Abstract
Introduction
Bortezomib (Velcade®) is a proteasome inhibitor that has shown important clinical efficacy either as a single agent or in combination with other cytostatic agents in multiple myeloma (MM). In the present protocol, bortezomib was combined with other active substances like bendamustine and prednisone (BPV), in order to assess the efficacy and toxicity of the combination therapy in patients with relapsed or refractory MM.
Methods
Between January 2005 and December 2011, 78 patients with relapsed or refractory MM were treated with bendamustine 60 (−120) mg/m2 on days 1 and 2, bortezomib 1.3 mg/m2 on days 1, 4, 8 and 11, and prednisone 100 mg on days 1, 2, 4, 8 and 11. The median number of prior therapies was 2 with a wide range of 1–9. Thirty-three patients had pre-existing severe thrombocytopenia and/or neutropenia (WHO grade 3 or 4).
Results
A median number of two (range 1–7) BPV treatment cycles were given to the patients. The majority of the patients (n = 54; 69 %) responded after at least one cycle of chemotherapy with 3 CR, 10 nCR, 10 VGPR and 31 PR. Median PFS and OS for patients without severe hematological toxicities due to previous treatments (n = 45) were 11 and 50 months, respectively. Outcome for these patients was significantly better than that for patients with severe hematological toxicities (grade 3 or 4, n = 33) with a PFS, and OS of 3 months (p < 0.05) and 5 months (p < 0.001), respectively. The regimen was well tolerated with few significant side effects in patients without severe hematological toxicities due to previous treatments.
Summary
These results indicate that the combination of bortezomib, bendamustine and prednisone is well tolerated in patients with relapsed or refractory MM.
Keywords: Multiple myeloma, Relapse/refractory, Bendamustine, Bortezomib, Prednisone
Introduction
Multiple myeloma (MM) is a generalized disease of malignant plasma cells, with an incidence of 4–5/100,000 person-years. Two-thirds of all newly diagnosed patients are over 65 years of age (Ferlay et al. 2004). The disease is incurable with conventional therapy (Alexanian and Dimopoulos 1994), and the median survival is between 3 and 5 years (Ferlay et al. 2004). The introduction of thalidomide, lenalidomide and bortezomib into standard therapy has had a positive effect on survival in patients with MM (Kumar et al. 2008).
Bendamustine is a bifunctional alkylating agent with low toxicity that produces both single- and double-strand breaks in DNA and shows only partial cross resistance with other alkylating drugs (Leoni et al. 2008). A prospective, randomized, phase III trial demonstrated that bendamustine (150 mg/m2 on days 1 and 2) in combination with prednisone (60 mg/m2 on days 1–4) was superior to the standard combination of melphalan/prednisone with respect to complete response rate, mean number of cycles to maximum response, time to treatment failure and quality of life in patients with newly diagnosed MM (Pönisch et al. 2006). In a phase I dose-escalation study of bendamustine in 31 patients with MM who had progressed after high-dose chemotherapy, the maximum tolerated dose (MTD) was 100 mg/m2 on days 1 and 2 per 28-day cycle. The overall response rate (ORR) was 55 % with a median progression-free survival (PFS) of 26 weeks (Knop et al. 2005). Fixed dose bendamustine (60 mg/m2, days 1, 8 and 15) has been used in combination with prednisolone and escalating thalidomide doses from 50 to 200 mg daily in a phase I study of relapsed myeloma patients (Pönisch et al. 2008). The ORR was 86 %, with a median PFS of 11 months. In contrast, a report of a similar schedule of bendamustine/thalidomide in combination with dexamethasone in 23 heavily pre-treated patients with a median of 5 previous lines of therapy as part of a compassionate use program resulted in an ORR of 26 % (Grey-Davies et al. 2012). The median PFS and overall survival (OS) were 3 and 13 months, respectively. A phase I/II study of the combination of bendamustine with lenalidomide and dexamethasone in 25 patients with advanced MM was published by Lentzsch et al. (2012). Maximum tolerated doses were as follows: bendamustine 75 mg/m2 (days 1 and 2), lenalidomide 10 mg (days 1–21) and dexamethasone 40 mg (weekly) of a 28-day cycle. The ORR was 52 % with 24 % very good partial response (VGPR) and 28 % partial response (PR).
Bortezomib (Velcade®) is a proteasome inhibitor with potent antimyeloma activity as a single agent (Richardson et al. 2003). The large randomized APEX trial demonstrated the superiority of bortezomib given intravenously on days 1, 4, 8 and 11 of a 21-day cycle over pulse dexamethasone in myeloma patients with relapsed/refractory disease (Richardson et al. 2005). The ORR was 38 %, and median time to progression (TTP) was 6.2 months, compared with only 18 % and 3.5 months with dexamethasone at the time of the first analysis.
