Abstract
Adaptive evolutionary change in only a few generations can increase the ability of non-native invasive species to spread, and yet adaptive divergence is rarely assessed in recently established populations. In this study, we experimentally test for evidence of fine-scale local adaptation in juvenile survival and growth among three populations of an invasive freshwater fish with reciprocal transplants and common-garden experiments. Despite intrinsic differences in habitat quality, in two of three populations we detected evidence of increased survival in ‘home’ versus ‘away’ environments with a Bayesian occupancy model fitted to mark–recapture data. We found support for the ‘local’ versus ‘foreign’ criterion of local adaptation as 14 of 15 pairwise comparisons of performance were consistent with local adaptation (p < 0.001). Patterns in growth were less clear, though we detected evidence of location- and population-level effects. Although the agents of divergent ecological selection are not known in this system, our results combine to indicate that adaptive divergence—reflected by higher relative survival of local individuals—can occur in a small number of generations and only a few kilometres apart on the landscape.
Keywords: divergent selection, contemporary evolution, invasive species, reciprocal transplant, immigrant inviability, Salmo trutta
1. Introduction
Theory predicts that in the absence of constraints, populations that experience divergent regimes of natural selection will evolve adaptations to local conditions [1–3]. Empirical evidence indicates that adaptive divergence of fitness-related traits can occur in a small number of generations [4,5] and at fine spatial scales [6]. As a poignant example, green turtle (Chelonia mydas) embryos display adaptive responses to contrasting thermal regimes among nesting beaches only a few kilometres apart [7]. Much of what we have learned about the conditions that promote adaptive divergence, and the speed and form of that divergence, has emerged from the opportunistic natural experiments afforded by biological invasions (reviewed by earlier studies [8,9]) or natural range expansions into new habitats [10,11]. It is increasingly clear that rapid evolutionary change can influence ecological dynamics in invaded ecosystems [12], and that adaptive evolution can create positive feedbacks on the ability of invaders to successfully disperse and colonize novel environments [13,14]. Despite its obvious importance, adaptation in recently established populations, and especially among vertebrates, is rarely assessed and even less frequently in situ.
In this study, we experimentally test for fine-scale local adaptation in three recently established populations of one of the world's worst alien invaders [15] with in situ reciprocal transplants and common-garden experiments. Brown trout (Salmo trutta) has been intentionally introduced to at least 42 countries on six continents [16]. Established populations can dramatically alter freshwater ecosystem functioning via trophic cascades [17], can influence the evolutionary trajectories of prey species [18], and are implicated in the extirpation of native fishes [19]. Brown trout populations in the native range display adaptation to local conditions at spatial scales in the hundreds of kilometres [20], and evidence from other related species suggests the potential to adapt to conditions at even finer spatial scales [21]. Although rapid development of reproductive isolation and divergence in neutral markers has been demonstrated in some established populations [22], no study has tested explicitly for adaptive divergence. This lack of understanding currently impairs the ability to accurately gauge the speed and potential for established populations of brown trout to further invade around the globe [23].
This study reports on the results of common-garden and in situ reciprocal transplant experiments designed to test simultaneously the home versus away and local versus foreign criteria of local adaptation [1]. Broadly speaking, these two criteria assess performance among and within environments. The home versus away criterion predicts that when local adaptation is present, individuals should perform better in their local conditions compared with foreign environments. Similarly, the local versus foreign criterion predicts that local individuals should consistently outperform foreign individuals within their home environments. Following the recommendations of earlier studies [1,21], we consider the local versus foreign criterion as diagnostic for the pattern of local adaptation as the home versus away criterion has the potential to confound local adaptation with inherent differences in habitat quality. Although evidence that satisfies both criteria intuitively is the most compelling, evidence for the local versus foreign criterion alone is as powerful as finding evidence for both [1].
2. Methods
(a). Study system
Brown trout, a member of the family Salmonidae, exhibits marked variability in its biology and ecology [24,25]. An archetypical life history of brown trout includes autumn spawning in streams and rivers followed by protracted embryonic and larval development in gravel substrate. Young fish emerge to claim and maintain feeding territories in the spring. The period of freshwater residence is a critical period in the life history of such salmonids and a determinant of performance later in life [26,27]. The native range of brown trout is Eurasian, but brown trout's reputation as a game fish led to widespread introductions around the globe [28]. The former British colony of Newfoundland was among the first North American locales to receive shipments of brown trout embryos from Europe in 1883 [29].
Middle Rocky Brook is a small, unproductive, high-gradient stream that flows from its headwaters into landlocked Windsor Lake, which was the site of first introduction of trout to Newfoundland from Europe [30]. Approximately 4.5 km away flows the Rennies River, which since 1884 has received plantings of fish from the Windsor Lake source. The introduction of fish to the Rennies River watershed is notable as it represents the first location in Newfoundland with a traversable connection to the ocean, and thus represents the initial colonizing source for other watersheds. The Waterford River runs 6 km from the Rennies, though the timing of trout establishment here is unknown. Past work suggests that the initial source of the Waterford trout population was ‘natural colonization’ from the Rennies River watershed [30]. In contrast to Middle Rocky Brook, both the Rennies and Waterford are larger and deeper, are more productive, and have a lower gradient (table 1). These populations currently display significant phenotype × habitat correlations that are consistent with responses to divergent selection [31], but the extent to which differences are adaptive is not known.
Table 1.
Abiotic and biotic characteristics of Middle Rocky Brook, Rennies River and Waterford River in Newfoundland, Canada (mean±s.d., temperature minimum and maximum range). Photos by C. Conway.
 Middle Rocky Brook Middle Rocky Brook |
 Rennies River Rennies River |
 Waterford River Waterford River |
|
|---|---|---|---|
| section length (m) | 386 | 935 | 725 |
| width (m) | 1.8 (0.81) | 6.5 (1.9) | 5.6 (0.30) |
| depth (cm) | 7.5 (0.14) | 23.2 (4.2) | 11.9 (20.2) |
| flow (m s−1) | 0.12 (0.04) | 0.29 (0.21) | 0.24 (0.08) |
| gradient (%) | 4.4 | 1.5 | 1.5 |
| conductivity (µS cm−1) | 43.6 (0.47) | 246.3 (17.1) | 299.3 (30.8) |
| pH | 5.8 (0.19) | 7.02 (0.08) | 7.41 (0.2) |
| temperature | 10.3 (1.5–21) | 11.7 (1.2–20) | 11.3 (0.9–21) |
| canopy cover (ranking 1–4) | 3.33 | 1.67 | 1 |
| conspecific density (g m−2) | 2.59 (2.5) | 2.21 (1.6) | 4.6 (2.6) |
| eels (Anguilla rostrata) | absent | present (0.018 fish m−2) | present (0.021 fish m−2) |
| brown trout age distribution | 0–2 | 0–5+ | 0–6+ |
(b). Experimental animals
We captured wild adult trout and created 23 families by crossing eight unique sires and dams at the Middle Rocky and Rennies locations, and seven unique parents at the Waterford. An eighth family originally created from the Waterford suffered 100 per cent mortality during early embryonic development. We assume that the parents selected for crossing were a random sample of spawning individuals and representative of the three populations. Non-genetic maternal effects can influence patterns of survival and performance in brown trout, which in part is mediated by differences in egg size and correlated traits [26,32]. Thus, we quantified the wet mass (mg) of 10 fertilized ova from each female, following Fleming & Ng [33]. Females from Middle Rocky Brook produced eggs that were on average (mean = 131.6 mg, s.d. = 18.6) approximately 20 per cent heavier than eggs produced by Rennies (107.2 ± 16.7) or Waterford (109.8 ± 31.8) females, and the differences were significant (ANOVA, p < 0.001). See the electronic supplementary material for additional details.
