Abstract
Human exposure to particulate matter (PM) air pollution has been linked with respiratory, cardiovascular, and neurodegenerative diseases, in addition to various cancers. Consistent among all of these associations is the hypothesis that PM induces inflammation and oxidative stress in the affected tissue. Consequently, a variety of assays have been developed to quantify the oxidative activity of PM as a means to characterize its ability to induced oxidative stress. The vast majority of these assays rely on high-volume, fixed-location sampling methods due to limitations in assay sensitivity and detection limit. As a result, our understanding of how personal exposure contributes to the intake of oxidative air pollution is limited. To further this understanding, we present a microfluidic paper-based analytical device (μPAD) for measuring PM oxidative activity on filters collected by personal sampling. The μPAD is inexpensive to fabricate and provides fast and sensitive analysis of aerosol oxidative activity. The oxidative activity measurement is based on the dithiothreitol assay (DTT assay), uses colorimetric detection, and can be completed in the field within 30 min following sample collection. The μPAD assay was validated against the traditional DTT assay using 13 extracted aerosol samples including urban aerosols, biomass burning PM, cigarette smoke and incense smoke. The results showed no significant differences in DTT consumption rate measured by the two methods. To demonstrate the utility of the approach, personal samples were collected to estimate human exposures to PM from indoor air, outdoor air on a clean day, and outdoor air on a wildfire-impacted day in Fort Collins, CO. Filter samples collected on the wildfire day gave the highest oxidative activity on a mass normalized basis, whereas typical ambient background air showed the lowest oxidative activity.
Introduction
Airborne particulate matter (PM) has a significant impact on human health.1-4 The World Health Organization (WHO) estimates that indoor and urban outdoor air pollution causes 3 million premature deaths worldwide per year with about half of the deaths from pneumonia in children under 5 years of age.5 The prevailing hypothesis regarding the toxic effects of PM centers on the ability of small particles to induce cellular oxidative stress through one of several pathways.6-8 Furthermore, ultrafine PM (particles with diameters less than 0.1 μm) can readily penetrate into the circulatory system, affecting tissues distant from the lungs.6-10 Because of the relationship between PM and oxidative stress, there has been significant interest in developing methods to measure the oxidative activity of aerosols. Historically, oxidative activity was estimated by measuring the presence of chemical species that could generate oxidative stress, such as transition metals, polycyclic aromatic hydrocarbons, and quinones. For example, several components of ambient PM (e.g., redox-active quinones) have been shown to catalyze the generation ROS in solution, which then react with tissues.11 Unfortunately, speciation methods are expensive and time-consuming, requiring laboratory-based instruments with long sample preparation and analysis times (e.g., chromatography, spectrometry etc.).2 Furthermore, these methods require a relatively large sample mass, and hence, a long sampling duration is needed to obtain sufficient sample for detection. Finally, the presence of chemicals capable of participating in oxidative reactions is a necessary but not sufficient criterion to determine overall PM oxidative activity.
As a result, there has been a push to develop improved, biologically-relevant approaches to estimate PM toxicity by measuring the oxidative activity rather than the composition of PM. The most common of these assays is the dithiothreitol (DTT) assay.12-22 In this assay, PM collected on a filter is extracted and reacted with reduced DTT. After a period of time, the remaining reduced DTT is reacted with Ellman’s reagent [5,5′-dithiobis(2-nitrobenzoic acid), DTNB], releasing a chromogenic compound (2-nitro-5-thiobenzoate, NTB2−) that can be measured using absorption spectroscopy. The DTT assay is considered biologically relevant because the rate of DTT consumption has been correlated with cellular oxidative stress both in vitro23, 24 and in vivo.25 Although the DTT assay has provided a wealth of information on particle oxidative activity, it relies on time-integrated sampling methods and requires a relatively large PM sample; thus, it is not suitable for on-line measurements and/or personal exposure assessment (i.e., the use of a lightweight, portable device that samples air within an individual’s breathing zone). We have recently addressed the need for on-line monitoring of aerosol oxidative activity by developing a microfluidic sensor coupled with a particle-into-liquid-sampler (PILS); this new system performs the DTT assay on-line and with a 3 min time resolution.26 Although the on-line method represents an improvement over the traditional DTT method in terms of sensitivity and speed, it is still limited to area-based exposure assessment since the PILS and associated equipment are too large for personal sampling.
Personal exposure assessment is considered the gold-standard for quantifying an individual’s risk from exposure. Personal samplers are designed to accompany an individual throughout their daily activities; these devices, therefore, can assimilate the dynamic exposures that an individual experiences as they move through various microenvironments each day.27 Personal exposure assessment is important because individuals experience a wide range of local microenvironments (e.g., at home, at work, in transit, etc.) in any given 24-hr period. Personal exposures may be assessed biologically (i.e., though biomarkers) or with the use of portable monitors worn within the breathing zone (personal samplers). The most common personal samplers for PM use filter media as the collection substrate, where a known volume of air is drawn through a filter over specific time period (usually 8-24 h) and collected samples are analyzed optically, gravimetrically, or chemically. Chemical analyses of filter extracts are typically done using sensitive but expensive laboratory-based equipment such as GC-MS28, 29 and HPLC-ECD/Fluorescence.30, 31 As a result, the entire process (sample collection, handling, storage, extraction and analysis) can take 1-2 weeks and incur significant costs. This ultimately limits the number of samples collected and analyzed, leading to a smaller-than-desired data set. Furthermore, sample handling and storage prior to analysis can result in decomposition of short-lived species.
The ideal personal exposure assessment method for oxidative activity would require short sampling durations and provide an analysis that is fast, sensitive, inexpensive, portable and easy to perform. Here, in an attempt to accomplish this goal, we developed a method using a microfluidic paper-based analytical device (μPAD) for measuring the oxidative activity of PM collected on personal filter samples. First introduced in 2007,32 μPADs are fabricated by creating hydrophobic barriers on paper using photolithography,32, 33 poly(dimethylsiloxane) printing,34 and/or wax printing.35-37 The barriers define hydrophilic channels designed to transport aqueous solutions by capillary action from a sample inlet to a detection zone. Detection is accomplished either colorimetrically38, 39 or electrochemically.40 A typical μPAD analysis reduces the required sample volume from milliliters to microliters and also enables sample analysis in the field due to the size and portability of the device.41 Most importantly, device fabrication is relatively simple and analysis costs can be very low (orders of magnitude less than traditional analytical methods), particularly when using wax printing and low cost colorimetric reagents.42 To date, μPADs have been used for quantitative and qualitative measurements in biological,43 environmental,42 and medical44 applications.
