Factors Contributing to Failure When Treating Patients With Chronic Hepatitis C Virus Infection
Abstract: Combination therapy with peginterferon and ribavirin is the current standard of care for patients with chronic hepatitis C virus (HCV) infection. This treatment yields sustained virologic response (SVR) in approximately half of all patients. Many demographic characteristics and medical comorbidities are associated with treatment failure. Treatment failure is more commonly observed in patients who experience severe adverse events that require peginterferon and/or ribavirin doses to be modified or interrupted. Measuring HCV RNA frequently during the course of treatment allows the various patterns of virologic response and nonresponse to be recognized. This allows the treating physician to more effectively manage the adverse events of therapy and to optimize treatment duration, enabling a higher percentage of patients to achieve SVR.
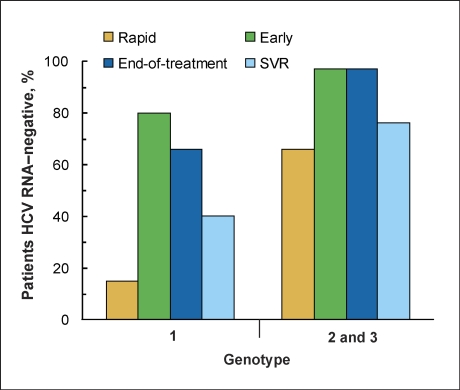
Combination therapy with peginterferon (PEG-IFN) and ribavirin (RBV) is the current standard of care for patients with chronic hepatitis C virus (HCV) infection.1–4 This regimen is highly effective, and the vast majority of treated patients, particularly those with HCV genotypes 2 or 3, exhibit some degree of virologic response. Approximately 66% of patients with HCV genotype 2 or 3 achieve rapid virologic response (RVR)—defined as having undetectable HCV RNA within 4 weeks of initiating treatment—97% have undetectable HCV RNA within 12–24 weeks of initiating treatment, and 76% achieve sustained virologic response (SVR).4,5 Although not quite as robust, a significant proportion of patients with HCV genotype 1 also respond to treatment: approximately 15% achieve RVR, and 80% achieve early virologic response (EVR), defined as having a 2 log lU/mL reduction in or undetectable HCV RNA within 12 weeks of initiating treatment. Thus, all patients with RVR also have EVR. After 24 weeks of treatment, 66% of HCV genotype 1 patients have undetectable HCV RNA and 40–45% achieve SVR.6 Thus, 97% of patients with HCV genotypes 2 or 3 and 80% of patients with genotype 1 exhibit some degree of antiviral response to treatment (Figure 1).
Figure 1.
Percentage of patients who achieve various virologie response patterns during treatment with peginterferon and ribavirin.
- SVR
- sustained virologic response
- HCV
- hepatitis C virus.
Unfortunately, only approximately half of all patients achieve SVR with 24–48 weeks of treatment with PEG-IFN and RBV.1,2 There are basically three reasons patients fail to achieve SVR: they do not develop undetectable HCV RNA levels during treatment; they develop virologic breakthrough after initially developing undetectable HCV RNA levels; and they relapse after treatment is stopped.7 Various pretreatment and ontreatment characteristics lead to treatment failure (Table 1).8–1 However, if these factors could be overcome, either during the initial treatment or during re-treatment, the percentage of patients with chronic HCV infection who could achieve SVR with PEG-IFN and RBV might increase substantially.7
Table 1.
Factors Related to Failure of Pegylated Interferon and Ribavirin in Patients With Chronic Hepatitis C
| Null response | Partial response | Breakthrough | Relapse | |
|---|---|---|---|---|
| Genotype 1 | ✓ | ✓ | ||
| High viral load | ✓ | ✓ | ||
| African-American race | ✓ | ✓ | ||
| HIV coinfection | ✓ | ✓ | ||
| Advanced fibrosis/cirrhosis | ✓ | ✓ | ||
| Delayed virologic response | ✓ | |||
| Noncompliance | ✓ | ✓ | ✓ | ✓ |
| Peginterferon dose reduction | ✓ | ✓ | ✓ | |
| Ribavirin dose reduction | ✓ | ✓ | ||
| Obesity | ? | ? | ? | |
| Insulin resistance | ? | ? | ? |
The factors that lead to failure when treating patients with chronic HCV infection will be discussed, as well as the ways these limitations may be overcome, either during the initial therapy or as part of a re-treatment strategy.
Patterns of Virologic Response
The various response patterns observed during HCV treatment are illustrated in Figure 2.8 The initial description of the nonresponder included all patients who did not develop undetectable HCV RNA levels during treat-ment.12 However, it is now apparent that nonresponse is composed of three distinct groups: null response, partial response, and breakthrough. Although not considered a pattern of nonresponse, relapse is another reason patients do not achieve SVR.11,13
Figure 2.
Patterns of virologic response that can be observed during treatment with peginterferon and ribavirin.
Adapted with permission from Shiffman.8
- HCV
- hepatitis C virus.
Null Response
Patients with a null response have less than a 2 log IU/mL decline in serum HCV RNA levels from the pretreatment baseline during treatment with PEG-IFN and RBV.7,13 This pattern of nonresponse occurs in approximately 20% of patients with HCV genotype 1 but appears to be very uncommon in patients with genotypes 2 or 3.7 Null response can be recognized within 4–12 weeks after treatment is initiated.7,13 Because patients with a null response do not have further declines in serum levels of HCV RNA with continued therapy, treatment should be discontinued as soon as this nonresponse pattern is recognized.7,13,14
Null response may occur in patients who are noncompliant or in those who develop severe adverse events of treatment and require that the dose of PEG-IFN be reduced or that treatment with PEG-IFN and/or RBV be temporarily or permanently discontinued.14–16 In contrast, simply reducing the RBV dose does not appear to enhance the likelihood of null response.17 Factors that are associated with an increased likelihood of intolerance to PEG-IFN and/or RBV and that enhance null response include advanced age, HIV coinfection, African-American race, and cirrhosis.8,18,19
Alternatively, some patients have a null response despite taking full doses of PEG-IFN and RBV. Such patients are simply resistant to the effects of these medications. Patients with an increased likelihood of null response, despite receiving full doses, include those with HCV genotype 1, high baseline viral load, African-American race, HIV coinfection, obesity, insulin resistance, and cirrhosis.1,2,20–23 Increasing the dose or dosing frequency of interferon (IFN), PEG-IFN, and/or RBV is unlikely to lead to virologic response and render HCV RNA levels undetectable.
Partial Virologic Response
Patients with partial virologic response have a 2 log IU/mL or more decline in serum HCV RNA levels from the pretreatment baseline within 12 weeks but do not develop undetectable HCV RNA levels after 24 weeks of treatment with PEG-IFN and RBV. This pattern of nonresponse occurs in approximately 15–20% of patients with HCV genotype 1 but only 3–5% of patients with HCV genotypes 2 and 3.4,6,7 Partial virologic response can be recognized within 12–24 weeks after treatment is initiated. Serum HCV RNA levels in patients with partial response do not continue to decline with continued therapy, and treatment should be discontinued after 24 weeks if the virus remains detectable.11–13
Factors associated with partial response are similar to those observed with null response. Thus, partial response is seen in patients with noncompliance or in those who develop severe adverse events and require that the dose of PEG-IFN be reduced.14–16 In contrast, simply reducing the RBV dose does not appear to enhance rates of partial response.17 The factors that are associated with an increased likelihood of intolerance to PEG-IFN and/or RBV and that increase the likelihood of partial response include advanced age, HIV coinfection, African-American race, and cirrhosis.8,18,19
Alternatively, some patients have a partial response despite receiving full doses of PEG-IFN and RBV. The reasons such patients have initial virologic response and achieve EVR but fail to develop undetectable HCV RNA levels remain unclear. Patients with an increased likelihood of partial response, despite receiving full-dose treatment, include those with genotype 1, high viral load, HIV coinfection, African-American race, obesity, insulin resistance, and cirrhosis. Because these patients have demonstrated virologic response to treatment, increasing the dose or dosing frequency of IFN or PEG-IFN may lead to virologic response and render their HCV RNA levels undetectable. This has been demonstrated in several studies.24–27
Virologic Breakthrough
Patients with virologic breakthrough initially develop undetectable HCV RNA levels in serum during treatment, although serum HCV RNA eventually reappears even with ongoing treatment.12,13 The most common reason for breakthrough is that PEG-IFN and/or RBV dosing was either prematurely terminated or temporarily interrupted, or the dose was reduced after the patient had developed undetectable HCV RNA levels.14–16,28 This occurred because either the patient was noncompliant or adverse events required the physician to alter the dosage of these medications. Once breakthrough has occurred, it is unlikely that the patient will again develop undetectable HCV RNA levels, even with continued treatment.28 Thus, treatment should be stopped once breakthrough has been established.
