Abstract
New longitudinal sleep data spanning ages 6–10 yr are presented and combined with previous data to analyze maturational trajectories of delta and theta EEG across ages 6–18 yr in non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. NREM delta power (DP) increased from age 6 to age 8 yr and then declined. Its highest rate of decline occurred between ages 12 and 16.5 yr. We attribute the delta EEG trajectories to changes in synaptic density. Whatever their neuronal underpinnings, these age curves can guide research into the molecular-genetic mechanisms that underlie adolescent brain development. The DP trajectories in NREM and REM sleep differed strikingly. DP in REM did not initially increase but declined steadily from age 6 to age 16 yr. We hypothesize that the DP decline in REM reflects maturation of the same brain arousal systems that eliminate delta waves in waking EEG. Whereas the DP age curves differed in NREM and REM sleep, theta age curves were similar in both, roughly paralleling the age trajectory of REM DP. The different maturational curves for NREM delta and theta indicate that they serve different brain functions despite having similar within-sleep dynamics and responses to sleep loss. Period-amplitude analysis of NREM and REM delta waveforms revealed that the age trends in DP were driven more by changes in wave amplitude rather than incidence. These data further document the powerful and complex link between sleep and brain maturation. Understanding this relationship would shed light on both brain development and the function of sleep.
Keywords: delta, slow wave, fast Fourier transform, puberty
long-standing evidence indicates that, during adolescence, the human brain undergoes a pervasive reorganization, which appears to be driven by synaptic elimination (13). A prominent electrophysiological manifestation of adolescent brain reorganization is a well-documented decline in the 1–4 Hz (delta) EEG of non-rapid eye movement (NREM) sleep (11, 17, 32, 37). In addition to its potential value as a marker of brain development, the NREM delta decline is a focus of basic sleep research because delta reflects recuperative processes of sleep (2, 11). Although less intensively studied than delta, NREM theta EEG, another major slow-wave component of NREM sleep, is similar to delta in its within-sleep dynamics, response to sleep loss (3, 21) and steep decline across adolescence (7, 22, 27, 30).
Previous reports (8, 16), presented longitudinal data from our 9- and 12-yr-old cohorts (C9 and C12), which together span ages 9–18 yr. These data modified our early finding that NREM delta EEG declines linearly across childhood and adolescence, (19). Instead, we found a curvilinear decline that accelerates between ages 12–16.5 yr. In this 4.5-yr period, NREM delta power falls by a remarkable ∼60% (7), indicating, according to our hypothesis, an extremely high rate of synaptic elimination.
Here, we report longitudinal findings from our newly completed 6-yr-old cohort (C6) to describe the maturational trajectories of NREM delta and theta power across ages 6–10 yr. We also combine these data with those from C9 and C12 to analyze age trajectories across 6–18 yr. Our previous reports were limited to the age trajectories of delta and theta power in NREM sleep. Here, we also include longitudinal data for delta and theta age trajectories in rapid eye movement (REM) sleep. We addressed the following questions: 1) What are the trajectories of NREM delta and theta power across ages 6–10 yr; specifically, does NREM delta power peak in the first decade as suggested by cross-sectional data (20)? 2) Do the EEG data for ages 6–10 yr support our earlier, more limited longitudinal evidence that NREM theta declines before NREM delta? (3) Do the C6 data contradict our previous finding that the steepest decline of NREM delta power occurs between 12 and 16.5 yr? 4) Do the maturational trajectories of delta and theta power in NREM sleep differ from those in REM sleep? and 5) How do the maturational trajectories of period-amplitude measures of delta wave amplitude, incidence, and mean frequency in NREM and REM sleep compare to the maturational trajectories for delta power?
METHODS
Subjects
We studied 98 subjects in three overlapping age cohorts. The youngest cohort (C6, n = 28, 11 female) began the experiment at approximately age 6 yr and was studied for 4 yr. The C9 cohort (n = 32, 16 female) entered the study at approximately age 9 yr and was studied for 7 yr. The C12 cohort (n = 38, 19 female) entered the study at approximately age 12 yr and was studied for 6 yr. Thus, the study spanned ages 6 to 18 yr, with C6 and C9 overlapping at ages 9–10 yr and C9 and C12 overlapping at ages 12–16 yr. Subjects who failed to complete 3 yr of study were not included in the analyses here, which are based on 92 of the 98 subjects originally recruited.
Subjects were recruited with newspaper advertisement and word of mouth. An interview with the parents screened the subjects for the following exclusion criteria: history of head injury that resulted in loss of consciousness, epilepsy, mental illnesses, and behavioral disorders, and use of prescription medication with central nervous system effects. Parents provided informed consent, and subjects older than 12 yr of age provided assent. Subjects were paid for their participation. The University of California Davis Institutional Review Board approved the protocol and all procedures.
Experiment Design
All-night sleep EEG was recorded semiannually in the subjects' homes in their habitual sleep environment. For the C6 cohort, sleep EEG was recorded for two consecutive nights on their current weekday sleep schedules. For the C9 and C12 cohorts, sleep EEG was recorded for four consecutive nights. On nights 1 and 2, subjects adhered to their current school-night sleep schedule. On nights 3 and 4, subjects maintained their school-night bedtimes but were asked to sleep as long as possible, up to 12 h. For the 5 days prior to the first night of recording, subjects maintained their current school-night bedtimes and rise times and refrained from napping. Actigraphy watches were used to monitor compliance. When subjects occasionally deviated from their stipulated schedule, recordings were rescheduled for a later date. School-night sleep schedules changed with age. We required only that subjects maintain their current school-night bed and wake schedules prior to and during the sleep EEG recordings.
EEG Recording
Gold-cup EEG electrodes were applied at Fz, Cz, C3, C4, O1, and either O2 or Pz with mastoid electrodes at A1 and A2. Electrooculogram (EOG) electrodes were applied at the right and left outer canthi and on the forehead. Signals were amplified and recorded on Grass H2O (200-Hz digitization rate) or Grass Aura (400-Hz digitization rate) ambulatory EEG recorders. As we reported previously (5), the two recorders have the same low-frequency filters and similar frequency-response curves over the EEG frequencies described here. Signals were recorded vs. reference and central vs. contralateral mastoid (C3-A2 or C4-A1) was obtained by subtraction.
