Abstract
Affinity monolith chromatography (AMC) is a type of liquid chromatography that uses a monolithic support and a biologically-related binding agent as a stationary phase. AMC is a powerful method for the selective separation, analysis or studies of specific target compounds in a sample. This review discusses the basic principles of AMC and recent developments or applications of this method, with particular emphasis being given to work that has appeared in the last five years. Various materials that have been used to prepare columns for AMC are examined, including organic monoliths, silica monoliths, agarose monoliths and cryogels. These supports have been used in AMC for formats that have ranged from traditional columns to disks, microcolumns and capillaries. Many binding agents have also been employed in AMC, such as antibodies, enzymes, proteins, lectins, immobilized metal-ions and dyes. Some applications that have been reported with these binding agents in AMC are bioaffinity chromatography, immunoaffinity chromatography or immunoextraction, immobilized metal-ion affinity chromatography, dye-ligand affinity chromatography, chiral separations and biointeraction studies. Examples are presented from fields that include analytical chemistry, pharmaceutical analysis, clinical testing and biotechnology. Current trends and possible future directions in AMC are also discussed.
Keywords: Affinity monolith chromatography, monolithic supports, affinity chromatography, bioaffinity chromatography, immunoaffinity chromatography, immobilized metal-ion affinity chromatography, dye-ligand affinity chromatography, biointeraction chromatography
Introduction
Affinity chromatography is an important separation approach for separating or analyzing specific target compounds in samples or for studying biological interactions. This method is a type of liquid chromatography that makes use of the selective and reversible binding that is present in many biological systems, such as the binding of an antibody with an antigen or the coupling of an enzyme with a substrate. Affinity chromatography utilizes these interactions by placing one of the binding partners within a column as the stationary phase and applying to this column the complementary partner, or target analyte [1–4].
Affinity columns can be used alone or in combination with other techniques for the selective isolation or analysis of the target [1–4]. In addition, experiments can be conducted to obtain information on the stoichiometry, thermodynamics and kinetics of the interaction that is taking place between the target and immobilized binding agent [1, 3, 5–8]. These features continue to make affinity chromatography a popular method and area of ongoing research in fields that range from the isolation of proteins, enzymes and antibodies to biochemical and medical research, molecular biology, pharmaceutical or clinical testing, biotechnology, environmental analysis, and biophysical measurements [1–8].
Because of the selective binding of affinity columns, it is not surprising that a growing number of applications have made use of these columns in biochemical separations and analytical chemistry. This work can be carried out by using binding agents that have been placed onto low performance supports or by using supports that are designed for HPLC [1–19]. Although most past reports with affinity chromatography have made use of particle-based supports [3, 4, 8, 19], there has been increasing interest in carrying out affinity-based separations with monolithic supports. This combination has lead to a method referred to as affinity monolith chromatography (AMC) [10, 13–15].
The initial development of AMC and applications in this field up to 2007–2008 have been discussed in previous reviews [10, 15]. Related articles have reviewed the use of AMC with carbohydrate-based monoliths [14] or in the specific area of bioaffinity chromatography [13]. This current review will build on these previous reports by focusing on more recent developments and applications of all AMC methods, with a particular emphasis being given to reports that have appeared in the last five years (i.e., 2008 to the present). To aid in the understanding of the applications or developments of this method and possible future directions in this field, the basic principles of AMC will also be discussed along with the types of supports and binding agents that have recently been used with this approach for chemical and biochemical analysis.
Principles of affinity chromatography
To fully understand AMC, it is necessary to first consider the basic principles of affinity chromatography. Figure 1 illustrates the format for sample application and elution that is most frequently employed in affinity chromatography and AMC. This approach, often referred to as the “on/off” mode of affinity chromatography, involves the use of two mobile phases (i.e., an application buffer and an elution buffer) to bind and separate a target analyte form other components in a sample. In this method, a sample containing the target analyte is injected onto the column and immobilized binding agent in the presence of the application buffer, which has an appropriate pH and ionic strength to promote binding between the column and the target [3, 20]. During this application step, the target will bind to the immobilized binding agent and the column while the other sample components will tend to be washed away as a non-retained peak. After the non-retained components have been eluted from the affinity column, a second mobile phase known as the “elution buffer” is used to release the target. This elution buffer may have a different pH or ionic strength from the application buffer, or may contain a competing agent that causes disruption of the binding between the retained target and the immobilized binding agent. As it elutes, the target can be collected for further use or, when used as part of an HPLC system, passed through an on-line detector for analysis. After the target has been removed, the column may then be regenerated by re-applying the original application buffer prior to the next application of the target and sample [3, 20].
Figure 1.
A typical on/off elution scheme used in affinity chromatography.
The on/off elution scheme shown in Figure 1 is often used in a situation in which there is strong and highly selective interactions between the target and the immobilized binding agent. For instance, this mode of affinity chromatography is commonly employed in cases where the immobilized binding agent is an antibody and the target is its antigen, a system for which the association equilibrium constant is often greater than 106 M−1 under physiological conditions [20]. The strong interactions in such a system requires an elution buffer that can later be passed through the column to disrupt the interactions between the target and binding agent and to elute the retained target from the column. As mentioned earlier, this may involve a general change in the mobile phase (e.g., an alteration in pH, ionic strength, polarity, or temperature), giving a technique known as “non-specific elution.” Alternatively, the elution buffer may contain an additive that competes with the target or immobilized binding agent for their sites of interaction, giving an approach referred to as “biospecific elution” [3, 20]. It is also possible with systems that have weaker binding to sometimes carry out affinity chromatography by utilizing isocratic conditions. This approach, referred to as “weak affinity chromatography”, employs the same mobile phase for elution as is used during application of the target [20–23]. Isocratic conditions can be utilized if the target binds to the immobilized agent with moderate-to-weak affinity, as occurs for an interaction that has an association equilibrium constant that is less than or equal to approximately 106 M−1 [20].
Another important factor to consider in any application of affinity chromatography is the type of chromatographic bed that is employed. Although this review will focus on monolithic supports, it is useful for the sake of comparison to also consider other support materials that have been used in the past for affinity chromatography. For instance, agarose and cellulose are carbohydrate supports that are frequently employed for preparative applications of this method or for the use of affinity columns for sample pretreatment [3, 18, 19]. These supports are attractive for such work because of their good stability over a wide pH range and their low non-specific binding for biological molecules. In addition, agarose has a large pore diameter and is useful in applications involving large biological compounds [18, 19]. Some alternatives to carbohydrate-based supports are modified silica particles [4, 8, 19], which have been the main materials used in HPLC-based affinity columns prior to the more recent development of monolithic supports for affinity separations [10, 13–15, 19]. The binding agent of interest is typically covalently attached to the support through various coupling techniques (e.g., amine- or sulfhydryl-based methods) [10, 15, 18]). Alternatively, the binding agent may be bound to the support through a secondary binding agent (e.g., the biospecific adsorption of antibodies to supports that containing antibody-binding proteins such as protein A or protein G) [16, 18].
The immobilized binding agent, or “affinity ligand”, is another vital factor to consider when performing any affinity chromatographic method. Most of the ligands that are used in affinity chromatography and AMC are obtained from a biological source, as is the case for antibodies, enzymes, transport proteins, and lectins [1–5, 16, 18]. However, affinity chromatography can also use synthetic ligands such as metal-ion chelates and biomimetic dyes [24–28]. The type of ligand that is employed is often used to divide affinity chromatography into several subcategories. Examples of these subcategories are bioaffinity chromatography, immunoaffinity chromatography (IAC), and immobilized metal-ion affinity chromatography (IMAC). The use of AMC in each of these areas will be examined later in this review.
Advantages of affinity monoliths
There are several reasons why the combination of monolithic supports with affinity chromatography has been of recent interest [9–15, 19]. The monoliths that are used in these applications consist of continuous bed supports that can, if prepared properly, display a higher external porosity than particle-based supports, allowing monoliths to exhibit increased permeability and lower back pressures in chromatographic systems [19, 29–31]. This difference is illustrated in Figure 2(a), in which affinity columns based on both organic monoliths and silica monoliths were shown to have significantly lower back pressures at a given flow when compared to affinity columns that were prepared using HPLC-grade silica particles [32]. This feature can be important in work where high flow rates and short analysis times are desirable, such as in high-throughput drug screening or rapid affinity-based binding assays [9, 10, 13, 15, 19].
Figure 2.
