Abstract
Objective
Determine whether being uninsured is associated with higher in-hospital postoperative mortality when undergoing surgery in the United States for a brain tumor.
Hypothesis
Uninsured patients may experience poor surgical outcomes due to suboptimal care, poor overall health, or impaired healthcare access.
Design
Retrospective cohort study utilizing the Nationwide Inpatient Sample (NIS), 1999 to 2008.
Setting
The NIS contains all inpatient records from a stratified sample of 20% of hospitals in 37 states.
Patients
28,582 patients, ages 18 to 65 years, who underwent craniotomy for a brain tumor. Three groups were studied: privately insured, Medicaid recipients, and uninsured.
Main Outcome Measure
In-hospital postoperative death. Associations between this outcome and insurance status were examined within the full cohort and within the subset of patients with no co-morbidity using Cox proportional hazards models. These models were stratified by hospital to control for any clustering effects that could arise from differing access to care.
Results
In the unadjusted analysis, the mortality rate for privately insured patients was 1.28% (95% confidence interval [CI], 1.13%-1.42%), compared to 2.60% for uninsured patients (CI, 1.87%-3.33%, P<.001), and 2.33% for Medicaid recipients (CI, 1.85%-2.82%, P<.001). After adjusting for patient characteristics and stratifying by hospital in patients with no co-morbidity, uninsured patients still had a higher risk of experiencing in-hospital death (hazard ratio 2.62, CI, 1.11-6.14, P=.027) compared with privately-insured patients. In this adjusted analysis, the disparity was not conclusively present in Medicaid recipients (hazard ratio 2.03, CI, 0.97-4.23, P=.06).
Conclusions
Uninsured patients who underwent craniotomy for a brain tumor experienced the highest in-hospital mortality. Differences in overall health or healthcare access do not fully account for this disparity.
INTRODUCTION
Inequalities in healthcare continue to be a matter of public concern. As new legislation is being developed to improve healthcare access in the United States (U.S.), an examination of factors that give rise to differences in health outcomes would be especially informative. Insurance status may be associated with differences in specialized medical and surgical care. For many surgical procedures, including hypophysectomy1, lung resection2, coronary artery bypass2, and gastrointestinal procedures2-3, non-privately insured patients fare worse post-operatively than privately insured patients. The mechanisms responsible for insurance-related disparities have not been identified, but public health efforts could be targeted more effectively if more about them were known.
Limited research has examined insurance-related disparities in patients undergoing surgery for brain tumors. Approximately 612,000 people in the U.S. have a diagnosis of a primary brain or central nervous system tumor.4 Malignant brain tumors cause 13,000 deaths annually5 and have a five year survival of about 35%.6 Existing therapies only modestly extend survival. Even small improvements on this prognosis might be meaningful, regardless of whether they are achieved by medical advances, or non-clinical means such as public health efforts.
We hypothesized that after craniotomy for brain cancer, uninsured patients experience higher rates of in-hospital death compared to privately insured patients. To evaluate this hypothesis, we analyzed in-hospital mortality rates in the Nationwide Inpatient Sample, the largest all-payer inpatient database. Because any disparity could be attributed to baseline differences in overall health, we decided to include a subset analysis of patients with no major co-morbidity. Furthermore, we employed methodology to control for possible patient clustering effects which could arise from differences in access to care.
DESIGN AND METHODS
Patient Population
This is a population-based retrospective cohort study of patients hospitalized for surgical treatment of brain cancer in the U.S. from 1999-2008. The data source was the Agency for Healthcare Research and Quality’s (AHRQ) Nationwide Inpatient Sample (NIS). The NIS contains all patient records from a stratified sample of 20% of nonfederal short-term hospitals in 37 states. The sample is representative of hospitals in these states along the parameters of region, urban versus rural locale, teaching status, bed size, and public ownership. The last 10 years of data were thought to be the most appropriate timeframe to analyze as they are most reflective of the current state of Medicaid recipients and the uninsured. Dramatic changes in the economy and government support programs for the poor occurred during the mid 1990s, and these changes could percolate into the ability to access healthcare through mechanisms that are not identified or understood. This publicly available, de-identified database was exempt from review by an institutional review board.
