Abstract
Recent evidence suggests that hepatocellular carcinoma (HCC) is organized by a subset of cells with stem cell features (cancer stem cells; CSCs). CSCs are considered a pivotal target for the eradication of cancer, and liver CSCs have been identified by the use of various stem cell markers. However, little information is known about the expression patterns and characteristics of marker-positive CSCs, hampering the development of personalized CSC-targeted therapy. Here, we show that CSC markers EpCAM and CD90 are independently expressed in liver cancer. In primary HCC, EpCAM+ and CD90+ cells resided distinctively, and gene-expression analysis of sorted cells suggested that EpCAM1 cells had features of epithelial cells, whereas CD90+ cells had those of vascular endothelial cells. Clinicopathological analysis indicated that the presence of EpCAM+ cells was associated with poorly differentiated morphology and high serum alpha-fetoprotein (AFP), whereas the presence of CD90+ cells was associated with a high incidence of distant organ metastasis. Serial xenotransplantation of EpCAM+/CD90+ cells from primary HCCs in immune-deficient mice revealed rapid growth of EpCAM+ cells in the subcutaneous lesion and a highly metastatic capacity of CD90+ cells in the lung. In cell lines, CD90+ cells showed abundant expression of c-Kit and in vitro chemosensitivity to imatinib mesylate. Furthermore, CD901 cells enhanced the motility of EpCAM+ cells when cocultured in vitro through the activation of transforming growth factor beta (TGF-β) signaling, whereas imatinib mesylate suppressed TGFB1 expression in CD90+ cells as well as CD90+ cell-induced motility of EpCAM+ cells.
Conclusion:
Our data suggest the discrete nature and potential interaction of EpCAM+ and CD90+ CSCs with specific gene-expression patterns and chemosensitivity to molecular targeted therapy. The presence of distinct CSCs may determine the clinical outcome of HCC.
The cancer stem cell (CSC) hypothesis, which suggests that a subset of cells bearing stem-cell–like features is indispensable for tumor development, has recently been put forward subsequent to advances in molecular and stem cell biology. Liver cancer, including hepatocellular carcinoma (HCC), is a leading cause of cancer death worldwide.1 Recent studies have shown the existence of CSCs in liver cancer cell lines and primary HCC specimens using various stem cell markers.2–7 Independently, we have identified novel HCC subtypes defined by the hepatic stem/progenitor cell markers, epithelial cell adhesion molecule (EpCAM) and alpha-fetoprotein (AFP), which correlate with distinct gene-expression signatures and prognosis.8,9 EpCAM+ HCC cells isolated from primary HCC and cell lines show CSC features, including tumorigenicity, invasiveness, and resistance to fluorouracil (5-FU).10 Similarly, other groups have shown that CD133+, CD90+, and CD13+ HCC cells are also CSCs, and that EpCAM, CD90, and CD133 are the only markers confirmed to enrich CSCs from primary HCCs thus far.3–5,10
Although EpCAM+, CD90+, and CD133+ cells show CSC features, such as high tumorigenicity, an invasive nature, and resistance to chemo- and radiation therapy, it remains unclear whether these cells represent an identical HCC population and whether they share similar or distinct characteristics. In this study, we used fluorescent-activated cell sorting (FACS), microarray, and immunohistochemistry (IHC) techniques to investigate the expression patterns of the representative liver CSC markers CD133, CD90, and EpCAM in a total of 340 HCC cases and 7 cases of mesenchymal liver tumors. We further explored gene and protein-expression patterns as well as tumorigenic capacity of sorted cells isolated from 15 primary HCCs and 7 liver cancer cell lines in an attempt to identify the molecular portraits of each cell type.
Materials and Methods
Clinical Specimens.
HCC samples were obtained with informed consent from patients who had undergone radical resection at the Liver Center in Kanazawa University Hospital (Kanazawa, Japan), and tissue acquisition procedures were approved by the ethics committee of Kanazawa University. A total of 102 formalin-fixed and paraffin-embedded HCC samples, obtained from 2001 to 2007, were used for IHC analyses. Fifteen fresh HCC samples were obtained between 2008 and 2012 from surgically resected specimens and an autopsy specimen and were used immediately to prepare single-cell suspensions and xenotransplantation (Table 1). Seven hepatic stromal tumors (three cavernous hemangioma, two hemangioendothelioma, and two angiomyolipoma) were formalin fixed and paraffin embedded and used for IHC analyses.
Table 1.
Clinicopathological Characteristics of HCC Cases Used for Xenotransplantation
| ID | Age/Sex | Etiology | Tumor Size (cm) | Histological Grade | AFP (ng/mL) | DCP (IU/mL) |
|---|---|---|---|---|---|---|
| P1 | 77/M | Alcohol | 12.0 | Moderate | 198 | 322 |
| P2 | 61/F | NBNC | 11.0 | Moderate | 12 | 3,291 |
| P3 | 66/M | NBNC | 2.2 | Moderate | 13 | 45 |
| P4 | 65/M | HCV | 4.2 | Poor | 13,700 | 25,977 |
| P5 | 52/M | HBV | 6.0 | Moderate | 29,830 | 1,177 |
| P6 | 60/M | HCV | 2.7 | Poor | 249 | 185 |
| P7 | 79/F | HBV | 4.0 | Poor | 46,410 | 384 |
| P8 | 77/F | NBNC | 5.5 | Moderate | 17,590 | 562 |
| P9 | 71/M | Alcohol | 7.0 | Poor | 3,814 | 607 |
| P10 | 51/M | HBV | 2.2 | Well | <10 | 21 |
| P11 | 71/M | Alcohol | 2.1 | Well | <10 | 11 |
| P12 | 60/M | HBV | 10.8 | Poor | 323 | 2,359 |
| P13 | 66/M | HCV | 2.8 | Moderate | 11 | 29 |
| P14 | 71/M | HCV | 7.2 | Moderate | 235,700 | 375,080 |
| P15 | 75/M | HBV | 5.5 | Poor | <10 | 97 |
Abbreviation: DCP, des-gamma-carboxy prothrombin.
Additional details of experimental procedures are available in the Supporting Information.
Results
EpCAM, CD133, and CD9 Expression in HCC.
