Abstract
We report the 11-year follow-up of a man with osteogenesis imperfecta type I who was treated with bisphosphonates and alfacalcidol. A 36-year-old Japanese man with osteogenesis imperfecta type I who had frequently experienced painful fragility fractures consulted our clinic because of chronic back pain. The patient had multiple morphometric vertebral fractures and a low bone mineral density (BMD) at the lumbar spine. The patient was treated with cyclical etidronate 200 mg, for 2 weeks every 3 months, plus alfacalcidol 1 μg daily, for 2 years; and alendronate 5 mg daily or 35 mg weekly, plus alfacalcidol 1 μg daily for 9 years. After 11 years of treatment, BMD at the lumbar spine increased by 6.4%, following a 20.3% reduction in serum alkaline phosphatase. Serum calcium, phosphorus, and intact parathyroid hormone levels remained within the normal ranges. Three clinical fractures occurred at two ribs and the metacarpus, and two morphometric vertebral fractures occurred at the thoracic spine during the 11-year treatment period, but the patient experienced no adverse effects. Thus, the present case report shows the long-term outcome and safety of bisphosphonate plus alfacalcidol treatment in a man with osteogenesis imperfecta type I.
Keywords: etidronate, alendronate, fragility fracture, bone mineral density, osteogenesis imperfecta
Introduction
Osteogenesis imperfecta is a heterogeneous group of genetic disorders that affect the integrity of connective tissue. The hallmark of osteogenesis imperfecta is bone fragility, although other manifestations, including dentigenesis imperfecta, blue sclera, easy bruising, joint laxity, and scoliosis, are also common among patients with the disease. The severity of osteogenesis imperfecta ranges from prenatal death to mild osteopenia without limb deformity.
The fracture rate decreases with maturation in patients with osteogenesis imperfecta.1,2 The clinical stability of the disease may be associated with bone maturation. Despite the reduced fracture rate, the bone mineral density (BMD) usually remains low in adults with osteogenesis imperfecta, and the optimal strategy for increasing BMD and preventing subsequent fractures in adults with osteogenesis imperfecta remains to be fully established. Although osteogenesis imperfecta is an inheritable disorder of bone formation, resulting in bone fragility, the activity of cancellous bone remodeling, bone resorption, and/or bone turnover are also increased.2–6 The efficacy of treatment with cyclical intravenous pamidronate for bone fragility in children with osteogenesis imperfecta has been established,7–12 and prevents fragile fractures without inhibiting bone growth. However, a recent report showed that treatment with oral alendronate for 2 years in pediatric patients with osteogenesis imperfecta decreased bone turnover and increased BMD at the lumbar spine, but was not associated with improved fracture outcome.13
Although the short-term effectiveness of bisphosphonates on BMD and a reduced incidence of fractures has been suggested in adults with osteogenesis imperfecta,14,15 very few reports have shown the long-term outcome of bisphosphonate treatment in adults with osteogenesis imperfecta. Combined treatment with bisphosphonates and alfacalcidol is more effective in increasing BMD or preventing fractures than single treatment with bisphosphonates alone in postmenopausal women and men with osteoporosis.16–18 We report the 11-year follow-up of a man with osteogenesis imperfecta type I19 who was treated with bisphosphonates and alfacalcidol.
Case report
A 36-year-old Japanese man consulted our clinic because of chronic back pain. The patient had experienced approximately 15 painful fractures of the forearm, elbow, hand, finger, clavicle, lower leg, ankle, and rib. The fracture frequency gradually decreased with maturation. Despite the experience of frequent fractures and existence of chronic back pain for years, he had never received treatment for these problems. His height was 1.70 m, his body weight was 62.5 kg, and his body mass index was 21.6 kg/m2 (Table 1). Three clinical criteria for osteogenesis imperfecta, ie, a history of fractures, blue sclera, and a positive family history, were present. However, neither deformities in the legs, such as anterolateral femoral bowing and anterior tibial bowing, nor marked deformities in the thoracic and lumbar spine were observed. The patient was diagnosed as having Sillence type I osteogenesis imperfecta.19 The patient had no past history of metabolic bone disease other than osteogenesis imperfecta, and had never taken medicine that affected bone metabolism. He was a current smoker, but he did not have any other clinical risk factors for fractures, such as a maternal history of hip fractures, an alcohol consumption ≥ 2 units daily, age ≥ 75 years, body mass index ≤ 18.5 kg/m2, or a history of steroid use.20
Table 1.