To minimize any potential maternal effects resulting from the observed differences in egg size, embryos were incubated at the family level in standard hatchery trays on a common source of flow-through water at ambient temperature. Except for the one family from the Waterford that experienced 100 per cent mortality, survival during incubation was high (greater than 90% in all families). Fish were removed from incubator trays when their yolk sac had been nearly absorbed and they exhibited free-swimming behaviour. Individuals were housed temporarily as separate families to monitor the potential mortality during this stage and introduced to artificial food. After acclimation to commercial dry salmon feed, families from each population were combined into adjacently positioned communal 1 m2 diameter tanks (one population per tank) as space limitations precluded separate rearing of families. Fish were kept on photoperiods that emulated natural day length (and adjusted periodically with seasonal changes), shared a common flow-through ambient water source and were fed ad libitum four to eight times daily.
In mid-July, 300 randomly chosen individuals from each of the three laboratory populations (hereafter ‘laboratory-born’) were lightly anaesthetized with MS-222, weighed to the nearest 0.0001 g on an analytical balance and photographed with a Nikon D300 (using 60 mm Micro Nikkor lens). Photographs were taken in a standardized position, and from the photos we quantified fork length to the nearest 0.5 mm in ImageJ (freely available at http://rsbweb.nih.gov/ij). Fish were then implanted with a passive integrated transponder (PIT) tag (length 8.4 mm, weight 64 mg, frequency 134.2 kHz; Biomark, Boise, ID) through a small vertical incision in the abdominal cavity and allowed to recover in common 1 m2 circular tanks. Mortality as a result of tagging was very low (less than 2%), and there were no signs of tag loss. In virtually all cases, fish were feeding and behaving normally within a few hours.
To investigate potential effects of laboratory rearing prior to release, we captured 100 wild individuals (hereafter ‘wild-born’) of the same age and approximate size from each location, and brought them to the laboratory for tagging and sampling (see above). We were able to collect only 65 wild-born individuals at the Middle Rocky location as many of the young-of-year trout were less than the threshold size for tagging (approx. 50 mm) and as a result 48 per cent were larger (greater than 65 mm) yearlings. We chose not to tag additional large wild fish in order to avoid further confounding of the effect of population origin (i.e. wild-born) and size. To be clear, wild-born fish were not transplanted among environments, but were used as a comparison to laboratory-born individuals within locations.
In addition to fish destined for release, 50 additional individuals from each of the Middle Rocky, Rennies and Waterford laboratory-born populations were measured, PIT tagged and held in the laboratory to facilitate the tracking of individual growth in a controlled common environment.
(c). Reciprocal transplants
At each location, approximately 100 individuals from each of the four experimental groups (local wild-born, local laboratory-born and two foreign laboratory-born) were released. Counting errors and mortality in transit led to minor differences in the number of individuals released in each group (table 2). However, we managed to tag only 65 wild-born individuals from the Middle Rocky Brook location as a result of naturally low numbers of wild fish available to sample.
Table 2.
Numbers of tagged brown trout released and percentage recovered during sampling bouts on approximately 70–100 DPR and approximately 325–370 DPR for three streams in Newfoundland, Canada to test for local adaptation. Fish were released into Middle Rocky Brook on 31 Jul 2009 and recovery attempts occurred on 7 Oct, 14 Oct, 23 Jun 2010, 24 Jun, 29 Jul and 4 Aug. Fish were released into Rennies on 4 Aug, 2009 and recoveries occurred on 5–6 Oct, 12 Oct, 23 Oct, 11 Nov, 25 Jun 2010, 27 Jul and 6–7 Aug. Fish were released in the Waterford on 7 Aug 2009 and recovered on 11 Oct, 13 Oct, 22–23 Oct, 26 Jun 2010, 28 Jul and 4–5 Aug.
| release location | population origin | number released | % recovery |
|
|---|---|---|---|---|
| autumn 2009 70–100 DPR |
summer 2010 325–370 DPR |
|||
| Middle Rocky Brook | Middle Rocky | 101 | 30 | 12 |
| Rennies | 100 | 16 | 4 | |
| Waterford | 100 | 22 | 8 | |
| wild | 65 | 34 | 4 | |
| Rennies | Middle Rocky | 100 | 9 | 2 |
| Rennies | 100 | 11 | 1 | |
| Waterford | 102 | 14 | 2 | |
| wild | 99 | 25 | 1 | |
| Waterford | Middle Rocky | 101 | 17 | 2 |
| Rennies | 98 | 12 | 3 | |
| Waterford | 100 | 18 | 3 | |
| wild | 100 | 28 | 5 | |
We conducted sampling to recapture tagged individuals beginning approximately 70 days post-release (DPR) and continuing to approximately 100 days DPR. We returned the following season to assess overwinter survival and performance (table 2; additional details in [34]). Our goal was to search the length of the experimental rivers in their entirety in order to maximize tag recovery and to accurately gauge relative apparent survival between groups. Each section of river (delineated by major habitat categories) was sampled in an upstream direction with electrofishing. Captured fish, regardless of size, were removed and temporarily stored in aerated containers until the upstream part of the section was reached. All fish, including potential predators (large brown trout and American eels) that may have ingested a tagged trout, were then scanned for PIT tags with handheld readers (Pocket Reader, Biomark). We conducted additional upstream electrofishing passes when tagged individuals were recovered and repeated the sampling of sections until subsequent passes yielded no additional tags.
Recovered tagged individuals were recorded by river section, lightly anaesthetized, weighed (0.1 g) and photographed in the field using the same protocol and equipment used prior to release. After individuals had recovered from handling and anaesthesia, they were released near their site of capture at the end of the day.
