Abstract
Bacteriophage particles contain both simple and complex macromolecular assemblages and machines that enable them to regulate the infection process under diverse environmental conditions with a broad range of bacterial hosts. Recent developments in cryo-electron tomography (cryo-ET) make it possible to observe the interactions of bacteriophages with their host cells under native-state conditions at unprecedented resolution and in three-dimensions. This review describes the application of cryo-ET to studies of bacteriophage attachment, genome ejection, assembly and egress. Current topics of investigation and future directions in the field are also discussed.
Keywords: Bacteriophage, Cryo-electron microscopy, Cryo-EM, Cryo-electron tomography, Cryo-ET, Sub-tomogram averaging
Introduction
Electron microscopy (EM) is one of the best direct imaging methods for studying the interactions between bacteriophages and bacterial cells. However, conventional EM carries the intrinsic disadvantage of requiring the use of heavy metal stains, fixatives, dehydration, and sectioning, all of which introduce preservation artifacts to the specimen and may limit the amount of useful structural information (Lucic et al., 2005, 2008). Plunge-freezing and high-pressure freezing methods were introduced in the 1980s and rapidly became the best methods to employ for the preservation of fine ultrastructure in biological specimens (Dubochet et al., 1983; Lepault et al., 1983; McDowall et al., 1983). Cryo-EM imaging of bacteriophages plunge frozen on EM grids, combined with icosahedral reconstruction methods has been used to solve the high-resolution three-dimensional (3D) structures of numerous bacteriophage capsids. This information has proven essential for determining common pathways of capsid maturation. However, for bacteriophage structures that either do not maintain a relationship with the icosahedral symmetry of the capsid or are present at one vertex of the capsid, reconstruction techniques that impose symmetry and, therefore limits to the structural information that may be resolved from the resultant 3D map. To this end, a number of groups developed approaches for reconstructing bacteriophages using algorithms that do not apply icosahedral averaging. Using phi-29 as a model system, Morais et al. (2001), introduced a method for the reconstruction of symmetry mismatches in tailed bacteriophages. This algorithm emerged as the basis implemented in most of the current asymmetric reconstruction techniques. These methods have been used to determine key bacteriophage structures, including the complete architecture of T7 (Agirrezabala et al., 2005), P22 (Chang et al., 2006), phi-29 (Tang et al., 2008), and Epsilon15 (Jiang et al., 2006). All together, these studies provided substantial information regarding the native structures of bacteriophage tail complexes and the DNA packaging and infection apparatus. The success of obtaining complete structures of bacteriophages in isolation provided greater impetus for determining the 3D structures of bacteriophage and the host cell during the infection process.
In order to visualize the complex 3D ultrastructure of entire eukaryotic and prokaryotic cells and pleiomorphic viruses, cryo-electron tomography (cryo-ET) has matured as an unparalleled imaging technology (Baumeister, 2004, 2005; Lucic et al., 2005; Murphy and Jensen, 2007; Yahav et al., 2011). Over the past few years, researchers have used cryo-ET to study the bacteriophage infection cycle and a number of the specific events that take place have been visualized at unmatched resolution (Table 1). This review highlights the history of biological EM, the development of cryo-EM and cryo-ET, and the recently published contributions to the field of bacteriophage biology that utilize cryo-ET as the main structural technique. With the goal of offering an overview of the broad range of applications of cryo-ET to the study of bacterial viruses, the structural studies discussed here answer questions regarding bacteriophage attachment, genome ejection, assembly and egress.
Table 1.
Overview of structural studies of bacteriophages that use cryo-electron tomography.
| Study | Phage | Host | Equipment | References |
|---|---|---|---|---|
| Genome ejection | T5 | Escherichia coli | Philips CM 120 Biofilter (120 kV) Philips CM 300 FEG (300 kV) | (Bohm et al., 2001) |
| Genome ejection | P-SSP7 | Prochlorococcus | JEOL JEM3200FSC (300 kV) | (Liu et al., 2010) |
| Initial interaction | P1 | Escherichia coli | FEI Tecnai G2 Polara (300 kV) | (Liu et al., 2011) |
| Initial interaction | ΦCbK/ΦCb13 | Caulobacter crescentus | FEI Tecnai G2 Polara (300 kV) FEI Tecnai G2 F30 (300 kV) | (Guerrero-Ferreira et al., 2011) |
| Whole cell/virus assembly | STIV | Sulfolobus solfataricus | FEI Tecnai G2 Polara (300 kV) | (Fu et al., 2011) |
| Whole virus | Φ12 | Pseudomonas syringae pv. phaseolicola | Tecnai F20 (200 kV) | (Hu et al., 2008) |
| Whole virus | Epsilon 15 | Salmonella anatum | JEOL JEM2200FS (200 kV) | (Murata et al., 2010) |
| Whole virus | BPP-1 | Bordetella spp. | FEI Tecnai G2 Polara (300 kV) | (Dai et al., 2010) |
| Whole virus | Φ6 | Pseudomonas syringae | FEI Tecnai-12 (120 kV) | (Nemecek et al., 2010) |
| Whole virus | Φ6 | Pseudomonas syringae | FEI Tecnai-12 (120 kV) | (Nemecek et al., 2011) |
| Whole virus/DNA ejection | Φ8a | Bacillus anthracis | FEI Tecnai G2 Polara (300 kV) | (Fu et al., 2011) |
| Whole virus/Initial interaction | Φ12 | Pseudomonas syringae pv. phaseolicola | Tecnai F20 (200 kV) JEOL JEM3200FSC (300 kV) | (Leo-Macias et al., 2011) |
Biological electron microscopy and the development of cryo-electron microscopy
Biological EM has a long history, which began with the development of the transmission electron microscope (TEM) in the 1930s and early 1940s (Bozzola and Russell, 1998). Hydrated specimens, such as cells, tissues, and purified macromolecules, require fixation, dehydration, and staining prior to their introduction into the high vacuum environment of the TEM. Two approaches were established to address the TEM imaging needs of the biological research community. First, negative-stain techniques were developed for the rapid preservation and staining of biological materials in suspension; and second, procedures were pioneered for embedding thicker specimens in resin for sectioning. Both methods are still widely used for processing samples prior to imaging, a brief summary of each is provided below (Bozzola and Russell, 1998).