Bortezomib combinations have been evaluated in a number of different settings in patients with relapsed/refractory MM. These include combinations with pegylated liposomal doxorubicin (Biehn et al. 2007; Orlowski et al. 2007; Bladé et al. 2008), melphalan (Berenson et al. 2006) and cyclophosphamide (Kropff et al. 2007). These generally produce high ORR, in the range of 50–80 % with complete response (CR) and near complete response (nCR) rates of 15–35 %. An escalation therapy with bortezomib, dexamethasone and bendamustine was investigated in a retrospective analysis of 50 patients with relapsed or refractory MM (Fenk et al. 2007). Bortezomib was given as monotherapy (1.3 mg/m2 on days 1, 4, 8 and 11) and was followed by the addition of dexamethasone (40 mg on days 1, 4, 8 and 11) in a first escalation step and bendamustine (50–100 mg/m2 on days 1 and 8) in a second escalation step for patients with less than a minor response (MR). In seven patients treated with the triple combination of bortezomib, dexamethasone and bendamustine, the response rates were 57 % PR and 29 % MR. Bendamustine, prednisone and bortezomib (BPV) was evaluated by our group in a retrospective analysis of 18 patients with newly diagnosed/untreated MM and renal insufficiency (GFR <35 ml/min) (Pönisch et al. 2012). The majority of the patients (n = 15; 83 %) responded after at least one cycle of chemotherapy with three stringent complete responses (sCR), five nCR, five VGPR and two PR. With a median follow-up of 17 months, PFS at 18 months was 57 % and OS was 61 %.
The combination of bendamustine, bortezomib and corticosteroids is currently being examined in four simultaneously conducted phase II studies in relapsed/refractory MM patients. The first results of these studies were recently published in abstract form with an ORR between 49 and 77 % in differing age populations, pre-treatments and treatment schedules (Berenson et al. 2011; Ludwig et al. 2012; Offidani et al. 2012; Rodon et al. 2012).
The success of a relapse therapy will depend strongly on events associated with the primary therapy, including the development of resistance and long-term side effects. In the case of primary therapy with strong stem-cell cytotoxicity, the reduced stem-cell reserve is likely to be associated with a significant increase in hematological toxicity in subsequent treatments. Protocols for relapsed MM patients include usually patients with only minor bone marrow dysfunction. However, many patients in everyday-care harbor marked pancytopenia (CTC-Criteria grade III and IV) due to stem-cell toxic pre-treatment and/or advanced disease. These patients generally do not meet phase I–III study inclusion criteria. Thus, results from studies including only “good risk” patients cannot necessarily be extended to all patients with advanced bone marrow dysfunction. In this retrospective analysis, we examine this issue by comparing efficacy and toxicities of BPV in patients with relapsed or refractory MM and normal or reduced/restricted bone marrow function.
Methods
Patients
Patients with relapsed or refractory MM who had been treated with a combination of bendamustine, prednisone and bortezomib in the Department of Hematology and Oncology of the University of Leipzig and in a hematology practice in Halle between January 2005 and December 2011 were included in this retrospective analysis. All patients had histologically or cytologically proven stage II or III MM according to the criteria of Durie and Salmon (1975), with measurable myeloma proteins in the serum and/or urine using protein electrophoresis. In contrast to other clinical studies, patients with pronounced pancytopenia due to stem-cell toxic pre-treatment or advanced disease were also included. Patients were allocated to two groups: group A comprised patients with normal bone marrow function and group B patients with impaired bone marrow function and pronounced pancytopenia (CTC-Criteria grade III and IV). Patients were excluded from this retrospective analysis if they had other secondary malignancies. For patients with pre-existent severe pancytopenia, cytological and cytogenetic bone marrow examination was performed in order to exclude myelodysplastic syndrome or secondary acute myeloid leukemia.
Treatment protocol
Bendamustine (60 mg/m2) was given as a 30-min infusion on days 1 and 2 in combination with prednisone (100 mg) given orally on days 1, 2, 4, 8 and 11, and bortezomib (1.3 mg/m2) as an intravenous push on days 1, 4, 8 and 11. Bendamustine dose was escalated to 80 mg/m2 in nine patients and 120 mg/m2 in three patients. In dialysis-dependent patients, bendamustine and bortezomib were given 30 min after the end of dialysis. Cycles were repeated every 21 days (from 1 to 7 cycles) until a maximum response, disease progression or death resulted. Maximum response was achieved if 3 weeks of therapy did not further reduce myeloma protein by more than 10 % in the serum and/or urine.
Definitions of response
Evaluation of response was based on the international uniform response criteria for multiple myeloma (Durie et al. 2006). In addition, the terms “near complete response” and “minor response” were applied to describe response. Under complete response (CR), two categories are listed: CR and stringent CR (sCR). Complete response was defined as a total resolution of measurable myeloma protein in the serum and urine by immunofixation, and <5 % plasma cells on bone marrow evaluation. sCR is a CR plus normal free light chain ratio and absence of clonal cells in bone marrow by immunohistochemistry or immunofluorescence. Near complete response (nCR), a subcategory of partial response, required the disappearance of myeloma protein in serum and urine using densitometry on protein electrophoresis, with positive immunofixation. A very good partial response was defined as at least a 90 % decrease in the serum myeloma protein level. Partial response was defined as at least 50 % decrease in the serum myeloma protein levels, and at least 90 % reduction in the monoclonal component, or a decrease to <200 mg/24 h in the urine myeloma protein levels. Minor response was defined as a reduction of myeloma protein in serum of 25–49 % and in urine of 50–89 %. A reduction in myeloma protein of 24 % or less was classified as stable disease (SD). Progressive disease (PD) was defined as an increase of 25 % or greater in myeloma protein.
