Abstract
Efavirenz (EFV) is one of the most commonly prescribed antiretrovirals for use in the treatment of human immunodeficiency virus (HIV) infection. EFV is extensively metabolized by cytochrome P450 to a number of oxygenated products; however, the pharmacologic activity and distribution of these metabolites in anatomic compartments have yet to be explored. The systemic distribution of EFV oxidative metabolites was examined in blood plasma, seminal plasma, and cerebrospinal fluid from subjects on an EFV-based regimen. The 8-hydroxy EFV metabolite was detected in blood plasma, seminal plasma, and cerebrospinal fluid, with median concentrations of 314.5 ng/ml, 358.5 ng/ml, and 3.37 ng/ml, respectively. In contrast, 7-hydroxy and 8,14-hydroxy EFV were only detected in blood plasma and seminal plasma with median concentrations of 8.84 ng/ml and 10.23 ng/ml, and 5.63 ng/ml and 5.43 ng/ml, respectively. Interestingly, protein-free concentrations of metabolites were only detectable in seminal plasma, where a novel dihdyroxylated metabolite of EFV was also detected. This accumulation of protein-free EFV metabolites was demonstrated to be the result of differential protein binding in seminal plasma compared with that of blood plasma. In addition, the oxidative metabolites of EFV did not present with any significant pharmacologic activity toward HIV-1 as measured using an HIV green fluorescent protein single-round infectivity assay. This study is the first to report the physiologic distribution of metabolites of an antiretroviral into biologic compartments that the virus is known to distribute and to examine their anti-HIV activity. These data suggest that the male genital tract may be a novel compartment that should be considered in the evaluation of drug metabolite exposure.
Introduction
Human immunodeficiency virus (HIV) infection distributes to a variety of anatomic sites including but not limited to the circulatory system, central nervous system (CNS), genital tracts, lymphatic system, and intracellular compartments. Antiretroviral drugs (ARVs) have been highly effective in the treatment of HIV through suppression of viral replication; however, there is a lack of understanding about ARV distribution beyond the blood plasma, which limits our ability to evaluate extravascular efficacy and/or toxicity. A major anatomic site of concern in the treatment of HIV is the CNS because the virus is known to distribute to this compartment yet many ARVs exhibit poor penetration into the cerebrospinal fluid (Letendre et al., 2008; Dellamonica et al., 2012). HIV-associated neurologic disease is relatively common, resulting in significant cognitive and neurologic impairment, but the etiology is incompletely understood (Resnick et al., 1988; Sacktor, 2002; McArthur, 2004; Valcour et al., 2012). The male genital tract has also shown poor ARV penetration (Kashuba et al., 1999; Avery et al., 2011) and is a compartment of concern in that it is the primary site of transmission through HIV-laden semen (Resnick et al., 1988; Stekler et al., 2008; UNAIDS, 2009). Although the ability of ARVs to penetrate these compartments is an important aspect of HIV therapy, in many cases a comprehensive understanding of the distribution of these drugs beyond the blood is lacking.
One of the better characterized drugs in this regard is the non-nucleoside reverse-transcriptase inhibitor efavirenz (EFV), which has been demonstrated to penetrate, though minimally, both the male genital tract and CNS. Recent reports have revealed that there exists a 150-fold total concentration gradient for EFV from blood plasma to cerebrospinal fluid (Best et al., 2011) and 20-fold from blood plasma to seminal plasma (Avery et al., 2011).
EFV is extensively metabolized by the cytochrome P450 (P450) superfamily of heme containing mono-oxygenases to yield several products including 8-hydroxyEFV (8-OH EFV), which is the major oxygenated metabolite of efavirenz and is formed by CYP2B6 (Ward et al., 2003; Bumpus et al., 2006). EFV is also metabolized to a lesser extent to 7-hydroxy efavirenz (7-OH EFV) and 8,14-hydroxy efavirenz (8,14-OH EFV), which are formed by CYP2A6 and CYP2B6, respectively (Mutlib et al., 1999; Bumpus et al., 2006; Ogburn et al., 2010). In addition, EFV is an autoinducer of metabolism in that it increases expression of CYP2B6 through activation of the constitutive androstane receptor (Faucette et al., 2007). These enzymes have been shown to be detectable in both the blood-brain barrier and the prostate, suggesting the possibility for local metabolism of EFV in the CNS and male genital tract (Finnström et al., 2001; Miksys and Tyndale, 2002; Kumagai et al., 2007).
Thus, the studies herein were designed to gain an understanding of the distribution of metabolites of EFV in the blood plasma, seminal plasma, and cerebrospinal fluid while also examining their anti-HIV activity. As such, these novel findings are reported: 1) mono-oxygenated and dioxygenated metabolites of EFV are present at similar levels in seminal plasma and blood plasma; 2) protein-free EFV metabolite concentrations were only present in seminal plasma and are presented with different degrees of protein binding compared with blood plasma; 3) mono-oxygenated and dioxygenated EFV metabolites are not pharmacologically active toward HIV; and 4) a previously unreported dihydroxylated metabolite of EFV that appears to be seminal plasma–specific has been detected.
Taken together, this study is the first, to the best of our knowledge, to report the physiologic distribution of metabolites of an ARV into relevant biologic compartments and to examine their anti-HIV activity. Further, these data suggest that the male genital tract may be a novel compartment that should be considered in working to gain a comprehensive understanding of drug metabolite exposure.
Materials and Methods
Subjects and Demographic Characteristics.
Archived blood plasma and cerebrospinal fluid samples were received from a previous study (Tovar-Y-Romo et al., 2012), and they were analyzed for total and protein-free EFV and EFV metabolite concentrations. The study involved 13 total participants on a once daily dosing regimen containing EFV, where paired blood plasma and cerebrospinal fluid samples were obtained. All subjects had been taking EFV for a minimum of 4 weeks before study enrollment. The 13 total research participants ranged in age from 37 to 71 years old; 2 were women, and 11 were men; 1 was European American, and 12 were African American.
