Abstract
The interplay of translation and mRNA turnover has helped unveil how the regulation of gene expression is a continuum in which events that occur during the “birth” of a transcript in the nucleus can have profound effects on subsequent steps in the cytoplasm. Exemplifying this continuum is nonsense-mediated mRNA decay (NMD), the process wherein a premature stop codon affects both translation and mRNA decay. Studies of NMD helped lead us to the therapeutic concept of treating a subset of patients suffering from multiple genetic disorders due to nonsense mutations with a single small molecule drug that modulates the translation termination process at a premature nonsense codon. Here we review both translation termination and NMD, and our subsequent efforts over the last fifteen years that led to the identification, characterization, and clinical testing of ataluren, a new therapeutic with the potential to treat a broad range of genetic disorders due to nonsense mutations.
Keywords: Nonsense codons, translation termination, release factors, premature termination, inherited disorders, nonsense codon readthrough, aminoglycosides, PTC124/ataluren
NONSENSE CODONS AND RELEASE FACTORS AND THEIR ROLE IN TRANSLATIONAL TERMINATION
In most genetic systems, 61 of the 64 possible triplet codons encode specific amino acids. The other three codons, UAA, UAG, and UGA, are noncoding or nonsense codons and promote translation termination (1). Two types of soluble “release factors” (RFs) respond to the nonsense codons (2). Class I RFs are tRNA mimics that recognize stop codons within the ribosomal A site and trigger the hydrolysis of the ester bond connecting the polypeptide chain and the tRNA in the P site. Class II RFs are GTPases that stimulate class I RF activity and confer GTP dependency upon the termination process (2). In prokaryotes, class I RF function is carried out by two distinct proteins. RF1 recognizes UAG and UAA, whereas RF2 recognizes UGA and UAA (2). In eukaryotes, translation termination is mediated cooperatively by eRF1 and eRF3-GTP, interacting proteins that execute class I and class II functions, respectively, while associating with the ribosome as a complex (2). With the exception of the GGQ motif that plays a key role in the hydrolysis of the nascent polypeptide, the sequences of prokaryotic RF1 and RF2 and eRF1 are not well conserved, implying their independent evolution (2).
eRF1 has a tRNA-like structure capable of recognizing all three nonsense codons, and association of the eRF1-eRF3-GTP complex with the ribosome triggers significant ribosomal conformational shifts (2). GTP hydrolysis by eRF3 appears to stimulate eRF1 release activity, possibly by positioning the eRF1 GGQ motif near the ribosome’s peptidyltransferase center (2). Although eukaryotic eRF1 and eRF3 had been thought to be sufficient for all aspects of termination, more recent studies show that dissociation and recycling of the termination complex is facilitated by the ABCE1 ATPase and initiation factors eIF3, eIF1, eIF1A, and eIF3j (3, 4). Additional factors that modulate the translation termination process have begun to be characterized. Some, such as the poly(A)-binding protein, a highly conserved polypeptide that associates with the 3′-poly(A) tail present on almost all eukaryotic mRNAs, appear to stimulate termination whereas others antagonize it (5, 6) (Figure 1a).
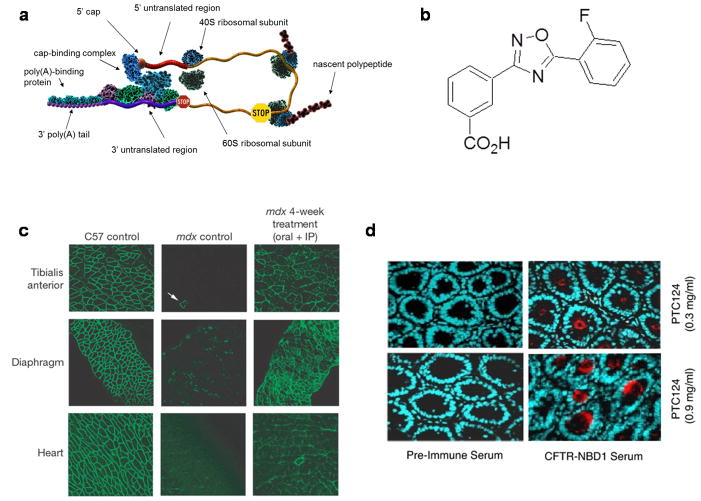
Figure 1. Ataluren treatment promotes dystrophin and CFTR synthesis in mouse models of nmDMD and nmCF.
a, Premature translation termination. The figure depicts a closed-loop mRNP in the process of being translated. Translational elongation doesn’t extend to the normal termination codon (red stop sign), but is instead halted by the premature termination codon (yellow stop sign), leading to the accumulation of a truncated polypeptide. b, Ataluren: 3-[5-(2-fluorophenyl)-[1,2,4]oxadiazol-3-yl]-benzoic acid (PTC124™) (71). c, Ataluren treatment of mdx mice rescues the dystrophic phenotype of affected muscles. Immunohistochemistry of indicated muscle cross-sections to visualize dystrophin in the tibialis anterior, diaphragm, and heart. The white arrow in the tibialis anterior negative control designates a revertant fibre. From (71). d, hCFTR expression in submucosal glands of intestinal tissues from Cftr−/− hCFTR-G542X mice treated with ataluren (PTC124). Samples from the duodenum of cftr −/− hCFTR G542X mice treated with 0.3 or 0.9 mg/ml ataluren in the Peptamen liquid diet. Samples were incubated with either preimmune or hCFTR-NBD1 serum as indicated. After incubation with a fluorescent secondary antibody, the samples were visualized by fluorescence microscopy. From (72).
THE NONSENSE-MEDIATED mRNA DECAY PATHWAY DEMONSTRATES THAT PREMATURE TERMINATION DIFFERS FROM NORMAL TERMINATION
Several lines of evidence indicate that premature translation termination (Figure 1a) differs from the normal termination process. The earliest observations showed that mRNAs containing a premature termination codon (PTC) were subject to accelerated degradation, a process that has been dubbed nonsense-mediated mRNA decay (NMD) (7–9). NMD is an evolutionarily conserved surveillance mechanism operative in all eukaryotic cells that severely dampens the expression of nonsense-containing mRNAs. NMD depends on nonsense codon recognition by the translation apparatus (7, 10, 11) and on a set of conserved factors that enable the discrimination of normal versus premature termination and promote rapid mRNA decay (12, 13). The proteins encoded by the UPF1, UPF2 (NMD2), and UPF3 genes are the principal NMD regulators in eukaryotes. Deleting or otherwise inactivating any of these genes results in the stabilization and increased accumulation of nonsense-containing mRNAs, with little or no effect on the abundance and stability of most wild-type transcripts (14, 15). Initial studies from yeast first suggested a mechanism whereby the NMD pathway discriminates normal from premature termination (15–17). The Upf proteins were shown to interact with eRF1 and eRF3 to form a termination surveillance complex (17, 18), a group of proteins thought to affect termination efficiency at a premature stop codon (18–20). Proteins within the surveillance complex were shown to monitor, and interact with, factors 3′ of the termination codon to determine whether the mRNA NMD pathway is to be activated (15–17). Factors that activated NMD included proteins such as Hrp1 that shuttled with the transcript from the nucleus but, as a consequence of premature termination, were not removed by a translating ribosome (15, 17). Thus, Hrp1 could serve as a mark for aberrant termination. Conversely, other RNA binding proteins could prevent activation of the NMD pathway (16).
