Abstract
Current models incompletely risk-stratify patients with acute chest pain. In this study, N-terminal proeB-type natriuretic peptide and cystatin C were incorporated into a contemporary chest pain triage algorithm in a clinically stratified population to improve acute coronary syndrome discrimination. Adult patients with chest pain presenting without myocardial infarction (n = 382) were prospectively enrolled from 2008 to 2009. After clinical risk stratification, N-terminal proeB-type natriuretic peptide and cystatin C were measured and standard care was performed. The primary end point was the result of a clinical stress test. The secondary end point was any major adverse cardiac event at 6 months. Associations were determined through multivariate stratified analyses. In the low-risk group, 76 of 78 patients with normal levels of the 2 biomarkers had normal stress test results (negative predictive value 97%). Normal biomarkers predicted normal stress test results with an odds ratio of 10.56 (p = 0.006). In contrast, 26 of 33 intermediate-risk patients with normal levels of the 2 biomarkers had normal stress test results (negative predictive value 79%). Biomarkers and stress test results were not associated in the intermediate-risk group (odds ratio 2.48, p = 0.09). There were 42 major adverse cardiac events in the overall cohort. No major adverse cardiac events occurred at 6 months in the low-risk subgroup that underwent stress testing. In conclusion, N-terminal proeB-type natriuretic peptide and cystatin C levels predict the results of stress tests in low-risk patients with chest pain but should not be substituted for stress testing in intermediate-risk patients. There is potential for their use in the early discharge of low-risk patients after clinical risk stratification.
Accurate and efficient risk stratification for patients with acute chest pain remains a significant problem.1,2,3 In this study, we evaluated low- and intermediate-risk patients with acute chest pain presenting to the emergency department (ED) by measuring cystatin C4,5 and N-terminal pro-B-type natriuretic peptide (NT-proBNP)6,7 to determine if these biomarkers could substitute for the results of stress testing, potentially leading to more rapid, accurate, and cost-effective triage to hospital admission or ED discharge. No previous study has attempted to correlate biomarkers with stress test results. Secondarily, we assessed whether these biomarkers provide incremental benefit above clinical risk stratification in this population.
Methods
Patients presenting to the ED at a large urban tertiary care medical center triaged with a chief complaint of chest pain were consecutively enrolled from August 2008 to June 2009. After obtaining informed consent, history and physical examination information was collected using a protocol-specific questionnaire. Each patient’s blood specimens were collected and stored. Patients were determined to be at high, intermediate, or low risk per Agency for Health Care Policy and Research (AHCPR) guidelines.8 The study was approved by the local institutional review board.
Eligible patients were aged ≥18 years with acute chest pain lasting ≥10 minutes and at low or intermediate risk per AHCPR guidelines without clinical, electrocardiographic, or biomarker evidence of myocardial infarction (MI). MI was defined per current guidelines to be a troponin I elevation ≥0.6 ng/ml along with the appropriate clinical, electrocar-diographic, or imaging criteria.9 Patients with electrocardiographic evidence of ST-segment elevation MI who underwent immediate cardiac catheterization without elevated troponin I were also counted in the MI group. Exclusion criteria included patients classified at high AHCPR risk, those with MIs, those without available blood samples, and those who failed to provide informed consent. Disagreements in classification of MI were resolved by the primary investigator. To allow adequate time to rule out acute MI, patients were observed for ≥9 hours in the chest pain or telemetry unit and until 2 sets of troponin I tests had been analyzed before enrollment.
Plasma was analyzed for troponin I, aliquoted, and stored at −20°C until testing for cystatin C and NT-proBNP. Conventional cardiac troponin I was measured by radial partition immunoassay using the Dade Stratus CS analyzer (Siemens Healthcare Diagnostics, Deerfield, Illinois). NT-proBNP was measured by chemiluminescent immunoassay on the Dade Dimension Xpand analyzer (Siemens Healthcare Diagnostics). Cystatin C was measured by immunoturbidimetry on the Roche DPP analyzer (Roche Diagnostics, Indianapolis, Indiana). An abnormal NT-proBNP level was defined as ≥125 pg/ml. An abnormal cystatin C level was defined as ≥1.19 mg/L. All patients included in the final analysis had NT-proBNP measured, but because of the lack of sufficient plasma sample, 37 patients did not have cystatin C measured.