Patients with breakthrough may be successfully re-treated with PEG-IFN and RBV, provided that the reason for the dose modification is identified and prevented during re-treatment.7,13 Patients with breakthrough do not require higher doses of PEG-IFN because they already developed undetectable HCV RNA levels with the given dose.
Relapse
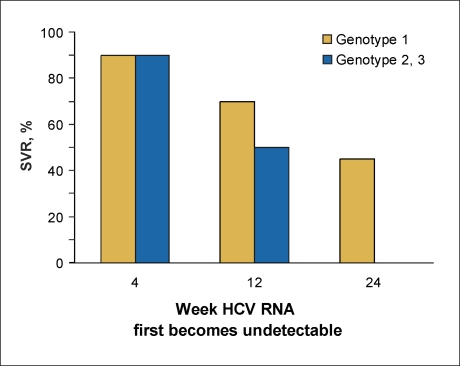
Patients with relapse developed and sustained undetectable HCV RNA levels in serum during treatment, although HCV RNA reappeared in serum after the discontinuation of treatment.11–13 In patients who remained on their full doses of PEG-IFN and RBV throughout the standard duration of therapy—48 weeks for patients with HCV genotype 1 and 24 weeks for patients with genotypes 2 or 3—the risk of relapse is directly related to the time at which the patient developed undetectable HCV RNA levels during treatment (Figure 3). Interestingly, this is independent of genotype. Patients who obtain undetectable HCV RNA levels within 4 weeks of initiating treatment have the lowest relapse rate, approximately 10%. Patients with HCV genotype 1 who developed undetectable HCV RNA levels at 12 weeks have a relapse rate of approximately 30%, and patients who do not develop undetectable HCV RNA levels until 24 weeks have a relapse rate of nearly 60%.6 Patients with genotypes 2 or 3 who obtain undetectable HCV RNA levels after 4 weeks have a relapse rate of approximately 50%.4 Thus, those patients with a delayed response to treatment, despite receiving full doses of medication, are at the highest risk for relapse (Figure 2). This occurs most commonly in patients with HCV genotype 1, high viral load, HIV coinfection, African-American race, obesity, insulin resistance, and cirrhosis.
Figure 3.
Sustained virologic response (SVR) based upon the time at which the patient first develops undetectable hepatitis C virus (HCV) RNA levels.
Recent studies demonstrated that relapse could be reduced in patients with HCV genotype 1 who obtained undetectable HCV RNA levels after 4–12 weeks if the treatment duration is prolonged from 48 to 72 weeks29–31 or if the RBV dose is increased.32 Thus, re-treatment of patients with prior relapse should include one or both of these strategies. Studies have not yet demonstrated that longer duration of therapy could reduce relapse in patients with genotypes 2 or 3 who developed undetectable HCV RNA levels after 4 weeks. However, relapse rates increased from 4-6% to 10-11% in patients with HCV genotype 2 or 3 when the treatment duration was reduced from 48 to 24 weeks.3 This suggests that patients with HCV genotype 2 or 3 who previously relapsed after 24 weeks of treatment could have a higher chance of achieving SVR if re-treated for up to 48 weeks.
Relapse may also occur in patients for whom the dose of PEG-IFN was reduced or dosing with PEG-IFN and/or RBV was temporarily interrupted or reduced either prior to or after the patient developed undetectable HCV RNA levels.14–16,28 Similar to patients with virologic breakthrough, those who relapse because of dose modification do not require higher doses of PEG-IFN because they already obtained undetectable HCV RNA levels at the dose previously given and may not require a longer duration of therapy. Rather, the reason for the dose modification should be identified and avoided during re-treatment. If successful, this could lead to SVR during re-treatment.
Factors That Contribute to Treatment Failure
For chronic HCV treatment to be successful, it is critical to monitor HCV RNA levels frequently during treatment until one of the various nonresponse patterns is recognized or the patient develops undetectable HCV RNA levels. If the patient develops an adverse event that requires dose reduction, HCV RNA levels should be assessed again to ensure that breakthrough has not occurred. HCV RNA levels should also be checked at the end of treatment and at 12 and/or 24 weeks after treatment has been discontinued to assess for SVR. A patient may fail treatment if the treating physician does not monitor HCV RNA levels frequently during treatment, fails to recognize which of the virologic nonresponse patterns has occurred, and/or fails to prolong therapy after a delayed virologic response. Other factors associated with increased treatment failure rates are addressed below.
Noncompliance
Missing doses of PEG-IFN and/or RBV within the first 12-24 weeks of treatment, especially prior to developing undetectable HCV RNA levels, is a frequent reason for failing HCV treatment. Educating patients regarding the need for compliance prior to initiating HCV treatment and questioning patients regarding missed doses during treatment is essential when monitoring a patient receiving HCV treatment or evaluating a patient who previously failed therapy for possible re-treatment.
Alcohol and Drug Use
Chronic excessive alcohol consumption and illicit drug use just prior to initiating and/or during HCV therapy enhance treatment failure and should be considered another manifestation of noncompliance. Alcohol abuse is common among patients with chronic hepatitis C. Nearly one-third of patients with HCV infection have a history of recent alcohol abuse.33 Patients who consume in excess of 30 g of alcohol on a chronic basis have a reduced SVR rate, even if they stop drinking just prior to the initiation of HCV treatment.33–36 Such patients have a higher likelihood of using alcohol to combat the adverse events associated with PEG-IFN and RBV, which is associated with a higher rate of premature treatment discontinuation.
Injection drug use is the most common risk factor associated with HCV infection.37 Hepatitis C is the most common infectious disease among drug users and exceeds 50% in some settings.37,38 Although some studies suggest that active drug users with chronic HCV can be successfully treated,38,39 it is probably much more appropriate to delay HCV treatment in these patients. Patients who actively use illicit drugs until just prior to initiating HCV therapy have been shown to return to drug use to combat the adverse events of treatment and have a higher rate of noncompliance.40
Prior to initiating PEG-IFN and RBV treatment, abstinence from alcohol and illicit drugs for at least 6 months and appropriate counseling for patients at risk for relapse have been advocated.33
Prior Treatment with Less Effective Medications
Many patients with nonresponse or relapse may have been treated with standard IFN, with or without RBV, many years ago. The combination of PEG-IFN and RBV is more effective at achieving SVR than either of these previous therapies.1,2 When re-treated with PEG-IFN and RBV, 50% of patients with prior relapse after treatment with standard IFN and RBV achieve SVR.25,41 In addition, approximately 15% of patients with prior nonresponse to standard IFN and RBV therapy and up to 30% of patients with prior nonresponse to standard IFN monotherapy achieve SVR when re-treated with PEG-IFN and RBV.16,25,41 The highest rate of response when retreated with a more effective therapy is observed among patients with partial response to the previous therapy.24,25
Adverse Events Leading to Dose Reductions
The adverse events associated with PEG-IFN and RBV require that treatment with these medications either be discontinued or interrupted, or that their doses be reduced in up to 20-25% of patients.1,2,42 The most common adverse events requiring dose modifications include severe flu-like symptoms, depression, anemia, neutropenia, and thrombocytopenia.42 PEG-IFN and RBV may cause many other adverse events, some of which may be severe or irreversible and may preclude further treatment. However, these are far less common.42
A retrospective analysis of data from a large prospective trial demonstrated that patients who take less than 80% of the cumulative total recommended doses of PEG-IFN and/or RBV have lower SVR rates than patients who are able to remain on the recommended doses of these medications.14,15 In contrast, a recent detailed analysis that evaluated PEG-IFN dose reductions independent of RBV dose reductions suggested that reducing the dose of PEG-IFN lowers the rate of SVR by increasing the null response and partial response rates. In contrast, reducing the RBV dose did not affect either virologic response or SVR, unless RBV dosing was temporarily interrupted or prematurely discontinued, especially if the dose reduction occurred after HCV RNA levels became undetectable.17,43
The management of anemia is one of the most controversial aspects of HCV therapy. Several studies clearly demonstrated that epoetin alfa44,45 or another hematologic growth factor45 can increase hemoglobin concentrations and improve the quality of life46 in patients who have developed severe anemia during PEG-IFN and RBV treatment. However, none of these studies demonstrated that this approach could reduce treatment failure rates. In addition, a recent randomized, controlled trial demonstrated that the routine use of epoetin alfa with PEG-IFN and weight-based dosing of RBV did not enhance EVR or virologic response rates or reduce relapse rates, given the same starting dose of RBV.47 Finally, the use of epoetin alfa to treat anemia arising with HCV treatment has been associated with the development of erythropoietin antibodies and pure red cell aplasia.48 As a result, the use of erythropoietic growth factors to treat this anemia cannot be routinely recommended in lieu of dose reduction.