(d). Estimating apparent survival and size-specific growth
(i). Home versus away
We estimated ‘home versus away’ performance with a hierarchical Bayesian occupancy model, fitted to individual recapture histories [35]. Each season was allowed to have a unique survival rate, modelled on a daily interval (‘autumn’ = 1 Oct–15 Nov, ‘winter’ = 15 Nov–1 May, ‘spring/summer’ = 1 May–15 Aug). We formulated a model that we felt captured the biology of the system. Specifically, within each season, we used the location of release as a proxy for environmental effects, experimental group (population origin, laboratory- or wild-born) as a proxy for genetic effects and a model that included both terms as a proxy for gene by environment interactions (G × E). Because of the large number of groups (location × origin), we treated group as a random effect within each season, assuming that they were distributed around a common mean. Survival in stream-dwelling salmonids can be influenced by individual traits such as body size [36] or condition [37]; therefore, we evaluated models that allowed individual covariates in survival. Survival was estimated while controlling for any effects of body size (i.e. length or weight) or condition (i.e. residuals from a log–log regression of length on weight) based on traits measured at the time of release. We interpret the body size covariate to capture both genetic differences and any lingering maternal effects among populations. Covariates were included in models individually to account for correlations between them, and their importance assessed via examination of posterior distributions. Posterior distributions with credible intervals not overlapping zero were included in final models. In addition, we compared models that allowed detection probability to be the same across sites to the performance of other models that allowed each site to have a unique detection probability. This allowed us to account for site-specific factors such as river width or depth that may have influenced detection. Note that this model assumes a closed system (i.e. that no emigration is possible). While this assumption is probably violated in our system, we attempted to select sites that would limit upstream passage (see the electronic supplementary material). Downstream passage was not restricted, but downstream habitats were sub-optimal, and we therefore assume that displacement downstream would be equivalent to mortality [38,39] (but see [40]).
We used linear models in a selection framework using AICc to test for differences in growth among groups and rearing locations. Analogous to our approach to quantify apparent survival, we fitted four ANOVAs with the following parameters (set as fixed factors): (i) population origin only (this includes wild-produced and laboratory-raised groups), (ii) location only, (iii) population × location and (iv) a null model with all predictors set to zero. Specific growth rates were estimated for individuals that survived and were recaptured in the autumn as few recaptures precluded robust comparisons of growth among groups and locations over longer time periods. Because organism growth rate varies as a function of size, we used a standardized mass-specific growth rate (Ω), following Ostrovsky [41], to quantify growth among groups while controlling for the effect of size:
where M1 and M2 are body mass (g) at the beginning and end of the experiment, respectively, time is the number of days between observations, and τ is the species-specific allometric coefficient for the relationship between growth rate and body mass. We set τ = 0.308 as the value is well established in brown trout [42]. Growth data were examined for normality and met parametric assumptions of ANOVA prior to fitting. Again, we biologically interpret the importance of the population grouping term as evidence of genetic control over growth, the location term to be the role of environmental influence on growth, and the interaction between population origin and location as evidence of underlying genetic control on growth norms of reaction.
(ii). Local versus foreign
Second, we quantified ‘local versus foreign’ performance using the log-odds ratio, following the approach of Fraser et al. [21]. For consistency with Fraser et al. [21], we based performance on the relative proportion of recaptured local versus foreign individuals during the autumn period (approx. 70–100 DPR). We included individuals not observed in the autumn, but detected the subsequent year—and thus that must have been alive in the autumn—as ‘recaptured’. Positive effect sizes were interpreted as evidence for local advantage, whereas negative effect sizes suggested the opposite. We treated both the wild-born and laboratory-born local groups within a location as ‘local’ when compared with other groups, and treated the wild-born individuals as ‘local’ when compared with their laboratory-born ‘local’ counterparts. We then compared the number of comparisons that were positive (i.e. suggestive of local advantage) out of all the pair-wise comparisons against the expected binomial probability of 50 : 50. Similarly, we quantified and compared the number of pair-wise comparisons of mean growth (generated from 10 000 bootstraps of the data) where local exceeded foreign growth rates with binomial tests. Data are available at Dryad (http://dx.doi.org/10.5061/dryad.pc14j).
3. Results
(a). Home versus away criterion
Of the 1166 total fish released, we recovered 198 individuals (19.2%) during sampling periods in the autumn (70–100 DPR) and 46 (4%) the following summer (325–370 DPR; table 2). As a caveat, we acknowledge that few recaptures in the period 325–370 DPR have the potential to influence the robustness of the results. That being said, the Bayesian occupancy model fitted to recapture histories over the course of the experiment (370 DPR) detected evidence in two of the populations that was consistent with higher apparent survival of individuals when reared in ‘home’ environments compared with when reared ‘away’ (figure 1). Median survival of Middle Rocky individuals was 6.5 per cent ± 0.05 (s.d.) in their home environment compared with 3.7 per cent ± 0.02 and 3.3 per cent±0.02 when reared away in the Rennies or Waterford environments, respectively. Rennies individuals survived best at ‘home’ (4.2% ± 0.03) and worse in the Waterford (3.9% ± 0.03) and Middle Rocky (2.4% ± 0.02). By contrast, Waterford individuals survived better at home (4.6% ± 0.03) than in Middle Rocky Brook (4.2% ± 0.02), but at higher rates away in the Rennies (6.3% ± 0.04). Respective survival of wild-born groups was 3.9 per cent ± 0.02, 5.1 per cent ± 0.03 and 5.8 per cent ± 0.03 in Middle Rocky, Rennies and Waterford. But as wild-born groups were not transplanted among locations comparisons of survival of wild versus laboratory-born individuals were done only within locations (see below). We observed that survival was independent of fish length or mass, but was positively related to individual condition (i.e. residuals from length–weight relationships). In other words, individuals that were heavy for their length survived at higher rates than individuals that were light for their length (see the electronic supplementary material, figure S1). We found evidence suggestive of site-specific detection probability as models with variable detection probability parameters were more supported by the data (deviance information criterion scores were more than 100 units better), yet had negligible impact on differences in survival rates and did not alter interpretation of survival among locations. Detection was highest in Middle Rocky (13.2%, 95% CI 10.9–15.9%), lowest in Rennies (3.1%, 2–4%) and intermediate in the Waterford (7.9%, 6.1–10.2%), which scales with stream size (Middle Rocky < Waterford < Rennies; table 1).
Figure 1.