Negative staining of small particles for TEM investigation began in the 1950s. Three independent research groups, Hall (1955); Huxley (1956), and Brenner and Horne (1959) used solutions of phosphotungstic acid to fix and stain virus particles. Some of the viruses first imaged by negative stain TEM include tomato bushy stunt virus (BSV), tobacco mosaic virus (TMV), and T2 bacteriophage. The technique of negative stain TEM has become very wide spread because of its speed and simplicity and that it allows one to determine the shape, size, and external structure of an isolated or purified specimen. Since then, many other heavy metal stains have been tested and are available for staining a wide range of specimen types. Single particle 3D reconstruction methods were originally adapted for and applied to particles that were negatively stained. However, even with its many benefits, the major disadvantages to using negative stain techniques are the loss of sample hydration, limitation of specimen size, and the lack of internal structure information.
In order to visualize the entire structure of thicker cells and tissues, it is necessary to preserve the specimen in such a way that it may be cut into thinner sections for viewing in the TEM. A number of methods were developed for the preservation of specimens prior to their embedment in polymer resins (Claude and Fullam, 1945; Glauert et al., 1956; Luft, 1961; Porter et al., 1945; Watson, 1958a, b); the basic steps are presented below. Prior to being embedded in resin, the macromolecules of the specimen need to be fixed and the water removed through dehydration. The most commonly used primary fixation scheme includes a combination of glutaraldehyde and paraformaldehyde at concentrations ranging from 0.5% to 4% for crosslinking the proteins followed by secondary fixation with 2%–4% osmium tetroxide that reacts with proteins and unsaturated lipids. After fixation, the specimen is dehydrated through a graded series of acetone or ethanol and is then suspended in a liquid polymer resin that is subsequently cured in an oven. Once thin sections are cut from the polymerized resin-specimen block, the sections are further stained with a combination of two heavy metal stains, uranyl acetate and lead citrate. This methodology can be adapted and modified for many specimens and procedures; however, the major limitations are that the specimen is chemically and physically altered through the introduction of fixatives, resins, and heavy metal stains.
Cryo-EM was born out of the desire to maintain biological specimen hydration for both direct imaging and electron diffraction in the TEM. The first frozen hydrated specimen imaged by electron diffraction in the TEM was catalase crystals by Taylor and Glaeser (1974). Here, they froze catalase crystals in a thin film of ice using liquid nitrogen as the cryogen. Several years later, cryogens other than liquid nitrogen were used to freeze samples and it was determined that the freezing rates were faster and limited the introduction of crystalline ice. Now, most aqueous samples are prepared for cryo-EM or cryo-ET by first applying a small aliquot of a suspension to an EM grid, blotting the grid to near dryness, and then rapidly plunge freezing it in a cryogen (typically liquid ethane or liquid propane) cooled to liquid nitrogen temperatures (~ −196°C). This method for plunge freezing grids preserves the biological sample in a thin layer of vitreous, non-crystalline ice in a near native state and eliminates artifacts that result from sample fixation, dehydration or staining (Dubochet et al., 1988; Lepault et al., 1983). Once prepared, a cryo-EM grid is stored under liquid nitrogen until it is transferred to a ‘cryo-stage.’ The cryo-stage is designed to maintain the specimen at close to liquid nitrogen temperatures while the specimen is imaged in the electron microscope.
There are a few key features to the electron microscopes used for cryo-ET studies of bacteriophages, whole bacterial cells, or the interactions between the two (Table 1). First, when thicker samples are imaged in the electron microscope, multiple scattering events result due to the limitation of the mean free path of the electrons (~200nm for 120 kV and ~350 nm for 300 kV) (Grimm et al., 1998). In order to overcome these limits, groups generally use a microscope with an intermediate accelerating voltage (200–300 kV) for improved sample penetration by the electron beam and/or an energy filter to remove noise-generating inelastically scattered electrons from the final image that is acquired on the CCD (Grimm et al., 1996). Specimens that are substantially thicker than 1 μm may be thinned by a number of methods prior to introduction into the microscope (Al-Amoudi et al., 2004; Marko et al., 2007). Electron tomography involves the specimen of interest being semi-automatically or automatically tilted to angles that range between ± 60◦ and ± 70◦ (Baumeister et al., 1999; Mastronarde, 2005; Suloway et al., 2009; Zheng et al., 2004). A series of individual 2D images are acquired of the area of interest over the tilt range at tilt intervals of 1–2◦. The ‘tilt series’ is then computationally processed to generate a 3D reconstruction of the specimen (Gilbert, 1972a,b; Kremer et al., 1996; Lee et al., 2008; Marabini et al., 1997). However, there is a physical limit of ~140◦ associated with the tilt range of the specimen holder and microscope goniometer, which creates what is known as a “missing wedge” of data in the 3D reconstruction. The missing wedge corresponds to the incomplete sampling of Fourier space, and may directly affect subsequent 3D alignment and averaging procedures. Primary data collection that incorporates dual axis tomography (Iancu et al., 2005; Mastronarde, 1997; Penczek et al., 1995) allows the investigator to acquire tilt series of the specimen along two orthogonal axes (i.e. sample rotated by 90◦). By sampling Fourier space along two axes at a tilt range of ± 60◦, one reduces the amount of unmeasured space, or the “missing wedge,” from ~33% (one axis) to 16% (two axes) (Iancu et al., 2005). This results in an increase in the isotropic resolution of the reconstructed data; consequently, improving data quality for downstream processing such as sub-tomogram averaging (see below), template-directed macromolecule identification (Bohm et al., 2000; Frangakis et al., 2002), and the selection or identification of a feature that was not observed along only one axis (Iancu et al., 2005; Mastronarde, 1997). However, there are two major limitations of dual axis cryo-ET. First, the low total electron dose used during image acquisition is further fractionated over a greater number of images, which may negatively impact image alignment during the reconstruction process. Second, additional improvements in the software for merging the two axes may be necessary to increase the quality of the final reconstruction.
In addition to better 3D reconstructions through dual axis tomography, algorithms have been developed for averaging sub-volumes of tomograms (Briggs et al., 2009; Leo-Macias et al., 2011; Schmid and Booth, 2008). Sub-tomogram averaging procedures generate isotropic, higher signal-to-noise structures of a complex of interest. It has proved to be especially powerful in studies of viral ultrastructure, specifically those of viral glycoproteins (Fontana et al., 2012; Liu et al., 2008; Zanetti et al., 2006; Zhu et al., 2008), lattice-forming structural proteins (Briggs et al., 2009; de Marco et al., 2010; Wright et al., 2007), and bacteriophage as presented below.