Selection of Patients
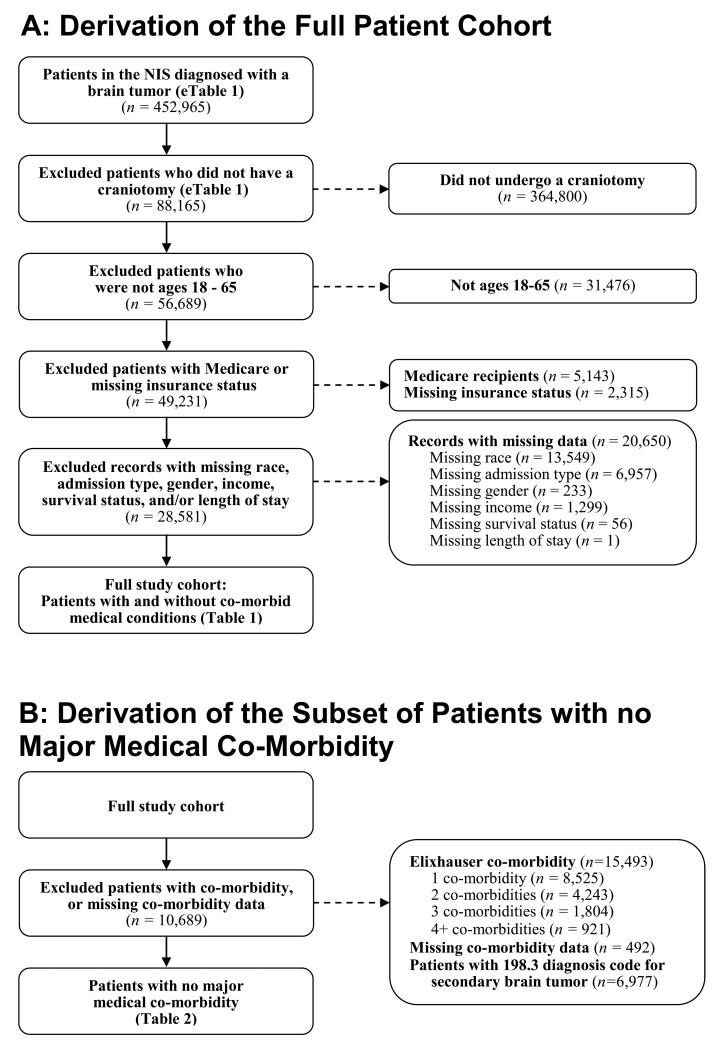
Patients ages 18-65 years were included if they had a diagnosis consistent with a brain tumor per International Statistical Classification of Diseases, version 9, Clinical Modifications (ICD-9-CM) codes; were admitted for a craniotomy per ICD-9-CM procedural codes; and had either private insurance, Medicaid, or no insurance (eMethods 1.1, eTable 1, Figure 1A). Patients over 65 were not included because age-related factors in this population could exert the dominant influence on post-operative prognosis. Patents under 18 were not included because younger patients are affected by different types of brain tumors than adults (eMethods 1.2). Patients with Medicare were excluded because individuals under 65 are not eligible for Medicare unless they are in a severe medical state: Eligibility for Medicare for individuals under 65 is limited to those who are permanently disabled (for more than 2 years), have end-stage renal disease, or have amyotrophic lateral sclerosis. Patients were excluded if data on insurance type, race, admission type, gender, income, length of stay, and/or survival status were missing (eMethods 1.3, eTable 2).
Figure 1.
A: Derivation of the final study cohort. A total of 78,210,598 patient records were available in the NIS database from 1999-2008. Of these, 28,581 patients underwent a craniotomy for a brain tumor; were ages 18-65; had either private insurance, Medicaid, or no insurance; and had no missing data.
B: For the subset analysis of patients with no major medical co-morbidity, a total of 17,892 records were excluded due to the presence of a co-morbidity, or missing co-morbidity data, leaving 10,689 patients with no major co-morbid condition.
Covariates
The primary variable of interest was insurance status, classified as private, Medicaid, or uninsured (eMethods 1.4). Patient characteristics which were studied included admission type, age, gender, race, income, malignant versus benign tumor characteristics, and year. Admission type was classified as emergent, urgent, or elective, as defined by AHRQ criteria. Race was assessed since prior studies showed that African-Americans have less access to neuro-oncologic care and poorer neurosurgical outcomes.7-10 Race was classified according to categories predefined by state inpatient databases from which the NIS derives. Available categories were: white, black, Hispanic, Asian/Pacific islander, Native American, other, or unspecified. Patients classified as “other” or “unspecified” were not analyzed since it is very difficult to infer the racial composition of this group. Income data from 1999-2002 were supplied as a range, and were merged into income quartile data from 2003-2008 (eMethods 1.5).
Role of Co-Morbid Conditions
For each medical record, we determined whether a major medical co-morbidity was present using the Elixhauser method.11 The Elixhauser method is designed to minimize the possibility of mislabeling as a co-morbidity a condition which was, in reality, related to the primary reason for the hospitalization (as reflected in the principal diagnosis), the severity of the principal diagnosis, or a complication of care. For example, a patient who was hospitalized for a kidney transplant could not be assigned renal failure as a co-morbidity under the Elixhauser method, since these two entities have a high likelihood of being related. Importantly, an Elixhauser co-morbidity must not relate directly to the diagnosis-related group assigned to the patient, thus maximizing the likelihood that the co-morbidity is a discreet and separate condition from the primary diagnosis. The Elixhauser method has been shown to capture most co-morbid conditions experienced among surgical admissions, and may be superior to the Charlson/Deyo method when administrative databases are used to study surgical populations.12-13 Elixhauser co-morbidities were determined for each patient through an established series of algorithms which are available in software from the AHRQ.14
Co-morbid medical conditions presented one possible confounding element because insurance companies may disproportionately enroll patients in good health (the insurance selection bias), and simultaneously these patients are less prone to die from their medical state than patients in poor health.15 It was problematic to control for the effects of co-morbid disease statistically for several reasons. First, the number of possible co-morbidities is very large, even with Elixhauser classifications. Second, the interactions of these conditions are complex, and probably lack consistency from patient to patient. Third, there is no information on the severity of each co-morbidity, or how well it was managed. Poor management of co-morbid conditions, and in particular hyperglycemia, has been shown to decrease survival of neurosurgical patients.16-17 For these reasons, it was felt that any attempt to model co-morbidity would require strong assumptions which are unlikely to hold, and whose violations could drastically influence any inference. Instead, an alternate approach was used to isolate the effects of insurance status from those of co-morbid conditions.
We asked if an insurance-related disparity observed in the full patient cohort would also be present in a subset of patients in which it is not likely for co-morbidity to be a confounding factor: those with no known co-morbid illness. The presence of a disparity in this subset would argue against differences in baseline health as being the causal mechanism for differing outcomes. Additionally, this subset would be relevant to study because in practice, patients with a high disease burden (aside from the intracranial pathology) are often excluded from neurosurgical consideration due to the added risks of operative intervention on an already precarious medical state. Therefore, the subset of patients with no Elixhauser co-morbidity was isolated and studied (eMethods 1.6, eTable 3, Figure 1B).