The objective of this work was to develop a μPAD for assessing PM oxidative activity on personal air samples using a variant of the traditional DTT assay. The μPAD was designed to conduct the DTT assay on a 2.5×2.5 cm device containing both sample and detection zones as shown in Figure 1A (step c). Unlike the traditional DTT assay, where PM from the filter is extracted into solution for subsequent reaction (causing sample dilution), in the μPAD, reduced DTT reacts directly with PM on surface of an air sampling filter. The remaining DTT is then eluted off the filter and onto the μPAD to react with Ellman’s reagent, forming a yellow product that is quantified by color intensity (Figure 1A, 1B). The μPAD DTT assay was optimized for reaction time and temperature using 1,4-naphthoquinone (1,4-NQ) as a standard oxidant. The sensitivity and detection limit of this assay (against the 1,4-NQ standard) may be modulated stoichiometrically by changing the level of DTT reactant presented to the sample. To test the μPAD performance for measuring aerosol oxidative activity, the device was validated against the traditional DTT assay with 13 extracted aerosol samples. No significant difference between the methods was observed for DTT consumption at the 95% confidence interval. Finally, to demonstrate that the μPAD can be applied for the measurement of aerosol oxidative activity for personal exposure, human exposures were assessed outdoors (a clean day and a smoky day, when ambient air quality was influenced by nearby wildfires, in Fort Collins, CO) and indoors (restaurant kitchen) using a personal filter sampler. Filters analyzed using the μPAD showed that samples collected on wildfire-impacted days gave higher relative oxidative activity than indoor samples and typical ambient background samples.
Figure 1.
(A) Steps to perform DTT assay on a microfluidic paper-based analytical device (μPAD). (B) Actual μPADs used for DTT detection. The left device measured high oxidative activity and, therefore, produced a low-intensity image (more DTT consumed); the right device measured low oxidative activity resulting in more color intensity at the detection zone (less DTT consumed). μPAD dimensions are 2.5×2.5 cm. The sample and detection zone diameters are 10 and 4 mm, respectively.
Experimental Section
Chemicals and Materials
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Ellman’s reagent [5,5′-dithiobis-(2-nitrobenzoic acid), or DTNB] was obtained from Alfa-Aesar (Ward Hill, MA). Poly(dimethyl siloxane) (PDMS) Sylgard 184 elastomer kit was purchased from Dow Corning (Midland, MI). A Xerox Phaser 8860 wax printer and Scanner Documate 3220 were purchased from Xerox (Norwalk, CT) for device printing and scanning, respectively. Whatman filter paper #1 was used as the base material for all μPAD devices. Phosphate buffer (100 mM pH7.4) was used to prepare standard 1,4-Naphthoquinone (1,4-NQ) and DTT solutions as well as elution buffer. The 1,4-NQ powder was dissolved in dimethyl sulfoxide prior to use.
Microfluidic Paper Analytic Device (PAD) Fabrication
There are two key steps associated with the DTT assay: the catalytic reaction of DTT with PM-generated ROS and the quenching reaction of remaining DTT with DTNB to produce a yellow product (NTB2−) for detection. The μPAD was designed to allow both reaction steps on a single device (step c in Figure 1). The pattern was created using Corel Draw (Corel Corporation, Mountain View, CA), and the device was fabricated by wax printing according to previously described methods.35 The wax printing fabrication technique is fast and inexpensive and does not require additional solvents or polymers.45 However, wax-based patterns are only amenable to aqueous flow and cannot be used with strong organic solvents. Following printing, wax-treated paper was placed on a hotplate at 110 °C for 2 min to allow the wax melt through the paper creating a hydrophobic barrier for directed sample flow. Each μPAD measures approximately 2.5×2.5 cm. The device includes a central sample zone connected to four detection zones via capillary flow channels. The sample zone (10 mm diameter) was designed to allow sufficient area to accommodate a 6 mm sample punch taken from a personal sampling filter. Four smaller areas (4 mm diameter) are each connected to the sample zone; three of these areas act as detection zones (for replicate measurement in a single device) and the fourth acts as a negative control. Immediately after fabrication, one side of the patterned paper was covered with Scotch® packing tape. This last step prevents any solution from leaking through the backside of the device during sample analysis. Violet-colored ink was used for the hydrophobic barrier around the detection zone because violet is complementary to yellow, providing increased contrast for subsequent DTT detection. The violet color in the area around detection zone was subtracted during image analysis using a color threshold adjustment with the imaging software. This process leaves only yellow product color for subsequent image intensity analysis.
Method Validation Using Filter Extracts
Method validation was carried out using extracted filter samples from biomass burning aerosol, urban PM, incense, and cigarette smoke. Filter extracts were chosen since they are amenable to both μPAD and traditional DTT assay analysis techniques. A total of 13 samples, representative of different PM sources and exposures were tested. Three PM2.5 samples were collected from the combustion of common vegetation found in North American wildfires using a high-volume filter sampler as part of the USDA’s third Fire Lab at Missoula Experiment study.46 Four PM2.5 samples of urban aerosol were collected on quartz filters over separate, integrated three-day sampling periods in Cleveland, OH during the winter of 2008 using a Thermo Anderson Hi-Volume Air Sampler (Windsor, NJ). The quartz filters were pre-baked in an oven at 550 °C for 12 h and wrapped in aluminum foil before use. Six samples of cigarette and incense aerosols were created in an aerosol chamber as described previously.26 Briefly, aerosol was generated in a ~1 m−3 chamber by burning the cigarette or incense for approximately 10 seconds. Filter holders (47 mm diameter) were placed in the chamber and sampled the aerosol for 4 hours at a flow of 10 L min−1. After sampling, all filters were stored at −20 °C prior to extraction.
