Abstract
Purpose
Lumbar posterior ring apophysis fracture (PRAF) is an uncommon disorder frequently accompanied by lumbar disc herniation (LDH). Over the years, there have constantly been published studies concerning this disorder. Due to its rarity, there is lack of an agreed treatment strategy, and lots of different opinions exist, including the choice of decompressive modalities, whether removal of apophyseal fragments or/and disc material, and the necessity of additional spinal fusion. The purpose of this review is to provide a collective opinion on the treatment of PRAF with LDH.
Methods
A MEDLINE search in the English language literature was performed from 1980 to 2012. To be included in the study, it was strictly necessary for each clinical article to provide information about the description of apophyseal fracture such as location, treatment methods and clinical outcome. The studies were mainly analyzed for general features, the related classifications and treatments.
Results
The literature searching yielded 19 articles reporting 366 patients experiencing 380 sites of fractures. All of them were case reports or case series. The classification systems of PRAF were various based on the morphology, mobilization, size or localization, and relationship between disc and fragment. The most used surgical options were posterior discectomy simultaneous excision of apophyseal fragments without spine fusion. Surgical treatment for PRAF with LDH had equally excellent clinical outcome compared with LDH alone.
Conclusions
The diverse features of apophyseal fracture lead to various modalities of classifications and operation options. Prior to operation, the surgeons should carefully make a plan to consider decompressive scope, removal of apophyseal fragment or/and disc and fusion or not. Because of methodological shortcomings in publications, it is not possible to definitively conclude what treatment modality is the best for the treatment of PRAF. More high-quality clinical studies are needed to draw more confirmable conclusions.
Keywords: Ring apophysis fracture, Lumbar disc herniation, Low back pain, Adolescent, Systematic review
Introduction
Lumbar posterior ring apophysis fracture (PRAF) is an uncommon disease typically found in adolescents and young adults, especially in young active athletes. It has been increasingly recognized as an important contribution of low back pain and neural symptom [1]. It is characterized by separation of osseous fragment at the posterior cephalad or caudad edge of the adjacent vertebral body, where fusion between ring apophysis and the adjacent vertebral body does not complete fully prior to the age of approximately 18–25 years [2]. A variety of terms are also referred to this entity, including “avulsed vertebral rim apophysis” [3], “limbus vertebral fracture” [4], “lumbar posterior marginal node” [5], “slipped vertebral epiphysis” [6], and so on. Just as the diversity of its terminology, different classifications have been developed on the basis of its different features. Over the years, many authors have emphasized the need for operative treatment when conservative therapy is ineffective. Due to its rarity and diversity of classification modality, there is lack of an agreed treatment strategy, and lots of different opinions exist, including the choice of decompressive modalities, whether removal of apophyseal fragments or/and disc, and the necessity of additional spinal fusion. Yet to the present date, it has not been fully reviewed as to the treatments available for PRAF with LDH. The primary objectives of this paper are to provide a collective opinion on the treatment of this entity.
Materials and methods
Searching strategy
A literature search was performed in electronic database PUBMED (from 1980 through 2012) using the keywords “ring apophysis fracture”, “limbus vertebral fracture”, “apophyseal separation”, “lumbar disc herniation”, “low back pain” with the limitations of English language and human subjects. All relevant articles were initially selected by the title and abstract. The articles in the reference lists were manually read to find the relevant additional supporting information and also included in this review. Two dependent reviewers performed this search.
All articles were in detail scrutinized for inclusion. The selected articles fulfilled the following inclusion criteria: (1) clinical studies that focused on the treatment of PRAF with LDH, (2) clinical articles including the description of ring apophysis fracture, such as location or classification or associated treatment. The case reports that consisted of few cases were also included due to its rare incidence.
Exclusion criteria were: (1) articles with duplicate information, (2) literatures not pertaining to surgical treatment of this disease, such as reviews, radiological and experimental studies.
Data extraction
After full text of each paper was found, the data reading and extraction of each article were done by the first author and checked for accuracy by the second author to minimize selection bias and errors. The discrepant data extracted from the same article were compared and discussed, and were resolved by the third author. All the extracted information was imported into an electronic spreadsheet-Microsoft Excel. A meta-analysis was not conducted because there was no study specialized in randomized controlled treatment of PRAF. There was too much clinical heterogeneity among these papers, therefore, we chose to provide a qualitative descriptive analysis.
Searching results
The search generated 60 hits. After screening titles and abstracts, 36 studies were excluded for not being clinical reports. After the full text of articles according to the inclusion criteria, another five studies were excluded. Three studies had data mixed with LDH treatment [7–9]. Overlap of patient material was found in three other studies by Epstein et al. [4, 10, 11]. Only the data from the last publication were used for the analysis [10]. Finally, 19 articles met our inclusion criteria and their demographics are summarized in Table 1. The information analyzed in each article included number of patients (with or without operation), sex, mean age (age range), surgical treatment with or without removal of bone fragment, follow-up, outcome, assessment standard and complication. These studies included a total of 366 patients experiencing 380 sites of fractures. All of them were retrospective case series or case reports.
Table 1.
Summary of clinical studies of included studies
| Study | Years | No of patients (OP/NOP) | M:F | Mean age (ranges, years)a | Surgical treatment | No of RBF | MFU (range, years)a | Outcome | Judgement standard | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| Akhaddar et al. [12] | 2011 | 87 (87/0) | 75:12 | 36.2 (18–54) | Hemi- or full laminectomy+discectomy | 32 | 5.6 (1.6–9.7) | Excellent:72.41 %; good = 25.29 %; fair = 2.3 % | Symptomatic relief and physical activities | Dural damage = 1; discitis = 1; disc recurrence = 4 |
| Farrokhi et al. [6] | 2009 | 2 (2/0) | 2:0 | 13.5 (13–14) | Extensive bilateral laminectomy+discectomy | 2 | NA | The symptoms completely relieved | Symptomatic relief | NA |
| Chang et al. [13] | 2008 | 27 (15/12) | 23:4 | 14.2 (8–18.3) | Laminectomy+discectomy | NA | 6.6 | NA | DeOrio’s classification [14] | NA |
| Matsumoto et al. [15] | 2007 | 18 (18/0) | 15:3 | 28.9 (11–69) | MED+discectomy | 18 | 1.76 (1–5) | Recovery rate was (86.3 + 11.4) % | JOA score | Disc recurrence = 1 |
| Shirado et al. [16] | 2005 | 32 (32/0) | 22:10 | 25.4 (16–39) | Laminectomy+discectomy | 11 | 4.7 (2.3–8.1) | Excellent:75 %; good = 22 %; fair = 3 % | Criteria described by Grevitt[17] | No complication |
| Molina et al. [18] | 2004 | 1 (1/0) | 1:0 | 17 | Laminectomy | 1 | 0.7 | Complete recovery of motor deficiency | Symptomatic relief | No complication |
| Asazuma et al. [19] | 2003 | 5 (5/0) | 3:2 | 17.4 (14–28) | PLIF | 5 | 4.3 (4–5) | Mean recovery rate was 89.4 % | JOA score | No complication |
| Mendez et al. [20] | 2002 | 23 (23/0) | 17:6 | 35 (22–58) | Hemi- or full laminectomy+discectomy | 23 | NA | 75 % excellent, 16 % good | NA | No complication |
