholangiography through the drain, the biloma was in communication with a patent common bile duct (Figure 1D); however, on subsequent cholangiography, the common bile duct was no longer visualized, concerning for development of a stricture (either ischemic in nature or from progression of her iatrogenic injury) (Figure 1E). A percutaneous transhepatic cholangiogram (PTC) was performed, a PTC drain was placed into the left hepatic duct, and a pigtail drain was placed into the biloma percutaneously, achieving internal control of her biliary fistula. Despite control of the fistula via the PTC drain, her jaundice progressed, and she also developed failure to thrive with significant malnutrition.
Given her refractory biliary drainage, progressive failure to thrive, jaundice, and malnutrition, she was taken to the operating room by a second surgeon for internal drainage of the chronic biloma via Roux-en-Y biliary cystenterostomy. Upon entering her abdomen, large omental varices were controlled with a LigaSure device. Dense periportal adhesions were encountered and dissected. The chronic biloma was identified, with the inferior wall of the biliary pseudocyst consisting of the hepatic flexure of the colon. A nickel-sized area of the cyst was excised, revealing a mature pseudocyst wall of sufficient thickness for biliary cystenterostomy. The existing pigtail catheter that had been percutaneously inserted into the biloma through the right flank was identified and left in situ. The end-to-side biliary cystenterostomy was fashioned using interrupted 4-0 PDS sutures. Her postoperative course was uncomplicated, and she was discharged home on postoperative day 9. 2 months postoperatively, a cholangiogram through her PTC catheter demonstrated flow of contrast into the roux limb (Figure 1F). All drains were removed after her final cholangiogram 2 months post-biliary cystenterostomy. Within 1 year of the operation, she had achieved significant weight gain, going from the 3rd to 10th percentile in weight. Four years from her original diagnosis, she continues to show no evidence of hepatoblastoma relapse.
3.0 Discussion
While rare, bilomas and biliary fistulae following liver resection for hepatoblastoma represent a unique management challenge due to the young age of the affected patient population and the potential delay of necessary adjuvant chemotherapy and surgical recovery. The rate of bile leak following pediatric liver resection is approximately 3.8%[7] and is reported to be between 3.6–8.1% in larger adult series[9–12]. The current management of bile leaks most often includes percutaneous drain placement and endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy and biliary stent placement.[13] Bile leaks refractory to this management may require percutaneous transhepatic cholangiography with catheter drainage (the so-called rendezevous procedure in which the endoscopic and transhepatic drains meet at the site of injury) or bile duct excision with hepaticoenterostomy.[13, 14] The patient reported herein failed catheter-based management. While ERCP has been proven to be safe in the neonatal and infant population[15], therapeutic ERCP with catheter-based intervention has not been extensively characterized in this population and was not thought possible or safe in this patient’s management. Simple ERCP with sphincterotomy was not thought to be helpful because the patient had developed an ischemic or iatrogenic stricture of the common bile duct during the chronic course of this complication. Resection of the affected duct with roux-en-Y hepaticojejunostomy was not deemed to be a safe option in this patient due to the high risk of bleeding from cavernous transformation of the portal vein in the setting of a reoperative field, and therefore roux-en-Y cyst-enterostomy was performed preferentially.
The biggest experience with biliary roux-en-Y cyst enterostomy exists in the historical management of choledochal cysts. Cyst enterostomy is no longer part of the surgical management of choledochal cysts due to the risk of malignant degeneration of the cyst with development of cholangiocarcinoma and a high rate of postoperative choledocholithiasis and cholangitis.[16, 17] However, a distinction exists between that situation and the current report because the development and malignant risk in choledochal cysts is thought to be secondary to chronic exposure to pancreatic digestive enzymes due to the presence of an abnormal configuration of the pancreatic and common bile ducts proximal to the ampulla of Vater resulting in a long common channel, i.e. pancreaticobiliary malunion, and also because biliary pseudocysts lack a true epithelial lining to undergo malignant transformation.[18] Reports in the European literature describe cyst jejunostomies performed in the setting of bilomas and biliary fistulae from hydatid liver cysts [19, 20] and an isolated case report in the Japanese literature exists detailing internal intestinal drainage for a chronic biloma complicating a Kasai portoenterostomy for biliary atresia.[21] In the current case, roux-en-Y cyst enterostomy rapidly remedied our patient’s yearlong struggle with biliocutaneous fistula, failure to thrive, and jaundice that was refractory to multiple attempts at noninvasive management. Nevertheless, when possible, we recommend extrahepatic bile duct excision with roux-en-Y hepaticojejunostomy for the treatment of iatrogenic bile duct injuries refractory to minimally invasive management. For patients in whom extensive dissection and excision of the extrahepatic bile duct poses prohibitive surgical risk, rouxen-Y cyst enterostomy represents a unique and efficacious surgical management option. These risks must be balanced against the risks of anastomotic stricture and cholangitis which may occur more frequently with the described operative approach.
Cyst enterostomy (e.g. internal intestinal drainage) is much more frequently employed, and is often highly successful, in the management of chronic pancreatic pseudocysts, which lack a true epithelial lining in a manner similar to the current case of a “biliary pseudocyst”.[22] Similar to the analogous pancreatic structure, the wall of a biliary pseudocyst is composed of fibrous or granulation tissue and may be of variable quality; therefore, performing an enteric anastomosis to this “neo-wall” may not always be possible, and was likely made possible by the chronic time course of the current patient’s complication. If the wall is not of sufficient quality to perform enteric anastomosis, the proximal bile ducts must be dissected and a roux-en-Y hepaticoenterostomy performed.