A similar model has also been proposed for mammalian cells. It posits that Upf1 binds to ribosome-bound release factors (eRFs) and activates NMD if it subsequently interacts with a Upf2:Upf3 heterodimer bound to a downstream exon junction complex (EJC). Specificity for premature termination follows from the notion that EJCs would be retained only if an initial round of translation stopped at an upstream PTC (13). In this case the EJC is the mark for aberrant termination. In this model it is thought that Upf1 and its regulator Smg-1 first form the SURF (Smg-1-Upf1-Release factors) complex with Smg-8 and Smg-9 (21). If a ribosome’s progression is halted by premature termination before it reaches and displaces the downstream EJC, then SURF-associated Upf1 is postulated to be able to interact with Upf2:Upf3 bound to the EJC. In turn, this can activate Upf1 phosphorylation by Smg-1, and activate the Upf1 ATPase and helicase activities (22). Activated and phosphorylated Upf1 is then thought to promote dissociation of the release factors and the ribosome and to interact with Smg-5–7 to initiate mRNA decay (13).
Since intron-less precursors of mRNAs are subject to NMD without prior splicing, proper EJC spacing, or EJC components alternate models for the activation of NMD need to be considered (23, 24). Thus, the initiation of NMD may also be influenced by the different efficiencies of premature and normal termination (25, 26), with the apparent inefficiency possibly reflecting reductions in ribosome and mRNP dissociation subsequent to peptide hydrolysis (25–28). These results have been incorporated into the faux-UTR model (25, 29–32) which suggests that proper termination and normal rates of mRNA decay require interactions between a terminating ribosome and a specific mRNP structure or set of factors localized 3′ to the normal stop codon (25, 29–31, 33).
TERMINATION AT NONSENSE CODONS IS NOT INEVITABLE: NONSENSE SUPPRESSION
UAA, UAG, and UGA codons can be functionally overridden by alterations in the translation process that affect the fidelity or efficiency of termination. This phenomenon is called nonsense suppression and it can occur as a consequence of mutations in the genes encoding specific components of the protein synthesis apparatus, cell- or development-specific translational regulatory mechanisms, or the presence of certain small molecules. Mutations that allow a tRNA to base-pair with a nonsense codon can promote nonsense suppression (34), but alterations in the structure of virtually any component of the translational apparatus that affects the fidelity or catalysis of translation termination can lead to misreading of termination codons by near-cognate tRNAs. Thus, substantial nonsense suppression has been observed in cells with altered structure or abundance of ribosomal RNAs, ribosomal proteins, elongation factors, or release factors (35–38). Mutations in the yeast UPF genes can also promote nonsense suppression (19, 39), but this phenomenon appears to be indirect (40). Nonsense suppression can also occur when termination, like other steps in protein synthesis, is subject to regulation (41).
Nonsense suppression can also be promoted by small molecules. More than four decades ago, it was recognized that streptomycin and other aminoglycosides could promote ribosomal misreading (42, 43). These drugs normally function by inhibiting bacterial protein synthesis at concentrations that do not affect comparable activity in eukaryotic cells. Gentamicin, for example, binds to 16S rRNA to inhibit both bacterial translation and growth (42, 44). However, at high concentrations, gentamicin and other members of the aminoglycoside class of antibiotics can also bind to eukaryotic 18S rRNA and promote low frequency readthrough of premature nonsense codons (45, 46). Crystallographic analyses of 30S ribosomal subunits complexed with mRNA and tRNA have have shown that the binding of a cognate tRNA induces significant A site structural changes, including reorientation of 16S rRNA basesthat monitor codon:anticodon base-pairing and help to exclude non-cognate tRNAs. Aminoglycosides, such as paromomycin, can bind near the A site and trigger similar rearrangements (47), negating the discrimination against near-cognate tRNAs and allowing misreading and nonsense suppression. The likely applicability of these experiments to eukaryotic 40S ribosomes is suggested by mutational analyses that indicate substantial conservation of prokaryotic and eukaryotic decoding sites (48).
THE GENERAL NOTION OF THERAPEUTIC NONSENSE SUPPRESSION
Nonsense mutations result in the occurrence of UAA, UAG, or UGA codons in the protein-coding region of the mRNA template, leading to the production of a truncated polypeptide product and, frequently, to mRNA destabilization through NMD (49). A frequent consequence of these combined effects is a severe depression of specific gene expression. Nonsense mutations have been implicated in numerous inherited diseases and several cancers (50). At least 2400 different genetic disorders have at least one causative nonsense allele. In general, it has been estimated that nonsense mutations account for 5 to 15% of the individual cases of genetic disorders, with a recent meta-analysis of the Human Gene Mutation Database (HGMD) estimating that nonsense mutations are responsible for approximately 11% of all known HGMD lesions causing inherited disorders and as many as 20% of the protein-coding region located, single base-pair mutations that cause disease (51). Moreover, some genes have considerably higher frequencies of nonsense mutations than missense mutations while others have precisely the opposite spectrum. Tumor suppressor genes comprise a significant representative of the former class. Since most but not all nonsense mutations trigger NMD (52), it is notable that those which do trigger NMD appear to be more likely to lead to disease phenotypes than those which do not (51). Likewise, because of the reductions in gene expression that they cause, nonsense mutations have been reported to come to clinical attention approximately threefold more frequently than missense mutations (53).
A substantial fraction of the genetic disorders that arise from nonsense mutations are disabling or fatal and have only palliative treatment options at best. Given the large number of individuals that are collectively afflicted by nonsense mutations, a therapeutic approach to nonsense suppression could be of considerable medical benefit. Of particular importance is the possibility that a drug capable of suppressing nonsense in a given gene would also be capable of having the same effect on nonsense mutations in a completely different gene. Thus, under ideal circumstances, a single drug could have the potential to treat hundreds, if not thousands, of different disorders where the only commonality would be their common origin from nonsense alleles.
Since the underlying basis for the manifestation of nonsense mutation-mediated disease is the combined effect of truncated translation and accelerated mRNA decay, possible approaches to this challenge would involve the development of small-molecule drugs or other agents capable of suppressing premature translation termination or antagonizing NMD, or both. Since increasing the stability of nonsense-containing transcripts does not always result in nonsense suppression and may be separable from it (19, 39), the favored approach would appear to be the modulation of premature translation termination. The feasibility of suppressing nonsense mutations by administration of small molecules is enhanced by the mechanistic differences between premature and normal termination. Furthermore, gene expression levels do not need to be restored to anywhere near normal levels in order to restore function. Minimally boosting expression levels to as little as 1–5 percent of normal levels can greatly reduce the severity of or eliminate the disease (54).
AMINOGLYCOSIDES PROMOTE NONSENSE SUPPRESSION
Aminoglycoside-mediated nonsense suppression activity has been demonstrated in numerous cell lines and cell-free extracts, including those derived from patients with genetic disorders (55–61), and led to evaluation of the aminoglycosides in preclinical model systems as potential therapeutics for diseases caused by nonsense mutations (62, 63). The preclinical results obtained with gentamicin therapy in mouse models led to the testing of comparable approaches in patients with disease caused by nonsense mutations (64–67). Topical application of gentamicin to the nasal mucosa for 14 days in nonsense mutation cystic fibrosis (nmCF) patients resulted in local CFTR protein production and corresponding functional improvements in chloride channel activity (66). In patients with nonsense mutation Duchenne muscular dystrophy (nmDMD), intravenous gentamicin administration showed evidence of nonsense mutation suppression as monitored by analyses of full-length dystrophin in muscle biopsies (65). A pilot study in patients with nonsense mutation hemophilia documented that intravenous gentamicin treatment could induce increases in clotting factors and thrombin generation with concomitant decreases in activated partial thromboplastin time (67).