| Martínez-Lage et al. [21] | 1998 | 1 (1/0) | 1:0 | 15 | Laminotomy+discectomy | 1 | 9 | Excellent | Symptomatic relief and physical activities | No complication |
| Talha et al. [22] | 1997 | 1 (1/0) | 1:0 | 18 | Wide laminectomy+discectomy+osteosynthesis | 1 | 0.75 | Complete relief of pain | Symptomatic relief | No complication |
| Baba et al. [23] | 1996 | 29 (29/0) | 19:10 | 16.5 (9–24) | Uni- or bilateral laminotomy+discectomy | 29 | 5.8 (1.3-9.5) | Grade A in 21; grade B in 8 | Symptomatic relief and physical activities | Temporary painful paraesthesia = 2 |
| Scarfo et al. [24] | 1996 | 26(26/0) | 17:9 | 34.3 (20–53) | Uni- or bilateral laminotomy+discectomy | 26 | 1 | Excellent | Symptomatic relief | No complication |
| Epstein et al. [10] | 1992 | 59 (56/3) | 38:21 | 33 | Hemi- or full laminectomy or laminotomy+discectomy | 33 | 1.7 | 42 excellent, 9 good, 4 fair, 1 poor | Symptomatic relief | No complication |
| Albeck et al. [1] | 1991 | 3 (3/0) | 1:2 | 17 (11–26) | Hemi-laminectomy+discectomy | 3 | 0.25 | No radiating pain | Symptomatic relief | NA |
| Savini et al. [25] | 1991 | 9 (9/0) | 7:2 | 27 (15–40) | Hemi- or full laminectomy or laminotomy+discectomy; Anterior approach. | 9 | 2–5.3 | Excellent | Symptomatic relief | Incomplete cauda syndrome = 1 |
| Takata et al. [26] | 1988 | 29 (25/4) | 19:10 | NA (11–58) | Posterior decompression+discectomy; anterior approach; chemonucleolysis. | 24 | 3 | All had good results | NA | NA |
| Fujita et al. [27] | 1986 | 1 (1/0) | 1:0 | 14 | NA | 1 | 0.5 | Excellent | Symptomatic relief | No complication |
| Laredo et al. [28] | 1986 | 12 (6/6) | 8:4 | NA (13–28) | Posterior decompression+discectomy | 4 | NA | Excellent | Symptomatic relief | NA |
| Techakapuch et al. [29] | 1981 | 1 (1/0) | 1:0 | 17 | Full laminectomy+discectomy | 1 | 5 | Pain resolved | Symptomatic relief | No complication |
OP operation, NOP nonoperation, NA not available, RBF removal of bone fragment, MFU mean follow-up, MED microendoscopic discectomy, PLIF posterior lumbar interbody fusion, JOA Japanese orthopaedic association
aThe values are given as the means, with the ranges in the parenthesis
General features
Age
Since the calcification and fusion process between ring apophysis and vertebral endplate may remain incomplete until the ages of 18–25, some studies thought the avulsion of this lesion was predominantly observed in the adolescents and young adults [4, 23, 26]. Takata et al. [26] found that about half of the patients with PRAF were in their second decade of life, and most of the others were in their 20s. This search of the literature indicated that this lesion was also occurred in patients in their 30s, 40s, and rarely 50s or older. Shirado et al. [16] reported 32 patients, 25 % were younger than 20 years, whereas 43.8 % were in the third decade, and 31.2 % in the fourth decade. Scarfo et al. [24] reported 26 cases, and the age of the patients at the onset of symptoms ranged from 20 to 53 years (mean, 34.3 years). The age of patients in the included articles ranged from 8 to 69 years. The mean age of patients ranged from 13.5 to 36.2 years.
Since the separation of ring apophysis initially occurs in growing patients due to the biomechanical week junction of the cartilage plate and the endplate, why are there detached bony fragments found in patients after skeletal maturity? There are two different explanations. Shirado et al. [16] and Epstein et al. [4] supported the view that the apophyseal fracture originally occurred in adult patients. Because they thought ring apophysis remained susceptible to fragmentation, and this detachment might be chronic in the more elderly patients. On the contrary, Savini et al. [25] believed that this separation in adults might be the result from the undetected or misdiagnosed fracture that occurred in the past when the patients were younger. The fusion of the cartilaginous rim delayed or had not happened.
Though there is no uniform agreement, these evidences can reasonably interpret phenomenon of occurrence of this disease in elder patients. These different findings suggested different pathogenesis of PRAF, which are discussed below.
Prevalence
The incidence of PRAF varies greatly at different ages. It was reported that patients with PRAF constitute 5.35–8.2 % among all ages of patients with LDH [15, 16, 30]. In children and adolescents, the frequency was reported variantly ranging from 5.8 to 28 % [13, 21]. The incidence was comparatively lower in the adult population. Akhaddar et al. [12] reported that 5.35 % of patients diagnosed with PRAF underwent surgery in a series of 1,625 consecutive LDH. 26 cases (11 %) were diagnosed in 237 adult LDH patients reported by Scarfo et al. [24]. These statistical data concerned the incidence of PRAF among the patients with LDH, however, the exact frequency in all population is still unknown. The true frequency of PRAF is difficult to estimate and easily underestimated, because the lesion cannot be obviously detected on plain radiograph and magnetic resonance imaging (MRI) [10, 31]. Other associated factors include many surgeons’ unfamiliarity with this entity or confusing it with ossification of posterior longitudinal ligament, intervertebral disc calcification, and posterior degenerative ridge osteophytes [1, 12].
Sex
The fractures are most frequent in males, and the male (271):female (95) ratio is 2.85:1, which may be explained by the greater liability to strenuous activity [1].
Affected location
PRAF occurs in all posterior rims of lumbar vertebral segments, including posterior superior or inferior endplates. Eighteen studies described the distribution of intervertebral disc levels of fracture, including 333 patients involving 342 fractures. The affected intervertebral disc levels included T12–L1 in 3 patients, L1–2 in 2, L2–3 in 3, L3–4 in 19, L4–5 in 146, and L5–S1 in 169. Therefore, the most susceptible intervertebral disc levels are L4–5 (42.7 %) and L5–S1 (49.4 %). The existence of upper intervertebral disc levels only constituted 7.9 % of all cases.
Fourteen papers mentioned in detail the posterior superior or inferior endplates where apophyseal fractures occurred. 211 patients presented 219 sites of fractures. The locations were T12 lower endplate in 2 cases, L1 lower endplate in 1, L2 lower endplate in 2, L3 lower endplate in 3, L4 upper endplate in 5, L4 lower endplate in 15, L5 upper endplate in 70, L5 lower endplate in 21 and S1 endplate in 90. Besides, ten cases of fractures extended across entire vertebral body, including one in L4 and nine in L5 [10]. Therefore, PRAF occurred in all lumbar vertebral segments and the most affected sites were posterior cephalad endplate of vertebra of L5 and S1. In the upper lumbar, caudad edge of vertebral body seemed susceptibly injured.
Multiple segments involving PRAF are rare, and 12 patients in five studies had two levels [10, 12, 24–26]. There were no data concerning isolated PRAF without LDH.
The most susceptible affected sites and disc levels of apophyseal fracture are identical with that of LDH. This consistency may somewhat indicate the pathogenesis of PRAF and the close relationship between these two diseases.
Pathogenesis
The mechanisms of detachment of posterior ring apophysis with herniated disc remain controversial. Several hypotheses have been introduced. Some authors thought separation of the ring apophysis was caused by trauma, because it commonly occurred in the active athletes (mostly sport-related or self-reported injury, such as weight lifting, gymnastics) [4, 21, 22, 25, 32]. Epstein et al. [4] showed that trauma played a significant etiologic role in up to one-half of the patients in their study. Mendez et al. [20] found 60 % of traumatic antecedents ranging from simple falls to strenuous activities. However, many patients had not recalled a history of recent trauma episode or just complained of the symptoms many years previously. The radiological appearance and histologic finding of this fracture were not consistent with a recent avulsion [26, 28].