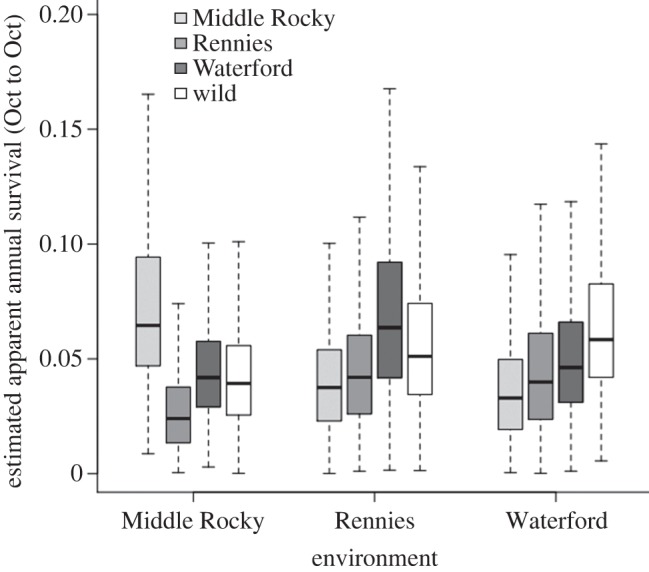
Apparent annual relative survival from a Bayesian occupancy model fitted to recapture histories of laboratory-born and wild-born juvenile brown trout reciprocally planted into local or foreign natural environments in Newfoundland, Canada. Survival estimates control for the positive effect of body condition (residuals from logged length–weight relationships). Lines represent median survival, boxes capture the 25th and 75th interquartile of the survival estimates, and the whiskers are 1.5 times the interquartile. See table 2 for recapture information.
Counter to predictions, growth rates were not highest for individuals when reared in local environments, but rather varied across locations consistent with intrinsic differences in habitat quality (table 1). In roughly 120 DPR, populations grew over twice as fast when reared in the Rennies and Waterford locations compared with the Middle Rocky, and faster yet in the common-laboratory environment (figure 2). Despite little evidence of local adaptation in growth, populations exhibited differential patterns of growth among locations, consistent with underlying gene × environment interactions (ANOVA with population × location term 17.4 likelihood units better than the next candidate model that contained only a location effect).
Figure 2.
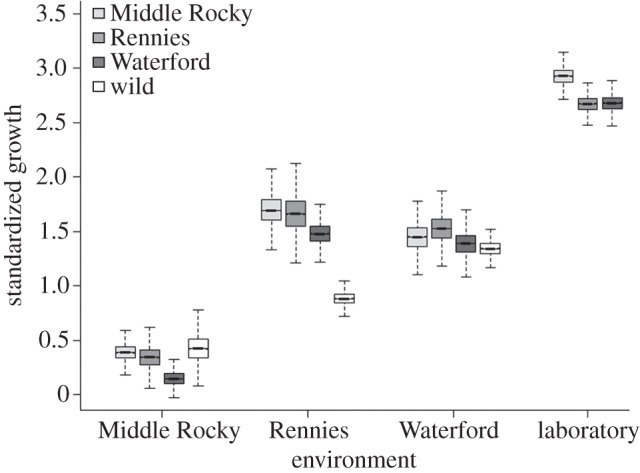
Size-adjusted specific growth (percentage change in mass per day) among experimental groups of brown trout reared in three wild environments (Middle Rocky, Rennies, Waterford) and one common-laboratory environment. Data represent 10 000 bootstraps of observed data, where the line represents median growth, boxes capture the 25th and 75th interquartile of the growth estimates, and the whiskers are 1.5 times the interquartile. Note that only the laboratory-born groups were reared in the common-laboratory environment.
(b). Local versus foreign criterion
We found complementary evidence of local advantage based on the local versus foreign criteria with effect sizes and log-odds ratios (figure 3) based on recovery data at 70–100 DPR (table 2). In 15 pair-wise comparisons of the frequencies of recaptured individuals that were of local versus foreign origin, we detected evidence consistent with local advantage and immigrant inviability in 94 per cent of cases, which is unlikely to have resulted from chance alone (p < 0.001). The one exception was the higher apparent survival of the Waterford group when reared in the Rennies. In addition, these comparisons indicated that wild-born individuals consistently outperformed both the foreign laboratory-born groups and their laboratory-born locally produced counterparts. We found congruent results when survival probabilities from the occupancy model were used as fitness proxies (12/15 positive effect sizes, p < 0.05).
Figure 3.
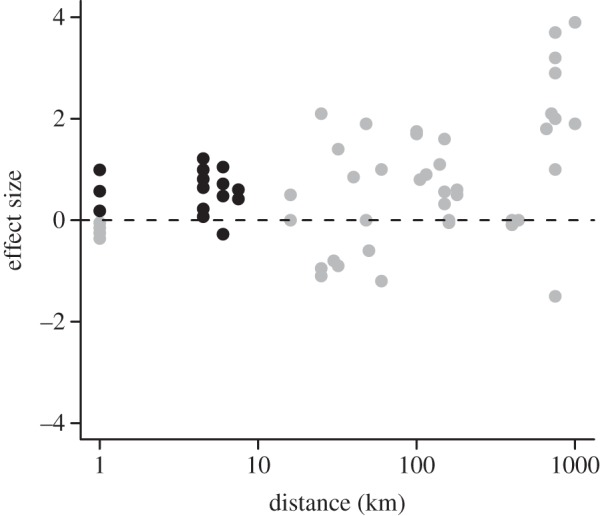
Effect size (log-odds ratio) of local versus foreign performance within environments as a function of spatial distance between populations (e.g. points at the 1000 km scale represent comparisons between populations that have evolved 1000 km apart from each other on the landscape). Grey points are data from figure 2 of Fraser et al. [21], and black points are data from this study. Points above the horizontal line are consistent with local adaptation, whereas points below suggest the opposite.
Again, patterns in growth were markedly less consistent than those based on survival (figure 2). Despite this variation, some evidence of local advantage in growth emerged. Laboratory-born Middle Rocky individuals when reared in their home stream grew 20 per cent and 65 per cent faster than Rennies and Waterford individuals, respectively. Wild-born fish in Middle Rocky grew faster than any group, including 10 per cent faster than their laboratory-born Middle Rocky counterparts. By contrast, laboratory-born local individuals in the Rennies and Waterford locations did not consistently outgrow foreign individuals. Curiously, we observed that growth of wild-born fish in Rennies and Waterford tended to be slower than either the foreign or local laboratory-born groups. Finally, Middle Rocky fish grew 8 per cent faster than the Rennies or Waterford populations in common-laboratory conditions.