Baseline characteristics of the patient
| Normal ranges | ||
|---|---|---|
| Age (year) | 36 | |
| Height (m) | 1.70 | |
| Body weight (kg) | 62.5 | |
| Body mass index (kg/m2) | 21.6 | |
| Lumbar spine BMD (g/cm2) | 0.562 | |
| % YAM in BMD | 54 | ≧ 80 |
| Serum | ||
| Calcium (mg/dL) | 9.8 | 8.4–10.2 |
| Phosphorus (mg/dL) | 3.9 | 2.5–4.5 |
| ALP (IU/L) | 359 | 100–340 |
| Intact PTH (pg/mL) | 18 | 10–66 |
| Urine | ||
| NTX (nmom BCE/mmol Cr) | 50.5 | 9.3–54.3* |
Note:
Reference range of healthy premenopausal women.
Abbreviations: BMD, bone mineral density; YAM, young adult mean; ALP, alkaline phosphatase; PTH, parathyroid hormone; NTX, cross-linked N-terminal telopeptides of type I collagen; BCE, bone collagen equivalent; Cr, creatinine.
To understand the pathogenesis of bone fragility and to determine an effective pharmacological treatment, radiographs of the thoracic and lumbar spine were taken, BMD was measured, and biochemical analyses were performed. Radiographs of the thoracic and lumbar spine showed the existence of morphometric vertebral fractures (at the 6th, 8th, and 10th thoracic vertebrae and the 2nd lumbar vertebra, Figure 1). Table 1 shows baseline BMD and biochemical markers. Lumbar spine (L1–L4) BMD measured using dual-energy x-ray absorptiometry with a Hologic QDR 1500 W device (Bedford, MA) was 0.562 g/cm2, corresponding to 54% of the young adult mean. The patient was diagnosed as having osteoporosis according to the Japanese criteria,21,22 ie, BMD > 70% of the young adult mean or 70%–80% of the young adult mean and a history of osteoporotic fractures. Serum calcium and phosphorus levels, measured using standard laboratory techniques, and the serum intact parathyroid hormone level, measured using a chemiluminescent immunoassay, were within the normal ranges. The serum alkaline phosphatase level, measured using standard laboratory techniques, and the urinary cross-linked N-terminal telopeptides of type I collagen (NTX) level (normal range 9.3–54.3 nmol bone collagen equivalent/mmol creatinine),23 measured using an enzyme-linked immunosorbent assay, were high above and within the normal ranges, respectively. Blood examinations revealed no other abnormalities. The active form of vitamin D3, vitamin K2, intramuscular elcatonin, and etidronate were available for the treatment of osteoporosis at that time in Japan.
Figure 1.

Radiographs of thoracic and lumbar spine.
Notes: Lateral views before treatment. Radiographs of thoracic and lumbar spine before treatment showed the existence of morphometric vertebral fractures at the 6th, 8th, and 10th thoracic vertebrae and the 2nd lumbar vertebra.
Abbreviation: T12, 12th thoracic spine.