Imaging whole virus (isolated particles)
Cryo-EM followed by icosahedral image reconstruction was used to determine the 3D organization of the nucleocapsid of the cystovirus Φ6 as well as Escherichia coli-expressed and assembled procapsid-related particles (Butcher et al., 1997). In a more recent study of another cystovirus, Φ12, cryo-ET was employed to study the overall substructure of the phage and the shape and distribution of the surface proteins (Hu et al., 2008). Cryo-ET was the method of choice to determine the structure of the surface proteins because their positions do not relate to the icosahedral symmetry of the nucleocapsid. The particles exhibit two discrete shells resulting from the nucleocapsid and the membranous envelope, respectively. Connections between the nucleocapsid and the inner surface of the envelope appear to center the nucleocapsid within the viral envelope. This study resolved the distribution of both elongated densities that maintained close associations with the membrane surface and donut-shaped densities set further away from the membrane surface (Fig. 1). It was previously determined that the cystovirus Φ6 does not have donut shaped complexes on its surface. The viral surface is instead exclusively decorated with horizontal spikes that are necessary for attachment to the host bacterium Pseudomonas syringae (Bamford et al., 1987). Therefore, cryo-ET was pivotal for elucidating the structural diversity of cystoviruses by establishing the presence of two asymmetrical surface densities. Moreover, this technique allows the initial description of a new kind of receptor binding protein, with a toroidal or donut-like shape (Fig. 1).
Fig. 1.
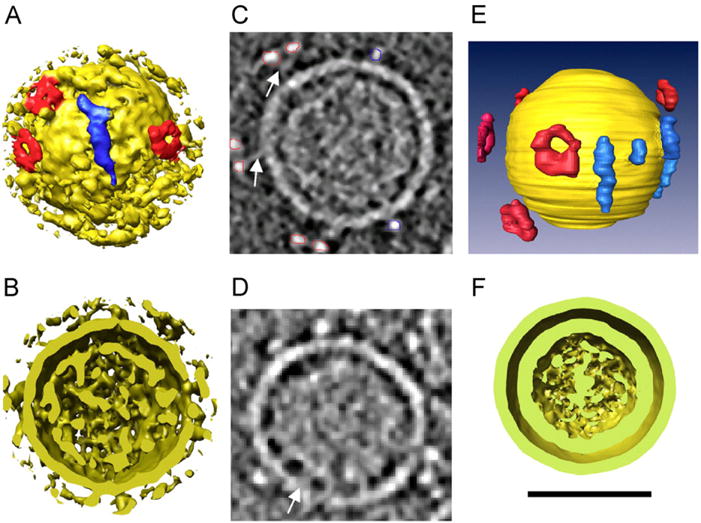
3D rendering of Φ12 bacteriophage structure. (A) Surface rendering of an individual virion depicting two types of protruding densities, labeled with red (donutshaped) and blue (elongated). (B) 7 nm thick section through a tomographic reconstruction of bacteriophage Φ12. The membrane and the two classes of protruding densities are clear. Red and blue lines represent the segmentation of donut-shaped and elongated protrusions, respectively. (C) 3D rendering resulting from the segmentation represented in panel ‘B’ with yellow label representing the membrane surface and red and blue the surface spikes. (D) Cross-section of the bacteriophage particle from panel ‘A’ illustrating two discrete shells. (E) Section through a Φ12 bacteriophage displaying the connection of the inner face of the envelope with the nucleocapsid (arrow). (F) Aligned average of 180 Φ12 bacteriophage particles clearly depicting two distinct shells. Reprinted from Virology, 372, Hu et al., Electron cryotomographic structure of cystovirus phi 12, 1–9, Copyright (2008), with permission from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To complement these findings, Leo-Macias et al. (2011) carried out sub-tomogram averaging of the surface complex of Φ12, averaging 744 copies of this surface density from 158 bacteriophage particles. Approximately five, randomly distributed, toroidal, surface complexes were found to be 13 nm from the membrane of the cystovirus. With a resolution of 2.6 nm, obtained by sub-tomogram averaging, the toroid was found to be 19 nm in diameter and to have six-fold symmetry. A 5 nm connecting density was also observed, that attaches the surface protein to the virus envelope. The authors proposed that the toroidal structure evolved as an alternative strategy for the cystovirus to infect additional cells.
Cryo-ET and sub-tomogram averaging were used by Nemecek et al. (2011) to establish the occupancy of the packaging NTPase (P4) and the RNA-dependent RNA polymerase (RdRP, P2) of bacteriophage Φ6 of P. syringae. 560 individual procapsids were extracted from three tomograms and aligned to an existing single particle reconstruction. With this approach, it was feasible to identify both P2 and P4 on individual procapsids, count individual molecules, and map their locations. Their results suggest that both subunits are randomly incorporated and that copy numbers may be determined by their individual rates of synthesis. Their evaluation of P2 and P4 locations also showed that these proteins do not tend to cluster or co-localize, suggesting that their activities are not physically coupled. In another study, cryo-EM and cryo-ET were applied to demonstrate the process of procapsid expansion in Φ6 (Nemecek et al., 2011). By using sub-tomogram averaging, this study classified individual vertices of compact and expanded Φ6. The aim was to visualize specific features on individual procapsids rather than averaging these features through single particle methods. The process of classifying all vertices extracted from compact and expanded forms from the same tomograms revealed four distinct classes of vertices: two of them were similar to the compact procapsid vertex and the other two to expansion intermediates.
Imaging attachment and DNA injection processes
In a structural study of T5 bound to receptor-containing proteoliposomes, Bohm et al. (2001) imaged the process of genome transfer. It is well established that during bacteriophage infection the viral genome is efficiently transferred into the bacterial host. The structural details of this process are, however, poorly understood. Cryo-ET revealed that after binding to the receptor, the tip of the bacteriophage tail passes through the lipid bilayer of the proteoliposome (Fig. 2) and undergoes a conformational change that increases its diameter from 2 to 4 nm and decreases its length from 50 to 23 nm.
Fig. 2.