Therefore, the second hypothetic mechanism of tension/shear stresses was elicited. Faizan et al. [33] used three-dimensional finite element pediatric lumbar models to investigate the effects of ossification of the ring on lumbar spine biomechanics. They found that increased stresses under repetitive extension might damage and weaken the ring apophysis in early stages of bone formation, and finally led to avulsion fracture with flexion. Sairyo et al. [34] also verified that apophyseal fracture was a fatigue phenomena that was caused by the higher compression stresses along with tension stresses in flexion. On the contrary, Takata et al. [26] postulated that the location of the fracture was more dependent on a locus of structural weakness than a position of flexion or extension.
Some authors believed that the common element in the pathogenesis of this disorder was probably degeneration of the intervertebral disc and vertebral cartilage [12, 24]. The degenerative disc acting as the triggering point facilitated the detachment under both axial and anteroposterior forces. This degenerative process may explain the coincident intervertebral disc levels of apophyseal fracture and disc herniation that mostly susceptibly affected, and the frequent association of PRAF with LDH in adults.
Given the evidence discussed above, multiple factors may affect the causes of PRAF. However, these hypotheses were just based on their own clinical observations or single factor test, more strict and reliable experiments combining degenerative and stress models should be performed. Besides, whether degenerated disc produces avulsion fracture or the latter resulted in annular disruption and disc herniation should also be verified.
Diagnosis
Clinical diagnosis
The most common clinical presentations of lumbar PRAF are generally similar to those observed in patients with LDH alone, including intractable low back pain and unilateral or bilateral radiculopathy [1, 10, 12, 13, 16, 28]. If spinal stenosis exists, intermittent claudication is complained [10, 23, 35]. The presenting symptoms and physical examination findings include paravertebral muscle spasm and tenderness, the presence of motor deficits or/and sensory disturbances, and the loss of a deep tendon reflex. Cauda equina dysfunction was not common [12, 16]. The straight-leg raising was limited to 60° or even less [16]. However, the symptoms of patients with PRAF are more severe than those with LDH alone [13]. It is may be due to the osseous compression.
Radiological diagnosis
Conventional radiography of the PRAF is often difficult to interpret, especially when the L5 or S1 vertebra is involved [1, 26]. The cartilaginous and/or small size of the fragment is not readily detectable [10]. The lateral lumbar film shows that an oblique bone defect is present at the posterior corner of the affected vertebral body and a limbus fragment displaced into spinal canal [4, 12, 26, 28] (Fig. 1). According to these previous reports, a range of 16–69 % of PRAF could be diagnosed by plain radiographs alone [4, 26, 36]. Diagnostic accuracy rate may be affected by the age of the patient, the affected level, the size and shape of the fragment.
Fig. 1.
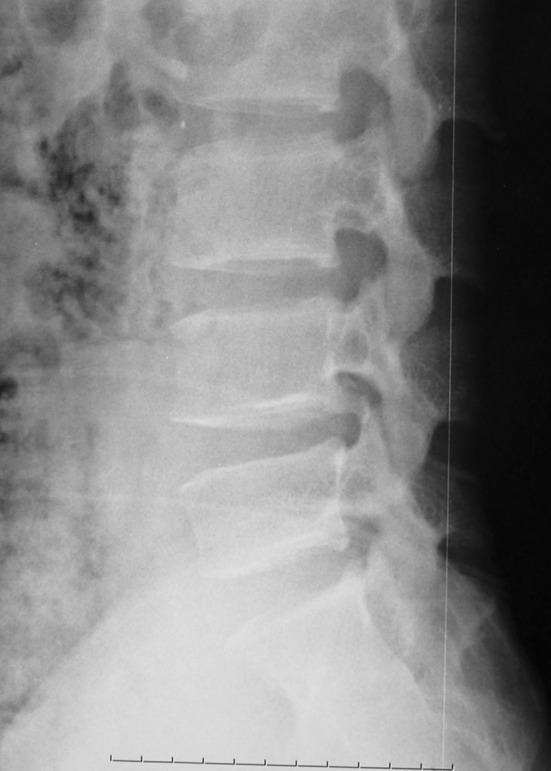
Plain radiograph shows a bony fragment displaced into spinal canal at the lower endplate of L4 vertebra
Computed tomography (CT) scan has the best performance for the demonstration of the size, shape and location of fracture [1, 20, 35] (Fig. 2). Almost all cases in these articles were eventually diagnosed according to CT findings. CT scan also helped differentiate the calcified or non-calcified fractures from disc herniation [10]. On the axial and sagittal reformatted images, CT scan offers superior visualization of the relationship between disc herniation and bony fragment [35, 36].
Fig. 2.
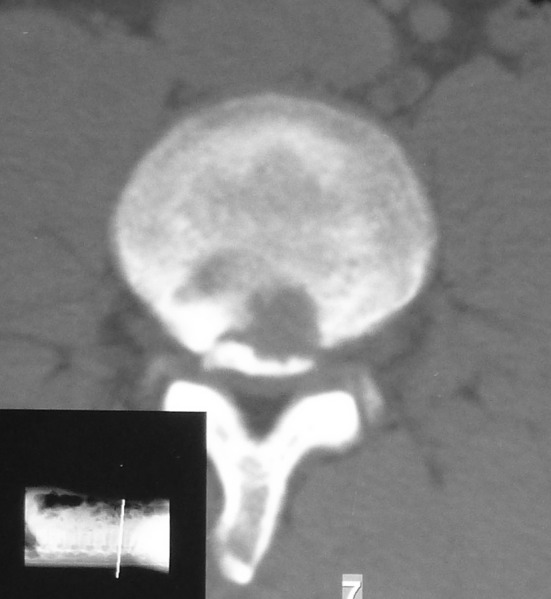
CT scan clearly demonstrates posterior detached bony fragment at the border of the posterior endplate of L4 and a round defect in the bone adjoining the fracture site
MRI is a less accurate method to visualize PRAF, because it is difficult to distinguish the bone fragment from the low signal intensity of the disc or the posterior longitudinal ligament [1, 12, 31, 37] (Fig. 3a, b). Fragments are more conspicuous on proton density or gradient echo sequences than T1 or T2 weighted images, unless a larger fracture containing marrow is present. Compared with the higher sensitivity of CT scanning, MRI only identifies 22 % of fractures [10].
Fig. 3.