Statistical Analysis of Outcomes
The outcome of interest was in-hospital death. Results were reported as hazard ratios (HR) comparing non-privately insured groups (Medicaid recipients and patients with no insurance) to privately insured patients. An unadjusted logistic regression was performed first on the full cohort, and next on the subset of patients with no co-morbidity. Subsequently, an adjusted analysis was performed on the subset of patients with no co-morbidity. A Cox proportional hazards model was used since time-to-event (death or discharge) can be derived from the length of the hospitalization (eMethods 1.7). The model was stratified by hospital, and adjusted for admission type, age, gender, race, income, tumor malignancy, and year. All tests were two-sided, with type I error rate set to 0.05. Models were checked via visual inspection and statistical tests for proportionality based on Schoenfeld residuals.18 R (Version 2.12.0, The R Project) was used for statistical analysis.
RESULTS
Study Cohort
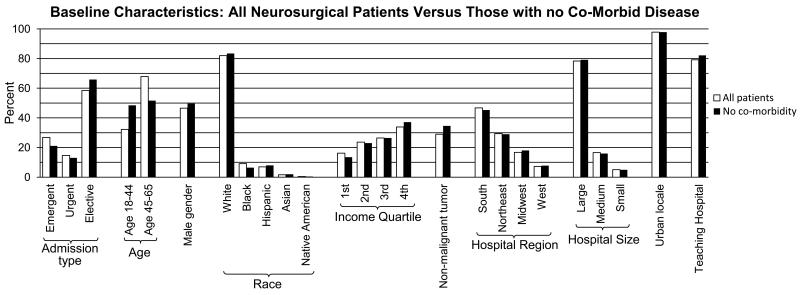
The full study cohort was comprised of 28,581 patients, of whom 81% had private insurance (n=23,051), 13% had Medicaid (n=3,685), and 6% were uninsured (n=1,845) (Figure 1A, Table 1). The average age of all patients was 49 years, with privately insured patients having an average age of 49 years, Medicaid recipients 45 years, and uninsured patients 48 years. Almost half of the patients were male (47%, n=13,305). Most patients were Caucasian (82%, n=23,431), followed by African Americans (9.1%, n=2,595), Hispanics (7.0%, n=1,990), Asian/Pacific Islander (1.6%, n=445), and Native Americans (0.42%, n=120), a distribution similar to the U.S. population from census data.19 Almost 80% of cases took place in teaching hospitals. All baseline characteristics differed significantly by insurance type as even small differences in these categories could be detected statistically due to the large number of patients. Baseline characteristics of the full cohort were similar to those of the subset with no co-morbidity (Table 2, Figure 2). A slightly higher percentage of the latter was insured privately (83%) compared to the full cohort (81%). Also, the average age of this subset was about 5 years younger than the full patient cohort as co-morbidity becomes more likely with age (Figure 2).
Table 1.
Characteristics of the full study cohort, by insurance status (%)
| Characteristic | Final Cohort (n = 28,581) |
Private (n = 23,051) |
Medicaid (n = 3,685) |
Uninsured (n = 1,845) |
|---|---|---|---|---|
| Insurance | ||||
| Private | 80.65 | |||
| Medicaid | 12.90 | |||
| Uninsured | 6.46 | |||
| Admission type | ||||
| Emergent | 26.77 | 23.17 | 40.43 | 44.39 |
| Urgent | 14.69 | 14.14 | 16.36 | 18.21 |
| Elective | 58.54 | 62.69 | 43.20 | 37.40 |
| Age (years) | ||||
| Mean age (SD) | 48.46 (11.18) | 49.02 (10.91) | 45.43 (12.11) | 47.59 (11.61) |
| Median age | 51 | 51 | 47 | 49 |
| Age 18-44 (%) | 32.21 | 30.20 | 43.85 | 34.15 |
| Age 45-65 (%) | 67.79 | 69.80 | 56.15 | 65.85 |
| Gender | ||||
| Male | 46.55 | 46.52 | 44.61 | 50.79 |
| Female | 53.45 | 53.48 | 55.39 | 49.21 |
| Race | ||||
| White | 81.98 | 86.55 | 62.55 | 63.69 |
| Black | 9.08 | 7.16 | 18.64 | 13.98 |
| Hispanic | 6.96 | 4.56 | 15.88 | 19.24 |
| Asian/Pacific Islander | 1.56 | 1.37 | 2.17 | 2.66 |
| Native American | 0.42 | 0.36 | 0.76 | 0.43 |
| Median Income Quartile | ||||
| 1 (lowest) | 16.16 | 12.76 | 32.46 | 26.07 |
| 2 | 23.58 | 22.07 | 30.26 | 29.05 |
| 3 | 26.45 | 27.21 | 22.50 | 24.82 |
| 4 (highest) | 33.81 | 37.95 | 14.79 | 20.05 |
| Tumor | ||||
| Non-malignant | 28.99 | 29.43 | 26.32 | 28.83 |
| Malignant | 71.01 | 70.57 | 73.68 | 71.17 |
| Region | ||||
| South | 46.74 | 44.35 | 49.77 | 70.57 |
| Northeast | 29.30 | 30.49 | 28.41 | 16.15 |
| Midwest | 16.71 | 17.78 | 14.19 | 8.35 |
| West | 7.25 | 7.37 | 7.63 | 4.93 |
| Hospital size | ||||
| Large | 78.34 | 78.73 | 78.40 | 73.39 |
| Medium | 16.59 | 16.01 | 18.24 | 20.49 |
| Small | 5.07 | 5.26 | 3.36 | 6.12 |
| Hospital location | ||||
| Rural | 2.17 | 2.14 | 2.23 | 2.49 |
| Urban | 97.83 | 97.86 | 97.77 | 97.51 |
| Hospital teaching status | ||||
| Nonteaching | 20.86 | 20.57 | 20.49 | 25.20 |
| Teaching | 79.14 | 79.43 | 79.51 | 74.80 |
Table 2.
