Abstract
Treg activation in response to environmental cues is necessary for regulatory T cells (Tregs) to suppress inflammation, but little is known about the transcription mechanisms controlling Treg activation. We report that despite the known proinflammatory role of the chromatin-remodelling factor BRG1 in CD4 cells, deleting Brg1 in all αβ T cell lineages led to fatal inflammation, which reflected essential roles of BRG1 in Tregs. Brg1 deletion impaired Treg activation, concomitant with the onset of the inflammation. Remarkably, as the inflammation progressed, Tregs became increasingly activated, but the activation levels could not catch up with the severity of inflammation. In vitro assays indicate that BRG1 regulates a subset of TCR target genes including multiple chemokine receptor genes. Finally, using a method that can create littermates bearing either a tissue-specific point mutation or deletion, we found the BRG1 ATPase activity partially dispensable for BRG1 function. Collectively, these data suggest that BRG1 acts in part via remodelling-independent functions to sensitize Tregs to inflammatory cues, thus allowing Tregs to promptly and effectively suppress autoimmunity.
Keywords: activation, brg1, immune, tolerance, Treg
Introduction
FoxP3+ regulatory T cells (Tregs) are potent suppressors of inflammatory responses (Sakaguchi et al, 2008; Benoist and Mathis, 2012). Precise regulation of Treg activity is essential for preventing autoimmunity while simultaneously permitting adequate immune response to pathogens. Various Treg properties are subject to regulation, including activation, migration, homoeostasis and functional specialization (Campbell and Koch, 2011; Yamaguchi et al, 2011; Josefowicz et al, 2012). This regulation is first suggested by the observation that normal mice harbour both naïve-like and effector/memory-like Treg subsets, the former homing to secondary lymphoid organs while the latter express activation markers (such as CD69 and ICOS) and traffic to non-lymphoid tissues (Huehn et al, 2004). Tregs produced from the thymus are all naïve-like, but a subset of them quickly acquires the effector/memory-like phenotype after entering secondary lymphoid organs, which occurs as a result of encounters with self-antigens and is presumably essential for peripheral tolerance (Lee et al, 2007). Tregs are subject to an additional layer of regulation in the presence of overt inflammation (due to, e.g., pathogen infection or organ transplant), which can involve Treg activation, expansion, differentiation and trafficking (Belkaid et al, 2002; Suffia et al, 2006; Belkaid and Tarbell, 2009; Zhang et al, 2009). Treg activation is crucial for Treg function both in vitro and in vivo (Takahashi et al, 1998; Thornton and Shevach, 1998; Park et al, 2010).
The transcription programs controlling such intricate Treg properties are under intense investigation. The best-defined transcription factor in Tregs is FOXP3, which is required for Treg development, proliferation, suppressor function and lineage stability; loss-of-function mutations in FoxP3 results in early-onset, aggressive, lethal inflammation in both humans and mice (Rudensky, 2011). FOXP3 acts by stabilizing and amplifying the expression of immunosuppressive genes while repressing proinflammatory genes. A number of other sequence-specific transcription factors have also been implicated in Treg development and function. These include the factors that promote FoxP3 expression during development or homoeostasis (such as FOXO, NF-kb, GATA3 and FOXP3 itself) and those that direct Treg functional specialization in response to inflammatory cues (such as T-BET, IRF4 and STAT3 selectively required for Tregs to suppress Th1-, Th2- and Th17-type inflammation, respectively) (Chaudhry et al, 2009; Koch et al, 2009; Long et al, 2009; Zheng et al, 2009; Kerdiles et al, 2010; Ouyang et al, 2010; Zheng et al, 2010; Ouyang and Li, 2011; Wang et al, 2011). Much less is known regarding the transcription programme that controls Treg activation, even though Treg activation is essential for Treg function. Available data indicate that the activation mechanisms in Tregs are divergent from conventional CD4 cells. For example, contrary to conventional CD4 cells, Tregs do not proliferate upon TCR stimulation alone; IL-2 is additionally required (Campbell and Ziegler, 2007). Furthermore, while TCR stimulation of Tregs is obligatory for Treg-suppressive function in vitro, pharmacological inhibition of multiple signal transduction pathways known to be downstream of TCR in conventional CD4 cells fails to disable Tregs (Hagness et al, 2012). Furthermore, in conventional CD4 cells, NFAT is localized in the cytoplasm in resting cells and moves to the nucleus upon TCR stimulation, where it drives T-cell activation and proliferation. In contrast, in Tregs, a fraction of NFAT is constitutively nuclear, and calcium influx is dispensable for Treg proliferation induced by TCR/IL-2 stimulation (Li et al, 2012a). Finally, we found that resting Tregs expressed detectable amounts of c-Fos and c-Jun messages, which was unexpectedly downregulated within 2 h following TCR stimulation (unpublished).
To regulate gene expression, sequence-specific transcription factors must act in conjunction with enzymes that modulate chromatin structure. Such enzymes fall into two major classes: histone-modifying enzymes that covalently modify histones to alter chromatin structure, and ATP-dependent chromatin remodellers that use energy of ATP hydrolysis to physically disrupt histone–DNA contact, thus loosening or moving nucleosomes (Narlikar et al, 2002; Clapier and Cairns, 2009). The prototypical mammalian chromatin remodeler is the BAF chromatin-remodelling complex related to the yeast Swi/Snf complex (Wang, 2003; Chi, 2004). The BAF complex contains ∼10 subunits, including the catalytic subunit BRG1. BRG1 is widely expressed, but its function is tissue-specific. For example, whereas BRG1 regulates the survival and CD4/CD8 expression in early thymocytes (Chi et al, 2002, 2003; Gebuhr et al, 2003; Jani et al, 2008), it promotes Th1/Th2 differentiation of conventional CD4 cells (Zhang and Boothby, 2006; Wurster and Pazin, 2008). Genome-wide mapping of BRG1-binding sites in conventional CD4 cells demonstrates that BRG1 often binds enhancer/promoters, and the binding patterns at some target genes vary according to the status of T-cell activation and/or effector lineage differentiation (De et al, 2011). Interestingly, using a genetic strategy that can produce littermates bearing either thymocyte-specific Brg1 deletion or a BRG1 ATPase point mutation (PM) that abolishes its chromatin-remodelling activity, we found that the BRG1 PM cannot fully recapitulate the defects in early T-cell development caused by Brg1 deletion. The data indicate that BRG1 harbours remodelling-independent activities sufficient for regulating the expression of some target genes in early thymocytes, but it is unclear whether such activities are of general importance (Jani et al, 2008). The role of BRG1 in Tregs is unknown. Here we report that BRG1 is essential for efficient Treg activation, and that BRG1 function is partially independent of its ATPase activity.
Results
Deletion of Brg1 from all αβ T-cell lineages results in late-onset inflammation in a fraction of mice
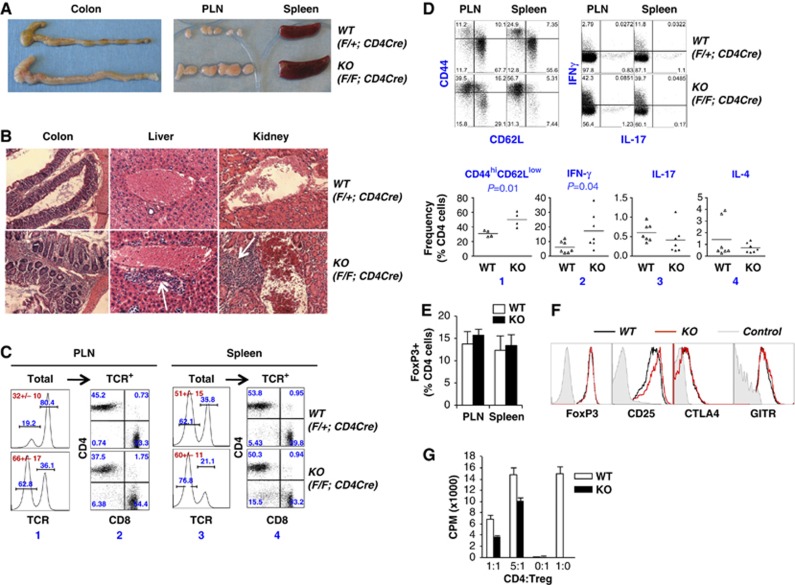
To study the role of Brg1 in T cells, we generated Brg1F/F; CD4Cre mice. PCR analysis confirmed that Brg1 was deleted in DP and peripheral T cells (not shown). Brg1 deletion did not grossly perturb T-cell development (Supplementary Figure S1) and young adults did not show any obvious phenotype. However, ∼20% of the mice eventually developed overt inflammation (by ∼5 months of age) characterized by rectal prolapse, colonic oedema, lymphadenopathy, splenomegaly and perivascular infiltration in multiple organs including liver and kidney (Figure 1A and B). In addition, in these mice, total lymphocyte count approximately doubled in peripheral lymph nodes (PLN) largely due to accumulation of B cells (Figure 1C, column 1), while the abundance of CD4 relative to CD8 cells remained unaltered (Figure 1C, column 2). B cells also accumulated in the spleen, albeit to a less extent (Figure 1C, column 3). Finally, effector/memory CD4 cells accumulated in the periphery and CD4 cells overproduced IFN-γ but not IL-4 or IL-17 (Figure 1D). By ∼7 months of age, these 20% of mice either died spontaneously or were euthanized for humane concerns. In contrast, the remaining 80% of the mice remained healthy.