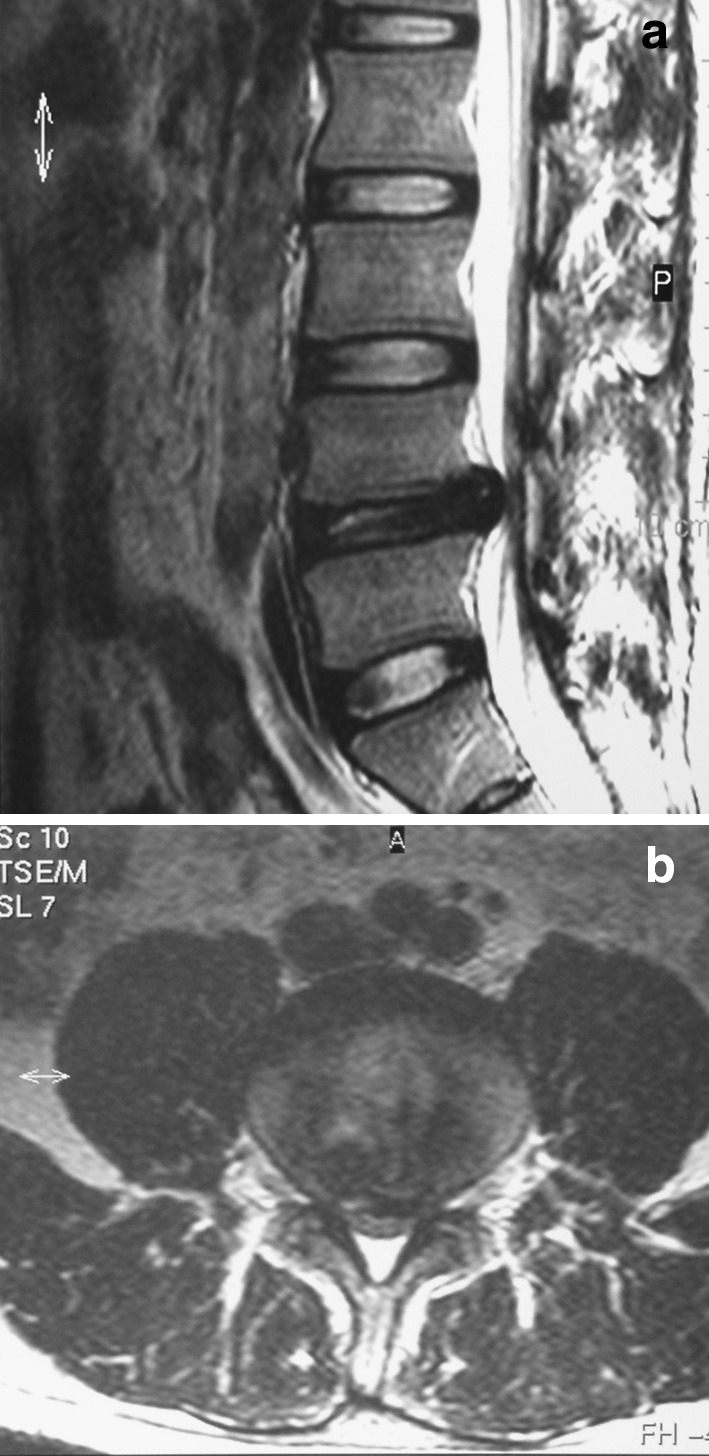
Sagittal section of T2-weighted (a) and axial section (b) of MRI demonstrate that the dual sac is severely compressed, but it is difficult to distinguish that the compressive material is apophyseal fragments or/and disc material
Classification
Preoperative comprehensive understanding of the location and type of PRAF is essential to guide the therapy. Therefore, the diverse features of bone fragment drive many surgeons to classify PRAF based on location, radiological and surgical findings (Table 2).
Table 2.
Chronologic list of PRAF classification systems
| Classification | Basis | Findings | Description | No of patients | Significance of classification |
|---|---|---|---|---|---|
| Takata et al. [26] | Morphology | CT scan | Type I: avulsion fractures of the posterior cortical rim | 10 | Type I, II and IV lesions most caused bilateral sciatica that needed extended decompression; Type III caused ipsilateral sciatica and the unilateral microsurgical approach was enough. |
| Type II: central cortical and cancellous fragments | 9 | ||||
| Type III: lateralized chip fracture bodies | 12 | ||||
| Epstein et al. [10] | Morphology | CT scan | Modified Takata classification: | ||
| Type I | 6 | ||||
| Type II | 6 | ||||
| Type III: Non-calcified: fracture of posterior cortical rim mimicking disc herniation | 15 | ||||
| Calcified: fracture of posterior calcified cortical rim | 19 | ||||
| Type IV: extending across entire vertebral bodies | 10 | ||||
| Scarfo et al. [24] | Relationship between disc and fragment | CT scan | Type I: median herniation with wide detachment of a large piece of apophysis | 14 | Type I caused bilateral sciatica, while type II caused ipsilateral symptom. |
| Type II: lateral disc herniation with detachment of a small piece of apophysis | 12 | ||||
| Shirado et al. [16] | Mobilization | Intraoperative observation | Immobile | 21 | Mobile bone should be excised while immobile one be retained. |
| Mobile | 11 | ||||
| Chang et al. [13] | Location and size | CT scan | Large-central | 13 | Smaller size has better clinical outcome than larger one when treated conservatively. |
| Small-central | 6 | ||||
| Large-lateral | 2 | ||||
| Small-lateral | 6 | ||||
| Akhaddar et al. [12] | Relationship between disc and fragment | Sagittal reconstruction CT scan | Stage A: disc material was displaced to the posterior margin of the bony fragment | 44 | In Stage B, bony fragment should be not removed, because it was herniated disc rather than the bone causing acute typical sciatica. |
| Stage B: disc material was displaced beyond the posterior margin of the bony fragment | 43 |
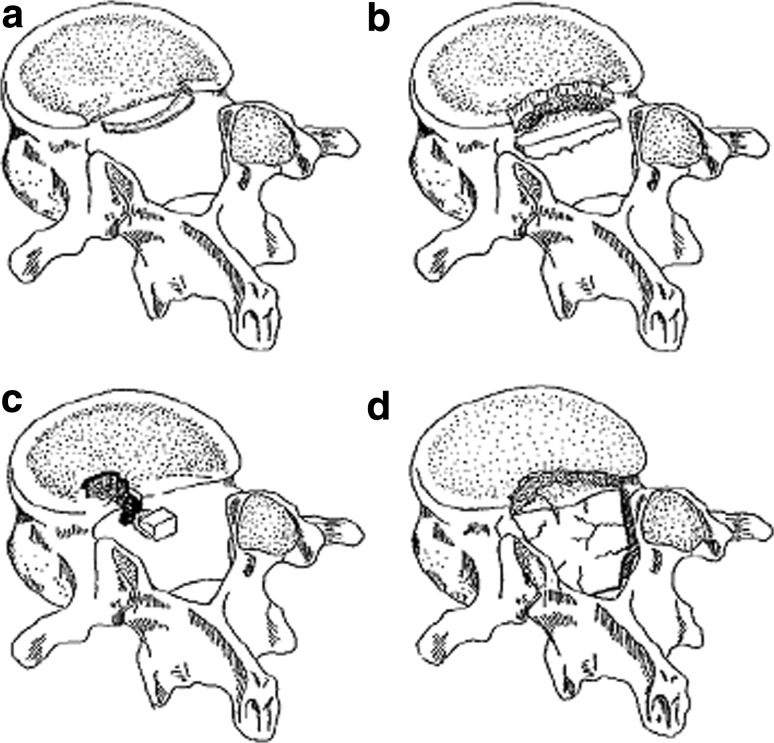
PRAFs were firstly classified morphologically into three types on the basis of CT scan by Takata et al. [26] (Fig. 4a–c). They found that there existed a correlation of the fracture type with the age. This classification was widely acknowledged and cited by many authors [12, 16, 20]. Epstein et al. [4, 10] modified it and added the type IV fracture (Fig. 4d). They classified type III into calcified or noncalcified type. Noncalcified type III fractures were easily misinterpreted as routine discs on both CT and MRI studies. They were first discovered intraoperatively, and finally diagnosed on pathologic evaluation [10].
Fig. 4.
Classification of PRAF. a Type I, an arcuate simple avulsion of the posterior cortex of the endplate without osseous defect. b Type II, an avulsion fracture of the central cortical and cancellous rim of posterior vertebra. c Type III, a more lateral localized fracture involving a larger amount of the vertebral body, resulting that osseous defect anterior to the fragment is larger than the fragment. d Type IV, a fracture spans full length of vertebral bodies between the endplates (schematic drawing was cited in Talha et al. [22], with reprint permission of Eur Spine J)
Scarfo et al. [24] classified PRAFs into two morphological types, considering the relationship of location between herniated disc and bone fragment. The size of bony fragment in spinal canal was also responsible for clinical symptoms except its location. Therefore, apophyseal fractures were classified into four types by the size (large or small) and the location (central or lateral) suggested by Chang et al. [13]. Based on CT imaging, the fracture occupying more than 50 % width of posterior wall of vertebral body was defined as large size, and less than 50 % width of posterior wall of vertebral body as smaller size.
