Abstract
Background:
Type 2 diabetic patients are faced with a higher risk of dyslipidemia and cardiovascular disorders. This study was undertaken to assess the effect of consumption of 1 cup cranberry juice by type 2 diabetic patients on serum paraoxonase-1 (PON-1) activity, apoA-1, apoB, glucose, and Lp(a).
Methods:
In a double-blind randomized clinical trial, 58 type 2 diabetic male patients were randomly divided to receive 1 cup cranberry juice (CJ) or placebo drink daily for 12 weeks. Fasting blood were obtained at beginning and at the end of study (12th week). Serum glucose and PON-1 activity were measured by enzymatic and colorimetric methods, respectively. ApoB, apoA-I, and Lp(a) were determined immunoturbidimetrically. The data were analyzed by SPSS version 16.
Results:
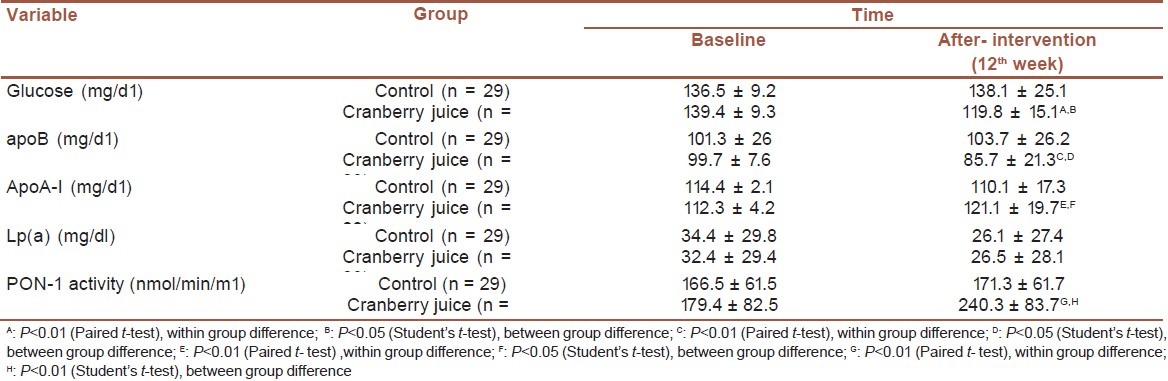
There were significant decrease in serum glucose and apoB (P>0.05 and P>0.01, respectively) and significant increase in serum apoA-1 and PON-1 activity (P>0.05 and P<0.01, respectively) at the end of study in CJ group compared with control group. In CJ group at the end of study, there were significant decrease in serum glucose and apoB (P<0.01 and P<0.01, respectively) and significant increase in serum apo A-1 and PON-1 activity (P<0.01 and P<0.01, respectively) compared with initial values. In CJ group, there was no significant change in Lp(a) at the end of study compared with initial values and also compared with control group.
Conclusion:
1 cup CJ for 12 weeks is effective in reducing serum glucose and apoB and increasing apoA-1 and PON-1 activity, so may have favorite effects on reducing CVD risk factors in type 2 diabetic male patients.