These proof-of-concept experiments demonstrated that small molecules that promote readthrough of premature termination codons have the potential to restore gene function in patients whose disease is caused by a nonsense mutation. However, the need for parenteral administration and the potential for serious renal and otic toxicities limit the clinical utility of long-term aminoglycoside therapy. Therefore, the discovery of orally bioavailable, non-toxic, low-molecular-weight compounds that promote readthrough of disease-causing nonsense mutations is an important objective. This has spurred the development of modified aminoglycosides (68), some of which have shown promise in mouse models (69). The need for improved approaches also propelled the identification and characterization of ataluren (PTC124) (Figure 1b; see below).
SCREENING FOR MOLECULES THAT PROMOTE READTHROUGH
Our understanding of the NMD pathway implied that premature and normal termination differed significantly and that the processes of translation termination and mRNA decay might be separable (19, 39, 70). We thus sought to identify small molecules that would be able to selectively read through premature nonsense codons, but not normal termination codons. Our goal was to identify a clinical candidate that could be taken orally and promote readthrough of nonsense mutations in multiple disease states independent of the specific transcript.
To identify compounds with the potential to suppress nonsense mutations in mammalian cells, we performed two high-throughput screens utilizing premature UGA luciferase (LUC) reporters in either stably transfected HEK293 cells or rabbit reticulocyte lysates (71). Compounds that manifested UGA readthrough activity with minimal cellular toxicity were characterized further for their relative potency and activity, including analyses of each compound’s dose-response relationship and ability to also read through UAA and UAG nonsense codons. These studies led to the identification of several lead compound classes that were then chemically optimized. The screening tier for the chemical optimization process included cell-based assays that initially assessed the expression of LUC nonsense alleles and then expanded to include disease-relevant assays, including nonsense suppression in cell-free translation systems, dystrophin production in cultured mdx myotubes (Figure 1c), pharmacologic activity in mdx mice as assessed by multiple endpoints, and chloride channel expression in transgenic mice harboring a cftr nonsense mutation (Figure 1d) (71, 72). Assays monitoring off-target effects were also performed. In vivo safety and suitability for pharmaceutical formulation were also evaluated (73). From over 3,500 compounds synthesized and evaluated, these analyses identified ataluren (PTC124™; 3-[5-(2-fluorophenyl)-[1,2,4]oxadiazol-3-yl]-benzoic acid) (Figure 1b) as a candidate for further development (71). This 284-Da, achiral, orally bioavailable compound has no structural similarity to aminoglycoside antibiotics or other clinically developed drugs.
CHARACTERIZATION OF ATALUREN PHARMACOLOGY
Ataluren promotes readthrough of each of the three nonsense codons. Correlating inversely with established termination efficiencies (61, 71), readthrough is highest at UGA, followed by UAG and then UAA. With all three stop codons, the minimal concentration of ataluren showing discernable readthrough is 0.01–0.1 μM, while the concentration achieving maximal activity is approximately 3 μM. Gentamicin, used for comparison, was only active at much higher concentrations (71). Continuous exposure to effective ataluren concentrations was shown to be important for maximizing activity in vitro. Readthrough of premature stop codons by ataluren in cell-based assays was rapidly reversed upon withdrawal of the drug. Ataluren was also shown to be effective in promoting readthrough of a premature UGA codon in synthetic LUC mRNA in a reticulocyte cell-free translation system, supporting the notion that of the drug targeted translation. Ataluren-enabled readthrough in cultured cells was unaffected by increased amounts of serum, indicating that protein binding does not reduce the drug’s effectiveness. Ataluren activity did not affect frameshift mutations and was unable to promote readthrough of multiple sequential premature stop codons. Importantly, ataluren-treated cells harboring a LUC reporter did not alter LUC mRNA levels, indicating that neither transcription nor mRNA stability was affected. Ataluren also had no significant effect on the levels of normal mRNAs or mRNAs that are endogenous NMD substrates (71).
Several approaches demonstrated that nonsense suppression in ataluren-treated cells, animals, and human subjects was selective for premature nonsense codons and did not lead to readthrough of normal stop codons. Two-dimensional gel electrophoretic comparisons of control cells with ataluren-treated cells did not produce any abnormally elongated proteins and a reporter gene capable of allowing detection of extended polypeptides that would result if readthrough of the normal stop codon occurred failed to manifest readthrough (71). Ataluren also did not promote readthrough of normal stop codons in vivo. Western blotting could not detect any ataluren-induced alterations in readthrough of normal stop codons in tissues from rats and dogs, or in PBMCs from patients (71).
ATALUREN NONSENSE SUPPRESSION ACTIVITY IN DISEASE MODELS
The general concept underlying therapeutic nonsense suppression is that a single drug may be beneficial in different diseases whose only commonality is a nonsense mutation. Accordingly, ataluren has been tested preclinically in diverse models of nonsense-mediated disease.
Nonsense Mutation Duchenne Muscular Dystrophy (nmDMD)
Duchenne muscular dystrophy (DMD) is a rare X-linked genetic disorder caused by mutations in the gene for dystrophin, a 427-kD plasma membrane protein of muscle fibers thought to play a role in protecting muscles from stress by linking the cytoskeleton to the extracellular matrix. Patients with DMD suffer from muscle weakness that leads to loss of ambulation during childhood or early adolescence (74). Approximately 13% of boys with DMD have dystrophin nonsense mutations (75).
The mdx mouse model, in which a UAA codon replaces a glutamine codon in exon 23 of the dystrophin gene (73), is used extensively for studying DMD. Ataluren treatment of cultured mdx myotubes yielded full-length dystrophin protein (Figure 1c) and ataluren-treated mdx mice had improved muscle function (71). A dosing regimen designed to maintain a trough ataluren concentration of approximately 2–10 μg/mL led to the production of full-length dystrophin protein, as demonstrated by western blotting, and to partial restoration of membrane-localized dystrophin in cardiac muscle and in all skeletal muscles examined (71). mdx mice treated with ataluren had dystrophin levels approximately 20% that of muscles from wild-type C57BL/6 mice.
The major functional deficit in dystrophic muscles is increased susceptibility to injury. This susceptibility results in repeated cycles of degeneration-regeneration, ongoing inflammation, and necrosis, with the eventual destruction of muscle (76). Thus, a good predictor of long-term therapeutic outcome may be protection from contraction-induced injury. Ataluren treatment was shown to improve protection against contraction-induced injury such that the decrement in force was not different from wild-type mice (71). Consistent with muscle improvement, the levels of creatine kinase (CK), a muscle-specific enzyme found at elevated levels in the serum after muscle is damaged (77), were also reduced with ataluren treatment. These data suggest that ataluren leads to decreased muscle fragility. Importantly, the ability of ataluren to suppress mdx nonsense mutations in mice and cultured myotubes has now been independently confirmed (78).
Nonsense Mutation Cystic Fibrosis (nmCF)
Cystic fibrosis (CF) is one of the most serious inherited diseases, with a total US prevalence of ~28,000 patients (79). CF is caused by mutations in the gene for the cystic fibrosis transmembrane conductance regulator (CFTR), a protein that acts as a chloride channel regulated by cyclic adenosine monophosphate (cAMP) (80). Loss of functional CFTR at membranes leads to abnormalities of cAMP-regulated chloride transport across epithelial cells on mucosal surfaces. This results in water transport abnormalities and viscous secretions in multiple organs, including the respiratory tract where these events lead to obstructive lung disease. Nonsense mutations account for ~10% of CF cases, resulting in a subpopulation of ~2,800 patients with nmCF in the United States. nmCF represents one of the most severe forms of the disease.
Ataluren activity was tested in a CF mouse model that expresses a human CFTR nonsense allele in the intestine (FABP-hCFTR-G542X) under control of the fatty acid binding protein promoter (72) (Figure 1d). In ataluren-treated cftr−/−FABP-hCFTR-G542X mice, the synthesis of CFTR was restored and its functional activity was demonstrated by increases in cAMP-stimulated transepithelial chloride currents (72).