The filter extraction procedure was described previously.47 Briefly, two 20-mm diameter punches from each filter sample were extracted in 5 mL of deionized water in a Nalgene Amber High Density Polyethylene (HDPE) bottle using sonication with heat (70 ± 5 °C) for 75 min. The aerosol extracts were syringe filtered (0.2 μm PTFE membrane, Millipore, Billerica, MA, USA) to remove insoluble materials. The filtered samples were kept in the dark at 4 °C until analysis.
The traditional DTT assay was performed according to previously described methods,20, 48 except that EDTA was not added to the reaction buffer for reasons described by Charrier et al.22 One milliliter of 0.1 M phosphate buffer (pH 7.4) was used as the reaction buffer. A 50 μL aliquot of 0.5 mM DTT was added to the buffer followed by 50 μL of the filtered aqueous aerosol extract. After mixing, the solution was incubated at 37 °C for 25 min. Then, 100 μL of 100 mM Ellman’s reagent was added to the reaction mixture to consume the remaining reduced DTT, forming a yellow colored solution of 2-nitro-5-thiobenzoate (NTB2−). Levels of NTB2− were then quantified by absorption at 412 nm using a Genesys UV10 spectrometer (Thermo Scientific, Waltham, MA).
Personal PM Exposure Sampling
Field validation was carried out using μPADs to analyze filter samples collected via personal sampling. Volunteers were asked to carry a backpack containing personal samplers for PM2.5 (PM size ≤ 2.5 μm) and PM10 (PM size ≤ 10 Dm). Personal sampling was conducted across a 24 h period and for three distinctly different events: an 8 h work shift in a restaurant kitchen, an 8 h outdoor exposure on a clean day, and an 8 h outdoor exposure on a smoky day, when the city of Fort Collins was impacted by the High Park wildfire of 2012. The remaining 16 h of each sampling day was spent indoors at the volunteer’s home in a relatively clean environment (free of other combustion or cooking sources). For each event, PM2.5 and PM10 samples were collected onto Teflon-coated glass fiber filters (Pallflex® T40A60, 37 mm, Pall Corporation, Ann Arbor, MI) using personal impactors (Personal Environmental Monitors, 761-203A or 761-200A, SKC, Inc.). A miniature sampling pump (Omni-400, BGI Incorporated, Waltham, MA) was used to draw air through each sampler at 4 L min−1. Filters were equilibrated at low relative humidity (RH< 40%) and weighed with a microbalance (Mettler-Toledo, model MX5) before and after sampling to determine collected aerosol mass prior to μPAD analysis of oxidative activity. The mass of PM collected on each filter is provided in Table S-1 (see Supporting Information).
DTT Assay on μPAD
Steps to perform the DTT assay on the μPAD for aerosol oxidative activity analysis are shown in Figure 1A. Samples for method validation (aerosol extract samples) and assay optimization (1,4-NQ solutions) were pipetted onto a 6 mm disk of standard filter paper (Whatman #1) that was created using a biopsy punch (Robbins Instruments, Chatham, NJ) from a larger sheet. A five microliter aliquot of aerosol extract or 1,4-NQ was added to the 6 mm punch followed by five microliters of DTT solution (0.5 mM in 100 mM phosphate buffer pH 7.4). The filter disk was then placed inside a Petri dish at room temperature (22 ± 2 °C) and allowed to react for 25 min. Next, the disk was placed in the sample zone of a μPAD and DTNB (5 mM, three aliquots of 0.4 μL at each detection zone) was pipetted onto three of the detection zones, while phosphate buffer (100 mM, pH 7.4) was deposited on the fourth zone as a negative control. The remaining reduced DTT from the punched filter paper was eluted with phosphate buffer (100 mM pH 7.4, three aliquots of 10 μL) allowing the remaining reduced DTT to flow into the detection zones and react with the DTNB to generate a yellow color. To prevent leakage and provide a uniform flow of a solution through the channel, a piece of polydimethylsiloxane (PDMS, Dow Corning) the same size as the paper device containing holes at the center and the detection zones was placed over the device prior to elution.42 A DTT calibration curve was constructed by adding varying DTT concentrations (0-0.7 mM, 5 μL) and phosphate buffer (5 μL) to the 6 mm filter disk. The measurement made without oxidant in this calibration was also used as a quality assurance blank. After 25 min, the remaining DTT was analyzed using the method described above. Quantification of the final yellow product was done by scanning the device using a desktop scanner (Xerox DocuMate 3220 Scanner, color photo setting, 600 dpi resolution) and analyzing the spot for yellow intensity using NIH ImageJ software as described previously.42, 43
Assay Optimization
For temperature optimization, 1,4-NQ was used as a standard oxidant. Two temperatures were compared: room temperature (22 ± 2 °C) and 37 °C. DTT (0.5 mM, 100 μL), 1,4-NQ (0-6 μg/mL, 50 μL) and phosphate buffer (350 μL) were mixed in a culture tube (5 mL, Fisher Scientific, Pittsburgh, PA) and allow to react for 25 min at either room temperature or 37 °C. An aliquot of the solution was then pipetted onto a 6 mm filter disk and analyzed using the μPAD as described above. For optimizing reaction time, different concentrations of 1,4-NQ (2-8 μg/mL, 5 μL) and DTT (0.5 mM, 5 μL) were pipetted onto a filter disk and allowed to react for 10, 20, or 25 min at room temperature. After the specified time, the DTT remaining on the filter punch was analyzed on the μPAD using the method described above.
Analysis of Personal Sampling Filters
As described above, a 6 mm punch was taken from each personal sampling filter. However, prior to analysis, each filter disk was treated with sodium dodecyl sulfate (1 mM SDS, 10 μL) to increase the hydrophillicity of the Teflon filter surface. After 15 minutes of SDS treatment, DTT (0.5 mM, 5 μL) and phosphate buffer (3 μL) were added to the filter disk. The reaction of DTT with PM was carried out for 25 min and then the remaining DTT was measured using the method described above. For each sample, a negative control was performed where the PM filter disk was analyzed without reacting with DTT. A DTT calibration curve for these samples was constructed in a similar manner to that for the extracted samples except that the SDS-modified blank Pallflex® filter was used as a filter disk instead of Whatman#1 filter. Blank filter punches, both from the laboratory and field, were routinely analyzed for quality control.