Characteristics of the subset of patients with no co-morbidity, by insurance status (%)
| Characteristic | Final Cohort (n = 10,689) |
Private (n = 8,866) |
Medicaid (n = 1,145) |
Uninsured (n = 678) |
|---|---|---|---|---|
| Insurance | ||||
| Private | 82.95 | |||
| Medicaid | 10.71 | |||
| Uninsured | 6.34 | |||
| Admission type | ||||
| Emergent | 21.12 | 18.23 | 32.84 | 39.23 |
| Urgent | 13.01 | 12.55 | 14.67 | 16.22 |
| Elective | 65.86 | 69.22 | 52.49 | 44.54 |
| Age (years) | ||||
| Average | 44.03 | 44.84 | 39.05 | 41.94 |
| Median | 45 | 46 | 38 | 42.5 |
| 18-44 | 48.40 | 45.53 | 66.72 | 55.01 |
| 45-65 | 51.60 | 54.47 | 33.28 | 44.99 |
| Gender | ||||
| Male | 49.65 | 49.64 | 47.77 | 52.95 |
| Female | 50.35 | 50.36 | 52.23 | 47.05 |
| Race | ||||
| White | 83.38 | 87.51 | 63.67 | 62.68 |
| Black | 6.39 | 5.13 | 13.71 | 10.47 |
| Hispanic | 7.91 | 5.39 | 18.60 | 22.86 |
| Asian/Pacific Islander | 1.92 | 1.61 | 3.14 | 3.83 |
| Native American | 0.39 | 0.35 | 0.87 | 0.15 |
| Median Income Quartile | ||||
| 1 (lowest) | 13.44 | 11.03 | 26.90 | 22.27 |
| 2 | 23.00 | 21.36 | 33.01 | 27.58 |
| 3 | 26.44 | 26.88 | 23.06 | 26.40 |
| 4 (highest) | 37.11 | 40.73 | 17.03 | 23.75 |
| Tumor | ||||
| Non-malignant | 34.56 | 34.93 | 31.62 | 34.66 |
| Malignant | 65.44 | 65.07 | 68.38 | 65.34 |
| Region | ||||
| South | 45.31 | 43.29 | 47.16 | 68.58 |
| Northeast | 28.92 | 29.72 | 29.78 | 16.96 |
| Midwest | 18.01 | 19.12 | 14.67 | 9.14 |
| West | 7.76 | 7.87 | 8.38 | 5.31 |
| Hospital size | ||||
| Large | 79.15 | 79.49 | 77.99 | 76.55 |
| Medium | 15.88 | 15.33 | 19.30 | 17.26 |
| Small | 4.98 | 5.18 | 2.71 | 6.19 |
| Hospital location | ||||
| Rural | 2.21 | 2.18 | 2.36 | 2.36 |
| Urban | 97.79 | 97.82 | 97.64 | 97.64 |
| Hospital teaching status | ||||
| Nonteaching | 17.88 | 17.55 | 17.90 | 22.12 |
| Teaching | 82.12 | 82.45 | 82.10 | 77.88 |
Figure 2.
By selecting for patients with no co-morbidity, there is minimal inadvertent selection for other characteristics. Patients with no co-morbidity have similar baseline characteristics compared to all neurosurgical patients. The main exception was age, which was about 5 years younger in patients with no co-morbidity compared to the full patient cohort, and was among the variables adjusted for in the final analysis.
Univariate Analyses of Postoperative Outcomes
In the unadjusted analysis of the full study cohort, lack of insurance and Medicaid receipt were associated with higher in-hospital post-operative mortality compared to private insurance (Table 3). The in-hospital mortality rate for privately insured patients was 1.28% (n=295, 95% confidence interval [CI], 1.13%-1.42%), compared to 2.60% for uninsured patients (n=48, CI 1.87%-3.33%, P < .001), and 2.33% for Medicaid recipients (n=86, CI, 1.85%-2.82%, P < .001). The overall in-hospital mortality rate was 1.50% (n=429, CI 1.36%-1.64%).
Table 3.
Unadjusted outcomes after surgery for brain tumors.