Nonsense Mutation Miyoshi Myopathy
Miyoshi myopathy is caused by the lack of dysferlin (81), a protein involved in muscle repair and in sealing breaks in the muscle plasma membrane. Studies of ataluren-mediated nonsense suppression were conducted in vehicle- and drug-treated myotubes derived from a healthy subject and from a Miyoshi myopathy patient with a nonsense mutation (R1905X) in the dysferlin gene (82). Ataluren-treated myotubes from patients expressed ~15% of the level of dysferlin detected in healthy subjects (Figure 2a,b), while no dysferlin was detected in vehicle-treated myotubes (82). Further in vitro studies demonstrated a functional benefit of ataluren-mediated dysferlin expression. When exposed to hypotonic solution, normal myotubes develop expanding membrane blebs, consistent with the presence of an intact muscle plasma membrane. In contrast, patient-derived myotubes did not bleb because of membrane breaks. Patient-dervied myotubes transfected with a recombinant adenovirus expressing full-length human dysferlin were rescued and able to bleb in response to hypotonic solution (82). Patient-dervied myotubes cultured with ataluren (10 μg/mL) were also able to bleb when exposed to hypotonic solution. Thus, ataluren treatment resulted in the expression of functional dysferlin protein in myotubes derived from a nonsense mutation Miyoshi myopathy patient.
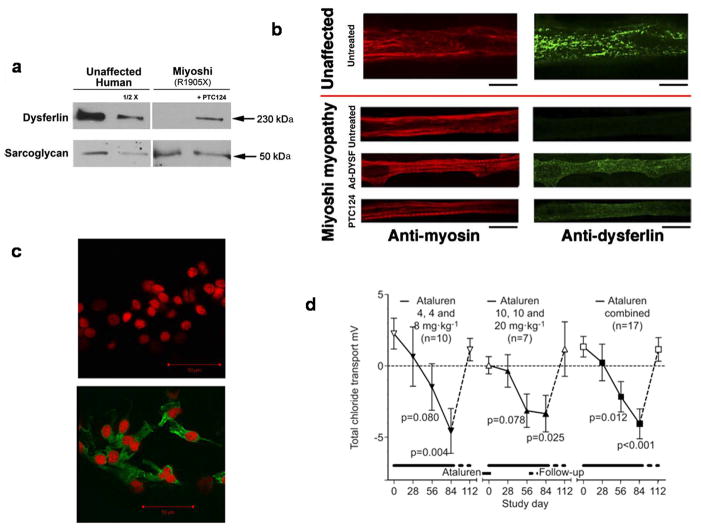
Figure 2. Ataluren-mediated dysferlin and CFTR expression.
a, Treatment of Miyoshi myopathy patient myotubes with ataluren yields ~15% of the levels of dysferlin present in unaffected myotubes. Comparisons employed western blotting and densitometry, and normalization to the levels of α-sarcoglycan in unaffected and Miyoshi myotubes. b, Dysferlin expression in myotubes from an unaffected human and from a Miyoshi patient. The Miyoshi myotubes do not express dysferlin unless infected with recombinant adenovirus that expresses full-length human dysferlin (Ad-DYSF) or treated with ataluren. Myotubes stained with either anti-myosin (red) or anti-dysferlin (green) antibodies. All scale bars represent 20 μm. From (82). c, Ataluren-mediated increase in expression of nasal epithelial cystic fibrosis transmembrane conductance regulator in a phase 2a CF patient. An immunofluorescence assay demonstrates that a patient from a phase 2a trial of ataluren efficacy in nmCF manifests full-length nasal epithelial cystic fibrosis transmembrane conductance regulator protein (green immunofluorescence) at the end of the trial (B), but not at its onset (A). Paired pre-and end-of-treatment immunostaining images are from a 17-year-old female patient with nmCF. She received ataluren 4, 4, 8 mg/kg for 14 days followed by 14 days off treatment, then received ataluren 10, 10, 20 mg/kg for 14 days. From (96). d, Restoration of chloride transport in phase 2a CF patients treated with ataluren. Seventeen nmCF patients were treated with ataluren (4, 4, and 8 or 10, 10, and 20 mgxkg−1) for 84 days, and followed up without drug for 28 days. Total chloride transport (mv) was monitored throughout the trial. Data are presented as mean±sem. p-values from paired t-test comparing on-study time point versus corresponding baseline. From (97).
Nonsense Mutation Hurler Syndrome
Hurler syndrome, the most severe form of mucopolysaccharidosis 1 (MPS1), is caused by the lack of the lysosomal enzyme iduronidase and results in the accumulation of glycosaminoglycans (GAGs) in tissues (83). Therefore, GAG levels can be monitored to assess the efficacy of treatment for this disease. In an animal model of Hurler syndrome, mice harbor a nonsense mutation in exon 9 of the IDUA gene that corresponds to the human W402X mutation. Ataluren treatment of these mice showed that the drug enabled readthrough of the premature stop codon present in the IDUA mRNA (D. Bedwell, unpublished results). As determined by dose-dependent changes of GAG levels in multiple tissues, ataluren-mediated readthrough resulted in increased iduronidase activity, with the greatest reduction of GAG levels occurring at 10 μg/mL ataluren.
Nonsense Mutation Carnitine Palmitoyltransferase 1A Deficiency
Carnitine palmitoyltransferase 1A (CPT1A) deficiency is a metabolic disorder in which the lack of CPT1A, a mitochondrial enzyme, prevents the conversion of long-chain fatty acids into energy, particularly during fasted the state (84). Ataluren (5 μM; 1.5 μg/mL) promotes expression of full-length CPT1A protein in fibroblasts derived from a patient with nonsense-mutation-mediated disease (85). Functional assays showed an increase in enzyme activity from 15% of wild-type levels in untreated cells to 45% of wild-type levels in ataluren-treated cells(85).
Nonsense Mutation Usher Syndrome
Usher type I syndrome is the most frequent cause of combined hereditary deaf-blindness (86). People with Usher type I syndrome have mutations in the USH1C gene, are born profoundly deaf, and begin to lose their vision in the first decade of life. At concentrations ranging from 2.5–10 μg/mL, ataluren increased expression and function of harmonin, the USH1C gene product, in tissue culture, retina cultures, and mice harboring a USH1C nonsense mutation (p.R31X) (87). Full-length protein was demonstrated by both immunofluorescence and western blotting and ataluren readthrough activity in primary retina explants led to an 8-fold increase in harmonin that was predominantly expressed in photoreceptor cells. Importantly, functional activity in ataluren-treated cells was demonstrated by the observation of actin filament bundling, which was not seen in untreated controls (87).
Nonsense Mutation Batten Disease
Neuronal ceroid lipofuscinoses, commonly known as Batten disease, comprise a group of neurodegenerative lysosomal storage disorders affecting both children and adults. The infantile form of the disease, caused by mutations in the CLN1 gene encoding palmitoyl-protein thioesterase-1 (PPT1), is particularly lethal and is caused by nonsense mutations in a high percentage of cases (88). Ataluren treatment of cultured cells from children with nonsense mutation Batten disease induced PPT1 enzymatic activity (89). Remarkably, although the ataluren-induced increase in PPT1 activity was modest, beneficial downstream effects on these cells were observed, including reduction of the levels of thioester (ceroid) load and suppression of apoptosis (89).