EEG Analysis
Using a computer display of the digitized EEG and EOG traces, each 20-s epoch was scored visually as waking, stage 1, NREM, REM, or movement. Scorers used Rechtschaffen and Kales (33) criteria modified by collapsing stages 2, 3, and 4 into a single NREM stage. Artifacts were marked independently of sleep stages. For each night, a second scorer checked the scoring, and a senior lab scientist reconciled any differences. Sleep duration data have been previously published (16).
For each night, the central signal (C3-A2 or C4-A1) with fewer artifacts was chosen for fast Fourier transform (FFT) analysis. EEG signals were analyzed with the FFT function of PASSPLUS (Delta Software, St. Louis, MO) using 5.12-s Welch tapered windows with 2.62 s of overlap to produce 8 windows per 20-s epoch. We report here age-related changes in delta (1–4 Hz) and theta (4–8 Hz) power. We also used PASSPLUS to determine the degree to which age-related changes in delta power were due to changes in wave incidence or wave amplitude by using the period-amplitude analysis (PAA) methods that we have previously described in detail (18). We used delta time in band (TIB) to estimate delta wave incidence. TIB is almost perfectly correlated with the actual number of half-waves but has somewhat higher within-subject reliability. Mean frequency was calculated as 0.5 times the number of half waves divided by time in band. We limited PAA to the delta band because theta frequencies are not effectively measured by either the zero-cross or zero-derivative PAA algorithms without prior band-pass filtering (23, 41)1.
Age trends in NREM and REM sleep were analyzed separately. School-night sleep durations progressively decreased across adolescence. These changes in total sleep time affect average power. Therefore, we limited the data analyzed to sleep durations commonly seen in 18-yr-olds. For NREM sleep, we analyzed all artifact-free epochs in the first 5 h of NREM sleep. For REM sleep, we analyzed artifact-free epochs in REM periods 2 and 3.
Statistical Analysis
Prior to statistical analyses, data from multiple nights were averaged within a subject for each semiannual recording. For the C6 cohort, data from nights 1 and 2 were averaged. For the C9 and C12 cohorts, data from nights 1, 2, and 3 were averaged. Night 3 was an extended night, but the first 5 h of NREM and REM periods 2 and 3 occurred prior to the period of sleep extension. Data from night 4 were excluded because sleep was extended on night 3. All statistical analyses used age as a continuous independent variable even though the figures display data averaged across subjects for each semiannual recording period.
We previously found, that for ages 9–18 yr, the age-related decline in delta and theta EEG power during NREM sleep could be described with a Gompertz growth function of the form power = D-A*e−e−C*(age−M). We use a negative A coefficient to describe decline rather than growth. The terms of the equation are as follows: D is the level of the upper asymptote, A is the difference between the upper and lower asymptotes, M is the age of most rapid decline, and C is the relative rate of decline at age M.
With additional data from 6 to 10 yr, we now describe the maturational pattern from age 6 to 18 yr for delta and theta EEG power not only in NREM sleep but also in REM sleep. We fit a Gompertz function using SAS nonlinear mixed-effect analysis with the D and A terms random, i.e., varying between subjects. We also separately analyzed the C6 data with SAS mixed-effect analysis to evaluate linear and quadratic trends across ages 6 to 10 yr. Maturational trends in period amplitude measures were analyzed with linear mixed-effect analysis. Mixed-effect analysis is well suited for longitudinal studies because it accounts for the inherent correlation between multiple observations on the same subject (35, 40).
RESULTS
Maturational Trajectory of Delta Power Within NREM Sleep
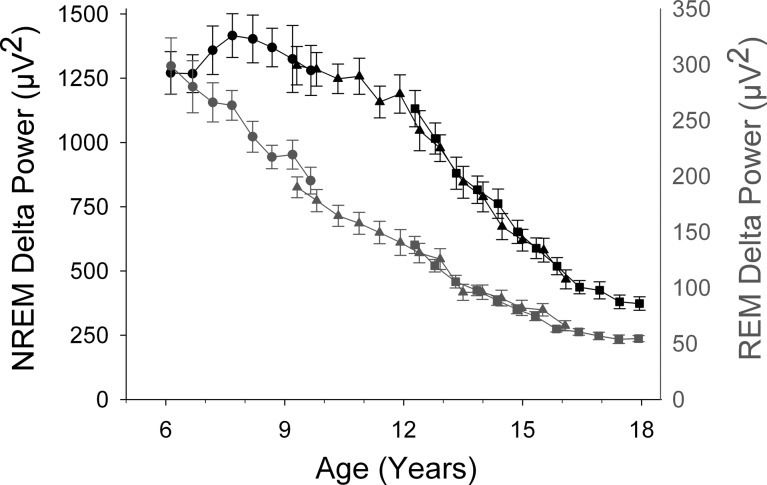
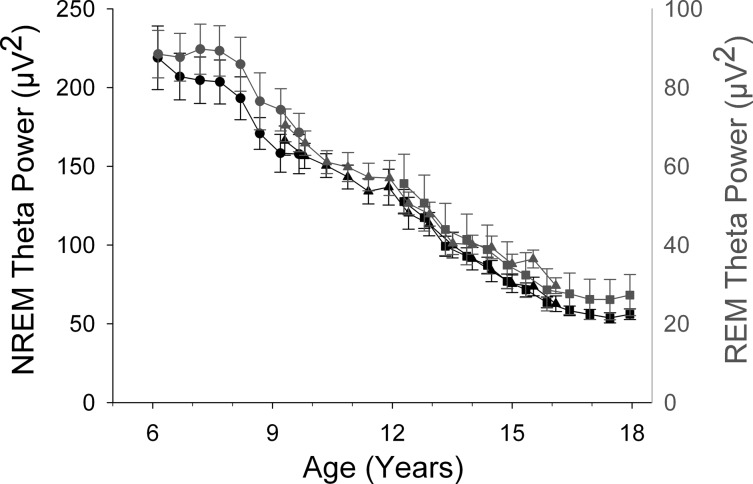
Figure 1 shows the age curves for NREM and REM delta power (DP) for ages 6–18 yr. We first discuss the NREM patterns found in C6 for ages 6–10 yr. DP increased from age 6 to about age 8 yr and then declined gradually. At age 10 yr, DP did not differ significantly from its level at age 6 and did not exhibit a significant linear trend across these ages (F1,148 = 0.98, P = 0.32). However, the curvature over 6–10 yr apparent in Fig. 1 produced a strong quadratic function: −42*(age-6)2+176*(age-6)+1222, with all coefficients significantly different from 0 at P < 0.001. We rearranged the quadratic equation to the form -A*(age-M)2 + C, where M is the age at which the quadratic peaks and C is delta power at the peak. Nonlinear mixed-effect analysis of this equation revealed a peak of 1404 μV2 occurring at 8.07 ± 0.17 yr (estimate ± SE) with a 95% confidence interval (CI) of 7.73–8.41 yr.