Comparison of (a) the back pressures of affinity columns containing alpha1-acid glycoprotein (AGP) that was immobilized to 300 Å pore size, 7 µm HPLC-grade silica particles, a silica monolith or a GMA/EDMA monolith; (b) comparison of the efficiencies, as represented by the total plate height (Htotal), for columns based on the same silica particles and silica monoliths as used in (a). All of these columns were prepared using the same type of AGP, the same general type of immobilization method, and the same sample application and elution conditions. The results in (a) are for injections of S-warfarin. The data in (b) have been adjusted to represent the back pressures that would be expected for a 10 cm × 4.6 mm I.D. column. (Adapted from Mallik R, Xuan H, Hage DS (2007) J Chromatogr A 1149:294–304. With permission.)
Monoliths are also available in several formats, including those based on organic polymers and silica or agarose monoliths, among others [10, 13, 15, 19, 29, 30] (Note: see Refs. [14, 19, 29–36] for more details on the pore structure and morphology of such materials). These monolithic supports generally contain relatively large, flow-through pores that allow the mobile phase to easily travel through the support. Also present are smaller side pores that provide most of the surface area for placing a stationary phase on the support, while also giving good mass transfer properties that allow an analyte to quickly reach this stationary phase for retention [19, 33]. As illustrated in Figure 2(b), an affinity monolith can provide lower plate heights and higher efficiencies than traditional HPLC-grade particulate supports [32], especially when used at high linear velocities and flow rates [10, 11, 15, 30]. This again results in supports that are attractive for use in high-throughput separations or fast analysis methods [10, 15].
Another advantage of monoliths is they can be prepared in a variety of formats. These formats include columns, such as used in Figure 2, as well as disks, capillaries and microchips. As a result, it has been possible to employ affinity monoliths in both traditional HPLC systems, in methods combining affinity columns with other techniques (e.g., mass spectrometry), and in microanalytical systems [9, 10, 13, 15]. Monoliths have also been combined with a variety of affinity ligands, allowing the resulting supports to be utilized in many types of biochemical separations [9–15, 19, 29]. Examples of specific applications that have used these features of monolith supports in AMC will be discussed in the following sections.
Organic-based affinity monoliths
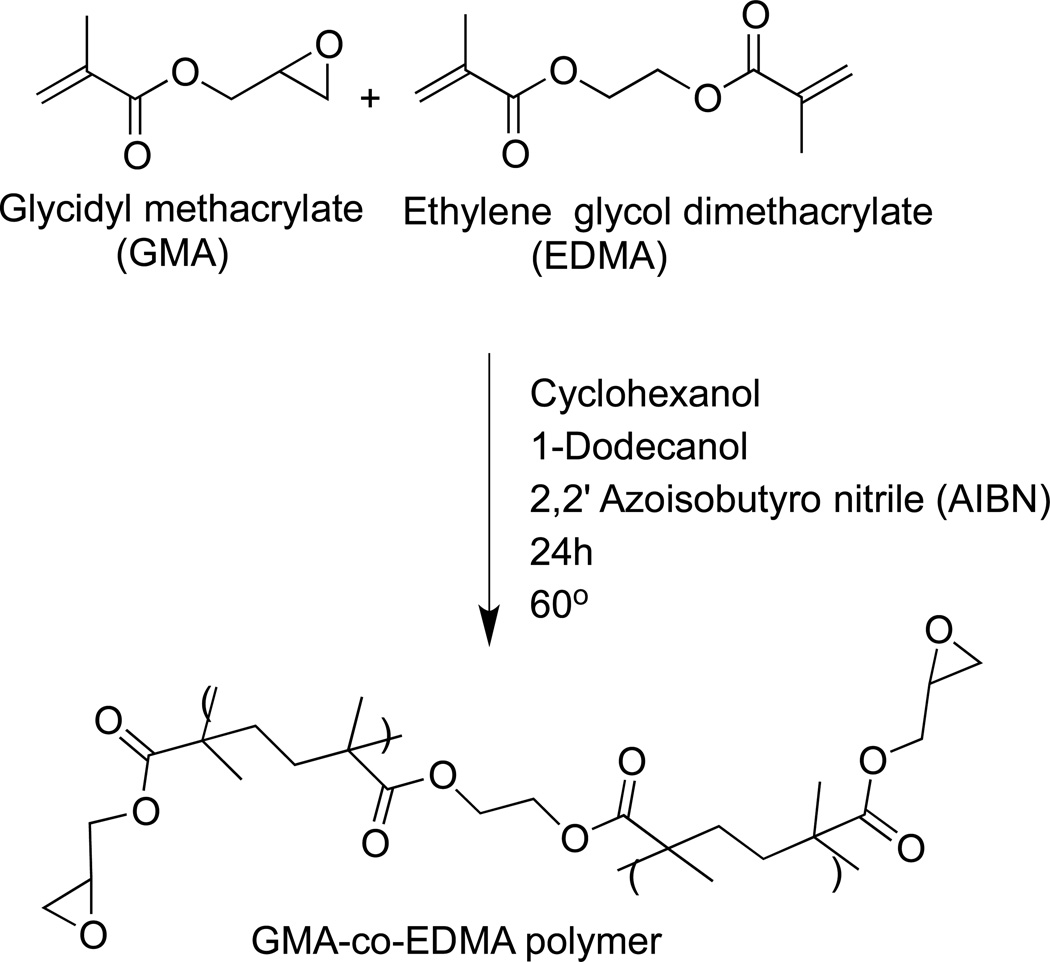
There are a variety of organic-based polymers that have been employed in monoliths for liquid-phase separations (e.g., see reviews in Refs. [33–35]). However, only a few of these have been used in AMC, and most work with organic-based affinity monoliths has been carried out by using co-polymers of glycidyl methacrylate (GMA) and ethylene glycol dimethacrylate (EDMA) [9–11, 13, 15, 29–31, 37]. There are several reasons for the popularity of GMA/EDMA monoliths for use with affinity ligands. First, GMA/EDMA monoliths are commercially available [9, 10, 13, 15]. Second, these materials are relatively easy to prepare in a hydrophilic form (e.g., after conversion of the epoxy groups on GMA into a diol form) that provides low non-specific binding for most biological agents [9, 10]. Third, there are various ways of modifying this type of monolith to make it suitable for ligand attachment. Fourth, these monoliths can be prepared with a variety of pore sizes, shapes, and surface areas. All of these features have lead to the creation of AMC supports in which GMA/EDMA has been employed with numerous binding agents and separation formats [10, 13, 15].
The solvents that are utilized to generate pores (or act as “porogens”) when preparing GMA/EDMA monoliths are usually cyclohexanol and 1-dodecanol. GMA/EDMA monoliths and related supports are often prepared by thermally initiated free radical polymerization [10, 13, 15]. However, there have also been cases in which photo-initiation has proven successful for the polymerization of organic monoliths in capillaries and within the channels of a microchip [38]. The general procedure for preparing a GMA/EDMA monolith for use in AMC is illustrated in Figure 3. The polymerization mixture is first mixed and then introduced into the desired casing (i.e., a capillary, disk, chip, or column). This mixture is next allowed to react for a given amount of time in the presence of a thermal initiator or photo-initiator. After the monolith has been formed, it is washed to remove any remaining reagents or soluble by-products and the porogenic solvents. Further reagents may then be passed through the monolith to activate the support and to immobilize a binding agent within this material [10, 15].
Figure 3.
Typical scheme for the thermal-initiated preparation of a GMA/EDMA monolith. (From Mallik R, Jiang T, Hage DS (2004) Anal Chem 76:7013–7022. With permission. Copyright 2005 American Chemical Society)
Many schemes have been reported for the immobilization of affinity ligands to monoliths [10, 13, 15]. Some common immobilization schemes that have been used for amine-containing ligands are illustrated in Figure 4, with each scheme beginning with the initial epoxy form of a GMA/EDMA monolith. One advantage to using GMA/EDMA monoliths in AMC is that the epoxy groups, as provided by GMA, can be directly used for the immobilization of various binding agents (e.g., proteins or other amine-containing ligands). This approach, known as the “epoxy method”, is relatively simple and fast to perform [9, 10, 39, 40]. However, the epoxy groups are susceptible to hydrolysis, which can result in lower amounts of immobilized ligand than other available amine-coupling methods [9]. The other methods shown in Figure 4 all involve the conversion of the epoxy groups into another activated form for ligand attachment. Examples of these other approaches include the Schiff base method (which uses an aldehyde-activated form of the support), as well as the carbonyldiimidazole (CDI) and disuccinimidyl carbonate (DSC) methods [9–11, 13, 15]. In this group of techniques, the Schiff base method has been found to give the highest relative activity for proteins such as HSA, followed by the DSC, CDI, and epoxy methods [11]. In addition, a hydrazide-activated surface and mild oxidation conditions have been used for the site-direct coupling of antibodies through their carbohydrate chains to GMA/EDMA monoliths [9].
Figure 4.