Results and Discussion
PAD Performance for DTT Detection
The ability of a μPAD to quantify DTT consumption was determined first. The linearity, reproducibility, sensitivity and detection limit were studied to demonstrate that the system provided effective analysis of reduced DTT. A plot of gray intensity for the DTT signal as a function of deposited DTT mass as well as apparent color obtained at different quantities of DTT is shown in Figure S-1 (see Supporting Information). Linearity in the range of 0 to 12.5 nmol DTT was obtained (y = 8.66x + 0.36, R2 = 0.99). The relative standard deviation from the analysis of all concentrations was in the range of 0.43-12.50% (n=3) demonstrating good repeatability of the system. Furthermore, to verify the repeatability and reliability of our method, we produced calibration curves with different devices on 5 separate days. The slopes and intercepts from these tests are shown below with 95% confidence intervals (Supporting information Figure S-2). The relative standard deviations for method slope and intercept are 6.81% and 21.09% respectively. The DTT detection limit, defined as the concentration giving a signal three times larger than the standard deviation of the blank (phosphate buffer), was 0.27 ± 0.05 nmol. These results demonstrate the ability to measure very low amounts of reduced DTT using a μPAD which is approximately 10-fold lower than published data using the traditional absorption spectrometry detection method.49
Optimization of DTT Assay
A typical dose-response curve for the μPAD DTT assay using 1,4-NQ as the oxidant is shown in Figure 2. Error bars in the curve represent within-sample replicates across the three detection zones on a single device. A linear signal decrease was observed, correlating with previously published colorimetric and electrochemical assays for DTT consumption.26 The optimal reaction time and temperature conditions for performing the μPAD DTT assay were also determined. As reported in the literature, the DTT assay is typically carried out at 37 °C. In this work, the DTT assay was performed at room temperature (22 ± 2 °C), in an effort to simplify the method. The comparison of assay results at room temperature (22 ± 2 °C) and 37 °C is shown in Figure 3A. At both temperatures, DTT reacts with 1,4-NQ at a similar rate.
Figure 2.
Typical response curve obtained from DTT assay plotted remaining DTT signal (gray intensity) as a function of 1,4-NQ model oxidant added to the assay (n=3).
Figure 3.
Optimization of DTT assay for analysis by μPAD using 1,4-NQ as a PM model oxidant (a) Reaction temperature study (b) Reaction time study.
The reaction duration was also studied. Method sensitivity increased as a function of reaction time with 25 min giving the steepest slope (Figure 3B). Further improvements at longer durations were not observed, primarily because the devices dried out consistently between 25 and 30 min. As a result, a 25 min reaction time was selected for further experiments. Longer drying times would be expected for more humid environments but no attempt was made to control this variable.
Analytical Figures of Merit for 1,4-NQ
Once an optimized protocol was obtained, the analytical figures of merit for the device were determined, again using 1,4-NQ as a standard oxidant. Changing the initial proportion of DTT shifted the dose response curve as shown in Figure 4. At low initial DTT (2.5 nmol), signal dropped quickly as a function of 1,4-NQ applied, providing the highest sensitivity of three conditions investigated. Unfortunately, this high sensitivity came at the cost of a small working range (0-20 ng of 1,4-NQ). Higher initial DTT (10 nmol) gave a lower sensitivity but provided a larger working range (0-140 ng of 1,4-NQ). The limit of detection, defined as the 1,4-NQ mass causing a decrease in the DTT signal three times the signal to noise ratio relative to the control, was found to depend on the starting amount of DTT. A lower initial DTT concentration provided a lower detection limit. Analytical figures of merit for DTT consumption are summarized in Table 1. Results shown here demonstrated that performance of the μPAD DTT assay can be adjusted by the choice of initial DTT amount. The assay sensitivity can also be modulated by changing the size of filter disk (from 3 to 10 mm diameter) and consequently changing PM mass on the filter as an alternative to varying the amount of DTT added (data not shown).
Figure 4.
The impact of initial DTT amount on the assay sensitivity using 1,4-NQ as a model oxidant (n=3).
Table 1.
Sensitivity, limit of detection (LOD), and linear range of DTT-μPAD assay for various DTT concentrations and using 1,4-NQ as a model oxidant.
| DTT Starting Amount (nmol) |
Sensitivity (Intensity/ngNQ) |
Limit of Detection (LODNQ) |
Linear Range (ngNQ) |
|---|---|---|---|
| 2.5 | 0.65 | 7.92 ± 0.30 | 0-20 |
| 5.0 | 0.32 | 9.38 ± 0.31 | 0-80 |
| 10.0 | 0.32 | 25.75 ± 0.04 | 0-140 |
Method Validation
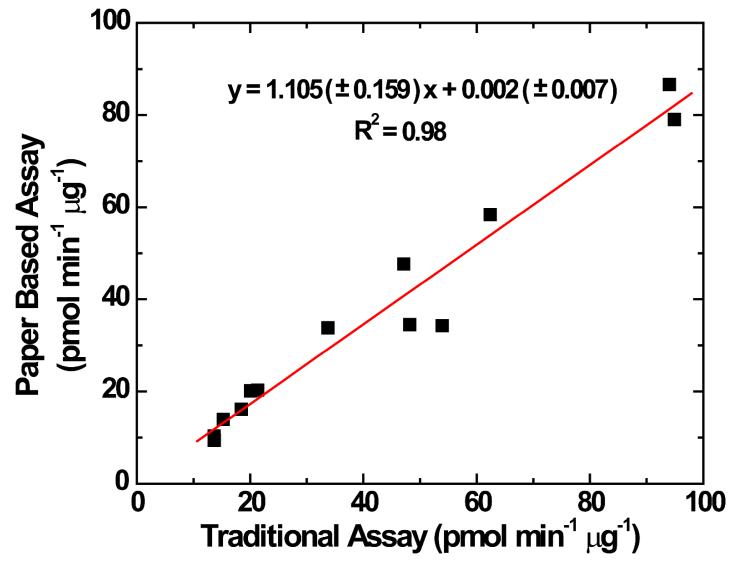
The μPAD was validated against the traditional DTT assay using 13 PM extracts taken from representative samples of urban dust, biomass burning, cigarette smoke and incense smoke. DTT consumption was measured using both methods and compared using linear regression (Figure 5). There was no significant difference in DTT consumption rate between the two methods based on a 95% confidence interval where the intercept and slope are not significantly different from 0 and 1, respectively (y = 1.105 (±0.159) x + 0.002 (±0.007), R2 = 0.98).50 The high degree of equivalence between the two methods shows that the μPAD DTT assay is appropriate for the analysis of oxidative activity from PM samples collected on filters. More importantly, however, the μPAD method requires approximately 10 times less aerosol mass and 100 times less reagent volume than the traditional DTT assay.7, 20, 48, 51 These improvements enable the analysis of PM oxidative activity on personal filter samples, which typically collect far less mass than fixed-location samplers (i.e., area-based sampling) or other high8volume techniques. We also compared the performance of the μPAD to our previously described microfluidic electrochemical sensor, with no difference in oxidative activity (DTT consumption rate) measured by the two methods (Supporting information Figure S-3). Differences between these three analytic methods (μPAD, electrochemical, traditional) are highlighted in Table S-2 in the Supporting Information.