Outcome of 11 years of treatment
Treatment with cyclical etidronate 200 mg daily for 2 weeks every 3 months plus alfacalcidol 1 μg daily was initiated. In Japan, nutritional calcium and vitamin D supplements are not widely available, and active vitamin D hormones, such as alfacalcidol, are used as an alternative. Nitrogen-containing bisphosphonates like alendronate and risedronate or anabolic agents like teriparatide were not available at the time when etidronate treatment was initiated. Daily (5 mg) and weekly (35 mg) alendronate became available one and 5 years after the start of etidronate treatment, respectively. The doses indicated in the parentheses above are the doses used in Japan for the treatment of postmenopausal women with osteoporosis and have been recognized as being safe and effective.24–27 BMD at the lumbar spine, serum calcium, phosphorus, alkaline phosphatase, and intact parathyroid hormone levels were evaluated every year during the 11-year period of treatment. The urinary NTX level was only measured once at one year after the start of treatment because of health insurance limitations. Radiographs of the thoracic and lumbar spine were also taken after 11 years of treatment to identify new vertebral fractures.
The patient’s chronic back pain decreased significantly at one year after the start of treatment with etidronate plus alfacalcidol. Figure 2 shows the longitudinal changes in lumbar spine BMD during the 11-year period of treatment. The lumbar spine BMD increased at one year after the start of etidronate plus alfacalcidol treatment but decreased at 2 years. Treatment was switched from etidronate plus alfacalcidol to daily alendronate plus alfacalcidol because of the possibility of development of generalized osteomalacia after long-term treatment with etidronate,28 and this treatment was continued for 3 years. Daily alendronate was then switched to weekly alendronate, and weekly alendronate plus alfacalcidol treatment was continued for 6 years. The increase in lumbar spine BMD from baseline was 2.8% at one year, 2.1% at 2 years, 4.6% at 3 years, 4.6% at 4 years, 5.0% at 5 years, 5.0% at 6 years, 5.3% at 7 years, 5.9% at 8 years, 6.4% at 9 years, 6.2% at 10 years, and 6.4% at 11 years.
Figure 2.

Changes in lumbar spine BMD during the 11-year period of treatment.
Notes: Treatment with cyclical etidronate plus alfacalcidol was initiated. The lumbar spine BMD increased at one year after the start of etidronate plus alfacalcidol treatment but decreased at 2 years. Treatment was switched from etidronate plus alfacalcidol to alendronate plus alfacalcidol, and this treatment was continued for 9 years. The increase in lumbar spine BMD from baseline was 2.8% at one year, 2.1% at 2 years, 4.6% at 3 years, 4.6% at 4 years, 5.0% at 5 years, 5.0% at 6 years, 5.3% at 7 years, 5.9% at 8 years, 6.4% at 9 years, 6.2% at 10 years, and 6.4% at 11 years.
Abbreviations: BMD, bone mineral density; ETD, etidronate; ALN, alendronate.
The urinary NTX level decreased from 50.5 to 22.8 nmol BCE/mmol Cr after one year of treatment. The reduction in urinary NTX level was 58.5%. Figure 3 shows the longitudinal changes in serum calcium, phosphorus, alkaline phosphatase, and intact parathyroid hormone levels during the 11-year period of treatment. The serum alkaline phosphatase level decreased at 1 and 2 years after the start of etidronate plus alfacalcidol treatment and was sustained thereafter for 9 years. The reduction in the serum alkaline phosphatase level was 16.2% at 1 year, 24.2% at 2 years, 23.7% at 3 years, 26.5% at 4 years, 26.7% at 5 years, 24.2% at 6 years, 20.6% at 7 years, 21.2% at 8 years, 21.7% at 9 years, 21.2% at 10 years, and 20.3% at 11 years. Serum calcium, phosphorus, and intact parathyroid hormone levels stayed within the normal ranges during the 11-year period of treatment. Table 2 shows the patient data after 11 years of treatment.
Figure 3.
Changes in biochemical markers during the 11-year period of treatment.