Evaluation of efficiency and toxicity
Patients were evaluated within 7 days of inclusion in the BPV protocol. A staging examination was performed in each patient, incorporating medical history, physical examination including a detailed neurological examination, determination of World Health Organization performance status, determination of laboratory parameters (including β2-microglobulin, serum protein, serum protein electrophoresis, myeloma typing of serum and urine, serum free light chain assay, serum creatinine, serum calcium and C-reactive protein), electrocardiogram, X-ray analysis and bone marrow biopsy. Myeloma protein concentration was determined by measuring the integral of the area under the myeloma protein curve using electrophoresis and calculating its portion of the total serum protein.
Response and side effects were evaluated by laboratory and clinical investigation. Toxicity was monitored throughout the treatment using the National Cancer Institute Common Toxicity version 2.0 (http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf). Patients were followed at 4-weekly intervals for the first three months after the end of treatment, and thereafter at 12-weekly intervals until disease progression.
Statistical methods
Descriptive statistical analyses were obtained for demographic and baseline variables. Patients were followed to a closeout date of at least December 31, 2011. All patients who commenced treatment were included in the analysis of OS and PFS. OS was measured from the time of inclusion in the protocol to the time of death, and PFS from the inclusion in the protocol to the time at which a relapse, progression or death was observed. OS and PFS were estimated using the Kaplan–Meier survival analysis and compared using the log rank test. Patients who received autologous stem-cell transplantation after BPV therapy were censored for the endpoint PFS on the first day of stem-cell mobilization cycle. p values were based on the Wilcoxon rank sum test. Categorical variables were compared using the χ2 test. p values were considered significant when <0.05.
Results
Patient characteristics
Seventy-eight patients with relapsed/refractory MM at stage II/III were enrolled in this retrospective analysis. All patients completed at least one cycle of BPV therapy and were available for analysis. Baseline demographics and disease characteristics are shown in Tables 1 and 2. In group A, 45 patients with normal bone marrow function and in group B 33 patients with restricted bone marrow function and pronounced thrombocytopenia and/or neutropenia (CTC-Criteria grade III and IV) were included. The latter patients had hypoplastic hematopoiesis in the bone marrow without dysplasia and with different degrees of infiltration by myeloma cells (between 10 and 90 %). Median age was 62 (range 31–81) years. Age, proportion of male/female, type of monoclonal protein, as well as Durie–Salmon stage, were comparable in both groups. Twenty-nine patients (37 %) had an advanced renal failure (DS stage III B). In our analysis, we included 36 patients (46 %) with a poor general condition (ECOG performance status grade 3/4). This reduced general condition was associated with severe pancytopenia in 23 of the 36 patients (64 %), compared to 10 of the 42 patients (24 %) with good performance status (p < 0.03). The median time from first diagnosis to start BPV therapy was 39 (range 1–183) months, the median number of previous treatments was 2 (range 1–9), the median duration of the last remission before beginning BPV therapy was 4 (range 0–51) months, and 39 patients (50 %) were refractory to the last therapy. A total of 43 subjects (55 %) had received prior autologous only (24 patients; 31 %) or autologous followed by allogeneic stem-cell transplantation (19 patients; 24 %).
Table 1.
Baselines characteristics and measurements for the 78 patients with relapsed/refractory multiple myeloma
| Parameter | Group A (n = 45) | Group B (n = 33) | p |
|---|---|---|---|
| Median age, years (range) | 60 (32–81) | 64 (31–74) | n.s. |
| Male, n (%) | 34 (76) | 19 (58) | n.s. |
| Female, n (%) | 11 (24) | 14 (42) | n.s. |
| ECOG PS | |||
| 0, n (%) | 0 | 0 | |
| 1, n (%) | 17 (38) | 1 (3) | |
| 2, n (%) | 15 (33) | 9 (27) | |
| 3, n (%) | 7 (16) | 9 (27) | |
| 4, n (%) | 6 (13) | 14 (42) | <0.03 |
| Type of monoclonal protein | |||
| IgG, n (%) | 20 (44) | 20 (61) | |
| IgA, n (%) | 8 (18) | 7 (21) | |
| Light chain only | 17 (38) | 6 (18) | n.s. |
| Durie–Salmon stage | |||
| IIA, n (%) | 1 (2) | 0 | |
| IIIA, n (%) | 30 (67) | 18 (55) | |
| IIIB, n (%) | 14 (31) | 15 (45) | n.s. |
| ISS stage | |||
| I, n (%) | 13 (29) | 0 | |
| II, n (%) | 9 (20) | 3 (9) | |
| III, n (%) | 23 (51) | 30 (91) | <0.001 |
| Chromosome 13, n = 26 (%) | 3/13 (23) | 6/13 (46) | n.s. |
Group A comprises patients with normal bone marrow function and group B patients with restricted bone marrow function and pronounced thrombocytopenia and/or neutropenia
Table 2.