On the basis of the observation during the surgical procedures, Shirado et al. [16] sorted the apophyseal fragments into immobile and mobile modalities, aiming to determine whether removal of the fragments or not. According to the finding by sagittal reconstruction CT scan, Akhaddar et al. [12] introduced another classification of Stage A and B based on the contribution to symptom by herniated disc or apophyseal fragment.
As summarized above, various classification schemes were evolved according to the diversified features of bony fragments. Each system has a significant role in guiding the surgeons to treat this disease (Table 2). However, most classifications are based on a single individual’s, or a comparatively small group’s, retrospective review of a case series. Therefore, the reliability and validity of each classification are suspected. Surgeon treating PRAF using different criteria may lead to different conclusions. Further systematic and reliable classifications should be developed.
Treatment
Because PRAFs are not common, most of the authors report a small number of patients. The mean age of patients in each study varies greatly. Diagnosis of PRAF in the series was all made based on combined evidences of clinical symptoms, physical examination and imaging findings. Different treatments have been reported according to the authors’ preferences and experiences. Included series were also heterogeneous in terms of follow-up duration and clinical result assessment criterion.
Conservative treatment
Few reports discussed the efficacy of nonoperative treatment for this entity in our systematic review. Laredo et al. [28] reported six of 12 patients treated conservatively. Takata et al. [26] treated conservatively four asymptomatic patients. Only three patients were treated nonoperatively in the series of 59 cases by Epstein et al. [10]. However, these papers had not provided enough information about the conservative indication, such as which types of apophyseal fracture should be treated conservatively or operatively. Chang et al. [13] reported 12 nonoperative adolescent patients. All six patients with central or lateral small fragments had excellent results, whereas three of the six patients with large apophyseal fragments had poor results and must be informed of a greater chance to have chronic back pain. Similarly, Baba et al. [23] suggested that patients with a small limbus bulge or non-calcified type III should be treated conservatively. Whereas, a large displaced fracture badly responded to conservative therapies.
The principle of conservative treatment is similar to treatment of LDH, consisting of bed rest, analgesic and nonsteroidal anti-inflammatory drugs, physical therapy and limitation of physical activities with lumbar braces [18]. How long conservative measures should be tried was not mentioned in all the papers. When this conservative therapy is ineffective or persistent back pain adversely compromises the patient’s day to day activities, regardless of existing neurological deficits, the need for operative treatment has been emphasized [25, 27].
Surgical treatment
The unique physiological natures of younger patients endow the treatment of PRAF with some distinctive characteristics and more careful consideration. The purpose of the operation is not only to relieve the neural decompression, but also to minimize the surgical trauma and avoid related complications to make them recover rapidly and return to school or other physical activities. Modalities of surgical treatment for PRAF consist of removal of disc or/and bony fragment via anterior or posterior approach with or without spinal fusion.
Anterior approach
It seems obviously that the complete removal of the compressive substances can be achieved via posterior or anterior approach. Only two studies reported six cases treated though anterior approach. Takata et al. [26] reported five out of 24 patients with PRAF treated in this way. However, the reason for this option and proper indication for the anterior approach had never been mentioned. Savini et al. [25] reported a woman with fracture of the T12 posteroinferior rim. Treatment included T12–L1 incomplete corporectomy and excision of the fragment, followed by anterior fusion. The effective decompression and excellent recovery were achieved at the last follow-up of 28 months. However, bone resection and discectomy from an anterior approach is not recommended for its extremely difficult manipulation and more complications [19].
Posterior approach
Decompression
A posterior approach is recommended as the best choice for resection of the compressive materials. And most authors had facilitated this approach to treat PRAF. To achieve this goal, the decompression was the primary process. The resection of lamina approaches to the compressive disc or apophyseal fragment. Different options are chosen to reach this purpose, such as MED, laminotomy and laminectomy.
The only study reporting patients treated with microendoscopic discectomy (MED) was published by Matsumoto et al. [15]. They treated 18 patients with PRAF and LDH. The patients with unilateral-type bony fragment and those with central-type were also compared in this study. The outcome of two groups showed that the mean recovery rate of Japanese orthopaedic association (JOA) score in the former group was statistically significantly higher than that in the latter group with the mean follow-up of 21.1 months. Therefore, the authors recommended that MED technique was more feasible in patients with the lateral-type, for its easy removal of lateral bony fragment and retraction of nerve roots. Though the MED technique minimizes invasion, its success requires the limited selection of candidate and proficient mastery of the surgical procedure.
Laminotomy or laminectomy was the mostly acceptable decompressive method used by most authors (Table 1). It was generally agreed in the literature that semi-laminotomy or laminectomy was indicated for posterolateral fracture, mostly the unilateral lesion (type III according to modified Takata classification and type II of Scarfo classification system) [10, 20, 23, 24]. However, the limited intervention was proved to be inadequate to relieve the symptoms for the large and base-abroad fragment causing canal or foraminal stenosis. Therefore, bilateral laminotomy or full laminectomy was required in cases of centrally located lesions (Types I, II and IV) suggested by many authors [6, 10, 20, 22–25]. For the fracture originated from the cephalad or caudad endplates, the extend resection of the superior or inferior adjacent lamina was necessary [10].
In summary, there are a number of strategies for decompression reported in the literature; however, the quality of evidence for each of treatment modalities is low, with no randomized studies. It is not possible to definitively conclude as to which decompressive option is the best. Even so, the scope of decompression of lamina is mainly dependent on multiple factors as follows: the size and location of bony fragment and herniated disc, the number of affected segments, the removal of the bony fragment or not and the skill to resect it.
Apophyseal fragment removal or not
It remains controversial whether the detached bony fragment should be resected simultaneously when the decompression and discectomy are done. Most authors advocated the simultaneous excision of apophyseal fragments when discectomy was performed and their clinical results were satisfactory [1, 6, 10, 13, 15, 19–22, 24–27, 29] (Table 1). The following observations may be the reasons for removal of apophyseal fragments. First, it is the existence of bony fragment that makes the crucial difference with LDH alone. The osseous material triggers the symptoms more severely [13]. Second, some authors thought that removal of the disc alone was not sufficient enough to relieve nerve impingement, because the fragment had a space occupying effect which necessitated its removal. If the fragment was untreated or unrecognized, the fracture could heal with residual bony spinal stenosis, which was labeled as congenital in origin due to unawareness [38].
However, some authors suggested that only discectomy and decompression were enough. 78 cases in three studies were treated without removal of bony fragment. Laredo et al. [28] reported two patients operated by discectomy without removal of the bony fragments, because the surgical procedure did not reveal their existence. Although the bony ridge was still obvious in postoperative radiographs, both of the patients greatly improved postoperatively. Shirado et al. [16] prospectively compared 11 patients treated by excision of mobile fragments with 21 patients with immobile bone retained. During the mean follow-up of 4.7 years, satisfactory results were obtained in both groups. They believed that removal of the detached fragment was not mandatory, as resection of the fragment did not influence the clinical results. Akhaddar et al. [12] supported this notion and divided PRAF into type I (with immobile fragments)/type II (with mobile fragments) and Stage A/B (Table 2). They found that the main cause of acute typical sciatica in type I PRAF seemed to be the herniated disc rather than the detached immobile apophyseal fragment, especially in Stage B. In this regard, the removal of the ring fragment was not always necessary. On the contrary, type II PRAF (with mobile fragments) must be excised, because mobile and sharp retropulsed particle could be displaced cephalad or caudal and henceforth might damage neural structures. In 55 cases the results were satisfactory without removal of bony fragment.