Notes: The patient was treated with cyclical etidronate plus alfacalcidol for 2 years and alendronate plus alfacalcidol for 9 years. The serum alkaline phosphatase level decreased at one and 2 years after the start of etidronate plus alfacalcidol treatment and was sustained thereafter for 9 years. The reduction in the serum alkaline phosphatase level was 16.2% at one year, 24.2% at 2 years, 23.7% at 3 years, 26.5% at 4 years, 26.7% at 5 years, 24.2% at 6 years, 20.6% at 7 years, 21.2% at 8 years, 21.7% at 9 years, 21.2% at 10 years, and 20.3% at 11 years. The serum calcium, phosphorus, and intact parathyroid hormone levels stayed within the normal ranges during the 11-year period of treatment. A: Calcium, B: Phosphorus, C: ALP, D: intact PTH.
Abbreviations: ALP, alkaline phosphatase; PTH, parathyroid hormone; ETD, etidronate; ALN, alendronate.
Table 2.
Patient data after 11 years of treatment
| Baseline | After 11 years | Percent changes | |
|---|---|---|---|
| Age (year) | 36 | 47 | |
| Height (m) | 1.70 | 1.68 | −1.2 |
| Body weight (kg) | 62.5 | 59.8 | −4.3 |
| Bone mass index (kg/m2) | 21.6 | 21.2 | −1.9 |
| Lumbar spine BMD (g/cm2) | 0.562 | 0.598 | +6.4 |
| Serum | |||
| Calcium (mg/dL) | 9.8 | 9.1 | −7.1 |
| Phosphorus (mg/dL) | 3.9 | 3.9 | 0.0 |
| ALP (IU/L) | 359 | 286 | −20.3 |
| Intact PTH (pg/mL) | 18 | 17 | −5.6 |
Abbreviations: BMD, bone mineral density; YAM, young adult mean; ALP, alkaline phosphatase; PTH, parathyroid hormone.
Radiographs of the thoracic and lumbar spine revealed the existence of morphometric vertebral fractures (at the 5th, 6th, 8th, 9th, and 10th thoracic vertebrae and the 2nd lumbar vertebra) after 11 years of treatment (Figure 4), suggesting the occurrence of new morphometric vertebral fractures (at the 5th and 9th thoracic vertebrae). During the 11-year period of treatment, three nonvertebral osteoporotic fractures occurred at two ribs and at the metacarpus. The patient experienced a 2 cm height loss after 11 years of treatment.
Figure 4.
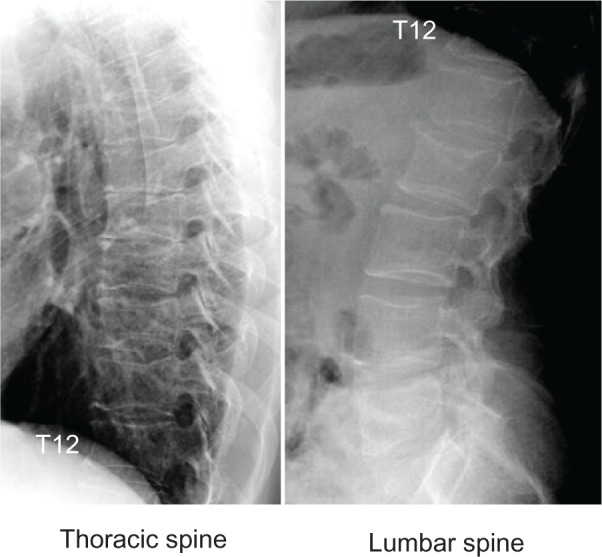
Radiographs of thoracic and lumbar spine.
Notes: Lateral views after 11 years of treatment. After 11 years of treatment, radiographs of the thoracic and lumbar spine revealed the existence of morphometric vertebral fractures (at the 5th, 6th, 8th, 9th, and 10th thoracic vertebrae and the 2nd lumbar vertebra), suggesting the occurrence of new morphometric vertebral fractures (at the 5th and 9th thoracic vertebrae).