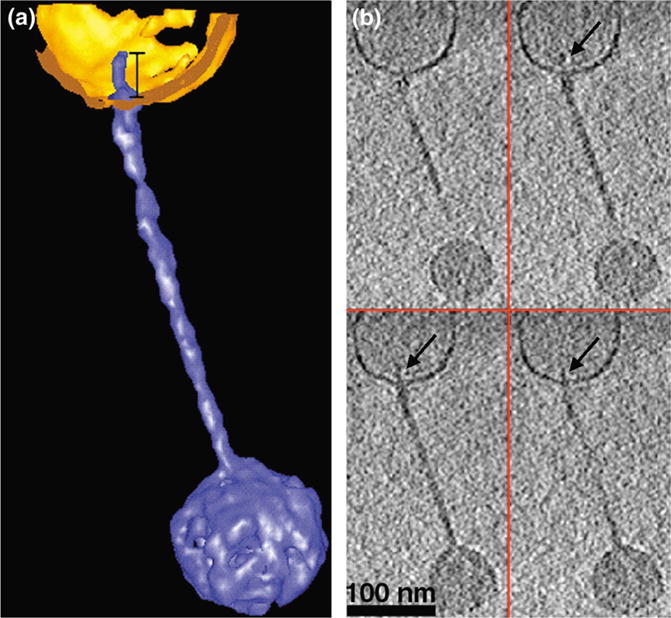
Binding of a T5 bacteriophage particle to a proteoliposome before DNA release. (a) Segmented 3D rendering of a T5 bacteriophage (depicted in blue) bound to a vesicle (displayed in gold) before DNA release. (b) Four XY slices (1.4 nm thick) through the same reconstruction depicted in (a). Arrows point to the tip of the bacteriophage tail inside the vesicle (Reprinted from Current Biology, 11/15, Böhm et al., FhuA-mediated phage genome transfer into liposomes: A cryo-electron tomography study, 1168–1175, Copyright (2001), with permission from Elsevier.) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Cryo-ET and sub-tomogram averaging methods were also used by Liu et al. (2011) to study P1 bacteriophage particles attached to E. coli mini-cells to determine the mechanism by which the bacteriophage interacts with the cell membrane of its host. A significant separation between the bacteriophage’s baseplate and the cell’s outer membrane was identified during the transition from an extended tail conformation to a contracted tail conformation. They determined that long tail fibers attach to the cell membrane in order to anchor the bacteriophage to the cell. Upon tail sheath contraction, the long tail fibers become extended, causing the baseplate to move farther away from the cell. This results in the movement of the bacteriophage’s head and tail tube closer to the cell, which causes the tail tube to puncture the cell envelope.
Other studies have also shown that structural alterations occur in bacteriophages upon binding to the host cell and those changes are associated with genome release. Liu et al. (2010) carried out structural analysis of bacteriophage P-SSP7 of Prochlorococcus marinus (a cyanobacterium) using cryo-ET. Their results indicate that during the process of infection, the tail fiber of P-SSP7 has the same conformation as that of empty bacteriophage particles. Furthermore, their results suggest that the tail-fibers induce a series of structural alterations in the portal vertex complex. The accumulation of such conformational changes results in the successful ejection, or release, of bacteriophage genomic material.
Imaging the interactions of bacteriophages with bacterial appendages
There are numerous examples in nature of how bacteriophage accessory filaments promote bacteriophage interactions with bacterial appendages and facilitate the infection of their hosts. Reports have shown that several bacteriophages utilize their tail filaments to adsorb to the bacterial flagellum. This interaction is very specific, as in the cases of coliphage chi (Schade et al., 1967) or Bacillus subtilis bacteriophage PBS1 (Raimondo et al., 1968). In both cases, the bacteriophage benefits from rotation of the flagellum, which allows the bacteriophage to access the sites of irreversible attachment and genome injection on the cell surface.
Recently, Guerrero-Ferreira et al. (2011) used cryo-ET and microbiology approaches to determine the role of a head filament during bacteriophage adsorption and the attachment of the ΦCbK/ΦCb13 tail to their host, Caulobacter crescentus. ΦCbK (and related bacteriophage ΦCb13) have an elongated head (60 nm in diameter and 200 nm in length), a non-contractile tail (290 nm in length) with tail filaments (Leonard et al., 1972, 1973) and an additional filament protruding from the head of the bacteriophage (Leonard et al., 1972). The study by Guerrero-Ferreira et al. (2011) concludes that the head filament is necessary for the initial interaction of the bacteriophage with the bacterial flagellum (Fig. 3). Flagellar rotation then promotes the translation of the bacteriophage towards the cell pole, where irreversible attachment occurs. In addition, 2D cryo-EM and microbiology techniques confirmed the site of attachment to be the pili portals located at the C. crescentus pole.
Fig. 3.
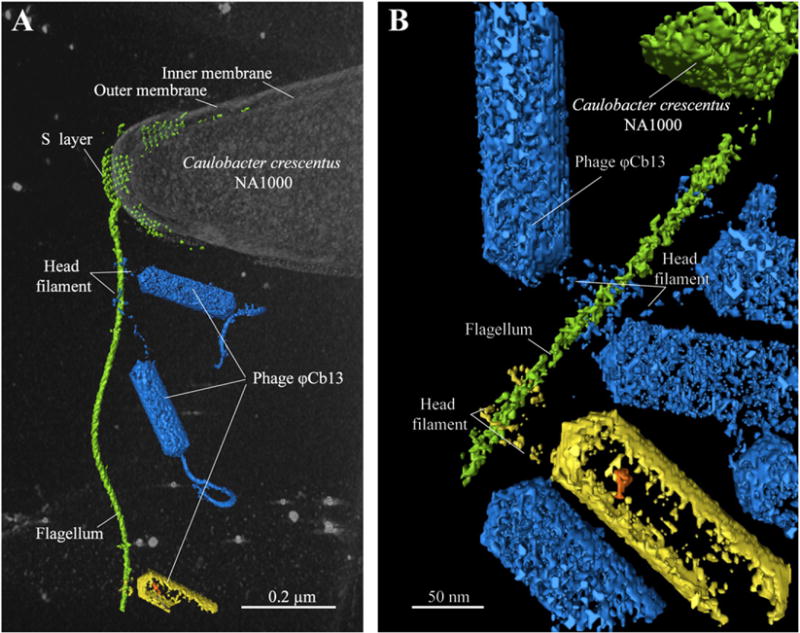
Segmented 3D volume from a cryo-electron tomogram of a ΦCb13-infected, C. crescentus cell illustrating head filament interacting with the flagellum (Reprinted from PNAS, 108/24, Guerrero-Ferreira et al., Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus, 9963–9968, Copyright (2011).
Imaging bacteriophage structural modifications during DNA ejection
A recent study by Fu et al. (2011) analyzed more than 2000 particles of bacteriophage 8a targeting spores of Bacillus anthracis. The results of this high throughput cryo-ET approach shed light on the multiple structural conformations that this bacteriophage goes through in order to release its genome from the capsid during infection. The group describes four distinct structural conformations that mainly vary in their genome content and tail length (Fig. 4). Structural changes are also observed in the baseplate in addition to changes in tail length during contraction. The authors concluded that these distinct states, which range from a genome-filled capsid and extended tail to an empty capsid and contracted tail, result in the translocation of the viral DNA from the bacteriophage 8a capsid into the cytoplasm of B. anthracis.