Keywords: Apo B, apoA-1, cranberry, glucose, paraoxonase-1, Lp(a), type 2 diabetes
INTRODUCTION
It is estimated that developing countries in Asia and in the Middle East, particularly in Persian Gulf states, will have the largest increase in the prevalence of diabetes by 2030, which is related to major shift in life style and nutrition transition in these countries.[1] The prevalence of type 2 diabetes (T2D) is reported to be more than 14% in Tehran, Iran, with an estimated incidence of new cases in about 1% of population per year.[2] There is high prevalence of Coronary Heart Disease (CHD) in this area too[1] and it has been shown that the Iranian population with diabetes has a high risk for Cardiovascular Disease (CVD), independent of traditional risk factors.[1,2] Decrease of High density lipoprotein cholesterol (HDL-c) and hypertriglyceridemia/hyperapo B was the dominant atherogenic dyslipoproteinemia in the diabetic patients, with a slight decrease to slight increase in Low density lipoprotein cholesterol (LDL-c),[3,4] and is characterized by higher LDL particle numbers with predominantly small, dense LDL due to increased secretion of apo B lipoprotein particles by the liver.[5,6] ApoB and apoA-I were better than LDL-C and HDL-c, respectively, in predicting cardiovascular risk in T2D.[7–10]
Also, increased concentration of lipoprotein(a) [Lp(a)] have been associated with higher risk of CVD in diabetic patients.[11,12] Paraoxonase-1 (PON-1) is an HDL- associated enzyme and has been implicated in many anti- atherogenic processes, such as inhibiting LDL and HDL from oxidation and reducing macrophage cholesterol biosynthesis, attenuating cholesterol and oxidized lipid influx, and stimulating macrophage cholesterol efflux.[13–15] PON-1 is dysfunctional due to glycation, reducing its ability to retard LDL and cell membrane oxidation and contributing to the inflammation typical of diabetes, leading to the excess atherosclerosis common in this disease.[16–19]
Cranberry has the greatest antioxidant content (flavonoids, phenolic acid, and the most notably the high molecular weight proanthocyanidins) of any fruit by fresh weight.[20–22] Two cities in Iran, Qazvin and Rodbar, have the highest production of cranberry in Iran predominantly in summer but cranberry juice (CJ) is more available in all seasons.[23]
Phytochemicals in cranberries could inhibit LDL oxidation and platelet aggregation, induce expression of LDL receptor, and reduce blood pressure.[24–27] To the best of our knowledge, the effects of cranberry on apoproteins, Lp(a), and PON-1 in type 2 diabetic subjects taking oral glucose- lowering drugs have not been reported. So, the aim of this study was the effects of CJ consumption on serum glucose, apoB, apoA-I, PON-1, and Lp(a) in type 2 diabetic male patients.
METHODS
Study design and Participants
A double-blind randomized clinical trial of parallel design was used. During a 2-week baseline period, participants continued their usual diet without any cranberry (Run-in period), then they were randomly divided to consume 1 cup (240 ml) CJ group or placebo drink (natural mineral water with strawberry flavor–Tazehnoush Company, Shiraz, Iran) (control group) for 12 weeks. Sixty nonsmoking T2D male patients were recruited from the Endocrine Research Center of Tehran University of Medical sciences (TUMS), Tehran, Iran, from January 2009 to September 2010. Patients were informed of their rights as volunteers in this study and signed approved consent forms. The procedures followed in this study were maintained in accordance with the Helsinki Declaration and the study was approved by the institutional review board of TUMS. This study registered in Iranian Registry of Clinical Trials (IRCT) and the IRCT ID is IRCT201111272709N23. Patients received no monetary incentive. Patients had been diagnosed with diabetes (fasting blood glucose ≥126 mg/dl or 2-hour postprandial glucose ≥200 mg/d1 within the previous 5 years.[19]
Inclusion criteria were Body Mass Index (BMI) <30 kg/m2, HbAlc <9%, serum triglyceride <400 mg/dl, and serum total cholesterol <240 mg/dl. Patients were included if they were taking oral hypoglycemic agents but not insulin. Patients were excluded if they had a recent (within previous 3 months) or past history of symptomatic heart disease, myocardial infarction, angina pectoris or stroke, surgery, liver, renal (plasma creatinine >1.62 mg/dl), or thyroid disease; use of steroids or drugs with unknown components, change in the use of medication for diabetes, hypertension, dyslipidemia or anti-platelet in the previous 2 months, pregnancy, or breastfeeding.
All patients were asked to maintain their usual diet and physical activity level and not to alter their lifestyle during the intervention.
The patients were followed up by telephone each week; patients who had no phone were instructed to return to the clinic every other week. Dietary intake was monitored by the same dietitian throughout the study and participants were asked to complete a 24-hour dietary recall questionnaire at the beginning, 6th and 12th weeks. Physical activity was measured by an International Physical Activity Questionnaire whose validity and reliability was examined in 12 countries,[28] at baseline and at the end of the 12 weeks intervention.