The literature also includes examples of nonsense mutation models where ataluren has not shown readthrough activity (90, 91). Reasons for the apparent inconsistencies include differences in the nonsense allele, the concentration of ataluren used, or the type of experimental system (e.g., transient versus stable transfection). Of interest in this regard is a recent study of the nonsense-suppressing activity of gentamicin in 66 different sequences that include a stop codon (92). Using a standardized, dual reporter assay, Floquet et al. observed that gentamicin-induced suppression varied more than tenfold depending on variations in the nucleotide sequences surrounding the termination codon. One report suggested that ataluren’s nonsense suppression activity may be an indirect consequence of the compound’s interaction with firefly luciferase (FLuc) (93). However, the Renilla luciferase (RLuc) reporter assay used in this study proved to be an unreliable gauge of nonsense suppression because it failed to respond significantly to G418, an established nonsense-suppressing aminoglycoside. Furthermore, it became apparent that the luciferase substrate utilized also governed the reported effects of compounds on luciferase inhibitory activity and that erroneous conclusions could be drawn depending on the luciferase reporter and substrate chosen (94). For example, a comparison of the luciferase Steady-Glo substrate versus Bright-Glo demonstrated a 60-fold difference between the respective ataluren IC50 values of luciferase inhibition (94). Detection of nonsense suppression when cells were treated with ataluren at 30 nM, well below the IC50 value of Bright-Go, indicates that the luciferase suppression activity shown previously cannot be due to luciferase inhibition (94). The multiple approaches utilized by us, our collaborators, and a number of independent investigators in preclinical and clinical studies argue strongly that ataluren manifests robust nonsense suppression activity (71, 72, 78, 82, 85, 87, 89, 95–97).
ATALUREN EVALUATION IN PHASE 1 SAFETY STUDIES
Safety and pharmacokinetic (PK) data from Phase 1 single- and multiple-dose studies in healthy volunteers (98) indicate that trough plasma concentrations in the target range of ~2–10 μg/mL can be achieved with an acceptable safety profile using a three times per day (TID) schedule of administration. The effects of food on drug absorption indicate that ataluren may be given with or without food, but it is recommended that ataluren be administered within 30 minutes of a meal. A Phase 1 absorption, metabolism, and excretion study in 7 male healthy volunteers (ages 19 to 50 years) indicated that ataluren has >55% bioavailability and is excreted by both hepatic and renal routes. The only detectable metabolite in systemic circulation is the acyl glucuronide metabolite.
Single doses of ataluren administered orally at dose levels ranging from 3 to 100 mg/kg were palatable and generally well tolerated (98). Mild adverse events of headache, dizziness, nausea, vomiting, diarrhea, and abdominal pain at dose levels of 150 and 200 mg/kg appeared to be coincident with achieving the Cmax. Elevations of serum ALT and aspartate aminotransferase (AST) were observed in one subject receiving a single dose of 200 mg/kg. With repeated doses through 50 mg/kg/dose twice per day (BID), reversible, low-grade (<2 times the upper limit of normal [ULN]) transaminase elevations without bilirubin increases were observed in some subjects. No notable renal abnormalities were observed.
NONSENSE SUPPRESSION PROOF OF CONCEPT CLINICAL STUDIES
A set of Phase 2a clinical studies in nmCF and nmDMD assessed whether ataluren is pharmacodynamically active in humans. Studies in 77 nmCF patients (ages 6–57 years (95–97) receiving oral ataluren for periods of 14 days through 12 weeks showed an ataluren safety profile consistent with Phase 1 studies and documented that ataluren can generate production of full-length, apically localized epithelial CFTR protein (Figure 2c). Paired pretreatment and end-of-treatment nasal brushing samples were analyzed for apical CFTR protein expression in 21 pediatric patients (96) manifesting a 17% improvement (p<0.01) in the overall proportion of epithelial cells expressing apical CFTR. On-treatment improvements in total chloride transport were followed by reversion to baseline values during the post-treatment period, indicating functional activity of the restored CFTR protein (95–97) (Figure 2d). In addition, patients treated with ataluren for three months manifested not only increased chloride channel activity, but also trends toward improvement in pulmonary function and statistically significant reductions in the number of coughs per day (97).
A Phase 2a study in 38 patients (ages 5–17 years) with nmDMD receiving oral ataluren for 28 days showed that ataluren was generally well tolerated and pharmacodynamically active, and that ataluren trough plasma concentrations active in mdx mice were consistently achieved at the mid- and high- dose levels. (Bönnemann, C., et al., personal communication). Pharmacological activity was measured by immunofluorescence evidence of an increase in C-terminal dystrophin expression in pre- and post-treatment biopsies of the extensor digitorum brevis muscle. Thirteen of 38 (34%) subjects demonstrated qualitative increases in post-treatment dystrophin expression appropriately localized in the sarcolemmal membrane.
PHASE 2B STUDIES OF ATALUREN IN NMDMD
A Phase 2b international, randomized, double-blind, placebo-controlled study (Study 007) evaluated the effects of 48 weeks of ataluren therapy on ambulatory ability in 174 patients ≥5 years of age with nmDMD. In this 3-arm study, patients were stratified based on age (<9 vs ≥9 years), use of corticosteroids (yes vs no), and their baseline 6-minute walk distance (6MWD) (<350 or ≥350 meters). Patients were randomized 1:1:1 to placebo, ataluren low dose, or ataluren high dose with all regimens given TID at morning, midday, and evening. The study hypothesis was that the mean change in 6MWD from baseline to 48 weeks would be 30 meters longer in at least one of the ataluren arms than in the placebo arm. The targeted treatment difference of 30 meters is consistent with 6MWD results for approved drugs used to treat other diseases with skeletal-muscle system involvement (99, 100) and falls within the range of estimates of the minimal clinically important difference (MCID) for 6MWD in idiopathic pulmonary fibrosis, diffuse parenchymal lung disease, and chronic obstructive pulmonary disease (101–103).
Study 007 showed that the mean change in 6MWD from baseline to Week 48 was 31.3 meters better for ataluren 10, 10, 20 mg/kg compared to placebo, consistent with the targeted 30-meter difference. In addition, patients receiving ataluren 10, 10, 20 mg/kg had a longer time to persistent 10% worsening in 6MWD compared to placebo (REF). The hazard ratio for ataluren 10, 10, 20 mg/kg versus placebo was 0.52, representing a 48% reduction in the risk of 10% 6MWD worsening. Slowing ambulatory decline represents an important clinical benefit in DMD, given that patients typically lose the ability to walk in their teenage years. Timed function tests of muscle function (stair climbing, stair descending, and walking/running 10 meters) revealed positive trends for ataluren 10, 10, 20 mg/kg compared to placebo, as evidenced by less decline over 48 weeks. Together, the 6MWT and the timed function tests provide complementary information and represent clinically meaningful endpoints in DMD (104). Positive trends favoring ataluren 10, 10, 20 mg/kg versus placebo were also seen for various other measures of physical functioning, including accidental fall frequency, myometric evaluation of muscle strength, activity and wheelchair use in the community setting, and patient-reported physical functioning as measured by the PedsQL. The consistency of these findings support an ataluren 10, 10, 20 mg/kg treatment effect on physical functioning. The higher dose (20-, 20-, 40-mg/kg) generally was not efficacious, suggesting an inverse or bell shaped dose-response relationship (see below).
PHASE 3 STUDIES OF ATALUREN IN NMCF
A Phase 3 double-blind study to assess ataluren safety and efficacy in nmCF enrolled patients ≥6 yrs of age to receive ataluren or placebo for 48 weeks. Patients were stratified by chronic inhaled antibiotic use, age, and %-predicted forced expiratory volume in 1 second (FEV1). Primary and important secondary endpoints were pulmonary function as measured by %-predicted FEV1 and pulmonary exacerbation rate, respectively. The difference between ataluren and placebo in mean relative change from baseline in %-predicted FEV1, the primary analysis, was 3.0% at Week 48 (personal communication from the Ataluren Study Group). The mean pulmonary exacerbation rate over 48 weeks was 23% lower for ataluren versus placebo.