Archived blood plasma and seminal plasma samples, obtained from an additional previous study (Cao et al., 2008), were analyzed for total and protein-free EFV and EFV metabolite concentrations. The original study involved six total research participants who provided paired blood and semen samples at multiple time points throughout a 5-day study. Research participants on 600 mg daily EFV were changed to a regimen of 100 mg EFV every 4 hours to establish near true steady-state conditions due to the estimated 40–55 hour EFV half-life and frequent dosing interval. Six men were enrolled, ranging in age from 33 to 48 years old; 1 was European American, and 5 were African American.
Each of these studies was approved by the Johns Hopkins Medicine institutional review board, and all research participants provided informed consent for participation.
Materials.
Efavirenz was obtained through the NIH Aids Research and Reagent Program (Germantown, MD). Synthetic 8-OH EFV, 7-OH EFV, and 8,14-OH EFV were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). A racemic 6-fluorinated analog of EFV (F-EFV) for use as an internal standard was synthesized by Dr. David Meyers (Johns Hopkins University School of Medicine Synthetic Core Facility located in the Department of Pharmacology and Molecular Sciences) using modifications of previously published methods (Radesca et al., 1997; U.S. FDA, 2001). EFV, 8-OH EFV, 7-OH EFV, and 8,14-OH EFV were dissolved in dimethylsulfoxide (DMSO) for infectivity assays and in acetonitrile for metabolism assays, and they were stored at −20°C. Concentrations of solvent vehicles were less than 0.1% in all experiments.
Separation of Protein Free from Protein Bound by Ultrafiltration.
Separation of the protein free from the protein bound was performed according to a method previously described elsewhere (Avery et al., 2011). Briefly, ultrafiltration of samples was performed using 96-well plates with a 10-kDa filter membrane (Millipore, Billerica, MA). Blank blood plasma for quality-control preparation and method development was obtained from Biologic Specialty Corporation (Colmar, PA). Blank seminal plasma was obtained from Bioreclamation, Inc. (Westbury, NY). To each well of the ultrafiltration plate, 100 μl of sample was added. Samples were incubated for 1 hour at 37°C. The plates were centrifuged at 15-minute intervals for 45 minutes at 37°C. At every 15-minute interval, the filtrate was collected in a v-bottom 96-well collection plate and extracted with methanol. Filtrate samples were then analyzed for EFV and EFV metabolite concentrations by ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). The free EFV concentration in the clinical samples evaluated at the three centrifugation time points was used to generate an EFV concentration versus centrifugation time curve for each clinical sample. The centrifugation time-adjusted free EFV concentration was determined by using a linear regression to estimate the concentration when time equals zero minutes (unperturbed precentrifugation sample). The percentage of protein binding was calculated as the centrifugation time-adjusted free drug concentration divided by the total drug concentration multiplied by 100.
Infectivity Assay.
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll-Paque PLUS (GE Healthcare Biosciences; Piscataway, NJ), according to the manufacturer’s instructions. Isolated cells were resuspended in a stimulation medium of Roswell Park Memorial Institute medium (RPMI) 1640 supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Grand Island, NY), 100 U/ml of interleukin-2 (proleukin), penicillin/streptomycin, and activated with 0.5 μg/ml of phytohemagglutinin (PHA) (Fisher Scientific; Pittsburgh, PA). Cell suspensions were incubated for 3 days at 37°C and 5% CO2. CD4+ T cells were isolated using the magnetic-activated cell sorting (MACS) CD4+ T cell isolation kit (Miltenyi Biotec, Cambridge, MA) according to the manufacturer’s instructions.
Inhibition of HIV-1 infection was measured by means of a modification of a previously established method that uses an HIV-1 reporter virus expressing an Env-green fluorescent fusion protein (GFP) (Zhang et al., 2004; Shen et al., 2008; Sampah et al., 2011; Jilek et al., 2012). Infections were performed in an assay medium containing RPMI 1640 + 50% FBS, 100 U/ml of interleukin-2, and penicillin/streptomycin. CD4+ T cells were seeded at 1 × 105 cells per well and incubated with EFV, 8-OH EFV, 7-OH EFV, or 8,14-OH EFV for 3 hours before infection. The DMSO solvent vehicle was less than 0.1% for all experiments.
Infection was performed via spinoculation (O’Doherty et al., 2000), incubated for 72 hours, and analyzed for infection by flow cytometry analysis of GFP expression. Infectivity for each sample [fu(SAMPLE X)] was characterized as the fraction of virus infection events affected by the drug (fa) relative to the fraction of virus infection events unaffected by drug (fu). Infectivity was determined relative to the positive control (HIV-GFP virus only, no drug) and negative control (no virus, no drug) for each sample set. The data from infectivity experiments is calculated as follows:
where fu = 1 − fa.
Metabolism Assays.
To analyze metabolite formation, EFV, 8-OH EFV, and 7-OH EFV were incubated at 5 μM or 20 μM with human liver microsomes (HLM) (50 donor pool; BD Biosciences, San Jose, CA) as well as cDNA-expressed individual human cytochromes P450 (CYPs 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4, 3A5, and 3A7; BD Biosciences). The final concentration of cDNA expressed P450s and HLM were 20 pmol/ml and 1 mg/mL, respectively. The final reaction volumes for assays performed using cDNA expressed P450s and HLM were 100 μl and 500 μl, respectively. After a 5-minute equilibration period in 100 mM potassium phosphate buffer (pH 7.4) at 37°C, the reaction was initiated by addition of an NADPH regenerating system (BD Biosciences). The reaction was incubated for 60 minutes at 37°C.
All reactions were terminated by the addition of acetonitrile. Metabolite products were extracted using a liquid:liquid extraction method, where 600 μl of 50 mM ammonium formate was added to the reaction mixture, followed by 900 μl of hexane:ethyl acetate (1:1). The reaction mixture was vortexed then centrifuged at 3000g for 10 minutes at 4°C; the organic layer was aliquoted into a separate tube. The organic layer was evaporated to dryness under nitrogen gas, the residue was reconstituted in 200 μl of methanol, and 5 μl was injected for UPLC-MS/MS analysis.