Data collection and measurement
Subjects were required to provide venous blood samples after fasting overnight for 12 to 14 hours on day 0 and at the end of the intervention (12th week). All samples were collected while the subjects rested in a supine position for 10 minutes. Serum glucose was measured with a Cobas MIRA analyzer (Roche Diagnostic, Basel, Switzerland) by enzymatic method (Pars Azmon Co., Tehran, Iran). ApoB, apoA-I, and Lp(a) were measured by immunoturbidimetry (Pars Azmon Co., Tehran, Iran) with a Cobas MIRA analyzer with calibration traceable to the International Federation of Clinical Chemistry Primary Standards.[6] PON-1 activity was measured by colorimetric method.[29] The intra-assay coefficients of variation for these assays (n = 10) were 1.1%, 1.2%, 1.2%, 1.4%, and 1.3% for PON-1, apoB, apoA-I, glucose, and Lp(a), respectively, and the inter-assay coefficient of variation (n = 10) were 1.3%, 1.4%, 1.4%, 1.5%, and 1.4%, respectively.
Statistical analysis
All data were expressed as the mean ± standard deviation. The level of significance was chosen as P<0.05. Statistical analyses were performed with SPSS version 16.0. The normal distribution of the variables was checked by the Kolmogorov-Smirnov test. In order to test whether the differences between the mean values of the items studied in both groups were significant, the student's t-test was used. Differences in the same diabetic patients, before and after 12 weeks of intervention, were evaluated by paired t-test. Diet records were analyzed using Food Processor II software. Repeated measurement ANOVA was used for comparison of means in different intervals of 24-hour dietary recall questionnaire.
RESULTS
Fifty-eight of 60 randomly selected T2D male patients (age: 54.8 ± 9.1, BMI: 28.8 ± 3.6) completed the study (2 ones could not adhere to the group meeting schedule and were not included in analyses). Baseline characteristics of the T2D patients confirmed that they were well matched for the inclusion criteria [Table 1]. There were no significant difference in BMI, waist circumference, and physical activity after the intervention compared with baseline values. There were no significant differences (the time × treatment interactions effect were not significant) in total energy and nutrients intake at beginning, 6 and 12 weeks [Table 2].
Table 1.
Baseline characteristics of the participants
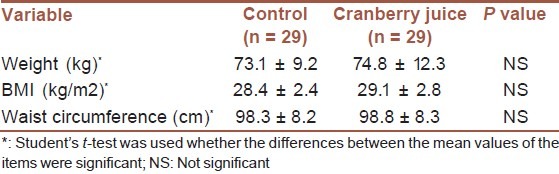
Table 2.
Daily total energy and dietary intakes at baseline, 6th, and 12th weeks of study

At the end of study in CJ group, there was a significant decrease in serum glucose and apo B either compared with initial values (P<0.01 and P<0.05, respectively) or comparing with control group (P<0.05 and P<0.05, respectively) [Table 3]. At the end of study in CJ group, there was significant increase in PON-1 activity and apoA-I either compared with initial values (P<0.01 and P<0.01, respectively) or comparing with control group (P<0.0001 and P<0.05, respectively) [Table 3]. There was no significant change in Lp(a) at the end of study compared with initial values and also compared with control group [Table 3].
Table 3.