After enrollment in the study, patients underwent standard clinical care as determined by their treating physicians. Telephone and medical chart follow-up occurred at 6 months. Measured end points included stress test results and any major adverse cardiac event (MACE). Stress tests included any noninvasive ischemic evaluation, including exercise treadmill testing, exercise or pharmacologic myocardial perfusion imaging, and exercise or pharmacologic echocardiography performed during the course of the index visit. All stress tests were performed per standard guideline-driven protocols.10,11 Stress test results were divided into normal, abnormal, or indeterminate on the basis of a review by a certified cardiologist. An abnormal stress test result was defined as electrocardiographic ST-segment depression >1 mm on exercise testing or abnormal myocardial perfusion on nuclear imaging. All interpretation and reports were on the basis of current consensus guidelines.10 This end point was chosen because of its use as the primary guideline-recommended ischemic evaluation for low- and intermediate-risk patients with chest pain. MACEs included the first occurrence of an MI, revascularization (percutaneous coronary intervention or coronary artery bypass grafting), or death from the time a patient left the ED (for discharge or admission) up to 6 months except for in-hospital MIs or staged percutaneous coronary intervention revascularization. Source documents of each MACE were reviewed by independent members of the team for accuracy (S.S.S. and K.S.). Reports of mortality were cross referenced with the Social Security Death Index.
Depending on the distribution, between-group differences for continuous variables were assessed using 2-sample Student’s t tests (expressed as mean ± SD) or Wilcoxon’s rank-sum tests (expressed as median [interquartile range]). Nominal variables are expressed as frequencies and percentages. For categorical variables, differences between groups were assessed using chi-square tests or Fisher’s exact tests as appropriate. The primary outcome measure of the relation of NT-proBNP or cystatin C to stress test results was expressed as an odds ratio (OR) using chi-square analyses. Each biomarker was analyzed individually for this relation, and then an analysis was performed with the 2 biomarkers. We stratified these analyses by low or intermediate risk using the Cochran-Mantel-Haenszel statistic to generate an adjusted OR. Secondary outcome measures are also expressed as ORs using chi-square or Fisher’s exact tests as appropriate. Sensitivity, specificity, positive predictive value, and negative predictive value (NPV) were generated using frequencies. Statistical significance was defined as a p value <0.05.
Results
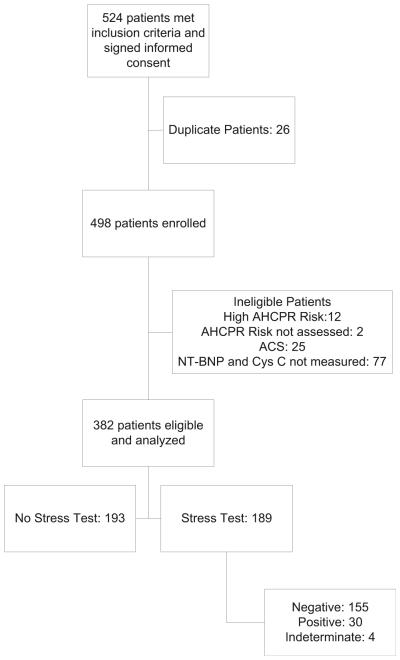
Of the 524 patients who initially consented to the study, 382 patients were eligible for final analysis (Figure 1). Of these patients, nearly half (n = 189) underwent stress testing during their hospital visits. Patients who underwent stress testing were more likely to be younger, female, and at low AHCPR risk. Stress testing was less likely to be performed in patients with histories of MI, revascularization, diabetes, or heart failure.
Figure 1.
Study flowchart. ACS = acute coronary syndrome; Cys C = cystatin C; NT-BNP = N-terminal proeB-type natriuretic peptide.