Depression and other psychiatric disorders common among patients with chronic HCV infection may be exacerbated during PEG-IFN and RBV treatment and may adversely affect outcome. Depression, anxiety, and insomnia are the most common neuropsychiatric adverse events associated with HCV treatment and frequently require PEG-IFN dose reduction or temporary discontinuation, enhancing the rate of null response, partial response, and virologic breakthrough.42 Aggressive management of these adverse events may enable patients to remain on full doses of PEG-IFN and may enhance virologic response.
Coinfection with HIV
The treatment of patients coinfected with HCV and HIV is complicated by a number of confounding conditions. The use of antiretroviral agents to treat HIV infection may cause direct hepatotoxicity and prevent HCV treatment from being initiated.49,50 Antiretroviral agents also cause myelosuppression, which may enhance the incidence of neutropenia and anemia associated with PEG-IFN and RBV treatment.51–53 Coinfected patients also have an increased incidence of neurocognitive dysfunction, psychiatric disorders, and substance abuse.18 All of these factors may increase the need to dose-reduce or prematurely discontinue PEG-IFN and RBV treatment, thereby enhancing the likelihood of null response, partial response, and virologic breakthrough.
Patients with HCV and HIV coinfection also have a higher incidence of null response, partial response, delayed response, and relapse, even if they remain on full doses of PEG-IFN and RBV for the recommended 24–48 weeks.21,51,53,54 Although controlled trials have not been performed, it is highly likely that prolonging therapy duration in patients with HCV and HIV coinfection will reduce the relapse rate in patients with delayed virologic response.
Advanced Age
Several studies have demonstrated that older patients have a higher failure rate after HCV treatment than younger patients.55–57 The apparent cause of the difference in failure rates is the occurrence of more frequent and more severe adverse events in older patients. Anemia appears to be particularly severe in older individuals and is, potentially, secondary to more severe bone marrow suppression by PEG-IFN.57 Whether hematologic growth factors could overcome the marrow suppressive effects of PEG-IFN in the elderly has not been specifically addressed.
The more severe adverse-event profile observed in older patients requires that the PEG-IFN and/or RBV dose be modified or prematurely discontinued more than twice as often than in younger patients. In patients older than 65 years of age, 29% were unable to remain on treatment compared to 20% of patients aged60–64 years. In contrast, only 11% of patients younger than 60 years of age discontinued treatment prematurely secondary to adverse events.57 This leads to a higher rate of null response, partial response, and virologic breakthrough in older patients.
Race
Three prospective, controlled trials demonstrated that African Americans with chronic HCV genotype 1 have significantly lower SVR rates when treated with PEG-IFN and RBV compared to white patients.22,58,59 In addition, a recent retrospective analysis showed that African Americans with HCV genotypes 2 or 3 also have SVR that is roughly 40–50% lower than that observed in white patients.60 The higher rate of treatment failure associated with African Americans appears to be secondary to a much higher rate of null response and partial virologic response.
In addition, African-American patients who respond to PEG-IFN and RBV treatment have a much slower decline in serum HCV RNA levels and a higher relapse rate than white patients.2,61 The possibility that prolonging therapy to 72 weeks or longer in African Americans who develop undetectable HCV RNA levels late during the standard course of treatment has not been specifically evaluated.
African Americans have a lower baseline white blood cell count and absolute neutrophil count (ANC) than white patients.22,58,59 Treatment with PEG-IFN and RBV leads to further declines in ANC and may require the PEG-IFN dose to be reduced. However, a recent study demonstrated that ANC in African Americans receiving PEG-IFN rarely declines below 500/mm3 and is not associated with an increased incidence of infection.58 Because reducing the PEG-IFN dose is associated with a higher risk of treatment failure, this strategy should be avoided in African Americans, if possible.
Advanced Fibrosis and Cirrhosis
Multiple clinical studies have demonstrated that patients with advanced fibrosis and cirrhosis have a treatment failure rate that is approximately 15–20% higher than patients with no or mild fibrosis.1,62–64 Furthermore, in a study of nonresponders re-treated with PEG-IFN and RBV, patients with cirrhosis had a lower SVR rate than patients with advanced fibrosis.65 Patients with cirrhosis have a higher incidence of thrombocytopenia, neutropenia, and anemia than patients with less advanced fibrosis.42 This leads to a higher rate of null response, partial response, virologic breakthrough, and relapse. However, a recent study also demonstrated that patients with cirrhosis have a higher rate of treatment failure, even in the absence of dose reduction.65
Obesity
Up to 20% of patients infected with HCV are classified as obese and have a body mass index (BMI) in excess of 30 kg/m2. Regardless of the type of PEG-IFN used, the SVR rate among obese patients is substantially lower than among patients with normal body weight.20 In contrast, dosing RBV by body weight, as opposed to a fixed dose of 800 mg daily, is associated with a higher SVR rate in obese patients.66 It is not currently known if the reduced SVR rate observed in obese patients is secondary to a higher rate of null response, partial response, relapse, or all three of these nonresponse patterns.
Three mechanisms have been proposed for the reduced chance of SVR in obese patients with chronic HCV infection. These include a decline in the bioavailability of IFN and/or RBV, an alteration in cytokine function secondary to the obese state, and the presence of insulin resistance.67 The possibility that higher doses of PEG-IFN and/or RBV could enhance SVR rates in obese patients with HCV genotype 1 and high baseline viral load is currently being explored in a randomized, controlled trial.
Insulin Resistance and Diabetes Mellitus
Insulin resistance is common among patients with chronic hepatitis C68 and may arise from disruption of the insulin signaling pathways by HCV proteins.69 An increased incidence of type 2 diabetes mellitus has also been linked to chronic hepatitis C. Patients with chronic HCV infection have a higher incidence of diabetes mellitus than patients with other chronic liver diseases, and patients with diabetes mellitus are nearly twice as likely to have chronic HCV infection compared to patients without diabetes mellitus.70
Insulin resistance has been shown to impair virologic response to antiviral therapy. Hyperinsulinemia hinders the ability of PEG-IFN to inhibit HCV replication in the replicon assay. In addition, patients with insulin resistance, as measured by the homeostasis model assessment-insulin resistance (HOMA-IR), are slightly more likely to fail HCV treatment than patients with a normal insulin index.23 These observations led some investigators to suggest that using an insulin-sensitizing agent along with PEG-IFN and RBV in patients with an elevated HOMA-IR score could reduce the risk of HCV treatment failure. However, it is currently unknown which of the various nonresponse patterns could be affected by insulin resistance or if this strategy would even be effective in enhancing SVR rates. Thus, the routine use of an insulin-sensitizing agent along with PEG-IFN and RBV to treat chronic HCV infection cannot be recommended at this time.
Conclusion
Multiple factors may contribute to treatment failure in patients receiving PEG-IFN and RBV for chronic HCV infection. Understanding and recognizing the various patterns of virologic response and nonresponse is critical to the success of therapy in these patients. This requires the frequent monitoring of the level of serum HCV RNA during the course of treatment. Although many patients currently fail HCV treatment, aggressive management of adverse events may reduce the frequency of null response, partial response, and virologic breakthrough, thereby enhancing virologic response and SVR rates. Prolonging HCV treatment in patients with late virologic response may reduce relapse rates and enhance SVR rates. In contrast, many factors associated with treatment failure cannot be corrected at the present time. The possibility that higher doses of PEG-IFN and/or RBV could enhance treatment responses and SVR rates is currently being explored in a randomized, controlled trial. The development of specific antiviral agents against HCV may enable patients with null response, partial response, and relapse to be more effectively treated in the future.