Prior antimyeloma treatment
| Parameter | Group A (n = 45) | Group B (n = 33) | p |
|---|---|---|---|
| Median time from diagnosis to start of BVP therapy, months (range) | 33 (2–98) | 45 (1–183) | n.s. |
| Median number of prior therapy lines (range) | 2 (1–9) | 3 (1–6) | n.s. |
| Type of previous therapy | |||
| Doxorubicin, n (%) | 36 (80) | 24 (73) | n.s. |
| Melphalan, n (%) | 39 (87) | 33 (100) | n.s. |
| Thalidomide, n (%) | 12 (27) | 19 (58) | <0.01 |
| Lenalidomide, n (%) | 7 (16) | 3 (9) | n.s. |
| Bortezomib, n (%) | 8 (18) | 6 (18) | n.s. |
| 1× autologous SCT, n (%) | 8 (18) | 7 (21) | n.s. |
| 2× autologous SCT, n (%) | 3 (7) | 6 (18) | n.s. |
| Auto/allo SCT, n (%) | 10 (22) | 9 (28) | n.s. |
| Median duration of previous remission, months (range) | 4 (0–51) | 4 (0–27) | n.s. |
| Pre-existing polyneuropathy | |||
| Grade 1 | 7 (16) | 7 (21) | n.s. |
| Grade 2 | 5 (11) | 3 (9) | n.s. |
| Patients refractory to last therapy, n (%) | 22 (49) | 17 (52) | n.s. |
Group A comprises patients with normal bone marrow function and group B patients with restricted bone marrow function
Response and survival
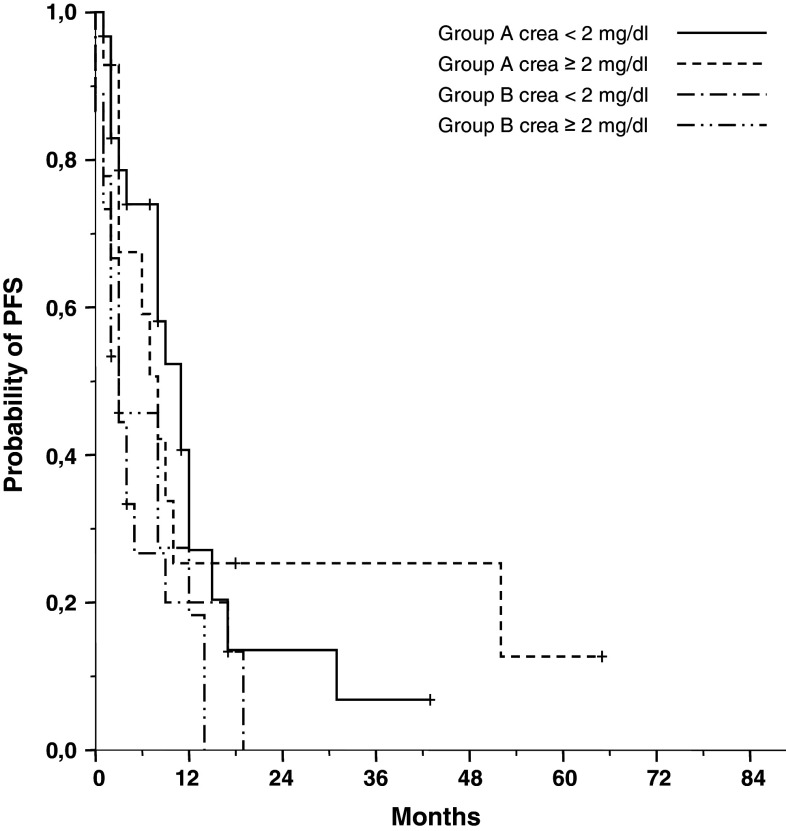
A median of 2 (range 1–7) BPV treatment cycles was given (Table 3). The majority of patients (n = 54; 69 %) responded after at least one cycle of chemotherapy with 3 CR, 10 nCR, 10 VGPR and 31 PR (Table 4). We also found no difference in ORR between group A (n = 34; 76 %) and group B (n = 20; 61 %). The myeloma protein decreased rapidly, the median interval from starting treatment to maximum response was 3 (range 3–18) weeks. Importantly, the responses of patients in group A were as fast as in group B with severe pre-existent pancytopenia (Fig. 1a, b).
Table 3.
Required number of treatment cycles, number of cycles to the first maximum response, progression-free survival and overall survival following treatment with bendamustine, prednisone and bortezomib (BPV) in 78 patients with relapsed or refractory multiple myeloma
| Parameter | Group A (n = 45) | Group B (n = 33) | p | ||
|---|---|---|---|---|---|
| Number of cycles (range) | 2 (1–6) | 2 (1–7) | n.s. | ||
| Number of cycle to first maximum response (range) | 1 (1–4) | 1 (1–6) | n.s. | ||
| Median PFS, months | 11 | 3 | p < 0.05 | ||
| (Creatinine < 2.0 mg/dl) | 11 | n.s. | 3 | n.s. | |
| (Creatinine ≥ 2.0 mg/dl) | 9 | 3 | |||
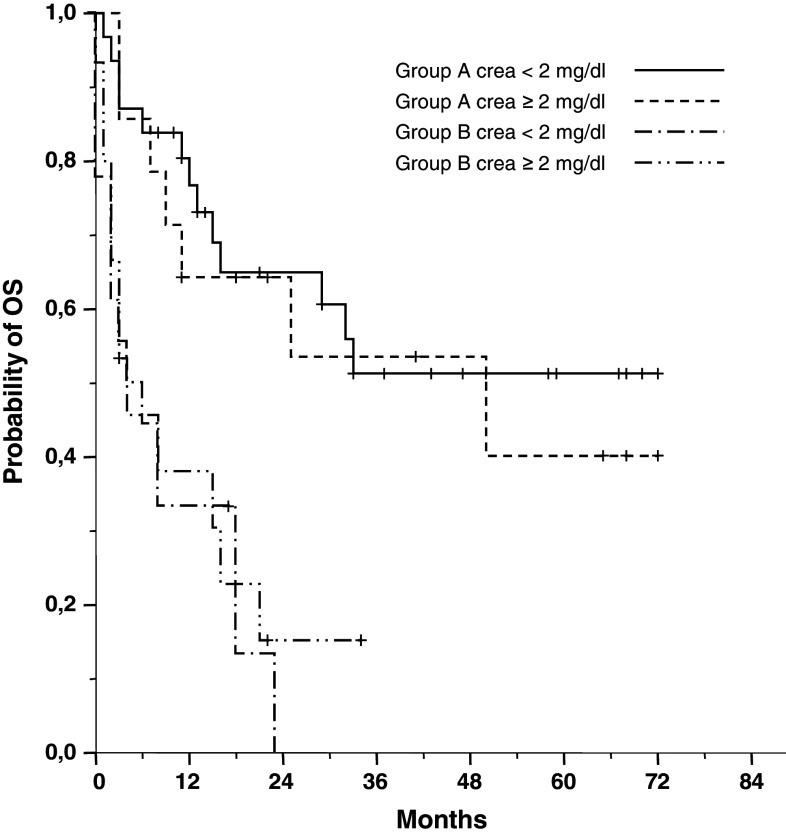
| Median OS, months | 50 | 5 | p < 0.001 | ||
| (Creatinine < 2.0 mg/dl) | n.r. | n.s. | 7 | n.s. | |
| (Creatinine ≥ 2.0 mg/dl) | 50 | 4 | |||
Group A comprises patients with normal bone marrow function and group B patients with restricted bone marrow function. n.r. not reached
Table 4.
Best confirmed response to treatment
| Best confirmed response | No. of patients (%) | ||
|---|---|---|---|
| Group A (n = 45) | Group B (n = 33) | Total (n = 78) | |
| Complete or partial response | 34 (76) | 20 (61) | 54 (69) |
| Complete response IF neg | 2 (4) | 1 (3) | 3 (4) |