Table 1 illustrates the baseline characteristics as stratified by AHCPR risk classification. Similar to the differences between patients who did and did not undergo stress testing, patients classified at low risk were younger and less likely to be men, to have hypertension, diabetes, heart failure, prior revascularization, to use aspirin, or to be prescribed typical cardiovascular medications.
Table 1.
Characteristics by Agency for Health Care Policy and Research classification
| Variable | n | Overall | Low AHCPR Risk (n = 191) | Intermediate AHCPR Risk (n = 191) | p Value |
|---|---|---|---|---|---|
| Men | 382 | 162 (42%) | 71 (37%) | 91 (48%) | 0.04 |
| Smokers | 345 | 137 (40%) | 70 (41%) | 67 (38%) | 0.58 |
| Previous MI | 369 | 59 (16%) | 1 (1%) | 58 (31%) | <0.001 |
| Previous percutaneous coronary intervention | 379 | 89 (24%) | 1 (1%) | 88 (47%) | <0.001 |
| Previous coronary artery bypass grafting | 382 | 44 (12%) | 0 (0) | 44 (23%) | <0.001 |
| History of heart failure | 376 | 56 (15%) | 3 (2%) | 53 (28%) | <0.001 |
| Previous stroke | 337 | 26 (8%) | 9 (5%) | 17 (10%) | 0.11 |
| Hypertension | 381 | 256 (67%) | 107 (56%) | 149 (78%) | <0.001 |
| Diabetes mellitus | 381 | 129 (34%) | 50 (26%) | 79 (41%) | 0.002 |
| Aspirin use (past 7 days) | 349 | 207 (59%) | 82 (49%) | 125 (69%) | <0.001 |
| Age (yrs) | 382 | 58.3 ± 13.5 | 52.3 ± 9.6 | 64.3 ± 14.2 | <0.001 |
| Body mass index (kg/m2) | 213 | 29.9 ± 7.5 | 30.8 ± 7.0 | 29.0 ± 7.9 | 0.07 |
| Heart rate (beats/min) | 345 | 82.4 ± 18.5 | 83.1 ± 17.3 | 81.7 ± 19.6 | 0.49 |
| Systolic blood pressure (mm Hg) | 340 | 140.3 ± 24.4 | 140.0 ± 22.1 | 140.6 ± 26.5 | 0.85 |
| Diastolic blood pressure (mm Hg) | 340 | 81.0 ± 15.8 | 82.1 ± 14.5 | 79.9 ± 16.9 | 0.20 |
| Antihypertensive medication | 317 | 218 (69%) | 88 (58%) | 130 (79%) | <0.001 |
| Antiplatelet therapy | 309 | 171 (55%) | 64 (43%) | 107 (67%) | <0.001 |
| Diabetes medication | 298 | 94 (32%) | 33 (22%) | 61 (40%) | <0.001 |
| Cholesterol medication | 302 | 129 (43%) | 38 (26%) | 91 (59%) | <0.001 |
| Creatinine (mg/dl) | 380 | 1.0 (0.4) | 1.0 (0.30) | 1.1 (0.50) | 0.002 |
| NT-proBNP (ng/ml) | 382 | 77.7 (322.4) | 35.1 (90.2) | 184.6 (1,064.0) | <0.001 |
| Cystatin C (mg/L) | 345 | 0.9 (0.4) | 0.8 (0.3) | 1.0 (0.5) | <0.001 |
Data are expressed as number (percentage), as mean ± SD, or as median (interquartile range).
In the stress test group (n = 189), there were 30 studies with abnormal results (15.9%). To confirm the accuracy of initial risk classification, intermediate-risk patients were statistically more likely to have abnormal stress test results compared to low-risk patients, with an unadjusted OR of 5.72 (95% confidence interval [CI] 2.38 to 13.75; Table 2).
Table 2.