References
- 1.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 3.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-a2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 4.Shiffman ML, Suter F, Bacon BR, et al. A randomized controlled trial evaluating 16 and 24 weeks of peginterferon alfa-2a plus ribavirin in patients with HCV genotypes 2 or 3. N Engl J Med. In press. [Google Scholar]
- 5.Shiffman ML, Pappas S, Nyberg S, et al. Peginterferon alfa-2A (PEGASYS) plus ribavirin (COPEGUS) for 16 or 24 weeks in patients with HCV genotype 2 or 3. Final results of the ACCELERATE trial. Presented at the European Association for the Study of Liver 41st Annual Meeting; April 26-30, 2006; Vienna, Austria. Abstract 734.
- 6.Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Shiffman ML. Chronic hepatitis C: treatment of pegylated interferon/ribavirin nonresponders. Curr Gastroenterol Rep. 2006;8:46–52. doi: 10.1007/s11894-006-0063-z. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman ML. Management of patients with chronic hepatitis C virus infection and previous nonresponse. Rev.Gastroenterol Disord. 2004;4(suppl 1):S22–S30. [PubMed] [Google Scholar]
- 9.Sethi A, Shiffman ML. Approach to the management of patients with chronic hepatitis C who failed to achieve sustained virologic response. Infect Dis Clin North Am. 2006;20:115–135. doi: 10.1016/j.idc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Shiffman ML. Retreatment of patients who do not respond to initial therapy for chronic hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S13–S16. doi: 10.3949/ccjm.71.suppl_3.s13. [DOI] [PubMed] [Google Scholar]
- 11.Shiffman ML. Retreatment of patients with chronic hepatitis C. Hepatology. 2002;36:S128–S134. doi: 10.1053/jhep.2002.36816. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay KL. Introduction to therapy of hepatitis C. Hepatology. 2002;36:S114–S120. doi: 10.1053/jhep.2002.36226. [DOI] [PubMed] [Google Scholar]
- 13.Sethi A, Shiffman ML. Approach to the management of patients with chronic hepatitis C who failed to achieve sustained virologic response. Clin Liver Dis. 2005;9:453–471. doi: 10.1016/j.cld.2005.05.002. vii-viii. [DOI] [PubMed] [Google Scholar]
- 14.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 15.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman ML, Di Bisceglie AM, Lindsay KL, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing pegin-terferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132:103–112. doi: 10.1053/j.gastro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Sulkowski MS, Thomas DL. Perspectives on HIV/hepatitis C virus co-infection, illicit drug use and mental illness. AIDS. 2005;19(suppl 3):S8–S12. doi: 10.1097/01.aids.0000192064.09281.48. [DOI] [PubMed] [Google Scholar]
- 19.Thabut D, Le CS, Thibault V, et al. Hepatitis C in 6,865 patients 65 yr or older: a severe and neglected curable disease? Am J Gastroenterol. 2006;101:1260–1267. doi: 10.1111/j.1572-0241.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- 20.Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639–644. doi: 10.1053/jhep.2003.50350. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CA, Shafran SD. Treatment of hepatitis C in HIV-coinfected patients. Ann Pharmacother. 2006;40:479–489. doi: 10.1345/aph.1G427. [DOI] [PubMed] [Google Scholar]
- 22.Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39:1702–1708. doi: 10.1002/hep.20212. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Gomez M, Del Mar V, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 24.Shiffman ML, Hofmann CM, Gabbay J, et al. Treatment of chronic hepatitis C in patients who failed interferon monotherapy: effects of higher doses of interferon and ribavirin combination therapy. Am J Gastroenterol. 2000;95:2928–2935. doi: 10.1111/j.1572-0241.2000.02321.x. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson IM, Gonzalez SA, Ahmed F, et al. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol. 2005;100:2453–2462. doi: 10.1111/j.1572-0241.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 26.Marcellin P, Jensen D. Retreatment with Pegasys in patients not responding to prior peginterferon alfa-2b/ribavirin (RBV) combination therapy-efficacy analysis of the 12-week induction period of the REPEAT study. Presented at 56th annual meeting of the American Association for the Study of Liver Diseases; November 11-15, 2005; San Francisco, California. Abstract LB04. Hepatology. 2005;42(suppl 1):749A–750A. [Google Scholar]
- 27.Kaiser S, Hass H, Gregor M. Successful retreatment of peginterferon nonresponder patients with chronic hepatitis C with high dose consensus interferon induction therapy. Presented at Digestive Disease Week; May 15-20, 2004; New Orleans, Louisiana. Abstract 125. Gastroenterology. 2004;126(suppl 2):A-668. [Google Scholar]
- 28.Bronowicki JP, Ouzan D, Asselah T, et al. Effect of ribavirin in genotype 1 patients with hepatitis C responding to pegylated interferon alfa-2a plus ribavirin. Gastroenterology. 2006;131:1040–1048. doi: 10.1053/j.gastro.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer JT, Nevens F, Bekkering FC, et al. Reduction of relapse rates by 18-month treatment in chronic hepatitis C. A Benelux randomized trial in 300 patients. J Hepatol. 2004;40:689–695. doi: 10.1016/j.jhep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Tapias JM, Diago M, Escartin P, et al. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131:451–460. doi: 10.1053/j.gastro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Berg T, von Wagner M, Nasser S, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–1097. doi: 10.1053/j.gastro.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Shiffman ML, Price A, Hubbard S, et al. Treatment of chronic hepatitis C virus (HCV) genotype 1 with peginterferon alfa-2b (PEGIFN) high weight based dose ribavirin (RVN) and epoetin alfa (EPO) enhances sustained virologic response (SVR). Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases; November 11-15, 2005; San Francisco, California. Abstract 55. Hepatology. 2005;42(suppl 1):217A. [Google Scholar]
- 33.Anand BS, Currie S, Dieperink E, et al. Alcohol use and treatment of hepatitis C virus: results of a national multicenter study. Gastroenterology. 2006;130:1607–1616. doi: 10.1053/j.gastro.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Loguercio C, Di PM, Di Marino MP, et al. Drinking habits of subjects with hepatitis C virus-related chronic liver disease: prevalence and effect on clinical, virological and pathological aspects. Alcohol Alcohol. 2000;35:296–301. doi: 10.1093/alcalc/35.3.296. [DOI] [PubMed] [Google Scholar]
- 35.Ohnishi K, Matsuo S, Matsutani K, et al. Interferon therapy for chronic hepatitis C in habitual drinkers: comparison with chronic hepatitis C in infrequent drinkers. Am J Gastroenterol. 1996;91:1374–1379. [PubMed] [Google Scholar]
- 36.Cheung RC, Currie S, Shen H, et al. Chronic hepatitis C in Latinos: natural history, treatment eligibility, acceptance, and outcomes. Am J Gastroenterol. 2005;100:2186–2193. doi: 10.1111/j.1572-0241.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 37.Sulkowski MS, Thomas DL. Epidemiology and natural history of hepatitis C virus infection in injection drug users: implications for treatment. Clin Infect Dis. 2005;40(suppl 5):S263–S269. doi: 10.1086/427440. [DOI] [PubMed] [Google Scholar]
- 38.Backmund M, Meyer K, Von ZM, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 39.Cournot M, Glibert A, Castel F, et al. Management of hepatitis C in active drugs users: experience of an addiction care hepatology unit. Gastroenterol Clin Biol. 2004;28:533–539. doi: 10.1016/s0399-8320(04)95008-7. [DOI] [PubMed] [Google Scholar]
- 40.Sylvestre DL, Clements BJ. The impact of negative prognostic factors on hepatitis C treatment outcomes in recovering injection drug user. Presented at the American Association for the Study of Liver Diseases Annual Meeting; November 2-5, 2005; Boston, Massachusetts. Abstract. Hepatology. 2002;36:A223. [Google Scholar]