Abbreviation: T12, 12th thoracic spine.
No adverse events, including hypercalcemia (alfacalcidol) and upper gastrointestinal tract symptoms, osteonecrosis of the jaw,29 or atypical fracture of the femur30 (bisphosphonates) were observed.
Discussion
Osteogenesis imperfecta is a congenital disease in which the main characteristic is bone fragility. Although fragile fractures occur frequently in children with osteogenesis imperfecta, the fracture rate decreases after adolescence because of the influences of sex hormones and maturation.1,2 Thus, the clinical stability of osteogenesis imperfecta is usually observed with age, and this is an important characteristic of the disease. The patient in this case report had been experiencing frequent fragile fractures, and the rate of these fractures, especially at the long bones, decreased with maturation. However, chronic back pain possibly associated with spinal osteoporosis and subsequent vertebral fractures persisted. Thus, effective treatment for this condition was considered to be necessary.
The osteoporosis in the present patient was surmised to be associated with a high bone turnover based on a high serum alkaline phosphatase level. Therefore, etidronate, an antiresorptive agent, was considered to be the first-line medicine at the time when treatment was initiated. Because combined treatment with bisphosphonates and alfacalcidol is more effective in increasing BMD or preventing fractures than single treatment with bisphosphonates alone,16–18 alfacalcidol was used in combination with bisphosphonates. As a result, the urinary NTX level decreased by 58.8% after one year of treatment, and the lumbar spine BMD increased by 6.4% following a 20.3% reduction in the serum alkaline phosphatase level. Studies in men with osteoporosis showed that etidronate treatment for 31 (range 18–45) months increased the lumbar spine BMD by 3.2% annually, while alendronate treatment for one year increased the lumbar spine BMD by 5.8%.31,32 Three clinical fractures occurred at two ribs and the metacarpus, and two morphometric vertebral fractures occurred at the thoracic spine during the 11-year treatment period. Furthermore, the patient experienced a 2 cm height loss. However, major nonvertebral fractures, including the hip, distal radius, and proximal humerus, did not occur. Whether the treatment applied in the patient was effective remains uncertain.
A histomorphometric study showed that osteoporosis in an adult man with osteogenesis imperfecta was associated with increased bone turnover according to bone marker measurements and increased bone resorption and decreased osteoblastic activity at the tissue level.33 The influence of bisphosphonates on osteoblast activity was a concern. Thus, anabolic agents such as teriparatide, which is now available in Japan, might be more effective for increasing the lumbar spine BMD and preventing fractures. However, the effectiveness of teriparatide has not been established in patients with osteogenesis imperfecta. Further studies are needed to confirm the effectiveness of teriparatide in adults with osteogenesis imperfecta.
Chronic back pain was significantly decreased by etidronate plus alfacalcidol treatment in the present patient with osteoporosis and morphometric vertebral fractures. However, the mechanism responsible for the pain relief effect of bisphosphonates remains uncertain. One possible mechanism may involve bone resorption, because the efficacy of bisphosphonates for bone pain in patients with avascular necrosis of the hip, osteoporotic vertebral fractures, skeletal metastases, or Paget’s disease of the bone has been demonstrated.34–38 Other possibilities may involve neuropeptides that are active during pain transmission, such as substance P and calcitonin gene-related peptide, and inflammatory cytokines, such as tumor necrosis factor-α.39–42 Bisphosphonates may suppress the production of substance P, calcitonin gene-related peptide, and tumor necrosis factor-α,43,44 possibly reducing back pain.