Figure 5.
Comparison of PM oxidative activity (DTT consumption rate, pmol min−1 Dg−1) between the traditional DTT assay and paper based DTT assay. Data represent aqueous extracts of 13 different aerosol samples.
Personal Exposure Assessment
A final evaluation of this new technology was conducted using real-world, personal filter sampling. Three sampling periods were selected, each representing a different aerosol source: ambient outdoor air (a typical clean day in Fort Collins, CO), a wildfire-impacted day (smoky day), and indoor aerosol from a restaurant kitchen. For these tests, Teflon-coated glass-fiber filters (Pallflex® T60A20) filters were used in place of Whatman #1 filter paper, since the former provide a lower pressure drop to accommodate the personal sampling pump at 4 L min−1 of flow. However, SDS treatment was required to increase the hydrophilicity of the Teflon filter coating to promote sample mixing and subsequent elution. Without SDS, aqueous sample surface tension prevented capillary flow through the Teflon-coated glass-fiber matrix. We compared SDS-treated Pallflex® filters and standard Whatman#1 filters and saw no significant difference in measured remaining DTT at the 95% confidence level using 11 different aerosol extracts (see Supporting Information Figure S-4).
PM oxidative activity was quantified on all personal filter samples (Figure 6). In this case, PM was not extracted from the filters, per se, but DTT solution was added to the filters, allowing reaction between DTT and the water-soluble fraction of PM and/or surface-confined reactive species on the PM. Aerosol oxidative activity tended to be higher in the PM2.5 size fraction (vs. PM10), but these differences were not statistically significant. This is likely because PM2.5 (a component of PM10) constituted the majority of collected sample mass.20, 52, 53 Typically, PM2.5 is more reactive than PM10 because it has higher surface area per unit mass. Also, PM2.5 is more likely to originate from anthropogenic sources (i.e., combustion) whereas PM10 has both anthropogenic and biogenic sources.54 Relative aerosol reactivity (DTT consumption rate normalized by filter mass gain) was highest from samples collected on a smoky day in Fort Collins during the 2012 High Park wildfire.55 This finding coincided with the poor air quality reported throughout the Rocky Mountain region during this time when the air quality index in Fort Collins was reported as unhealthy for sensitive groups. In comparison to outdoor samples from other cities, the oxidative activity of PM collected on a clean day Fort Collins (5.5-6.3 pmol min−1 Dg−1) was substantially lower than values reported for ambient air in Los Angeles and in Mexico City (~20-50 pmol min−1 Dg−1). The oxidative activity on the smoky day Fort Collins (31.6-43.5 pmol min−1 Dg−1) was comparable to these urban environments, all of which are known to contain relatively higher levels of air pollution.15, 20
Figure 6.
Oxidative activity of personal PM2.5 (red bar) and PM10 (green bar) samples collected in various environments. Outdoor (clean day): PM collected on a typical day in Fort Collins, CO. Outdoor (smoky day): PM collected on a smoky day when the High Park wildfire (10 miles from Fort Collins, CO) was active. Indoor (Kitchen): PM collected in a restaurant kitchen.
Samples collected from the restaurant kitchen had higher oxidative activity than for samples collected outdoors on a relatively pollution-free day. This result was also expected, since cooking is a known source of indoor air pollution that can produce toxic PAHs, aldehydes, and organic acids.56-59 Outdoor PM levels in Fort Collins are typically below national ambient air quality standards set by the U.S. EPA.60 However, for each of the personal samples, we cannot rule out the contribution from the air in each volunteer’s home for these comparisons. Although we attempted to reduce sources of indoor air pollution that may be encountered at home (e.g., volunteers were asked to refrain from cooking and cleaning), we did not eliminate or control these exposures. Rather, the intent of the personal sampling campaign was to demonstrate applicability of the μPAD method for personal sampling and to investigate potential variability in 24-hr measures of PM oxidative activity when measured within the breathing-zone of individuals. Further investigation into how specific sources contribute to the inhalation of aerosol on a personal level is a promising avenue of future research (which has been enabled by the development of the μPAD technology described here).
These results demonstrate the use of an inexpensive μPAD DTT assay to measure differences in PM reactivity based on personal sampling. Applications of this technology, however, are not limited to personal sampling but can also be applied to any filter-based method (e.g., area or high-volume sampling).