Fig. 1.
Comparison of the maturational trajectories for average delta (1–4 Hz) EEG power in non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. Average (± SE) delta power is plotted against age for the C6 (●), C9 (▲), and C12 (■) cohorts. Note the different scales for the NREM delta power (black) and REM delta power (gray) axes. NREM delta power showed a complex maturational trend with an initial increase to a peak at about age 8 yr and a very steep decline between ages 12 and 16.5 yr. In contrast, REM delta power declined steadily until its decline decelerated at about age 16 yr.
Combining the C6 with the C9 and C12 data allowed us to examine the DP trajectory across ages 6–18 yr. A Gompertz function fit to these data showed the age of most rapid decline as 13.32 ± 0.12 yr with a 95% CI of 13.08 − 13.56 yr. We previously reported that the decline in NREM DP was particularly rapid across ages 12–16.5 and suggested that this was the most rapid period of DP decline. However, because the C6 data peaked at 8 yr, it was possible that the decline between 8 and 12 yr would be equally or more steep. The analyses in Table 1 show this was not the case. The linear decline of DP across ages 12–16.5 yr was 2.5 times greater than the decline across ages 8–12 yr.
Table 1.
Comparison of age-related rates of decline of EEG power between ages 8 to 12 yr and 12 to 16.5 yr
| Frequency | Age Range | Intercept | Slope | Slope SE | Slope 95% CI |
|---|---|---|---|---|---|
| NREM delta | |||||
| 8 to 12 | 1405 | −64.6 | 18.8 | −27.4 to −101.8 | |
| 12 to16.5 | 1136 | −164 | 8.0 | −148 to −179 | |
| NREM theta | |||||
| 8 to 12 | 188 | −15.2 | 2.3 | −10.7 to −19.8 | |
| 12 to16.5 | 128 | −17.0 | 1.1 | −14.9 to −19.1 | |
| REM delta | |||||
| 8 to 12 | 221 | −21.0 | 2.5 | −16.0 to −25.9 | |
| 12 to16.5 | 132 | −18.0 | 1.0 | −16.0 to −20.0 | |
| REM theta | |||||
| 8 to 12 | 80.2 | −6.59 | 1.07 | −4.48 to −8.71 | |
| 12 to16.5 | 53.9 | −6.50 | 0.39 | −5.74 to −7.25 |
Estimates of intercept and slope, SEs, and confidence intervals (CI) are from linear mixed effect analysis. Only for non-rapid eye movement delta (NREM delta) is the decline significantly steeper between 12 and 16.5 yr than between 8 and 12 yr (i.e., CIs do not overlap). REM, rapid eye movement.
To determine whether the accelerated decline between 12 and 16.5 yr was specific to NREM delta among the EEG measures, we performed the same analyses on delta power in REM sleep and theta power in both REM and NREM (Table 1). Only NREM DP manifested an accelerated decline between 12 and 16.5 yr.
Maturational Trajectory of Delta Power in REM Sleep
Figure 1 shows that the maturational trajectory of DP in REM sleep differed strikingly from its NREM trajectory. Between ages 6 and 10 yr, REM DP did not show the significant curvature (quadratic F1,154 = 0.50, P = 0.48) found for NREM DP. Instead, REM DP declined linearly from 295 μV2 at age 6 at a rate of 29 μV2/yr (F1,155 = 45.1, P < 0.0001). This decline continued to age 16–17 yr, when it slowed noticeably. A Gompertz function estimated the age of most rapid DP decline in REM sleep at 6.48 ± 0.95 yr (95% CI = 4.59–8.36 yr), much earlier than the corresponding value of 13.32 yr for NREM DP (95% CI = 13.08–13.56 yr).
Period-Amplitude Measures of Delta Waveform Trajectories
Delta waveforms in NREM sleep.