| Characteristic | All patients | Patients with no co-morbidity | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. | In-Hospital Mortality (%) |
P-Value | No. | In-Hospital Mortality (%) |
P- Value |
|
| Overall | 28,581 | 1.50 | 10,689 | 0.89 | ||
| Insurance | ||||||
| Private | 23,051 | 1.28 | <.001 | 8,866 | 0.73 | .001 |
| Medicaid | 3,685 | 2.33 | 1,145 | 1.57 | ||
| Uninsured | 1,845 | 2.60 | 678 | 1.77 | ||
| Admission type | ||||||
| Emergent | 7,650 | 2.73 | <.001 | 2,258 | 1.86 | <.001 |
| Urgent | 4,199 | 2.17 | 1,391 | 1.37 | ||
| Elective | 16,732 | 0.77 | 7,040 | 0.48 | ||
| Age (years) | ||||||
| 18-44 | 9,207 | 1.18 | .002 | 5,174 | 0.70 | .039 |
| 45-65 | 19,374 | 1.65 | 5,515 | 1.07 | ||
| Gender | ||||||
| Male | 13,305 | 1.77 | .001 | 5,307 | 1.04 | .106 |
| Female | 15,276 | 1.27 | 5,382 | 0.74 | ||
| Race | ||||||
| White | 23,431 | 1.37 | .002 | 8,913 | 0.73 | <.001 |
| Black | 2,595 | 2.24 | 683 | 2.05 | ||
| Hispanic | 1,990 | 2.01 | 846 | 1.30 | ||
| Asian/Pacific Islander |
445 | 2.02 | 205 | 2.44 | ||
| Native American | 120 | 0.83 | 42 | 0.00 | ||
| Median Income Quartile | ||||||
| 1 (lowest) | 4,618 | 1.88 | .12 | 1,437 | 1.04 | .151 |
| 2 | 6,739 | 1.50 | 2,459 | 1.22 | ||
| 3 | 7,560 | 1.40 | 2,826 | 0.71 | ||
| 4 (highest) | 9,664 | 1.40 | 3,967 | 0.76 | ||
| Tumor | ||||||
| Non-malignant | 8,286 | 0.77 | <.001 | 3,694 | 0.49 | .001 |
| Malignant | 20,295 | 1.80 | 6,995 | 1.10 | ||
| Region | ||||||
| South | 13,360 | 1.61 | .39 | 4,843 | 1.03 | .489 |
| Northeast | 8,374 | 1.43 | 3,091 | 0.78 | ||
| Midwest | 4,775 | 1.47 | 1,925 | 0.83 | ||
| West | 2,072 | 1.16 | 830 | 0.60 | ||
| Hospital size | ||||||
| Large | 22,391 | 1.42 | .003 | 8,460 | 0.83 | .369 |
| Medium | 4,741 | 2.02 | 1,697 | 1.18 | ||
| Small | 1,449 | 1.10 | 532 | 0.94 | ||
| Hospital location | ||||||
| Rural | 621 | 1.13 | .44 | 236 | 0.85 | .945 |
| Urban | 27,960 | 1.51 | 10,453 | 0.89 | ||
| Hospital teaching status | ||||||
| Nonteaching | 5,962 | 1.90 | .005 | 1,911 | 1.05 | .417 |
| Teaching | 22,619 | 1.40 | 8,778 | 0.85 | ||
Boldface type indicates a P value < .05
This insurance-related disparity was also present in the subset of patients with no known co-morbid illness (Table 3). The mortality rate for privately insured patients was 0.73% (n=65, CI, 0.55%-0.91%), compared to 1.77% for uninsured patients (n=12, CI, 0.77%-2.76%, P=.005) and 1.57% for Medicaid recipients (n=18, CI, 0.85%-2.29%, P=.004). The overall in-hospital mortality rate of this subset was 0.89% (n=95, CI 0.71%-1.07%).
In the full patient cohort, other factors were also associated with in-hospital postoperative mortality, including admission type, age, gender, race, tumor malignancy, hospital size, and hospital teaching status. However, in the subset of patients with no co-morbid disease, only admission type, age, race, and tumor malignancy, continued to be associated with in-hospital postoperative mortality (Table 3). Patients admitted non-electively experienced higher in-hospital mortality rates compared with elective admissions (1.37% for urgent and 1.86% for emergent admissions, versus 0.48% for elective admissions, P < .001). Race was also associated with in-hospital mortality (P < .001). Whites experienced a 0.73% mortality rate, whereas Hispanics and blacks experienced 1.30% and 2.05% mortality rates respectively. Additionally, there was an association between malignant tumors and post-operative death (P = .001).
Multivariate Analysis of Postoperative Outcomes in Patients with no Co-morbidity
The subset of patients with no co-morbidity was further examined to determine if insurance-related disparities would be present in a multivariate analysis. Using a Cox proportional hazards model, controlling for patient demographics, admission type, tumor characteristics, and year, and after stratification by hospital; uninsured status was still associated with higher in-hospital mortality rates than private insurance, with an adjusted HR of 2.62 (CI, 1.11-6.14) (Table 4). In the adjusted analysis, Medicaid receipt was not convincingly associated with higher in-hospital mortality compared to private insurance. The Medicaid adjusted hazard ratio was 2.03 (CI, 0.97-4.23) (Table 4).
Table 4.
In-hospital mortality after surgery for a brain tumor.
| Covariate | Unadjusted outcomes, OR (95% CI) | Adjusted outcomes, HR (95% CI) | ||
|---|---|---|---|---|
|
| ||||
| All patients (n = 28,581) |
Patients with no co-morbidity (n = 10,689) |
Patients with no co-morbidity (n = 10,689) |
Patients with no co-morbidity in teaching hospitals (n = 8,778) |
|
| Insurance type (versus private) |
||||
| Medicaid | 1.84 (1.45 - 2.35) a | 2.16 (1.28 - 3.66) c | 2.03 (0.97 - 4.23) | 1.63 (0.69 - 3.85) |
| Uninsured | 2.06 (1.51 - 2.81) b | 2.44 (1.31 - 4.54) d | 2.62 (1.11 - 6.14) e | 3.55 (1.41 - 8.95) f |
| Admission Type (versus emergent) |
||||
| Urgent | - | - | 0.66 (0.30 - 1.43) | 0.51 (0.21 - 1.25) |
| Elective | - | - | 0.69 (0.37 - 1.26) | 0.60 (0.29 - 1.23) |
| Age | - | - | 1.01 (0.99 - 1.03) | 1.00 (0.98 - 1.03) |
| Female Gender (versus male) |
- | - | 0.88 (0.53 - 1.47) | 0.97 (0.55 - 1.71) |
| Race (versus White) |
||||
| Black | - | - | 1.12 (0.44 - 2.87) | 0.80 (0.27 - 2.38) |
| Others | - | - | 0.83 (0.35 - 1.95) | 1.01 (0.39 - 2.56) |
| Income quartile | - | - | 0.97 (0.73 - 1.28) | 0.99 (0.72 - 1.35) |
| Malignant Tumor (versus benign) |
- | - | 1.32 (0.70 - 2.48) | 1.22 (0.61 - 2.42) |
| Year | - | - | 0.99 (0.89 - 1.11) | 0.99 (0.87 - 1.12) |
OR indicates odds ratios; HR indicates hazard ratios; CI indicates confidence interval. Boldface type indicates P < .05. Compared to patients with private insurance:
P < .001
P < .001
P = .004
P = .005
P = .027
P = .002
A post-hoc subset analysis was performed on patients with no co-morbidity in teaching hospitals. Teaching hospitals account for about 80% of neurosurgical procedures and provide training for future generations of medical and surgical practitioners. Within teaching hospitals, uninsured patients still experienced higher in-hospital mortality than privately insured patients (adjusted HR 3.55, CI, 1.41-8.95). But Medicaid receipt was not associated with higher in-hospital mortality (adjusted HR 1.63, CI, 0.69-3.85). Non-teaching hospitals could not be analyzed reliably because they represented such a small portion of the sample size.