Stress test results and biomarker levels by Agency for Health Care Policy and Research classification
| Variable | Overall | Low Risk | Intermediate Risk | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Stress test results abnormal | 30/184 (16%) | 8 (7.1%) | 22 (30.6%) | 5.72 | 2.38–13.75 | <0.001 |
| NT-proBNP level abnormal | 158/382 (41%) | 45 (23.6%) | 113 (59.2%) | 4.70 | 3.02–7.31 | <0.001 |
| Cystatin C level abnormal | 83/345 (24%) | 19 (11.5%) | 64 (35.8%) | 4.31 | 2.44–7.59 | <0.001 |
| Either abnormal | 172/353 (49%) | 49 (28.8%) | 123 (67.2%) | 5.06 | 3.22–7.97 | <0.001 |
Overall, 158 of 382 patients (41%) had elevated NT-proBNP levels. In contrast, 83 of 345 patients (24%) had abnormal cystatin C levels. Either value was abnormal in 172 patients (49%). When comparing by AHCPR risk classification, individually, NT-proBNP and cystatin C were statistically more likely to be elevated in intermediate-risk patients (Table 2). Likewise, elevation of either NT-proBNP or cystatin C was statistically more likely to be present in the intermediate-risk group compared to the low-risk group (OR 5.06, 95% CI 3.22 to 7.97).
In the stress test group (n = 189), 58 patients had either elevated cystatin C or NT-proBNP levels (30.7%; Table 3), with 19 having an abnormal stress test results. In contrast, 102 of 111 patients with normal cystatin C and NT-proBNP levels had normal stress test results, yielding an NPV of 92%. Therefore, biomarkers predicted the stress test results with an OR of 5.52 (95% CI 2.3 to 13.2, p <0.0001).
Table 3.
Biomarker levels by stress test rsults and Agency for Health Care Policy and Research risk classification
| Abnormal Values | Stress Test Results Abnormal | Sensitivity | Specificity | PPV | NPV | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|---|---|
| Low AHCPR risk | ||||||||
| NT-proBNP | 3/21 (14%) | 37.5% | 82.3% | 14.3% | 94.5% | 2.87 | 0.63–13.09 | 0.160 |
| Cystatin C | 3/8 (38%) | 42.9% | 94.6% | 37.5% | 95.6% | 13.05 | 2.27–74.90 | <0.001 |
| Either | 5/23 (22%) | 71.4% | 80.8% | 21.7% | 97.4% | 10.56 | 1.89–58.86 | 0.006 |
| Intermediate AHCPR risk | ||||||||
| NT-proBNP | 13/29 (45%) | 59.1% | 68.0% | 44.8% | 79.1% | 3.07 | 1.09–8.66 | 0.032 |
| Cystatin C | 6/19 (32%) | 30.0% | 72.3% | 31.6% | 70.8% | 1.12 | 0.36–3.54 | 0.845 |
| Either | 14/35 (40%) | 66.7% | 55.3% | 40.0% | 78.8% | 2.48 | 0.845–7.25 | 0.094 |
| Overall | ||||||||
| NT-proBNP | 16/50 (32%) | 53.3% | 77.9% | 32.0% | 89.6% | 4.03 | 1.79–9.09 | <0.001 |
| Cystatin C | 9/27 (33%) | 33.3% | 87.1% | 33.3% | 87.1% | 3.36 | 1.31–8.62 | 0.009 |
| Either | 19/58 (33%) | 67.9% | 72.3% | 32.8% | 91.9% | 5.52 | 2.30–13.24 | <0.001 |
PPV = positive predictive value.
Further analysis divided the 189 patients who underwent stress testing by risk classification and analyzed its relation to biomarkers. In the group at low AHCPR risk (n = 101), either marker was abnormal in 23 patients. Of these, 5 patients had abnormal stress test. However, in the 78 patients with normal biomarker levels, only 2 patients had abnormal stress test results (NPV 97.4%). In this stratified group, the OR for the biomarker level predicting the stress test result was 10.56 (95% CI 1.9 to 58.9, p = 0.006). In contrast, in the intermediate-risk group that underwent stress testing, 35 of 68 patients had abnormal NT-proBNP or cystatin C levels. Of the patients with abnormal biomarker levels, 14 had abnormal stress test results, but 26 of 33 patients with normal biomarker levels had normal stress test results (NPV 79%). In contrast to the low-risk population, the association between biomarker levels and stress test results did not reach statistical significance (OR 2.48, 95% CI 0.845 to 7.25) in the intermediate-risk group.