Fig. 4.
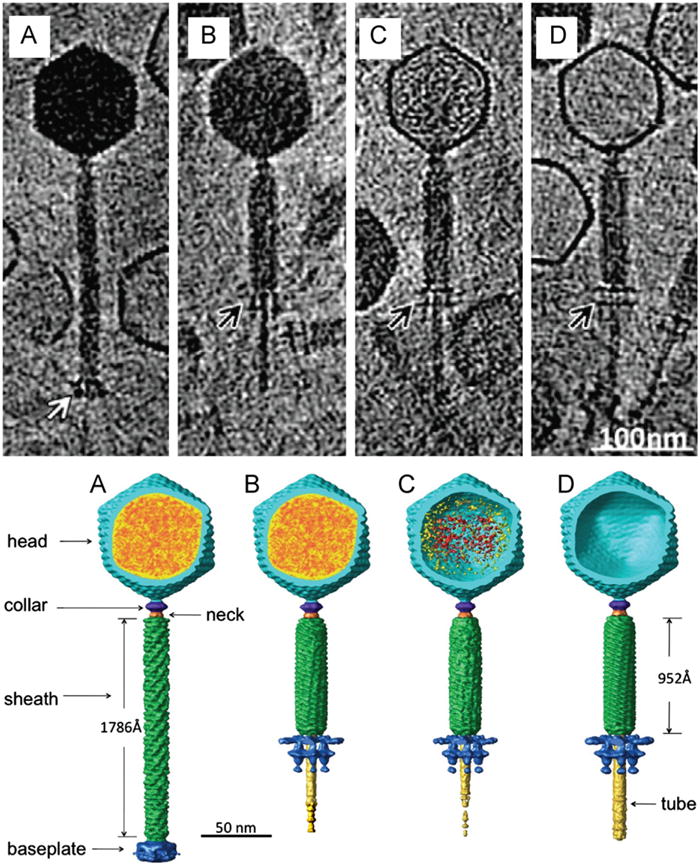
The top panel depicts cryo-ET of representative bacteriophage 8a particles at each of the four states of DNA ejection. Differences in genome content and tail sheath length are evident. Notice the difference in baseplate morphology indicated by the arrows. The bottom panel depicts the corresponding segmented volumes of the four states, indicating viral components and size differences between the extended and contracted tail. Modified from Fu et al. (2011). Reprinted from Virology, 410/2, Fu et al. The mechanism of DNA ejection in the Bacillus anthracis spore-binding phage 8a revealed by cryo-electron tomography, 141–148, Copyright (2011), with permission from Elsevier.
No apparent difference was observed in the head structure when comparing states 1 (Fig. 4A: DNA-filled capsid, extended tail) and 2 (Fig. 4B: DNA-filled capsid, contracted tail). The heads of bacteriophages in state 3 (Fig. 4C: partially DNA-filled capsid, contracted tail) contain densities that either corresponds to genome or protein. These densities were completely absent in state 4 (Fig. 4D: empty capsid, contracted tail) virions. The portal protein is clearly visible in each of the four states, although its morphology is more easily depicted in bacteriophage particles in state 4 (empty, contracted-tailed bacteriophages). The portal has a typical wide-end inside the bacteriophage head, a central region and a central channel sized for double-stranded DNA. A unique feature of the bacteriophage 8a portal is an extra goblet-like structure that extends from the 8a “portal crown domain” region toward the center of the capsid suggesting that it may play a role in DNA translocation.
Ultrastructure applied to study bacteriophage evolution
In addition to elucidating viral functions by biochemical methods and microbiological assays, the evaluation of virus architecture by high-resolution structural methods has allowed the clarification of existing long-range phylogenic relationships in viral lineages (Ravantti et al., 2003). An example is bacteriophage PRD1, which has an icosahedral protein coat. The major coat protein, P3, has three interlocking subunits, each with two eight-stranded viral jelly rolls normal to the viral capsid, and putative membrane-interacting regions. The P3 structure closely resembles the equivalent protein in human adenovirus. Both viruses have similar overall architecture, with identical capsid lattices and attachment proteins at their vertices. Although the two dsDNA viruses infect hosts from very different domains of life, their striking similarities from major coat protein through capsid architecture strongly suggest an evolutionary relationship (Benson et al., 1999). A common ancestor has also been proposed for these two viruses (Martin et al., 2001). High-throughput cryo-ET, combined with sub-tomogram averaging, are ideal complementary approaches to genome-based methods to address questions regarding the evolution of viral ultrastructure in bacteriophage lineages.
Conclusions
The observation of frozen, hydrated biological specimens through cryo-ET is a unique, alternative approach for the structural analysis of cellular structures in their native states (Baumeister, 2005; Lucic et al., 2005). This review illustrates the enormous potential of cryo-ET as a tool for elucidating the mechanisms of viral infection, assembly and egress. Information from cryo-ET studies has been pivotal in the field of bacteriophage biology, not only in combination with molecular approaches but also as a stand-alone method.
As noted earlier, the principle of cryo-ET requires that a number of images of a region of interest be acquired from different tilt angles. In order to reduce irradiation damage, a low total electron dose has to be distributed among all the images. This dose fractionation results in an inevitably low signal to noise ratio. Usually, the application of a defined defocus to the images achieves adequate phase contrast. However, this comes with a loss of low spatial frequency information. Zernike phase contrast in TEM has been used recently to obtain phase contrast without sacrificing resolution (Danev and Nagayama, 2001) and its application has been extended and combined successfully with cryo-ET protocols (Danev et al., 2010; Hosogi et al., 2011). Thus, the use of this technology will also benefit ultrastructural studies of bacteria–bacteriophage interactions.
Further developments in the areas of correlative microscopes will complement the application of cryo-EM and cryo-ET in life sciences. A problem that arises in cryo-EM and cryo-ET is the difficulty to locate regions of interest on a grid due to the low contrast of the sample. In addition, the use of fluorescence microscopy to localize specific biological processes as they occur can be complemented with cryo-ET to allow for a higher resolution analysis of the process (Kukulski et al., 2011; Sartori et al., 2007). Integration of low resolution and high resolution microscopy techniques in a correlative approach allows for the understanding of processes in the context of whole cells and at the same time the investigation of the macromolecular complexes involved in these processes (Muller-Reichert et al., 2007; Plitzko et al., 2009; van Driel et al., 2009).