COMMENT
Among brain tumor patients with no other major medical condition, uninsured patients (but not necessarily Medicaid recipients) were found to have higher in-hospital mortality than privately-insured patients, a disparity which was very pronounced in teaching hospitals. These findings further reinforce prior data showing insurance-related disparities in medical and surgical settings.2,20-22
These insurance-related disparities might be explained by one of 3 possible mechanisms. i) insurance status could influence health outcomes by affecting a patient’s overall state of health20, ii) the ability to access care (affecting the acuity of disease presentation)23-26, or iii) the quality of treatment that is delivered.27-28 With regard to the first possibility: We tried to control for the effects of co-morbid disease as best as possible by studying the subset of patients with no recorded co-morbidity. However, the reliability of co-morbidity data for uninsured patients remains questionable: Uninsured patients are less likely to see a physician regularly, and there is probably a tendency for medical conditions to be undiagnosed in this group. This may be problematic because hospital records often depend on what patients know and say when asked about their medical history. Furthermore, diagnosing some co-morbidities in the acute setting is not always straightforward—high dose steroids interfere with blood glucose levels, and blood pressures are routinely labile following craniotomy, for example. With regard to the second possibility: There is no direct measure of disease severity in the NIS, but there was an indirect indication that uninsured patients may present with more advanced stages of brain cancer. Uninsured patients tended to undergo biopsy procedures more often than privately insured patients or Medicaid recipients (eMethods 1.8, eTable 4). This maygive some insight about disease progression because, biopsy without surgery may be reserved for larger, more invasive tumors, particularly eloquent cortex, in sicker patients. This is reflected in a higher mortality rate in patients who undergo biopsy procedures alone (eMethods 1.8, eTable 4). Third, it is possible that hospitals provide different care to uninsured patients, but our study does not prove it, and the available data do not support unambiguous conclusions or policy statements in this regard. We think it is important to mention this possibility to the extent that it might prompt providers to reflect on this question of the quality of care that we provide to our patients.29
The phenomenon of statistical clustering is unlikely to explain the insurance-related disparity observed in this study. A disparity may seem to be present if patients with a certain insurance status disproportionately seek care at hospitals which have poor overall outcomes (which arise independently of factors related to the insurance status of their patients). Alternatively, both the diagnosis of an operative brain tumor and lack of insurance could make it far more likely that a patient will be transferred to a tertiary care center, which may skew the population being cared for at teaching hospitals. This possible clustering effect, where both the exposure (insurance type) and outcome of interest (in-hospital mortality) are associated with the same hospital type, may result in statistical confounding.30-31 A difference in outcomes that arises only from such clustering effects would not be genuine evidence of unequal care, although it may indicate variations in hospital choice or impaired access to high-quality institutions. To minimize the possibility of confounding by clustering, we used a model which was stratified by hospital. In this model, the insurance-related disparity was still present, suggesting that clustering did not account for the disparity.
Putting our results in context with data from other medical specialties, there are several points to be made. First, insurance-related disparities are not unique to the field of neurosurgery. Uninsured patients fare worse than privately-insured patients in the settings of critical illness32(higher chance of having life support withdrawn), ischemic or hemorrhagic stroke21,33 (higher mortality and neurological impairment), myocardial infarction21 (higher mortality), and physical trauma (higher mortality).34-35 However, it is not clear that enrolling in a state-funded health plan would help the uninsured, because Medicaid recipients also seem to experience a similar disparity in other settings, including pneumonia21, appendicitis36-37, abdominal aortic aneurysm repair,38-39 limb-threatening ischemia39, and surgery for colorectal carcinoma3. Of note, this Medicaid disparity was also present in our full cohort, but was not convincingly present in the adjusted analysis of patients with no co-morbid disease, especially in teaching hospitals. This observation led us to wonder if differences in baseline health contribute to the Medicaid disparity, and moreover, what would have happened if prior studies had analyzed patients with no co-morbidity separately. Finally, there is the question of a disparity’s “effect size.” With the advanced state of modern medicine, catastrophic outcomes are infrequent. In this setting, many of the high hazard ratios reported in past studies, and in this study, originate from small absolute percentage differences. Although professional ethics compel us to be concerned with all inequalities, the question is whether correcting “small effect size” disparities should take priority over other promising strategies for improving public health.