Serum glucose, apoB, apoA-I, Lp(a), and PON-1 activity at baseline and after the intervention
DISCUSSION
The results of this double-blind randomized clinical trial demonstrate that intake of 1 cup CJ for 12 weeks leads to significant increase in apoA-I and PON-1 activity and significant decrease in serum glucose and apo B in T2D patients. It seems that diet full of antioxidant and phytochemical (e.g., DASH diets) have beneficial effects on c-reactive protein, hepatic function tests,[29] increasing insulin sensitivity[30] and HDL-c, decreasing serum glucose and LDL-c,[31] and finally is inversely associated with incidence of T2D.[31] Our finding about significant decrease in serum glucose in CJ group was consistent with Wilson et al.'s reports.[25,33] One plausible explanation for this observation may be a delay in the gastric uptake of glucose or distribution of glucose to insulin-sensitive tissues following CJ consumption.[33] The ingredients in CJ block and even overcome the oxidative stress most probably by an indirect effect of decreasing postprandial glucose and triglyceride which are responsible for inducing oxidative stress as well as a direct in vivo antioxidant mechanism.[34] CJ is better source of quality antioxidants compared with cranberry fruit and also have more lipoprotein-bound antioxidant activity compared with cranberry fruit.[34] The quality of antioxidants in a food or beverage is the result of the chemical composition and is independent of the quantity of antioxidant present.[34] CJ antioxidants have the ability to bind to lower density lipoproteins and to protect them from oxidation, increase in plasma antioxidant capacity, and increase in HDL-c. This is one mechanism by which CJ can protect against heart disease.[27,35] CJ is known to be a rich source of quercetin and myricetin glycosides, as well as larger proanthocyanidin polymers[19,21,25] and also contain acetylsalicylic acid that possess anti-inflammatory activity.[27] Quercetin has been demonstrated to inhibit gastric uptake of glucose in the porcine model. Quercetin and myricetin has also been demonstrated to inhibit GLUT4-mediated glucose uptake by rat adipocytes,[20,25] inhibit aldose reductase,[36] α-amylase,[37] and α-glucosidase activity in vitro.[38] On the other hand, two studies reported inconsistent results with our study but these two studies used CJ concentrate powder capsules equivalent to 240 ml CJ and the possibility that the heat processing necessary to convert the CJ to a powder had altered its bioactivity was raised by authors as a possible explanation for their result.[20,36]
Because of significant decrease of serum glucose due to CJ, the possible necessity of changing drug regimen must be considered in future studies if diabetic patients had been advised to consume CJ. Future studies may wish to examine the effect that CJ has on the absorption and distribution kinetics of glucose in this regard.
The significant decrease in apoB and significant increase in apoA-I in our study was a favorite result for decreasing the CVD risk. However, the effects of CJ on serum lipoprotein has been examined but on apoproteins have not been reported. Phytochemicals in cranberries could inhibit LDL oxidation and induce expression LDL receptors,[24] increase in cholesterol uptake by hepatocyte,[26] increase binding and excretion of bile acids.[39]
Other studies found a strong relationship between the elevation of plasma HDL-C and apoA-I, the change in plasma apoA-I concentration explaining almost half (approximately 48%) of the variation in HDL-c concentration during the course of the intervention. This relationship could either reflect a decrease in the clearance of HDL particles and/or an increase in the synthesis of apoA-I during the course of the intervention.
Although studies are needed to further investigate these mechanisms, the latter explanation could be the result of an increased production of apoA-I, an effect that could be similar to the one reported following the consumption of food rich in polyphenolic compounds, as are cranberry.[27,40]
Although oxidized apoA-I was not measured in the present study, a possible reduction in apoA-I oxidation following CJ consumption could also have contributed to increase in serum apoA-I concentration.[27,41] Furthermore, a possible indirect effect of cranberry antioxidants on the activity of the adenosine triphosphate-binding cassette transporter A-I and scavenger receptor class B type 1 expression in macrophages cannot be excluded.[27,42] However, increasing LDL-c and decreasing HDL-C are well-established risk factors for CVD,[43,44] but apoA-I and apoB are stronger predictors for CVD.[43] Decrease of apoB in this study means that small, dense LDL were significantly less than control group and CVD risk is lower in T2D patients consuming CJ. We did not see significant change in serum Lp(a). To date, we are unaware of any study demonstrating the impact of cranberry or other natural source of antioxidant on serum Lp(a) in T2D. Optimization of glycemic control does not affect serum Lp(a) levels in patients with T2D,[11,12] which was concordant with our study.