4. Discussion
Taken as a whole, our results indicate that established trout populations in Newfoundland—recently descended from common ancestors—have in several generations undergone adaptive evolutionary divergence. Local fish exhibited higher survival, and in some cases higher growth, than foreign individuals within an environment, and two of three populations survive better at home than when reared away. These results are congruent with theoretical predictions that divergent natural selection should, in the absence of substantial gene flow, lead to local adaptation [3]. The extent to which local adaptation is common in nature is unclear and potentially biased by the use of study systems that already reveal marked divergence. Tests for local adaptation are frequently conducted on highly phenotypically divergent populations, ‘eco-types’, or incipient biological species where adaptation is essentially a foregone conclusion [2]. By contrast, the extent of phenotypic divergence in the populations of trout we examined was not dramatic [31], yet evidence of adaptation to local conditions was detected nonetheless.
Despite the widespread use of salmonids as classic models for the power of natural selection to drive adaptive divergence [43–46], few studies have directly tested for local adaptation in nature [20,21]. Reciprocal transplant and common-garden experiments are effective ways to test for local adaptation [1–3], yet fully replicated reciprocal transplants using salmonids are exceedingly rare (for an elegant example, see [47]). No study we are aware of has used a three-population design, even though replication of n > 2 populations is necessary to show that differences are unlikely to be explained by population × habitat interactions unrelated to divergent selection (discussed by Kawecki & Ebert [1]). Non-genetic maternal effects can influence offspring performance in nature [48]; however, our results are unlikely to reflect maternal contributions as the beneficial effects of egg size erode quickly with age [32]. We attempted to minimize the influence of any lingering maternal effects by rearing individuals for several months in common-laboratory conditions prior to their release into the wild, and controlled for individual body size and condition on survival. However, the potential for adaptive long-lasting maternal effects cannot entirely be ruled out. In addition, we compared performance in the wild of laboratory-born local groups with wild-born counterparts within an environment. Our findings of generally higher survival of wild-born individuals are consistent with growing evidence that salmonids can quickly adapt to laboratory conditions, which in turn can influence fitness in the wild [49,50].
Two of three populations, Middle Rocky Brook and Rennies, survived at higher rates at home than when reared away, despite intrinsic differences in habitat quality that may have served to mask this pattern. Laboratory-born Middle Rocky individuals survived better at home than when reared in either Rennies or Waterford, whereas laboratory-born Rennies individuals survived at comparable rates at home and in Waterford, but lower in Middle Rocky, and the laboratory-born Waterford individuals survived comparably well regardless of environment. These patterns may reflect, in part, parallel evolution to similar ecological selection in Rennies and Waterford as the biotic and abiotic are more similar to each other than to Middle Rocky Brook (table 1). Indeed, similar environments often yield more similar patterns of ecological selection [3]. This pattern is consistent with other taxa where the degree of adaptive divergence scales with differences in ecological conditions and strength of divergent selection [51,52]. An additional (but not mutually exclusive) explanation is that the homogenizing effect of gene flow between the Rennies and Waterford locations may result in similar survival responses to similar conditions. Indeed, gene flow (in the form of sea-going dispersers) is possible only between the Rennies and Waterford populations as they have traversable connections to the ocean, whereas Middle Rocky Brook is entirely landlocked.
In contrast to the above, our results clearly satisfy the ‘local versus foreign’ criterion, which is diagnostic of local adaption [1,21]. Within a location, local individuals outperformed foreign groups in 14 of 15 and 12 of 15 pair-wise comparisons based on the frequency of recaptures and apparent survival estimates from the Bayesian occupancy model as proxies for fitness, respectively. These results are consistent with local adaptation and immigrant inviability, and are unlikely to reflect chance alone. By placing our results into the context of the review by Fraser et al. [21], we observed that the magnitude of our effect sizes was generally as large as those at similar spatial scales and that our results filled a spatial gap of examination (figure 2). The magnitude of the effect sizes may reflect the recent origins of these populations and an ongoing invasion process, and is suggestive of strong divergent selection. The majority of studies reported by Fraser et al. [21] were conducted on anadromous populations situated more than 50 km apart on the landscape, whereas we examined adaptation in situ among landlocked and facultatively anadromous populations less than 10 km apart. Why studies have, until now, focused at larger spatial scales is not clear, but perhaps reflect assumptions about the scale at which adaptation can operate. The results presented here, combined with evidence by Meier et al. [20], indicate that adaptation at fine spatial scales can occur.
We observed that wild-born individuals tended to survive at higher rates than their laboratory-born counterparts within an environment, which is consistent with current literature [53,54]. The differential performance between laboratory-born and wild-born groups may reflect behavioural responses to early-life-history rearing conditions, rather than genetic effects per se [55]. Prior to sampling and tagging, wild-born groups would have had the opportunity to acquire feeding territories in Middle Rocky Brook, Rennies and Waterford. The acquisition and maintenance of feeding territories are fundamental for juvenile survival in stream-dwelling salmonids [26,56], and prior residency of territories can influence the outcome of competitive interactions [57,58]. In addition, prior residency may have influenced dispersal patterns of laboratory-born fish. Dispersal in closely related Atlantic salmon varies as a function of local stream density, implying competition for limited areas for feeding [59]. As in many capture–mark–recapture experiments, our estimates of survival are confounded with emigration, and we cannot rule out the possibility that individuals that were not recaptured, and thus were presumed dead, moved outside the experimental sections. Our experimental streams and locations of releases were specifically chosen to minimize movement, but controlling for downstream emigration was not feasible (for details, see the electronic supplementary material). Ultimately, we assume that if individuals were displaced downstream it was the result of competitive exclusion [25,38,39] consistent with our goal of quantifying relative performance among released groups.
In contrast to survival, patterns with growth rate as a proxy for fitness or performance were less clear. This suggests caution in using non-survival-based metrics of fitness for tests of adaptation. That being said, we did observe patterns consistent with growth adaptation in some comparisons; laboratory-born and wild-born Middle Rocky Brook individuals grew faster than foreign groups in their local environment. However, these patterns were not consistent enough in other locations to warrant robust conclusions. Growth in the home versus away criterion revealed evidence of potential gene × environment interactions, but interactions were not consistent with patterns of adaptation. The more than doubling of average growth observed in Rennies and Waterford compared with Middle Rocky Brook did not translate to higher average survival (mean survival of all groups among locations was 4.8% ± 0.02, 5.3% ± 0.01 and 4.9% ± 0.01), again evidence that growth and survival are not equivalent fitness proxies. Laboratory-born fish survived at lower rates than wild-born fish in Rennies and Waterford, despite faster growth than the wild-born groups. The rapid growth by the laboratory-born groups may represent compensatory responses to conditions conducive for growth in Rennies and Waterford, but may have come at a cost to survival. Indeed, compensatory growth patterns in salmonids appear to be heritable, under selection, and capable of evolving [36,60]. Finally, we observed that growth of the Middle Rocky Brook population was faster than individuals from the Rennies or Waterford populations when reared in the common-laboratory environment. This finding again suggests underlying genetic differences in growth potential among populations, though we cannot rule out lingering maternal effects as Middle Rocky Brook individuals were originally spawned from significantly larger eggs.