The results for %-predicted FEV1 and pulmonary exacerbation rate were especially strong in patients not chronically taking inhaled antibiotics (6.7% difference in mean relative change in %-predicted FEV1, 43% lower pulmonary exacerbation rate over 48 weeks). In this study, several inhaled antibiotics were used chronically by patients, including tobramycin, colistin, and aztreonam. However, analyses of the effects of these different inhaled antibiotics on %-predicted FEV1 and pulmonary exacerbation rate results indicated that tobramycin antagonized ataluren’s effect. Consistent with this view, tobramycin also antagonized ataluren-induced readthrough in in vitro nonsense suppression assays.
Ataluren demonstrated an excellent safety profile. The safety profiles were similar for ataluren and placebo, other than cases of reversible Grade 3–4 creatinine elevations, which were associated with nephrotoxic concomitant medications.
ATALUREN’S BELL-SHAPED DOSE RESPONSE RELATIONSHIP AND ITS IMPLICATIONS FOR MECHANISM OF ACTION
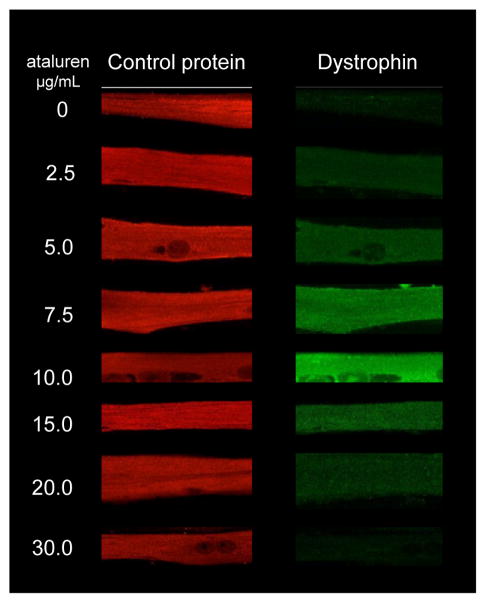
Analysis of the Phase 2b DMD clinical data suggests an inverse, or bell shaped dose-response curve. Given that ataluren is an example of a new drug class that modulates translation termination at a premature stop codon, the finding of an unusual dose-response relationship in the clinic warranted a more complete characterization of its dose-response properties. Accordingly, ataluren’s dose-response was studied further in cultured mouse myotubes taken from the mdx mouse model. The results of this analysis demonstrated that optimal ataluren activity occurred at 10 μg/mL, with less efficacy observed at higher concentrations. No cytotoxicity was observed at any concentration. Similar results were obtained for dystrophin expression in cultured human myotubes derived from biopsy samples taken from a nmDMD patient who participated in the Phase 2a study (Figure 3).
Figure 3. Ataluren manifests a bell-shaped dose response.
Myotubes from a phase 2a nmDMD patient were cultured in the presence of different concentrations of ataluren and assayed for the accumulation of dystrophin and a control protein by immunofluorescence assays.
A bell-shaped dose-response curve was also observed in the mouse nmHurler syndrome model. Ataluren increased iduronidase activity in cultured mouse embryonic fibroblasts from IDUA-W392X mice, with optimal efficacy at 10 μg/mL and less efficacy at higher concentrations. No cytotoxicity was observed at any concentration. Furthermore, ataluren increased iduronidase activity in vivo in IDUA-W392X mice, with better efficacy at lower doses. Reduced activity at higher ataluren concentrations was not due to reduced plasma ataluren concentrations.
Studies of aminoglycoside antibiotics capable of enabling premature stop codon readthrough have also manifested bell-shaped dose-response relationships (46, 55, 105). For example, studies of geneticin (G418) in nonsense mutation ataxia telangiectasia cell lines and gentamicin in cultured skin fibroblasts from patients with nonsense mutation Stüve Wiedemann Syndrome demonstrated that lower doses of aminoglycoside enabled premature stop codon readthrough, but that such activity decreased at higher doses (55, 105). Likewise, an evaluation of the activity of paromomycin in monkey COS-7 cells transfected with a chloramphenicol acetyl transferase construct containing a premature stop showed that premature stop codon readthrough activity was maximal between 750 and 1000 μg/mL and was lower at higher doses. Notably, no toxicity was observed at any of the doses tested (46).
Although ataluren’s target has yet to be identified, it is plausible that the drug could affect premature translation termination directly. For example, ataluren could associate with the ribosome or any of the regulatory factors involved in the termination process, thereby altering the competition between regulatory factors and near-cognate tRNAs for binding to the ribosomal A site. Numerous experiments in nonclinical model systems have shown that mutations that alter the structures of ribosomal proteins, ribosomal RNAs, or regulatory factors, can promote premature stop codon readthrough. Likewise, as discussed above, aminoglycosides promote premature stop codon readthrough by binding to ribosomal RNA.
Consistent with results in model systems, and with aminoglycosides, ataluren-mediated inhibition of a major termination-related activity would reduce the fidelity of termination and allow for premature stop codon readthrough. Premature termination might be selectively affected because it is kinetically compromised and would therefore be more sensitive to a reduction in activity than normal termination or because ataluren might target a translational component utilized only for premature termination.
Clearly, the simplest explanation for ataluren’s mechanism of action is that it behaves in a manner similar to that of the aminoglycosides. In this model, ataluren’s association with the ribosome at low doses would lead to occupancy of a high-affinity binding site and consequent alteration of A site fidelity, possibly by base-flipping. However, at higher doses, ataluren would occupy a second ribosomal site of lower affinity, and interaction with the second site would preclude the loss of A site fidelity that leads to premature stop codon readthrough. In short, the bell-shaped dose-response seen in cell cultures and mice and the dose-response seen in Study 007 patients would be attributable to a common mechanism.
Collectively, our results, and those of others, provide multiple, independent demonstrations of ataluren’s nonsense suppressing activity. This compound is the first small molecule that was discovered and developed specifically to treat diseases due to a nonsense mutation, and its development offers the prospect of validating nonsense suppression as a potential therapeutic approach in a large number of genetic disorders. Ataluren relies on small-molecule pharmacology and thus is more convenient for patients compared to alternative therapies such as biologics, antisense, gene therapy, and RNA interference, which generally cannot be delivered orally. Successful development of ataluren suggests continued research in a large and diverse group of genetic disorders for which the primary disease commonality is the presence of a nonsense mutation. As such, this approach is among the first to test the model of personalized medicine, in which the focus shifts from treatment of a disease to treatment of a specific molecular defect.
Acknowledgments
This work was supported by PTC Therapeutics, Inc. Additional support came from grants to A.J. from the National Institutes of Health (R37 GM27757) and the Human Frontiers Science Program Organization (RGP0018).
Footnotes
DISCLOSURE STATEMENT
All of the authors are paid employees or consultants of PTC Therapeutics Inc.