- 41.Krawitt EL, Ashikaga T, Gordon SR, et al. Peginterferon alfa-2b and ribavirin for treatment-refractory chronic hepatitis C. J Hepatol. 2005;43:243–249. doi: 10.1016/j.jhep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Shiffman ML. Side effects of medical therapy for chronic hepatitis C. Ann Hepatol. 2004;3:5–10. [PubMed] [Google Scholar]
- 43.Reddy KR, Shiffman ML, Morgan TR, et al. Impact of ribavirin dose reductions in hepatitis C virus genotype 1 patients completing peginterferon alfa-2a/ribavirin treatment. Clin Gastroenterol Hepatol. 2006;5:124–129. doi: 10.1016/j.cgh.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Dieterich DT, Wasserman R, Brau N, et al. Once-weekly epoetin alfa improves anemia and facilitates maintenance of ribavirin dosing in hepatitis C virus-infected patients receiving ribavirin plus interferon alfa. Am J Gastroenterol. 2003;98:2491–2499. doi: 10.1111/j.1572-0241.2003.08700.x. [DOI] [PubMed] [Google Scholar]
- 45.Afdhal NH, Dieterich DT, Pockros PJ, et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–1311. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 46.Pockros PJ, Carithers R, Desmond P, et al. Efficacy and safety of two-dose regimens of peginterferon alpha-2a compared with interferon alpha-2a in chronic hepatitis C: a multicenter, randomized controlled trial. Am J Gastroenterol. 2004;99:1298–1305. doi: 10.1111/j.1572-0241.2004.30306.x. [DOI] [PubMed] [Google Scholar]
- 47.Collantes RS, Younossi ZM. The use of growth factors to manage the hematologic side effects of PEG-interferon alfa and ribavirin. J Clin Gastroenterol. 2005;39(suppl 1):S9–13. doi: 10.1097/01.mcg.0000142583.00102.45. [DOI] [PubMed] [Google Scholar]
- 48.Stravitz RT, Chung H, Sterling RK, et al. Antibody-mediated pure red cell aplasia due to epoetin alfa during antiviral therapy of chronic hepatitis C. Am J Gastroenterol. 2005;100:1415–1419. doi: 10.1111/j.1572-0241.2005.41910.x. [DOI] [PubMed] [Google Scholar]
- 49.Adeyemi OM. Hepatitis C in HIV-positive patients—treatment and liver disease outcomes. J Clin Gastroenterol. 2007;41:75–87. doi: 10.1097/01.mcg.0000225592.16448.f3. [DOI] [PubMed] [Google Scholar]
- 50.Lai AR, Tashima KT, Taylor LE. Antiretroviral medication considerations for individuals coinfected with HIV and hepatitis C virus. Aids Patient Care STDS. 2006;20:678–692. doi: 10.1089/apc.2006.20.678. [DOI] [PubMed] [Google Scholar]
- 51.O'Leary JG, Chung RT. Management of hepatitis C virus coinfection in HIV-infected persons. AIDS Read. 2006;16:313–320. [PubMed] [Google Scholar]
- 52.Soriano V, Nunez M, Camino N, et al. Hepatitis C virus-RNA clearance in HIV-coinfected patients with chronic hepatitis C treated with pegylated interferon plus ribavirin. Antivir Ther. 2004;9:505–509. [PubMed] [Google Scholar]
- 53.Hoefs J, Aulakh VS. Treatment of chronic HCV infection in special populations. Int J Med Sci. 2006;3:69–74. doi: 10.7150/ijms.3.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 55.Uka K, Suzuki F, Akuta N, et al. Efficacy of interferon monotherapy in young adult patients with chronic hepatitis C virus infection. J Gastroenterol. 2006;41:470–475. doi: 10.1007/s00535-006-1793-2. [DOI] [PubMed] [Google Scholar]
- 56.Hiramatsu N, Oze T, Kurashige N, et al. Should aged patients with chronic hepatitis C be treated with interferon and ribavirin combination therapy? Hepatol Res. 2006;35:185–189. doi: 10.1016/j.hepres.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Nudo CG, Wong P, Hilzenrat N, Deschenes M. Elderly patients are at greater risk of cytopenia during antiviral therapy for hepatitis C. Can J Gastroenterol. 2006;20:589–592. doi: 10.1155/2006/357259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conjeevaram HS, Fried MW, Jeffers LJ, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites [erratum in N Engl J Med. 2004;351:1268] N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 60.Shiffman ML, Mihas AA, Mallwala F, et al. Treatment of chronic hepatitis C virus in African Americans with genotypes 2 and 3. Am J Gastroenterol. 2007;102:761–766. doi: 10.1111/j.1572-0241.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 61.Layden-Almer JE, Ribeiro RM, Wiley T, et al. Viral dynamics and response differences in HCV-infected African American and white patients treated with IFN and ribavirin. Hepatology. 2003;37:1343–1350. doi: 10.1053/jhep.2003.50217. [DOI] [PubMed] [Google Scholar]
- 62.Marrache F, Consigny Y, Ripault MP, et al. Safety and efficacy of peginterferon plus ribavirin in patients with chronic hepatitis C and bridging fibrosis or cirrhosis. J Viral Hepat. 2005;12:421–428. doi: 10.1111/j.1365-2893.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 63.Bruno S, Camma C, Di MV, et al. Peginterferon alfa-2b plus ribavirin for naive patients with genotype 1 chronic hepatitis C: a randomized controlled trial. J Hepatol. 2004;41:474–481. doi: 10.1016/j.jhep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Lee SS, Bain VG, Peltekian K, et al. Treating chronic hepatitis C with pegylated interferon alfa-2a (40 KD) and ribavirin in clinical practice. Aliment Pharmacol Ther. 2006;23:397–408. doi: 10.1111/j.1365-2036.2006.02748.x. [DOI] [PubMed] [Google Scholar]
- 65.Everson GT, Hoefs JC, Seeff LB, et al. Impact of disease severity on outcome of antiviral therapy for chronic hepatitis C: lessons from the HALT-C trial. Hepatology. 2006;44:1675–1684. doi: 10.1002/hep.21440. [DOI] [PubMed] [Google Scholar]
- 66.Jacobson IM, Brown R, Freilich B, et al. Response to peginterferon alfa-2b and ribavirin for chronic hepatitis C in patients with body weight >125 kg; results from the WIN-R trial. Presented at the 57th Annual Meeting of the American Association for the Study of Liver Diseases; October 27-31, 2006; Boston, Massachusetts. Abstract 369. Hepatology. 2007;44(suppl 1):328A. [Google Scholar]
- 67.Charlton MR, Pockros PJ, Harrison SA. Impact of obesity on treatment of chronic hepatitis C. Hepatology. 2006;43:1177–1186. doi: 10.1002/hep.21239. [DOI] [PubMed] [Google Scholar]
- 68.Romera M, Corpas R, Romero GM. Insulin resistance as a non-invasive method for the assessment of fibrosis in patients with hepatitis C: a comparative study of biochemical methods. Rev Esp Enferm Dig. 2006;98:161–169. doi: 10.4321/s1130-01082006000300002. [DOI] [PubMed] [Google Scholar]
- 69.Koike K. Hepatitis C virus infection can present with metabolic disease by inducing insulin resistance. Intervirology. 2006;49:51–57. doi: 10.1159/000087263. [DOI] [PubMed] [Google Scholar]
- 70.Zein CO, Levy C, Basu A, Zein NN. Chronic hepatitis C and type II diabetes mellitus: a prospective cross-sectional study. Am J Gastroenterol. 2005;100:48–55. doi: 10.1111/j.1572-0241.2005.40429.x. [DOI] [PubMed] [Google Scholar]
Managing Side Effects Related to Treatment for Chronic Hepatitis C
Abstract: Treatment for chronic hepatitis C has improved substantially in recent years, yet nearly half of all patients treated with interferon (IFN)-based therapy still do not achieve sustained virologic response. Many reasons exist for treatment failure and may include both viral and host factors. Adverse effects to antiviral therapy, including hematologic and neuropsychiatric effects, are important contributors to dose reduction and treatment discontinuation, and contribute to poor adherence. These adverse treatment effects are, therefore, an important cause of treatment failure in patients undergoing combination antiviral therapy for chronic hepatitis C. Multiple strategies exist to aid clinicians in the treatment of patients who have shown no response to IFN-based therapy or have relapsed after its completion. Managing or reversing treatment-related adverse events during re-treatment is an important step in preventing subsequent treatment failure.