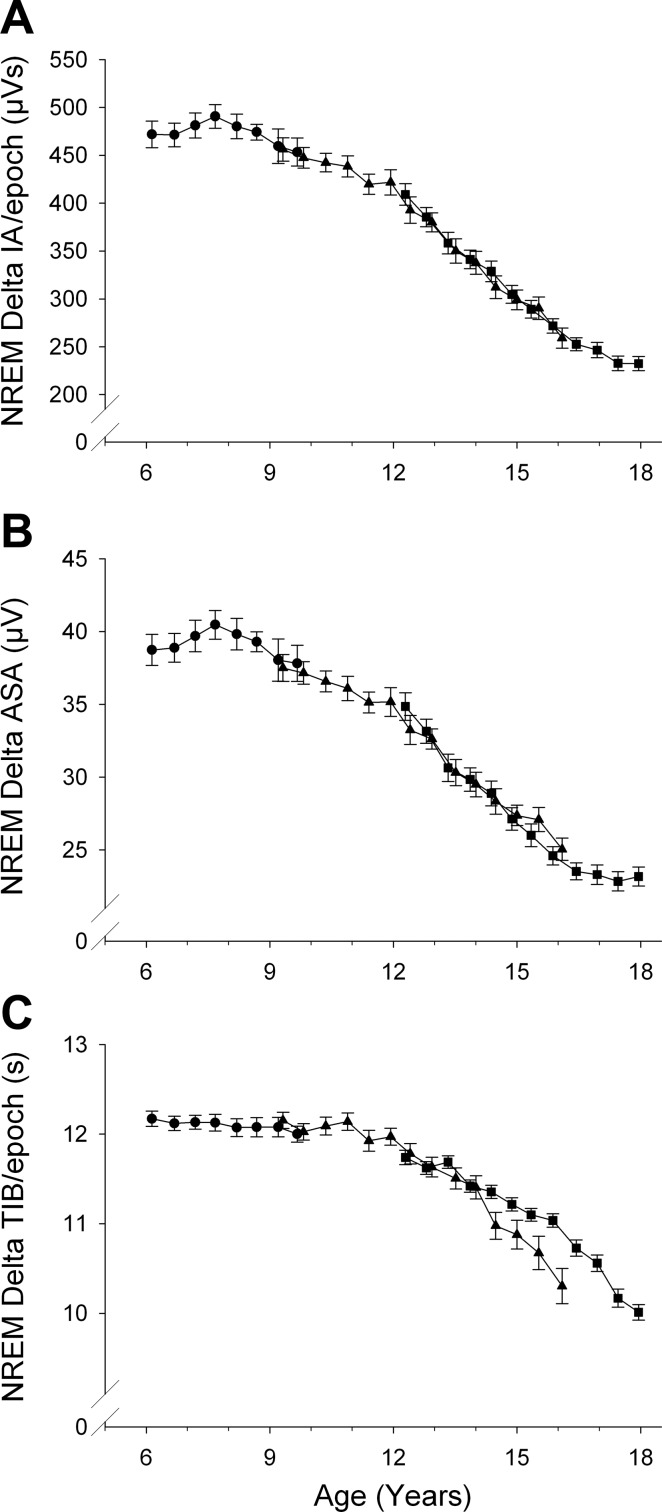
Period amplitude (PA) analysis can estimate separately average wave amplitudes (ASA) and wave incidence (TIB, or time in band). In addition, one can compute their product, integrated amplitude (IA), which is the homolog of FFT power. Both integrated amplitude and FFT power represent the combined effects of wave amplitudes and incidence. Figure 2 summarizes the PA-measured changes in delta waveforms in NREM sleep. Each of the PA measures of NREM delta changed significantly across adolescence at high F values (Table 2). As expected, the maturational trend for NREM delta IA (Fig. 2A) closely paralleled that for NREM delta power (Fig. 1).
Fig. 2.
Period-amplitude measures of delta (1–4 Hz) EEG during NREM sleep. A: maturational trend for NREM delta integrated amplitude (IA) closely resembled that for NREM delta power. IA is the product of average sample amplitude (ASA) and time in band (TIB). The maturational change in ASA (B) largely determined the trend in IA. TIB (C) remained constant between ages 6 and 11 before declining. We could not find a basis for the C9 C12 cohort difference in TIB in late adolescence. It was not due to a few outlying observations and probably represents sampling variation.
Table 2.
Age-related change in period amplitude measures of delta (1–4 Hz) EEG activity during the first 5 h of NREM sleep
| PAA Measure | Age 6 Intercept | Slope (per year) | F | P |
|---|---|---|---|---|
| IA, μVs | 552 | −27.9 | 2471 | <0.0001 |
| TIB, s | 13.0 | −0.243 | 1634 | <0.0001 |
| ASA, μV | 44.0 | −1.84 | 1739 | <0.0001 |
| Mean freq., Hz | 1.93 | 0.0191 | 1422 | <0.0001 |
Shown are results of linear mixed-effect analysis. PAA, periodamplitude analysis; IA, integrated amplitude; TIB, time in band; ASA, average wave amplitude; Freq, frequency.
Figure 2B shows that the maturational decline in NREM delta IA (and, presumably, in DP) was heavily determined by the decline in wave amplitude. Thus, the curve for NREM ASA paralleled the curves for both DP and IA. ASA increased from age 6 to peak at about age 7.5 yr and then slowly declined between ages 8 and 12 yr. After age 12, ASA declined steeply between ages 12 and 16.5–17 yr, when its rate of decline slowed.
NREM delta wave incidence, estimated as TIB/20-s epoch of NREM sleep, showed a different age trajectory. TIB showed little change across ages 6–12 yr. At about age 12 yr, delta wave incidence began a steady decline that continued to age 17.5 yr (Fig. 2C). The deceleration in its rate of decline toward the end of this age range was less pronounced for TIB than for the declines in amplitude and FFT power. The relative linear decline was greater for ASA than TIB: ASA declined at 1.84 μV/yr or 4.2%/yr, whereas TIB decreased by 0.24 s/yr or 1.9%/yr.
In an analysis similar to that performed for NREM DP, we compared the rates of change between 8 and 12 yr and between 12 and 16.5 yr for period amplitude measures. The slopes for NREM delta wave amplitude, incidence, and mean frequency were each significantly greater across 12–16.5 yr than across 8–12 yr.
Delta waveforms in REM sleep.