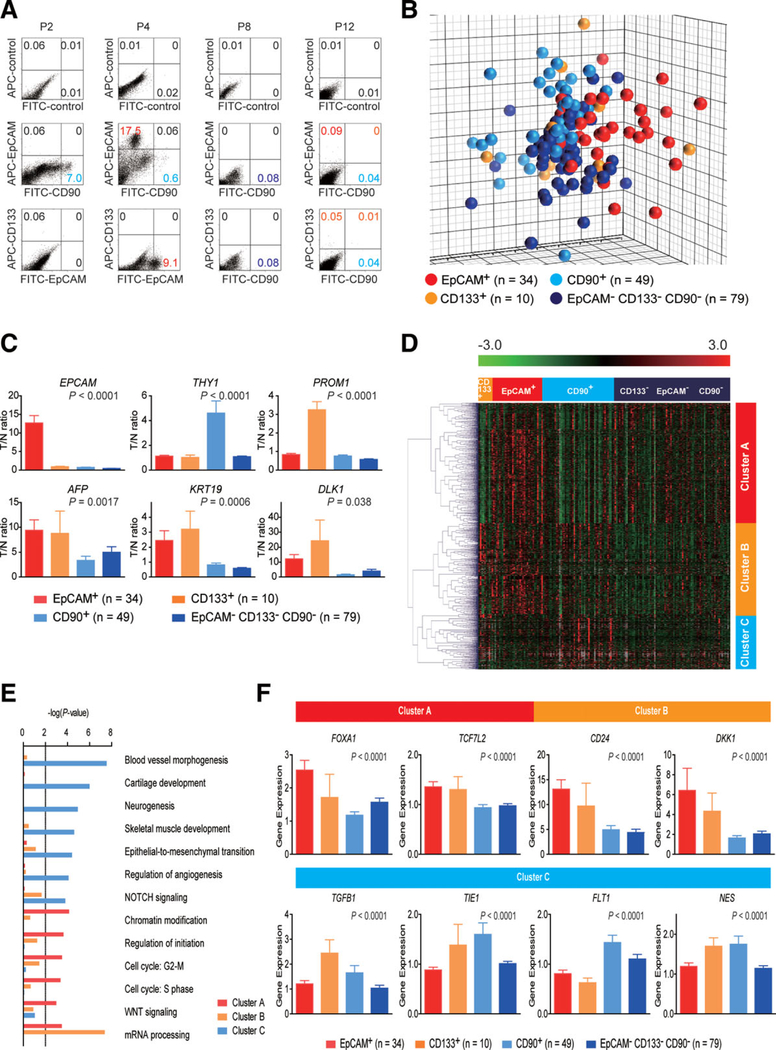
We first evaluated the frequencies of three representative CSC markers (EpCAM+, CD90+, and CD133+ cells) in 12 fresh primary HCC cases surgically resected by FACS (representative data shown in Fig. 1A). Clinicopathological characteristics of primary HCC cases are shown in Table 1. We noted that frequency of EpCAM+, CD90+, and CD133+ cells varied between individuals. Abundant CD90+ (7.0%), but almost no EpCAM+, cells (0.06%, comparable to the isotype control) were detected in P2, whereas few CD90+ (0.6%), but abundant EpCAM+, cells (17.5%) were detected in P4. Very small populations of EpCAM+(0.09%), CD90+ (0.04%), and CD133+ cells (0.05%) were found in P12, but they were almost nonexistent in P8, except for CD90+ cells (0.08%) (Fig. 1A). We further evaluated the expression of EpCAM, CD90, and CD133 in xenografts obtained from surgically resected samples (P13 and P15) and an autopsy sample (P14). As a whole, compared to the isotype control, 7 of 15 HCCs contained definite EpCAM+ cells (46.7%), whereas only 3 HCCs contained definite CD133+ cells (20%) (Table 2). CD90+ cells were detected at variable frequencies in all 15 HCCs analyzed.
Fig. 1.
Gene-expression profiles of CSC marker-positive HCCs. (A) FACS analysis of primary HCCs stained with fluorescent-labeled Abs against EpCAM, CD90, or CD133. (B) Multidimensional scaling analysis of 172 HCC cases characterized by the expression patterns of EpCAM, CD133, and CD90. Red, EpCAM+ CD90− CD133− (n = 34); orange, EpCAM− CD90 CD133+ (n = 10); light blue, EpCAM− CD90+ CD133− (n = 49); blue, EpCAM− CD90− CD133− (n = 79). HCC specimens were clustered in specific groups with statistical significance (P < 0.001). (C) Expression patterns of well-known hepatic stem/progenitor markers in each HCC subtype, as analyzed by microarray. Red bar, EpCAM+; orange bar, CD133+; light blue bar, CD90+; blue bar, EpCAM− CD90− CD133−. (D) Hierarchical cluster analysis based on 1,561 EpCAM/CD90/ CD133-coregulated genes in 172 HCC cases. Each cell in the matrix represents the expression level of a gene in an individual sample. Red and green cells depict high and low expression levels, respectively, as indicated by the scale bar. (E) Pathway analysis of EpCAM/CD90/CD133coregulated genes. Canonical signaling pathways activated in cluster A (red bar), cluster B (orange bar), or cluster C (light blue bar) with statistical significance (P < 0.01) are shown. (F) Expression patterns of representative genes differentially expressed in EpCAM/CD90/CD133 HCC subtypes. Red bar, EpCAM+; orange bar, CD133+; light blue bar, CD90+; blue bar, EpCAM− CD133− CD90−.
Table 2.