Significant increase in serum PON-1 activity in CJ group compared with control group could be a favorite and beneficial effect on reducing of CVD complication in T2D patients. Connelly et al. reported that in T2D patients, serum PON-1 is decreased and was inversely associated with glucose concentration which was concordant with results of our study.[14] Furthermore, increased serum PON1 activity in our study could reduce the inflammation and oxidative stress associated with atherosclerosis, inhibit the oxidation of LDL (and cell membrane), reducing macrophage cholesterol efflux biosynthesis, negatively associated with HDL peroxide and positively associated with HDL antioxidative ability.[13–19] All these appear to be important in the inflammatory response in artery that prevents atherogenesis.[19] PON-1 reduces monocyte adhesion to endothelial cells and macrophage chemotaxis attributable to oxidized phospholipids.[13–15] PON-1 also appears to have a phospholipase A2 activity, resulting in the release of lysophosphatidylcholine from macrophages which produces a dose-dependent decrease in cholesterol biosynthesis in macrophages.[15,45,46] This may be beneficial effect in reducing the risk of CVD in diabetic patients in CJ group. Finally, we conclude PON-1 and apoA-I increasing concomitant with apoB and glucose decreasing effect due to 1 cup CJ consumption for 12 weeks, which reinforces the notion that health benefits can be derived from consuming CJ as an antioxidant-rich (e.g., flavonoid) food in type 2 diabetic patients.
ACKNOWLEDGMENTS
The authors would like to thank the subjects who participated in this study, without whom no clinical research would be possible.
Footnotes
Source of Support: This study was funded by Tehran University of Medical Sciences and was registered with project number 699
Conflict of Interest: None declared.
REFERENCES
- 1.Hadaegh F, Fahimfar N, Khalili D, Sheikholeslami F, Azizi F. New and known type2 diabetes as coronary heart disease equivalent: Results from 7.6 year follow up in a middle east population. Cardiovasc Diabetol. 2010;9:84. doi: 10.1186/1475-2840-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harati H, Hadaegh F, Saadat N, Azizi F. Population-based incidence of Type 2 diabetes and its associated risk factors: Results from a six-year cohort study in Iran. BMC Public Health. 2009;9:186. doi: 10.1186/1471-2458-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlen EM, Lanne T, Engvall J, Lindstrom T, Grodzinsky E, Nystrom FH. Carotid intima- media thickness and apolipoprotein B/apolipoprotein A-I ratio in middle- aged patients with Type2 diabetes. Diabet Med. 2009;26:384–90. doi: 10.1111/j.1464-5491.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 4.Harder H, Dinesen B, Astrup A. The effect of a rapid weight loss on lipid profile and glycemic control in obese type 2 diabetic patients. Int J Obes Relat Metab Disord. 2004;28:180–2. doi: 10.1038/sj.ijo.0802529. [DOI] [PubMed] [Google Scholar]
- 5.Williams K, Tchernof A, Hunt KJ, Wagenknecht LE, Haffner SM, Sniderman AD. Diabetes, abdominal adiposity and atherogenic dyslipoproteinemia in women compared with men. Diabetes. 2008;57:3289–96. doi: 10.2337/db08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastelein JP, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, et al. Lipids, apoproteins and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117:3002–9. doi: 10.1161/CIRCULATIONAHA.107.713438. [DOI] [PubMed] [Google Scholar]
- 7.Charton- Menyes V, Betteridge DJ, Colhoun H, Fuller J, France M, Hitman GA, et al. Apolipoproteins, cardiovascular risk and statin response in type 2 diabetes: The Collaborative Atrovastatin Diabetes Study (CARDS) Diabetologia. 2009;52:218–25. doi: 10.1007/s00125-008-1176-8. [DOI] [PubMed] [Google Scholar]