UPLC-MS/MS Detection of EFV and Metabolites.
EFV and EFV metabolites were detected and quantified using a UPLC-MS/MS assay performed on an AB Sciex QTRAP 5500 (AB Sciex, Foster City, CA) interfaced with an Acquity UPLC (Waters Inc., Milford, MA). EFV and EFV metabolite samples were analyzed according to a modification of a previously established method (Avery et al., 2010). EFV and EFV metabolites were resolved using a reverse-phase UPLC column (2.1 × 50 mm; Acquity UPLC BEH C18) with a flow rate of 0.5 mL/min by gradient elution: mobile phase A (MPA) of 0.1% formic acid in water; mobile phase B (MPB) of 0.1% formic acid in acetonitrile. At initial conditions, the samples were injected at solvent conditions of 70% MPA and 30% MPB. From 0.4 minutes to 4.5 minutes, MPB is increased to 85% and then 100% MPA at 4.6 minutes to elute EFV and EFV metabolites. The column conditions are equilibrated to 85% MPA and 15% MPB from 4.8 minutes to 5.5 minutes.
EFV and EFV metabolites were detected via negative-ion multiple-reaction monitoring (MRM). The assay was linear from 0.5–500 ng/ml with assay characteristics consistent with the U.S. Food and Drug Administration Guidance for Industry (2001), where all calibration standards and quality controls were linear with a percentage deviation and coefficient of variation of ≤15%. A fluorinated analog of EFV (F-EFV) was employed as the internal standard, as previously described elsewhere (Avery et al., 2010). EFV, 8-OH EFV, 7-OH EFV, 8,14-OH EFV, and F-EFV were detected via the MRM transitions: m/z 314.0 > 244.1, m/z 329.9 > 162.0, m/z 329.9 > 188.9, m/z 346.0 > 262.0, and m/z 298.0 > 227.9, respectively.
Results
Quantitation of EFV Metabolite Concentrations in Blood Plasma, Seminal Plasma, and Cerebrospinal Fluid.
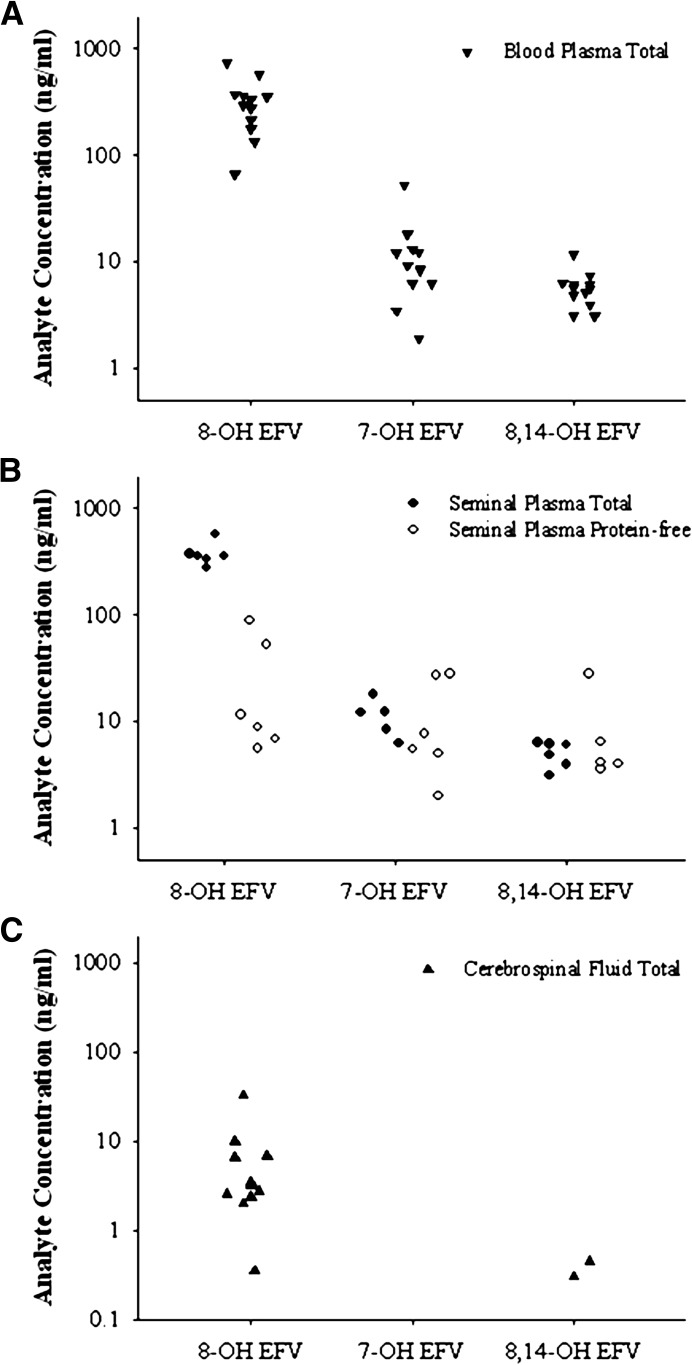
Total concentrations of EFV were previously established in the paired blood plasma and seminal plasma samples with median (interquartile range [IQR]) concentrations of 2360 ng/ml (1530–4120) and 95 ng/ml (70–206), respectively (Avery et al., 2011). Total concentrations of EFV were previously measured in the paired blood plasma and cerebrospinal fluid samples with median (IQR) concentrations of 2170 ng/ml (1896–2520) and 19 ng/ml (7–24) (Tovar-Y-Romo et al., 2012). To measure the concentrations of 8-OH EFV, 7-OH EFV, and 8,14-OH EFV in blood plasma, seminal plasma, and cerebrospinal fluid, we developed a novel UPLC-MS/MS assay. Using this assay, we determined that 8-OH EFV, the predominant metabolite of EFV, was detectable in blood plasma, seminal plasma, and cerebrospinal fluid with median (IQR) concentrations of 314.5 ng/ml (206–362.3), 358.5 ng/ml (340–368.8), and 3.37 ng/ml (2.58–6.54), respectively. In contrast, 7-OH EFV was only detected in blood plasma and seminal plasma, with median (IQR) concentrations of 8.84 ng/ml (6.21–12.48) and 10.23 ng/ml (8.26–12.23), respectively. The 8,14-OH EFV metabolite was quantifiable in blood plasma and seminal plasma with median (IQR) concentrations of 5.63 ng/ml (4.58–6.16) and 5.43 ng/ml (4.15–6.10). Only 2 out of 13 subjects had detectable 8,14-OH EFV in the cerebrospinal fluid, with concentrations of 0.375 and 0.444 ng/ml (Fig. 1). Qualitatively, it was also found that N- and O-linked glucuronidated metabolites of EFV were present in both blood plasma and seminal plasma and that the abundance of these glucuronide conjugates was similar in the two compartments (unpublished data).