Tumorigenic Capacity of Unsorted, EpCAM+, EpCAM−, CD90+, and CD90− Cells From Primary HCCs and Xenografts
| Sample | CD133 (%) | CD90 (%) | EpCAM (%) | Cell Surface Marker | Number of Cells | Tumor Formation |
|
|---|---|---|---|---|---|---|---|
| 2M | 3M | ||||||
| P1 | 0 | 3.1 | 0 | Unsorted | 1 × 107 | 0/5 | 0/5 |
| CD90+ | 1 × 105 | 0/5 | 0/5 | ||||
| CD90− | 1 × 105 | 0/5 | 0/5 | ||||
| P2 | 0.06 | 7.0 | 0.06 | Unsorted | 1 × 107 | 0/5 | 0/5 |
| CD90+ | 1 × 105 | 0/5 | 0/5 | ||||
| CD90− | 1 × 105 | 0/5 | 0/5 | ||||
| P3 | 0 | 1.3 | 0 | Unsorted | 1 × 106 | 0/2 | 0/2 |
| CD90+ | 1 × 104 | 0/4 | 0/4 | ||||
| CD90− | 1 × 104 | 0/4 | 0/4 | ||||
| P4 | 0 | 0.6 | 17.5 | Unsorted | 1 × 106 | ¾ | 4/4 |
| EpCAM+ | 1 × 103 | 0/3 | 2/3 | ||||
| 1 × 104 | ¾ | 4/4 | |||||
| 1 × 105 | 3/3 | 3/3 | |||||
| CD90+ | 1 × 103 | 0/3 | 0/3 | ||||
| 1 × 104 | 0/4 | 0/4 | |||||
| 1 × 105 | 0/3 | 0/3 | |||||
| EpCAM− | 1 × 103 | 0/3 | 0/3 | ||||
| CD90− | |||||||
| 1 × 104 | 0/4 | 0/4 | |||||
| 1 × 105 | 0/3 | 0/3 | |||||
| P5 | 0 | 0.8 | 29.7 | Unsorted | 1 × 106 | 0/5 | 0/5 |
| EpCAM+ | 1 × 105 | 0/5 | 0/5 | ||||
| CD90+ | 1 × 105 | 0/5 | 0/5 | ||||
| EpCAM− | 1 × 105 | 0/5 | 0/5 | ||||
| CD90− | |||||||
| P6 | 0 | 0.7 | 0 | Unsorted | 1 × 106 | 0/2 | 0/2 |
| CD90+ | 1 × 104 | 0/4 | 0/4 | ||||
| CD90− | 1 × 104 | 0/4 | 0/4 | ||||
| P7 | 1.38 | 4.5 | 4.4 | Unsorted | 1 × 106 | 2/2 | 2/2 |
| EpCAM+ | 2 × 102 | 0/3 | 0/3 | ||||
| 1 × 103 | 0/3 | 1/3 | |||||
| 1 × 104 | 2/4 | 4/4 | |||||
| CD90+ | 2 × 102 | 0/3 | 0/3 | ||||
| 1 × 103 | 0/3 | 0/3 | |||||
| 1 × 104 | 0/4 | 0/4 | |||||
| EpCAM− | 1 × 103 | 0/3 | 0/3 | ||||
| CD90− | |||||||
| 1 × 104 | 0/3 | 0/3 | |||||
| 1 × 105 | 0/4 | 0/4 | |||||
| P8 | 0 | 0.08 | 0 | Unsorted | 1 × 105 | 0/4 | 0/4 |
| CD90+ | 1 × 103 | 0/3 | 0/3 | ||||
| CD90− | 1 × 105 | 0/3 | 0/3 | ||||
| P9 | 0 | 0.26 | 0 | Unsorted | 1 × 105 | 0/4 | 0/4 |
| CD90+ | 1 × 103 | 0/3 | 0/3 | ||||
| CD90− | 1 × 105 | 0/3 | 0/3 | ||||
| P10 | 0 | 0.78 | 0 | Unsorted | 1 × 104 | 0/4 | 0/4 |
| CD90+ | 1 × 103 | 0/3 | 0/3 | ||||
| CD90− | 1 × 104 | 0/3 | 0/3 | ||||
| P11 | 0 | 0.1 | 1.54 | Unsorted | 5 × 104 | 0/2 | 0/2 |
| EpCAM+ | 1 × 103 | 0/3 | 0/3 | ||||
| CD90+ | 1 × 103 | 0/3 | 0/3 | ||||
| EpCAM− | 1 × 104 | 0/3 | 0/3 | ||||
| CD90− | |||||||
| P12 | 0.06 | 0.05 | 0.09 | Unsorted | 1 × 105 | 0/3 | 3/3 |
| CD90+ | 1 × 103 | 0/4 | ¼ | ||||
| CD90− | 1 × 103 | 0/4 | ¼ | ||||
| 1 × 104 | 0/3 | 3/3 | |||||
| P13 | 0 | 0.03 | 67.7 | EpCAM+ | 5 × 105 | 4/4 | NA |
| 5 × 104 | 3/3 | NA | |||||
| 5 × 103 | 3/3 | NA | |||||
| EpCAM− | 5 × 105 | 0/4 | NA | ||||
| 5 × 104 | 0/3 | NA | |||||
| 5 × 103 | 0/3 | NA | |||||
| P14 | 24.0 | 0.06 | 3.1 | EpCAM+ | 5 × 103 | 4/5 | NA |
| EpCAM− | 5 × 103 | 2/5 | NA | ||||
| P15 | 0 | 2.45 | 0 | CD90+ | 5 × 104 | ¾ | NA |
| 5 × 103 | 1/3 | NA | |||||
| 5 × 102 | 1/3 | NA | |||||
| CD90− | 5 × 104 | 2/4 | NA | ||||
| 5 × 103 | 1/3 | NA | |||||
| 5 × 102 | 0/3 | NA | |||||
NA, not available.
To explore the status of these CSC marker-positive cells in HCC in a large cohort, we utilized oligo-DNA microarray data from 238 HCC cases (GEO accession no.: GSE5975) to evaluate the expression of EPCAM (encoding EpCAM and CD326), THY1 (encoding CD90), and PROM1 (encoding CD133) in whole HCC tissues and nontumor (NT) tissues. Because previous studies demonstrated that CD133+ and CD90+ cells were detected at low frequency (~13.6% by CD133 staining and ~ 6.2% by CD90 staining) in HCC, but were almost nonexistent in NT liver (4, 5),4,5 we utilized tumor/nontumor (T/N) gene-expression ratios to detect the existence of marker-positive CSCs in tumor. Accordingly, we showed that a 2-fold cutoff of T/N ratios of EPCAM successfully stratifies HCC samples with EpCAM+ liver CSCs.9,10
A total of 95 (39.9%), 110 (46.2%), and 31 (13.0%) of the 238 HCC cases were thus regarded as EpCAM+, CD90+, and CD133+ HCCs (T/N ratios: ≥ 2.0), respectively. As observed in the FACS data described above, we detected co-expression of EpCAM and CD90 in 45 HCCs (18.9%), EpCAM and CD133 in five HCCs (2%), CD90 and CD133 in five HCCs (2%), and EpCAM, CD90, and CD133 in 11 HCCs (4.6%). To clarify the characteristics of gene-expression signatures specific to stem cell marker expression status, we selected 172 HCC cases expressing a single CSC marker (34 EpCAM+ CD90− CD133−, 49 EpCAM− CD90+ CD133, and 10 EpCAM− CD90−CD133+) or all marker-negative HCCs (79 EpCAM− CD90− CD133−). A class-comparison analysis with univariate F tests and a global permutation test (×10,000) yielded a total of 1,561 differentially expressed genes. Multidimensional scaling (MDS) analysis using this gene set indicated that HCC specimens were clustered in specific groups with statistical significance (P < 0.001). Close examination of MDS plots revealed three major HCC subtype clusters: all marker-negative HCCs (blue spheres); EpCAM single-positive HCCs (red spheres); and CD90 single-positive HCCs (light blue spheres). CD133+ HCCs (orange spheres) were rare, relatively scattered, and not clustered (Fig. 1B).