Figure 1.
Phenotype of Brg1F/F; CD4Cre mice (KO) versus Brg1-sufficient littermates (WT). (A–D) Inflammatory disorder in ∼5-month-old KO mice. (A) Gross appearance of colon, peripheral LN and spleen. (B) H & E staining of tissue sections of colon, liver and kidney. Arrows indicate foci of cell infiltration. (C) Cell composition of LN and spleen. Cells were stained for CD4, CD8 and TCR before TCR+ cells were analysed for CD4/CD8 expression. The numbers in red within the plots are total cellularity (+/− s.d.) averaged from at least three mice. (D) Lymphocytes were either stained for CD4, CD44 and CD62L cells to quantify the abundance of effector/memory (CD44high CD62Llow) CD4 cells (top left), or stimulated with PMA and ionomycin for 4–6 h for the analysis of cytokine induction in CD4 cells (top right). The scatter plot at the bottom summarizes the data from splenic CD4 cells. (E–G) No apparent defects in Brg1 KO Tregs. (E) Treg frequencies in ∼5-month-old mice. The data are averaged from five mice. (F) Expression of Treg markers in ∼5-month-old mice. (G) Brg1 KO did not impair the ability of Tregs to suppress CD4 cell proliferation in vitro. Tregs were isolated from ∼4-week-old Brg1F/F; CD4Cre mice. Naïve CD4 cells from WT mice were co-cultured with equal numbers of or five-fold less Tregs in the presence of mitogenic stimuli. Proliferation of CD4 cells was measured by H3-thymidine incorporation. Data are averaged from five independent experiments.
Brg1 is not essential for Treg development or in vitro suppression
Given the known roles of Brg1 in promoting Th1/Th2 differentiation, it was unexpected that Brg1 deletion in T cells would lead to hyperinflammation rather than immune deficiency. We reasoned that since Brg1 deletion in DP cells using the CD4Cre transgene depleted BRG1 not only in conventional T cells, which would impair immune responses, but also in Tregs, which might impair Treg development/function and enhance the immune responses, Brg1 deletion in all T cells might have conflicting effects on inflammation, with the balance tipped towards hyperinflammation in some mice. Thus, we examined Tregs in Brg1F/F; CD4Cre mice. Tregs were present in normal numbers and expressed normal levels of FOXP3, CD25, GITR and CTLA4 (Figure 1E and F), suggesting Brg1 was dispensable for Treg development. To determine whether Brg1 KO Tregs were functionally defective, we measured the ability of Tregs to inhibit the proliferation of CD4 cells in vitro. To avoid potential confounding effects of hyperinflammation on Treg function, Brg1 KO Tregs were isolated from ∼4-week-old Brg1F/F; CD4Cre mice lacking overt inflammation. Tregs were co-cultured with naïve WT CD4 cells in the presence of irradiated antigen-presenting cells and anti-CD3, and the proliferation measured by H3-thymidine incorporation. As expected, Tregs did not proliferate while CD4 cells showed robust proliferation, incorporating high amounts (∼5000, c.p.m.) of H3-thymidine, which was inhibited by WT Tregs in a dose-dependent manner (Figure 1G, white bars). Importantly, Brg1 KO Tregs were even more effective than WT Tregs in suppressing CD4 cell proliferation in this assay (Figure 1G, black bars), indicating the inflammation in vivo is not due to a defect in Treg-suppressive function as measured by this assay. Thus, despite the inflammation in Brg1F/F; CD4Cre mice, there was no obvious defect in Treg development or function.
Treg-specific Brg1 deletion produces early-onset, aggressive, fatal inflammation
To further explore the mechanism of autoimmunity in Brg1F/F; CD4Cre mice, we deleted Brg1 selectively in Tregs. Without the antagonizing effects of Brg1 deletion in conventional effector T cells, the Treg-specific deletion should produce a more severe phenotype than that in Brg1F/F; CD4Cre mice, if the inflammation in Brg1F/F; CD4Cre mice indeed reflected a role of Brg1 in Tregs.
To delete Brg1 in Tregs, we crossed Brg1F/F mice to a Cre-deleter line bearing an Ires-Cre-YFP cassette knocked into the 3′ UTR of the FoxP3 locus, which causes Cre-YFP fusion protein to be co-expressed with FOXP3 (Rubtsov et al, 2008). Since FoxP3 is located on the X-chromosome, which undergoes random inactivation, Brg1 was deleted in all Tregs only in Brg1F/F; FoxP3YFP-Cre hemizygous males and Brg1F/F; FoxP3YFP-Cre/YFP-Cre homozygous females (termed ‘KO’ thereafter), whereas the Brg1F/F;FoxP3YFP-Cre/YFP-Cre/+ heterozygous females were mosaic, containing not only YFP+ but also YFP− Tregs and were thus healthy (see further).
We found that the KO mice invariably developed an early-onset, aggressive, fatal inflammatory disorder more severe than that in Brg1F/F; CD4Cre mice and reminiscent of that seen in Scurfy mice bearing a germline FoxP3 mutation that blocks Treg development (Godfrey et al, 1991). Specifically, starting as early as 2 weeks of age, the mice frequently began to show appreciable runting, blepharitis, alopecia, increased abdominal girth, palpable lymph nodes and pale foot pads, which became increasingly obvious and penetrant with age. Adult mice were severely runted (Figure 2A and D) and 50% of mice died within 50 days after birth, the mortality rate reaching nearly 100% by 200 days (Figure 2C), which is less severe than scurfy mice that died within 3–6 weeks of age in our hands. Liver necrosis was dramatic (Figure 2A), as was lymph nodes and spleen enlargement (Figure 2A and E). Erythrocytes were visibly depleted in both bone marrow and blood, consistent with profound anaemia (Figure 2A and F). Perivascular cellular infiltration was apparent in multiple tissues (Figure 2B) with concordant accumulation of CD8 as well as B and CD4 cells in the liver (Figure 2G, right). In addition, CD8 cells accumulated in the spleen (Figure 2G, middle). The thymi were extremely small and depleted of DP cells, presumably due to distress-induced apoptosis (Figure 2H). Effector/memory CD4 cells dramatically accumulated in spleen and lymph nodes (Figure 3A and C). The CD4 cells of any single mouse overproduced one or more of the three lineage-specific cytokines (IFN-γ, IL-4 and IL-17), which suggests that Brg1 deficiency in Tregs impaired the function of each of the three effector Treg subsets, thus pointing to a fundamental role of Brg1 in Treg function (Figure 3B and C). Finally, CD4 cells were hyperproliferative (Figure 4E). These data confirm that Brg1 is essential for Treg-mediated immune suppression.
Figure 2.
Effects of Treg-specific Brg1 deletion. Brg1F/F; FoxP3YFP-Cre males and Brg1F/F; FoxP3YFP-Cre/YFP-Cre females (KO) were compared with Brg1-sufficient, gender-matched littermates (WT) that carried one or two functional Brg alleles. (A, B) Gross anatomy (A) and histology (B) of ∼30-day-old males. The ears in (A) were from males different from that shown at the top of panel A. The white and red arrows in (A) indicate necrotic spots on liver and enlarge spleen, respectively. (C) Survival of males. (D) Body weight of 40-day-old males. The symbols represent individual mice. (E–H) The weight of spleen relative to that of the whole body (E), haematocrit (F), lymphocyte cellularity (G) and thymocyte composition (H) in ∼30-day-old males. In H, thymocytes were stained with TCRβ, CD4 and CD8 antibodies before the TCR−/lo and TCRhi subsets were analysed for CD4/CD8 expression.
Figure 3.