Fig. 1.
Distribution of EFV and hydroxylated EFV metabolites into blood plasma, seminal plasma, and cerebrospinal fluid. Total concentrations of 8-OH EFV, 7-OH EFV, and 8,14-OH EFV were analyzed in subjects on a regular EFV-based regimen and were found to be detectable in blood plasma (A, ▾), seminal plasma (B, ●), and cerebrospinal fluid (C, ▴). Protein-free (○) concentrations of 8-OH EFV, 7-OH EFV, and 8,14-OH EFV were only detectable in seminal plasma (B).
To measure the protein-free concentrations of the EFV metabolites, an established ultrafiltration method (Avery et al., 2011) was used. Protein-free concentrations of EFV metabolites were only detectable in seminal plasma with median (IQR) concentrations of 10.16 ng/ml (7.29–42.36) for 8-OH EFV, 6.55 ng/ml (5.14–22.18) for 7-OH EFV, and 4.04 ng/ml (3.98–5.80) for 8,14-OH EFV (Fig. 1). As an explanation for the differences observed in both the total concentration gradients and the detection of protein-free metabolites only in seminal plasma, protein binding was examined for each metabolite. Because protein-free concentrations were not detectable at concentrations greater than the lower limit of quantitation (0.5 ng/ml) in the blood plasma from the clinical samples, concentrations of EFV metabolites were added to blank lots of blood plasma to provide a comparison with seminal plasma. The median protein binding of EFV, 8-OH EFV, 7-OH EFV, and 8,14-OH EFV in blood plasma was 99.82, 99.97, 99.62, and 99.61%, respectively. The median protein binding of EFV, 8-OH EFV, 7-OH EFV, and 8,14-OH EFV in seminal plasma was 95.10, 96.20, 38.09, and 15.42%, respectively.
Formation of Dihydroxylated Metabolites of EFV.
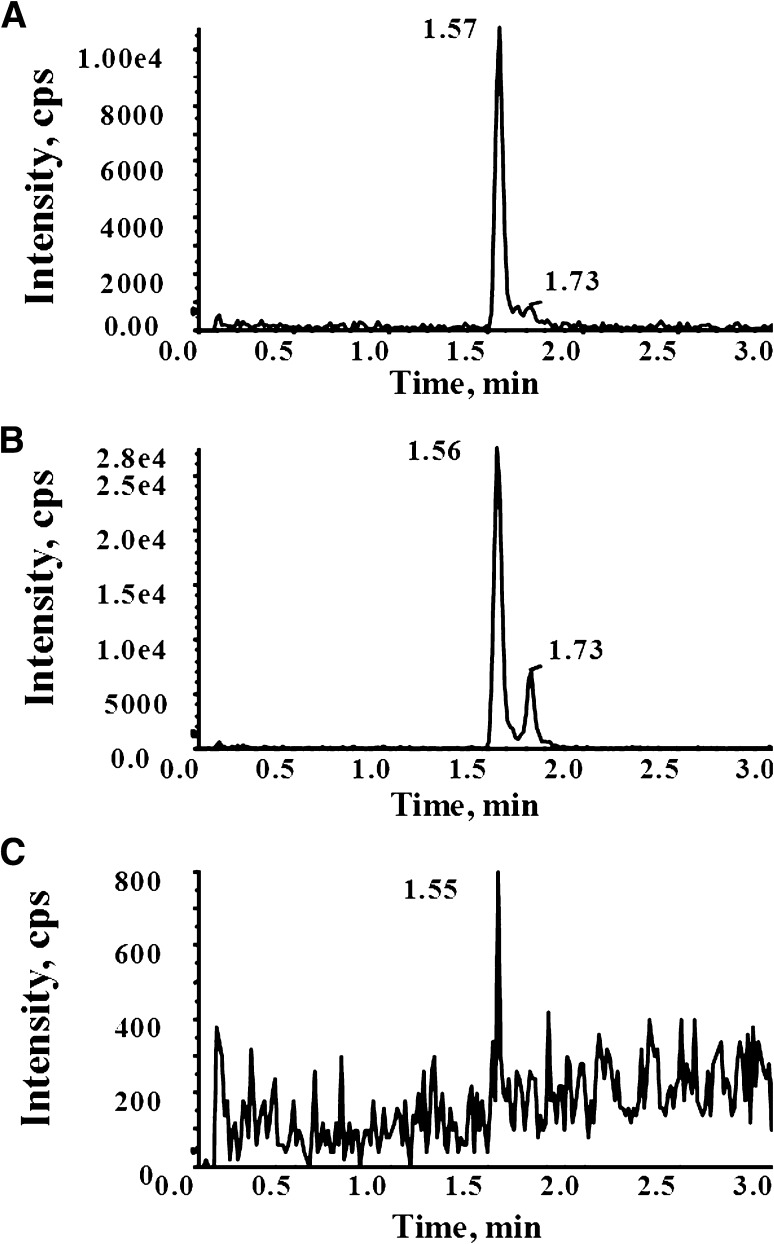
Interestingly, analysis of the metabolite profiles in each anatomic compartment revealed that in addition to 8,14-OH EFV (Fig. 2; retention time 1.56 minutes) a second dihydroxylated metabolite (Fig. 2; 1.73 minutes) was detectable above the background in seminal plasma. Previous studies examining the oxidative metabolism of EFV had identified 8,14-OH EFV as the sole dihydroxylated metabolite of EFV in humans (Mutlib et al., 1999; Ward et al., 2003). A recent study has identified a second dihydroxylated metabolite of EFV that was observed in vivo and in vitro after incubation with either 7- or 8-OH EFV as a substrate (Ogburn et al., 2010). With this in mind, to investigate the formation of this potentially novel metabolite, in vitro studies were performed using human liver microsomes with EFV, 7-OH EFV, or 8-OH EFV as the substrate. As shown in Fig. 3, the 1.56- and 1.73-minute dihydroxylated metabolites were only detectable in the reactions containing 8-OH EFV as the substrate, suggesting that formation of these metabolites involved oxygen insertion at the 8-position of EFV. By contrast, a third metabolite was detectable at 1.92 minutes after incubation with EFV, 7-OH EFV, and 8-OH EFV, suggesting that formation of this metabolite may involve oxygen insertion at both the 7-position and 8-position of EFV.