Implications for Personal Exposure Assessment
A microfluidic paper-based analytical device was developed to measure aerosol oxidative activity in the context of personal exposure assessment. Unlike current methods for PM oxidative activity that require whole-filter extracts and expensive laboratory equipment, the μPAD DTT assay analyzes oxidative activity directly from a filter without substantial handling and extraction steps. In terms of instrumentation for μPAD analyses, only a scanner and pipettor are required. These tools are substantially less expensive and more portable than even the most basic laboratory spectrophotometers, making on-site analysis possible. Furthermore, Shen et al. recently developed a quantitative detection method for colorimetric μPADs using only a smartphone, which would eliminate the need for the scanner described here.61
Several limitations of this method are also worth noting. Although widely-used and commonly accepted as a standard method for assessing aerosol oxidative activity, the DTT assay has inherent limitations. For example, the DTT assay cannot account for immune-mediated reactivity (e.g., PM-induced release of ROS by macrophages) or enzymatic forms of PM reactivity (e.g., redox-active metabolites of stable, parent compounds), both of which may be relevant mechanisms for PM-induced toxicity. The DTT assay may also be less sensitive to certain types redox-active species.22 Second, our μPAD method, as currently described, accounts only for water-soluble and surface-available (i.e., insoluble species present on the surface of particles) fractions of PM; our method may not fully account for insoluble components of PM that can contribute to DTT reactivity.62 Although the μPAD developed here uses an aqueous-based analysis, we envision future μPAD designs that incorporate alternatives to wax-based flow channels (such as SU8 or other polymeric materials) that are resistant to chemical solvents and, thus, amenable to various organic solvents/chemistries.
The μPAD system developed here shows promise for personal exposure studies where a high number of samples are needed to better understand the impacts of oxidative PM on human health. The low cost and ease-of-use of this technology should also enable greater sample throughput (i.e., to support larger sample sizes given fixed resources) and application in more challenging sampling environments (i.e., the developing world). Finally, this technology has promising implications for more sustainable chemistry, as it can be implemented at reduced cost and with reduced reagent use relative to the current state-of-the-art.
Supplementary Material
Acknowledgements
This work was supported by grant ES019264 from the National Institute of Environmental Health Sciences. Y.S. gratefully acknowledges the financial support from the Royal Thai Government Scholarship, Ministry of Science and Technology. The authors wish to thank Dr. Jeffrey Collett, Jr. for providing the extracted aerosol samples and Ms. Taylor Carpenter for her assistance with gravimetric analysis of personal filter samples.
Footnotes
Supporting Information
This section contains filter mass data from the personal sampling campaign, μPAD calibration and quality assurance data, and comparative data for μPAD, electrochemical, and traditional DTT assays. This information is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Nel A. Air pollution-related illness: Effects of particles. Science. 2005;308(5723):804–806. doi: 10.1126/science.1108752. DOI 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 2.Poschl U. Atmospheric aerosols: Composition, transformation, climate and health effects. Angew Chem Int Edit. 2005;44(46):7520–7540. doi: 10.1002/anie.200501122. DOI DOI 10.1002/anie.200501122. [DOI] [PubMed] [Google Scholar]
- 3.Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (ufps) and implications in epidemiologic research. Environ. Health Perspect. 2005;113(8):947–955. doi: 10.1289/ehp.7939. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandstrom T, Blomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009;6(1):36–44. doi: 10.1038/ncpcardio1399. DOI 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- 5.Air quality and health . World Health Organization Fact Sheets. Vol. 2011. Geneva: 2011. http://www.who.int/mediacentre/factsheets/fs313/en/ [Google Scholar]
- 6.Araujo JA, Barajas B, Kleinman M, Wang XP, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ. Res. 2008;102(5):589–596. doi: 10.1161/CIRCRESAHA.107.164970. DOI 10.1161/circresaha.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang MY, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003;111(4):455–460. doi: 10.1289/ehp.6000. DOI 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limbach LK, Wick P, Manser P, Grass RN, Bruinink A, Stark WJ. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007;41(11):4158–4163. doi: 10.1021/es062629t. DOI 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008;44(9):1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. DOI 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman I, MacNee W. Lung glutathione and oxidative stress: Implications in cigarette smoke-induced airway disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999;277(6):L1067–L1088. doi: 10.1152/ajplung.1999.277.6.L1067. DOI. [DOI] [PubMed] [Google Scholar]
- 11.Vidrio E, Phuah CH, Dillner AM, Anastasio C. Generation of hydroxyl radicals from ambient fine particles in a surrogate lung fluid solution. Environ. Sci. Technol. 2009;43(3):922–927. doi: 10.1021/es801653u. DOI Doi 10.1021/Es801653u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung MY, Lazaro RA, Lim D, Jackson J, Lyon J, Rendulic D, Hasson AS. Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts. Environ. Sci. Technol. 2006;40(16):4880–4886. doi: 10.1021/es0515957. DOI 10.1021/es0515957. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Polidori A, Arhami M, Shafer MM, Schauer JJ, Cho A, Sioutas C. Redox activity and chemical speciation of size fractioned pm in the communities of the los angeles-long beach harbor. Atmos. Chem. Phys. 2008;8(21):6439–6451. DOI. [Google Scholar]