Examples of covalent immobilization methods that have been used to attach proteins and other amine-containing agents to GMA/EDMA monoliths: (a) the epoxy method, (b) the Schiff base method, (c) the carbonyldiimidazole (CDI) method, and (d) the disuccinimidyl carbonate (DSC) method.
The binding agent is often immobilized within an activated GMA/EDMA monolith by passing through this support a solution of the binding agent in an appropriate buffer [10, 15]. The final quantity of the immobilized binding agent can be adjusted by varying the concentration of the applied ligand, the immobilization method, or the buffer composition and pH [9, 10]. It is also possible to alter the relative amounts of the porogenic solvents that are used to generate the monolith. This last approach has been found to have a significant influence on the pore size and accessible surface area that is available for immobilization in GMA/EDMA monoliths. As an example, it has been shown that altering the ratio of dodecanol-to-cyclohexanol that is used in the polymerization mixture can have a large effect on the amount of antibodies that can later be attached to GMA/EDMA monoliths for use in immunoextraction [9].
Ligands that have been immobilized onto GMA/EDMA monoliths have included antibodies, antibody-binding proteins (e.g., protein A or protein G), lectins, avidin, L-histidine, trypsin, peptides and serum albumins [9–1, 13–15, 41–44]. A recent example involved the immobilization of trypsin to an epoxy-containing monolith to create an enzyme reactor for protein analysis [43]. Another report used a GMA/EDMA monolith that contained immobilized monomeric avidin for binding to biotin-labeled agents. This monolith was used in combination with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for the enrichment and analysis of biotinylated proteins and peptides [44]. One disadvantage to using GMA/EDMA monoliths is that they tend to have a lower surface area compared to conventional particulate supports or silica monoliths; this difference, in turn, can limit the amount of ligand that can be immobilized onto these supports [10, 13].
In spite of the popularity of GMA/EDMA monoliths in AMC, there have several reports that have considered and used other organic polymers for this purpose. For instance, one study used glyceryl methacrylate (GMM) in place of GMA as a co-polymer with EDMA for use in immobilizing lectins [45]. An alternative cross-linking agent to EDMA that has been used with GMA is trimethylolpropane trimethacrylate (TRIM) [13, 46], which has been recently employed in the creation of GMA/TRIM monoliths that contain human serum albumin (HSA) as a chiral stationary phase [47]. Co-polymers based on GMA and divinylbenzene (DVB) have been utilized for the creation of immobilized trypsin enzyme reactors [48] and with immobilized chelating agents plus Cu2+ [49] or Fe3+ [50] in IMAC. In addition, monoliths based on DVB [51] or EDMA [52] have been used to entrap metal oxide nanoparticles for the capture of phosphopeptides. The use of co-polymers of 2-hydroxyethyl methacrylate (HEMA), piperazine diacrylamide (PDA) and (+)-N,N-diallyltartardiamide (DATD) have also been examined as an alternative to GMA/EDMA monoliths in AMC [13, 53, 54].
Inorganic-based affinity monoliths
Monoliths comprised of inorganic polymers like silica (see Figure 5) [55–57] have also been considered for use in AMC [10, 13, 15]. Silica monoliths were introduced for HPLC by Tanaka and co-workers in 1996 [56]. Silica monoliths are prepared by the sol-gel method and generally use a starting sol that consists of tetramethoxysilane (TMOS) and polyethylene oxide (PEO) in water. The PEO in water undergoes hydrolysis and poly-condensation, during which there is phase separation and gelation that produces flow-through pores and smaller side pores. After ageing, the resulting silica monolith is dried and heated. This final form is later encased in a cladding material to produce the final column [57].
Figure 5.
Example of a silica monolith. (From Vervoort N, Saito H, Nakanishi K, Desmet G (2005) Anal Chem 77:3986–3992. With permission. Copyright 2005 American Chemical Society)
There have been several examples in which silica monoliths have been employed in AMC. Two reports used HSA and alpha1-acid glycoprotein (AGP) that were coupled to silica monoliths for use in chiral separations [32, 58]. Other binding agents that have been used in combination with silica monoliths are enzymes, iminodiacetic acid (for use in chelating metalions), trypsin and 3-(2-aminoethylamino)propyl ligands [59–62]. Another recent example used silica monoliths that contained immobilized serum transport proteins to estimate the dissociation rate constants for various drugs from these binding agents [63].
There are several advantages in using silica monoliths for AMC. These advantages again include the good mass transfer properties and low back pressures of these materials when compared to particulate supports [57]. In addition, commercially-available silica monoliths can be modified for use in AMC [32, 58]. Another important advantage arises from the fact that silica monoliths have the same surface chemistry as silica particles, so similar immobilization methods and conditions can be employed with these two types of supports [10, 15]. Silica monoliths also suffer from the same limitations as silica particles, such as their limited pH stability [10, 19]. However, the different pore structure and morphology of silica monoliths compared to GMA/EDMA supports tends to give silica monoliths higher accessible surface areas for protein immobilization, allowing for an increase in the amount of protein that can be attached within such materials [10, 32, 58].
The immobilization methods that are used with silica monoliths can often based on methods that have been adapted from silica particles, and include procedures similar to many of those shown in Figure 4 for GMA/EDMA monoliths. This list includes the epoxy method and Schiff base method [10], as well as the use of hydrazide-activated silica and mild oxidation conditions for immobilizing carbohydrate-containing agents such as AGP [32]. Another option that can be sometimes employed with small silica monoliths (e.g., filled capillaries) is to include the affinity ligand in the reaction mixture during the sol-gel process. This approach leads to the encapsulation of the ligand within the support, but can result in some ligand denaturation due to alcoholic by-products that are released during the polymerization process [13]. However, work has also been conducted with a protein-compatible silane for the sol-gel method that can avoid the release of these by-products and minimize such denaturation [64].
Other types of monoliths for AMC
Agarose has long been a popular support in affinity chromatography [1, 2, 18], so it is not surprising that research has also been conducted in making monolithic supports from agarose [14, 19]. Agarose is a polysaccharide containing repeating units of D-galactose and 3,6-anhydro-L-galactose. This material is hydrophilic in nature and has low non-specific binding for most proteins and biological ligands. Agarose also has good chemical stability over a broad pH range. The major limitation for agarose-based supports is their lack of high mechanical stability, which limits their use in HPLC unless some crosslinking of the support is employed [19]. An advantage to using agarose in monoliths is that these supports are fairly easy to prepare in a variety of shapes and sizes [14, 19].
The preparation of agarose monoliths occurs through several steps. The first step involves dissolving the agarose in water and heating the solution to 90–100°C. The agarose solution is then mixed with a water-immiscible organic solvent. This mixture is shaken to form an emulsion and poured into a mold such as a column. The monolith is allowed to cool and take the shape of the mold. The end result is a monolith that is a continuous unit and that contains flow–through pores [14].
One application for agarose monoliths involved the use of an immobilized derivative of NAD+ for purifying bovine lactate dehydrogenase (see Figure 6) [30]. A second paper used an agarose monolith with immobilized antibodies for β-galactosidase, which was then used to retain intracellular β-galactosidase from samples of E. coli [65]. Related applications have involved the use of agarose monoliths in biosensors based on immobilized lactase, glucose oxidase or acetylcholinesterase, and the immobilization of antibodies in agarose monoliths for trapping specific target analytes [14].
Figure 6.
Purification of lactate dehydrogenase using a 6.0 cm × 16 mm I.D. agarose monolith that contained immobilized NAD+. These results were obtained for the application of a 50 ml sample to the column at 60 cm/h. The sample was applied and the column was washed using a pH 7 buffer, while the retained target was eluted by using a similar buffer with 1 mM NADH added as a competing agent. (From Gustavsson PE, Larsson PO (1999) J Chromatogr A 832:29–39. With permission.)
Cryogels are another group of monoliths that have been used in AMC [10, 15, 66–72]. These supports can have good chemical and physical stability and have been reported to be cost-effective to use [66]. Cryogels are gel matrices that are formed in the presence of moderately frozen solutions of monomeric or polymeric precursors. These conditions can provide interconnected macropores that allow for relatively fast diffusion as analytes pass through this type of material. Cryogel monoliths can be prepared in the form of either columns or membranes [10]. Like other types of monoliths, cryogels often allow the use of high flow rates, making it possible to pass through large sample volumes in a short amount of time [66, 67]. One possible limitation of a cryogel is its relatively low surface area, which could limit the amount of binding agent that can be immobilized to this support for AMC [30].