We examined the expression of representative hepatic stem/progenitor cell markers AFP, KRT19, and DLK1 in HCCs with regard to the gene-expression status of each CSC marker (Fig. 1C). All three markers were up-regulated in EpCAM+ and CD133+ HCCs, compared with all marker-negative HCCs, consistent with previous findings.10,11 However, we found no significant overexpression of AFP, KRT19, and DLK1 in CD90+ and all marker-negative HCCs.
Hierarchical cluster analyses revealed three main gene clusters that were up-regulated in EpCAM+ HCCs (cluster A, 706 genes), EpCAM+ or CD133+ HCCs (cluster B, 530 genes), and CD90+ or CD133+ HCCs (cluster C, 325 genes) (Fig. 1D). Pathway analysis indicated that the enriched genes in cluster A (red bar) were associated with chromatin modification, cell-cycle regulation, and Wnt/β-catenin signaling (Fig. 1E). Genes associated with messenger RNA processing were enriched in clusters A (red bar) and B (orange bar). Surprisingly, genes in cluster C were significantly associated with pathways involved in blood-vessel morphogenesis, angiogenesis, neurogenesis, and epithelial mesenchymal transition (EMT) (light blue bar). Close examination of genes in each cluster suggested that known hepatic transcription factors (FOXA1), Wnt regulators (TCF7L2 and DKK1), and a hepatic stem cell marker (CD24) were dominantly up-regulated in EpCAM+ and CD133+ HCCs (Fig. 1F). By contrast, genes associated with blood-vessel morphogenesis (TIE1 and FLT1), EMT (TGFB1), and neurogenesis (NES) were activated dominantly in CD90+ HCCs and CD133+ HCCs.
CD90+ HCC Cells Share Features With Mesenchymal Vascular Endothelial Cells.
Because CD133+ HCCs were relatively rare and constituted only 13% (microarray cohort) to 20% (FACS cohort) of all HCC samples analyzed, we focused on the characterization of EpCAM and CD90. To clarify the cell identity of EpCAM+ or CD90+ cells in primary HCCs, we performed IHC analysis of 18 needle-biopsy specimens of premalignant dysplastic nodules (DNs), 102 surgically resected HCCs, and corresponding NT liver tissues. When examining the expression of EpCAM and CD90 in cirrhotic liver tissue by double-color IHC analysis, we found that EpCAM+ cells and CD90+ cells were distinctively located and not colocalized (Supporting Fig. 1A). Immunoreactivity (IR) to anti-CD90 antibodies (Abs) was detected in vascular endothelial cells (VECs), inflammatory cells, fibroblasts, and neurons, but not in hepatocytes or cholangiocytes, in the cirrhotic liver (Supporting Fig. 1B, panels a,b). IR to anti-EpCAM Abs was detected in hepatic progenitors adjacent to the periportal area and bile duct epithelial cells in liver cirrhosis (Supporting Fig. 1B, panels c,d).
IR to anti-EpCAM Abs was detected in 37 of 102 surgically resected HCCs (Fig. 2A, panel b), but not in 18 DNs (Fig. 2A, panel a). By contrast, no tumor epithelial cells (TECs) showing IR to anti-CD90 Abs were found in any of the 18 DNs or 102 HCCs examined (Fig. 2A, panels c,d). However, we identified CD90+ cells that were morphologically similar to VECs or fibroblasts within the tumor nodule in 37 of the 102 surgically resected HCC tissues (≥5% positive staining in a given area). IR to anti-CD90 Abs was also detected in hepatic mesenchymal tumors (Supporting Fig. 1C, panels a-c), indicating that CD90 is also a marker of liver stromal tumors.
Fig. 2.

Distinct EpCAM+ and CD90+ cell populations in HCC. (A) Representative images of EpCAM and CD90 staining in dysplastic nodule (panels a,c) and HCC (panels b,d) by IHC analysis (scale bar, 50 μm). EpCAM (panels a,b) and CD90 (panels c,d) immunostaining is depicted. (B) Upper panel: representative images of EpCAM (red) and CD90 (brown) double staining in HCC by IHC (scale bar, 50 μm). Lower panel: representative images of EpCAM (green) and CD90 (red) staining with 4’6-diamidino-phenylindole (DAPI) (blue) in HCC by IF (scale bar, 50 μm). (C) qPCR analysis of sorted EpCAM+ (red bar), CD90+ (orange bar), or EpCAM− CD90− (blue bar) derived from a representative primary HCC. Experiments were performed in triplicate, and data are shown as mean ± standard error of the mean.
Double-color IHC and immunofluorescence (IF) analysis confirmed the distinct expression of EpCAM and CD90 in HCC (Fig. 2B), consistent with the FACS data (Fig. 1A). Quantitative real-time polymerase chain reaction (qPCR) analysis of sorted EpCAM+, CD90+, and EpCAM− CD90− cells after CD45+ cell depletion indicated that the hepatic stem/progenitor markers, AFP and KRT19, were up-regulated in EpCAM+ cells (red bar), whereas the mesenchymal markers, KIT and FLT1, were up-regulated in CD90+ cells (orange bar), compared with EpCAM− CD90− cells (blue bar) (Fig. 2C). The hepatocyte marker, CYP3A4, was down-regulated in EpCAM+ cells and not detected in CD90+ cells, compared with EpCAM− CD90− cells. POU5F1 and BMI1 were equally up-regulated in both EpCAM+ and CD90+ cells, compared with EpCAM− CD90− cells.
EpCAM and CD90 were independently and distinctively expressed in different cellular lineages, so we evaluated the staining of EpCAM and CD90 separately and analyzed the clinicopathological characteristics of surgically resected HCC cases. HCCs were regarded marker positive if ≥5% positive staining was detected in a given area. The existence of EpCAM+ cells (≥5%) was characterized by poorly differentiated morphology and high serum AFP values with a tendency for portal vein invasion, whereas the existence of CD90+ cells (≥5%) was associated with poorly differentiated morphology and a tendency for large tumor size (Supporting Tables 2 and 3). Notably, the existence of CD90+ cells was associated with a high incidence of distant organ metastasis, including lung, bone, and adrenal gland, within 2 years after surgery, whereas EpCAM+ cell abundance appeared unrelated to distant organ metastasis.