Fig. 2.
Physiologic formation of dihydoxylated EFV metabolites. The production of a second di-OH EFV metabolite (1.73-minute retention time), distinct from 8,14-OH EFV (1.56-minute retention time) was determined in the clinical matrices from subjects on a steady-state EFV-containing regimen. The panels depict the MRM transition of m/z 346 > 262 for: (A) blood plasma, (B) seminal plasma, and (C) cerebrospinal fluid from an individual subject.
Fig. 3.
Formation of dihydoxylated EFV metabolites from human liver microsomes. Production of dihydroxylated EFV metabolite after substrate incubation with human liver microsomes. The panels depict the common MRM transition of m/z 346 > 262 for human liver microsomes incubated with (A) 20 μM EFV, (B) 20 μM 8-OH EFV, and (C) 20 μM 7-OH EFV.
To probe the structures of these three dihydroxylated products, tandem mass spectrometry (MS/MS) was performed (Fig. 4). The fragmentation pattern of the 1.56-minute retention time metabolite is commensurate with that of 8,14-OH EFV as previously described elsewhere (Mutlib et al., 1999). We propose that the m/z 282.0, 262.0, 232.0, and 167.7 fragment ions of the 1.73-minute retention-time metabolites result from the loss of CH2O2 and H2O, C4H4O2, C5H6O3, and C7H11OF3, respectively. The daughter ions 302.0, 273.9, 262.0, and 210.0 of the 1.92-minute retention-time metabolite are proposed to correspond to the loss of CO2, C3H4O2, C4H4O2, and C6H7F3, respectively. The three observed dihydroxylated products exhibit highly similar fragmentation patterns; however, the fragment ion of m/z 210.0 for the 1.92-minute retention-time metabolite may indicate dihydroxylation on the aromatic ring as opposed to both the aromatic ring and the cyclopropyl ring. Thus, this metabolite may be a 7,8-OH EFV product as previously hypothesized (Ogburn et al., 2010).
Fig. 4.
Fragmentation patterns of dihydroxylated metabolites of EFV. The MS/MS fragmentation patterns were determined for each distinct dihydroxylated EFV metabolite with retention times of (A) 1.57 minutes, (B) 1.73 minutes, and (C) 1.92 minutes.
In contrast, the m/z 168.0 fragment of the 1.73-minute retention-time metabolite suggests hydroxylation on the cyclopropyl ring similar to 8,14-OH EFV. Such a metabolite has yet to be reported. With this in mind, we employed cDNA-expressed P450 to identify the enzyme(s) involved in the formation of this product. The panel of P450s tested included CYPs 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4, 3A5, and 3A7. The dihydroxylated metabolites retained at 1.56 and 1.73 minutes were solely formed using 8-OH EFV as a substrate, and the formation of these products was primarily catalyzed by CYP2B6 as well as CYP1A1 to a lesser extent. The dihydroxylated metabolite retained at 1.92 minutes was formed primarily by CYP2B6 with 8-OH EFV as a substrate. In addition, this product was also detected in incubations containing CYP2B6 and CYP2C8 with substrates EFV and 7-OH EFV, respectively (Fig. 5). CYP2B6 has been previously demonstrated to be responsible for metabolizing EFV to 8-OH EFV and 8,14-OH EFV (Ward et al., 2003). To summarize the formation of dihydroxylated metabolites, we propose the schematic in Fig. 6, consistent with previously determined oxidative metabolism of EFV (Mutlib et al., 1999; Ward et al., 2003; Ogburn et al., 2010).
Fig. 5.
Contribution of individual CYPs to the formation of EFV metabolites. Individual cDNA-expressed CYPs were incubated with 5 μM EFV, 8-OH EFV, or 7-OH EFV and analyzed by UPLC-MS/MS for the formation of dihdyroxylated products of EFV at (A) 1.57 minutes, (B) 1.74 minutes, and (C) 1.92 minutes. The data are presented as mean ± standard deviation (SD) of n = 3.
Fig. 6.
Proposed schematic of EFV mono-oxidative and dioxidative metabolism. EFV metabolism to 8-hydroxy-EFV catalyzed by CYP2B6 was previously established by Ward et al. (2003). EFV metabolism to 7-hydroxy-EFV catalyzed by CYP2A6 and to 8-14-dihydroxy-EFV catalyzed by CYP2B6 was previously established by Ogburn et al. (2010).
HIV-Inhibitory Activity of EFV Metabolites.
Because we were able to detect metabolites of EFV in extravascular compartments where HIV can distribute, we used a single-round infectivity assay (Zhang et al., 2004; Shen et al., 2008; Sampah et al., 2011; Jilek et al., 2012) to investigate the anti-HIV activity of synthetic 8-OH EFV, 7-OH EFV, and 8,14-OH EFV. Data demonstrating whether metabolites of EFV contribute to the inhibition of HIV activity of EFV have yet to be reported. Compared with the experimentally observed IC50 of 0.36 ng/ml for EFV, 8-OH EFV, 7-OH EFV, and 8,14-OH EFV had IC50 values of 42.25 ng/ml, 44.68 ng/ml, and 2238.4 ng/ml, respectively. Our experimentally determined IC50 value for EFV is comparable with the previously established wild-type in vitro IC50 for EFV of 0.51 ng/mL (Parkin et al., 2004).