Cryogel monoliths have been used in separations involving plasmids, proteins and even cells [67]. Antibodies, concanavalin A, dyes, and metal-ion chelates have all been coupled to cryogel monoliths and used in applications such as the purification and depletion of proteins and enzymes from samples [66, 68–71]. For instance, a cryogel comprised of HEMA and containing the immobilized dye Cibacron Blue F3GA was utilized for the purification of human interferon (see Figure 7) [66]. Another report used a similar cryogel for the depletion of albumin from human serum [72].
Figure 7.
Components used to prepare a cryogel comprised of 2-hydroxyethyl methacrylate (HEMA) and containing the immobilized dye Cibacron Blue F3GA (Adopted from Dogan A, Ozkara S, Sari M M, Uzun L, Denizli A (2012) J Chromatogr B 893–894:69–76. With permission.)
Monoliths in bioaffinity chromatography
Bioaffinity chromatography is a type of affinity chromatography that employs a biologically-related ligand as the stationary phase [16]. Some examples of binding agents that can be used in this method are immunoglobulin-binding proteins, enzymes, and lectins (note: antibodies and serum proteins, two other groups of biologically-related binding agents, will be discussed separately in later sections) [10, 13]. Two binding agents from this group that have been employed in AMC are protein A and protein G [12, 41, 73]. These proteins are used to bind immunoglobulins and antibodies. Protein A and protein G are found on the surface of certain bacterial cells and bind to the Fc region of antibodies. Protein A is produced by Staphylococcus aureus and protein G is produced by Streptococci bacteria [16, 18]. There have been a number of examples in which protein A and protein G have been used in affinity monoliths [13, 15]. Protein A has been immobilized onto a GMA/EDMA monolith for the purification of IgG-class antibodies [42] and was used to bind IgG from microliter samples of human serum [12]. In addition, a GMA/EDMA monolith was modified with protein G and used in combination with an anion-exchange monolith disk for the rapid analysis of IgG, transferrin, and insulin in cell cultures [74].
Enzymes are another class of binding agents that can be used in AMC, as well as in related applications such as enzyme reactors and enzyme-based biosensors [14, 43, 75–79]. For instance, dihydrofolate reductase has been placed in an affinity monolith for screening potential inhibitors for this enzyme [15]. The use of affinity monoliths for enzyme reactors have been of particular interest [13, 15]. This is because the good mass transfer properties, low back pressures, and reasonable surface areas of these materials have made it possible to obtain high digestion efficiencies and good immobilization yields for enzymes [13]. Two recent examples involved the use of silica monoliths that contained immobilized trypsin for the digestion of proteins [76, 77]. This type of bioreactor has been coupled online with liquid chromatography-electrospray ionization tandem mass spectrometry for the analysis and identification of proteins [76, 77]. Another report used trypsin immobilized within a monolith based on GMA/DVB and placed into pipette tips. These supports were then used for the microwave-assisted tryptic digestion of proteins within only a few minutes [48]. Other reports have used trypsin with GMA/EDMA monoliths [43] and various enzymes within agarose-based monoliths [14].
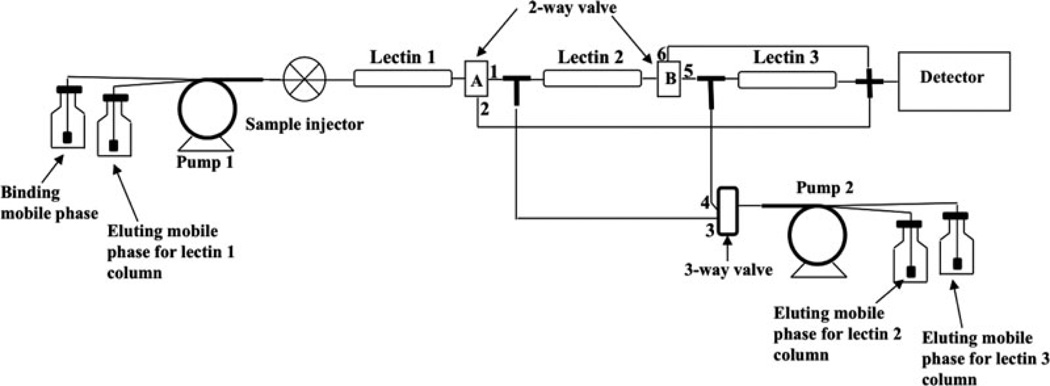
Lectins have also been utilized as binding agents in bioaffinity chromatography [16, 18]. Lectins are non-enzymatic and non-immune system proteins that can recognize and bind carbohydrate residues. Typical applications of lectins in AMC have involved their use in the isolation of biological molecules such as glycoproteins and glycolipids. Concanavalin A (Con A) and wheat germ agglutinin (WGA) are two lectins that are commonly employed in this type of research [13, 15]. A recent example used Con A, WGA, and Ricinus communis agglutinin-I (RCA-I) immobilized to three, tandem monoliths made from co-polymers of GMM and EDMA; these lectin GMM/EDMA monoliths were then used for capturing glycoproteomics from samples representing breast cancer and disease-free human serum (see Figure 8) [45]. Another example used Pisum sativum agglutinin (PSA) that was immobilized to a GMA/EDMA monolith for the separation of several glycoproteins (i.e., turkey ovalbumin, chicken ovalbumin and ovomucoid) [13].
Figure 8.
An HPLC system for capturing and concentrating glycoproteins using tandem lectin columns based on monolithic supports. (From Selvaraju S, El Rassi Z (2012) J Sep Sci 35:1785–1795. With permission.)
Monoliths in immunoaffinity chromatography (IAC)
Affinity columns that make use of immobilized antibodies or related agents produce a special type of bioaffinity chromatography that is known as immunoaffinity chromatography (IAC) [17, 80]. Many IAC methods have been developed in the past for the isolation and purification of hormones, enzymes, peptides, viruses and other biologically-relevant substances [17, 80–86]. Immunoextraction is a special type of immunoaffinity chromatography in which an affinity column is used to isolate compounds from a sample prior to analysis by a second method [17, 80].
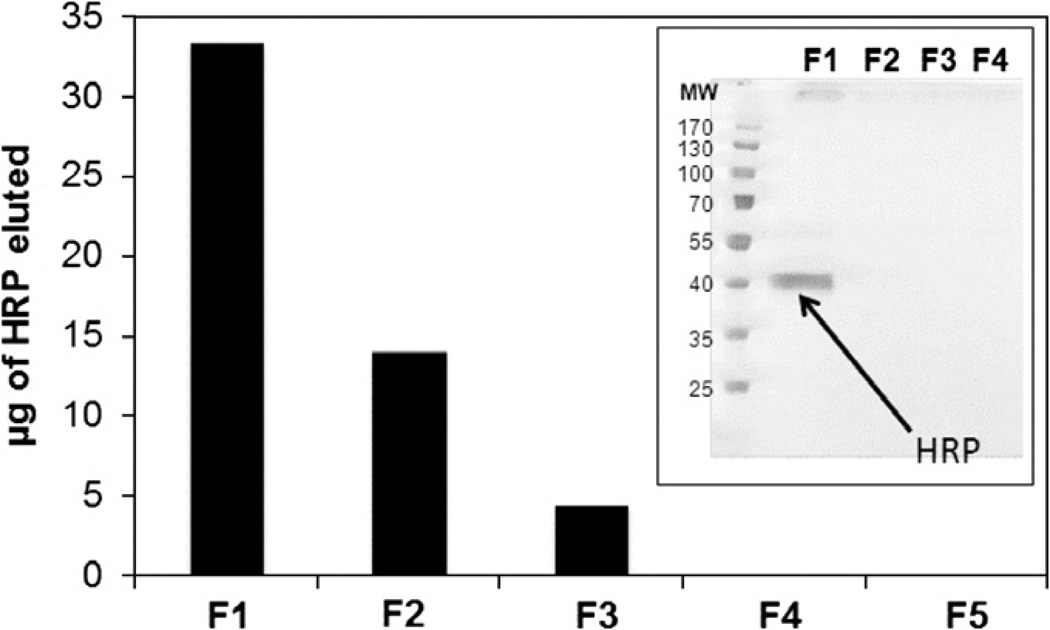
Immunoaffinity monoliths have been used to retain analytes that have included testosterone, diuron, aflatoxin B1, bisphenol A, myoglobin, and N-terminal pronatriuretic peptide [13, 15]. One specific example of immunoextraction involved the immobilization of IgG-class antibodies through various methods to GMA/EDMA monoliths. The final optimized conditions were then used to prepare GMA/EDMA disks for use in the ultrafast immunoextraction of small solutes in as little as 100 ms [9]. A second recent application used a GMA/EDMA monolith and additional polymer-based monoliths for the immobilization of anti-haptoglobin antibodies and other antibodies, with the goal of producing hydrophilic monoliths that could be used as immunosorbents [87]. Another report used GMA/EDMA monoliths and immobilized antihorseradish peroxidase (HRP) polyclonal antibodies to optimize and create immunosorbents (see Figure 9) [88]. There have also been several other examples in which antibodies or aptamers have been immobilized onto GMA/EDMA monoliths for applications such as protein extractions [10, 13, 15, 89, 90].