- 14.McWhinney RD, Gao SS, Zhou SM, Abbatt JPD. Evaluation of the effects of ozone oxidation on redox-cycling activity of two-stroke engine exhaust particles. Environ. Sci. Technol. 2011;45(6):2131–2136. doi: 10.1021/es102874d. DOI 10.1021/es102874d. [DOI] [PubMed] [Google Scholar]
- 15.Mugica V, Ortiz E, Molina L, De Vizcaya-Ruiz A, Nebot A, Quintana R, Aguilar J, Alcantara E. Pm composition and source reconciliation in mexico city. Atmos. Environ. 2009;43(32):5068–5074. DOI 10.1016/j.atmosenv.2009.06.051. [Google Scholar]
- 16.Rattanavaraha W, Rosen E, Zhang HF, Li QF, Pantong K, Kamens RM. The reactive oxidant potential of different types of aged atmospheric particles: An outdoor chamber study. Atmos. Environ. 2011;45(23):3848–3855. DOI 10.1016/j.atmosenv.2011.04.002. [Google Scholar]
- 17.Verma V, Ning Z, Cho AK, Schauer JJ, Shafer MM, Sioutas C. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos. Environ. 2009;43(40):6360–6368. DOI 10.1016/j.atmosenv.2009.09.019. [Google Scholar]
- 18.Verma V, Pakbin P, Cheung KL, Cho AK, Schauer JJ, Shafer MM, Kleinman MT, Sioutas C. Physicochemical and oxidative characteristics of semi-volatile components of quasi-ultrafine particles in an urban atmosphere. Atmos. Environ. 2011;45(4):1025–1033. DOI DOI 10.1016/j.atmosenv.2010.10.044. [Google Scholar]
- 19.Verma V, Polidori A, Schauer JJ, Shafer MM, Cassee FR, Sioutas C. Physicochemical and toxicological profiles of particulate matter in los angeles during the october 2007 southern california wildfires. Environ. Sci. Technol. 2009;43(3):954–960. doi: 10.1021/es8021667. DOI 10.1021/es8021667. [DOI] [PubMed] [Google Scholar]
- 20.Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, Eiguren-Fernandez A, Froines JR. Redox activity of airborne particulate matter at different sites in the los angeles basin. Environ. Res. 2005;99(1):40–7. doi: 10.1016/j.envres.2005.01.003. DOI 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Arellanes C, Curtis DB, Paulson SE. Probing the source of hydrogen peroxide associated with coarse mode aerosol particles in southern california. Environ. Sci. Technol. 2010;44(11):4070–4075. doi: 10.1021/es100593k. DOI 10.1021/es100593k. [DOI] [PubMed] [Google Scholar]
- 22.Charrier J, Anastasio C. On dithiothreitol (dtt) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. Discuss. 2012;12:11317–11350. doi: 10.5194/acpd-12-11317-2012. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma: A paradigm for the role of oxidative stress in pm-induced adverse health effects. Clin. Immunol. 2003;109(3):250–265. doi: 10.1016/j.clim.2003.08.006. DOI. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003;111(4):455–60. doi: 10.1289/ehp.6000. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, Wang MY, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, Harkema JR, Nel AE. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ. Health Perspect. 2009;117(7):1116–1123. doi: 10.1289/ehp.0800319. DOI 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sameenoi Y, Koehler K, Shapiro J, Boonsong K, Sun Y, Collett J, Volckens J, Henry CS. Microfluidic electrochemical sensor for on-line monitoring of aerosol oxidative activity. J. Am. Chem. Soc. 2012;134(25):10562–10568. doi: 10.1021/ja3031104. DOI 10.1021/ja3031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Air pollution, the automobile, and public health. National Academy Press; Washington, D.C.: 1988. [PubMed] [Google Scholar]
- 28.Jeng HA, Pan CH, Diawara N, Chang-Chien GP, Lin WY, Huang CT, Ho CK, Wu MT. Polycyclic aromatic hydrocarbon-induced oxidative stress and lipid peroxidation in relation to immunological alteration. Occup. Environ. Med. 2011;68(9):653–658. doi: 10.1136/oem.2010.055020. DOI 10.1136/oem.2010.055020. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Han I-K, Hu M, Shao M, Zhang J, Tang X. Personal exposure to particulate pahs and anthraquinone and oxidative DNA damages in humans. Chemosphere. 2010;81(10):1280–1285. doi: 10.1016/j.chemosphere.2010.08.055. DOI 10.1016/j.chemosphere.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 30.Ruchirawat M, Navasumrit P, Settachan D, Tuntaviroon J, Buthbumrung N, Sharma S. Measurement of genotoxic air pollutant exposures in street vendors and school children in and near bangkok. Toxicol. Appl. Pharmacol. 2005;206(2):207–214. doi: 10.1016/j.taap.2004.11.025. DOI 10.1016/j.taap.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Ruchirawat M, Mahidol C, Tangjarukij C, Pui-ock S, Jensen O, Kampeerawipakorn O, Tuntaviroon J, Aramphongphan A, Autrup H. Exposure to genotoxins present in ambient air in bangkok, thailand — particle associated polycyclic aromatic hydrocarbons and biomarkers. Sci. Total Environ. 2002;287(1-2):121–132. doi: 10.1016/s0048-9697(01)01008-7. DOI 10.1016/s0048-9697(01)01008-7. [DOI] [PubMed] [Google Scholar]
- 32.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem.-Int. Edit. 2007;46(8):1318–1320. doi: 10.1002/anie.200603817. DOI 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008;80(10):3699–3707. doi: 10.1021/ac800112r. DOI 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruzewicz DA, Reches M, Whitesides GM. Low-cost printing of poly(dimethylsiloxane) barriers to define microchannels in paper. Anal. Chem. 2008;80(9):3387–3392. doi: 10.1021/ac702605a. DOI 10.1021/ac702605a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Shi WW, Jiang L, Qin JH, Lin BC. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis. 2009;30(9):1497–1500. doi: 10.1002/elps.200800563. DOI 10.1002/elps.200800563. [DOI] [PubMed] [Google Scholar]
- 36.Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009;81(16):7091–7095. doi: 10.1021/ac901071p. DOI 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Shi WW, Qin JH, Lin BC. Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal. Chem. 2010;82(1):329–335. doi: 10.1021/ac9020193. DOI 10.1021/ac9020193. [DOI] [PubMed] [Google Scholar]