How long patients with osteoporosis may continue to receive bisphosphonate treatment remains to be established. The prolonged suppression of remodeling is associated with accumulation of microdamage, advanced glycation products, and increased tissue mineral density, while stopping treatment results in the re-emergence of remodeling.45 Seeman inferred that stopping antiresorptive treatment was more likely to do net harm than continuing the treatment.45 Thus, antiresorptive treatment should be continued as long as possible in patients with a risk of fractures. Patients with a low BMD and prevalent vertebral fractures after 5 years of alendronate treatment are recommended to continue treatment for more than 5 years.46–48 The present patient continued treatment with bisphosphonates and alfacalcidol for 11 years. However, no adverse events, including hypercalcemia, upper gastrointestinal tract symptoms, osteonecrosis of the jaw, and atypical fractures of the femur, were observed, suggesting the safety of long-term treatment with bisphosphonates plus alfacalcidol.
Conclusion
This case report showed the long-term outcome and safety of bisphosphonate plus alfacalcidol treatment in a man with osteogenesis imperfecta type I. However, three clinical nonvertebral fractures and two morphometric vertebral fractures occurred during the 11-year treatment period. Thus, it would be of interest to examine the effectiveness of anabolic agents like teriparatide on BMD and the fracture incidence in men with osteogenesis imperfecta, because osteogenesis imperfecta is basically an inheritable disorder of bone formation that results in bone fragility.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.King JD, Bobechko WP. Osteogenesis imperfecta. An orthopaedic description and surgical review. J Bone Joint Surg Br. 1971;53:72–89. [Google Scholar]
- 2.Albright JA, Grunt JA. Studies of patients with osteogenesis imperfecta. Bone Joint Surg Am. 1971;53:1415–1425. [PubMed] [Google Scholar]
- 3.Baron R, Gertner JM, Lang R, et al. Increased bone turnover with decreased bone formation by osteoblasts in children with osteogenesis imperfecta tarda. Pediatr Res. 1983;17:204–207. doi: 10.1203/00006450-198303000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Riley FC, Jowsey J, Brown DM. Osteogenesis imperfecta: morphologic and biochemical studies of connective tissue. Pediatr Res. 1973;7:757–768. doi: 10.1203/00006450-197309000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Rauch F, Travers R, Parfitt AM, et al. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone. 2000;26:581–589. doi: 10.1016/s8756-3282(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 6.Brenner RE, Vetter U, Bollen AM, et al. Bone resorption assessed by immunoassay of urinary cross-linked collagen peptides in patients with osteogenesis imperfecta. J Bone Miner Res. 1994;9:993–997. doi: 10.1002/jbmr.5650090706. [DOI] [PubMed] [Google Scholar]
- 7.Astrom E, Soderhall S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child. 2002;86:356–364. doi: 10.1136/adc.86.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glorieux FH, Bishop NJ, Plotkin H, et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 9.Glorieux FH. Bisphosphonate therapy for severe osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2000;13:989–992. [PubMed] [Google Scholar]
- 10.Gonzalez E, Pavia C, Ros J, et al. Efficacy of low dose schedule pamidronate infusion in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2001;14:529–533. doi: 10.1515/jpem.2001.14.5.529. [DOI] [PubMed] [Google Scholar]
- 11.Lee YS, Low SL, Lim LA, et al. Cyclic pamidronate infusion improves bone mineralisation and reduces fracture incidence in osteogenesis imperfecta. Eur J Pediatr. 2001;160:641–644. doi: 10.1007/s004310100844. [DOI] [PubMed] [Google Scholar]