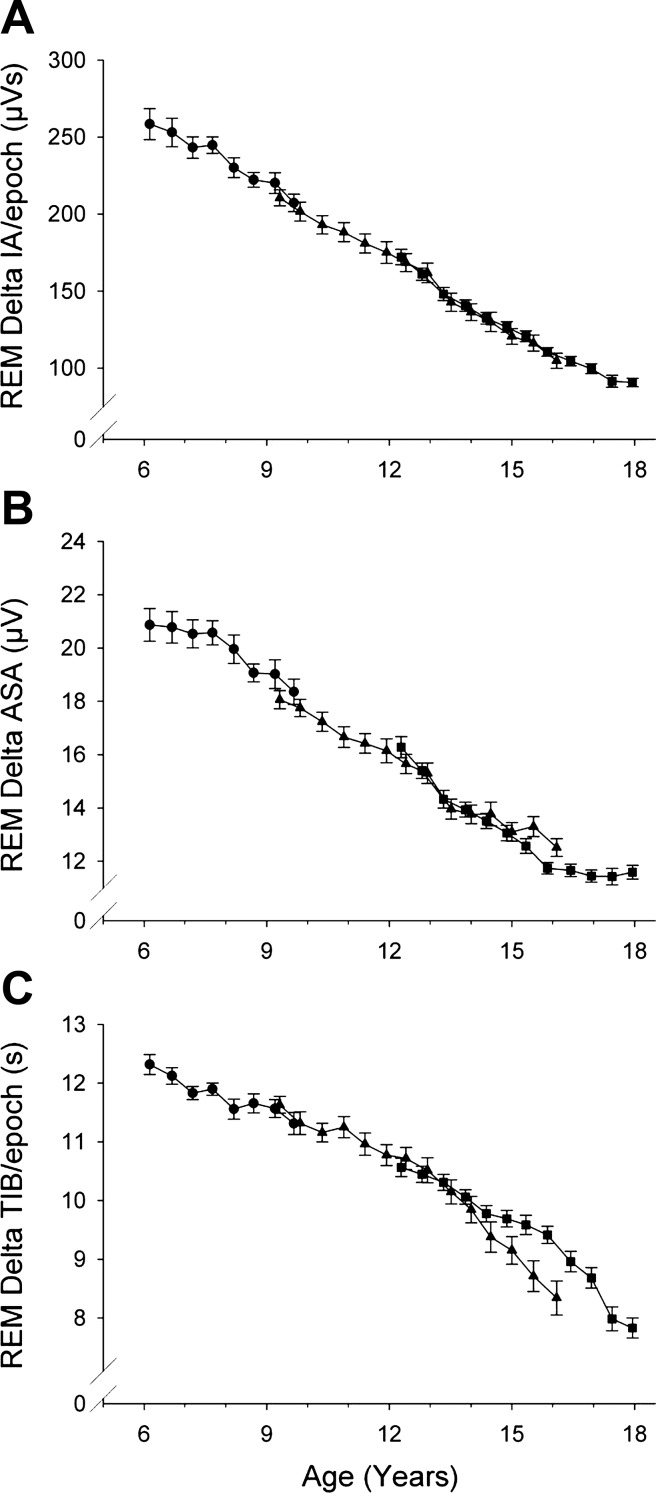
Each of the PA measures of REM delta changed significantly across adolescence (Table 3). As was true for NREM sleep, the age curve for delta IA in REM sleep (Fig. 3A) closely resembled the curve for REM DP (Fig. 1). The rate of decline in REM delta wave amplitude (4.0%/yr) (Fig. 3B) was virtually identical to that in NREM sleep (4.2%/yr). However, delta wave incidence (TIB, Fig. 3C) in REM sleep declined more with age (3.3%/yr) than in NREM sleep (1.9%/yr).
Table 3.
Age-related change in period amplitude measures of delta (1–4 Hz) EEG activity during cycles 2 and 3 of REM sleep
| PAA Measure | Age 6 Intercept | Slope (per year) | F | P |
|---|---|---|---|---|
| IA, μVs | 262 | −15.0 | 3261 | <0.0001 |
| TIB, s | 13.1 | −0.434 | 2177 | <0.0001 |
| ASA, μV | 21.1 | −0.854 | 1895 | <0.0001 |
| Mean freq., Hz | 2.26 | 0.0247 | 1095 | <0.0001 |
Shown are results of linear mixed effect analysis.
Fig. 3.
Period-amplitude measures of delta (1–4 Hz) EEG during REM sleep (format as in Fig. 2). In contrast to NREM, delta ASA and delta TIB showed similar maturational trajectories in REM sleep.
Delta mean frequency in NREM and REM sleep.
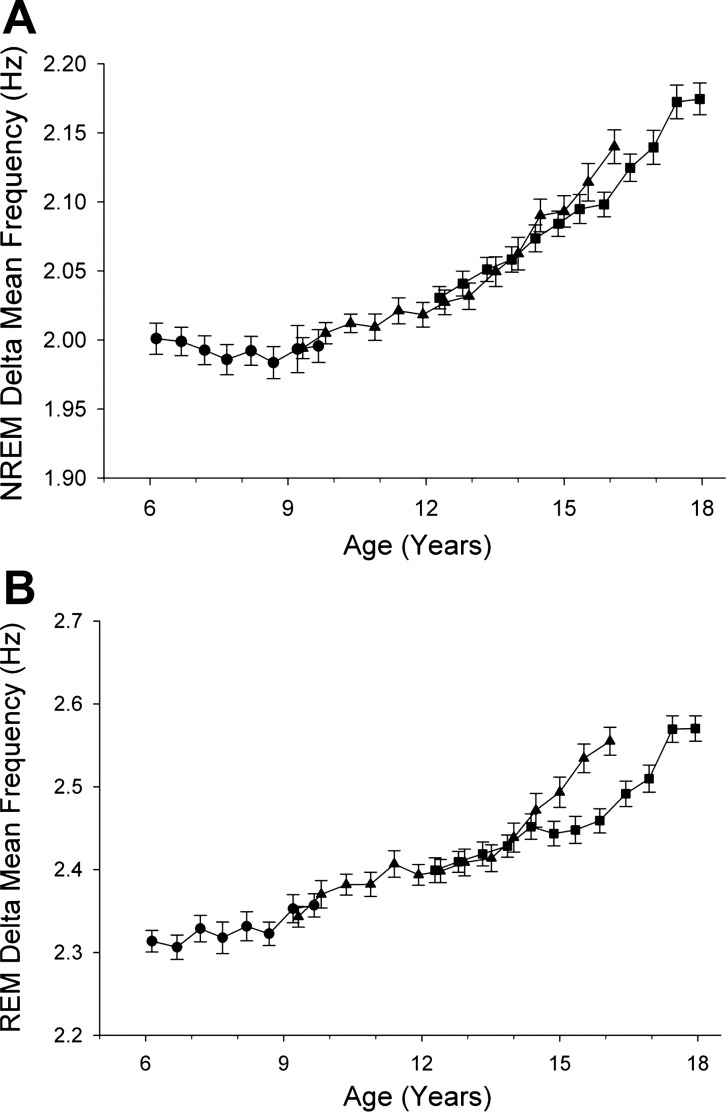
The changes in delta wave amplitude and incidence were accompanied by changes in delta wave mean frequency (Fig. 4). For the EEG of both NREM and REM sleep, mean frequency of delta waves increased significantly (P < 0.0001) from age 6 to 18 yr; however, the maturational patterns differed for NREM and REM. Between ages 6 and 10 yr, mean delta frequency in NREM sleep did not change (P = 0.93). For REM sleep EEG, mean frequency increased significantly by 0.017 Hz/yr (P < 0.0001) between ages 6 and 10 yr in the C6 cohort. From age 10 to 18 yr, delta mean frequency increased steeply and significantly (P < 0.0001) for both NREM and REM sleep.