Figure 9.
Binding and elution of horseradish peroxidase (HRP) when using an anti-HRP monolith in a small pipette-based column. F1–F5 represent the fractions of the retained HRP that were eluted from the monolith when applying successive 150 µL aliquots of methanol to the column. The gel results were obtained by SDSPAGE without the use of a reducing agent. (From Faye C, Chamieh J, Moreu T, Granier F, Favre K, Dugas V, Demesmay C (2012) Anal Biochem 420:147–154. With permission.)
Monoliths in immobilized metal-ion affinity chromatography (IMAC)
IMAC is a type of affinity chromatography that makes use of the specific interactions that can occur between immobilized metal-ions and targets such as amino acids, peptides, proteins or nucleic acids [25]. The immobilized metal-ions frequently consist of Co2+, Zn2+ or Ni2+ ions, among others. Chelating agents that have been used to immobilize metal-ions in columns for traditional IMAC are iminodiacetic acid (IDA), nitrilotriacetate, carboxymethylated aspartic acid, and tris(carboxymethyl)ethylenediamine [15, 25]. Of these, the most common chelating agent that has been used in combination with monoliths is IDA. Amino acid residues on proteins and peptides that are able to bind to the immobilized metal-ions are generally residues such as histidine, tryptophan and cysteine, although other types of amino acids or modified residues may also be involved [25].
Several types of monoliths have been employed in IMAC, ranging from silica monoliths to polymethacrylate-based supports and cryogels [49, 50, 60, 91, 92]. One previous application utilized a silica monolith capillary column that was functionalized with IDA and Fe3+. This capillary column was used to capture phosphopeptides from samples of α-casein [60]. Another application employed cryogel monoliths and utilized N-methacryloyl-(L)-histidine methyl ester as a chelating agent for Cu2+ ions. This monolith was used for the rapid purification of cytochrome C from rat liver homogenate [93]. A separate report involved a commercial GMA/EDMA disk that was coupled with IDA-Cu2+ for use in plasmid DNA purification [94].
A number of recent articles have explored an interesting variation of IMAC known as metal oxide affinity chromatography (MOAC), in which metal oxide particles are used as ligands for the isolation of metal-binding analytes (e.g., targets containing phosphate groups) [95]. In one such report, titanium dioxide and zirconium dioxide microparticles and nanoparticles were placed within highly porous poly(divinylbenzene) monoliths and tested for use in the isolation of phosphopeptides obtained from digests of α-casein, β-casein and phosphorylated extracellular signal regulated kinase 1 (ERK1) [51]. Titanium dioxide particles have also been placed within monoliths prepared with EDMA and through emulsion photopolymerization for the isolation of phosphopeptides form digests of α-casein and β-casein [52]. Another report used a coating of iron oxide particles on GMA/EDMA monoliths that had been modified to contain quaternary amine groups, with the resulting support then being used to extract phosphopeptides from digests of α-casein and β-casein as model systems [96].
Monoliths in dye-ligand affinity chromatography
Dye-ligand affinity chromatography is a type of affinity chromatography that employs a synthetic dye as the immobilized binding agent. There are several advantages in utilizing dyes as the stationary phase for an affinity column, including their good stability, ease of preparation, low cost and high binding capacity [27]. Often these binding agents are based on triazine dyes like Cibracron Blue 3GA. There have been several reports in which these dyes have been used in combination with monolithic supports such as agarose and cryogel-based monoliths [15, 97, 98]. One paper utilized superporous agarose beds containing Cibracron Blue 3GA for the purification of lactate dehydrogenase from a crude bovine heart extract [97]. Another example utilized Cibracron Blue 3GA immobilized to a poly(hydroxyethyl methacrylate) cryogel for the rapid removal of high abundant proteins such as albumin from human serum (see Figure 7) [72].
Monoliths in biointeraction chromatography
Biointeraction chromatography is a special type of affinity chromatography in which this method is used to study a biological interaction [7, 8]. One method that can be used for this purpose is frontal analysis. In frontal analysis, the analyte is continuously applied to a column that contains the immobilized binding agent until a characteristic breakthrough curve is formed [8]. If the analyte and binding agent have relatively fast interactions on the time scale of the experiment, the mean position of this breakthrough curve can then be used to obtain information on the association equilibrium constants and the binding capacity for the analyte on the column [6, 8]. In one recent report, affinity silica monoliths were evaluated by frontal analysis to examine interactions between the drug carbamazepine and the proteins HSA and AGP to aid in the development of chiral stationary phases based on these proteins [32, 58]. A similar study used GMA/EDMA monoliths to examine the binding of naproxen to immobilized bovine serum albumin [99]. Frontal analysis has been further employed with mass spectrometry as a technique for screening the binding of small molecule libraries against immobilized enzymes, as has been done with dihydrofolate reductase contained in a sol-gel in a fused silica capillary [100].
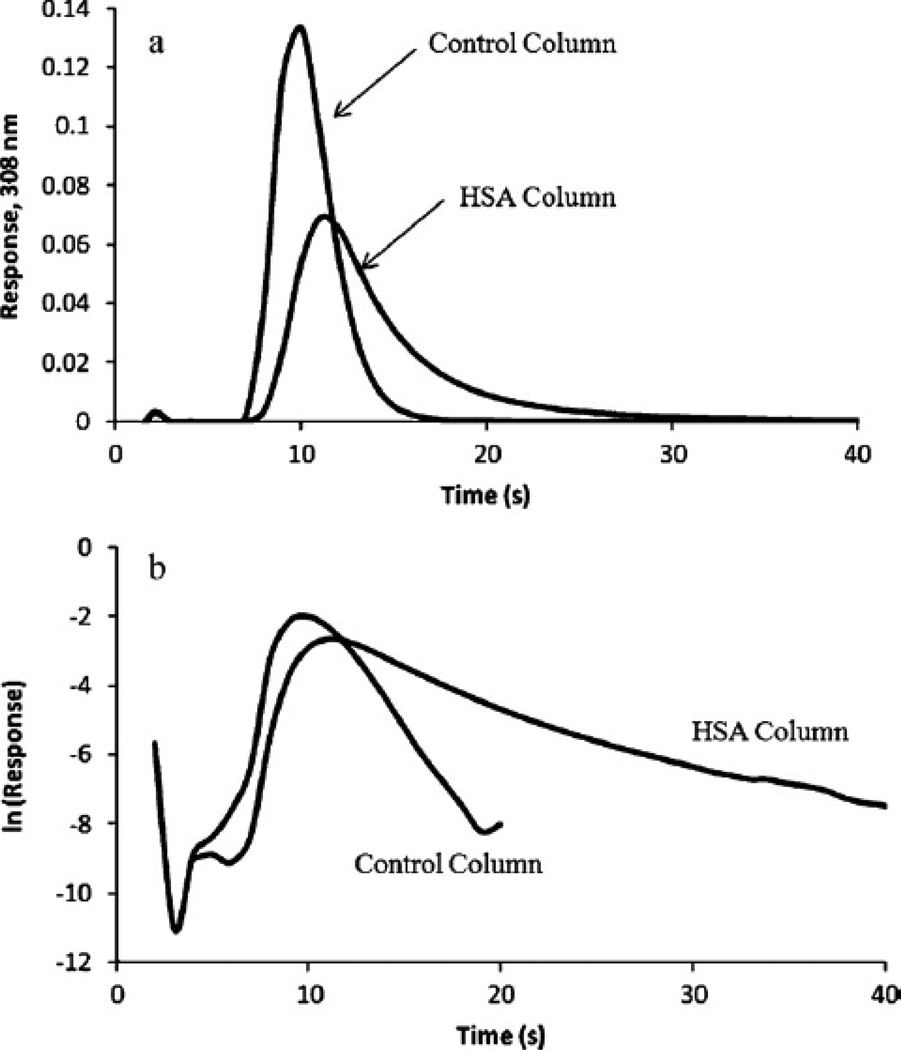
Zonal elution is a second technique that has been used in AMC for examining the binding of analytes with immobilized binding agents [15]. Zonal elution involves the injection of a small plug of analyte onto an affinity column while the peak position or peak shape is examined as the experimental conditions are varied [7, 8]. In previous studies, zonal elution measurements were used to compare the relative binding activities of GMA/EDMA monoliths that contained HSA which had been immobilized by several different methods [11]. The same approach has been used to examine the binding of several drug enantiomers to silica monoliths that contain HSA or AGP and to compare the behavior of these columns with particulate supports or GMA/EDMA monoliths that contained the same binding agents [32, 58]. Silica monoliths as small as 1–3 mm in length have been created and tested for use in high-throughput screening for the retention and band-broadening of drugs on immobilized HSA supports [101]. Similar silica monolith microcolumns containing immobilized HSA have been used in a modified zonal elution format for the rapid determination of protein dissociation rates for drugs such as warfarin, diazepam, and imipramine (see Figure 10) [102].