CD4 cells in 25- to 50-day-old KO mice. (A) Accumulation of effector memory (CD44high CD62Llow) CD4 cells in LN and spleen. (B) Enhanced cytokine induction in CD4 cells stimulated with ionomycin (1 μM) and PMA (1 ng/ml) for 5 h. (C) Summary of data from experiments represented in (A) and (B).
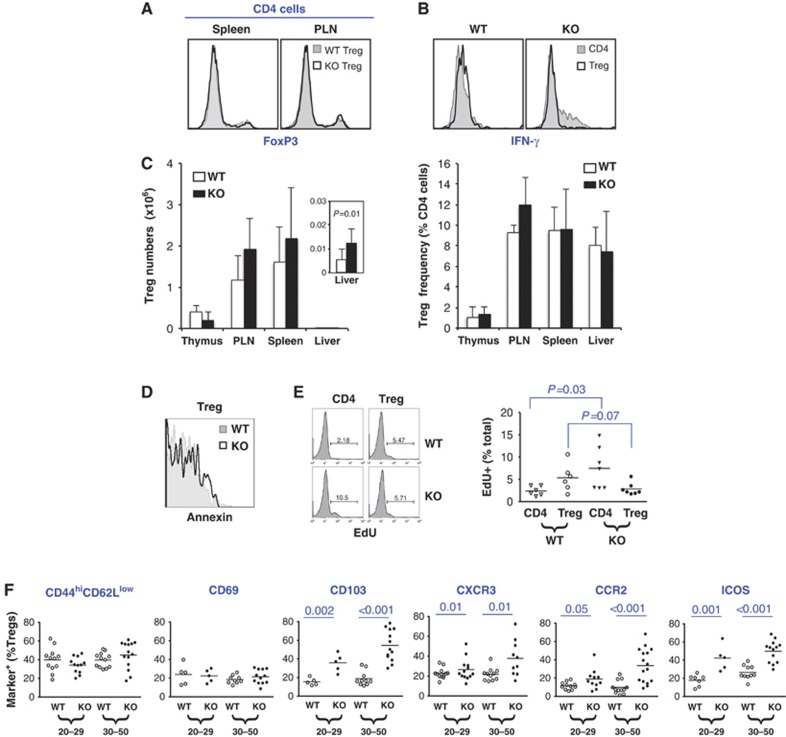
Figure 4.
Tregs in 25- to 50-day-old KO mice. (A) Normal FOXP3 expression. Cells were stained for CD4 and FoxP3 before analysis. (B) KO Tregs did not express IFN-γ in hyperinflammatory environment whereas CD4 cells from the same mouse overproduced IFN-γ as expected (right). Cells were stimulated with ionomycin (1 μM) and PMA (1 ng/ml) for 5 h before staining for intracellular FOXP3 and IFN-γ. (C) Absolute numbers of Tregs (left) and their abundance relative to CD4 cells (right) in various organs. The inset is a blown-up view of the cellularity in the liver. (D) Normal viability. Splenic lymphocytes were stained with CD4APC and annexin–PE before analysis of annexin expression in CD4+YFP+ Tregs. (E) Proliferation. Mice were exposed to the BrdU analogue EdU for 3 days. Splenic conventional CD4 cells (CD4) and Tregs were FACS-purified based on YFP expression before analysis of EdU incorporation. (F) Expression of activation markers (including chemokine receptors) in splenic Tregs. Mice were separated into two age groups (25–29 versus 30–50 days old) based on distinct mortality rates (see Figure 2C).
Brg1 deletion impairs Treg activation in response to self-antigens: analysis of Tregs at different stages of inflammation in mice with Treg-specific Brg1 deletion
We next investigated the mechanisms whereby Brg1 promotes Treg function. We first analysed Tregs in 25–50-day-old Brg1F/F; FoxP3YFP-Cre males, which had developed overt inflammation. We found that Brg1 KO Tregs expressed normal levels of FOXP3 (Figure 4A), lacked aberrant expression of proinflammatory cytokine IFN-γ, IL-4 or IL-17, were not converted to conventional CD4 cells (Figure 4B; data unpublished), and their numbers and frequencies in lymphoid organs were comparable to that in the littermate controls (Figure 4C). Interestingly, there was a two-fold increase in total Treg numbers in the liver, suggesting some Brg1 KO Tregs had migrated to the liver in response to inflammation (Figure 4C, left, inset) although the proportions of the Tregs relative to conventional CD4 cells remained unaltered (Figure 4C, right) due to the aforementioned accumulation of conventional CD4 cells (Figure 2G, right). Finally, Brg1 deficiency did not affect Treg viability (Figure 4D) although Treg proliferation seemed marginally (albeit statistically insignificantly) impaired (from 5.3±3.1 in WT to 2.8±1.4 in KO, P=0.07; Figure 4E). These data do not reveal a clear defect in Brg1 KO Treg.
As Treg activation is essential for Treg function, we examined the activation status of Brg1 KO Tregs by analysing effector/memory-like Tregs (CD44highCD62Llow) and multiple activation markers (CD103, CXCR3, CCR2, ICOS and CD69). Given the severe inflammation, the mutant mice should harbour more activated Tregs, and such cells should become more abundant with age. We therefore analysed both young (day 20–29) and old (day 30–50) mice; both groups had severe inflammation but the young mice were mostly viable while the old ones showed a high mortality rate (Figure 2C). As compared with WT mice, there was no increase in the frequencies of effector/memory-like or CD69+ Tregs even in the old KO mice, suggesting that Brg1 deficiency indeed impaired Treg activation (Figure 4F). However, remaining markers (CD103, CCR2, CXCR3 and ICOS) were all elevated in the KO mice relative to the WT mice (Figure 4F; this upregulation of trafficking molecules is consistent with the aforementioned increase in Treg abundance in the liver shown in Figure 4C, left). The data, however, are difficult to interpret because of the severe inflammation in the KO mice; it is possible that without the inflammation, marker expression might be below the WT level, which would reveal a role of BRG1 in Treg activation. To address this, we analysed Tregs in very young (∼10-day-old) Brg1 KO mice (Brg1F/F; FoxP3YFP-Cre males and Brg1F/F; FoxP3YFP-Cre/YFP-Cre females), where outward manifestations of inflammation were unappreciable, although hepatic cellular infiltration (Figure 5A and B) and splenic accumulation of effector/memory CD4 cells (Figure 5C) had emerged. Remarkably, despite the weak inflammation, the activation markers in the Brg1 KO Tregs were indeed expressed below the WT level (Figure 5D). Curiously, the ‘effector/memory-like’ (CD44HighCD62Llow) Tregs were only marginally decreased. However, these cells in the 10-day-old mice were paradoxically more abundant than in 25–50-day-old mice (∼60–70% versus ∼40%; compare Figure 5D with 4F), suggesting that in 10-day-old mice, the CD44HighCD62Llow Treg pool was heavily contaminated with non-effector/memory-like cells, which might have obscured the depletion of effector/memory-like cells in the KO mice. Finally, in agreement with defective expression of inflammatory chemokine receptors, Treg trafficking to inflamed liver seemed impaired in these mice: despite the onset of hepatitis as manifested by the cellular infiltration in the liver, hepatic Treg numbers in these mice were not increased (2300±500 compared with 2900±600 in WT mice; Figure 5E, top) and so the abundance of hepatic Tregs relative to total CD4 cells was decreased due to infiltration/proliferation of conventional CD4 cells (from 16.6±2.2 down to 5.1±0.2%; Figure 5E, bottom). Thus, in the very young KO mice, despite the mild inflammation, Tregs were hypoactivated relative to that in the WT mice, demonstrating a role of BRG1 in Treg activation.
Figure 5.
Treg hypoactivation in ∼10-day-old KO mice. (A) Cell infiltration (arrow) in liver in Brg1 KO mice. (B) Numbers of lymphocytes recovered from the liver. The inset is a blown-up view of the CD4 and CD8 cellularity. (C) Accumulation of effector/memory CD4 cells in the spleen. A representative experiment was shown at the top, and the data summarized at the bottom. (D) Defective Treg activation. Shown are the frequencies of splenic Tregs expressing various surface markers. (E) Relative depletion of Tregs in the liver.
Defective Treg activation revealed in mosaic females
To corroborate the above data, we examined the mosaic Brg1F/F; FoxP3YFP-Cre/+ females. These mice were healthy, harbouring both YFP+ and YFP− Tregs (Figure 6A, left), with Brg1 specifically deleted in the YFP+ subset (Figure 6B), thus allowing for comparison of WT and KO Tregs under identical, noninflammatory conditions.