As antiviral therapy has improved, rates of sustained virologic response (SVR) have increased: 6-12% with interferon (IFN) monotherapy, 42% with IFN and ribavirin (RBV) combination therapy, and 55% with pegylated IFN (PEG-IFN) and RBV.1–4 Despite these advances, approximately half of patients either do not respond to initial IFN-based therapy or relapse after treatment.5 Re-treatment with PEG-IFN and RBV is a common strategy and yields SVR rates of 6–23%.6–13 Numerous factors have been shown to contribute to treatment failure in chronic hepatitis C, including lack of patient adherence, numerous viral- and host-related factors, and treatment-related adverse events. Importantly, these factors may be fixed and unchangeable or may be correctable prior to the initiation of re-treatment.14–16
Treatment-related adverse events, a major contributor to impaired antiviral response, are common and include myalgias, headache, and other flu-like symptoms, as well as dermatologic, gastrointestinal, neuro-psychiatric, and hematologic effects.1,17 Some adverse events associated with IFN-based therapy, such as anemia, neuropsychiatric effects, and flu-like symptoms, compromise quality of life and may necessitate dose reductions.1,17–21 Some evidence suggests that these adverse events, particularly influenza-like symptoms, are more severe with PEG-IFN than with standard IFN combination therapy.2
Treatment-related adverse effects cause a significant disruption in the personal and professional lives of patients, with fatigue, flu-like symptoms, and depression reported as the most troubling effects.22 In one study, 80% of patients surveyed reported moderate to severe adverse effects associated with treatment.22 In addition, 31% of patients were forced to quit their jobs because of physical or psychological symptoms, and one-fifth reported a significant impairment in their interpersonal relationships. Approximately one-third of patients reported having depression during treatment.
Discontinuation of therapy due to adverse events is also common, occurring in up to 27% of patients in clinical trials. Dose reductions have also been reported in up to one-third of patients.1,2,23 Importantly, treatment-related adverse effects related to PEG-IFN may worsen with time, especially after the first 24 weeks of therapy.24 Effective management of treatment-related adverse events is, therefore, essential for improving adherence and increasing the likelihood of SVR.22,25
The Impact of Adherence
Adherence to therapy is a correctable variable that can be changed to favorably improve treatment outcomes. Importantly, treatment-related adverse effects are the most common reason for lack of adherence to antiviral therapy.25 Patients who receive less than their optimal course of therapy are less likely to achieve SVR (Figure 1).24,26 In a retrospective analysis of a large clinical trial, patients who adhered to at least 80% of their IFN and RBV doses for at least 80% of their prescribed treatment duration were more likely to achieve SVR (Figure 2).25,27 In another study, early adherence (defined as occurring within the first 12 weeks of therapy) to at least 80% of PEG-IFN and RBV doses was also found to significantly improve outcomes.28 The early virologic response rate in these patients was 80%; however, patients with treatment durations of less than 80% had early virologic response rates of only 50%.28 Maintaining the prescribed dose of RBV is especially crucial to optimizing treatment outcomes. Decreasing the RBV dose to below 10.6 mg/kg daily early in the course of therapy has been linked to decreased SVR rates.2,26 Conversely, the likelihood of achieving SVR increases as the RBV dose increases.2
Figure 1.
Effect of dose reduction on pegylated interferon (PEG-IFN) alfa-2a and ribavirin (RBV) response. The incidence of dose reductions among patients who cleared the virus at the end of treatment and after follow-up.
Reprinted with permission from Aspinall and Pockros.24
Figure 2.
Sustained virologic response (SVR) rates in patients with chronic hepatitis C, according to adherence to both pegylated interferon alfa-2b and ribavirin.
≥80/≥80/≥80=adherent to ≥80% of IFN alfa-2b dose/adherent to ≥80% of ribavirin dose/adherent for ≥80% of prescribed treatment duration (48 weeks); <80/<80/>80, adherent to <80% of IFN alfa-2b dose/adherent to <80% of ribavirin dose/adherent for ≥80% of prescribed treatment duration; ITT=intent to treat.
*P=.057 vs original intent-to-treat analysis; †P=.065 vs less adherent group; ‡P=.046 vs original ITT analysis; §P=.08 vs less adherent group.
Reprinted with permission from Manns27 and created with data from Manns et al.2 and McHutchison et al.25
Common Treatment-related Adverse Effects
General management strategies are in place for all patients undergoing hepatitis C virus (HCV) therapy (Table 1).17 Patients should be educated about treatment-related adverse effects that may occur with combination therapy, especially effects that impair quality of life.17 Patients should also be monitored regularly, and dose reductions and treatment holidays should be implemented when necessary. Access to medical services and the involvement of an interdisciplinary team may help manage adverse effects.24
Table 1.
General Strategies for the Management of Adverse Events
|
Data from Fried.17
As in registration trials, the careful selection of patients based on the risk of adverse events is an important first safeguard in clinical practice; however, in clinical practice, antiviral agents are prescribed more often to patients who do not meet the strict inclusion criteria of registration trials.17 As a result, clinicians need to recognize that the severity and frequency of adverse events may differ between patients in these two treatment settings.17
Flu-like Syndrome
Patients treated with IFN-based regimens may experience a flu-like syndrome 6–8 hours after the initiation of treatment, with symptoms such as chills, fever, malaise, myalgias, arthralgias, tachycardia, and anorexia.29 In most patients, these symptoms persist for 48 hours after injection, but in some patients, they do not occur until 24–48 hours thereafter. Flu-like symptoms usually occur and are most severe during the first 2–3 weeks of therapy, with a decline in severity as treatment is continued. Management is supportive; acetaminophen (≤2 g/24 hr) and nonsteroidal anti-inflammatory drugs have been effective in controlling these symptoms, as have adequate sleep and hydration.24,30
Dermatologic Symptoms
Combination antiviral therapy for chronic hepatitis C has been associated with dermatologic side effects such as RBV-mediated pruritus.24 This macular-papular rash does not respond well to topical therapy but resolves with the cessation of therapy. RBV should be discontinued if the rash spreads to the face, as it may cause periorbital edema.24
Injection-site reactions occur in the vast majority of patients and may be managed by rotating the site of injection. Large, red, or tender injection-site reactions should be examined for the development of an abscess.24
Hematologic Complications
Cytopenias are common during combination ther-apy1,2,31,32 and are the most frequent laboratory anomalies causing dose reductions and discontinuations in major clinical trials (Table 2).1,2 Both standard IFN and PEG-IFN give rise to pancytopenia, which is mediated by dose-dependant bone marrow suppression,33,34 and both therapies contribute to the development of anemia by inhibiting the production of erythropoietin (EPO).35 RBV, specifically, is associated with a reversible hemolytic anemia caused by oxidative damage.36
Table 2.
Frequency of Dose Reduction of Pegylated Interferon/Ribavirin Therapy Secondary to Cytopenias in Chronic Hepatitis C
| Cytopenia | Dose reduction, % patients | Reference |
|---|---|---|
| Anemia | 9–22% | Manns et al2 Fried et al1 |
| Neutropenia | 20% (pegylated interferon alfa-2a) | Fried et al1 |
| 18% (pegylated interferon alfa-2b) | Manns et al2 | |
| Thrombocytopenia | 4–6% (pegylated interferon) | Fried et al1 |
Adapted with permission from Collantes and Younossie.31
Anemia
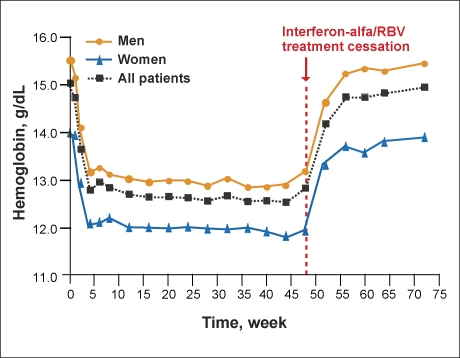
Both IFN and RBV decrease hemoglobin concentrations in patients with chronic hepatitis C.34 Therapy with standard IFN or PEG-IFN plus RBV results in average hemoglobin decreases of 2–3 g/dL early in treatment (Figure 3) that may persist until the cessation of treatment (Figure 4).34 Treatment-related anemia also increases fatigue and has a significant impact on quality of life.17 Declines in hemoglobin levels during RBV therapy are independently associated with renal function, baseline hemoglobin levels, and older age.34 In addition, anemia associated with either IFN or RBV is more pronounced in HIV/HCV-coinfected patients, as these patients have numerous risk factors for the development of anemia.37,38
Figure 3.
Magnitude of hemoglobin decreases in patients treated with interferon alfa-2b and ribavirin.
Adapted with permission from Sulkowski et al.34
Figure 4.
Time course of hemoglobin changes during treatment with interferon alfa-2b and ribavirin (RBV) in patients with chronic hepatitis C.