- 12.Plotkin H, Rauch F, Bishop NJ, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000;85:1846–1850. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 13.Ward LM, Rauch F, Whyte MP, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96:355–364. doi: 10.1210/jc.2010-0636. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto J, Matsu K, Takeda T, et al. Effects of treatment with etidronate and alfacalcidol for osteogenesis imperfecta type I: a case report. J Orthop Sci. 2003;8:243–247. doi: 10.1007/s007760300042. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto J, Takeda T, Sato Y. Effect of treatment with alendronate in osteogenesis imperfecta type I: a case report. Keio J Med. 2004;53:251–255. doi: 10.2302/kjm.53.251. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto J, Takeda T, Ichimura S, et al. Effects of cyclical etidronate with alfacalcidol on lumbar bone mineral density, bone resorption, and back pain in postmenopausal women with osteoporosis. J Orthop Sci. 2003;8:532–537. doi: 10.1007/s00776-003-0655-5. [DOI] [PubMed] [Google Scholar]
- 17.Orimo H, Nakamura T, Fukunaga M, et al. Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: the Japanese Osteoporosis Intervention Trial (JOINT)-02. Curr Med Res Opin. 2011;27:1273–1284. doi: 10.1185/03007995.2011.580341. [DOI] [PubMed] [Google Scholar]
- 18.Ringe JD, Farahmand P, Schacht E, et al. Superiority of a combined treatment of alendronate and alfacalcidol compared to the combination of alendronate and plain vitamin D or alfacalcidol alone in established postmenopausal or male osteoporosis (AAC-Trial) Rheumatol Int. 2007;27:425–434. doi: 10.1007/s00296-006-0288-z. [DOI] [PubMed] [Google Scholar]
- 19.Sillence DO. Osteogenesis imperfecta nosology and genetics. Ann NY Acad Sci. 1988;543:1–15. doi: 10.1111/j.1749-6632.1988.tb55311.x. [DOI] [PubMed] [Google Scholar]
- 20.Orimo H. [Japanese guideline for prevention and treatment of osteoporosis] Life Science. 2011 Japanese. [Google Scholar]
- 21.Orimo H, Sugioka Y, Fukunaga M, et al. Diagnostic criteria of primary osteoporosis. J Bone Miner Metab. 1998;16:139–150. [Google Scholar]
- 22.Orimo H, Hayashi Y, Fukunaga M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 23.Nishizawa Y, Nakamura T, Ohta H, et al. Committee on the Guidelines for the Use of Biochemical Markers of Bone Turnover in Osteoporosis Japan Osteoporosis Society. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004) J Bone Miner Metab. 2005;23:97–104. doi: 10.1007/s00774-004-0547-6. [DOI] [PubMed] [Google Scholar]
- 24.Kushida K, Shiraki M, Nakamura T, et al. The efficacy of alendronate in reducing the risk of vertebral fracture in Japanese patients with osteoporosis: A randomized, double-blind, active-controlled, double-dummy trial. Curr Ther Res Clin Exp. 2002;63:606–620. [Google Scholar]
- 25.Kushida K, Shiraki M, Nakamura T, et al. Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study. J Bone Miner Metab. 2004;22:462–468. doi: 10.1007/s00774-004-0508-0. [DOI] [PubMed] [Google Scholar]
- 26.Shiraki M, Kushida K, Fukunaga M, et al. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. Osteoporos Int. 1999;10:183–192. doi: 10.1007/s001980050214. [DOI] [PubMed] [Google Scholar]
- 27.Uchida S, Taniguchi T, Shimizu T, et al. Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab. 2005;23:382–388. doi: 10.1007/s00774-005-0616-5. [DOI] [PubMed] [Google Scholar]
- 28.Cortet B, Béra-Louville A, Gauthier P, et al. Comparative efficacy and safety study of etidronate and alendronate in postmenopausal osteoporosis. Effect of adding hormone replacement therapy. Joint Bone Spine. 2001;68:410–415. doi: 10.1016/s1297-319x(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 29.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 30.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 31.Anderson FH, Francis RM, Bishop JC, et al. Effect of intermittent cyclical disodium etidronate therapy on bone mineral density in men with vertebral fractures. Age Ageing. 1997;26:359–365. doi: 10.1093/ageing/26.5.359. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto J, Takeda T, Sato Y, et al. Comparison of the effect of alendronate on lumbar bone mineral density and bone turnover in men and postmenopausal women with osteoporosis. Clin Rheumatol. 2007;26:161–167. doi: 10.1007/s10067-006-0252-z. [DOI] [PubMed] [Google Scholar]