| Near complete response IF pos | 7 (16) | 3 (9) | 10 (13) |
| Very good partial response | 5 (11) | 5 (15) | 10 (13) |
| Partial response | 20 (44) | 11 (33) | 31 (40) |
| Minor response | 6 (13) | 3 (9) | 9 (12) |
| No change | 5 (11) | 4 (12) | 9 (12) |
| Progressive disease | 0 | 6 (18) | 6 (8) |
Group A comprises patients with normal bone marrow function and group B patients with restricted bone marrow function
Fig. 1.
Cumulative percentage of hematological response after each BPV cycle: a comprises 45 patients with normal bone marrow function; b comprises 33 patients with restricted bone marrow function
Treatment was continued to the point of maximum response in 38 (49 %) of the 78 patients. Sixteen patients (group A: 14; group B: 2) with PR or better discontinued BPV therapy after the second cycle and received autologous (n = 10; 13 %) or autologous/allogenic (n = 6; 8 %) stem-cell transplantation. Twenty-four patients terminated prematurely: 15 patients (19 %) terminated because of SD or PD after 2 cycles of BPV therapy, and 9 (12 %) patients with pre-existent pancytopenia terminated because of aggravated neutropenia and/or thrombocytopenia during BPV therapy. Nine patients (12 %) died during BPV treatment on disease progression (n = 5) and lethal infection associated with severe pre-existent neutropenia (n = 4). A follow-up of surviving patients at a median of 34 months revealed median PFS and OS for patients without severe hematological toxicities due to previous treatments (group A) to be 11 months and 50 months, respectively (Fig. 2). Outcome for these patients was significantly better than that of patients with severe pre-existent hematological toxicities (group B) with a median PFS, and OS of 3 months (p < 0.05) and 5 months (p < 0.001), respectively (Table 3). There was no significant difference in ORR, PFS and OS between patients with normal renal function and patients with advanced renal failure in either group A or group B.
Fig. 2.
Progression-free survival in 78 patients with advanced multiple myeloma treated with BPV: group A comprises 45 patients with normal bone marrow function; group B comprises 33 patients with restricted bone marrow function
Toxicity
There was a substantial difference in hematological toxicity between group A (patients with normal bone marrow function) and group B (patients with restricted bone marrow function and pre-existent severe pancytopenia) (Table 5). In group A, grade 3/4 anemia occurred in only two patients, and grade 3/4 leukocytopenia and neutropenia in seven patients. Moderate to severe infections were seen in seven patients, four of whom required hospitalization. While most infections occurred during the first two cycles of BPV therapy, patients developed leukopenia and neutropenia after the second cycle. During the 3-week treatment cycles, 19 patients experienced transient grade 3/4 thrombocytopenia between cycle days 12 and 18 (Fig. 3).
Table 5.