Adapted with permission from Sulkowski et al.34
Anemia has been shown to lead to dose reductions, reported in 9–22% of patients in two large clinical trials,2,17 and tends to occur within the first 2–4 weeks of therapy.17 Decreasing the RBV dose to more than 10.6 mg/kg daily early in the course of treatment decreases the likelihood of SVR, so it is especially important to manage anemia early on.26 A pharmacokinetic analysis showed that higher RBV serum concentrations at 4 weeks were associated with an increased viral response at 24 weeks.39 In a study conducted in 1,530 patients receiving combination therapy, RBV doses greater than 10.6 mg/kg were associated with higher rates of viral eradication than lower doses.2 Further, in another study, patients receiving RBV 1,000–1,200 mg daily had higher SVR rates than those receiving 800 mg daily.40
Until recently, the only available strategy for managing treatment-related anemia was RBV dose reduction or treatment discontinuation (Table 3).41 However, this strategy was associated with decreased SVR rates.2,25,42,43 Data from recent studies suggest that recombinant human EPO (rHuEPO, or epoetin alfa) should be considered for the management of treatment-related anemia during hepatitis C therapy.41,42,44 In an open-label, prospective pilot study of 56 patients with chronic hepatitis C treated with IFN and RBV, mean hemoglobin concentrations at the end of the study were 12.7 (± 1.7) g/dL in patients given epoetin alfa compared to 13.0 (± 1.4) g/dL in patients not given such treatment. (The difference between the groups was nonsignificant).35 However, in a multicenter, placebo-controlled trial of epoetin alfa, 185 HCV-infected patients who developed anemia (hemoglobin ≤12 g/dL) during combination therapy were treated with epoetin alfa (40,000 U once weekly) or placebo.41 At the end of an 8-week double-blind treatment phase, the RBV dose was maintained in 88% of patients receiving epoetin alfa and 60% of those receiving placebo. Mean hemoglobin concentrations increased by 2.2 (± 1.3) g/dL with epoetin alfa and by 0.1 (± 1.0) g/dL with placebo from randomization to the end of the double-blind treatment phase.41 Results were similar in 64 HCV-infected patients who had hemoglobin concentrations of 12 g/dL or less during the first 24 weeks of IFN and RBV therapy; 83% of the patients receiving epoetin alfa (40,000 U once weekly) maintained RBV dosage of at least 800 mg daily by the end of the study, compared to 54% of patients receiving standard anemia management care (ie, RBV dose reduction/discontinuation or transfusion; P=.022).44
Table 3.
Ribavirin Dose Reduction Guidelines
| Measured hemoglobin concentration | Ribavirin dose reduction |
|---|---|
| <10 g/dL |
|
| <8.5 g/dL | Discontinue ribavirin in patients receiving any pegylated interferon-based regimen |
Data from Sulkowski.42
In patients who did not respond to a prior IFN-based regimen, rHuEPO can be given as PEG-IFN and RBV therapy is initiated or as soon as hemoglobin decreases occur to potentially prevent dose reductions or treatment discontinuations and to increase the likelihood that retreatment will yield SVR.15 Although rHuEPO has been shown to improve hemoglobin levels, more research is needed to determine its impact on SVR rates.
Neutropenia
IFN is commonly associated with decreases in neutrophil counts, as a result of bone marrow suppression.45 In a study of 119 chronic HCV patients treated with IFN-alfa/RBV, neutrophil counts fell by an average of 34%.45 Neutropenia associated with PEG-IFN in patients with HCV and cirrhosis can occur within the first 2 weeks of therapy and increases the risk of septic complications.1,20 Treatment with PEG-IFN has been shown to induce neutropenia to a greater extent than treatment with standard IFN.17 However, evidence suggests that neutropenia in patients who receive IFN/RBV or PEG-IFN/RBV treatment is not associated with increased risk of serious infection.1,40,42,45
Most patients with treatment-related neutropenia can be safely managed with dose modification.42 The manufacturer's recommendations state that in patients with an absolute neutrophil count (ANC) lower than 750/mm3, PEG-IFN alfa-2b dosage should be reduced by 50% and PEG-IFN alfa-2a dosage should be reduced to 135 µg once weekly. In contrast, the recommendations for patients with an ANC lower than 500/mm3 state that PEG-IFN alfa-2b dosage should be discontinued permanently and PEG-IFN alfa-2a dosage should be discontinued until the ANC increases to 1,000/mm3 and then restarted at 90 µg.1,40,42,45 Neutrophil counts decrease rapidly during the first 2 weeks after the initiation of PEG-IFN therapy and then typically stabilize during the following 4 weeks, as steady-state concentrations of PEG-IFN are reached; furthermore, neutrophil counts rapidly return to baseline levels after the discontinuation of treatment.17 Additionally, the use of filgrastim (human granulocyte colony-stimulating factor produced by recombinant DNA technology) dosed at 300 µg 2 or 3 times per week may aid in preventing dose reduction or treatment discontinuation.15,42,46 Of note, the efficacy of filgrastim to correct treatment-related neutropenia has not been investigated in clinical trials,15 and the drug is not approved for use in patients with HCV who receive PEG-IFN-based therapy.42
Thrombocytopenia
The use of IFN in patients with HCV—especially in those with advanced fibrosis or cirrhosis—is associated with a quick, sustained reduction in peripheral platelet count.42,47 According to the manufacturer's recommendations, PEG-IFN alfa-2b dosage should be reduced by 50% in patients with a platelet count of less than 80,000/mm3 and should be permanently discontinued in patients with a platelet count of less than 50,000/mm3. In contrast, PEG-IFN alfa-2a dosage should be reduced to 135 µg in patients with a platelet count of less than 50,000/mm3 and should be permanently discontinued in patients with a platelet count of less than 25,000/mm3.42 However, many hepatologists believe that it is unnecessary to reduce dosage or discontinue treatment until the platelet count drops to 25,000-30,000/mm3.15 Eltrombopag, an oral platelet growth factor, is currently being investigated and has been shown to increase platelet counts in subjects with HCV.48 Nevertheless, at present, effective therapy is not available to correct treatment-related thrombocytopenia.15
Fatigue
Fatigue is one of the most common side effects of combination antiviral therapy for chronic hepatitis C and occurs in up to 64% of patients in clinical trials2,43,49 and in up to 74% of patients in a recent survey.22 Fatigue, measured by the Fatigue Severity Scale, has also been shown to be an independent predictor of premature drug discontinuation in patients receiving combination therapy.50 Patients should be told to expect fatigue and encouraged to plan accordingly. Injections of PEG-IFN may be given prior to a weekend to minimize workplace disruption. Although methylphenidate and amphetamine salts—drugs normally given for attention-deficit/hyperactivity disorder—are used in clinical practice in patients experiencing treatment-related fatigue, their role and efficacy remain poorly understood. Severe fatigue may also occur as a symptom of depression and may respond to antidepressant medication such as selective serotonin reuptake inhibitors.24
Neuropsychiatric Effects
Adverse neuropsychiatric events linked to antiviral treatment include the development of an acute confusional state, a depressive syndrome, and/or manic conditions (eg, irritability, agitation, euphoria).121 Interactions between neuropsychiatric adverse events may be complex and difficult to manage.17,52–54 Furthermore, other adverse events associated with IFN-based therapy (eg, insomnia, fatigue) could potentially exacerbate neuropsych-iatric symptoms.17,55,56 In patients with a history of alcoholism, neuropsychiatric adverse events can escalate alcohol abuse.57,58
Treatment-induced psychiatric events may compromise quality of life and lead to dose reduction and treatment discontinuation.59 Psychiatric adverse events can occur shortly after the initiation of IFN therapy and, at other times, as a result of ongoing treatment 60–62 or after the end of treatment.63
Depression
Treatment-related depression is common, may lower adherence, and may affect viral outcomes.64 Depression is a well-described adverse effect of IFN therapy, occurring in 20-50% of patients.2,17,43,65 Depression is also a common indication for dose reduction and may, therefore, lower SVR rates.2,17 The exact mechanism of IFN-medi-ated depression is unknown, but it may involve the action of IFN on glucocorticoid and serotonin 1A receptors, both of which have been implicated in the pathogenesis of depression.66,67 Reductions in serotonin levels, mediated by tryptophan depletion, has been shown to correlate with the development of depression in patients undergoing IFN therapy.68
Treatment with IFN has been shown to precipitate mania and hypomania, although these symptoms had previously been considered to be infrequent.69,70 Recent data, however, suggest that these symptoms may be more common than previously thought. A European study found that up to 20% of patients undergoing IFN-based therapy for chronic hepatitis C showed manic or hypomanic symptoms at some point during treatment.71 A recent prospective study was undertaken to analyze the rates of neuropsychiatric side effects in 99 patients undergoing IFN-based treatment for chronic hepatitis C. Psychiatric adverse events occurred in approximately one-third of patients and included 3 cases of mania (3%) and 15 cases of irritable hypomania (50%). Importantly, there were also 12 cases (40%) of a depressive mixed state.72 Care should be taken to differentiate between IFN-induced unipolar depression and depression in a mixed-mania state, considering the differences in the management of the two states. The development of mania has generally been an indication for the discontinuation of IFN, followed by prompt psychiatric referral for mood stabilizers.73
Patients who experience treatment-related depression may still achieve viral response. In a recent study of 98 consecutive treatment-naive chronic hepatitis C patients, 39% experienced psychiatric side effects (ie, mood disorders), mainly within the first 12 weeks of therapy (87%). However, the rate of dose reductions did not differ between patients who experienced psychiatric symptoms and patients who did not (46% vs 37%; P=NS). In addition, SVR rates were similar between the groups. Early detection of mood disorders with appropriate intervention can maximize treatment outcomes in patients experiencing neuropsychiatric symptomatology.74
Patients receiving IFN should, therefore, be assessed for depression at least every 2 weeks using validated depression scales.3,59 Patients should be advised of the risk of treatment-related depression and instructed on symptom recognition.59 Repeated psychiatric assessment during and after treatment and interdisciplinary treatment involving both physicians and psychiatrists could yield important benefits.63 Figure 5 presents an algorithm for the evaluation and management of patients who experience depression while undergoing IFN therapy.51
Figure 5.