Figure 6.
Tregs in mosaic Brg1F/F; FoxP3YFP-Cre/+ (F/F) adult females and the littermate control Brg1F/+; FoxP3YFP-Cre/+ (F/+) females. (A) YFP versus CD25 expression in total CD4 cells. (B) qPCR measuring Brg1F abundance in Tregs. (C) Viability of YFP+ Tregs. Splenic Tregs were stained for CD4 and annexin before analysis of annexin expression in CD4+YFP+ Tregs. (D–F) Abundance (D), proliferation (E) and activation (F) of YFP− versus YFP+ Tregs.
We analysed Brg1F/F; FoxP3YFP-Cre/+ females together with the heterozygous Brg1F/+; FoxP3YFP-Cre/+ female littermate controls. We found that Brg1 deletion in Brg1F/F; FoxP3YFP-Cre/+ females did not affect Treg viability (Figure 6C) but slightly depleted Tregs relative to the YFP− subset (Figure 6D, groups 3 versus 4). This depletion is consistent with impaired Treg homeostatic proliferation, as evidenced by a moderate decrease in EdU incorporation (Figure 6E). Importantly, Brg1 deletion impaired effector/memory-like Treg production and induction of all the activation markers (Figure 6F). This activation defect may also explain the impaired homoeostatic proliferation, as the latter is presumably dependent on TCR signalling and hence activation. Interestingly, in Brg1F/+; FoxP3YFP-Cre/+ females, Treg proliferation (Figure 6E) and induction of some markers (CD69 and ICOS; Figure 6F) seemed marginally impaired in YFP+ Tregs as compared with the YFP− Treg subset, although the differences were statistically insignificant. Since the YFP+ cells in these mice contained only one copy of Brg1, the data raised the possibility that both copies of Brg1 is required for optimal Treg activation. These data reinforce the notion that BRG1 promotes Treg activation in response to self-antigens.
Defective Treg activation during ConA-induced inflammation
We next determined whether BRG1 also promotes Treg activation in response to pathological inflammatory cues, using as a model system concanavalin A (Con A)-induced hepatitis (Tiegs, 2007). Con A triggers acute Th1-type hepatitis and induces the expression of multiple CXCR3 ligands in the liver. In response, Tregs quickly express CXCR3 (perhaps due to stimulation by Con A in combination with IFN-γ) and migrate to the liver to restrain the inflammation (Erhardt et al, 2011). Since Brg1 KO Tregs must not be exposed to inflammation prior to Con A injection, we performed the experiments in Brg1F/F; FoxP3YFP-Cre/+ females. In the experiments, the YFP− (WT) Tregs could theoretically serve as an internal control for YFP+, Brg1-deficient Tregs. However, as the former cells were YFP−FoxP3+, a combination of FOXP3 staining and YFP fluorescence was needed for their identification, which proved technically difficult especially for the cells isolated from the liver (not shown). One option would be to use CD25 to identify the YFP− Tregs. However, CD25 is also expressed in conventional CD4 cells where its expression is presumably subject to influences by Con A, thus confounding data interpretation. We therefore used the Brg1F/+FoxP3YFP-Cre/+ female littermates as controls, as these mice contained Brg1-sufficient Tregs marked by YFP, which bypassed the need for FOXP3 or CD25 staining.
Our analysis was focused on CXCR3+ Tregs, the central player in this hepatitis model (Erhardt et al, 2011). As expected from Figure 6F, prior to Con A injection, the frequencies of Brg1 KO YFP+ CXCR3+ Tregs were ∼2-fold lower in Brg1F/F; FoxP3YFP-Cre/+ mosaic females than the WT counterpart in the littermate controls (Figure 7A, left and Figure 7B, top). We then injected Con A and analysed CXCR3 induction 48 h thereafter, at a time when Treg numbers in the liver had peaked (Supplementary Figure S2). Before Con A injection, CXCR3 was expressed in ∼21% of YFP+ WT Tregs in the spleen, and the value was increased to ∼42% following Con A injection (Figure 7A; Figure 7B, top). In other words, the YFP+ WT Tregs that lacked CXCR3 expression comprised 79% of total Tregs before Con A injection, but only 58% after the injection. This indicates that 21% (79–58%) of YFP– Tregs had upregulated CXCR3 following Con A injection. Since the CXCR3− Treg subset comprised 79% of YFP+ Tregs before Con A injection, Con A must have induced CXCR3 on 27% (21/79) of CXCR3− YFP+ WT Tregs. In contrast, for the KO counterpart, CXCR3 was expressed on ∼8 and ∼17% YFP+ KO Tregs before and after Con A injection, respectively, indicating Con A had induced CXCR3 in only 10% of CXCR3− YFP+ KO Tregs as compared with 27% of WT counterparts (Figure 7A and Figure 7B, top). Thus, Brg1 deletion led to a 2.7-fold reduction in the CXCR3 induction frequency in CXCR3− YFP+ Tregs. Furthermore, surface CXCR3 abundance (as measured by MFI) on the Brg1 KO Tregs that had managed to express CXCR3 was lower (∼1.7-fold on average) at the single cell level both before and after Con A injection (Figure 7A and Figure 7B, bottom). Thus, Brg1 deficiency compromised both the probability and magnitude of CXCR3 induction in the resting state and following Con A stimulation. As a result, at 48 h following Con A injection, there were ∼109 000 CXCR3+YFP+ WT Tregs in the spleen, but only ∼28 000 KO counterparts (Figure 7C), indicating a four-fold reduction in the absolute numbers of KO Tregs after Con A injection in the spleen. CXCR3 is crucial for Treg trafficking to the liver during Con A-induced hepatitis. The fact that fewer CXCR3+YFP+ KO Tregs were present in the spleen predicts that fewer CXCR3+YFP+ KO Tregs can be recruited to the liver during the hepatitis. Indeed, before Con A injection, ∼600 and ∼200 CXCR3+ WT and KO YFP+ Tregs were present in the liver, respectively (Figure 7C, top). Two days following Con A injection, the numbers of hepatic WT CXCR3+ YFP+ Tregs had increased from 600±380 to 7350±3080, suggesting ∼6750 WT YFP+ CXCR3+ Tregs had been recruited during this time (Figure 7C, top), and these new recruits largely account for the increase in the numbers of total YFP+ cells in the liver (from 2000 to 11 300, Figure 7C, bottom), as expected. In contrast, following Con A injection, KO YFP+ CXCR3+ Tregs were increased by only ∼730 (from 230±130 to 960±540; Figure 7C, top). Thus, nine-fold (6750 versus 730) fewer YFP+ CXCR3+ KO Tregs were recruited to the liver, confirming our prediction. Interestingly, this nine-fold reduction in the number of recruited KO Tregs was disproportional to the aforementioned four-fold reduction in the numbers of YFP+ CXCR3+ KO Tregs in the spleen. Since the KO CXCR3+ Tregs showed impaired surface CXCR3 expression (in the liver as in the spleen; Figure 7B, bottom), the lowered CXCR3 expression presumably impeded trafficking of CXCR3+ Tregs. The impediment in the trafficking of the CXCR3+ Tregs may explain the disproportional reduction in the hepatic versus splenic KO CXCR3+ Tregs. Perhaps for the same reason, at the basal state before Con A injection, hepatic KO CXCR3+ Tregs (200 cells versus 600 WT counterparts) were also disproportionally depleted as compared to the splenic Tregs (12 600 KO versus 29 400 WT cells). These data demonstrate that Brg1 deletion impaired Treg activation in response to pathological inflammatory cues, just as it impaired Treg activation by self-antigens.
Figure 7.
Brg1 KO impaired CXCR3 induction and Treg trafficking following Con A stimulation. (A) CXCR3 expression in YFP+ Tregs in the spleen (top) and liver (bottom) in the absence (left) or 2 days after (right) ConA injection. WT and KO Tregs were YFP+ cells in Brg1F/+; FoxP3YFP-Cre/+ and Brg1F/F; FoxP3YFP-Cre/+ females, respectively. (B) The data in (A) were summarized from multiple experiments to show the percentages of CXCR3+ subset among total YFP+ Tregs (top) and the mean fluorescence intensity (MFI) of CXCR3 expression in the CXCR3+ subset (bottom). (C) Same as B, except that the absolute numbers of Tregs are displayed.