For all patients included in the study, biomarker and MACE data were available for 332 patients (Table 4). In these patients, there were 42 events at 6 months after leaving the ED (whether discharged or admitted), a rate of 12.7%, with most consisting of revascularizations. Either NT-proBNP or cystatin C level was abnormal in 26 of these patients (62%), compared to 16 with normal biomarker levels (38%). As seen in Table 4, the association between biomarker levels and events did not reach statistical significance, regardless of risk stratification. In the subgroup of patients who underwent stress tests, there were no events in the low-risk group. The intermediate-risk group had 14 events: 7 marker positive and stress test positive, 5 biomarker negative and stress test positive, 0 biomarker negative and stress test positive, and 2 biomarker negative and stress test negative.
Table 4.
MACE frequencies classified by risk and by biomarker
| AHCPR Risk Classification | n | Biomarker Level Normal | Biomarker Level Abnormal | OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| All | 42 | 16 | 26 | 1.79 | 0.92–3.47 | 0.08 |
| Low | 5 | 2 | 3 | 3.78 | 0.61–23.42 | 0.15 |
| Intermediate | 37 | 14 | 23 | 0.72 | 0.34–1.53 | 0.39 |
This table lists the number of MACEs stratified by AHCPR risk and by either normal or abnormal biomarker level (either cystatin C or NT-proBNP).
Discussion
In this cohort of 382 patients with acute chest pain at low to intermediate AHCPR risk, we evaluated the diagnostic utility of 2 relevant cardiac biomarkers (NT-proBNP and cystatin C) and showed that they may be useful in the triage of low-risk patients but do not substitute for stress testing in intermediate-risk patients. Few studies have evaluated the potential niche for biomarkers in low- and intermediate-risk patients that are heavy on resource utilization but lack clear algorithms for efficient and reliable triage.
When patients were stratified by low and intermediate risk, the NPV was 98% in the low-risk group but decreased to 79% in the intermediate-risk group. Low-risk patients with normal biomarkers were 10 times less likely to have abnormal stress test results compared to a patient at similar risk with elevated biomarkers. In addition, no low-risk patients who underwent stress tests (regardless of their results) had events at 6 months. This provides evidence that simply drawing a panel of admission biomarkers, including cardiac troponin, NT-proBNP, and cystatin C, after clinical risk stratification may allow more accurate discrimination between those patients needing further ischemia evaluation and those who can be discharged without the current guideline recommended stress testing within 24 to 72 hours of discharge.12
No previous study has associated biomarker results with the outcomes of stress tests, focusing instead on prognostic rather than diagnostic utility in high-risk populations. 13-17 We purposefully chose a pure clinical risk classification scheme that predated the use of troponin,8 favoring a mechanism for immediate-risk stratification on arrival to the ED. The AHCPR guideline accurately identifies a low-risk population with a 2.5% event rate at 30 days,18 a rate similar to that in the low-risk cohort derived from risk stratification schemes incorporating conventional cardiac troponin.19,20
If patients with low AHCPR risk and normal NT-proBNP and cystatin C levels can be confidently discharged earlier from the ED, there is tremendous potential for cost savings individually and for the health care system. Although stress tests have long been established as an effective method of refining clinical risk stratification, using biomarkers as a substitute may provide a less expensive and speedier alternative. Currently, an estimated $6 billion are spent annually on negative chest pain evaluations.21 These costs increase in low-risk patients, who likely do not have acute coronary syndromes.22 With improved diagnostic classification, at a very modest cost, we have the opportunity to reallocate these resources to those patients with actual pathology.