We evaluated the characteristics of EpCAM+ or CD90+ cells in seven representative HCC cell lines. Morphologically, all EpCAM+ cell lines (HuH1, HuH7, and Hep3B) showed a polygonal, epithelial cell shape, whereas three of four CD90+ cell lines (HLE, HLF, and SK-Hep-1) showed a spindle cell shape (Fig. 3A). EpCAM+ cells were detected in 11.5%, 57.7%, and 99.6% of sorted HuH1, HuH7, and Hep3B cells, respectively. A small CD90+ cell population (0.66%) was observed in PLC/PRL/5, whereas 91.3%, 10.8%, and 59.0% of CD90+ cells were detected in HLE, HLF, and SK-Hep-1, respectively. Compared with primary HCCs, only EpCAM+ or CD90+ cells were detected in liver cancer cell lines under normal culture conditions (Fig. 3B), suggesting that these cell lines contain a relatively pure cell population most likely obtained by clonal selection through the establishment process.
Fig. 3.
Characteristics of HCC cell lines defined by EpCAM and CD90. (A) Representative photomicrographs of EpCAM+CD90− and EpCAM− CD90+ HCC cell lines. (B) Representative FACS data of EpCAM+CD90− and EpCAM− CD90+ HCC cell lines stained with fluorescein isothiocyanate (FITC)-EpCAM and APC-CD90 Abs. (C) Heat-map images of seven HCC cell lines based on 890 EpCAM/CD90-coregulated genes. Each cell in the matrix represents the expression level of a gene in an individual sample. Red and green cells depict high and low expression levels, respectively, as indicated by the scale bar. (D and E) Pathway analysis of EpCAM/CD90-coregulated genes. Canonical signaling pathways activated in cluster I (orange bar) or II (blue bar) with statistical significance (P < 0.01) are shown. (F) qPCR of representative differentially expressed genes identified by microarray analysis (C) in seven HCC cell lines.
A class-comparison analysis with univariate t tests and a global permutation test (×10,000) of microarray data yielded two main gene clusters up-regulated in EpCAM+ cell lines (HuH1, HuH7, and Hep3B) (cluster I, 524 genes) or in CD90+ cell lines (HLE, HLF, and SK-Hep-1) (cluster II, 366 genes) (Fig. 3C). PLC/PRL/5 showed intermediate gene-expression patterns between EpCAM+ and CD90+ cell lines using this gene set. Pathway analysis indicated that the genes enriched in cluster II were mainly associated with blood-vessel morpho- and angiogenesis (Fig. 3D). By contrast, the enriched genes in cluster I were significantly associated with known hepatocyte functions (P < 0.01) (Fig. 3E). In addition, we identified that the enriched genes in cluster II were significantly associated with neurogenesis, skeletal muscle development, and EMT.
We used qPCR to validate that known hepatic stem cell (HSC) and hepatocyte markers, such as AFP, EPCAM, ALB, and HNF4A genes, were up-regulated in EpCAM+ cell lines, but not detected in CD90+ cell lines (Fig. 3F). By contrast, genes associated with mesenchymal lineages and EMT, such as KIT, TWIST1, CD44, and THY1, were strongly up-regulated in CD90+ cell lines.
Unique Tumorigenicity and Metastasis Capacity of Distinct CSCs Defined by EpCAM and CD90.
We investigated the tumorigenic capacity of EpCAM+ or CD90+ cells by subcutaneously (SC) injecting 1 × 105 sorted cells of four HCC cell lines (HuH1, HuH7, HLE, and HLF) into nonobese diabetic, severe combined immunodeficient (NOD/ SCID) mice. We excluded Hep3B cells for the evaluation of tumorigenicity because almost 100% of cells were EpCAM positive. We further excluded SK-Hep-1 cells from the analysis because they potentially originated from endothelial cells.12 The highly tumorigenic capacities of EpCAM+ and CD90+ cells were reproduced in HuH1, HuH7, and HLF cell lines, compared with marker-negative cells (Fig. 4A). However, HLE cells did not produce SC tumors, even 12 months after transplantation, in NOD/SCID mice. EpCAM+ cells from HuH1 and HuH7 formed larger tumors more rapidly than CD90+ cells from HLF (Fig. 4B). IHC analyses indicated that EpCAM+ cells did not produce CD90+ cells and vice versa in these cell lines in vivo (Fig. 4C). CD90+ cells showed a high metastatic capacity, whereas EpCAM+ cells showed no metastasis to the lung when SC tumor volume reached approximately 2,000 (HuH1 and HuH7) or 700 mm3 (HLF) (Fig. 4D). The high metastatic capacity of PLC/PRL/5 cells, which contain a small population of CD90+ cells, was also confirmed after SC injection into NOD/SCID mice (data not shown). CD90+ cells could divide to generate both CD90+ and CD90 cells, and CD90+ cells showed a high capacity to invade and form spheroids with overexpression of TWIST1 and TWIST2, which are known to activate EMT programs in HLF cells (Supporting Fig. 2A–D).
Fig. 4.

Distinct tumorigenic/metastatic capacities of HCC cell lines defined by EpCAM and CD90. (A) Tumorigenicity of 1 × 105 cells sorted by anti-EpCAM (HuH1 and HuH7) or anti-CD90 (HLE and HLF) Abs. Data are generated from 8 mice/cell line. (B) Tumorigenic ability of EpCAM+ and CD90+ sorted cells in NOD/SCID mice. Aggressive tumor growth in the SC lesion was observed in EpCAM+ HuH1 or HuH7 cells, compared with CD90+ HLE or HLF cells. EpCAM+ (1 × 105) or CD90+ cells were injected. Tumor-volume curves are depicted as mean ± standard deviation of 4 mice/group. (C) Histological analysis of EpCAM+ or CD90+ cell-derived xenografts. Hematoxylin and eosin (H&E) staining of a SC tumor (upper panels) and IHC of the tumor with anti-EpCAM (middle panels) or anti-CD90 Abs (bottom panels) are shown (scale bar, 50 μm). (D) Metastasis was evaluated macroscopically and microscopically in the left and right lobes of the lung separately in each mouse (n = 4) (scale bar, 100 μm).
We next evaluated the tumorigenic/metastatic capacity of CD45− tumor cells using 12 fresh primary HCC specimens (P1-P12) that had been surgically resected (Table 2). We further evaluated the tumorigenicity of EpCAM/CD90 sorted cells obtained from xenografts derived from primary HCCs (Supporting Fig. 3A). Of these, we confirmed the tumorigenicity of cancer cells obtained from six primary HCCs after SC injection into NOD/SCID mice within 3 months after transplantation (Fig. 5A; Table 2; Supporting Fig. 3B). EpCAM+ cells derived from four HCCs (P4, P7, P13, and P14) showed highly tumorigenic capacities, compared with EpCAM− cells. CD90+ cells derived from two HCCs showed equal (P12) or more-tumorigenic capacities (P15), compared with CD90 cells. Tumorigenicity of EpCAM+ cells was observed in three hepatitis C virus (HCV)-related HCCs and an hepatitis B virus (HBV)-related HCC, whereas tumorigenicity of CD90+ cells was observed in two HBV-related HCCs (Tables 1 and 2).