Discussion
EFV is one of the most commonly used ARVs in the treatment of HIV. Although EFV is extensively metabolized, there is a lack of understanding of the distribution and pharmacologic effect of the metabolites of EFV. This study is the first to demonstrate the physiologic distribution of ARV metabolites into relevant biologic compartments and to examine the role that metabolism may play in antiviral activity. Concentrations of 8-OH EFV, 7-OH EFV, and 8,14-OH EFV were detectable in biologic matrices of the blood plasma, seminal plasma, and cerebrospinal fluid of patients on an antiretroviral regimen containing EFV.
Interestingly, EFV has been previously demonstrated to induce compartment-specific toxicities, including hepatotoxicity, CNS toxicity, and decreased semen quality (Sulkowski et al., 2002; Martín-Carbonero et al., 2003; Dieterich et al., 2004; Ciccarelli et al., 2011). Recently, we demonstrated for the first time that 8-OH EFV has the ability to induce cytotoxicity via stimulation of mitochondrial dysfunction and activation of stress-activated signaling pathways (Bumpus, 2011). Subsequently, toxicity of 8-OH EFV to dendritic cells of the CNS has also been reported (Tovar-y-Romo et al., 2012). Although the mechanisms underlying the adverse effects of EFV on semen quality have yet to be fully elucidated, it has been reported that treatment with EFV results in decreased motile spermatozoa and vitality, suggested to be the result of mitochondrial toxicity or direct toxicity to the cells producing spermatozoa (van Leeuwen et al., 2008; Lambert-Niclot et al., 2011). Because 8-OH EFV has been reported to play a causal role in certain compartment-specific toxicities (Bumpus, 2011; Tovar-y-Romo et al., 2012), understanding the clinical exposure of monohydroxylated and dihydroxylated metabolites of EFV is of importance.
We reported previously that protein-free concentrations of EFV are in equilibrium in the blood plasma and seminal plasma as a result of different degrees of protein binding in each compartment, creating the very large total drug concentration gradients (Avery et al., 2011). This suggests that EFV distributes into the male genital tract by mechanisms of passive diffusion. By contrast, for the monohydroxylated and dihydroxylated metabolites of EFV, we have demonstrated in this study that the protein-free metabolites do not exist in equilibrium in the two compartments, but rather are found at measurable concentrations only in the seminal plasma; they are below assay detection limits, if present at all, in blood plasma and cerebrospinal fluid. Although the protein-free concentrations of EFV metabolites were not detectable in the blood plasma samples, the protein-free gradients may be estimated using the in vitro determinations of metabolite protein binding in blood plasma and the median total concentration determined for each metabolite. Protein-free 8-OH EFV, 7-OH EFV, and 8,14-OH EFV may therefore have seminal plasma:blood plasma free-drug concentration gradients as high as 145-, 188-, and 209-fold, respectively. One possible explanation for these gradients would involve EFV metabolites being potential substrates for human drug transporters at the blood-testis barrier, causing an influx of these molecules within the compartment. In the event of local metabolism of EFV, this could result in reduced compartment-specific concentrations of the parent compound, resulting in reduced pharmacologic effect or an increased concentration of metabolites that may be pharmacologically active with a desirable effect or may induce local toxicity.
While the protein binding of 8-OH EFV is similar to EFV in both blood plasma and seminal plasma, the protein binding of 7-OH EFV and 8,14-OH EFV was found to be significantly reduced compared with the parent compound. These differences suggest that chemical modification by hydroxylation impacts the affinity with which the compounds bind to plasma proteins, which may impact the overall distribution of each compound. If monohydroxylated and dihydroxylated metabolites of EFV are primarily transported by passive diffusion, accumulation may be the result of protein binding, lipophilicity partitioning (logP), and/or ion trapping (Kashuba et al., 1999).
Seminal plasma, composed primarily of secretions from the prostate glands and seminal vesicles, is the main fluid assessed when analyzing the male genital tract (Klemmt and Scialli, 2005). Weak acids have previously been demonstrated to accumulate in prostatic fluid by ion-trapping mechanisms (Winningham et al., 1968). Mono-oxygenation or dioxygenation of a compound can impact both the acid-dissociation constant and lipophilicity (partition coefficient, logP) of a molecule, which can have a marked impact on the compound’s disposition (Mack and Bönisch, 1979; Kem et al., 2004). In oxidative metabolism, the logP is typically shown to reduce for a compound as it is transformed to be more hydrophilic (Manners et al., 1988), which also impacts the acidity and basicity (pKa) of a compound. For EFV metabolites, it is our hypothesis that the observed seminal plasma accumulation is the result of these fundamental chemical changes to EFV resulting from primary oxidative metabolism.
To date, it has yet to be demonstrated whether the metabolites of EFV contribute to the anti-HIV activity of EFV. Using an HIV pseudovirus with an env-GFP fusion protein, we have analyzed the impact of 8-OH EFV, 7-OH EFV, and 8,14-OH EFV on viral infectivity. Based on the clinical concentrations observed for 8-OH EFV and 7-OH EFV, these results suggest 8-OH EFV and 7-OH EFV are minimally effective at inhibiting viral replication. Although the clinically observed concentrations were greater than our experimentally determined IC50, particularly for 8-OH EFV, because of the high degree of protein binding there is insufficient protein-free drug available in biologic matrices to contribute to the pharmacologic activity of EFV.