Figure 10.
Use of silica monoliths in small columns containing immobilized HSA or an inert control support to estimate the dissociation rate of warfarin from HSA. The results in (a) show the chromatograms that were obtained for 100 µL injections of racemic warfarin at 4 ml/min onto 1 mm × 4.6 mm I.D. monoliths. The data in (b) show the natural logarithms of the same chromatograms, with the slopes of the tailing edge for the peak on the HSA column being used to estimate the dissociation rate constant for warfarin from this protein. (From Yoo MJ, Hage DS (2011) J Chromatogr A 1218:2072–2078. With permission.)
Monoliths in chiral separations
Another group of applications that involve affinity chromatography are chiral separations. Examples of some chiral stationary phases are those that are based on serum transport proteins such as HSA and AGP [7, 15]. Both of these proteins are inherently chiral, but HSA tends to bind anionic and neutral compounds while AGP typically binds to cationic species [15]. In one report, a GMA/EDMA monolith was prepared via a thermal-initiated polymerization reaction, followed by immobilization of HSA through the epoxy, Schiff base, CDI and DSC methods. It was found that the Schiff base method gave the highest total of immobilized protein for such a column, leading to improved retention for chiral solutes such as R/S-warfarin and D/L-tryptophan [11]. Other studies have used silica monoliths that contained AGP or HSA as chiral stationary phases. A silica monolith containing AGP was used to separate R/S–warfarin and R/S-propranolol (see Figure 11) [32], while a silica monolith containing HAS was used to separate R/S-warfarin and D/L-tryptophan [58]. In these last two cases, a comparison was also made between the performance seen for these columns and the behavior of equivalent columns that contained alternative supports like silica particles or GMA/EDMA monoliths [32, 58].
Figure 11.
Typical chiral separation obtained for injections of R/S-propranolol at several flow rates onto a 10 cm × 4.6 mm I.D. silica monolith containing immobilized AGP. The mobile phase was pH 7.4, 0.067 M phosphate buffer containing 3% isopropanol. (From Mallik R, Xuan H, Hage DS (2007) J Chromatogr A 1149:294–304. With permission.)
Conclusions & Future Directions
This review examined the basic principles and recent applications of AMC. Advantages of using monoliths over traditional affinity supports include the good efficiency and low back pressures of such materials, as well as their ability to be made in a variety of formats. A variety of materials have been used to make columns and supports for AMC. Organic monoliths based on GMA/EDMA have been particularly popular in this method. However, silica monoliths, agarose monoliths and cryogels have also been utilized. These supports have been used in formats that have ranged from traditional columns to disks, microcolumns, capillaries, and microchips [10, 13–15, 47, 101, 102].
An area of future growth that is expected for AMC is the modification and adaption of new types of monoliths for work with affinity ligands. This trend is expected to include the modification of existing, alternative types of organic polymers to provide a broader range of pore sizes, surface areas and morphologies that can be used in this method. The need for supports with high accessible surface areas for ligand immobilization and the creation of suitable binding capacities or binding properties is further expected to grow as research continues in the utilization of AMC in miniaturized analytical systems [9, 10, 13, 15, 101, 102]. The creation of composite materials for AMC is also expected to be of interest, as is illustrated by the recent work that combines organic monoliths with metal oxide particles for the isolation of phosphopeptides [51, 52, 96]. The possible use of hybrids of silica and organic monolith supports, as has been reported for other chromatographic methods [103, 104], is another combination which may create supports with unique properties for AMC.
There have already been many types of binding agents that have been used with monolithic supports in AMC. These binding agents have included antibodies, enzymes, proteins, peptides, lectins, immobilized metal-ions and dyes. The result has been a variety of applications that have been reported for AMC that are based on methods such as bioaffinity chromatography, IAC or immunoextraction, IMAC, dye-ligand affinity chromatography, and chiral separations. However, future growth in this area is expected as there are still many types of binding agents that have not yet been adapted for work with affinity monoliths. For example, the number of reports that have used AMC for dye-ligand affinity chromatography is still small considering the widespread use and importance of this separation method with traditional affinity supports in the areas of protein and enzyme purification for biochemistry and biopharmaceuticals [27]. The same is true in the use of AMC for chiral separations, which is an important method when used with more traditional HPLC-based affinity supports for pharmaceutical and clinical analysis [7, 15, 16]. Other binding agents that may offer additional areas of future growth in AMC include antibody-mimics such as aptamers or molecularly-imprinted polymers (MIPs) [13, 27, 105–107].
As alternative supports and affinity ligands are used in AMC, it is anticipated that more work will also be needed in the characterization of these supports and in better understanding the unique properties and behavior they may offer in liquid-phase separations. Such developments are already occurring in AMC as this method is being used with microscale analytical devices [9, 10, 13, 15], hybrid materials [51, 52, 96], and biointeraction chromatography [10, 32, 58, 100–102]. The combined efforts that are expected to occur in these future practical and theoretical developments should help to increase the use of AMC in fields that include analytical chemistry, pharmaceutical analysis, clinical testing and research, and biotechnology, to name a few. Given the wide range of supports, formats and binding agents that can already be employed in AMC, it is expected that the use of this method will continue to grow in these and other fields in the coming years.
Acknowledgments
This work was supported in part by the National Institutes of Health under grant R01 GM044931, the National Science Foundation (NSF) REU program and the NSF/EPSCoR program under grant EPS-1004094.
References
- 1.Hage DS, editor. Handbook of Affinity Chromatography. 2nd edn. Boca Raton: CRC Press; 2006. [Google Scholar]
- 2.Cuatrecasas P, Wilchek M, Anfinsen CB. Proc Natl Acad Sci USA. 1968;68:636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters RR. Anal Chem. 1985;57:1099A–1114A. doi: 10.1021/ac00288a001. [DOI] [PubMed] [Google Scholar]
- 4.Larsson PO. Methods Enzymol. 1987;104:212–223. doi: 10.1016/s0076-6879(84)04091-x. [DOI] [PubMed] [Google Scholar]
- 5.Schiel JE, Mallik R, Soman S, Joseph KS, Hage DS. J Sep Sci. 2006;29:719–737. doi: 10.1002/jssc.200500501. [DOI] [PubMed] [Google Scholar]