- 33.Iwamoto J, Takeda T, Ichimura S. Increased bone resorption with decreased activity and increased recruitment of osteoblasts in osteogenesis imperfecta type I. J Bone Miner Metab. 2002;20:174–179. doi: 10.1007/s007740200025. [DOI] [PubMed] [Google Scholar]
- 34.Agarwala S, Sule A, Pai BU, et al. Alendronate in the treatment of avascular necrosis of the hip. Rheumatology (Oxford) 2002;41:346–347. doi: 10.1093/rheumatology/41.3.346-a. [DOI] [PubMed] [Google Scholar]
- 35.Gangji V, Appelboom T. Analgesic effect of intravenous pamidronate on chronic back pain due to osteoporotic vertebral fractures. Clin Rheumatol. 1999;18:266–267. doi: 10.1007/s100670050099. [DOI] [PubMed] [Google Scholar]
- 36.Glover D, Lipton A, Keller A, et al. Intravenous pamidronate disodium treatment of bone metastases in patients with breast cancer. A doseseeking study. Cancer. 1994;74:2949–2955. doi: 10.1002/1097-0142(19941201)74:11<2949::aid-cncr2820741110>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med. 1996;335:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 38.Siris ES, Chines AA, Altman RD, et al. Risedronate in the treatment of Paget’s disease of bone: an open label, multicenter study. J Bone Miner Res. 1998;13:1032–1038. doi: 10.1359/jbmr.1998.13.6.1032. [DOI] [PubMed] [Google Scholar]
- 39.Brown MF, Hukkanen MV, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147–153. doi: 10.1302/0301-620x.79b1.6814. [DOI] [PubMed] [Google Scholar]
- 40.Hartman JM, Berger A, Baker K, et al. POWER (Premenopausal, Osteoporosis, Women, Alendronate, Depression) Study Group. Quality of life and pain in premenopausal women with major depressive disorder: the POWER study. Health Qual Life Outcomes. 2006;4:2. doi: 10.1186/1477-7525-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohtori S, Inoue G, Koshi T, et al. Sensory innervation of lumbar vertebral bodies in rats. Spine (Phila Pa 1976) 2007;32:1498–1502. doi: 10.1097/BRS.0b013e318067dbf8. [DOI] [PubMed] [Google Scholar]
- 42.Ohtori S, Inoue G, Koshi T, et al. Characteristics of sensory dorsal root ganglia neurons innervating the lumbar vertebral body in rats. J Pain. 2007;8:483–488. doi: 10.1016/j.jpain.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi M, Franchi S, Ferrario P, et al. Effects of the bisphosphonate ibandronate on hyperalgesia, substance P, and cytokine levels in a rat model of persistent inflammatory pain. Eur J Pain. 2008;12:284–292. doi: 10.1016/j.ejpain.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Orita S, Ohtori S, Koshi T, et al. The effects of risedronate and exercise on osteoporotic lumbar rat vertebrae and their sensory innervation. Spine. 2010;35:1974–1982. doi: 10.1097/BRS.0b013e3181d5959e. [DOI] [PubMed] [Google Scholar]
- 45.Seeman E. To stop or not to stop, that is the question. Osteoporos Int. 2009;20:187–195. doi: 10.1007/s00198-008-0813-x. [DOI] [PubMed] [Google Scholar]
- 46.Compstom JE, Bilezikian JP. Bisphosphonate therapy for osteoporosis: the long and short of it. J Bone Miner Res. 2012;27:240–242. doi: 10.1002/jbmr.1542. [DOI] [PubMed] [Google Scholar]
- 47.Whitaker M, Guo J, Kehoe T, et al. Bisphosphonates for osteoporosis – where do we go from here? N Engl J Med. 2012;366:2048–2051. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
- 48.Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis – for whom and for how long? N Engl J Med. 2012;366:2051–2503. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]