Uninsured patients are a very heterogeneous group, and should not necessarily be thought of as being poor. Uninsured patients usually fall into one of several categories: (1) poor and/or unemployed people who cannot afford insurance; (2) individuals who qualify for Medicaid but have not yet signed up for it; (3) young, healthy individuals who choose to forgo insurance; or (4) self-employed people at a broad range of incomes who are deterred by the high cost of individual insurance. This last group of patients is probably not small, as reflected by the fact that 1 of 5 uninsured patients in our study were in the highest income quartile. Also, state-specific policies and procedures may give rise to regional variations in the demographics of the uninsured. Some states in the South make it extremely hard to enroll in Medicaid, and 71% of this study’s uninsured patients came from the South. In contrast, Massachusetts has a universal insurance law which covers almost everyone, except for a few mostly affluent, self-employed people who choose to pay a fine for not enrolling. These variations in patient demographics underscore the need for adjusting for socioeconomic variables such as income status and geographic location in studies of the uninsured.
For this study, a retrospective analysis performed with the NIS database was the best available design. The NIS presented several advantages over other data sources. Its massive size was critical for a study of rare outcomes such as in-hospital mortality. It is the only database with information on all patients regardless of payer, including those with no payer.40 The NIS is amenable to statistical analyses which are stratified at the hospital level because its sampling method is based at the hospital level (not patient-level). Finally, the possibilities for a prospective design were limited by the low overall incidence of surgically resectable brain tumors, the rarity of in-hospital mortality as an outcome, and the difficulty of obtaining a representative sample of the entire country. There are 4 limitations which could not be overcome: First, Medicaid and privately-insured groups are heterogeneous in terms of what medical procedures they cover. Medicaid coverage varies by state, and private insurance varies by plan. Even some privately-insured patients may not have adequate coverage for major surgery. Second, the patient follow-up interval was confined to the time between admission and discharge, although deaths which occurred immediately after discharge would also have reflected the quality of post-operative care. Third, no detailed information is recorded for tumor histology, anatomic location, grade, or stage, although such factors are related to treatment choice and mortality risk. Fourth, uninsured patients sometimes enroll in Medicaid during their hospitalization, and the rates at which this occurs may vary by state. The NIS classifies them as Medicaid recipients, although the more appropriate classification may be “uninsured.” It is difficult to separate these patients from other Medicaid recipients (eMethods 1.9, eFigure 1).
CONCLUSION
Uninsured patients undergoing craniotomy for a brain tumor experience worse outcomes than privately-insured patients, and this difference is very pronounced in teaching hospitals. This variation in post-operative outcomes remains unexplained by hospital characteristics, including clustering effects, co-morbid disease, or socioeconomic variations. This study did not exclude the possibilities that co-morbid conditions are underdiagnosed in uninsured patients, or that uninsured patients are presenting with more advanced stages of disease.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support
Funding sources: The Doris Duke Charitable Foundation supplied a grant to the Johns Hopkins School of Medicine to fund ENM. HA was funded by the VSBfonds and the Prins Bernhard Cultuurfonds. RTS is funded partially by T32 AG000247-16. The Robert Wood Johnson Foundation supported the work of AQH.
Footnotes
Disclosures: The authors disclose no financial relationship with, or financial interest in, anything pertaining to this research.
The authors disclose no conflicts of interest in anything pertaining to this research study.
Author contributions
Eric Momin had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Momin, Adams, Shinohara, Frangakis, Quiñones-Hinojosa,
Analysis and interpretation of data: Momin, Adams, Shinohara, Frangakis
Drafting of the manuscript: Momin, Adams, Shinohara, Frangakis, Quiñones-Hinojosa,
Critical revision of the manuscript for important intellectual content: Adams, Shinohara, Frangakis, Quiñones-Hinojosa
Statistical Analysis: Momin, Shinohara, Frangakis
Supervision: Adams, Quiñones-Hinojosa
REFERENCES
- 1.Barker FG, 2nd, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003 Oct;88(10):4709, 4719. doi: 10.1210/jc.2003-030461. [DOI] [PubMed] [Google Scholar]
- 2.Lapar DJ, Bhamidipati CM, Mery CM, et al. Primary Payer Status Affects Mortality for Major Surgical Operations. Ann Surg. 2010 Jul 19; doi: 10.1097/SLA.0b013e3181e8fd75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelz RR, Gimotty PA, Polsky D, Norman S, Fraker D, DeMichele A. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer. 2004 Nov 15;101(10):2187–2194. doi: 10.1002/cncr.20624. [DOI] [PubMed] [Google Scholar]
- 4.Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010 Jun;12(6):520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society . Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- 6.CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2007. Hinesdale, IL: 2010. [Google Scholar]