As for this discrepancy, both viewpoints had their supporters, but there was lack of solid evidence. These previous studies did have several limitations with low quality. First, the low frequency of this disease limited surgeons to design and conduct clinical prospective randomized controlled trials with large sample. Second, the diverse characteristics of apophyseal fragment induced the different classifications of types of PRAF, and different results and conclusions might be induced. Third, the ages of enrolled patients differed greatly. The fracture in younger patient was cartilaginous or non-calcified, it continued ossification or healed with adjacent rim of vertebral body similar to that commonly seen at other sites of avulsion, whereas in adults, the size of bone fragment would never continue to grow. In this regard, in the study of Shirado et al. [16], though the quality of evidence in the study was more higher, the excised mobile fragments were usually large and occurred in younger patients, while the immobile fragments retained were often small and exclusively noted in adults. Therefore, there was no inclination to conclude that removal of all kinds of the detached fragments was not mandatory. Fourth, what was the prominent cause of the patients’ clinical symptoms was relatively difficult to determine. Akhaddar et al. [12] used sagittal reconstruction CT to determine the prominent cause of the patients’ clinical symptoms. Though it could demonstrate the relationship between the bony fragment and the displacement of disc material, the most convincing evidence may be obtained with the observation during the operative process. After discectomy, the tension of the nerve roots should be examined to see if the compression still existed as a result of the bone. This manipulation can be achieved by extensive laminectomy transversely or longitudinally, but may also affect spinal stability and the patient’s recovery.
Because the bone fragment is less pliable than the disc material, the safe removal is a great challenge and technique demanding. Preoperative understanding of the type and location of apophyseal fragment is essential. For the lateralized types of lesions, unilateral decompression was conducted to remove them through a transaxillary route, whereas the lesion located centrally or wider base-abroad required bilateral or extended decompression [7, 10, 23]. The compressive lesion was resected in en bloc and/or in a piecemeal resection fashion with a down biting curette, tamp and mallet technique or microdrill [10, 24]. A shoe-shaped double-ended impactor was recommended for impacting and separating the fragment from the posterior vertebral body margin to protect both root sleeves and the dural sac [19].
Though these difficulties and limitations exist, it is necessary to further exploit the indication of retaining bony fragment to minimize surgical trauma. Therefore, from these authors’ experiences, the reasonable decision should be made after systematic consideration according to these factors, such as the mobilization, size, location of fragment or its contribution to neurologic symptoms and different surgical technique.
Herniated disc removal or not
In most cases, PRAF accompanies LDH. The preoperative MRI and postoperative histological findings showed the disc material was degenerative, especially in adult patients [12, 24, 25, 37]. In some cases, protruded disc was found to directly compress the neural roots or dural sac. Even it was large-central apophyseal fragment instead of herniated disc that caused canal narrowing and root entrapment, discogenic pain was responsible for chronic back pain [13]. Therefore, almost all of the authors recommend removal of disc [6, 10, 12, 13, 15, 16, 19–26, 28, 29] (Table 1).
However, few authors advocated that discectomy should be considered in relation to age and MRI findings. Molina et al. [18] reported a case of a posterior margin fracture in the L5. The disc was not excised because MRI did not show injured or degenerative disc. At the last evaluation of 8 months, the patient had no functional deficiency and lumbar stability. Liquois et al. [39] also suggested discectomy was not performed in children in the absence of degenerative disc disease. In their series of ten cases of ages ranging from 10 to 15 years, seven had a surgical treatment including six of resection of the avulsed fragment and one of total discectomy. After the mean follow-up of 5 years, all children had a good result without sciatica recurrence and there was no complication.
According to these two reports, it seems that there is no necessity to remove the disc when there is no abnormal signal in MRI findings, especially for the children suffering from mild trauma whose nucleus pulposus are more elasticity and liquid than the usual degenerated disc found in the adult patients [29]. However, both articles are case reports with lower quality of evidence. Besides, the incidence of PRAF with normal disc is extremely rare and the long-term success rate should be evaluated.
Fusion or not
The majority of the authors agreed that fusion should not be performed routinely [10, 12, 13, 15, 16]. There have been a few reports concerning the use of spinal fusion on treatment of PRAF. In the study of Baba et al. [23], posterolateral fusion was performed in three patients, including two patients who needed more than 50 % medial facetectomy and one patient who had a significant instability preoperatively at the level of involvement. All of them achieved solid bony union at 8 months after surgery. Talha et al. [22] suggested that if a wide bilateral laminectomy was performed, posterolateral arthrodesis was necessary to avoid secondary instability. Asazuma et al. [19] firstly performed posterior lumbar interbody fusion (PLIF) in five adolescent cases to treat compression of the root sleeves, as well as spinal instability. The scores represented a recovery of 80.3–100 % (mean, 89.4 %) after a 4- to 5-year follow-up. Bony union was seen in conventional radiographs for each patient. Kuh et al. [8] reported similar results in 13 patients with PLIF. Though they achieved good results, it is impossible to draw conclusions from such a small series without a comparison group as to whether the method of fusion is necessary. As to the surgical treatment of other spinal diseases, the mostly accepted indications for spinal fusion are limited to preoperative segmental instability, multiple level laminectomy or extended facetectomy in more than 50 % of the facet joints [19, 23, 40].
In short, the most used surgical options for PRAF with LDH were posterior discectomy simultaneous excision of apophyseal fragments without spine fusion. In fact, these four topics are more related to each other rather than isolated, though we discussed them separately. For example, whether resection of herniated disc or/and detached apophyseal fragment determines the scope of decompression, which in turn affects the postoperative spinal stability remains to be seen. The surgeons should integrally consider these factors before the surgical intervention. Figure 5 shows the process to treat a patient with PRAF and LDH and the influencing factors for choice of each of the options.
Fig. 5.
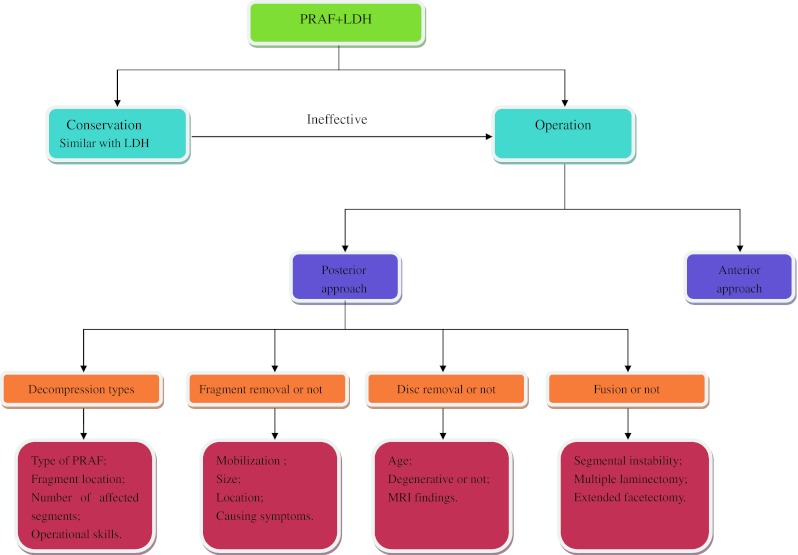
Flow chart of the process to treat a patient with PRAF and LDH
Clinical results and postoperative complications
The most common outcome measures were assessed by symptomatic relief (including neurological recovery and physical activities) or JOA score recovery rate. Most adolescent and adult cases surgically treated for PRAF showed good to excellent results or their symptoms were satisfactorily relieved. However, these positive results are mainly based on short- or mid-term outcome varying from only 3 months to 6.6 years. Therefore, long-term clinical follow-up should be observed, especially for the adolescent and younger adults.