In the discussion above, we have assessed Con A-induced Treg recruitment based on the absolute numbers of recruited Tregs rather than fold increases in hepatic Treg abundance relative to the basal levels, as the fold change is confounded by the basal number of CXCR3+ Tregs and thus cannot directly and accurately represent the effect of Con A. It is also noteworthy that the activation and trafficking defects in Brg1 KO Tregs were not caused by the presence of the WT Tregs in the mosaic mice; the WT Tregs could compete with the KO Tregs thus helping reveal their intrinsic defects, but could never create such defects.
Brg1 regulates TCR target genes in vitro
The experiments described above were carried out in vivo. To directly test the role of BRG1 in Treg activation, YFP+ KO Tregs lacking prior inflammatory exposure were isolated from Brg1F/F; FoxP3YFP-Cre/+ mosaic females and stimulated with anti-CD3/CD28-coated beads in the presence of IL-2. YFP+ Tregs from Brg1-sufficient mosaic females were used as a control. To ensure the Tregs were not contaminated with conventional CD4 cells, Tregs were purified to near homogeneity using anti-CD25 magnetic beads followed by electronic sorting for YFP+ cells. Ideally, the experiment should use Tregs lacking activation marker expression, but this is not feasible because even ‘naïve’ (CD103− CD44low CD62Lhigh) Tregs can express activation markers (Huehn et al, 2004).
Tregs were stimulated for 2, 12 or 48 h before analysis of gene expression. We examined all the activation marker genes that we have characterized in vivo as well as multiple known or suspected TCR target genes in Tregs (McHugh et al, 2002; Park et al, 2010; Moran et al, 2011), but only 10 genes showed reproducible changes in our hands (Figure 8A). The expression levels of these genes before TCR stimulation were comparable between WT and KO Tregs, and TCR stimulation induced all these genes in WT Tregs, but with different kinetics (Figure 8A). Thus, Nur77 induction was rapid and transient, reaching the plateau within 2 h after stimulation but had returned to baseline by 48 h, whereas the induction of the remaining genes was not detectable at 2 h, variable at 12 h and became stable at 48 h. Importantly, Brg1 KO impaired the induction of all chemokine receptor genes (Cxcr3, Cxcr5, Ccr2, Ccr6 and Ccr8), Gadd45γ and Socs2 (Figure 8A). The effects mirror the activation defects in vivo, arguing for a role of BRG1 in facilitating Treg activation in response to TCR signalling. However, the in vitro data must be interpreted with caution as it was unable to fully recapitulate the in vivo phenomena: while Brg1 KO impaired Icos induction in vivo, BRG1 was dispensable for Icos induction in vitro (Figure 8A). Furthermore, increasing the dose of anti-CD3/CD28 or addition of IFN-γ failed to rescue the activation defects (preliminary data).
Figure 8.
BRG1 regulated TCR target genes in vitro. (A) YFP+ Tregs isolated from Brg1F/+; FoxP3YFP-Cre/+ (WT) or Brg1F/F; FoxP3YFP-Cre/+ (KO) females were untreated (−) or stimulated (+) with anti-CD3/CD28 for 2 h (for Nur77) or 48 h (for the remaining genes). mRNA was quantified by RT-PCR, normalized to β-actin mRNA and plotted relative to that in activated WT Tregs, the latter set as 100 (dotted line). Data were pooled from 2–3 independent trials. Gad, Gadd45γ. (B) BRG1 binds the Cxcr3 and Ccr2 loci. Freshly isolated YFP+ Brg1-sufficient Tregs were subjected to ChIP assays using J1 anti-BRG1 antibody or rabbit IgG control, and the abundance of the precipitated DNA sequences was plotted relative to that in the input; IgG-precipitated DNA was often below the detection limit. RNA Pol N (De et al, 2011) and Gapdh served as a positive and negative control, respectively. The locus structure is depicted at the left, where the blue arrows indicate the positions of the PCR amplicons in the ChIP assays. A replica experiment is shown in Supplementary Figure S3B.
As mentioned above, the expression levels of TCR target genes were comparable between freshly isolated WT and Brg1 KO Tregs, which apparently contradicts the fact that the former Treg population harboured ∼20% of Tregs expressing markers such as Cxcr3, Ccr2 and Icos while the latter only ∼10% (Figure 6F). However, RT–PCR quantified the RNA in the entire Treg population, including both the marker+ and the marker− subsets. The latter subset, which constituted the majority of Treg population, might contain basal levels of RNA detectable by RT–PCR. Furthermore, the former, marker+ subset only weakly expressed the markers even in WT Tregs. Thus, the overall difference in mRNA abundance between freshly isolated WT and Brg1 KO populations might be <2-fold, which would be difficult to detect by RT–PCR.
A potential caveat in the experiment described above is that YFP+ WT Tregs in the Brg1-sufficient mosaic females harboured two-fold more effector/memory-like cells than the YFP+ Brg1 KO Tregs in Brg1F/F; FoxP3YFP-Cre/+ mosaic females (Figure 6F). Since effector/memory-like cells presumably react more robustly to TCR stimulation, the apparent defect in TCR signalling in Brg1 KO Tregs may simply reflect the depletion in effector/memory-like Tregs. However, the two-fold reduction in the effector/memory-like cells could not account for the more severe defects in the induction of some of the BRG1-dependent genes (e.g., Cxcr3, Cxcr5, Ccr2, Ccr6 and Gadd45γ). Furthermore, the induction of these five genes was similarly defective in YFP+ Tregs isolated from ∼21-day-old Brg1F/F; FoxP3YFP-Cre males (Supplementary Figure S3A), where overt inflammation had developed and the abundance of effector/memory-like Tregs was comparable to WT mice (Figure 4F). The data thus confirm the roles of BRG1 in TCR signalling at least for these five genes. We conclude that BRG1 regulates a subset of TCR target genes in vitro.
Brg1 binds the Cxcr3 and Ccr2 loci in Tregs
We sought to determine whether BRG1 directly regulates the target genes. We focused on Cxcr3 and Ccr2 because their expression was strongly BRG1-dependent both in vitro and in vivo. Several BRG1-binding sites have been mapped at each gene in conventional CD4 cells, including regions ∼3- and ∼30-kb upstream of the Cxcr3 and Ccr2 transcription start sites, respectively (Figure 8B, left) (De et al, 2011). We consistently detected BRG1 at both sites in YFP+ Tregs (Figure 8B, right; Supplementary Figure S3B), which suggests that BRG1 directly regulates Cxcr3 and Ccr2 in Tregs.
BRG1 ATPase activity is partially dispensable for BRG1 function
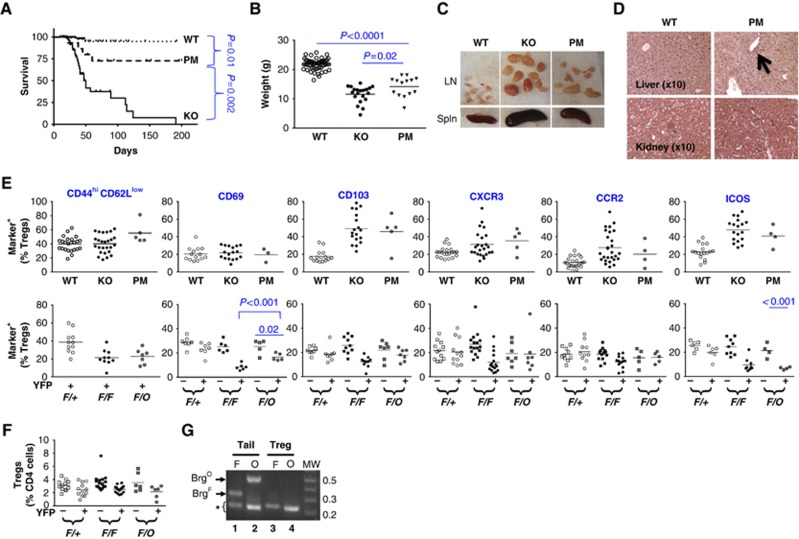
Using a genetic method that can produce littermates bearing either Brg1 KO or Brg1 ATPase-dead K/R point mutation (PM), the former completely eliminating BRG1 function while the latter selectively abolishes ATP-dependent remodelling, we previously found that BRG1 harbours non-classical, remodelling-independent function (RIF) during early T-cell development (Jani et al, 2008). To explore the role of RIF in Tregs, we compared the phenotypes of Treg-specific KO versus PM mice. We found dramatic differences in the mortality rates: while the KO mice mostly died by 120 days, ∼75% PM littermates survived at least 200 days (Figure 9A). Furthermore, although PM mice (14.2±3.5 g on day 40) were very runted as compared with the WT controls (21.8±2.0; P<0.001), they were heavier than the KO littermates (11.6±2.8; P=0.02) (Figure 9B). In addition, in PM mice, lymphadenopathy, splenomegaly and liver inflammation were mild and kidney inflammation undetectable (Figure 9C and D). Remarkably, despite the absence of severe inflammation in the PM mice, Tregs in the PM were as hyperactivated as the KO littermates (Figure 9E, top), suggesting that Tregs in the PM mice remained highly sensitive to inflammatory signals. Indeed, even in mosaic mice, under noninflammatory condition, Tregs bearing the Brg1 PM showed normal expression of CD103, CXCR3 or CCR2 (Figure 9E, bottom), and Treg abundance was not significantly reduced (Figure 9F). However, in the mosaic mice, Brg1 PM did deplete effector/memory-like Tregs and impair CD69 and ICOS expression to the same extent as Brg1 KO (Figure 9E, bottom), indicating that classic ATP-dependent chromatin remodelling is important for these aspects of Treg activation (only) under strictly physiological condition. Of note, the discrepancy in the phenotypes caused by Brg1 PM versus KO is not an artifact resulting from inefficient Brg1F deletion in the PM mice (Figure 9G). Finally, while the mice deleting Brg1 in all αβ T cells (using the CD4Cre deleter) developed late-onset inflammation as described before (Figure 1), the littermates bearing the BRG1 PM in these T cells showed little phenotype (Supplementary Figure S4), consistent with the phenotype of mice bearing Treg-specific PM. Collectively, these data demonstrate that BRG1 ATPase activity is partially dispensable for BRG1 to suppress autoimmunity, thus exposing the role of RIF in immune tolerance.