Fig. 5.

Tumorigenic/metastatic capacities of EpCAM+ and CD90+ cells in primary HCC. (A) Representative NOD/SCID mice with SC tumors (white arrows) from EpCAM+ P4 or P7 cells (left and middle panels) and CD90+ or CD90− P12 cells (right panel). (B) FACS analysis of CD90 and EpCAM staining in primary HCCs and the corresponding secondary tumors developed in NOD/SCID mice. Unsorted cells (1 × 106 cells in P4 and P7 or 1 × 105 cells in P12) were SC injected to evaluate the frequency of each marker-positive cell in primary and secondary tumors. (C) IHC analysis of EpCAM and CD90 in primary HCCs P4, P7, and P12 (scale bar, 50 μm). (D) FACS analysis of VEGFR1 (Alexa488) and CD105 (APC) in primary HCC P12. (E) Hematoxylin and eosin staining of lung tissues in P4 and P12 (scale bar, 200 μm). (F) Frequency of lung metastasis in NOD/SCID mice SC transplanted using unsorted primary HCC cells.
Using unsorted cells, we compared the frequency of EpCAM+ and CD90+ cells in primary and xenograft tumors and found that EpCAM+ cells remained, but CD90+ cells disappeared, in secondary tumors derived from P4 or P7, whereas EpCAM+ cells disappeared, but CD90+ cells remained, in secondary tumors derived from P12 (Fig. 5B). Morphologically, tumorigenic EpCAM+ cells showed an epithelial cell shape, whereas CD90+ cells showed a mesenchymal VEC shape (Fig. 5C and Supporting Fig. 3C). FACS analysis indicated that P12 HCC cells showed abundant expression of vascular endothelial growth factor receptor (VEGFR) 1 and a vascular endothelial marker endoglin (CD105) (Fig. 5D). By contrast, P4 and P7 HCC cells did not express these vascular endothelial markers (data not shown). Lung metastasis was detected in NOD/SCID mice transplanted with P12 HCC cells, but not in mice transplanted with P4 and P7 HCC cells (Fig. 5E,F).
Taken together, these results suggest that the tumorigenic and metastatic capability of primary HCC may depend on the presence of distinct EpCAM+ or CD90+ CSCs. EpCAM+ cells were associated with a high tumorigenic capacity with hepatic epithelial stem cell features, whereas CD90+ cells were related to the metastatic propensity with VEC features.
Suppression of Lung Metastasis Mediated by CD90+ CSCs by Imatinib Mesylate.
We previously demonstrated that Wnt/β-catenin signaling inhibitors could successfully attenuate the tumorigenic capacity of EpCAM+ CSCs in HCC.8,10 To explore the potential molecular targets activated in CD90+ CSCs, we investigated the expression of the known VEC markers, CD105, VEGFR1 (encoded by FLT1), and c-Kit (encoded by KIT), in cell lines and showed that they were abundantly expressed in CD90+ cell lines, but not EpCAM+ cell lines (Fig. 6A). No expression of VEGFR2 was detected in this set of cell lines, suggesting that molecular reagents specifically targeting VEGFR2 may have no effects on CD90+ CSCs. CD44, a stem cell marker that functionally regulates redox status and is a potential target of CD90+ CSCs, was also abundantly expressed in CD90+ cell lines (Supporting Fig. 4A), consistent with previous data.5,13 No significant difference was detected in the expression of the hematopoietic marker, CD34, or ABCG2 between EpCAM+ and CD90+ cell lines (Supporting Fig. 4A).
Fig. 6.

Suppression of lung metastasis mediated by CD90+ CSCs by imatinib mesylate. (A) FACS analysis of seven HCC cell lines stained by APC-CD105, Alexa 488/VEGFR1, APC/VEGFR2, and Alexa 488/c-Kit Abs or isotype control. (B) Tumorigenicity of 5 × 105 HuH7 cells and 2.5 × 105 HuH7 cells plus 2.5 × 105 HLF cells treated with imatinib mesylate or control phosphate-buffered saline (PBS) (200 μL/mouse) orally ingested three times per week (100 mg/kg) for 2 weeks. Data are generated from 5 mice per condition. (C) IHC analysis of EpCAM in lung metastasis detected in NOD/SCID mice SC injected with 2.5 × 105 HuH7 cells and 2.5 × 105 HLF cells. Metastasis was evaluated macro- and microscopically in the left and right lobes of the lung separately in each mouse (n = 5) (scale bar, 100 μm). (D) Cell motility of HuH7 cells cocultured with HuH7, HLF, or HLF cells with imatinib mesylate (10 μM) was monitored in a real-time manner by time-lapse image analysis. HuH7 and HLF cells were labeled with the lipophilic fluorescence tracer, Dil (indicated as red) or DiD (indicated as blue), and incubated in a μ-Slide eight-well chamber overnight. Silicone inserts were detached and the culture media replaced with Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, including 0.1% dimethyl sulfoxide (DMSO) (control) or 10 μM of imatinib mesylate dissolved in DMSO (final concentration 0.1%). Immediately after the medium change, cells were cultured at 37°C in 5% CO2 and time-lapse images were captured for 72 hours. (E) qPCR analysis of TGFB1 in HuH7 (white bar), HLF (gray bar), and HLF cells pretreated with imatinib mesylate for 24 hours. (F) Smad3 and its phosphorylation evaluated by western blotting. HuH7 cells and HLF cells were harvested in cell culture inserts and treated with DMSO (0.1%) or imatinib mesylate (10 μM) for 24 hours. Cell culture inserts were washed with PBS, cocultured with HuH7 cells for 8 hours, and then removed. HuH7 cells were lysed using radioimmunoprecipitation assay buffer for western blotting. (A) HuH7 cells cocultured with HuH7 cells. (B) HuH7 cells cocultured with HLF cells. (C) HuH7 cells cocultured with HLF cells pretreated with imatinib mesylate.
Among these molecular targets, we focused on the characterization of c-Kit because the c-Kit tyrosine kinase inhibitor, imatinib mesylate, is readily available, is widely used for the treatment of gastrointestinal stromal tumor with activation of c-Kit, and may have potential antitumor activity against a subset of HCC.14 We explored the effect of imatinib mesylate on HCC cell lines and found that treatment with 10 μM reduced cell proliferation and spheroid formation in CD90+ cell lines, but had no effect on EpCAM+ cell lines (Supporting Fig. S4B,C).