An alternate explanation for this patient’s refractory bile leak could be that the common bile duct was occluded preoperatively by the tumor and that the bile leak did not heal despite minimally invasive management secondary to this original occlusion. If the common bile duct had been entirely occluded by tumor, liver transplantation may have been necessary in the early management of this patient’s hepatoblastoma. Since there was no definitive evidence of common bile duct occlusion until the cholangiogram performed just prior to the roux-en-Y cyst enterostomy, we feel that this was more likely due to development of an ischemic stricture.
In conclusion, surgical complications in the pediatric cancer patient often pose unique challenges that require innovative operative management strategies. Although somewhat of a last resort, roux-en-Y cyst enterostomy represents a viable management strategy in highly selected cases of chronic bile leak that are refractory to minimally invasive or endoscopic management and may rapidly reverse the malnutrition that accompanies these complications.
Acknowledgements
The Vanderbilt Institutional Review Board approved this report (IRB#100734).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflicts of interest to report.
References
- 1.Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008;13:812–820. doi: 10.1634/theoncologist.2008-0011. [DOI] [PubMed] [Google Scholar]
- 2.Meyers RL. Tumors of the liver in children. Surg Oncol. 2007;16:195–203. doi: 10.1016/j.suronc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Czauderna P, Otte JB, Aronson DC, et al. Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL) Eur J Cancer. 2005;41:1031–1036. doi: 10.1016/j.ejca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Tiao GM, Bobey N, Allen S, et al. The current management of hepatoblastom: a combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr. 2005;146:204–211. doi: 10.1016/j.jpeds.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Towu E, Kiely E, Pierro A, et al. Outcome and complications after resection of hepatoblastoma. J Pediatr Surg. 2004;39:199–202. doi: 10.1016/j.jpedsurg.2003.10.013. discussion 199–202, [DOI] [PubMed] [Google Scholar]
- 6.Schnater JM, Aronson DC, Plaschkes J, et al. Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer. 2002;94:1111–1120. [PubMed] [Google Scholar]
- 7.Tannuri AC, Tannuri U, Gibelli NE, et al. Surgical treatment of hepatic tumors in children: lessons learned from liver transplantation. J Pediatr Surg. 2009;44:2083–2087. doi: 10.1016/j.jpedsurg.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard J, Brown J, Shafford E, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–3828. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita Y, Hamatsu T, Rikimaru T, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233:45–50. doi: 10.1097/00000658-200101000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagano Y, Togo S, Tanaka K, et al. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695–698. doi: 10.1007/s00268-003-6907-x. [DOI] [PubMed] [Google Scholar]
- 11.Lo CM, Fan ST, Liu CL, et al. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg. 1998;133:156–161. doi: 10.1001/archsurg.133.2.156. [DOI] [PubMed] [Google Scholar]
- 12.Capussotti L, Ferrero A, Vigano L, et al. Bile leakage and liver resection: Where is the risk? Arch Surg. 2006;141:690–694. doi: 10.1001/archsurg.141.7.690. discussion 695, [DOI] [PubMed] [Google Scholar]
- 13.Ciftci AO, Karnak I, Senocak ME, et al. Surgical injury of the biliary tract in children. Eur J Pediatr Surg. 2000;10:100–105. doi: 10.1055/s-2008-1072335. [DOI] [PubMed] [Google Scholar]
- 14.El Idrissi-Lamghari A, Olivie D, Boudjema K, et al. Successful treatment of intrahepatic biloma by combined percutaneous and endoscopic technique: an intrabiloma "rendezvous". Gastrointest Endosc. 2006;63:721–723. doi: 10.1016/j.gie.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Aabakken L, Aagenaes I, Sanengen T, et al. Utility of ERCP in neonatal and infant cholestasis. J Laparoendosc Adv Surg Tech A. 2009;19:431–436. doi: 10.1089/lap.2008.0272. [DOI] [PubMed] [Google Scholar]
- 16.Chijiiwa K, Koga A. Surgical management and long-term follow-up of patients with choledochal cysts. Am J Surg. 1993;165:238–242. doi: 10.1016/s0002-9610(05)80518-5. [DOI] [PubMed] [Google Scholar]
- 17.Todani T, Watanabe Y, Narusue M, et al. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–269. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 18.Kamisawa T, Takuma K, Anjiki H, et al. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol. 2009;7:S84–S88. doi: 10.1016/j.cgh.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Mentha G, Morel P, Buhler L, et al. [Surgical treatment of hepatic hydatidosis] Schweiz Med Wochenschr. 1991;121:1231–1237. [PubMed] [Google Scholar]
- 20.Brezean I, Catrina E, Aldoescu S, et al. "The fate" of a peri-cystic-jejunostomy in the treatment of the hydatid liver cysts. Chirurgia (Bucur) 2006;101:81–82. [PubMed] [Google Scholar]
- 21.Shigeru O, Kazuaki T, Koji H, et al. Internal intestinal drainage for postoperative biloma in a patient with biliary atresia. Japanese Journal of Pediatric Surgery. 2002;34:1426–1429. [Google Scholar]
- 22.Bergman S, Melvin WS. Operative and nonoperative management of pancreatic pseudocysts. Surg Clin North Am. 2007;87:1447–1460. doi: 10.1016/j.suc.2007.09.003. ix, [DOI] [PubMed] [Google Scholar]