- 7.Curry WT, Jr., Carter BS, Barker FG., 2nd Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988-2004. Neurosurgery. 2010 Mar;66(3):427–437. doi: 10.1227/01.NEU.0000365265.10141.8E. discussion 437-428. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee D, Kosztowski T, Zaidi HA, et al. Disparities in access to pediatric neurooncological surgery in the United States. Pediatrics. 2009 Oct;124(4):e688–696. doi: 10.1542/peds.2009-0377. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee D, Zaidi HA, Kosztowski T, et al. Disparities in access to neuro-oncologic care in the United States. Arch Surg. 2010 Mar;145(3):247–253. doi: 10.1001/archsurg.2009.288. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D, Zaidi HA, Kosztowski T, et al. Predictors of access to pituitary tumor resection in the United States, 1988-2005. Eur J Endocrinol. 2009 Aug;161(2):259–265. doi: 10.1530/EJE-09-0043. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004 Apr;42(4):355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 13.Stukenborg GJ, Wagner DP, Connors AF., Jr. Comparison of the performance of two comorbidity measures, with and without information from prior hospitalizations. Med Care. 2001 Jul;39(7):727–739. doi: 10.1097/00005650-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Kruzikas D. Comorbidity Software Documentation: HCUP Methods Series Report # 2004-01. [Accessed May 2, 2011]. http://www.hcup-us.ahrq.gov/reports/methods. [Google Scholar]
- 15.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005 Oct 12;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 16.McGirt MJ, Chaichana KL, Gathinji M, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008 Aug;63(2):286–291. doi: 10.1227/01.NEU.0000315282.61035.48. discussion 291. [DOI] [PubMed] [Google Scholar]
- 17.Chaichana KL, McGirt MJ, Woodworth GF, et al. Persistent outpatient hyperglycemia is independently associated with survival, recurrence and malignant degeneration following surgery for hemispheric low grade gliomas. Neurol Res. 2010 May;32(4):442–448. doi: 10.1179/174313209X431101. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982 Apr 1;69(1):239–241. [Google Scholar]
- 19.United States Census Bureau . Overview of race and Hispanic origin: Census 2000 brief. Mar, 2001. [Google Scholar]
- 20.Franks P, Clancy CM, Gold MR. Health insurance and mortality. Evidence from a national cohort. JAMA. 1993 Aug 11;270(6):737–741. [PubMed] [Google Scholar]
- 21.Hasan O, Orav EJ, Hicks LS. Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010 Oct;5(8):452–459. doi: 10.1002/jhm.687. [DOI] [PubMed] [Google Scholar]
- 22.Sada MJ, French WJ, Carlisle DM, Chandra NC, Gore JM, Rogers WJ. Influence of payor on use of invasive cardiac procedures and patient outcome after myocardial infarction in the United States. Participants in the National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998 Jun;31(7):1474–1480. doi: 10.1016/s0735-1097(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 23.Brown DL, Schneider DL, Colbert R, Guss D. Influence of insurance coverage on delays in seeking emergency care in patients with acute chest pain. Am J Cardiol. 1998 Aug 1;82(3):395–398. doi: 10.1016/s0002-9149(98)00338-5. [DOI] [PubMed] [Google Scholar]
- 24.Hafner-Eaton C. Physician utilization disparities between the uninsured and insured. Comparisons of the chronically ill, acutely ill, and well nonelderly populations. JAMA. 1993 Feb 10;269(6):787–792. [PubMed] [Google Scholar]
- 25.Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000 Dec 1;89(11):2202–2213. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999 Aug 18;91(16):1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 27.Landon BE, Schneider EC, Normand SL, Scholle SH, Pawlson LG, Epstein AM. Quality of care in Medicaid managed care and commercial health plans. JAMA. 2007 Oct 10;298(14):1674–1681. doi: 10.1001/jama.298.14.1674. [DOI] [PubMed] [Google Scholar]
- 28.Asch SM, Kerr EA, Keesey J, et al. Who is at greatest risk for receiving poor-quality health care? N Engl J Med. 2006 Mar 16;354(11):1147–1156. doi: 10.1056/NEJMsa044464. [DOI] [PubMed] [Google Scholar]
- 29.Manfuso J. Unequal outcomes. Dome Magazine. 2011 Mar;62(3):1–2. [Google Scholar]
- 30.Localio ARB, J A, Ten Have TR. Confounding due to cluster in multicenter studies-causes and cures. Health Serv Outcomes Resh Meth. 2002;3(3-4):195–210. [Google Scholar]
- 31.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001 Jul 17;135(2):112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 32.Fowler RA, Noyahr LA, Thornton JD, et al. An official American Thoracic Society systematic review: the association between health insurance status and access, care delivery, and outcomes for patients who are critically ill. Am J Respir Crit Care Med. 2010 May 1;181(9):1003–1011. doi: 10.1164/rccm.200902-0281ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen JJ, Washington EL. Disparities in outcomes among patients with stroke associated with insurance status. Stroke. 2007 Mar;38(3):1010–1016. doi: 10.1161/01.STR.0000257312.12989.af. [DOI] [PubMed] [Google Scholar]
- 34.Haider AH, Chang DC, Efron DT, Haut ER, Crandall M, Cornwell EE., 3rd Race and insurance status as risk factors for trauma mortality. Arch Surg. 2008 Oct;143(10):945–949. doi: 10.1001/archsurg.143.10.945. [DOI] [PubMed] [Google Scholar]
- 35.Maybury RS, Bolorunduro OB, Villegas C, et al. Pedestrians struck by motor vehicles further worsen race- and insurance-based disparities in trauma outcomes: the case for inner-city pedestrian injury prevention programs. Surgery. 2010 Aug;148(2):202–208. doi: 10.1016/j.surg.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Braveman P, Schaaf VM, Egerter S, Bennett T, Schecter W. Insurance-related differences in the risk of ruptured appendix. N Engl J Med. 1994 Aug 18;331(7):444–449. doi: 10.1056/NEJM199408183310706. [DOI] [PubMed] [Google Scholar]
- 37.Smink DS, Fishman SJ, Kleinman K, Finkelstein JA. Effects of race, insurance status, and hospital volume on perforated appendicitis in children. Pediatrics. 2005 Apr;115(4):920–925. doi: 10.1542/peds.2004-1363. [DOI] [PubMed] [Google Scholar]
- 38.Boxer LK, Dimick JB, Wainess RM, et al. Payer status is related to differences in access and outcomes of abdominal aortic aneurysm repair in the United States. Surgery. 2003 Aug;134(2):142–145. doi: 10.1067/msy.2003.214. [DOI] [PubMed] [Google Scholar]
- 39.Giacovelli JK, Egorova N, Nowygrod R, Gelijns A, Kent KC, Morrissey NJ. Insurance status predicts access to care and outcomes of vascular disease. J Vasc Surg. 2008 Oct;48(4):905–911. doi: 10.1016/j.jvs.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002 May-Jun;5(3):143–151. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.