Fig. 4.
Maturation of delta (1–4 Hz) mean frequency during NREM sleep (A) and REM sleep (B). In NREM sleep, delta mean frequency was unchanged between ages 6 and 10 yr and then increased steadily. In REM sleep, delta mean frequency increased across ages 6 to 18 yr.
Maturational trajectories of theta power in NREM and REM sleep.
In contrast to delta power, theta power showed similar age trends in NREM and REM sleep (Fig. 5). In both sleep states, theta power changed little between ages 6 and 7.5 yr. From about age 7.5 yr, NREM and REM theta power decreased steadily to age 16–17 yr, when its decline slowed. A Gompertz function fit to NREM theta estimated the age of most rapid decline at 10.43 ± 0.55 yr (95% CI 9.33–11.52 yr). This estimate was significantly earlier than the estimate of 13.32 yr for the age of most rapid NREM delta decline. The Gompertz function for theta power in REM sleep had an estimated age of most rapid decline (10.98 ± 0.16 yr) that was similar to that in NREM. Furthermore, the 95% CI (10.7–11.3 yr) for age of most rapid decline in REM overlapped the 95% CI for NREM. Separate analysis across ages 6–10 yr for NREM theta power showed a linear decline (F1,148 = 40.7, P < 0.0001) without significant curvature (quadratic component P = 0.49). REM theta power across ages 6–10 yr also showed a significant linear decline (F1,155 = 30.2, P < 0.0001). In addition, REM theta power over this age range showed a nonsignificant trend toward curvature across ages 6–10 yr (quadratic component P = 0.059).
Fig. 5.
Comparison of the maturational trajectories for theta (4–8 Hz) FFT power in NREM sleep and REM sleep. The format is the same as in Fig. 1. In contrast to the different age trajectories for delta power in NREM and REM sleep, theta power showed similar maturational trends in NREM and REM sleep.
DISCUSSION
To facilitate this discussion, we first briefly outline the conceptual models we use to interpret these results. We proposed that NREM sleep reflects processes in which changes produced by waking brain activity are reversed and that the intensity of reversal is proportional to high-amplitude EEG slow waves (11). The amount of NREM reversal needed is determined by prior wake duration and the intensity of brain activity during waking, with intensity indexed by cerebral metabolic rate (CMR). Subsequently, we proposed (20) that age changes in CMR are proportional to the age changes in cortical synaptic density observed by Huttenlocher (28), i.e., that higher levels of synaptic density produce more intense neuronal activity and, hence, higher levels of waking CMR. This increases the need for sleep recuperation, i.e., NREM delta. The model of Tononi and Cirelli (39) postulates that the homeostatic need for neuronal recovery during sleep results from an increase in synaptic “weight” during waking. Synaptic weight is reversed by “synaptic downscaling” during NREM delta sleep. Whatever the underlying neuronal mechanisms, the increase in NREM delta EEG in infancy and early childhood should reflect synaptic proliferation and its stimulating effects on CMR (and synaptic “weight”); conversely, the decline in NREM delta power over adolescence is driven by synaptic elimination, indirectly through its effects on CMR and synaptic “weight”, and directly through its influence on EEG amplitude.
Age trajectory of DP in NREM sleep.
The C6 data reveal that DP increased from ages 6 to 8 yr and then declined. We interpret this result as indicating that postnatal synaptic proliferation continues to age 8 yr, when synaptic elimination supervenes. The finding that delta power peaks in the second half of the first decade of life is consistent with our earlier cross-sectional findings (20). They showed that the ontogenetic curves for delta amplitude parallel the curves for synaptic density and CMR and that all three brain measures peak in the first decade of life and then decline steeply across adolescence (20).
Our previous analysis of C9 and C12 data suggested that the DP decline is steepest between 12 and 16.5 yr. We used the C6 data to test the possibility that a faster decline occurs earlier, i.e., between 8 and 12 yr. Our analyses ruled out this possibility. Between 12 and 16.5 yr, NREM DP declines 2.5 times faster than between 8 and 12 yr, indicating that the highest rate of synaptic elimination occurs between ages 12 and 16.5 yr. The DP data presented here are for recordings at electrodes C3 or C4. Similar analyses for Fz and Cz also show the steepest delta decline across 12–16.5 yr. This was not true for O1; the occipital lobe completes synaptic elimination prior to adolescence (29). Our topographic results are also consistent with recent findings by Kurth, Huber, and colleagues who recorded high-density EEG in subjects ranging in age from 2.4 to 19.4 yr (32). Their data also show an earlier maturation in the occipital regions and the general “back to front” maturational pattern reported with structural MRI (26). Furthermore, a direct comparison of structural MRI and EEG provides data consistent with our hypothesis that maturational changes in sleep EEG provide good markers of the brain changes involved in adolescent brain maturation (4).
In fact, we would argue that current methods of sleep EEG analysis are superior to current measurements of structural MRI for evaluating adolescent brain maturation. Thus, structural MRI has not identified the high rate of change between ages 12 and 16.5 yr (25, 31, 34). Some of these imaging studies may have lacked sufficient age resolution because they were cross-sectional. However, longitudinal MRI studies also have not detected accelerated maturational change between 12 and 16.5 yr. The resolution of these studies may have been limited by relatively few repeated measurements. Our data are based on eight multiple-night sleep recordings at 6-mo intervals in C6 and 12 to 14 multiple-night recordings at 6-mo intervals in the older cohorts. In addition, the extremely high within-subject reliability of computer-measured sleep EEG (36, 42) probably added to the resolution of these age changes.
We emphasize the high rate of maturational change between 12 and 16.5 yr because it could guide future research. Assuming that these EEG changes are driven by genetic programs, investigators could seek developmental (apoptotic?) genes whose expression is initiated around age 8 yr, increases around age 12 and decreases at about age 16.5 yr. The high rate of NREM delta maturation across ages 12–16.5 yr also recommends it as a fruitful period for studies of cognitive development: this age period seems to mark a major transition from child to adult levels of cognitive “power” (c.f. discussion in Ref. 13).