Using our highly sensitive UPLC-MS/MS method, we have demonstrated the production of three distinct dihydroxylated metabolites, with proposed fragmentation patterns and mechanism of formation based on in vitro and in vivo characterization. The metabolism of EFV to 8-OH EFV, 7-OH EFV, and 8,14-OH EFV was originally established by Mutlib et al. (1999), who identified 17 potential metabolites of EFV by use of high-pressure liquid chromatography (HPLC) and NMR. More recently, Ogburn et al. (2010) described an observed dihydroxylated metabolite from EFV after incubation with 7-OH EFV and 8-OH EFV that was primarily formed by CYP 2B6. Consistent with their results, we propose this metabolite to be a 7,8-dihydroxylated metabolite. In addition, a third dihydroxylated metabolite of EFV was also detected, which shared fragmentation patterns similar to 8,14-OH EFV, indicating this metabolite may also be hydroxylated on the cyclopropyl ring of EFV. Because the HPLC methods used in prior analyses did not have the sensitivity and resolution of our methodology, it is likely they did not have the ability to distinguish or separate multiple dihydroxylated metabolites. Many of the fragmentation ions proposed by Mutlib et al. (1999) that are characteristic of 8,14-OH EFV are also characteristic of the two novel dihydroxylated metabolites of EFV proposed here and by Ogburn et al. (2010), suggesting that they were not able to differentiate between the multiple products. In addition, our results have also shown that the primary dihydroxylated metabolite of 8,14-OH EFV in vivo differs from the primary metabolite of 7,8-OH EFV in vitro using HLMs. This is consistent with Mutlib et al. (1999), as 8,14-OH EFV was originally isolated and characterized from in vivo samples of the urine of guinea pigs.
In summary, we have established the distribution of the primary oxidative metabolites of EFV, 8-OH EFV, 7-OH EFV, and 8,14-OH EFV, and defined the role of protein binding in extravascular compartments. We have characterized the concentration profiles of EFV metabolites in three anatomic compartments relevant to HIV infection: the blood plasma of the circulatory system, the seminal plasma of the male genital tract, and the cerebrospinal fluid of the CNS. Interestingly, we have shown how there is significant accumulation in the seminal plasma of the hydroxylated metabolites of EFV, which may impact local pharmacology and/or toxicology. Future work may involve examination of the potential for local toxicity in compartments such as the male genital tract, where we have found oxidative metabolites to accumulate, including 8-OH EFV, which has been demonstrated to induce compartment-specific mechanisms of toxicity (Bumpus, 2011; Tovar-Y-Romo et al., 2012). Taken together, this study is the first to demonstrate the anatomic distribution of metabolites of an ARV into relevant biologic compartments and to examine their anti-HIV activity. These results suggest there may be important compartmental differences in EFV metabolite distribution, and the male genital tract may be a novel compartment in understanding the local drug distribution, efficacy, and toxicity of oxidative metabolites.
Abbreviations
- ARV
antiretroviral drug
- CNS
central nervous system
- DMSO
dimethylsulfoxide
- EFV
efavirenz
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HIV
human immunodeficiency virus
- HLM
human liver microsome
- HPLC
high-pressure liquid chromatography
- IL-2
interleukin-2
- IQR
interquartile range
- logP
lipophilicity partitioning
- MACS
magnetic-activated cell sorting
- MPA
mobile phase A
- MPB
mobile phase B
- MS/MS
tandem mass spectrometry
- 7-OH EFV
7-hydroxy efavirenz
- 8-OH EFV
8-hydroxy efavirenz
- 8,14-OH EFV
8,14-dihydroxy efavirenz
- P450
cytochrome P450
- PBMC
peripheral blood mononuclear cell
- PHA
phytohemagglutinin
- RPMI medium
Roswell Park Memorial Institute medium
- UPLC-MS/MS
ultraperformance liquid chromatography-tandem mass spectrometry
Authorship Contributions
Participated in research design: Avery, Bumpus.
Conducted experiments: Avery, VanAusdall.
Contributed new reagents or analytic tools: Hendrix.
Performed data analysis: Avery, VanAusdall, Bumpus.
Wrote or contributed to the writing of the manuscript: Avery, VanAusdall, Hendrix, Bumpus.
Footnotes
This work was supported by a pharmacology/toxicology research starter grant from the Pharmaceutical Research and Manufacturers of America Foundation (N.N.B.); by the National Institutes of Health [Grant 1S10 RR 27733]; and by the Pendleton Foundation Trust (C.W.H.).