There have been a few reports in the literature concerning the surgical clinical outcome of PRAF associated with LDH compared with LDH alone (Table 3). The results showed that there was no difference in clinical outcomes between these two groups. In the studies of Shirado et al. [16] and Akhaddar et al. [12], the mean ages of surgical intervention for PRAF patients were individually 13 and 8 years younger than those with LDH alone. Besides, the rate of surgical intervention was found significantly higher in patients with PRAF compared with LDH alone (56 % of patients with PRAF vs. 25 % of patients without PRAF) [13].
Table 3.
Surgical clinical outcomes of PRAF associated with LDH compared with LDH alone
| References | No of patients | Mean age (years) | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| W/ PRAF | W/O PRAF | W/ PRAF | W/O PRAF | p value | W/ PRAF | W/O PRAF | p value | |
| Shirado et al. [16]. | 32 | 132 | 25.4 | 38.6 | <0.05 | Excellent: 75 %, good = 22 %. | Excellent: 77 %, good = 20 % | >0.05 |
| Matsumoto et al. [15] | 18 | 18 | 28.9 | 29.1 | >0.05 | Recovery rate = 86.3 %. | Recovery rate = 85.2 % | >0.05 |
| Akhaddar et al. [12] | 87 | 89 | 36.2 | 44.3 | < 0.05 | Excellent: 63 %, good = 22 %. | Excellent: 70 %, good = 18 % | >0.05 |
W/PRAF with PRAF, W/O PRAF without PRAF
There may be many clinical factors that influence postoperative outcome such as age, the types of apophyseal fragment, preoperative symptoms or associated diseases, different surgical techniques and postoperative management. However, Epstein et al. [10] thought that the postoperative outcomes were mainly dependent on the severity of the preoperative deficit, but independent of associated LDH, spondylosis, stenosis, or type of PRAF.
Postoperative complications found in PRAF patients were similar to those in LDH, included dural damage, temporary painful paraesthesia, deep wound infection and discitis and disc recurrence [12, 15, 23, 25].
Therefore, patients with PRAF and LDH are inclined to surgical intervention, and have equal clinical outcomes and post-operative complications compared with LDH alone.
Limitation
This review has several shortcomings. First, because PRAF is not common, few studies were designed with adequate methodological quality and authors reported a small number of patients without prospective randomized controlled studies. Therefore, definite answers to best treatment options could not be obtained. Second, included studies were greatly heterogeneous in terms of patients’ quantities, classifications of apophyseal fracture, severity of the preoperative deficit, treatment modalities, ranging of follow-up and assessment standards, which were determined as the variables of the present review. Because of methodological variance in publications included in this systematic review, different results may be produced with high risk of bias. For the above mentioned reasons, it is difficult to definitively conclude what is the best type of treatment for PRAF. Third, though the physiological natures are different between the younger and older patients, the enrollment of patients was mixed, and most studies did not focus their treatment separately. Therefore, we did not individually compare the surgical efficacy between the younger and older patients. Based on these limitations, it is not possible to definitively conclude what treatment modality is the best for the treatment of PRAF. A larger number of high-level clinical studies are needed.
Conclusion
In summary, PRAF is a rare entity occurring in the age range of 10s to 50s or above. It occurs in all posterior rims of lumbar vertebral segments. CT is the best diagnostic performance for detection of bone fragments compared with plain radiograph and MRI. The diverse features of bone fragment lead to various modalities of classification and operation. Though the widely acceptable surgical options are posterior discectomy simultaneous excision of apophyseal fragments without spine fusion, the surgeons should carefully consider these factors such as decompressive scope, removal of apophyseal fragment/disc or not and fusion or not. Because of methodological shortcomings in publications included in this systematic review, it is not possible to definitively conclude what treatment modality is the best for the treatment of PRAF. More high-quality clinical studies are needed to draw more confirmable conclusions.
Conflict of interest
None.
Footnotes
X. Wu and H. Du equally contributed to this article.
References
- 1.Albeck MJ, Madsen FF, Wagner A, Gjerris F. Fracture of the lumbar vertebral ring apophysis imitating disc herniation. Acta Neurochir (Wien) 1991;113:52–56. doi: 10.1007/BF01402115. [DOI] [PubMed] [Google Scholar]
- 2.Bick EM, Copel JW. The ring apophysis of the human vertebra; contribution to human osteogeny II. J Bone Joint Surg Am. 1951;33(A):783–787. [PubMed] [Google Scholar]
- 3.Gooding CA, Hurwitz ME. Avulsed vertebral rim apophysis in a child. Pediatr Radiol. 1974;2:265–268. doi: 10.1007/BF00972702. [DOI] [PubMed] [Google Scholar]
- 4.Epstein NE, Epstein JA. Limbus lumbar vertebral fractures in 27 adolescents and adults. Spine (Phila Pa 1976) 1991;16(8):962–966. doi: 10.1097/00007632-199108000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Leroux JL, Fuentes JM, Baixas P, Benezech J, Chertok P, Blotman F. Lumbar posterior marginal node (LPMN) in adults. Report of fifteen cases. Spine (Phila Pa 1976) 1992;17(12):1505–1508. doi: 10.1097/00007632-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Farrokhi MR, Masoudi MS. Slipped vertebral epiphysis (report of 2 cases) J Res Med Sci. 2009;14:63–66. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LH, Chang CH, Lee ZL, Niu CC, Lai PL, Tan CF, Chen WJ. Intervertebral disc herniation in adolescents. Chang Gung Med J. 2004;27:22–28. [PubMed] [Google Scholar]
- 8.Kuh SU, Kim YS, Cho YE, Yoon YS, Jin BH, Kim KS, Chin DK. Surgical treatments for lumbar disc disease in adolescent patients; chemonucleolysis/microsurgical discectomy/PLIF with cages. Yonsei Med J. 2005;46:125–132. doi: 10.3349/ymj.2005.46.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smorgick Y, Floman Y, Millgram MA, Anekstein Y, Pekarsky I, Mirovsky Y. Mid- to long-term outcome of disc excision in adolescent disc herniation. Spine J. 2006;6:380–384. doi: 10.1016/j.spinee.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Epstein NE. Lumbar surgery for 56 limbus fractures emphasizing noncalcified type III lesions. Spine (Phila Pa 1976) 1992;17(12):1489–1496. doi: 10.1097/00007632-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Epstein NE, Epstein JA, Mauri T. Treatment of fractures of the vertebral limbus and spinal stenosis in five adolescents and five adults. Neurosurgery. 1989;24:595–604. doi: 10.1227/00006123-198904000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Akhaddar A, Belfquih H, Oukabli M, Boucetta M. Posterior ring apophysis separation combined with lumbar disc herniation in adults: a 10-year experience in the surgical management of 87 cases. J Neurosurg Spine. 2011;14:475–483. doi: 10.3171/2010.11.SPINE10392. [DOI] [PubMed] [Google Scholar]