Several trials have advocated the wholesale use of coronary computed tomography as a quick way to discharge patients with chest pain from the ED.23-25 However, the high NPV of this modality and potential for a quicker ED visit needs to be balanced with the radiation risk and cost of doing this procedure on most (especially low-risk) patients with chest pain. As another alternative, a recent analysis suggested potential discharge without further ischemic evaluation in low-risk patients on the basis of clinical risk stratification alone.26 Although we believe that risk stratification is the cornerstone of diagnostic evaluation in patients with acute chest pain, the subsequent addition of NT-proBNP and cystatin C incrementally increases NPV, favoring more likely adoption of this strategy in the contemporary health care and medicolegal climate.
Aside from NT-proBNP and cystatin C, another intriguing new biomarker for ischemia or early infarction is high-sensitivity troponin.27 Recent studies have confirmed a high NPV for this assay in excluding acute coronary syndromes applied across an entire ED cohort of patients with chest pain.28,29 Another study revealed the benefit of this test in the context of a clinically risk stratified population at 30 days.30 Longer follow-up, such as the 6 months used in our study, is certainly needed.
The primary end point of a clinical stress test comprised a variety of modalities, each having its own sensitivity and specificity profile. However, this end point was specifically chosen because our aim was to evaluate whether the biomarkers had the ability to substitute for noninvasive testing as currently used in the clinical algorithm for these patients. Although certainly a subject of further inquiry, this particular study was not designed or powered to look at clinical outcomes. Although data were collected at 6 months, event rates were expectedly low. Furthermore, previous studies have demonstrated the association of worse outcome with abnormal NT-proBNP or cystatin C levels, providing evidence for prognostic implications. We instead sought to demonstrate clinical utility and diagnostic significance of that association by using stress test results as a surrogate. This preliminary study provides evidence for the importance of a larger randomized study to evaluate clinical outcomes.
Acknowledgment
Dr. Nisenbaum gratefully acknowledges the support of the Ontario Ministry of Health and Long-Term Care, Toronto, Ontario, Canada. The views expressed in this publication are the views of the investigators and do not necessarily reflect the views of the Ontario Ministry of Health and Long-Term Care.
This study was sponsored partly by Radiometer (Brønshøj, Denmark). See page 497 for disclosure information.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States 1999-2008. NCHS Data Brief. 2010;43:1–8. [PubMed] [Google Scholar]
- 2.Lee TH, Goldman L. Evaluation of the patient with acute chest pain. N Engl J Med. 2010;342:1187–1195. doi: 10.1056/NEJM200004203421607. [DOI] [PubMed] [Google Scholar]
- 3.Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–1170. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 4.Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation. 2004;110:2342–2348. doi: 10.1161/01.CIR.0000145166.44942.E0. [DOI] [PubMed] [Google Scholar]
- 5.Akerblom Å , Wallentin L, Siegbahn A, Becker RC, Budaj A, Buck K, Giannitsis E, Horrow J, Husted S, Katus HA, Steg PG, Storey RF, Åsenblad N, James SK. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST-elevation and non-ST-elevation acute coronary syndromes: results from the Platelet Inhibition and Patient Outcomes study. Clin Chem. 2012;58:190–199. doi: 10.1373/clinchem.2011.171520. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, Hall C, McCabe CH, Braunwald E. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004;44:1988–1995. doi: 10.1016/j.jacc.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 7.Staub D, Jonas N, Zellweger MJ, Nusbaumer C, Wild D, Pfisterer ME, Mueller-Brand J, Perruchoud AP, Mueller C. Use of N-terminal pro-B-type natriuretic peptide to detect myocardial ischemia. Am J Med. 2005;118:1287. doi: 10.1016/j.amjmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E, Jones RH, Mark DB, Brown J, Brown L, Cheitlin MD, Concannon CA, Cowan M, Edwards C, Fuster V, Goldman L, Green LA, Grines CL, Lytle BW, McCauley KM, Mushlin AI, Rose GC, Smith EE, III, Swain JA, Topol EJ, Willerson JT. Agency for Health Care Policy and Research. Diagnosing and managing unstable angina. Circulation. 1994;90:613–622. doi: 10.1161/01.cir.90.1.613. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 10.