- 38.Dungchai W, Chailapakul O, Henry CS. Use of multiple colorimetric indicators for paper-based microfluidic devices. Analytica Chimica Acta. 2010;674(2):227–33. doi: 10.1016/j.aca.2010.06.019. DOI 10.1016/j.aca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed Engl. 2007;46(8):1318–20. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dungchai W, Chailapakul O, Henry CS. Electrochemical detection for paper-based microfluidics. Anal Chem. 2009;81(14):5821–6. doi: 10.1021/ac9007573. DOI 10.1021/ac9007573. [DOI] [PubMed] [Google Scholar]
- 41.Zhao WA, van den Berg A. Lab on paper. Lab. Chip. 2008;8(12):1988–1991. doi: 10.1039/b814043j. DOI 10.1039/b814043j. [DOI] [PubMed] [Google Scholar]
- 42.Mentele MM, Cunningham J, Koehler K, Volckens J, Henry CS. Microfluidic paper-based analytical device for particulate metals. Anal. Chem. 2012;84(10):4474–4480. doi: 10.1021/ac300309c. DOI 10.1021/ac300309c. [DOI] [PubMed] [Google Scholar]
- 43.Jokerst JC, Adkins JA, Bisha B, Mentele MM, Goodridge LD, Henry CS. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal. Chem. 2012;84(6):2900–2907. doi: 10.1021/ac203466y. DOI 10.1021/ac203466y. [DOI] [PubMed] [Google Scholar]
- 44.Vella SJ, Beattie P, Cademartiri R, Laromaine A, Martinez AW, Phillips ST, Mirica KA, Whitesides GM. Measuring markers of liver function using a micropatterned paper device designed for blood from a fingerstick. Anal. Chem. 2012;84(6):2883–2891. doi: 10.1021/ac203434x. DOI 10.1021/ac203434x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010;82(1):3–10. doi: 10.1021/ac9013989. DOI 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 46.Hennigan CJ, Miracolo MA, Engelhart GJ, May AA, Presto AA, Lee T, Sullivan AP, McMeeking GR, Coe H, Wold CE, Hao WM, Gilman JB, Kuster WC, de Gouw J, Schichtel BA, Collett JL, Jr, Kreidenweis SM, Robinson AL. Chemical and physical transformations of organic aerosol from the photo-oxidation of open biomass burning emissions in an environmental chamber. Atmos. Chem. Phys. Discuss. 2011;11:11995–12037. DOI. [Google Scholar]
- 47.Baumann K, Ift F, Zhao JZ, Chameides WL. Discrete measurements of reactive gases and fine particle mass and composition during the 1999 atlanta supersite experiment. J. Geophys. Res. Atmos. 2003;108(D7) DOI 10.1029/2001jd001210. [Google Scholar]
- 48.Li QF, Wyatt A, Kamens RM. Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos. Environ. 2009;43(5):1037–1042. DOI 10.1016/j.atmosenv.2008.11.018. [Google Scholar]
- 49.Hansen RE, Østergaard H, Nørgaard P, Winther JR. Quantification of protein thiols and dithiols in the picomolar range using sodium borohydride and 4,4′-dithiodipyridine. Anal. Biochem. 2007;363(1):77–82. doi: 10.1016/j.ab.2007.01.002. DOI 10.1016/j.ab.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Miller JC. Statistics for analytical chemistry. E. Horwood Halsted Press; Chichester, England: 1988. [Google Scholar]
- 51.Kumagai Y, Koide S, Taguchi K, Endo A, Nakai Y, Yoshikawa T, Shimojo N. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002;15(4):483–489. doi: 10.1021/tx0100993. DOI Doi 10.1021/Tx0100993. [DOI] [PubMed] [Google Scholar]
- 52.Wallace LA, Emmerich SJ, Howard-Reed C. Source strengths of ultrafine and fine particles due to cooking with a gas stove. Environ. Sci. Technol. 2004;38(8):2304–2311. doi: 10.1021/es0306260. DOI 10.1021/es0306260. [DOI] [PubMed] [Google Scholar]
- 53.Li CS, Lin WH, Jenq FT. Size distributions of submicrometer aerosols from cooking. Environ. Int. 1993;19(2):147–154. DOI 10.1016/0160-4120(93)90365-o. [Google Scholar]
- 54.Koike E, Kobayashi T. Chemical and biological oxidative effects of carbon black nanoparticles. Chemosphere. 2006;65(6):946–951. doi: 10.1016/j.chemosphere.2006.03.078. DOI 10.1016/j.chemosphere.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 55. [accessed on 06/21/12];Faq on how to protect your respiratory health in fort collins during high park fire incident. http://www.fcgov.com/airquality/pdf/_20120611_FAQ_FINAL_HighParkFire.pdf.
- 56.Fullana A, Carbonell-Barrachina AA, Sidhu S. Comparison of volatile aldehydes present in the cooking fumes of extra virgin olive, olive, and canola oils. J. Agric. Food Chem. 2004;52(16):5207–5214. doi: 10.1021/jf035241f. DOI 10.1021/jf035241f. [DOI] [PubMed] [Google Scholar]
- 57.Hung HS, Wu WJ, Cheng YW, Wu TC, Chang KL, Lee H. Association of cooking oil fumes exposure with lung cancer: Involvement of inhibitor of apoptosis proteins in cell survival and proliferation in vitro. Mut. Res. Gen. Tox. Env. Mutagen. 2007;628(2):107–116. doi: 10.1016/j.mrgentox.2006.12.005. DOI 10.1016/j.mrgentox.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Lin JM, Liou SJ. Aliphatic aldehydes produced by heating chinese cooking oils. Bull. Environ. Contam. Toxicol. 2000;64(6):817–824. doi: 10.1007/s0012800076. DOI. [DOI] [PubMed] [Google Scholar]
- 59.Yang SC, Jenq SN, Kang ZC, Lee H. Identification of benzo a pyrene 7,8-diol 9,10-epoxide n2-deoxyguanasine in human lung adenocarcinoma cells exposed to cooking oil fumes from frying fish under domestic conditions. Chem. Res. Toxicol. 2000;13(10):1046–1050. doi: 10.1021/tx0000419. DOI 10.1021/tx0000419. [DOI] [PubMed] [Google Scholar]
- 60. [acessed on 06/03/12];Fort collins air quality report 2010. http://www.fcgov.com/airquality/pdf/AAQR-2010.pdf?1312821338.
- 61.Shen L, Hagen JA, Papautsky I. Point-of-care colorimetric detection with a smartphone. Lab. Chip. 2012;12(21):4240–4243. doi: 10.1039/c2lc40741h. DOI. [DOI] [PubMed] [Google Scholar]
- 62.Verma V, Rico-Martinez R, Kotra N, King L, Liu J, Snell TW, Weber RJ. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic sub-fractions to the reactive oxygen species-generating potential of fine ambient aerosols. Environ. Sci. Technol. 2012 doi: 10.1021/es302484r. DOI 10.1021/es302484r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.