Incidence of hematological and non-hematological toxicities following treatment with bendamustine, prednisone and bortezomib (BPV)
| Parameter | No. of patients (%) | ||
|---|---|---|---|
| Group A (n = 45) | Group B (n = 33) | Total (n = 78) | |
| Hematological | |||
| Anemia | |||
| Grade 3 | 1 (2) | 11 (33) | 12 (15) |
| Grade 4 | 1 (2) | 5 (15) | 6 (8) |
| Thrombocytopenia | |||
| Grade 3 | 11 (24) | 8 (24) | 19 (24) |
| Grade 4 | 8 (18) | 22 (67) | 30 (38) |
| Leukocytopenia | |||
| Grade 3 | 7 (16) | 10 (30) | 17 (22) |
| Grade 4 | 0 | 14 (42) | 14 (18) |
| Neutropenia | |||
| Grade 3 | 7 (16) | 9 (27) | 16 (21) |
| Grade 4 | 0 | 14 (40) | 14 (18) |
| Non-hematological | |||
| Infection | |||
| Grade 2 | 3 (7) | 5 (15) | 8 (10) |
| Grade 3 | 2 (4) | 4 (12) | 6 (8) |
| Grade 4 | 2 (4) | 0 | 2 (3) |
| Grade 5 | 0 | 4 (12) | 4 (5) |
| Neuropathy | |||
| Grade 1 | 0 | 1 (3) | 1 (1) |
| Grade 2 | 1 (2) | 1 (3) | 2 (3) |
| Grade 3 | 0 | 0 | 0 |
| Thrombosis and embolism | |||
| Grade 2 | 0 | 0 | 0 |
| Grade 3 | 0 | 0 | 0 |
| Skin rash | |||
| Grade 2 | 0 | 0 | 0 |
| Grade 3 | 0 | 0 | 0 |
| Nausea and vomiting | |||
| Grade 2 | 0 | 1 (3) | 1 (1) |
| Grade 3 | 0 | 0 | 0 |
Group A comprises patients with normal bone marrow function and group B patients with restricted bone marrow function
Fig. 3.
Overall survival in 78 patients with advanced multiple myeloma treated with BPV: group A comprises 45 patients with normal bone marrow function; group B comprises 33 patients with restricted bone marrow function
The majority of patients with restricted bone marrow function (group B) developed an increasing severity of the pre-existent anemia, thrombocytopenia and neutropenia during BPV treatment. Nine such patients discontinued BPV therapy because of prolonged grade 4 neutropenia and/or thrombocytopenia after the first cycle. The incidence of grade 2–5 infections in group B (39 %) was considerably higher than in group A (16 %). Respiratory tract infections with septicemia associated with severe pre-existent neutropenia caused death in four patients during treatment with BPV. The most common severe side effect in group B was grade 3/4 thrombocytopenia in 91 % of the patients.
Grade 2 nausea and vomiting was noted in only one patient in the first cycle and treatment-related polyneuropathy grade 1/2 in only three patients. In 22 patients with pre-existing neuropathy, there was no significant worsening in clinical symptoms. No patients developed skin rash, and in the absence of anticoagulant prophylaxis, no patient experienced deep vein thrombosis or pulmonary thromboembolism.
Discussion
The vast majority of MM patients develop a relapse after either conventional chemotherapy or high-dose therapy with autologous stem-cell transplantation.
Treatment options are limited, and MM patients with relapsed/refractory disease are frequently not considered candidates for intensive strategies as they have usually been heavily treated and/or are of advanced age. The availability of new substances such as the proteasome inhibitor bortezomib or the immunomodulatory agents as thalidomide or lenalidomide has significantly improved the outlook for patients with relapsed/refractory disease. Bortezomib is an effective agent for patients with relapsed MM producing a response rate of approximately 40 % (Richardson et al. 2005). Over the last few years, a combination of bortezomib, corticosteroids and conventional chemotherapy has been suggested to increase the response rate in advanced MM. In this retrospective analysis, we treated relapsed/refractory MM patients with a combination of bortezomib, bendamustine and prednisone. Our data demonstrates that bortezomib in combination with bendamustine and prednisone is highly active in patients with relapsed or refractory MM. Due to the high efficacy of our treatment, only a short duration of therapy was necessary to achieve a maximum response.
The resulting ORR of 76 % and CR/nCR rate of 20 % in patients with normal bone marrow function (group A) compares very favorably with those reported for other bortezomib/corticosteroid-containing combination regimens tested. For example, for the combination with pegylated liposomal doxorubicin (Biehn et al. 2007; Orlowski et al. 2007; Bladé et al. 2008), melphalan (Berenson et al. 2006), cyclophosphamide (Kropff et al. 2007) and bendamustine (Berenson et al. 2011; Ludwig et al. 2012; Offidani et al. 2012; Rodon et al. 2012), response rates ranged from 45 to 80 % and CR/nCR rates from 3 to 35 %.
Bortezomib and bendamustine are effective single agents for MM patients with renal failure, resulting in a myeloma response rate of approximately 50 % (Ludwig et al. 2007; San-Miguel et al. 2008; Preiss et al. 2003). In our analysis, the efficacy of the combination of bendamustine and prednisone with bortezomib does not appear to be substantially different in patients with moderate/severe renal impairment compared to that in patients with no/mild renal impairment.
We observed severe hematological side effects in half of our patients who initially had normal bone marrow function. Leukocytopenia was transient, and neither dose reduction nor administration of granulocyte colony-stimulating factor was required.
Although the majority of patients experienced mild, moderate or occasionally severe infections during the first BPV cycle, these were due to the underlying disease and the neutropenia that occurred after the second cycle did not lead to a higher incidence of infection. As expected and consistent with other bortezomib-containing regimens (Berenson et al. 2006; Kropff et al. 2007; Orlowski et al. 2007), grade 3/4 thrombocytopenia (42 %) was the most frequently reported adverse effect in our trial.