LITERATURE CITED
- 1.Nirenberg M, Leder P, Bernfield M, Brimacombe R, Trupin J, et al. Proc Natl Acad Sci USA. 1965;53:1161–8. doi: 10.1073/pnas.53.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngman EM, McDonald ME, Green R. Ann Rev Microbiol. 2008;62:353–73. doi: 10.1146/annurev.micro.61.080706.093323. [DOI] [PubMed] [Google Scholar]
- 3.Pisarev AV, Hellen CU, Pestova TV. Cell. 2007;131:286–99. doi: 10.1016/j.cell.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker T, Franckenberg S, Wickles S, Shoemaker CJ, Anger AM, et al. Nature. 2012;482:501–6. doi: 10.1038/nature10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosson B, Couturier A, Chabelskaya S, Kiktev D, Inge-Vechtomov S, et al. Mol Cell Biol. 2002;22:3301–15. doi: 10.1128/MCB.22.10.3301-3315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Roy F, Salehzada T, Bisbal C, Dougherty JP, Peltz SW. Nat Struct Mol Biol. 2005;12:505–12. doi: 10.1038/nsmb944. [DOI] [PubMed] [Google Scholar]
- 7.Losson R, Lacroute F. Proc Natl Acad Sci USA. 1979;76:5134–7. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daar IO, Maquat LE. Mol Cell Biol. 1988;8:802–13. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltz SW, Brown AH, Jacobson A. Genes Dev. 1993;7:1737–54. doi: 10.1101/gad.7.9.1737. [DOI] [PubMed] [Google Scholar]
- 10.Belgrader P, Cheng J, Maquat LE. Proc Natl Acad Sci USA. 1993;90:482–6. doi: 10.1073/pnas.90.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaba A, Jacobson A, Sachs MS. Mol Cell. 2005;20:449–60. doi: 10.1016/j.molcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson A, Izaurralde E. In: Translational Control in Biology and Medicine. Mathews MB, Sonenberg N, Hershey JWB, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 659–91. [Google Scholar]
- 13.Isken O, Maquat LE. Genes Dev. 2007;21:1833–56. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 14.He F, Brown AH, Jacobson A. Mol Cell Biol. 1997;17:1580–94. doi: 10.1128/mcb.17.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez CI, Bhattacharya A, Wang W, Peltz SW. Gene. 2001;274:15–25. doi: 10.1016/s0378-1119(01)00552-2. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Echevarria MJ, Peltz SW. Cell. 2000;101:741–51. doi: 10.1016/s0092-8674(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez CI, Ruiz-Echevarria MJ, Vasudevan S, Henry MF, Peltz SW. Mol Cell. 2000;5:489–99. doi: 10.1016/s1097-2765(00)80443-8. [DOI] [PubMed] [Google Scholar]
- 18.Czaplinski K, Ruiz-Echevarria MJ, Paushkin SV, Han X, Weng Y, et al. Genes Dev. 1998;12:1665–77. doi: 10.1101/gad.12.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng Y, Czaplinski K, Peltz SW. Mol Cell Biol. 1996;16:5477–90. doi: 10.1128/mcb.16.10.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng Y, Czaplinski K, Peltz SW. Mol Cell Biol. 1996;16:5491–506. doi: 10.1128/mcb.16.10.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita A, Izumi N, Kashima I, Ohnishi T, Saari B, et al. Genes Dev. 2009;23:1091–105. doi: 10.1101/gad.1767209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Jayachandran U, Bonneau F, Fiorini F, Basquin C, et al. Mol Cell. 2011;41:693–703. doi: 10.1016/j.molcel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Wen J, Brogna S. EMBO J. 2010;29:1537–51. doi: 10.1038/emboj.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBlanc JJ, Beemon KL. J Virol. 2004;78:5139–46. doi: 10.1128/JVI.78.10.5139-5146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amrani N, Ganesan R, Kervestin S, Mangus DA, Ghosh S, Jacobson A. Nature. 2004;432:112–8. doi: 10.1038/nature03060. [DOI] [PubMed] [Google Scholar]
- 26.Peixeiro I, Inacio A, Barbosa C, Silva AL, Liebhaber SA, Romao L. Nucleic Acids Res. 2011;40:1160–73. doi: 10.1093/nar/gkr820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S, Ganesan R, Amrani N, Jacobson A. RNA. 2010;16:1832–47. doi: 10.1261/rna.1987710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franks TM, Singh G, Lykke-Andersen J. Cell. 2010;143:938–50. doi: 10.1016/j.cell.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eberle AB, Stalder L, Mathys H, Orozco RZ, Muhlemann O. PLoS Biol. 2008;6:e92. doi: 10.1371/journal.pbio.0060092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov PV, Gehring NH, Kunz JB, Hentze MW, Kulozik AE. EMBO J. 2008;27:736–47. doi: 10.1038/emboj.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh G, Rebbapragada I, Lykke-Andersen J. PLoS Biol. 2008;6:e111. doi: 10.1371/journal.pbio.0060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amrani N, Sachs MS, Jacobson A. Nat Rev Mol Cell Biol. 2006;7:415–25. doi: 10.1038/nrm1942. [DOI] [PubMed] [Google Scholar]
- 33.Kervestin S, Li C, Buckingham R, Jacobson A. Biochimie. 2012;94:1560–71. doi: 10.1016/j.biochi.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman HM, Abelson J, Landy A, Brenner S, Smith JD. Nature. 1968;217:1019–24. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- 35.Stansfield I, Kushnirov VV, Jones KM, Tuite MF. Eur J Biochem. 1997;245:557–63. doi: 10.1111/j.1432-1033.1997.00557.x. [DOI] [PubMed] [Google Scholar]
- 36.Baxter-Roshek JL, Petrov AN, Dinman JD. PLoS One. 2007;2:e174. doi: 10.1371/journal.pone.0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirthi N, Roy-Chaudhuri B, Kelley T, Culver GM. RNA. 2006;12:2080–91. doi: 10.1261/rna.302006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandbaken MG, Culbertson MR. Genetics. 1988;120:923–34. doi: 10.1093/genetics/120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maderazo AB, He F, Mangus DA, Jacobson A. Mol Cell Biol. 2000;20:4591–603. doi: 10.1128/mcb.20.13.4591-4603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansson MJ, Jacobson A. Genes Dev. 2010;24:1491–5. doi: 10.1101/gad.1930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janzen DM, Frolova L, Geballe AP. Mol Cell Biol. 2002;22:8562–70. doi: 10.1128/MCB.22.24.8562-8570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorini L, Kataja E. Proc Natl Acad Sci USA. 1964;51:487–93. doi: 10.1073/pnas.51.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermann T. Cell Mol Life Sci. 2007;64:1841–52. doi: 10.1007/s00018-007-7034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshizawa S, Fourmy D, Puglisi JD. EMBO J. 1998;17:6437–48. doi: 10.1093/emboj/17.22.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manuvakhova M, Keeling K, Bedwell DM. RNA. 2000;6:1044–55. doi: 10.1017/s1355838200000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke JF, Mogg AE. Nucleic Acids Res. 1985;13:6265–72. doi: 10.1093/nar/13.17.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogle JM, Brodersen DE, Clemons WM, Jr, Tarry MJ, Carter AP, Ramakrishnan V. Science. 2001;292:897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- 48.Fan-Minogue H, Bedwell DM. RNA. 2008;14:148–57. doi: 10.1261/rna.805208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendell JT, Dietz HC. Cell. 2001;107:411–4. doi: 10.1016/s0092-8674(01)00583-9. [DOI] [PubMed] [Google Scholar]
- 50.Culbertson MR. Trends Genet. 1999;15:74–80. doi: 10.1016/s0168-9525(98)01658-8. [DOI] [PubMed] [Google Scholar]
- 51.Mort M, Ivanov D, Cooper DN, Chuzhanova NA. Hum Mutation. 2008;29:1037–347. doi: 10.1002/humu.20763. [DOI] [PubMed] [Google Scholar]
- 52.Nagy E, Maquat LE. Trends Biochem Sci. 1998;23:198–9. doi: 10.1016/s0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- 53.Krawczak M, Ball EV, Cooper DN. Am J Hum Genet. 1998;63:474–88. doi: 10.1086/301965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerem E. Curr Opin Pulm Med. 2004;10:547–52. doi: 10.1097/01.mcp.0000141247.22078.46. [DOI] [PubMed] [Google Scholar]