The patterns in survival and growth we observed are likely to be the result of divergent ecological selection; however, the proximate mechanisms that favour local over foreign individuals are not known. Unfortunately, ecological agents of selection are often difficult to illuminate in reciprocal transplant designs [43,54]. Predation can promote adaptive divergence [61] and may have played a mechanistic role in shaping the outcomes we observed. For predation to play an important role, populations would need to differ in their susceptibility to predators. Body shape and cryptic morphology differ in these populations [31], but the link to predator avoidance is not known. Notwithstanding extensive sampling of potential fish predators, we documented only one case of predation: cannibalism of a tagged Waterford individual by an untagged yearling brown trout in Middle Rocky Brook. The lack of tag recoveries in predators is not surprising as predation is notoriously difficult to observe and quantify in the wild [61,62]. Based on water temperatures during the experiment, ingested tags would probably have spent only around 20 h in the digestive system of predators according to gastric evacuation models [63]. In addition to cannibalism, Rennies and Waterford contain populations of American eels (Anguilla rostrata), which are known predators on small salmonids [64]. Yet no ingested tags were found in captured eels, and overall the average apparent survival was equal among locations, irrespective of potential predators.
The evidence for rapid evolution following naturalization to new environments by non-native species is now incontrovertible (reviewed by Sax et al. [65]). Our results contribute to the mounting evidence that evolutionary change in colonizing species is adaptive and frequently driven by divergent ecological selection across the landscape [3]. Rapid evolutionary change can increase the capacity of invading species to colonize new habitats (reviewed by recent studies [23,66]) and spread further. For example, introduced toads in Australia [14] and Chinook salmon in New Zealand [13] display adaptive evolution in dispersal and reproductive traits tied to population vital rates. Native species can also evolve quickly [9], but what remains to be seen is whether adaptive responses by natives will be sufficient to allow them to persist [67] or even flourish [68] in an increasingly homogenized global biotic community.
Acknowledgements
All measurements, tagging and housing of the experimental animals were conducted in accordance with the guidelines provided by the Canadian Council on Animal Care and with approval (09-10-IF) of Memorial University's Institutional Animal Care Committee.
We thank C. Conway, K. Oke, B. Wringe and D. Hauser for their help in the laboratory and recapturing fish in the wild. P.A.H.W. was supported by the Institute of Biodiversity and Ecosystem Sustainability and by the Atlantic Salmon Federation. The research was supported by an NSERC Discovery Grant to I.A.F. We thank G. Perry and C. Bourgeois of Department of Fisheries and Oceans Canada for their assistance acquiring permits for the reciprocal plants. The manuscript was improved by the thoughtful comments of three anonymous reviewers.
References
- 1.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241 10.1111/j.1461-0248.2004.00684.x (doi:10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 2.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Nosil P. 2012. Ecological speciation, p. 280 New York, NY: Oxford University Press [Google Scholar]
- 4.Hendry AP, Kinnison MT. 1999. The pace of modern life: measuring rates of contemporary microevolution. Evolution 53, 1637–1653 10.2307/2640428 (doi:10.2307/2640428) [DOI] [PubMed] [Google Scholar]
- 5.Reznick DN, Shaw SH, Rodd FH, Shaw RG. 1997. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275, 1934–1937 10.1126/science.275.5308.1934 (doi:10.1126/science.275.5308.1934) [DOI] [PubMed] [Google Scholar]
- 6.Losos JB, Warheit KI, Schoener TW. 1997. Adaptive differentiation following experimental island colonization in Anolis lizards. Nature 387, 70–73 10.1038/387070a0 (doi:10.1038/387070a0) [DOI] [Google Scholar]
- 7.Weber SB, Broderick AC, Groothuis TGG, Ellick J, Godley BJ, Blount JD. 2012. Fine-scale thermal adaptation in a green turtle nesting population. Proc. R. Soc. B 279, 1077–1084 10.1098/rspb.2011.1238 (doi:10.1098/rspb.2011.1238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reznick DN, Ghalambor CK. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112, 183–198 10.1023/A:1013352109042 (doi:10.1023/A:1013352109042) [DOI] [PubMed] [Google Scholar]
- 9.Westley PAH. 2011. What invasive species reveal about the rate and form of contemporary phenotypic change in nature. Am. Nat. 177, 496–509 10.1086/658902 (doi:10.1086/658902) [DOI] [PubMed] [Google Scholar]
- 10.Yeh PJ. 2004. Rapid evolution of a sexually selected trait following population establishment in a novel habitat. Evolution 58, 166–174 [DOI] [PubMed] [Google Scholar]
- 11.Eroukhmanoff F, Hargeby A, Svensson EI. 2009. Rapid adaptive divergence between ecotypes of an aquatic isopod inferred from Fst–Qst analysis. Mol. Ecol. 18, 4912–4923 10.1111/j.1365-294X.2009.04408.x (doi:10.1111/j.1365-294X.2009.04408.x) [DOI] [PubMed] [Google Scholar]
- 12.Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127 10.1111/j.1461-0248.2005.00812.x (doi:10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 13.Kinnison MT, Unwin MJ, Quinn TP. 2008. Eco-evolutionary versus habitat contributions to invasion in salmon: experimental evaluation in the wild. Mol. Ecol. 17, 405–414 10.1111/j.1365-294X.2007.03495.x (doi:10.1111/j.1365-294X.2007.03495.x) [DOI] [PubMed] [Google Scholar]
- 14.Phillips BL, Brown GP, Webb JK, Shine R. 2006. Invasion and the evolution of speed in toads. Nature 439, 803. 10.1038/439803a (doi:10.1038/439803a) [DOI] [PubMed] [Google Scholar]