Figure 9.
Phenotype of mice bearing Treg-specific Brg1 PM. (A–D) Survival (A), body weight at 40 days of age (B) and various organs (C, D) at ∼30 days of age. All mice were males. A and B are identical to Figure 2C and Figure 2D, respectively, except for the inclusion of Brg1F/O; FoxP3YFP-Cre point mutants (PM). Arrow in D indicates marginal infiltration in the liver. (E) Treg activation in 20- to 50-day-old mice (top) and in adult mosaic mice (bottom), which are identical to Figure 4F and Figure 6F, respectively, except for the inclusion of the PM mice. Note that in the plots at the top, the mice are not separated into different age groups as in Figure 4F due to limiting sample sizes of the PM mice. F/O, mosaic PM females (Brg1F/O; FoxP3YFP-Cre/+). (F) Treg abundance in adult mosaic females. The plot is identical to Figure 6D except for the inclusion of PM mice (Brg1F/O; FoxP3YFP-Cre/+). (G) PCR analysis of Brg1F (F) and Brg1O (O) in tail (lanes 1–2) and YFP+ Tregs (lanes 3–4) from Brg1F/O; FoxP3YFP-Cre/+ mosaic females. The two alleles were detected by a primer pair flanking the 3′-LoxP site in Brg1F and that amplifying a fragment specifically present in Brg1O, respectively. The asterisk indicates co-amplified internal controls. The data indicate efficient Brg1F deletion concomitant with Brg1O conversion. The assays were performed essentially as described (Jani et al, 2008).
Discussion
This study demonstrates that BRG1 promotes Treg activation and immune tolerance, which overweighs its proinflammatory role in conventional T cells. Brg1 KO Tregs can be partially activated by persistent, severe inflammation, but this activation is not sufficient to stop the inflammation. Finally, the classic ATP-dependent chromatin-remodelling activity is partially dispensable for BRG1 to promote Treg activation and immune tolerance.
Facilitation of Treg activation by BRG1: biological importance, potential mechanisms and cell-type specificity
KO Tregs were invariably hypoactivated relative to WT Tregs under comparable conditions, whether in the 10-day-old mice, in the normal or inflamed mosaic mice, or in vitro following TCR stimulation. These data reveal an activation defect in the KO Tregs. While the full spectrum of the defect in vivo remains to be determined, which is technically challenging because the defects should be analysed at the single cell level as mentioned in the Results section, it is already clear that Brg1 KO impaired the induction of multiple types of functionally important genes, including chemokine receptor genes, Cd103 and Icos, which are involved in Treg trafficking, retention and the suppressive potential, respectively (Suffia et al, 2005; Campbell and Koch, 2011; Kornete et al, 2012). In addition, Brg1 KO impaired Treg proliferation in vivo, which may also be secondary to a defect in Treg activation. Finally, in vitro assays suggest that Brg1 regulated several other TCR target genes, including Gadd45γ and the FoxP3-dependent gene Socs2 (Sugimoto et al, 2006). Thus, Brg1 KO compromised multiple activation-dependent events in Tregs. These various defects may conspire to cause or at least contribute to the onset of the inflammation. Remarkably, as the inflammation in the KO mice persisted and progressed, Brg1-decifient Tregs became increasingly activated. However, CXCR3 and CCR2 expression in 20–29-day-old mice was only marginally above the WT level despite the severe inflammation in these mice, and furthermore, the abundance of effector/memory-like and CD69+ Tregs remained at the WT level even in 30–50-day-old mice. Thus, although strong and persistent inflammatory cues could activate KO Tregs to some degrees, the activation level was disproportional to the severity of the inflammation. Indeed, this level was not higher than in the Tregs in the PM mice lacking severe inflammation, again arguing that the activation level in the KO mice failed to ‘match’ the severity of inflammation. While this activation level might suffice to control mild inflammation, it is presumably insufficient to ‘catch up with’ and stop the ongoing severe inflammation in these mice. Furthermore, even if Tregs could be fully activated by the severe inflammation, it may be too late to benefit the mice, as inflammation-induced tissue damages might have become irreversible in 20–50-day-old mice. We therefore think that BRG1 promotes immune tolerance at least in part by facilitating Treg activation in response to environmental cues, thus allowing Tregs to promptly and robustly react to incipient inflammation and nip it in the bud; while the KO Tregs eventually became somewhat activated following persistent and strong provocation, this response would be too late and too weak to control the already severe inflammation. Obviously, the activation defects do not exclude additional defects that might contribute to the autoimmune disease in the KO mice, and future studies are needed to identify such potential additional defects. Of note, while the BAF complex associates with FOXP3 (Li et al, 2006, 2007; Rudra et al, 2012), the significance of this interaction is unclear, as Brg1 deletion did not affect FOXP3-dependent processes such as Treg development, expression of Treg signature genes and prevention of Tregs conversion into effector T cells.
Our in vitro assays suggest that BRG1 regulates a subset of TCR target genes in Tregs. Multiple mechanisms that are not mutually exclusive might be involved in this regulation. In conventional CD4 cells, BRG1 is rapidly (within minutes) recruited to nuclear matrix upon TCR signalling, suggesting that BRG1 might exert an acute, global, nonspecific effect on the nuclear architecture that might influence the induction of all TCR target genes (Zhao et al, 1998). In addition, BRG1 binds both the Cxcr3 and Ccr2 loci in Tregs, suggesting that BRG1 can directly and specifically regulate some of the target genes. Finally, BRG1 might act indirectly, by fine-tuning the expression of intermediate molecules in the TCR signalling pathways, and this fine-tuning may be required for the optimal induction of some BRG1 target genes. Thus, BRG1 may use distinct mechanisms to regulate different target genes.
The roles of BRG1 in Treg activation may be divergent from that in conventional CD4 cells, given the divergence in the activation mechanisms between the two cell types (see Introduction). Indeed, although Brg1 RNAi impairs Th1/Th2 differentiation in vitro, it does not seem to impair CD4 cell proliferation, a hallmark of activation (Zhang and Boothby, 2006; Wurster and Pazin, 2008). Furthermore, while BRG1 promotes Th1/Th2 differentiation in CD4 cells by remodelling Il4 and Ifng chromatin structure, respectively, remodelling activities were partially dispensable for BRG1 to promote Treg function, reinforcing the notion about cell-type specificity in BRG1 action.