The ORR did not differ significantly between patients with reduced bone marrow function (group B: 61 %) and those with normal hematopoiesis (group A: 76 %). However, the treatment frequently had to be interrupted prematurely because of aggravation of hematological toxicity in those patients with pre-existing reduced bone marrow function. This resulted in a significantly shorter PFS and OS. Despite the fact that BPV has proven to be effective even in the presence of severe hematological toxicities due to previous treatments, there was only a limited benefit in this situation. No previous studies have explored bendamustine in combination with bortezomib in such an unfavorable patient population. A retrospective analysis of another bendamustine-containing regimen (combined with thalidomide and dexamethasone) in similar heavily pre-treated patients reported comparable results with an ORR of 26 %, a median PFS of 3 months and an OS of 13 months (Grey-Davies et al. 2012).
The non-hematological toxicity of the BPV therapy was low and could be managed with simple medical intervention that did not require interruption or delay in treatment for the vast majority of patients. None of our 78 patients experienced bendamustine-related moderate or severe skin rash as seen in other lymphoma patients (Herold et al. 2006). A mild or moderate treatment-emergent polyneuropathy was found in three patients only, which is lower than in other clinical trials (Mateos et al. 2006; Dimopoulos et al. 2009).
In conclusion, BPV therapy was well tolerated in patients with relapsed/refractory MM, and the response rate was approximately 70 %. The high efficacy and the favorable toxicity profile of BPV warrant further evaluation in clinical trials.
Conflict of interest
None.
References
- Alexanian R, Dimopoulos M (1994) The treatment of multiple myeloma. N Engl J Med 330:484–489 [DOI] [PubMed] [Google Scholar]
- Berenson JR, Yang HH, Sadler K, Jarutirasarn SG, Vescio RA, Mapes R, Purner M, Lee SP, Wilson J, Morrison B, Adams J, Schenkein D, Swift R (2006) Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol 24:937–944 [DOI] [PubMed] [Google Scholar]
- Berenson JR, Yellin O, Bessudo A, Boccia RV, Noga SJ, Gravenor DS, Patel-Donnelly D, Siegel RS, Kewalramani T, Gorak EJ, Swift RA, Mayo D (2011) Bendamustine combined with bortezomib has efficacy in patients with relapsed or refractory multiple myeloma: a phase 1/2 study blood. ASH Annual Meeting Abstracts 118:1857 [Google Scholar]
- Biehn SE, Moore DT, Voorhees PM, Garcia RA, Lehman MJ, Dees EC, Orlowski RZ (2007) Extended follow-up of outcome measures in multiple myeloma patients treated on a phase I study with bortezomib and pegylated liposomal doxorubicin. Ann Hematol 86:211–216 [DOI] [PubMed] [Google Scholar]
- Bladé J, Sonneveld P, San Miguel JF, Sutherland HJ, Hajek R, Nagler A, Spencer A, Robak T, Cibeira MT, Zhuang SH, Harousseau JL, Orlowski RZ (2008) Pegylated liposomal doxorubicin plus bortezomib in relapsed or refractory multiple myeloma: efficacy and safety in patients with renal function impairment. Clin Lymphoma Myeloma 6:352–355 [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kastritis E, Kropff M, Petrucci MT, Delforge M, Alexeeva J, Schots R, Masszi T, Mateos MV, Deraedt W, Liu K, Cakana A, van de Velde H, San Miguel JF (2009) VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol 27:6086–6093 [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV, International Myeloma Working Group (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–1473 [DOI] [PubMed] [Google Scholar]
- Durie BG, Salmon SE (1975) A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 36:842–854 [DOI] [PubMed] [Google Scholar]
- Fenk R, Michael M, Zohren F, Graef T, Czibere A, Bruns I, Neumann F, Fenk B, Haas R, Kobbe G (2007) Escalation therapy with bortezomib, dexamethasone and bendamustine for patients with relapsed or refractory multiple myeloma. Leuk Lymphoma 48:2345–2351 [DOI] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin DM (2004) GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. IARC Cancer Base No 5 version 2.0. IARCPress, Lyon
- Grey-Davies E, Bosworth JL, Boyd KD, Ebdon C, Saso R, Chitnavis D, Mercieca JE, Morgan GJ, Davies FE (2012) Bendamustine, thalidomide and dexamethasone is an effective salvage regimen for advanced stage multiple myeloma. Br J Haematol 156:552–555 [DOI] [PubMed] [Google Scholar]
- Herold M, Schulze A, Niederwieser D, Franke A, Fricke HJ, Richter P, Freund M, Ismer B, Dachselt K, Boewer C, Schirmer V, Weniger J, Pasold R, Winkelmann C, Klinkenstein C, Schulze M, Arzberger H, Bremer K, Hahnfeld S, Schwarzer A, Muller C, Muller C (2006) Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non-Hodgkin’s lymphoma and mantle cell lymphoma: results of a randomised phase III trial (OSHO# 19). J Cancer Res Clin Oncol 132:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop S, Straka C, Haen M, Schwedes R, Hebart H, Einsele H (2005) The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemotherapy. Haematologica 90:1287–1288 [PubMed] [Google Scholar]