- 8.Sierra- Johnson J, Fisher RM, Romero- Corral A, Somers VK, Lopes- Jimenes F, Ohrvik J, et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/ apolipoprotein A-I in predicting coronary heart disease mortality: Findings from a multi-ethnic US population. Eur Heart J. 2009;30:710–7. doi: 10.1093/eurheartj/ehn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson A, Danesh J. Association between apolipoprotein B, apolipoprotein A-I, the apolipoprotein B/A-I ratio and coronary heart disease: A literature- based meta- analysis of prospective studies. J Intern Med. 2006;259:481–92. doi: 10.1111/j.1365-2796.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 10.Canoui- Poitrine F, Luc G, Bard JM, Ferrieres J, Yarnell J, Arveiler D, et al. Relative contribution of lipids and apolipoproteins to incident coronary heart disease and ischemic stroke: The PRIME study. Cerebrovasc Dis. 2010;30:252–9. doi: 10.1159/000319067. [DOI] [PubMed] [Google Scholar]
- 11.Mora S, Kamstrup PR, Rifai N, Nordestgaad BG, Buring JE, Ridker PM. Lipoprotein (a) and risk of type 2 diabetes. Clin Chem. 2010;56:1257–60. doi: 10.1373/clinchem.2010.146779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakhjavani M, Morteza A, Esteghamati A, Khalilzadeh O, Zandieh A, Safari R. Serum lipoprotein (a) levels are greater in female than male patients with type-2 diabetes. Lipids. 2011;46:349–56. doi: 10.1007/s11745-010-3513-1. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Gu Q. Protective effect of paraoxonase-1 of high- density lipoprotein in type 2 diubetic patients with hephropathy. Nephrology (Carlton) 2009;14:514–20. doi: 10.1111/j.1440-1797.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- 14.Connelly PW, Zinman B, Maguire GF, Mamakeesick M, Harris SB, Hegele RA. Association of the novel cardiovascular risk factors paraoxonase-1 and cystatin C in type2 diabetes. J lipid Res. 2009;50:1216–22. doi: 10.1194/jlr.P800070-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: Continuing issues. Curr Opin Lipidol. 2004;15:261–7. doi: 10.1097/00041433-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Mackness B, Mackness M. Anti-inflammatory properties of paraoxonase-1 in atherosclerosis. Adv Exp Med Biol. 2010;660:143–51. doi: 10.1007/978-1-60761-350-3_13. [DOI] [PubMed] [Google Scholar]
- 17.Jornayvaz FR, Brulhart-Meynet MC, James Rw. Myeloperoxidase and paraoxonase-1 in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2009;19:613–9. doi: 10.1016/j.numecd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Poh R, Muniandy S. Paraoxonase-1 activity as a predictor of cardiovascular disease in type 2 diabetes. Southeast Asian J Trop Med Public Health. 2010;41:1231–46. [PubMed] [Google Scholar]
- 19.Jayakumari N, Thejaseebai G. High prevalence of low serum paraoxonase -1 in subjects with coronary artery disease. J Clin Biochem Nutr. 2009;45:278–84. doi: 10.3164/jcbn.08-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TI, Chan Yc, Lin CW, Lee WJ, Sheu WH. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet Med. 2008;25:1473–7. doi: 10.1111/j.1464-5491.2008.02588.x. [DOI] [PubMed] [Google Scholar]
- 21.Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: Fruits. J Agric Food Chem. 2001;49:5315–21. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 22.Mckay Dl, Blumberg JB. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr Rev. 2007;65:490–502. doi: 10.1111/j.1753-4887.2007.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 23.Rahbar M, Diba K. In vitro activity of cranberry extract against etiological agents of urinary tract infections. Afr J Pharm Pharmacol. 2010;4:286–8. [Google Scholar]
- 24.Kim MJ, Jung HN, Nam Kim KN, Kwak HK. Effects of cranberry powder on serum lipid profiles and biomarkers of oxidative stress in rats fed an atherogenic diet. Nutr Res Practice. 2008;2:158–64. doi: 10.4162/nrp.2008.2.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson T, Singh AP, Vorsa N, Goettl CD, Kittleson KM, Roe CM, et al. Human glycemic response and phenolic content of unsweetened cranberry juice. J Med Food. 2008;11:46–54. doi: 10.1089/jmf.2007.531. [DOI] [PubMed] [Google Scholar]
- 26.Neto CC. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–64. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 27.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Favourable impact of low- calorie cranberry juice consumption on plasma HDL- Cholesterol concentrations in men. Br J Nutr. 2006;96:357–64. doi: 10.1079/bjn20061814. [DOI] [PubMed] [Google Scholar]