Remodelling Independent Function (RIF) of BRG1
BRG1 is generally assumed to act by ATP-dependent remodelling of nucleosome structure. However, we previously found that eliminating this remodelling activity fails to completely abolish BRG1 function during early T-cell development: while the remodelling activity is essential for BRG1 to promote survival and development of early thymocytes, it is dispensable for BRG1 to regulate CD4 expression during DN to DP transition (Jani et al, 2008). We now demonstrate that as a whole, the remodelling activity was partially dispensable for BRG1 to promote Treg function and suppress autoimmunity, because overall, the BRG1 ATPase PM mice displayed an intermediate phenotype between the WT and KO mice. Interestingly, different components of the phenotype were differentially affected by the point mutation, although for the most part, the defects in the PM mice were rather weak. Specifically, while severe runting was present in both KO and PM mice, lifespan reduction (within the time window analysed), liver inflammation, lymphadenopathy and splenomegaly were dramatic in KO but mild in the PM mice, and furthermore, kidney inflammation was totally absent in the PM mice. Thus, chromatin remodelling by BRG1 is essential for BRG1 to regulate body weight, partially/largely dispensable for controlling lifespan, liver inflammation, lymphadenopathy and splenomegaly, and fully dispensable for repressing kidney inflammation. At the transcription level, the PM impaired the induction of some (Icos and Cd69) but not other (Cd103, Cxcr3 and Ccr2) activation markers in mosaic mice, indicating that RIF is necessary and sufficient for BRG1 to regulate a subset of target genes. This conclusion has been reached before when we showed, as mentioned above, that RIF alone can regulate CD4 expression during DN→DP transition (Jani et al, 2008), but our data in the current paper reveal a significant role of RIF in immune tolerance, thus highlighting for the first time the biological importance of RIF. Of note, the role of RIF may have been underestimated in the current study, because the defects in the PM mice were compared to the Brg1-sufficient control mice some of which carried two copies of the functional Brg1 allele in Tregs (see Figure 2 legend), whereas in the PM mice, the ATPase-dead BRG1 mutant was expressed from the single-copy mutant allele (Jani et al, 2008). Thus, the BRG1 abundance in the control mice was perhaps higher than that of the ATPase-dead mutant in the PM mice, which might have exaggerated the defects in the PM mice, leading to the overestimation of the importance of the BRG1 ATPase activity. The molecular basis of RIF is unclear, but may reflect a role of BRG1 in organizing nuclear architecture, as suggested by rapid targeting of BRG1 to nuclear matrix following TCR stimulation (Zhao et al, 1998). Such global organization might entail the functions of the BAF complex in facilitating actin polymerization (Rando et al, 2002) and large-scale chromatin movement (Hu et al, 2008), which are conceivably independent of the traditional nucleosome remodelling activity of BRG1.
Interesting, the H3K27 demethylase Jmjd3 is recently shown to promote Ifng expression and Th1 differentiation independently of its catalytic activity, because point mutations that abolish Jmjd3 catalytic activity do not affect its function (Miller et al, 2010). Similarly, P53 point mutants unable to induce cell cycle arrest, apoptosis or senescence unexpectedly retain substantial tumour-suppressing activities (Li et al, 2012b). In general, proteins are multi-functional, and our genetic strategy of producing littermates bearing either KO or PM is useful for dissecting these functions.
Materials and methods
Mice
Floxed Brg1 (Brg1F) mice (Chi et al, 2003) and BrgO mice bearing conditional Brg1 PM (Jani et al, 2008) were mated with CD4Cre (Lee et al, 2001) or FoxP3YFP-Cre (Rubtsov et al, 2008) mice to mutate Brg1 in all αβ T-cell lineages or Tregs only, respectively. The mice were maintained on the C57BL/6 background.
Histology
Tissues were fixed in the Prefer fixative. Paraffin embedding and H&E staining were performed by the histology service at Yale Research Histology.
Cytokine production in T cells in vitro
Lymphocytes were isolated from spleen and lymph nodes in complete RPMI. About 0.2 million cells were incubated for 5 h at 37°C with PMA (1 ng/ml), ionomycin (1 μM) and Golgistop (3 mM) before the analysis. Cells incubated with Golgistop alone were used as a negative control. Intracellular staining of cytokines was performed according to the protocol from Ebioscience.
EdU incorporation
For the experiments in Figure 4E, EdU (300 μg, Invitogen) was injected i.p. once a day for three consecutive days to ∼30-day-old KO (Brg1F/F; FoxP3YFP-Cre) mice or littermate controls. On day four, lymphocytes were harvested from the spleen, CD4 cells magnetically enriched, stained with anti-CD4-PerCP before purification of conventional CD4 cells and Tregs via electronic sorting based on YFP expression. EdU incorporation was then determined using the Click-it EdU staining (Invitrogen). The separation of CD4 cells and Tregs before EdU staining was necessary because the Click-it chemistry quenches the YFP fluorescence. The Edu incorporation in Tregs from the mosaic mice (Figure 5E) was similarly determined, except that the CD4 cells were stained with anti-CD4-PerCP together with anti-CD25-FITC before sorting and subsequent EdU staining of YFP−CD25+ and YFP+ CD25+Tregs.
Treg-suppressive function in vitro
PLNs and spleens were harvested from ∼6-week-old mice and cells pooled. Naïve CD4 cells (from WT mice) and Tregs (from WT and Brg1F/F; CD4Cre mice) were sorted using CD4+ CD25− CD45RBhi and CD4+ CD25+ CD45RBlow as markers, respectively. The purity of sorted cells exceeded 95%, and 95–98% of the sorted ‘Tregs’ expressed Foxp3 (not shown). About 2 × 104 naïve CD4 cells were mixed with varying numbers of Tregs (CD4+CD25+) in the presence of 8 × 104 irradiated antigen-presenting cells, the latter prepared by removing CD4 and CD8 cells from splenocytes and lymph node cells followed by lethal X-ray irradiation. Cells were stimulated with soluble α-CD3 (1 μg/ml) for 48 h. 3H-thymidine (1 μCi) was then added, and 3H incorporation measured 24 h thereafter using a β-counter.
Hepatitis induction and lymphocyte analysis
Six-week-old Brg1F/F; FoxP3YFP-Cre/+ or Brg1F/+; FoxP3YFP-Cre/+ females were injected via the tail vein with 1 mg/ml Concanavalin A Type IV (Sigma 11028-71-0, diluted in PBS) at 10 μg/g body weight. Mice were sacrificed 48 h later and lymphocytes collected from lymph nodes, spleen and liver. Liver lymphocytes were isolated as described (Zenewicz et al, 2007). Briefly, liver tissues were mechanically disrupted in 5 ml RPMI and digested for 40 min with 0.01 mg Collagenase IV (Sigma C-5138) and 0.2 μg DNase I (Sigma D4527). The samples were washed with 45 ml RPMI and debris removed by centrifugation at 300 r.p.m. for 3 min. The cells were pelleted by centrifugation at 1600, r.p.m. for 10 min, and resuspended in 1 ml RPMI media plus 4 ml gradient buffer, the latter comprising Optiprep, Tricine buffer (30 mM Tricinc-NaOH pH 7.4, 0.85% NaCl) and PBS at 2:1:1.4 ratio (volume). Cells in the gradient buffer were transferred to 15 ml conical tubes, overlaid with 1 ml RPMI media and centrifuged for 20 min at 1650, r.p.m. at 4°C without acceleration or brake. Cells at the interface were collected and washed with 15 ml RPMI media. Contaminating RBC were then lysed before analysis of the lymphocytes.
Treg activation in vitro
CD25+ CD4 cells from adult Brg1F/F; FoxP3YFP-Cre/+ or Brg1F/+; FoxP3YFP-Cre/+ females were first magnetically isolated with a Treg purification kit (Invitrogen, cat no 113.63D) prior to FACS sorting for YFP+ subset, the purity of sorted cells exceeding 99%; the sorting step was included to ensure that Tregs were not contaminated with CD25+ conventional CD4 cells. About 0.1–0.2 million sorted cells were incubated with equal numbers of anti-CD3/CD28 dynal beads (Invitrogen) in the presence of 2 U/μl recombinant IL-2 (Peprotech). Cells were harvested at indicated times and RNA purified with the RNAeasy kit (Qiagen). Target genes were quantified by real-time, one-step RT–PCR using the QuantiTect SYBR Green RT–PCR kit (Qiagen). The primer sequences are available upon request.
BRG1 ChIP
YFP+ Tregs were isolated from Brg1-sufficient adults as described above. BRG1 ChIP was performed essentially as described (Jani et al, 2008). Briefly, cells were washed once in PBS and then fixed with 6 μg/ml dimethyl adipimidate for 90 min at room temperature. Cells were washed again with PBS and then fixed with 1% paraformaldehyde at room temperature for 5 min. Cells were resuspended in RIPA (0.8 M NaCl) at a final concentration of 40 million cells/ml. Fixed cells were sonicated for 45–60 min (30′ on/30′ off, at the high intensity) in a Bioruptor sonicator to shear chromatin to ∼200 basepair fragments. One to two million cells were diluted with equal volume of RIPA (0 M NaCl), and chromatin precipitated. ChIP DNA and input DNA were quantified by real-time PCR. Primers for amplifying Cxcr3- and Ccr2-binding sites are: (Cxcr3) 5′-TTGGCTAGGTAGTTGCCTCTGACCTT-3′ and 5′-TGCCCAGTTAGTGGCTTGCTC-3′; (Ccr2F) 5′-AGGAGTGGGAGGTCAGAGGAAGT-3′ and 5′-TGCCACAGAAGGGAGGTGCTCAT-3′.