We further explored the effect of imatinib mesylate in vivo. Because EpCAM+ and CD90+ cells reside in the primary HCC, but not in established cell lines, we SC injected HuH7 and HLF cell lines to generate tumors organized by EpCAM+ and CD90+ CSCs. Interestingly, when HLF cells were coinjected with HuH7 cells, EpCAM+ cells could metastasize to the lung, whereas SC primary tumors showed no difference in size (Fig. 6B,C). Furthermore, although imatinib mesylate treatment had little effect on the size of primary SC tumors, it significantly suppressed lung metastasis in primary tumors (Fig. 6C). These data suggest that CD90+ cells are not only metastatic to the distant organ, but also help the metastasis of CD90− cells, including EpCAM+ cells, which originally have no distant metastatic capacity. Our data further suggest that imatinib mesylate can inhibit distant organ metastasis by suppressing CD90+ metastatic CSCs, albeit with little effect on EpCAM+ tumorigenic epithelial stem-like CSCs.
To explore the potential mechanism of how CD90+ cells dictate the metastasis of EpCAM+ cells, we utilized coculture systems and time-lapse image analysis. Wound-healing analysis clearly indicated that motility of HuH7 cells was enhanced when HLF cells were cocultured, and this effect was abolished by imatinib mesylate treatment (Fig. 6D; see Supporting Videos 1–3). HLF cells abundantly expressed TGFB1, compared with HuH7 cells, and its expression was dramatically suppressed by imatinib mesylate treatment (Fig. 6E). Mothers against decapentaplegic homolog 3 (Smad3) phosphorylation was augmented in HuH7 cells when cocultured with HLF cells, and this effect was attenuated when cocultured with HLF cells pretreated with imatinib mesylate.
Taken together, our data suggest that liver CSCs are not a single entity. Liver CSCs defined by different markers show unique features of tumorigenicity/metastasis with phenotypes closely associated with committed liver lineages. These distinct CSCs may collaborate to enhance tumorigenicity and metastasis of HCCs.
Discussion
The current investigation demonstrates that CSC marker expression status may be a key determinant of cancer phenotypes, in terms of metastatic propensity and chemosensitivity, to certain molecularly targeted therapies. EpCAM appears to be an epithelial tumorigenic CSC marker, whereas CD90 seems to be a mesenchymal metastatic CSC marker associated with expression of c-Kit and chemosensitivity to imatinib mesylate. Imatinib mesylate may be effective in inhibiting metastasis, but has little effect on primary EpCAM+ HCC cell growth.
We investigated the frequency of three CSC markers (EpCAM, CD90, and CD133) in 15 primary HCCs with a confirmed cell viability of ≥70% and found that three HCCs contained CD133+ cells, seven HCCs contained EpCAM+ cells, and all HCCs contained CD90+ cells. Among them, we confirmed the perpetuation of CD133+ cells derived from three HCCs (P7, P12, and P14; data not shown), EpCAM+ cells derived from four HCCs (P4, P7, P13, and P14), and CD90+ cells derived from two HCCs (P12 and P15). Recent studies showed that at least 8 of 21 HCCs (38%)4 and 13 of 13 HCCs (100%)5 contained tumorigenic CD133+ or CD90+ CSCs, respectively. Recent IHC and tissue microarray studies also demonstrated that CD133+ and CD90+ cells were detected in 24.8% (≥1% of tumor cells) and 32.2% (≥5% of tumor cells) of HCC cases examined, respectively.15,16
One possible explanation of the comparatively low frequency of CD133+ liver CSCs identified in our study is that we used the monoclonal Ab CD133/2, whereas Ma et al. used CD133/1. Another possible explanation is the difference of etiology related to hepatocarcinogenesis. We examined tumorigenicity using 15 HCCs (five HBV related, four HCV related, three non-B, non-C hepatitis [NBNC] related, and three alcohol related) and identified that tumorigenic CSCs were only obtained from HBV- or HCV-related cases. Previous liver CSC studies were performed using HBV-related HCCs,4,5 and a recent study showed that HBV X may play a role in generating EpCAM+ CSCs.17 The role of hepatitis virus infection on the generation of CSCs is still unclear and should be clarified in future studies.
We were unable to confirm the tumorigenicity of CD90+ cells in 13 of 15 HCCs, but we observed abundant CD90+ cells in more-advanced HCCs by IHC (data not shown). Tumorigenic CD90+ cells may emerge at a later stage of hepatocarcinogenesis, and the majority of CD90+ cells in early HCCs may be cancer-associated VECs without tumorigenic capacity. Furthermore, we identified tumorigenic CD90+ cells only from HBV-related HCCs, and a recent study suggested that expression of CD90 was associated with HBV infection.16 We could not detect the small population of CD90+ HuH7 and Hep3B cells reported on by Yang et al. However, because we identified a small population of CD90+ HuH7 cells after treatment with 5-FU (manuscript in preparation), it is conceivable that different cellular stress statuses may explain the observed differences between our findings and those of Yang et al.
The majority of CSC markers discovered thus far are almost identical to those found in healthy tissue stem cells or embryonic stem cells. However, with regard to the liver, the characteristics of healthy hepatic stem/progenitor cells isolated using different stem cell markers are currently under investigation. A recent article examined the characteristics of EpCAM+ and CD90+ oval cells isolated from 2-acetylaminofluorene/ partial hepatectomy or D-galactosamine-treated rats.18 Interestingly, EpCAM+ and CD90+ oval cells represent two distinct populations: The former expresses classical oval cell markers, such as AFP, OV-1, and cytokeratin-19 (CK-19), whereas the latter expresses desmin and alpha smooth muscle actin, but not AFP, OV-1, or CK-19, which indicates that CD90+ populations are more likely to be mesenchymal cells. Another study has demonstrated that mesenchymal cells can interact with HSCs to regulate cell-fate decision.19 We found that EpCAM+ and CD90+ cells isolated from liver cancer are distinct in terms of gene- and protein-expression patterns in both primary liver cancers and cell lines. Furthermore, these distinct CSCs can interact to regulate the tumorigenicity and metastasis of HCC. Molecular characteristics of EpCAM+/CD90+ CSCs may potentially reflect the cellular context of healthy stem or progenitor cells.
Although our study strongly indicates that abundant CD90+ cells in a tumor is a risk for distant metastasis in liver cancer, the cell identity and role of CD90+ cells remains elusive. As our IHC, FACS, and xenotransplantation assays revealed, some CD90+ cells in liver cancer may be cancer-associated VECs or fibroblasts that cannot perpetuate in the xenograft. Recent findings have suggested the importance of stromal cells in tumorigenesis and cancer metastasis,20−22 so it is possible that these cells may help TECs invade and intravasate into blood vessels, thus playing crucial roles in metastasis.