- 6.Loun B, Hage DS. Anal Chem. 1994;66:3814–3822. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 7.Patel S, Wainer IW. In: Handbook of Affinity Chromatography. 2nd edn. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 21. [Google Scholar]
- 8.Schiel JE, Joseph KS, Hage DS. In: Advances in Chromatography. N Grinsberg N, Grushka E, editors. Vol. 48. New York: Taylor & Francis; 2009. Chap 4. [Google Scholar]
- 9.Jiang T, Mallik R, Hage DS. Anal Chem. 2005;77:2362–2372. doi: 10.1021/ac0483668. [DOI] [PubMed] [Google Scholar]
- 10.Mallik R, Hage DS. J Sep Sci. 2006;29:1686–1704. doi: 10.1002/jssc.200600152. [DOI] [PubMed] [Google Scholar]
- 11.Mallik R, Jiang T, Hage DS. Anal Chem. 2004;76:7013–7022. doi: 10.1021/ac049001q. [DOI] [PubMed] [Google Scholar]
- 12.Pan Z, Zou H, Mo W, Huang X, Wu R. Anal Chim Acta. 2002;466:141–150. [Google Scholar]
- 13.Tetala KKR, Van Beek TA. J Sep Sci. 2010;33:422–438. doi: 10.1002/jssc.200900635. [DOI] [PubMed] [Google Scholar]
- 14.Gustavsson PE, Larsson PO. J Chromatogr Lib. 2003;67:121–141. [Google Scholar]
- 15.Yoo MJ, Hage DS. In: Monolithic Chromatography and its Modern Applications. Wang P, editor. St Albans: ILM; 2010. Chap 1. [Google Scholar]
- 16.Hage DS, Bian M, Burks R, Karle E, Ohnamacht C, Wa C. In: Handbook of Affinity Chromatography. 2nd edn. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 5. [Google Scholar]
- 17.Hage DS. J Chromatogr. 1998;715:3–28. doi: 10.1016/s0378-4347(97)00621-x. [DOI] [PubMed] [Google Scholar]
- 18.Hermanson GT, Mallia AK, Smith PK. Immobilized Affinity Ligand Techniques. New York: Academic Press; 1992. pp. 137–279. [Google Scholar]
- 19.Gustavsson PE, Larsson PO. In: Handbook of Affinity Chromatography. 2nd edn. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 2. [Google Scholar]
- 20.Hage DS, Xuan H, Nelson MA. In: Handbook of Affinity Chromatography. 2nd edn. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 4. [Google Scholar]
- 21.Ohlson S, Lundblad A, Zopf D. Anal Biochem. 1988;169:204–208. doi: 10.1016/0003-2697(88)90275-8. [DOI] [PubMed] [Google Scholar]
- 22.Strandh M, Andersson HS, Ohlson S. Methods Mol Biol. 2000;147:7–23. doi: 10.1007/978-1-60327-261-2_2. [DOI] [PubMed] [Google Scholar]
- 23.Hage DS. In: Encyclopedia of Chromatography. 3rd edn. Cazes J, editor. New York: Taylor & Francis; 2010. pp. 33–36. [Google Scholar]
- 24.Liu XC, Scouten W. In: Handbook of Affinity Chromatography. 2nd ed. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 8. [Google Scholar]
- 25.Todorova D, Vijayalakshmi MA. In: Handbook of Affinity Chromatography. 2nd ed. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap. 10. [Google Scholar]
- 26.Block H, Maertens B, Spriestersbach A, Brinker N, Kubicek J, Fabis R, Labahn J, Schäfer F. Methods Enzymol. 2009;463:439–73. doi: 10.1016/S0076-6879(09)63027-5. [DOI] [PubMed] [Google Scholar]
- 27.Labrou NE, Mazitsos K, Clonis YD. In: Handbook of Affinity Chromatography. 2nd edn. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 9. [Google Scholar]
- 28.Denizli A, Pikin E. J Biochem Biophys Methods. 2001;49:391–416. doi: 10.1016/s0165-022x(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 29.Ikegami T, Tanaka N. Curr Opin Chem Biol. 2004;8:527–533. doi: 10.1016/j.cbpa.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Gustavsson PE, Larsson PO. J Chromatogr A. 1999;832:29–39. doi: 10.1016/s0021-9673(98)00979-0. [DOI] [PubMed] [Google Scholar]
- 31.Guiochon G. J Chromatogr A. 2007;1168:101–168. doi: 10.1016/j.chroma.2007.05.090. [DOI] [PubMed] [Google Scholar]
- 32.Mallik R, Xuan H, Hage DS. J Chromatogr A. 2007;1149:294–304. doi: 10.1016/j.chroma.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jandera P, Urban J. In: Monolithic Chromatography and its Modern Applications. Wang P, editor. St Albans: ILM; 2010. Chap 2. [Google Scholar]
- 34.Coufal P, Bosakova Z, Sirc J, Pacakova V, Stulik K. In: Monolithic Chromatography and its Modern Applications. Wang P, editor. St Albans: ILM; 2010. Chap 4. [Google Scholar]
- 35.Greiderer A, Clark Ligon SC, Jr, Huck CW, Bonn GK. In: Monolithic Chromatography and its Modern Applications. Wang P, editor. St Albans: ILM; 2010. Chap 7. [Google Scholar]
- 36.Zhong H, Yang Q. In: Monolithic Chromatography and its Modern Applications. Wang P, editor. St Albans: ILM; 2010. Chap 3. [Google Scholar]
- 37.Nordberg A, Hilder EF. Anal Bioanal Chem. 2009;394:71–84. doi: 10.1007/s00216-009-2636-9. [DOI] [PubMed] [Google Scholar]
- 38.Mao X, Luo Y, Dai Z, Wang K, Du Y, Lin B. Anal Chem. 2004;76:6941–6947. doi: 10.1021/ac049270g. [DOI] [PubMed] [Google Scholar]
- 39.Kalashnikova IL, Tennikova TB. Anal Chem. 2007;79:5173–5180. doi: 10.1021/ac0700629. [DOI] [PubMed] [Google Scholar]
- 40.Plantonova GA, Pankova GA, Ill'ina IY, Vlasosv GP, Tennikova TB. J Chromatogr A. 1999;852:129–140. doi: 10.1016/s0021-9673(99)00672-x. [DOI] [PubMed] [Google Scholar]
- 41.Calleri E, Marrubini G, Brustotti G, Massolini G, Caccialanza G. J Pharm Biomed Anal. 2007;44:396–403. doi: 10.1016/j.jpba.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Luo Q, Zou H, Zhang Q, Xiao X, Ni J. Biotech Bioeng. 2002;80:481–489. doi: 10.1002/bit.10391. [DOI] [PubMed] [Google Scholar]
- 43.Zacharis CK, Kalaitzantonakis EA, Podgornik A, Theodoridis G. J Chromatogr A. 2007;1144:126–134. doi: 10.1016/j.chroma.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 44.Spross J, Sinz A. Anal Bioanal Chem. 2012;402:2395–2405. doi: 10.1007/s00216-011-5670-3. [DOI] [PubMed] [Google Scholar]
- 45.Selvaraju S, El Rassi Z. J Sep Sci. 2012;35:1785–1795. doi: 10.1002/jssc.201200230. [DOI] [PubMed] [Google Scholar]
- 46.Zhong H, El Rassi Z. J Sep Sci. 2009;32:10–20. doi: 10.1002/jssc.200800546. [DOI] [PubMed] [Google Scholar]
- 47.Pfaunmiller EL. MS Thesis. Lincoln, NE: University of Nebraska; 2011. [Google Scholar]
- 48.Hahn HW, Rainer M, Ringer T, Huck CW, Bonn GK. J Proteome Res. 2009;8:4225–4230. doi: 10.1021/pr900188x. [DOI] [PubMed] [Google Scholar]
- 49.Rainer M, Najam-ul-Haq M, Bakry R, Huck CW, Bonn GK. J Proteome Res. 2007;6:382–386. doi: 10.1021/pr060426y. [DOI] [PubMed] [Google Scholar]
- 50.Aprilita NH, Huck CW, Bakry R, Fuerstein I, Stecher G, Morandell S, Huang HL, Statsyk T, Huber LA, Bonn GK. J Proteome Res. 2005;4:2312–2319. doi: 10.1021/pr050224m. [DOI] [PubMed] [Google Scholar]
- 51.Rainer M, Sonderegger H, Bakry R, Huck CW, Morandell S, Huber LA, Gjerder DT, Bonn GK. Proteomics. 2008;8:4593–4602. doi: 10.1002/pmic.200800448. [DOI] [PubMed] [Google Scholar]
- 52.Liang SS, Chen SH. J Chromatogr A. 2009;1216:2282–2287. doi: 10.1016/j.chroma.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 53.Tetala KKR, Chen B, Visser GM, Maruska A, Kornysova O, Van Beek TA, Sudholter EJR. J Biochem Biophys Methods. 2007;70:63–69. doi: 10.1016/j.jbbm.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Tetala KKR, Chen B, Visser GM, Van Beek TA. J Sep Sci. 2007;30:2828–2835. doi: 10.1002/jssc.200700356. [DOI] [PubMed] [Google Scholar]
- 55.Vervoort N, Saito H, Nakanishi K, Desmet G. Anal Chem. 2005;77:3986–3992. doi: 10.1021/ac0502798. [DOI] [PubMed] [Google Scholar]
- 56.Minakuchi H, Nakanishi K, Soga N, Ishizuka N, Tanaka N. Anal Chem. 1996;68:3498–3501. doi: 10.1021/ac960281m. [DOI] [PubMed] [Google Scholar]
- 57.Cabrera K. J Sep Sci. 2004;27:843–852. doi: 10.1002/jssc.200401827. [DOI] [PubMed] [Google Scholar]