Sample size and statistical analysis
Unless otherwise noted in the figure legends, for any experiment, at least three independent experiments were performed, and at least three mice total per group were analysed. P-values were calculated by t-test.
Supplementary Material
Acknowledgments
We thank Drs Carmen Booth, Daniel Campbell, Mike Pazin, David Hudnall and members of the Flavell lab (especially Drs Adam William, William O’Connor and Lauren Zenewicz) for advice and technical assistance, and Drs Chris Wilson and Alexander Rudensky for Cre-deleter lines. The study was supported by NIH 1RO1 AI063554-01 (TC), NIH P30 AR053495 (TC) and by NIH MSTP TG T32GM07205 (BHC).
Author contribution: BHC and HH analyzed mice with Treg-specific Brg1 mutation, while AJ and YW analyzed mice with T-cell-specific Brg1 mutations. RF and TC directed the study. TC conceived the project and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL (2002) CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420: 502–507 [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Tarbell KV (2009) Arming Treg cells at the inflammatory site. Immunity 30: 322–323 [DOI] [PubMed] [Google Scholar]
- Benoist C, Mathis D (2012) Treg cells, life history, and diversity. Cold Spring Harb Perspect Biol 4: a007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Koch MA (2011) Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 11: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Ziegler SF (2007) FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol 7: 305–310 [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY (2009) CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326: 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi T (2004) A BAF-centred view of the immune system. Nat Rev Immunol 4: 965–977 [DOI] [PubMed] [Google Scholar]
- Chi TH, Wan M, Lee PP, Akashi K, Metzger D, Chambon P, Wilson CB, Crabtree GR (2003) Sequential roles of Brg, the ATPase subunit of BAF chromatin remodeling complexes, in thymocyte development. Immunity 19: 169–182 [DOI] [PubMed] [Google Scholar]
- Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, Crabtree GR (2002) Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature 418: 195–199 [DOI] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78: 273–304 [DOI] [PubMed] [Google Scholar]
- De S, Wurster AL, Precht P, Wood WH, Becker KG, Pazin MJ (2011) Dynamic BRG1 recruitment during T helper differentiation and activation reveals distal regulatory elements. Mol Cell Biol 31: 1512–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt A, Wegscheid C, Claass B, Carambia A, Herkel J, Mittrucker HW, Panzer U, Tiegs G (2011) CXCR3 deficiency exacerbates liver disease and abrogates tolerance in a mouse model of immune-mediated hepatitis. J Immunol 186: 5284–5293 [DOI] [PubMed] [Google Scholar]
- Gebuhr TC, Kovalev GI, Bultman S, Godfrey V, Su L, Magnuson T (2003) The role of Brg1, a catalytic subunit of mammalian chromatin-remodeling complexes, in T cell development. J Exp Med 198: 1937–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey VL, Wilkinson JE, Russell LB (1991) X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol 138: 1379–1387 [PMC free article] [PubMed] [Google Scholar]
- Hagness M, Henjum K, Landskron J, Brudvik KW, Bjornbeth BA, Foss A, Tasken K, Aandahl EM (2012) Kinetics and activation requirements of contact-dependent immune suppression by human regulatory T cells. J Immunol 188: 5459–5466 [DOI] [PubMed] [Google Scholar]
- Hu Q, Kwon YS, Nunez E, Cardamone MD, Hutt KR, Ohgi KA, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG, Fu XD (2008) Enhancing nuclear receptor-induced transcription requires nuclear motor and LSD1-dependent gene networking in interchromatin granules. Proc Natl Acad Sci USA 105: 19199–19204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A (2004) Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med 199: 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani A, Wan M, Zhang J, Cui K, Wu J, Preston-Hurlburt P, Khatri R, Zhao K, Chi T (2008) A novel genetic strategy reveals unexpected roles of the Swi-Snf-like chromatin-remodeling BAF complex in thymocyte development. J Exp Med 205: 2813–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM (2010) Foxo transcription factors control regulatory T cell development and function. Immunity 33: 890–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornete M, Sgouroudis E, Piccirillo CA (2012) ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol 188: 1064–1074 [DOI] [PubMed] [Google Scholar]
- Lee JH, Kang SG, Kim CH (2007) FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J Immunol 178: 301–311 [DOI] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB (2001) A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15: 763–774 [DOI] [PubMed] [Google Scholar]
- Li B, Samanta A, Song X, Furuuchi K, Iacono KT, Kennedy S, Katsumata M, Saouaf SJ, Greene MI (2006) FOXP3 ensembles in T-cell regulation. Immunol Rev 212: 99–113 [DOI] [PubMed] [Google Scholar]
- Li B, Saouaf SJ, Samanta A, Shen Y, Hancock WW, Greene MI (2007) Biochemistry and therapeutic implications of mechanisms involved in FOXP3 activity in immune suppression. Curr Opin Immunol 19: 583–588 [DOI] [PubMed] [Google Scholar]
- Li Q, Shakya A, Guo X, Zhang H, Tantin D, Jensen PE, Chen X (2012a) Constitutive nuclear localization of NFAT in Foxp3+ regulatory T cells independent of calcineurin activity. J Immunol 188: 4268–4277 [DOI] [PubMed] [Google Scholar]
- Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W (2012b) Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149: 1269–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S (2009) Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 31: 921–931 [DOI] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC (2002) CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16: 311–323 [DOI] [PubMed] [Google Scholar]
- Miller SA, Mohn SE, Weinmann AS (2010) Jmjd3 and UTX play a demethylase-independent role in chromatin remodeling to regulate T-box family member-dependent gene expression. Mol Cell 40: 594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA (2011) T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208: 1279–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell 108: 475–487 [DOI] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO (2010) Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol 11: 618–627 [DOI] [PubMed] [Google Scholar]
- Ouyang W, Li MO (2011) Foxo: in command of T lymphocyte homeostasis and tolerance. Trends Immunol 32: 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Mathur R, Long M, Hosh N, Hao L, Hayden MS, Ghosh S (2010) T regulatory cells maintain intestinal homeostasis by suppressing gammadelta T cells. Immunity 33: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ, Zhao K, Janmey P, Crabtree GR (2002) Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc Natl Acad Sci USA 99: 2824–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr, Muller W, Rudensky AY (2008) Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558 [DOI] [PubMed] [Google Scholar]
- Rudensky AY (2011) Regulatory T cells and Foxp3. Immunol Rev 241: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra D, Deroos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, Leslie C, Shaffer SA, Goodlett DR, Rudensky AY (2012) Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 13: 1010–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133: 775–787 [DOI] [PubMed] [Google Scholar]
- Suffia I, Reckling SK, Salay G, Belkaid Y (2005) A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol 174: 5444–5455 [DOI] [PubMed] [Google Scholar]
- Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y (2006) Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med 203: 777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S (2006) Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol 18: 1197–1209 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S (1998) Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 10: 1969–1980 [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM (1998) CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188: 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G (2007) Cellular and cytokine-mediated mechanisms of inflammation and its modulation in immune-mediated liver injury. Z Gastroenterol 45: 63–70 [DOI] [PubMed] [Google Scholar]
- Wang W (2003) The SWI/SNF family of ATP-dependent chromatin remodelers: similar mechanisms for diverse functions. Curr Top Microbiol Immunol 274: 143–169 [DOI] [PubMed] [Google Scholar]
- Wang Y, Su MA, Wan YY (2011) An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35: 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster AL, Pazin MJ (2008) BRG1-mediated chromatin remodeling regulates differentiation and gene expression of T helper cells. Mol Cell Biol 28: 7274–7285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wing JB, Sakaguchi S (2011) Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Sem Immunol 23: 424–430 [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27: 647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Boothby M (2006) T helper type 1-specific Brg1 recruitment and remodeling of nucleosomes positioned at the IFN-gamma promoter are Stat4 dependent. J Exp Med 203: 1493–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS (2009) Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 30: 458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A, Crabtree GR (1998) Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell 95: 625–636 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY (2009) Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458: 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY (2010) Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.