Another possibility is that CD90+ cells are cancer cells with features of fibroblasts (having undergone EMT) or VECs (having undergone vasculogenic mimicry; VM) that can invade, intravasate, and metastasize cells to distant organs. Recently, two groups reported that a subset of tumor VECs originate from glioblastoma CSCs.23,24 We successfully confirmed the tumorigenicity and metastatic capacity of CD90+ cells that were morphologically identical to VECs from primary HCCs that could perpetuate in the xenograft. However, a recent study demonstrated that CD90+ HCC cells express glypican-3, a marker detected in hepatic epithelial cells.25 Further studies are warranted to clarify the nature and role of CD90+ HCC cells.
In our study, CD90+ cells expressed the endothelial marker, c-Kit, CD105, and VEGFR1, and a mesenchymal VEC morphology and high metastatic capacity were confirmed in both primary liver cancer and cell lines. We further confirmed that CD90+ liver cancer cells showed chemosensitivity to imatinib mesylate, suggesting that cancer cells committed to mesenchymal endothelial lineages could be eradicated by the compound. Although imatinib mesylate treatment had little effect on the size of primary tumors originated from both EpCAM+ and CD90+ CSCs, it significantly suppressed lung metastasis in vivo. These data are consistent with a recent phase II study demonstrating the tolerable toxicity, but limited efficacy, of imatinib mesylate alone for unresectable HCC patients. Eligibility of imatinib mesylate for advanced HCC patients may be restricted to the HCC subtypes organized by CD90+ CSCs with a highly metastatic capacity and VEC features. Therefore, a combination of compounds targeting EpCAM+ tumorigenic CSCs as well as CD90+ metastatic CSCs may be required for the eradication of HCC and should be tested in the future.
Supplementary Material
Acknowledgments:
The authors thank Ms. Nami Nishiyama and Ms. Mikiko Nakamura for their excellent technical assistance.
This study was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (23590967), a grant from the Japanese Society of Gastroenterology, a grant from the Ministry of Health, Labor, and Welfare, and a grant from the National Cancer Center Research and Development Fund (23-B-5) of Japan. X.W.W. is supported by the Intramural Research Program of the Center for Cancer Research, U.S. National Cancer Institute.
Abbreviations:
- 5-FU
fluorouracil
- Abs
antibodies
- AFP
alpha-fetoprotein
- CK-19
cytokeratin-19
- CSC
cancer stem cell
- DNs
dysplastic nodules
- EMT
epithelial mesenchymal transition
- EpCAM
epithelial cell adhesion molecule
- FACS
fluorescent-activated cell sorting
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HSCs
hepatic stem cells
- IF
immunofluorescence
- IHC
immunohistochemistry
- IR
immunoreactivity
- MDS
multidimensional scaling
- NBNC
non-B, non-C hepatitis
- NOD/SCID
nonobese diabetic, severe combined immunodeficient
- NT
nontumor
- OV-1
ovalbumin 1
- qPCR
quantitative real-time polymerase chain reaction
- SC
subcutaneous
- Smad3
Mothers against decapentaplegic homolog 3
- TECs
tumor epithelial cells
- TGF-β
transforming growth factor beta
- T/N
tumor/nontumor
- VECs
vascular endothelial cells
- VM
vasculogenic mimicry
- VEGFR
vascular endothelial growth factor receptor
References
- 1.Tsai WL, Chung RT. Viral hepatocarcinogenesis. Oncogene 2010;29: 2309–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. HEPATOLOGY 2006;44:240–251. [DOI] [PubMed] [Google Scholar]
- 3.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest 2010;120:3326–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, et al. miR130b promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 2010;7:694–707. [DOI] [PubMed] [Google Scholar]
- 5.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008;13:153–166. [DOI] [PubMed] [Google Scholar]
- 6.Zen Y, Fujii T, Yoshikawa S, Takamura H, Tani T, Ohta T, Nakanuma Y. Histological and culture studies with respect to ABCG2 expression support the existence of a cancer cell hierarchy in human hepatocellular carcinoma. Am J Pathol 2007;170:1750–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 2011;9: 50–63. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res 2007;67:10831–10839. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. .EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res 2008;68:1451–1461. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009;136: 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007;132:2542–2556. [DOI] [PubMed] [Google Scholar]
- 12.Heffelfinger SC, Hawkins HH, Barrish J, Taylor L, Darlington GJ. SK HEP-1: a human cell line of endothelial origin. In Vitro Cell Dev Biol 1992;28A:136–142. [DOI] [PubMed] [Google Scholar]
- 13.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 2011;19:387–400. [DOI] [PubMed] [Google Scholar]
- 14.Ramadori G, Fuzesi L, Grabbe E, Pieler T, Armbrust T. Successful treatment of hepatocellular carcinoma with the tyrosine kinase inhibitor imatinib in a patient with liver cirrhosis. Anticancer Drugs 2004;15: 405–409. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. HEPATOLOGY 2011;54: 1707–1717. [DOI] [PubMed] [Google Scholar]
- 16.Lu JW, Chang JG, Yeh KT, Chen RM, Tsai JJ, Hu RM. Overexpression of Thy1/CD90 in human hepatocellular carcinoma is associated with HBV infection and poor prognosis. Acta Histochem 2011;113: 833–838. [DOI] [PubMed] [Google Scholar]
- 17.Arzumanyan A, Friedman T, Ng IO, Clayton MM, Lian Z, Feitelson MA. Does the hepatitis B antigen HBx promote the appearance of liver cancer stem cells? Cancer Res 2011;71:3701–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. HEPATOLOGY 2008;47:636–647. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Yao HL, Cui CB, Wauthier E, Barbier C, Costello MJ, et al. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. HEPATOLOGY 2010;52:1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dome B, Timar J, Ladanyi A, Paku S, Renyi-Vamos F, Klepetko W, et al. Circulating endothelial cells, bone marrow-derived endothelial progenitor cells and proangiogenic hematopoietic cells in cancer: from biology to therapy. Crit Rev Oncol Hematol 2009;69:108–124. [DOI] [PubMed] [Google Scholar]
- 21.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557–563. [DOI] [PubMed] [Google Scholar]
- 22.Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 2008;68:4331–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010;468:824–828. [DOI] [PubMed] [Google Scholar]
- 24.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010;468:829–833. [DOI] [PubMed] [Google Scholar]
- 25.Ho DW, Yang ZF, Yi K, Lam CT, Ng MN, Yu WC, et al. Gene expression profiling of liver cancer stem cells by RNA-sequencing. PLoS One 2012;7:e37159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.