Any search for the mechanisms driving the sleep EEG changes of adolescence must consider possible links to pubertal development. Despite the existence of a sex difference in onset of the DP decline (6), it has been difficult to demonstrate a relation between pubertal change and the adolescent sleep EEG changes when age is controlled. We recently identified such a relationship in our C9 and C12 subjects. In both sexes, earlier pubertal development predicted significantly earlier DP decline (8). Further research is needed to determine whether this finding indicates that neuroendocrine systems drive sleep EEG maturation or whether both are time-linked to some master neurodevelopmental program.
It is important to note that NREM delta power continues to decline after adolescence at a slow but still appreciable rate (15, 22, 38). The delta decline during adulthood has traditionally been ascribed to aging (degenerative brain change). We have summarized evidence (14) arguing against two qualitatively different causes: maturational in adolescence and aging in adults. Others disagree (30). The resolution of this question will be important for an understanding of brain ontogeny.
Age trajectory of DP in REM sleep.
Baker et al. (1) noted that in addition to NREM DP, DP within REM sleep declines during adolescence. Our data expand on their finding and show that the age trajectory of DP in REM sleep differs markedly from its NREM trajectory (Fig. 1). Interestingly, the DP trajectory in REM more closely resembles the theta power trajectories in both NREM and REM sleep than it does the DP trajectory in NREM. This finding suggests an interpretation of the functional significance of REM DP. Neurophysiologically, REM sleep is a state of partial arousal with many elements common to waking. The age-associated decline of delta power in REM sleep might, therefore, be produced by maturation of the same arousal systems that underlie the clinically observed disappearance of delta waves from the waking EEG during the first ∼15 yr of life (24).
Age trajectory of theta power.
Preliminary evidence from the C9 and C12 cohorts indicated that NREM theta power declines earlier than does NREM delta power (7). This finding is robustly confirmed by the C6 results. These different age trajectories of delta and theta EEG in NREM sleep pose a conundrum because, as we noted in our introduction, NREM delta and theta power otherwise share important biological properties: both decline across NREM sleep (5), increase after sleep deprivation (3), are decreased following napping (44), and decline strongly across childhood-adolescence (7). However, their different age trajectories indicate they have different functional correlates. Here, we hypothesize a broad functional difference based on the similarity of the delta age trajectory in REM sleep to the theta trajectories in both NREM and REM. If we are correct in hypothesizing that the age-related decline in REM delta power reflects maturation of cortical arousal systems, the similarity of the age curves of theta power to the REM delta trajectory suggests that theta EEG during NREM sleep might also reflect the recuperation of cortical arousal systems. It is well established that the waking state requires input from the brain stem reticular activating system (RAS). In producing the waking state, RAS stimulation must increase the responsivity of large populations of cortical neurons. This effect may itself produce a need for sleep recuperation carried out during NREM theta. Thus, we speculate that the EEG slow waves of NREM sleep reflect two general restorative processes. One (NREM theta) reverses neuronal changes produced by waking itself. The second (NREM delta) reverses some of the changes produced during waking in plastic neuronal systems that perform information processing and memory formation.
Maturational changes in delta waveforms.
Period-amplitude analysis showed that the maturational decline in NREM delta wave amplitude was greater than the decline in delta incidence. In addition, the age curves for amplitude more closely paralleled the curves for FFT power than did the curves for wave incidence. In REM sleep, the differences between the decline rates of delta wave amplitude and incidence were less marked than in NREM sleep. These changes in amplitude and incidence were accompanied by a strong age-dependent increase in average delta wave frequency. This age trajectory for mean frequency differed in NREM and REM sleep for the C6, but not the two older cohorts. The different maturational patterns of delta wave amplitude and incidence in NREM sleep raise the possibility that they reflect different aspects of the hypothesized processes of sleep recuperation. Further interpretation would require knowledge of the cellular and molecular processes involved.
These observations suggest that normal brain maturation could provide a useful experimental model for elucidating the mechanisms that underlie EEG oscillations. Standard theory holds that the amplitude of EEG waves is determined by synchronous, slow changes in the membrane potentials of large populations of cortical neurons (10). Therefore, the decreasing neuronal connectivity produced by synaptic elimination could decrease the size of the cortical populations whose potentials oscillate synchronously, decreasing EEG amplitudes (13). Wave incidence is thought to be driven by subcortical pacemakers (9). Despite its plausibility, we still lack mechanistic proof for this model. Such evidence might be obtained by using the maturational changes in nonhuman primate sleep as a research model.
Perspectives and Significance
This paper marks the completion of data collection in a 10-yr longitudinal study of child-adolescent sleep EEG. This study has contributed new observations on sleep durations, the age trajectories of NREM, and REM delta and theta EEG, the emergence of daytime sleepiness, sex differences, and the temporal link between EEG and pubertal maturation. Future publications will describe the trajectories of other EEG frequencies and overall EEG spectra. With the support of National Institute of Mental Health, the unique data set we have collected will be archived on the National Institutes of Health National Database for Autism Research. The data, to be available to all researchers, will include raw and analyzed sleep EEG, height, weight, and Tanner stage at each measurement point. The data can be used to verify our findings or to perform entirely new EEG and statistical analyses. It is already clear that the sleep EEG changes robustly with adolescent brain development. Understanding these electrophysiological brain changes will shed light on the mechanisms of postnatal brain maturation, which, in turn, could provide new insights into the function of sleep. Moreover, since major mental illness often begins in adolescence, developmental sleep EEG changes are clinically significant. Observations on sleep EEG stimulated the “synaptic pruning” hypothesis of schizophrenia (13) and the complex patterns of postnatal sleep EEG maturation gave rise to the hypothesis that the 1% incidence of schizophrenia reflects a stochastic error in adolescent brain development rather than the frequency of a specific abnormal gene (12), a possibility that recently received experimental support (43).