References
- Avery LB, Bakshi RP, Cao YJ, Hendrix CW. (2011) The male genital tract is not a pharmacological sanctuary from efavirenz. Clin Pharmacol Ther 90:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery LB, Parsons TL, Meyers DJ, Hubbard WC. (2010) A highly sensitive ultra performance liquid chromatography-tandem mass spectrometric (UPLC-MS/MS) technique for quantitation of protein free and bound efavirenz (EFV) in human seminal and blood plasma. J Chromatogr B Analyt Technol Biomed Life Sci 878:3217–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best BM, Koopmans PP, Letendre SL, Capparelli EV, Rossi SS, Clifford DB, Collier AC, Gelman BB, Mbeo G, McCutchan JA, et al. CHARTER Group (2011) Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother 66:354–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus NN. (2011) Efavirenz and 8-hydroxyefavirenz induce cell death via a JNK- and BimEL-dependent mechanism in primary human hepatocytes. Toxicol Appl Pharmacol 257:227–234 [DOI] [PubMed] [Google Scholar]
- Bumpus NN, Kent UM, Hollenberg PF. (2006) Metabolism of efavirenz and 8-hydroxyefavirenz by P450 2B6 leads to inactivation by two distinct mechanisms. J Pharmacol Exp Ther 318:345–351 [DOI] [PubMed] [Google Scholar]
- Cao YJ, Ndovi TT, Parsons TL, Guidos AM, Caffo B, Hendrix CW. (2008) Effect of semen sampling frequency on seminal antiretroviral drug concentration. Clin Pharmacol Ther 83:848–856 [DOI] [PubMed] [Google Scholar]
- Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, Tamburrini E, Cauda R, De Luca A, Silveri MC. (2011) Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 76:1403–1409 [DOI] [PubMed] [Google Scholar]
- Dellamonica P, Di Perri G, Garraffo R. (2012) NNRTIs: pharmacological data. Med Mal Infect 42:287–295 [DOI] [PubMed] [Google Scholar]
- Dieterich DT, Robinson PA, Love J, Stern JO. (2004) Drug-induced liver injury associated with the use of nonnucleoside reverse-transcriptase inhibitors. Clin Infect Dis 38 (Suppl 2):S80–S89 [DOI] [PubMed] [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H. (2007) Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 320:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnström N, Bjelfman C, Söderström TG, Smith G, Egevad L, Norlén BJ, Wolf CR, Rane A. (2001) Detection of cytochrome P450 mRNA transcripts in prostate samples by RT-PCR. Eur J Clin Invest 31:880–886 [DOI] [PubMed] [Google Scholar]
- Jilek BL, Zarr M, Sampah ME, Rabi SA, Bullen CK, Lai J, Shen L, Siliciano RF. (2012) A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat Med 18:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) (2009) 2008 Report on the Global AIDS Epidemic, World Health Organization, Geneva [Google Scholar]
- Kashuba ADM, Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. (1999) Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother 43:1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Prokai L, Papke RL, Cao X, LeFrancois S, Wildeboer K, Prokai-Tatrai K, Porter-Papke J, Soti F. (2004) Hydroxy metabolites of the Alzheimer’s drug candidate 3-[(2,4-dimethoxy)benzylidene]-anabaseine dihydrochloride (GTS-21): their molecular properties, interactions with brain nicotinic receptors, and brain penetration. Mol Pharmacol 65:56–67 [DOI] [PubMed] [Google Scholar]
- Klemmt L, Scialli AR. (2005) The transport of chemicals in semen. Birth Defects Res B Dev Reprod Toxicol 74:119–131 [DOI] [PubMed] [Google Scholar]
- Kumagai J, Fujimura T, Takahashi S, Urano T, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Blumberg B, Inoue S. (2007) Cytochrome P450 2B6 is a growth-inhibitory and prognostic factor for prostate cancer. Prostate 67:1029–1037 [DOI] [PubMed] [Google Scholar]
- Lambert-Niclot S, Poirot C, Tubiana R, Houssaini A, Soulié C, Dominguez S, Schubert B, Prades M, Bonmarchand M, Calvez V, et al. (2011) Effect of antiretroviral drugs on the quality of semen. J Med Virol 83:1391–1394 [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, et al. CHARTER Group (2008) Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Carbonero L, Núñez M, González-Lahoz J, Soriano V. (2003) Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials 4:115–120 [DOI] [PubMed] [Google Scholar]
- Mack F, Bönisch H. (1979) Dissociation constants and lipophilicity of catecholamines and related compounds. Naunyn Schmiedebergs Arch Pharmacol 310:1–9 [DOI] [PubMed] [Google Scholar]
- Manners CN, Payling DW, Smith DA. (1988) Distribution coefficient, a convenient term for the relation of predictable physico-chemical properties to metabolic processes. Xenobiotica 18:331–350 [DOI] [PubMed] [Google Scholar]
- McArthur JC. (2004) HIV dementia: an evolving disease. J Neuroimmunol 157:3–10 [DOI] [PubMed] [Google Scholar]
- Miksys SL, Tyndale RF. (2002) Drug-metabolizing cytochrome P450s in the brain. J Psychiatry Neurosci 27:406–415 [PMC free article] [PubMed] [Google Scholar]
- Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS, Christ DD. (1999) Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos 27:1319–1333 [PubMed] [Google Scholar]
- O’Doherty U, Swiggard WJ, Malim MH. (2000) Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol 74:10074–10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. (2010) Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos 38:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ. (2004) Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother 48:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radesca LA, Lo YS, Moore JR, Pierce ME. (1997) Synthesis of HIV-1 reverse transcriptase inhibitor DMP 266. Synth Commun 27:4373–4384 [Google Scholar]
- Resnick L, Berger JR, Shapshak P, Tourtellotte WW. (1988) Early penetration of the blood-brain-barrier by HIV. Neurology 38:9–14 [DOI] [PubMed] [Google Scholar]
- Sacktor N. (2002) The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol 8 (Suppl 2):115–121 [DOI] [PubMed] [Google Scholar]
- Sampah MES, Shen L, Jilek BL, Siliciano RF. (2011) Dose-response curve slope is a missing dimension in the analysis of HIV-1 drug resistance. Proc Natl Acad Sci USA 108:7613–7618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Peterson S, Sedaghat AR, McMahon MA, Callender M, Zhang H, Zhou Y, Pitt E, Anderson KS, Acosta EP, Siliciano RF. (2008) Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med 14:762–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekler J, Sycks BJ, Holte S, Maenza J, Stevens CE, Dragavon J, Collier AC, Coombs RW. (2008) HIV dynamics in seminal plasma during primary HIV infection. AIDS Res Hum Retroviruses 24:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. (2002) Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology 35:182–189 [DOI] [PubMed] [Google Scholar]
- Tovar-Y-Romo LB, Bumpus NN, Pomerantz D, Avery LB, Sacktor N, McArthur J, Haughey NJ. (2012) Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther 343:696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research (CDER), U.S. Food and Drug Administration (FDA) (2001) Guidance for industry: bioanalytical method validation. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, et al. RV254/SEARCH 010 Study Group (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen E, Wit FW, Repping S, Eeftinck Schattenkerk JKM, Reiss P, van der Veen F, Prins JM. (2008) Effects of antiretroviral therapy on semen quality. AIDS 22:637–642 [DOI] [PubMed] [Google Scholar]
- Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. (2003) The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 306:287–300 [DOI] [PubMed] [Google Scholar]
- Winningham DG, Nemoy NJ, Stamey TA. (1968) Diffusion of antibiotics from plasma into prostatic fluid. Nature 219:139–143 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou Y, Alcock C, Kiefer T, Monie D, Siliciano J, Li Q, Pham P, Cofrancesco J, Persaud D, Siliciano RF. (2004) Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol 78:1718–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]