- 28.Craig CL, Marshall Al, Sjostrom M, Bauman AE, Booth ML, Ainsworft BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sport Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 29.Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The dietary approaches to stop hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. 2011;141:1083–8. doi: 10.3945/jn.110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinderliter AL, Babyak MA, Sherwood A, Blumenthal JA. The DASH diet and insulin sensitivity. Curr Hypertens Rep. 2011;13:67–73. doi: 10.1007/s11906-010-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azadbakht L, Fard NR, Karimi M, Baghaei MH, Surkan PJ, Rahimi M, et al. Effects of the dietary approaches to stop hypertension(DASH) eating plan on cardiovascular risks among type 2 diabetes patients: A randomized crossover clinical trial. Diabetes Care. 2011;34:55–7. doi: 10.2337/dc10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase / arylesterase.Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–6. [PubMed] [Google Scholar]
- 33.Wilson T, Meyers SL, Singh AP, Limburg PJ, Vorsa N. Favorable glycemic response of type 2 diabetics to low- calorie cranberry juice. J Food Sci. 2008;73:241–5. doi: 10.1111/j.1750-3841.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 34.Vinson JA, Bose P, Proch J, AL Kharrat H, Samman N. Cranberries and cranberry products: Powerful in vitro, ex vivo and in vivo sources of antioxidants. J Agric Food Chem. 2008;56:5884–91. doi: 10.1021/jf073309b. [DOI] [PubMed] [Google Scholar]
- 35.Ruel G, Pomerleau S, Couture P, Lamarche B, Couillard C. Changes in plasma antioxidant capacity and oxidized low-density lipoprotein levels in men after short-term CJ consumption. Metabolism. 2005;54:856–61. doi: 10.1016/j.metabol.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Chambers BK, Camire EM. Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care. 2003;26:2695–6. doi: 10.2337/diacare.26.9.2695-a. [DOI] [PubMed] [Google Scholar]
- 37.Pinto Mda S, Ghaedian R, Shinde R, Shetty K. Potential of cranberry powder for management of hyperglycemia using in vitro models. J Med Food. 2010;13:1036–44. doi: 10.1089/jmf.2009.0225. [DOI] [PubMed] [Google Scholar]
- 38.Apostolidis E, Kwon YI, Shetty K. Potential of cranberry- based herbal synergies for diabetes and hypertension management. Asia Pac J Clin Nutr. 2006;15:433–41. [PubMed] [Google Scholar]
- 39.Kahlon TS, Smith GE. In vitro binding of bile acids by blueberries, plums, prunes, strawberries, cherries, cranberries and apples. Food Chem. 2007;100:1182–7. [Google Scholar]
- 40.Kalgaonkar S, Gross HB, Yokoyama W, Keen CL. Effects of a flavonol- rich diet on select cardiovascular parameters in a Golden Syrian hamster model. J Med Food. 2010;13:108–15. doi: 10.1089/jmf.2008.0295. [DOI] [PubMed] [Google Scholar]
- 41.Francis GA. High density lipoprotein oxidation: In vitro susceptibility and potential in vivo consequences. Biochim Biophys Acta. 2000;1483:217–35. doi: 10.1016/s1388-1981(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 42.Singaraja RR, Fievet C, Castro G. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shidfar F, Froghifar N, Vafa MR, Rjab A, Hosseini S, Shidfar S, et al. The effects of tomato consumption on serum glucose, apolipoprotein B, apolipoprotein A-I, homocysteine and blood pressure in type 2 diabetic patients. Int J Food Sci Nutr. 2001;62:289–94. doi: 10.3109/09637486.2010.529072. [DOI] [PubMed] [Google Scholar]
- 44.Shakouri Mahmoudabadi MM, Djalali M, Djazayery SA, Keshavarz SA, Eshraghian MR, Saboor-Yaraghi A, et al. J Res Med Sci. 2011;16:361–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Stefanovic A, Kotur-stevuljevic J, Spasic C, Vekic J, Zeljkovic A, Spasojevic-Kalimanovska B, et al. HDL2 particles are associated with hyperglycaemia, lower PON1 activity and oxidative stress in type 2 diabetes mellitus. Clin Biochem. 2010;43:1230–5. doi: 10.1016/j.clinbiochem.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Lu C, Gao Y, Zhou H, Tian H. The relationship between PON1 activity as well as oxLDL levels and coronary artery lesions in CHD patients with diabetes mellitus or impaired fasting glucose. Coron Artery Dis. 2008;19:565–73. doi: 10.1097/MCA.0b013e3283109206. [DOI] [PubMed] [Google Scholar]