- 15.Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the world's worst invasive alien species: a selection from the global invasive species database. Gland, Switzerland: IUCN [Google Scholar]
- 16.Froese R, Pauly D. 2000. FishBase 2000: concepts, design and data sources. Laguna, The Philippines: ICLARM [Google Scholar]
- 17.Townsend CR. 1996. Invasion biology and ecological impacts of brown trout (Salmo trutta) in New Zealand. Biol. Conserv. 78, 13–22 10.1016/0006-3207(96)00014-6 (doi:10.1016/0006-3207(96)00014-6) [DOI] [Google Scholar]
- 18.McIntosh AR, Townsend CR. 1994. Interpopulation variation in mayfly anti-predator tactics: differential effects of contrasting predatory fish. Ecology 75, 2078–2090 10.2307/1941612 (doi:10.2307/1941612) [DOI] [Google Scholar]
- 19.McDowall RM. 2006. Crying wolf, crying foul, or crying shame: alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev. Fish Biol. Fish. 16, 233–422 10.1007/s11160-006-9017-7 (doi:10.1007/s11160-006-9017-7) [DOI] [Google Scholar]
- 20.Meier K, Hansen MM, Bekkevold D, Skaala Ø, Mensberg K. 2011. An assessment of the spatial scale of local adaptation in brown trout (Salmo trutta L.): footprints of selection at microsatellite DNA loci. Heredity 106, 488–499 10.1038/hdy.2010.164 (doi:10.1038/hdy.2010.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser DJ, Weir LK, Bernatchez L, Hansen MM, Taylor EB. 2011. Extent and scale of local adaptation in salmonid fishes: review and meta-analysis. Heredity 106, 404–420 10.1038/hdy.2010.167 (doi:10.1038/hdy.2010.167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayllon F, Davaine P, Beall E, Garcia-Vazquez E. 2006. Dispersal and rapid evolution in brown trout colonizing virgin Subantarctic ecosystems. J. Evol. Biol. 19, 1352–1358 10.1111/j.1420-9101.2005.01075.x (doi:10.1111/j.1420-9101.2005.01075.x) [DOI] [PubMed] [Google Scholar]
- 23.Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P. 2006. A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol. Evol. 21, 130–135 10.1016/j.tree.2005.10.012 (doi:10.1016/j.tree.2005.10.012) [DOI] [PubMed] [Google Scholar]
- 24.Jonsson B, Jonsson N. 2011. Ecology of Atlantic salmon and brown trout: habitat as a template for life histories. New York, NY: Springer [Google Scholar]
- 25.Elliott JM. 1994. Quantitative ecology and the brown trout. New York, NY: Oxford University Press [Google Scholar]
- 26.Einum S, Fleming IA. 2000. Selection against late emergence and small offspring in Atlantic salmon (Salmo salar). Evolution 54, 628–639 [DOI] [PubMed] [Google Scholar]
- 27.Quinn TP. 2005. The behavior and ecology of Pacific salmon and trout. Seattle, WA: University of Washington Press [Google Scholar]
- 28.MacCrimmon HR, Marshall TL. 1968. World distribution of brown trout, Salmo trutta. J. Fish. Res. Board Can. 25, 2527–2548 10.1139/f68-225 (doi:10.1139/f68-225) [DOI] [Google Scholar]
- 29.Maitland JRG. 1887. The history of Howietoun. Stirling, UK: J. R. Guy, Howietoun Fishery [Google Scholar]
- 30.Westley PAH, Fleming IA. 2011. Landscape factors that shape a slow and persistent aquatic invasion: brown trout in Newfoundland 1883–2010. Divers. Distrib. 17, 566–579 10.1111/j.1472-4642.2011.00751.x (doi:10.1111/j.1472-4642.2011.00751.x) [DOI] [Google Scholar]
- 31.Westley PAH, Conway CM, Fleming IA. 2012. Phenotypic divergence of exotic fish populations is shaped by spatial proximity and habitat differences across an invaded landscape. Evol. Ecol. Res. 14, 147–167 [Google Scholar]
- 32.Einum S, Fleming IA. 1999. Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proc. R. Soc. Lond. B 266, 2095–2100 10.1098/rspb.1999.0893 (doi:10.1098/rspb.1999.0893) [DOI] [Google Scholar]
- 33.Fleming IA, Ng S. 1987. Evaluation of techniques for fixing, preserving and measuring salmon eggs. Can. J. Fish. Aquat. Sci. 44, 1957–1962 10.1139/f87-240 (doi:10.1139/f87-240) [DOI] [Google Scholar]
- 34.Westley PAH. 2012. Biological invasions as fortuitous experiments in nature: ecology, evolution, and phenotypic plasticity of non-native brown trout (Salmo trutta) in Newfoundland. St John's, Canada: Memorial University of Newfoundland [Google Scholar]
- 35.Royle JA, Dorazio RM. 2008. Hierarchical modeling and inference in ecology: the analysis of data from populations, metapopulations and communities. San Diego, CA: Academic Press [Google Scholar]
- 36.Carlson SM, Hendry AP, Letcher BH. 2004. Natural selection acting on body size, growth rate and compensatory growth: an empirical test in a wild trout population. Evol. Ecol. Res. 6, 955–973 [Google Scholar]
- 37.Hendry AP, Letcher BH, Gries G. 2003. Estimating natural selection acting on stream-dwelling Atlantic salmon: implications for the restoration of extirpated populations. Conserv. Biol. 17, 795–805 10.1046/j.1523-1739.2003.02075.x (doi:10.1046/j.1523-1739.2003.02075.x) [DOI] [Google Scholar]
- 38.Chapman DW. 1966. Food and space as regulators of salmonid populations in streams. Am. Nat. 100, 345–357 10.1086/282427 (doi:10.1086/282427) [DOI] [Google Scholar]
- 39.Bujold V, Cunjak RA, Dietrich JP, Courtemanche DA. 2004. Drifters versus residents: assessing size and age differences in Atlantic salmon (Salmo salar) fry. Can. J. Fish. Aquat. Sci. 61, 273–282 10.1139/f03-162 (doi:10.1139/f03-162) [DOI] [Google Scholar]
- 40.Kahler TH, Roni P, Quinn TP. 2001. Summer movement and growth of juvenile anadromous salmonids in small western Washington streams. Can. J. Fish. Aquat. Sci. 58, 1947–1956 10.1139/f01-134 (doi:10.1139/f01-134) [DOI] [Google Scholar]
- 41.Ostrovsky I. 1995. The parabolic pattern of animal growth: determination of equation parameters and their temperature dependencies. Freshwater Biol. 33, 357–371 10.1111/j.1365-2427.1995.tb00398.x (doi:10.1111/j.1365-2427.1995.tb00398.x) [DOI] [Google Scholar]
- 42.Elliott JM, Hurley MA, Fryer RJ. 1995. A new, improved growth model for brown trout, Salmo trutta. Funct. Ecol. 9, 290–298 10.2307/2390576 (doi:10.2307/2390576) [DOI] [Google Scholar]