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA. American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Nuclear Cardiology, American College of Radiology, American Heart Association, American Society of Echocardiography, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Nuclear Medicine. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119:561–587. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40:1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 12.Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, Kontos MC, McCord J, Miller TD, Morise A, Newby LK, Ruberg FL, Scordo KA, Thompson PD. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–1776. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omland T, Persson A, Ng L, O’Brien R, Karlsson T, Herlitz J, Hartford M, Caidahl K. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 14.Jernberg T, Stridsberg M, Venge P, Lindahl B. N-terminal pro brain natriuretic peptide on admission for early risk stratification of patients with chest pain and no ST-segment elevation. J Am Coll Cardiol. 2002;40:437–445. doi: 10.1016/s0735-1097(02)01986-1. [DOI] [PubMed] [Google Scholar]
- 15.Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, Jarolim P, White HD, Fox KA, Califf RM, Braunwald E. Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294:2866–2871. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 16.Kilic T, Oner G, Ural E, Yumuk Z, Sahin T, Bildirici U, Acar E, Celikyurt U, Kozdag G, Ural D. Comparison of the long-term prognostic value of cystatin C to other indicators of renal function, markers of inflammation and systolic dysfunction among patients with acute coronary syndrome. Atherosclerosis. 2009;207:552–558. doi: 10.1016/j.atherosclerosis.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Bhardwaj A, Truong QA, Peacock WF, Yeo KT, Storrow A, Thomas S, Curtis KM, Foote RS, Lee HK, Miller KF, Januzzi JL., Jr A multicenter comparison of established and emerging cardiac biomarkers for the diagnostic evaluation of chest pain in the emergency department. Am Heart J. 2011;162:276–282. doi: 10.1016/j.ahj.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Farkouh ME, Aneja A, Reeder GS, Smars PA, Bansilal S, Lennon RJ, Wiste HJ, Razzouk L, Traverse K, Holmes DR, Jr, Mathew V. Clinical risk stratification in the emergency department predicts long-term cardiovascular outcomes in a population-based cohort presenting with acute chest pain: primary results of the Olmsted County chest pain study. Medicine (Baltimore) 2009;88:307–313. doi: 10.1097/MD.0b013e3181b98782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 20.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. GRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 21.Storrow AB, Gibler WB. Chest pain centers: diagnosis of acute coronary syndromes. Ann Emerg Med. 2000;35:449–461. [PubMed] [Google Scholar]
- 22.Khare RK, Powell ES, Venkatesh AK, Courtney DM. Diagnostic uncertainty and costs associated with current emergency department evaluation of low risk chest pain. Crit Pathw Cardiol. 2008;7:191–196. doi: 10.1097/HPC.0b013e318176faa1. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O’Neil BJ, Shaw LJ, Shen MY, Valeti US, Raff GL. CT-STAT Investigators. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–1422. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 24.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE. ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penumetsa SC, Mallidi J, Friderici JL, Hiser W, Rothberg MB. Outcomes of patients admitted for observation of chest pain. Arch Intern Med. 2012;172:873–877. doi: 10.1001/archinternmed.2012.940. [DOI] [PubMed] [Google Scholar]
- 27.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 28.Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, Wibberley C, Nuttall M, Mackway-Jones K. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58:1332–1339. doi: 10.1016/j.jacc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Aldous SJ, Richards M, Cullen L, Troughton R, Than M. Diagnostic and prognostic utility of early measurement with high-sensitivity troponin T assay in patients presenting with chest pain. CMAJ. 2012;184:e260–e268. doi: 10.1503/cmaj.110773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Than M, Cullen L, Aldous S, Parsonage WA, Reid CM, Greenslade J, Flaws D, Hammett CJ, Beam DM, Ardagh MW, Troughton R, Brown AF, George P, Florkowski CM, Kline JA, Peacock WF, Maisel AS, Lim SH, Lamanna A, Richards AM. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT Trial. J Am Coll Cardiol. 2012;59:2091–2098. doi: 10.1016/j.jacc.2012.02.035. [DOI] [PubMed] [Google Scholar]