- Kropff M, Bisping G, Schuck E, Liebisch P, Lang N, Hentrich M, Dechow T, Kröger N, Salwender H, Metzner B, Sezer O, Engelhardt M, Wolf HH, Einsele H, Volpert S, Heinecke A, Berdel WE, Kienast J, Deutsche Studiengruppe Multiples Myelom (2007) Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol 138:330–337 [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 111:2516–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentzsch S, O’Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL, Burt S, Boyiadzis M, Roodman GD, Mapara MY, Agha M, Waas J, Shuai Y, Normolle D, Zonder JA (2012) Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood 119:4608–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, Elliott G, Niemeyer CC (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 14:309–317 [DOI] [PubMed] [Google Scholar]
- Ludwig H, Drach J, Graf H, Lang A, Meran JG (2007) Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica 92:1411–1414 [DOI] [PubMed] [Google Scholar]
- Ludwig H, Pour L, Kasparu H, Greil R, Linkesch W, Thaler J, Leitgeb C, Rauch E, Heintel D, Zojer N, Weissmann A, Adam Z (2012) Bortezomib-Bendamustine-Dexamethasone (BBD) in patients with relapsed/refractory myeloma and different risk profiles namely, cytogenetics, previous exposure to bortezomib or to lenalidomide. Haematologica 97 (s1):347 (abstract n. 847) [Google Scholar]
- Mateos MV, Hernández JM, Hernández MT, Gutiérrez NC, Palomera L, Fuertes M, Díaz-Mediavilla J, Lahuerta JJ, de la Rubia J, Terol MJ, Sureda A, Bargay J, Ribas P, de Arriba F, Alegre A, Oriol A, Carrera D, García-Laraña J, García-Sanz R, Bladé J, Prósper F, Mateo G, Esseltine DL, van de Velde H, San Miguel JF (2006) Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood 108:2165–2172 [DOI] [PubMed] [Google Scholar]
- Offidani M, Corvatta L, Caraffa P, Liberati AM, Alesiani F, Brunori M, Caravita di Toritto T, Gentili S, Attolico I, Mele A, Pulini S, Ballanti S, Galimberti S, Gozzetti A, Coppetelli U, Ledda A, Leoni P (2012) Bendamustine, bortezomib and dexamethasone (BVD) combination fort he treatment of relapsed/refractory multiple myeloma: An interim analysis of a phase II study. Haematologica 97 (s1):353 (abstract n. 858)22058220 [Google Scholar]
- Orlowski RZ, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, San Miguel J, Robak T, Dmoszynska A, Horvath N, Spicka I, Sutherland HJ, Suvorov AN, Zhuang SH, Parekh T, Xiu L, Yuan Z, Rackoff W, Harousseau JL (2007) Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol 25:3892–3901 [DOI] [PubMed] [Google Scholar]
- Pönisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, Dachselt K, Richter P, Schirmer V, Schulze A, Subert R, Harksel B, Grobe N, Stelzer E, Schulze M, Bittrich A, Freund M, Pasold R, Friedrich T, Helbig W, Niederwieser D (2006) Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone—a randomized phase III study of the East German Study Group of Haematology and Oncology (OSHO). J Cancer Res Clin Oncol 132:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pönisch W, Rozanski M, Goldschmidt H, Hoffmann FA, Boldt T, Schwarzer A, Ritter U, Rohrberg R, Schwalbe E, Uhlig J, Zehrfeld T, Schirmer V, Haas A, Kreibich U, Niederwieser D, East German Study Group of Haematology, Oncology (OSHO) (2008) Combined bendamustine prednisolone and thalidomide for refractory or relapsed multiple myeloma after autologous stem-cell transplantation or conventional chemotherapy: results of a Phase I clinical trial. Br J Haematol 143:191–200 [DOI] [PubMed] [Google Scholar]
- Pönisch W, Andrea M, Wagner I, Hammerschmidt D, Kreibich U, Schwarzer A, Zehrfeld T, Schwarz M, Winkelmann C, Petros S, Bachmann A, Lindner T, Niederwieser D (2012) Successful treatment of patients with newly diagnosed/untreated multiple myeloma and advanced renal failure using bortezomib in combination with bendamustine and prednisone. J Cancer Res Clin Oncol 138:1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss R, Teichert J, Pönisch W, Niederwieser D, Matthias M, Merkle KH (2003) Pharmacokinetics and toxicity profile of bendamustine in multiple myeloma patients with end-stage renal disease. Hematol J 4(Suppl. 1):26312872151 [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348:2609–2617 [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Bladé J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC, Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352:2487–2498 [DOI] [PubMed] [Google Scholar]
- Rodon P, Hulin C, Pegourie B, Tiab M, Anglaret B, Benboubker L, Jardel H, Decaux O, Kolb B, Roussel M, Garderet L, Leleu X, Royer B, Banos A, Benramdane R, Cony-Makhoul P, Dib M, Fontan J, Stoppa A, Traullé C, Vilque J, Moreau P, Mathiot C, Avet-Loiseau H (2012) Bendamustine, bortezomib and dexamethasone (BVD) in elderly patients with relapsed/refractory Multiple myeloma: The Intergroupe Francophone du Myelome (IFM) 2009-01 protocol. Haematologica 97 (s1):342 (abstract n. 835) [Google Scholar]
- San-Miguel JF, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, Bladé J, Boccadoro M, Cavenagh JD, Neuwirth R, Boral AL, Esseltine DL, Anderson KC (2008) Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia 22:842–849 [DOI] [PubMed] [Google Scholar]