Bacteriophage biology developed as a means to the search for antibacterial therapies, the development of modern molecular biology and the generation of tools for recombinant DNA technology and genetic engineering. However, a great deal of information has been missing in regard to the complex structural relationships that exist between bacteria and bacteriophages. Now, bacteriophage biology has at its disposal a powerful new tool for studying cellular ultrastructure and infrastructure: cryo-ET. This technology is rapidly evolving and has already been used to examine several of the basic stages of the bacteriophage infection cycle. Combining the strengths of bacteriophage biology with cryo-ET will open new frontiers in bacteriophage biology.
Acknowledgments
The authors thank Dr. Ian Molineux for the invitation to submit a minireview article to Virology and for critical reading of the manuscript. We are grateful to Drs. Jens Holl and Gabriella Kiss for valuable help with manuscript preparation and helpful discussions. This work was supported in part by the Emory University, the Children’s Healthcare of Atlanta, the Georgia Research Alliance, and the HFSP Grant RGP0051 to E.R.W.
References
- Agirrezabala X, Martin-Benito J, Valle M, Gonzalez JM, Valencia A, Valpuesta JM, Carrascosa JL. Structure of the connector of bacteriophage T7 at 8 A resolution: structural homologies of a basic component of a DNA translocating machinery. J Mol Biol. 2005;347:895–902. doi: 10.1016/j.jmb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Al-Amoudi A, Chang JJ, Leforestier A, McDowall A, Salamin LM, Norlen LP, Richter K, Blanc NS, Studer D, Dubochet J. Cryo-electron microscopy of vitreous sections. EMBO J. 2004;23:3583–3588. doi: 10.1038/sj.emboj.7600366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford DH, Romantschuk M, Somerharju PJ. Membrane fusion in prokaryotes: bacteriophage phi 6 membrane fuses with the Pseudomonas syringae outer membrane. EMBO J. 1987;6:1467–1473. doi: 10.1002/j.1460-2075.1987.tb02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister W. Mapping molecular landscapes inside cells. Biol Chem. 2004;385:865–872. doi: 10.1515/BC.2004.113. [DOI] [PubMed] [Google Scholar]
- Baumeister W. From proteomic inventory to architecture. FEBS Lett. 2005;579:933–937. doi: 10.1016/j.febslet.2004.10.102. [DOI] [PubMed] [Google Scholar]
- Baumeister W, Grimm R, Walz J. Electron tomography of molecules and cells. Trends Cell Biol. 1999;9:81–85. doi: 10.1016/s0962-8924(98)01423-8. [DOI] [PubMed] [Google Scholar]
- Benson SD, Bamford JKH, Bamford DH, Burnett RM. Viral evolution revealed by bacteriophage prd1 and human adenovirus coat protein structures. Cell. 1999;98:825–833. doi: 10.1016/s0092-8674(00)81516-0. [DOI] [PubMed] [Google Scholar]
- Bohm J, Frangakis AS, Hegerl R, Nickell S, Typke D, Baumeister W. Toward detecting and identifying macromolecules in a cellular context: template matching applied to electron tomograms. Proc Nat Acad Sci USA. 2000;97:14245–14250. doi: 10.1073/pnas.230282097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm J, Lambert O, Frangakis AS, Letellier L, Baumeister W, Rigaud JL. FhuA-mediated phage genome transfer into liposomes: a cryo-electron tomography study. Curr Biol. 2001;11:1168–1175. doi: 10.1016/s0960-9822(01)00349-9. [DOI] [PubMed] [Google Scholar]
- Bozzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. 2nd. Jones & Bartlett Learning; Subdury, MA, USA: 1998. [Google Scholar]
- Brenner S, Horne RW. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Briggs JA, Riches JD, Glass B, Bartonova V, Zanetti G, Krausslich HG. Structure and assembly of immature HIV. Proc Nat Acad Sci USA. 2009;106:11090–11095. doi: 10.1073/pnas.0903535106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SJ, Dokland T, Ojala PM, Bamford DH, Fuller SD. Intermediates in the assembly pathway of the double-stranded RNA virus phi6. EMBO J. 1997;16:4477–4487. doi: 10.1093/emboj/16.14.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Weigele P, King J, Chiu W, Jiang W. Cryo-EM asymmetric reconstruction of bacteriophage P22 reveals organization of its DNA packaging and infecting machinery. Structure. 2006;14:1073–1082. doi: 10.1016/j.str.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Claude A, Fullam EF. An electron microscope study of isolated mitochondria: method and preliminary results. J Exp Med. 1945;81:51–62. doi: 10.1084/jem.81.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Hodes A, Hui WH, Gingery M, Miller JF, Zhou ZH. Three-dimensional structure of tropism-switching Bordetella bacteriophage. Proc Nat Acad Sci USA. 2010;107:4347–4352. doi: 10.1073/pnas.0915008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danev R, Kanamaru S, Marko M, Nagayama K. Zernike phase contrast cryo-electron tomography. J Struct Biol. 2010;171:174–181. doi: 10.1016/j.jsb.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Danev R, Nagayama K. Transmission electron microscopy with Zernike phase plate. Ultramicroscopy. 2001;88:243–252. doi: 10.1016/s0304-3991(01)00088-2. [DOI] [PubMed] [Google Scholar]
- de Marco A, Muller B, Glass B, Riches JD, Krausslich HG, Briggs JA. Structural analysis of HIV-1 maturation using cryo-electron tomography. PLoS Pathog. 2010;6:e1001215. doi: 10.1371/journal.ppat.1001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J, Adrian M, Chang JJ, Homo JC, Lepault J, McDowall AW, Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988;21:129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Dubochet J, McDowall AW, Menge B, Schmid EN, Lickfeld KG. Electron microscopy of frozen-hydrated bacteria. J Bacteriol. 1983;155:381–390. doi: 10.1128/jb.155.1.381-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J, Cardone G, Heymann JB, Winkler DC, Steven AC. Structural changes in Influenza virus at low pH characterized by cryo-electron tomography. J Virol. 2012;86:2919–2929. doi: 10.1128/JVI.06698-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangakis AS, Bohm J, Forster F, Nickell S, Nicastro D, Typke D, Hegerl R, Baumeister W. Identification of macromolecular complexes in cryoelectron tomograms of phantom cells. Proc Nat Acad Sci USA. 2002;99:14153–14158. doi: 10.1073/pnas.172520299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Walter MH, Paredes A, Morais MC, Liu J. The mechanism of DNA ejection in the Bacillus anthracis spore-binding phage 8a revealed by cryo-electron tomography. Virology. 2011;421:141–148. doi: 10.1016/j.virol.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. Iterative methods for the three-dimensional reconstruction of an object from projections. J Theor Biol. 1972a;36:105–117. doi: 10.1016/0022-5193(72)90180-4. [DOI] [PubMed] [Google Scholar]