- 13.Chang CH, Lee ZL, Chen WJ, Tan CF, Chen LH. Clinical significance of ring apophysis fracture in adolescent lumbar disc herniation. Spine (Phila Pa 1976) 2008;33(16):1750–1754. doi: 10.1097/BRS.0b013e31817d1d12. [DOI] [PubMed] [Google Scholar]
- 14.DeOrio JK, Bianco AJ. Lumbar disc excision in children and adolescents. J Bone Joint Surg Am. 1982;64:991–996. [PubMed] [Google Scholar]
- 15.Matsumoto M, Watanabe K, Tuji T, Ishii K, Takaishi H, Nakamura M, Chiba K, Toyama Y. Microendoscopic discectomy for lumbar disc herniation with bony fragment due to apophyseal separation. Minim Invasive Neurosurg. 2007;50:335–339. doi: 10.1055/s-2007-993202. [DOI] [PubMed] [Google Scholar]
- 16.Shirado O, Yamazaki Y, Takeda N, Minami A. Lumbar disc herniation associated with separation of the ring apophysis: is removal of the detached apophyses mandatory to achieve satisfactory results? Clin Orthop Relat Res. 2005;431:120–128. doi: 10.1097/01.blo.0000150457.47232.fd. [DOI] [PubMed] [Google Scholar]
- 17.Grevitt MP, Gardner AD, Spilsbury J, Shackleford IM, Baskerville R, Pursell LM, Hassaan A, Mulholland RC. The Graf stabilisation system: early results in 50 patients. Eur Spine J. 1995;4(3):169–175. doi: 10.1007/BF00298241. [DOI] [PubMed] [Google Scholar]
- 18.Molina V, Court C, Dagher G, Pourjamasb B, Nordin JY. Fracture of the posterior margin of the lumbar spine: case report after an acute, unique, and severe trauma. Spine (Phila Pa 1976) 2004;29(24):E565–E567. doi: 10.1097/01.brs.0000148151.23560.c4. [DOI] [PubMed] [Google Scholar]
- 19.Asazuma T, Nobuta M, Sato M, Yamagishi M, Fujikawa K. Lumbar disc herniation associated with separation of the posterior ring apophysis: analysis of five surgical cases and review of the literature. Acta Neurochir (Wien) 2003;145(461–466):466. doi: 10.1007/s00701-003-0044-z. [DOI] [PubMed] [Google Scholar]
- 20.Mendez JS, Huete IL, Tagle PM. Limbus lumbar and sacral vertebral fractures. Neurol Res. 2002;24:139–144. doi: 10.1179/016164102101199675. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Lage JF, Poza M, Arcas P. Avulsed lumbar vertebral rim plate in an adolescent: trauma or malformation? Childs Nerv Syst. 1998;14:131–134. doi: 10.1007/s003810050195. [DOI] [PubMed] [Google Scholar]
- 22.Talha A, Cronier P, Toulemonde JL, Namour A. Fracture of the vertebral limbus. Eur Spine J. 1997;6:347–350. doi: 10.1007/BF01142684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba H, Uchida K, Furusawa N, Maezawa Y, Azuchi M, Kamitani K, Annen S, Imura S, Tomita K. Posterior limbus vertebral lesions causing lumbosacral radiculopathy and the cauda equina syndrome. Spinal Cord. 1996;34:427–432. doi: 10.1038/sc.1996.76. [DOI] [PubMed] [Google Scholar]
- 24.Scarfo GB, Muzii VF, Mariottini A, Bolognini A, Cartolari R. Posterior retroextramarginal disc hernia (PREMDH): definition, diagnosis, and treatment. Surg Neurol. 1996;46:205–211. doi: 10.1016/0090-3019(96)00154-1. [DOI] [PubMed] [Google Scholar]
- 25.Savini R, Di Silvestre M, Gargiulo G, Picci P. Posterior lumbar apophyseal fractures. Spine (Phila Pa 1976) 1991;16(9):1118–1123. doi: 10.1097/00007632-199109000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Takata K, Inoue S, Takahashi K, Ohtsuka Y. Fracture of the posterior margin of a lumbar vertebral body. J Bone Joint Surg Am. 1988;70:589–594. [PubMed] [Google Scholar]
- 27.Fujita K, Shinmei M, Hashimoto K, Shimomura Y. Posterior dislocation of the sacral apophyseal ring. A case report. Am J Sports Med. 1986;14:243–245. doi: 10.1177/036354658601400313. [DOI] [PubMed] [Google Scholar]
- 28.Laredo JD, Bard M, Chretien J, Kahn MF. Lumbar posterior marginal intra-osseous cartilaginous node. Skeletal Radiol. 1986;15:201–208. doi: 10.1007/BF00354061. [DOI] [PubMed] [Google Scholar]
- 29.Techakapuch S. Rupture of the lumbar cartilage plate into the spinal canal in an adolescent. A case report. J Bone Joint Surg Am. 1981;63:481–482. [PubMed] [Google Scholar]
- 30.Yang IK, Bahk YW, Choi KH, Paik MW, Shinn KS. Posterior lumbar apophyseal ring fractures: a report of 20 cases. Neuroradiology. 1994;36:453–455. doi: 10.1007/BF00593682. [DOI] [PubMed] [Google Scholar]
- 31.Peh WC, Griffith JF, Yip DK, Leong JC. Magnetic resonance imaging of lumbar vertebral apophyseal ring fractures. Australas Radiol. 1998;42:34–37. doi: 10.1111/j.1440-1673.1998.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 32.Ehni G, Schneider SJ. Posterior lumbar vertebral rim fracture and associated disc protrusion in adolescence. J Neurosurg. 1988;68:912–916. doi: 10.3171/jns.1988.68.6.0912. [DOI] [PubMed] [Google Scholar]
- 33.Faizan A, Sairyo K, Goel VK, Biyani A, Ebraheim N. Biomechanical rationale of ossification of the secondary ossification center on apophyseal bony ring fracture: a biomechanical study. Clin Biomech (Bristol, Avon) 2007;22(10):1063–1067. doi: 10.1016/j.clinbiomech.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Sairyo K, Goel VK, Masuda A, Vishnubhotla S, Faizan A, Biyani A, Ebraheim N, Yonekura D, Murakami R, Terai T. Three-dimensional finite element analysis of the pediatric lumbar spine. Part I: pathomechanism of apophyseal bony ring fracture. Eur Spine J. 2006;15:923–929. doi: 10.1007/s00586-005-1026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mupparapu M, Vuppalapati A, Mozaffari E. Radiographic diagnosis of limbus vertebra on a lateral cephalometric film: report of a case. Dentomaxillofac Radiol. 2002;31:328–330. doi: 10.1038/sj.dmfr.4600698. [DOI] [PubMed] [Google Scholar]
- 36.Dietemann JL, Runge M, Badoz A, Dosch JC, Beaujeux R, Bonneville JF, Wackenheim A. Radiology of posterior lumbar apophyseal ring fractures: report of 13 cases. Neuroradiology. 1988;30:337–344. doi: 10.1007/BF00328185. [DOI] [PubMed] [Google Scholar]
- 37.Ikata T, Morita T, Katoh S, Tachibana K, Maoka H. Lesions of the lumbar posterior end plate in children and adolescents. An MRI study. J Bone Joint Surg Br. 1995;77:951–955. [PubMed] [Google Scholar]
- 38.Banerian KG, Wang AM, Samberg LC, Kerr HH, Wesolowski DP. Association of vertebral end plate fracture with pediatric lumbar intervertebral disk herniation: value of CT and MR imaging. Radiology. 1990;177:763–765. doi: 10.1148/radiology.177.3.2243985. [DOI] [PubMed] [Google Scholar]
- 39.Liquois F, Demay P, Filipe G. Sciatica caused by avulsion of the vertebral limbus in children. Rev Chir Orthop Reparatrice Appar Mot. 1997;83:210–216. [PubMed] [Google Scholar]
- 40.Dang L, Liu Z. A review of current treatment for lumbar disc herniation in children and adolescents. Eur Spine J. 2010;19:205–214. doi: 10.1007/s00586-009-1202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]