- 55.Lai CH, Chun HH, Nahas SA, Mitui M, Gamo KM, et al. Proc Natl Acad Sci U S A. 2004;101:15676–81. doi: 10.1073/pnas.0405155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keeling KM, Bedwell DM. J Mol Med. 2002;80:367–76. doi: 10.1007/s00109-001-0317-z. [DOI] [PubMed] [Google Scholar]
- 57.Sleat DE, Sohar I, Gin RM, Lobel P. Eur J Paediatr Neurol. 2001;5(Suppl A):57–62. doi: 10.1053/ejpn.2000.0436. [DOI] [PubMed] [Google Scholar]
- 58.Howard M, Frizzell RA, Bedwell DM. Nat Med. 1996;2:467–9. doi: 10.1038/nm0496-467. [DOI] [PubMed] [Google Scholar]
- 59.Howard MT, Anderson CB, Fass U, Khatri S, Gesteland RF, et al. Ann Neurol. 2004;55:422–6. doi: 10.1002/ana.20052. [DOI] [PubMed] [Google Scholar]
- 60.Bedwell DM, Kaenjak A, Benos DJ, Bebok Z, Bubien JK, et al. Nat Med. 1997;3:1280–4. doi: 10.1038/nm1197-1280. [DOI] [PubMed] [Google Scholar]
- 61.Bidou L, Hatin I, Perez N, Allamand V, Panthier JJ, Rousset JP. Gene Ther. 2004;11:619–27. doi: 10.1038/sj.gt.3302211. [DOI] [PubMed] [Google Scholar]
- 62.Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL. J Clin Invest. 1999;104:375–81. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du M, Jones JR, Lanier J, Keeling KM, Lindsey JR, et al. J Mol Med. 2002;80:595–604. doi: 10.1007/s00109-002-0363-1. [DOI] [PubMed] [Google Scholar]
- 64.Clancy JP, Bebok Z, Ruiz F, King C, Jones J, et al. Am J Respir Crit Care Med. 2001;163:1683–92. doi: 10.1164/ajrccm.163.7.2004001. [DOI] [PubMed] [Google Scholar]
- 65.Politano L, Nigro G, Nigro V, Piluso G, Papparella S, et al. Acta Myol. 2003;22:15–21. [PubMed] [Google Scholar]
- 66.Wilschanski M, Yahav Y, Yaacov Y, Blau H, Bentur L, et al. N Eng J Med. 2003;349:1433–41. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 67.James PD, Raut S, Rivard GE, Poon MC, Warner M, et al. Blood. 2005;106:3043–8. doi: 10.1182/blood-2005-03-1307. [DOI] [PubMed] [Google Scholar]
- 68.Nudelman I, Rebibo-Sabbah A, Cherniavsky M, Belakhov V, Hainrichson M, et al. J Med Chem. 2009;52:2836–45. doi: 10.1021/jm801640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang D, Belakhov V, Kandasamy J, Baasov T, Li SC, et al. Mol Genet Metab. 2012;105:116–25. doi: 10.1016/j.ymgme.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, Czaplinski K, Rao Y, Peltz SW. EMBO J. 2001;20:880–90. doi: 10.1093/emboj/20.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, et al. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 72.Du M, Liu X, Welch EM, Hirawat S, Peltz SW, Bedwell DM. Proc Natl Acad Sci USA. 2008;105:2064–9. doi: 10.1073/pnas.0711795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. Science. 1989;244:1578–80. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 74.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, et al. Lancet Neurol. 9:77–93. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 75.Dent KM, Dunn DM, von Niederhausern AC, Aoyagi AT, Kerr L, et al. Am J Med Genet A. 2005;134:295–8. doi: 10.1002/ajmg.a.30617. [DOI] [PubMed] [Google Scholar]
- 76.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Proc Natl Acad Sci USA. 1993;90:3710–4. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Florence JM, Fox PT, Planer GJ, Brooke MH. Neurology. 1985;35:758–61. doi: 10.1212/wnl.35.5.758. [DOI] [PubMed] [Google Scholar]
- 78.Kayali R, Ku JM, Khitrov G, Jung ME, Prikhodko O, Bertoni C. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds223. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, et al. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, et al. Cell. 1990;63:827–34. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 81.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, et al. Nature. 2003;423:168–72. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 82.Wang B, Yang Z, Brisson BK, Feng H, Zhang Z, et al. J Appl Physiol. 2010;109:901–5. doi: 10.1152/japplphysiol.01366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ballabio A, Gieselmann V. Biochim Biophys Acta. 2009;1793:684–96. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Bonnefront JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Mol Aspects Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Tan L, Narayan SB, Chen J, Meyers GD, Bennett MJ. J Inherit Metab Dis. 2011;34:443–7. doi: 10.1007/s10545-010-9265-5. [DOI] [PubMed] [Google Scholar]
- 86.Reiners J, Nagel-Wolfrum K, Jurgens K, Marker T, Wolfrum U. Exp Eye Res. 2006;83:97–119. doi: 10.1016/j.exer.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Goldmann T, Overlack N, Wolfrum U, Nagel-Wolfrum K. Hum Gene Ther. 2011;22:537–47. doi: 10.1089/hum.2010.067. [DOI] [PubMed] [Google Scholar]
- 88.Cotman SL, Staropoli JF. Clin Lipidol. 2012;7:79–91. doi: 10.2217/clp.11.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarkar C, Zhang Z, Mukherjee AB. Mol Genet Metab. 2011;104:338–45. doi: 10.1016/j.ymgme.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dranchak PK, Di Pietro E, Snowden A, Oesch N, Braverman NE, et al. J Cell Biochem. 2011;112:1250–8. doi: 10.1002/jcb.22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harmer SC, Mohal JS, Kemp D, Tinker A. Biochem J. 2012;443:635–42. doi: 10.1042/BJ20111912. [DOI] [PubMed] [Google Scholar]
- 92.Floquet C, Hatin I, Rousset JP, Bidou L. PLoS Genet. 2012;8:e1002608. doi: 10.1371/journal.pgen.1002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Auld DS, Thorne N, Maguire WF, Inglese J. Proc Natl Acad Sci USA. 2009;106:3585–90. doi: 10.1073/pnas.0813345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peltz SW, Welch EM, Jacobson A, Trotta CR, Naryshkin N, et al. Proc Natl Acad Sci USA. 2009;106:E64. doi: 10.1073/pnas.0901936106. author reply E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerem E, Hirawat S, Armoni S, Yaakov Y, Shoseyov D, et al. Lancet. 2008;372:719–27. doi: 10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
- 96.Sermet-Gaudelus I, Boeck KD, Casimir GJ, Vermeulen F, Leal T, et al. Am J Respir Crit Care Med. 2010;182:1262–72. doi: 10.1164/rccm.201001-0137OC. [DOI] [PubMed] [Google Scholar]
- 97.Wilschanski M, Miller LL, Shoseyov D, Blau H, Rivlin J, et al. Eur Respir J. 2011;38:59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]
- 98.Hirawat S, Welch EM, Elfring GL, Northcutt VJ, Paushkin S, et al. J Clin Pharmacol. 2007;47:430–44. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- 99.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, et al. Genet Med. 2006;8:465–73. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 100.van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, et al. N Engl J Med. 362:1396–406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 101.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Eur Respir J. 2008;32:637–43. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- 102.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Respir Med. 2009;103:1430–5. doi: 10.1016/j.rmed.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 103.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, et al. Am J Respir Crit Care Med. 183:1231–7. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 104.Mazzone E, Martinelli D, Berardinelli A, Messina S, D’Amico A, et al. Neuromuscul Disord. 2010;20:712–6. doi: 10.1016/j.nmd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 105.Bellais S, Le Goff C, Dagoneau N, Munnich A, Cormier-Daire V. Eur J Hum Genet. 2010;18:130–2. doi: 10.1038/ejhg.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]