- Gilbert PF. The reconstruction of a three-dimensional structure from projections and its application to electron microscopy II. Direct methods. Proc R Soc London B Biol Sci. 1972b;182:89–102. doi: 10.1098/rspb.1972.0068. [DOI] [PubMed] [Google Scholar]
- Glauert AM, Glauert RH, Rogers GE. A new embedding medium for electron microscopy. Nature. 1956;178:803. doi: 10.1038/178803a0. [DOI] [PubMed] [Google Scholar]
- Grimm R, Singh H, Rachel R, Typke D, Zillig W, Baumeister W. Electron tomography of ice-embedded prokaryotic cells. Biophys J. 1998;74:1031–1042. doi: 10.1016/S0006-3495(98)74028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm R, Typke D, Barmann M, Baumeister W. Determination of the inelastic mean free path in ice by examination of tilted vesicles and automated most probable loss imaging. Ultramicroscopy. 1996;63:169–179. doi: 10.1016/0304-3991(96)00035-6. [DOI] [PubMed] [Google Scholar]
- Guerrero-Ferreira RC, Viollier PH, Ely B, Poindexter JS, Georgieva M, Jensen GJ, Wright ER. Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc Nat Acad Sci USA. 2011;108:9963–9968. doi: 10.1073/pnas.1012388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CE. Electron densitometry of stained virus particles. J Biophys Biochem Cytol. 1955;1:1–12. doi: 10.1083/jcb.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogi N, Shigematsu H, Terashima H, Homma M, Nagayama K. Zernike phase contrast cryo-electron tomography of sodium-driven flagellar hookbasal bodies from Vibrio alginolyticus. J Struct Biol. 2011;173:67–76. doi: 10.1016/j.jsb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Hu GB, Wei H, Rice WJ, Stokes DL, Gottlieb P. Electron cryo-tomographic structure of cystovirus phi 12. Virology. 2008;372:1–9. doi: 10.1016/j.virol.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley HE. Some observations on the structure of tobacco mosaic virus. In: Sjoöstrand FS, Rhodin J, editors. Proceedings of the Stockholm Conference on Electron Microscopy. Almquist & Wiksell; Stockholm: 1956. pp. 260–261. [Google Scholar]
- Iancu CV, Wright ER, Benjamin J, Tivol WF, Dias DP, Murphy GE, Morrison RC, Heymann JB, Jensen GJ. A “flip-flop” rotation stage for routine dual-axis electron cryotomography. J Struct Biol. 2005;151:288–297. doi: 10.1016/j.jsb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Jiang W, Chang J, Jakana J, Weigele P, King J, Chiu W. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature. 2006;439:612–616. doi: 10.1038/nature04487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kukulski W, Schorb M, Welsch S, Picco A, Kaksonen M, Briggs JA. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J Cell Biol. 2011;192:111–119. doi: 10.1083/jcb.201009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Fahimian BP, Iancu CV, Suloway C, Murphy GE, Wright ER, Castano-Diez D, Jensen GJ, Miao J. Radiation dose reduction and image enhancement in biological imaging through equally-sloped tomography. J Struct Biol. 2008;164:221–227. doi: 10.1016/j.jsb.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo-Macias A, Katz G, Wei H, Alimova A, Katz A, Rice WJ, Diaz-Avalos R, Hu GB, Stokes DL, Gottlieb P. Toroidal surface complexes of bacteriophage varphi12 are responsible for host-cell attachment. Virology. 2011;414:103–109. doi: 10.1016/j.virol.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KR, Kleinschmidt AK, Agabian-Keshishian N, Shapiro L, Maizel JV., Jr Structural studies on the capsid of Caulobacter crescentus bacteriophage phiCbK. J Mol Biol. 1972;71:201–216. doi: 10.1016/0022-2836(72)90346-4. [DOI] [PubMed] [Google Scholar]
- Leonard KR, Kleinschmidt AK, Lake JA. Caulobacter crescentus bacteriophage phiCbK: structure and in vitro self-assembly of the tail. J Mol Biol. 1973;81:349–365. doi: 10.1016/0022-2836(73)90146-0. [DOI] [PubMed] [Google Scholar]
- Lepault J, Booy FP, Dubochet J. Electron microscopy of frozen biological suspensions. J Microsc. 1983;129:89–102. doi: 10.1111/j.1365-2818.1983.tb04163.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455:109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen CY, Shiomi D, Niki H, Margolin W. Visualization of bacteriophage P1 infection by cryo-electron tomography of tiny Escherichia coli. Virology. 2011;417:304–311. doi: 10.1016/j.virol.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Q, Murata K, Baker ML, Sullivan MB, Fu C, Dougherty MT, Schmid MF, Osburne MS, Chisholm SW, Chiu W. Structural changes in a marine podovirus associated with release of its genome into Prochlorococcus. Nat Struct Mol Biol. 2010;17:830–836. doi: 10.1038/nsmb.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucic V, Forster F, Baumeister W. Structural studies by electron tomgraphy: from cells to molecules. Annu Rev Biochem. 2005;74:833–865. doi: 10.1146/annurev.biochem.73.011303.074112. [DOI] [PubMed] [Google Scholar]
- Lucic V, Leis A, Baumeister W. Cryo-electron tomography of cells: connecting structure and function. Histochem Cell Biol. 2008;130:185–196. doi: 10.1007/s00418-008-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft JH. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabini R, Rietzel E, Schroeder R, Herman GT, Carazo JM. Three-dimensional reconstruction from reduced sets of very noisy images acquired following a single-axis tilt schema: application of a new three-dimensional reconstruction algorithm and objective comparison with weighted backprojection. J Struct Biol. 1997;120:363–371. doi: 10.1006/jsbi.1997.3923. [DOI] [PubMed] [Google Scholar]
- Marko M, Hsieh C, Schalek R, Frank J, Mannella C. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat Methods. 2007;4:215–217. doi: 10.1038/nmeth1014. [DOI] [PubMed] [Google Scholar]