GRANTS
United States Public Health Services grant R01MH062521 supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.F. and I.G.C. conception and design of research; I.F. and I.G.C. interpreted results of experiments; I.F. drafted manuscript; I.F. and I.G.C. edited and revised manuscript; I.F. and I.G.C. approved final version of manuscript; I.G.C. performed experiments; I.G.C. analyzed data; I.G.C. prepared figures.
ACKNOWLEDGMENTS
We thank the research associates and student assistants who helped collect and analyze the data over the course of this longitudinal project. We also thank the participants and their families. Adair McPherson, PhD helped edit the many versions of this manuscript. Dr. Adair McPherson made helpful comments on several versions of this manuscript.
Footnotes
In addition to 1–4 Hz, we examined the PAA data separately for 1–2 Hz because some investigators have found that these frequencies show the highest agreement with FFT delta results (23, 41). In our data, the 1–2 Hz band included ∼70% of the FFT power. Because the age curve for 1–2 Hz was nearly identical to the 1–4 Hz curve, we present the latter, more inclusive data.
REFERENCES
- 1. Baker FC, Turlington SR, Colrain I. Developmental changes in the sleep electroencephalogram of adolescent boys and girls. J Sleep Res 21: 59–67, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borbély AA. A two process model of sleep regulation. Hum Neurobiol 1: 195–204, 1982 [PubMed] [Google Scholar]
- 3. Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep-deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol 51: 483–493, 1981 [DOI] [PubMed] [Google Scholar]
- 4. Buchmann A, Ringli M, Kurth S, Schaerer M, Geiger A, Jenni OG, Huber R. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex 21: 607–615, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Campbell IG, Darchia N, Higgins LM, Dykan IV, Davis NM, de Bie E, Feinberg I. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during non-rapid eye movement sleep. Sleep 34: 83–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell IG, Darchia N, Khaw WY, Higgins LM, Feinberg I. Sleep EEG evidence of sex differences in adolescent brain maturation. Sleep 28: 637–643, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci USA 106: 5177–5180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell IG, Grimm KJ, de Bie E, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc Natl Acad Sci USA 109: 5740–5743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci 13: 9–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elul R. The genesis of the EEG. Int Rev Neurobiol 15: 227–272, 1972 [DOI] [PubMed] [Google Scholar]
- 11. Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res 10: 283–306, 1974 [DOI] [PubMed] [Google Scholar]
- 12. Feinberg I. Schizophrenia as an emergent disorder of late brain maturation. In: Neurodevelopmental and Adult Psychopathology, edited by Keshavan MS, Murray R. Cambridge, MA: Cambridge University Press, 1997, p. 237–252 [Google Scholar]
- 13. Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 17: 319–334, 1982/1983 [DOI] [PubMed] [Google Scholar]
- 14. Feinberg I. Slow-wave sleep and release of growth hormone. JAMA 284: 2717–2718, 2000 [PubMed] [Google Scholar]
- 15. Feinberg I, Carlson VR. Sleep variables as a function of age in man. Arch Gen Psychiatry 18: 239–250, 1968 [Google Scholar]
- 16. Feinberg I, Davis NM, de Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol 302: R533–R540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feinberg I, Hibi S, Carlson VR. Changes in EEG amplitude during sleep with age. In: The Aging Brain and Senile Dementia, edited by Nandy K and Sherwin I. New York: Plenum Press, 1977, p. 85–98. [Google Scholar]
- 18. Feinberg I, March JD, Fein G, Floyd TC, Walker JM, Price L. Period and amplitude analysis of 0.5–3 c/sec activity in NREM sleep of young adults. Electroencephalogr Clin Neurophysiol 44: 202–213, 1978 [DOI] [PubMed] [Google Scholar]
- 19. Feinberg I, March JD, Flach K, Maloney T, Chern WJ, Travis F. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (delta) electroencephalogram of human sleep. Brain Dysfunction 3: 183–192, 1990 [Google Scholar]
- 20. Feinberg I, Thode HC, Jr, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol 142: 149–161, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Finelli L, Borbély AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci 13: 2282–2290, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res 10: 165–172, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Geering BA, Achermann P, Eggimann F, Borbély AA. Period-amplitude analysis and power spectral analysis: a comparison based on all-night sleep EEG recordings. J Sleep Res 2: 121–129, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Gibbs F, Gibbs E. Atlas of Electroencephalography. Reading, MA: Addison-Wesley, 1958 [Google Scholar]
- 25. Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2: 861–863, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hetta J, Almqvist M, Liljenberg B, Roos B. Epidemiological aspects on relations between psychiatric and somatic symptoms and sleep disturbances. Sleep Res 16: 356, 1987 [Google Scholar]
- 28. Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res 163: 195–205, 1979 [DOI] [PubMed] [Google Scholar]
- 29. Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett 33: 247–252, 1982 [DOI] [PubMed] [Google Scholar]
- 30. Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep 27: 774–783, 2004 [PubMed] [Google Scholar]
- 31. Jernigan TL, Trauner DA, Hesselink JR, Tallal PA Maturation of human cerebrum observed in vivo during adolescence. Brain 114: 2037–2049, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci 30: 13211–13219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington, D. C.: Public Health Services, U.S. Government Printing Office, 1968 [Google Scholar]
- 34. Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28: 3586–3594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 23: 323–355, 1998 [Google Scholar]
- 36. Tan X, Campbell IG, Feinberg I. Internight reliability and benchmark values for computer analyses of non-rapid eye movement (NREM) and REM EEG in normal young adult and elderly subjects. Clin Neurophysiol 112: 1540–1552, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep 33: 801–809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarokh L, Van Reen E, LeBourgeois M, Seifer R, Carskadon MA. Sleep EEG provides evidence that cortical changes persist into late adolescence. Sleep 34: 1385–1393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull 62: 143–150, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology. Cambridge, MA: Cambridge University Press, 2003 [Google Scholar]
- 41. Uchida S, Feinberg I, March JD, Atsumi Y, Maloney T. A comparison of period amplitude analysis and FFT power spectral analysis of all-night human sleep EEG. Physiol Behav 67: 121–131, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Van Dongen HPA, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep 28: 479–496, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 320: 539–543, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Werth E, Dijk DJ, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol Regul Integr Comp Physiol 271: R501–R510, 1996 [DOI] [PubMed] [Google Scholar]