- 58.Mallik R, Hage DS. J Pharm Biomed Anal. 2008;46:820–830. doi: 10.1016/j.jpba.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong X, Dong J, Ou J, Zhu Y, Zou H. Electrophoresis. 2007;28:2606–2612. doi: 10.1002/elps.200600605. [DOI] [PubMed] [Google Scholar]
- 60.Feng S, Pan C, Jiang X, Xu S, Zhou H, Ye M, Zou H. Proteomics. 2007;7:351–360. doi: 10.1002/pmic.200600045. [DOI] [PubMed] [Google Scholar]
- 61.Kato M, Inuzuka K, Sakai-kato K, Toyo'oka T. Anal Chem. 2005;77:1813–1818. doi: 10.1021/ac048388u. [DOI] [PubMed] [Google Scholar]
- 62.Huang G, Zeng W, Lian Q, Xie Z. J Sep Sci. 2008;31:2244–2251. doi: 10.1002/jssc.200800009. [DOI] [PubMed] [Google Scholar]
- 63.Yoo MJ, Hage DS. J Sep Sci. 2011;34:2255–2263. doi: 10.1002/jssc.201100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodgson RJ, Chen Y, Zhang Z, Tleugabulova D, Long H, Zhao X, Organ M, Brook MA, Brennan JD. Anal Chem. 2004;76:2780–2790. doi: 10.1021/ac0352124. [DOI] [PubMed] [Google Scholar]
- 65.Nandakumar MP, Palsson E, Gustavsson PE, Larsson PO, Mattiasson B. Biosep. 1999;9:193–202. doi: 10.1023/a:1008117827057. [DOI] [PubMed] [Google Scholar]
- 66.Dogan A, Ozkara S, Sari MM, Uzun L, Denizli A. J Chromatogr B. 2012;893–894:69–76. doi: 10.1016/j.jchromb.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 67.Arvidsson P, Plieva FM, Saviina IN, Lozinsky VI, Fexby S, Bulow L, Galaev IY, Mattiasson B. J Chromatogr A. 2002;977:27–38. doi: 10.1016/s0021-9673(02)01114-7. [DOI] [PubMed] [Google Scholar]
- 68.Lozinski V, Plieva FM, Glaev IY, Mattiason B. Biosep. 2002;10:163–188. doi: 10.1023/a:1016386902611. [DOI] [PubMed] [Google Scholar]
- 69.Dainiak MB, Galaey IY, Mattiasson B. J Chromatogr A. 2006;1123:145–150. doi: 10.1016/j.chroma.2006.05.089. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Schaochuan S, He X, Yun K, Yao SJ. Biochem Eng J. 2008;42:237–242. [Google Scholar]
- 71.Derazshamshir A, Ergun B, Pesint G, Odabasi M. J Appl Polym Sci. 2008;109:2905–2913. [Google Scholar]
- 72.Andac M, Galaer I, Denizli A. J Sep Sci. 2012;35:1173–1182. doi: 10.1002/jssc.201101020. [DOI] [PubMed] [Google Scholar]
- 73.Ostryanina N, Ill'ina I, Tennikova T. J Chromatogr B. 2002;770:35–43. doi: 10.1016/s1570-0232(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 74.Ralla K, Anton F, Scheper T, Kasper C. J Chromatogr A. 2009;1216:2671–2675. doi: 10.1016/j.chroma.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 75.Nicoli R, Bartolini M, Rudaz S, Andrisano V, Veuthey JL. J Chromatogr A. 2008;1206:2–10. doi: 10.1016/j.chroma.2008.05.080. [DOI] [PubMed] [Google Scholar]
- 76.Temporini C, Perani E, Mancini F, Bartolini M, Calleri E, Lubda D, Felix G, Andrisano V, Massolini G. J Chromatogr A. 2006;1120:121–131. doi: 10.1016/j.chroma.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 77.Calleri E, Temporini C, Perani E, Stella C, Rudaz S, Lubda D, Mellerio G, Veuthey JL, Caccialanza G, Massolini G. J Chromatogr A. 2004;1045:99–109. doi: 10.1016/j.chroma.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 78.Svec F. Electrophoresis. 2006;27:947–961. doi: 10.1002/elps.200500661. [DOI] [PubMed] [Google Scholar]
- 79.Peterson DS, Rohr T, Svec F, Frechet JMJ. J Proteome Res. 2002;1:563–568. doi: 10.1021/pr0255452. [DOI] [PubMed] [Google Scholar]
- 80.Hage DS, Phillips TM. In: Handbook of Affinity Chromatography. 2nd edn. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 6. [Google Scholar]
- 81.Colon LA, Li L. In: Advances in Chromatography. Grushaka E, Grinberg N, editors. New York: Dekker; 2008. Chap 8. [Google Scholar]
- 82.Xu L, Lee HK. J Chromatogr A. 2008;1195:78–84. doi: 10.1016/j.chroma.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 83.Plieva FM, Savina IN, Deraz S, Andersson J, Galaev IY, Mattiasson B. J Chromatrogr B. 2004;807:129–137. doi: 10.1016/j.jchromb.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 84.Plieva FM, Andersson J, Galaev IY, Mattiasson B. J Sep Sci. 2004;27:828–836. doi: 10.1002/jssc.200401836. [DOI] [PubMed] [Google Scholar]
- 85.Arvidsson P, Plieva FM, Savina IN, Lozinski VI, Fexby S, Bulow L, Galaev IY, Mattiasson B. J Chromatogr A. 2002;977:27–38. doi: 10.1016/s0021-9673(02)01114-7. [DOI] [PubMed] [Google Scholar]
- 86.Lozinsky VI, Vainerman ES, Titova EF, Belavtseva EM, Rogozhin SV. Colloid Polym Sci. 1984;262:769–774. [Google Scholar]
- 87.Gunasena DN, El Rassi Z. J Sep Sci. 2011;34:2097–2105. doi: 10.1002/jssc.201100353. [DOI] [PubMed] [Google Scholar]
- 88.Faye C, Chamieh J, Moreu T, Granier F, Favre K, Dugas V, Demesmay C. Anal Biochem. 2012;420:147–154. doi: 10.1016/j.ab.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 89.Li L, Wang J, Zhou S, Zhao M. Anal Chim Acta. 2008;620:1–7. doi: 10.1016/j.aca.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 90.Yang W, Sun X, Pan T, Wooleey A T. Electrophoresis. 2008;29:3429–3435. doi: 10.1002/elps.200700704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peterka M, Jarc M, Banjac M, Frankovic V, Bencina K, Merhar M, Gaberc-Porekar V, Menart V, Strancar A, Podgornik A. J Chromatogr A. 2006;1109:80–85. doi: 10.1016/j.chroma.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 92.Efremenko E, Votchitseva Y, Plieva F, Galaev I, Mattiason B. Appl Microbiol Biotech. 2006;70:558–563. doi: 10.1007/s00253-005-0103-x. [DOI] [PubMed] [Google Scholar]
- 93.Cimen D, Denizli A. Colloids Surf B Biointerfaces. 2012;93:29–35. doi: 10.1016/j.colsurfb.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 94.Shin MJ, Tan L, Jeong MH, Kim JH, Choe WS. J Chromatogr A. 2011;1218:5273–5378. doi: 10.1016/j.chroma.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 95.Leitner A. Trends Anal Chem. 2010;29:177–185. [Google Scholar]
- 96.Krenkova J, Foret F. J Sep Sci. 2011;34:2106–2112. doi: 10.1002/jssc.201100256. [DOI] [PubMed] [Google Scholar]
- 97.Gustavsson PE, Larsson PO. J Chromatogr A. 2001;925:69–78. doi: 10.1016/s0021-9673(01)01027-5. [DOI] [PubMed] [Google Scholar]
- 98.Uzun L, Yavuz H, Say R, Ersoz A, Denizli A. Ind Eng Chem Res. 2004;43:6507–6513. [Google Scholar]
- 99.Zacharis CK, Kalaitzantonakis EA, Podgornik A, Theodoridis G. J Chromatogr A. 2007;1144:126–134. doi: 10.1016/j.chroma.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 100.Hodgson RJ, Chen Y, Zhang Z, Tleugabulova D, Long H, Zhao X, Organ X, Brook MA, Brennan JD. Anal Chem. 2004;76:2780–2790. doi: 10.1021/ac0352124. [DOI] [PubMed] [Google Scholar]
- 101.Yoo MJ, Hage DS. J Sep Sci. 2009;32:2776–2785. doi: 10.1002/jssc.200900346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoo MJ, Hage DS. J Chromatogr A. 2011;1218:2072–2078. doi: 10.1016/j.chroma.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colon LA, Li L. In: Advances in Chromatography. Grushka E, Grinberg N, editors. Vol 48. New York: Marcel Dekker; 2008. Chap 8. [Google Scholar]
- 104.Xu L, Lee HK. J Chromatogr A. 2008;1195:78–84. doi: 10.1016/j.chroma.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Q, Li XF, Shao Y, Le XC. Anal Chem. 2008;80:7586–5793. doi: 10.1021/ac801206s. [DOI] [PubMed] [Google Scholar]
- 106.Zhao Q, Li XF, Shao Y, Le XC. Anal Chem. 2008;80:3915–3920. doi: 10.1021/ac702567x. [DOI] [PubMed] [Google Scholar]
- 107.Haupt K. In: Handbook of Affinity Chromatography. 2nd edn. Hage DS, editor. Boca Raton: CRC Press; 2006. Chap 30. [Google Scholar]