- Martin CS, Burnett RM, de Haas F, Heinkel R, Rutten T, Fuller SD, Butcher SJ, Bamford DH. Combined EM/X-ray imaging yields a quasi-atomic model of the adenovirus-related bacteriophage PRD1 and shows key capsid and membrane interactions. Structure. 2001;9:917–930. doi: 10.1016/s0969-2126(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Dual-axis tomography: an approach with alignment methods that preserve resolution. J Struct Biol. 1997;120:343–352. doi: 10.1006/jsbi.1997.3919. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- McDowall AW, Chang JJ, Freeman R, Lepault J, Walter CA, Dubochet J. Electron microscopy of frozen hydrated sections of vitreous ice and vitrified biological samples. J Microsc. 1983;131:1–9. doi: 10.1111/j.1365-2818.1983.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Morais MC, Tao Y, Olson NH, Grimes S, Jardine PJ, Anderson DL, Baker TS, Rossmann MG. Cryoelectron-microscopy image reconstruction of symmetry mismatches in bacteriophage phi29. J Struct Biol. 2001;135:38–46. doi: 10.1006/jsbi.2001.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Reichert T, Srayko M, Hyman A, O’Toole ET, McDonald K. Correlative light and electron microscopy of early Caenorhabditis elegans embryos in mitosis. Methods Cell Biol. 2007;79:101–119. doi: 10.1016/S0091-679X(06)79004-5. [DOI] [PubMed] [Google Scholar]
- Murata K, Liu X, Danev R, Jakana J, Schmid MF, King J, Nagayama K, Chiu W. Zernike phase contrast cryo-electron microscopy and tomography for structure determination at nanometer and subnanometer resolutions. Structure. 2010;18:903–912. doi: 10.1016/j.str.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GE, Jensen GJ. Electron cryotomography. Biotechniques. 2007;43(4):413–415. doi: 10.2144/000112568. 417 passim. [DOI] [PubMed] [Google Scholar]
- Nemecek D, Cheng N, Qiao J, Mindich L, Steven AC, Heymann JB. Stepwise expansion of the bacteriophage Φ6 procapsid: possible packaging intermediates. J Mol Biol. 2011;414:260–271. doi: 10.1016/j.jmb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecek D, Heymann JB, Qiao J, Mindich L, Steven AC. Cryo-electron tomography of bacteriophage [phi]6 procapsids shows random occupancy of the binding sites for RNA polymerase and packaging NTPase. J Struct Biol. 2010;171:389–396. doi: 10.1016/j.jsb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penczek P, Marko M, Buttle K, Frank J. Double-tilt electron tomography. Ultramicroscopy. 1995;60:393–410. doi: 10.1016/0304-3991(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Plitzko JM, Rigort A, Leis A. Correlative cryo-light microscopy and cryo-electron tomography: from cellular territories to molecular landscapes. Curr Opin Biotechnol. 2009;20:83–89. doi: 10.1016/j.copbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Porter KR, Claude A, Fullam EF. A study of tissue culture cells by electron microscopy: methods and preliminary observations. J Exp Med. 1945;81:233–246. doi: 10.1084/jem.81.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo LM, Lundh NP, Martinez RJ. Primary adsorption site of phage PBS1: the flagellum of Bacillus subtilis. J Virol. 1968;2:256–264. doi: 10.1128/jvi.2.3.256-264.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravantti JJ, Gaidelyte A, Bamford DH, Bamford JKH. Comparative analysis of bacterial viruses Bam35, infecting a gram-positive host, and PRD1, infecting gram-negative hosts, demonstrates a viral lineage. Virology. 2003;313:401–414. doi: 10.1016/s0042-6822(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Sartori A, Gatz R, Beck F, Rigort A, Baumeister W, Plitzko JM. Correlative microscopy: bridging the gap between fluorescence light microscopy and cryo-electron tomography. J Struct Biol. 2007;160:135–145. doi: 10.1016/j.jsb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Schade SZ, Adler J, Ris H. How bacteriophage chi attacks motile bacteria. J Virol. 1967;1:599–609. doi: 10.1128/jvi.1.3.599-609.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MF, Booth CR. Methods for aligning and for averaging 3D volumes with missing data. J Struct Biol. 2008;161:243–248. doi: 10.1016/j.jsb.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suloway C, Shi J, Cheng A, Pulokas J, Carragher B, Potter CS, Zheng SQ, Agard DA, Jensen GJ. Fully automated, sequential tilt-series acquisition with Leginon. J Struct Biol. 2009;167:11–18. doi: 10.1016/j.jsb.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Olson N, Jardine PJ, Grimes S, Anderson DL, Baker TS. DNA poised for release in bacteriophage phi29. Structure. 2008;16:935–943. doi: 10.1016/j.str.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Glaeser RM. Electron diffraction of frozen, hydrated protein crystals. Science. 1974;186:1036–1037. doi: 10.1126/science.186.4168.1036. [DOI] [PubMed] [Google Scholar]
- van Driel LF, Valentijn JA, Valentijn KM, Koning RI, Koster AJ. Tools for correlative cryo-fluorescence microscopy and cryo-electron tomography applied to whole mitochondria in human endothelial cells. Eur J Cell Biol. 2009;88:669–684. doi: 10.1016/j.ejcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Watson ML. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958a;4:475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ML. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958b;4:727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ER, Schooler JB, Ding HJ, Kieffer C, Fillmore C, Sundquist WI, Jensen GJ. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 2007;26:2218–2226. doi: 10.1038/sj.emboj.7601664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav T, Maimon T, Grossman E, Dahan I, Medalia O. Cryo-electron tomography: gaining insight into cellular processes by structural approaches. Curr Opin Struct Biol. 2011;21:670–677. doi: 10.1016/j.sbi.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Zanetti G, Briggs JA, Grunewald K, Sattentau QJ, Fuller SD. Cryo-electron tomographic structure of an immunodeficiency virus envelope complex in situ. PLoS Pathog. 2006;2:e83. doi: 10.1371/journal.ppat.0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QS, Braunfeld MB, Sedat JW, Agard DA. An improved strategy for automated electron microscopic tomography. J Struct Biol. 2004;147:91–101. doi: 10.1016/j.jsb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Zhu P, Winkler H, Chertova E, Taylor KA, Roux KH. Cryoelectron tomography of HIV-1 envelope spikes: further evidence for tripod-like legs. PLoS Pathog. 2008;4:e1000203. doi: 10.1371/journal.ppat.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]