- 43.Quinn TP, Kinnison MT, Unwin MJ. 2001. Evolution of chinook salmon (Oncorhynchus tshawytscha) populations in New Zealand: pattern, rate, and process. Genetica 112, 493–513 10.1023/A:1013348024063 (doi:10.1023/A:1013348024063) [DOI] [PubMed] [Google Scholar]
- 44.Hendry AP, Stearns SC. 2004. Evolution illuminated. New York, NY: Oxford University Press [Google Scholar]
- 45.Taylor EB. 1991. A review of local adaptation in salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture 98, 185–207 10.1016/0044-8486(91)90383-I (doi:10.1016/0044-8486(91)90383-I) [DOI] [Google Scholar]
- 46.Garcia de Leaniz C, et al. 2007. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biol. Rev. 82, 173–211 10.1111/j.1469-185X.2006.00004.x (doi:10.1111/j.1469-185X.2006.00004.x) [DOI] [PubMed] [Google Scholar]
- 47.Mayama H, Nomura T, Ohkuma K. 1989. Reciprocal transplatation experiment of Masu salmon (Oncorhynchus masou) population. II. Comparison of seaward migrations and adult returns of local stock and transplanted stock of masu salmon. Sci. Repts Hokkaido Salmon Hatchery 43, 99–113 [Google Scholar]
- 48.Einum S, Fleming IA. 2000. Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature 405, 565–567 10.1038/35014600 (doi:10.1038/35014600) [DOI] [PubMed] [Google Scholar]
- 49.Christie MR, Marine ML, French RA, Blouin MS. 2012. Genetic adaptation to captivity can occur in a single generation. Proc. Natl Acad. Sci. USA 109, 238–242 10.1073/pnas.1111073109 (doi:10.1073/pnas.1111073109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araki H, Berejikian BA, Ford MJ, Blouin MS. 2008. Fitness of hatchery-reared salmonids in the wild. Evol. Appl. 1, 342–355 10.1111/j.1752-4571.2008.00026.x (doi:10.1111/j.1752-4571.2008.00026.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendry AP, Taylor EB, McPhail JD. 2002. Adaptive divergence and the balance between selection and gene flow: lake and stream stickleback in the misty system. Evolution 56, 1199–1216 [DOI] [PubMed] [Google Scholar]
- 52.Moore JS, Hendry AP. 2005. Both selection and gene flow are necessary to explain adaptive divergence: evidence from clinal variation in stream stickleback. Evol. Ecol. Res. 7, 871–886 [Google Scholar]
- 53.Fleming IA, Hindar K, Mjølnerød IB, Jonsson B, Balstad T, Lamberg A. 2000. Lifetime success and interactions of farm salmon invading a native population. Proc. R. Soc. Lond. B 267, 1517–1523 10.1098/rspb.2000.1173 (doi:10.1098/rspb.2000.1173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGinnity P, et al. 2003. Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc. R. Soc. Lond. B 270, 2443–2450 10.1098/rspb.2003.2520 (doi:10.1098/rspb.2003.2520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleming IA, Lamberg A, Jonsson B. 1997. Effects of early experience on the reproductive performance of Atlantic salmon. Behav. Ecol. 8, 470–480 10.1093/beheco/8.5.470 (doi:10.1093/beheco/8.5.470) [DOI] [Google Scholar]
- 56.Gibson RJ. 1993. The Atlantic salmon in freshwater: spawning, rearing and production. Rev. Fish Biol. Fisheries 3, 39–73 10.1007/BF00043297 (doi:10.1007/BF00043297) [DOI] [Google Scholar]
- 57.Rhodes JS, Quinn TP. 1998. Factors affecting the outcome of territorial contests between hatchery and naturally reared coho salmon parr in the laboratory. J. Fish Biol. 53, 1220–1230 10.1111/j.1095-8649.1998.tb00243.x (doi:10.1111/j.1095-8649.1998.tb00243.x) [DOI] [Google Scholar]
- 58.Metcalfe NB, Valdimarsson SK, Morgan IJ. 2003. The relative roles of domestication, rearing environment, prior residence and body size in deciding territorial contests between hatchery and wild juvenile salmon. J. Appl. Ecol. 40, 535–544 10.1046/j.1365-2664.2003.00815.x (doi:10.1046/j.1365-2664.2003.00815.x) [DOI] [Google Scholar]
- 59.Einum S, Nislow KH. 2005. Local-scale density-dependent survival of mobile organisms in continuous habitats: an experimental test using Atlantic salmon. Oecologia 143, 203–210 10.1007/s00442-004-1793-y (doi:10.1007/s00442-004-1793-y) [DOI] [PubMed] [Google Scholar]
- 60.Morris MRJ, Fraser DJ, Eddington J, Hutchings JA. 2010. Hybridization effects on phenotypic plasticity: experimental compensatory growth in farmed wild Atlantic salmon. Evol. Appl. 4, 444–458 10.1111/j.1752-4571.2010.00159.x (doi:10.1111/j.1752-4571.2010.00159.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nosil P, Crespi BJ. 2006. Experimental evidence that predation promotes divergence in adaptive radiation. Proc. Natl Acad. Sci. USA 103, 9090–9095 10.1073/pnas.0601575103 (doi:10.1073/pnas.0601575103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlson SM, Rich HB, Jr, Quinn TP. 2009. Does variation in selection imposed by bears drive divergence among populations in the size and shape of sockeye salmon? Evolution 63, 1244–1261 10.1111/j.1558-5646.2009.00643.x (doi:10.1111/j.1558-5646.2009.00643.x) [DOI] [PubMed] [Google Scholar]
- 63.Ruggerone GT. 1989. Gastric evacuation of single and multiple meals by piscivorous coho salmon, Oncorhynchus kisutch. Environ. Biol. Fishes 26, 143–147 10.1007/BF00001030 (doi:10.1007/BF00001030) [DOI] [Google Scholar]
- 64.Godfrey H. 1957. Feeding of eels in four New Brunswick salmon streams. Prog. Rep. Atlantic Coast Stations, Fisheries Res. Board Can. 67, 19–22 [Google Scholar]
- 65.Sax DF, Stachowicz JJ, Gaines SD. 2005. Species invasions: insights into ecology, evolution, and biogeography. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 66.Kinnison MT, Hairston NG. 2007. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 21, 444–454 10.1111/j.1365-2435.2007.01278.x (doi:10.1111/j.1365-2435.2007.01278.x) [DOI] [Google Scholar]
- 67.Phillips BL, Shine R. 2006. An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proc. R. Soc. B 273, 1545–1550 10.1098/rspb.2006.3479 (doi:10.1098/rspb.2006.3479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carroll SP, Loye JE, Dingle H, Mathieson M, Famula TR, Zalucki MP. 2005. And the beak shall inherit: evolution in response to invasion. Ecol. Lett. 8, 944–951 10.1111/j.1461-0248.2005.00800.x (doi:10.1111/j.1461-0248.2005.00800.x) [DOI] [PubMed] [Google Scholar]