Algorithm for the treatment of interferon-induced depression.
- BDI
- Beck Depression Inventory
- CES-D
- Center for Epidemiological Studies Depression Rating Scale
- SRI
- serotonin reuptake inhibitor
- Zung SDS
- Zung Self-Rating Depression Scale Index score.
Adapted with permission from Raison et al.51
Antidepressant treatment for IFN-induced depression is effective in approximately 80% of patients, and response is rapid with fairly small doses.75,76 Most patients with IFN-related depression respond to treatment with selective serotonin reuptake inhibitors, often allowing the continuation of treatment without dose reduction.59,75,77,78 IFN-based therapy can be successfully initiated in patients who have responded to antidepressant therapy, provided that the patient is continually monitored by a mental healthcare professional.59,79
The choice of selective serotonin reuptake inhibitor should be based on primary symptomatology. For example, fluoxetine or sertraline, which may be more stimulating than paroxetine and fluvoxamine, may be preferred in patients with diminished cognition or fatigue.80,81 Little data exist on the effectiveness of other antidepressants (eg, venlafaxine, bupropion, nefazodone) in patients with chronic hepatitis C.81 Tricyclic antidepressants may be sedating and, thus, generally should not be prescribed in this patient population.81 IFN therapy should be discontinued, and immediate psychiatric assessments should be made in patients with worsening depression or psychotic/suicidal tendencies.59,82 Because IFN-related psychiatric adverse events may persist after the end of treatment, continued assessment is warranted.63
Strategies and Side Effects Related to Managing Treatment Failure
Managing treatment-related side effects is a crucial step in improving treatment outcomes in patients receiving combination therapy. In patients undergoing re-treatment after failing earlier treatment, it is also important to administer an appropriate and effective therapeutic regimen.5 Logically, to achieve SVR, the re-treatment regimen must be more effective than the initial therapy.5,16,83 Re-treatment with the initial pharmacologic regimen will likely lead to the same outcome, unless dose reduction or premature treatment discontinuation contributed to treatment failure.5,83
Regimens containing both PEG-IFN and consensus IFN (CIFN) have been shown to be effective in clinical trials. Re-treatment with PEG-IFN has been widely studied and is associated with SVR rates of approximately 6–23%.6–13 Using CIFN to re-treat patients who failed earlier therapy has also been under recent investigation.84–88 Although the optimal CIFN regimen has not yet been determined, the combination of high-dose CIFN and RBV is a promising option for the re-treatment of patients who have not responded to or who have relapsed from previous IFN-based therapy.
Tables 4 and 5 list common adverse effects and the frequencies of their occurrences in clinical trials in the retreatment of patients who failed earlier IFN-based therapy for chronic hepatitis C.
Table 4.
Common Adverse Effects of Pegylated Interferon and Ribavirin Combination Therapy and Incidence in Treatment-Naive Patients
| Adverse effect | Incidence |
|---|---|
| Dermatologic | |
| Injection-site reaction | 36–58% |
| Pruritus | 21–26% |
| Constitutional | |
| Arthralgia | 25–34% |
| Fatigue | 47–64% |
| Headache | 47–62% |
| Myalgia | 37–56% |
| Pyrexia | 39–43% |
| Rigors | 24–48% |
| Neuropsychiatric | |
| Depression | 15–30% |
| Irritability | 24–35% |
| Insomnia | 33–40% |
| Gastrointestinal | |
| Anorexia | 15–32% |
| Diarrhea | 16–22% |
| Nausea | 29–43% |
| Hematologic | |
| Anemia | 3–22% |
| Neutropenia | 3–20% |
| Thrombocytopenia | 4–6% |
Table 5.
Adverse Effects and Incidence in Patients Re-treated With Consensus Interferon
| Adverse effect | Incidence |
|---|---|
| Hematologic | |
| Neutropenia | 16–27% |
| Anemia | 8–28% |
| Flu-like symptoms | 16–85% |
| Gastrointestinal disturbance | 26–44% |
| Skin disorders | 32–38% |
| Neuropsychiatric disorders | 18–26% |
Conclusion
The treatment of chronic hepatitis C is still associated with a high failure rate, and SVR rates upon re-treatment have been disappointingly low. Treatment-related side effects—flu-like symptoms, hematologic disorders, skin and gastrointestinal symptoms, and neuropsychiatric symptoms—are common and may lead to dose reduction or treatment discontinuation. The management of side effects is, therefore, crucial to preserve SVR rates in patients undergoing treatment. Proper patient selection is an important first step in minimizing the possibility of treatment failure, and patients who undergo treatment should be educated on the nature and frequency of common side effects. Constitutional symptoms of IFN-based therapy—including myalgias, arthralgias, headache, nausea, and diarrhea—may compromise quality of life but can be managed effectively with acetaminophen, nonsteroidal anti-inflammatory drugs, anti-emetics, and antimotility agents. Anemia and neutropenia are frequent and common indications for dose reductions; however, anemia can be effectively managed with erythropoietin and neutropenia can be managed with filgrastim. Depression is a common indication for treatment discontinuation, but it responds quickly to intervention with a selective serotonin reuptake inhibitor. Finally, using an effective antiviral re-treatment strategy is also important. Regimens containing PEG-IFN or CIFN have shown promise in re-treating patients who failed prior therapy.
References
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 4.Poynard T, Bedossa P, Chevallier M, et al. A comparison of three interferon alfa-2b regimens for the long-term treatment of chronic non-A, non-B hepatitis. Multicenter Study Group. N Engl J Med. 1995;332:1457–1462. doi: 10.1056/NEJM199506013322201. [DOI] [PubMed] [Google Scholar]
- 5.Keeffe EB. Chronic hepatitis C: management of treatment failures. Clin Gastroenterol Hepatol. 2005;3:S102–S105. doi: 10.1016/s1542-3565(05)00698-1. [DOI] [PubMed] [Google Scholar]
- 6.Gaglio P, Choi J, Zimmerman D, et al. Weight based ribavirin in combination with pegylated interferon alpha 2-b does not improve SVR in HCV infected patients who failed prior therapy: results in 454 patients. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases; November 11-15, 2005; San Francisco, California. Abstract 59. Hepatology. 2005;42(suppl 1):219A. [Google Scholar]
- 7.Jacobson IM, Gonzalez SA, Ahmed F, et al. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol. 2005;100:2453–2462. doi: 10.1111/j.1572-0241.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 8.Lawitz EJ, Bala NS, Becker S, et al. Pegylated interferon alfa 2b and ribavirin for hepatitis C patients who were nonresponders to previous therapy. Presented at Digestive Disease Week 2003; May 17-22, 2003; Orlando, Florida. Abstract 1293. Gastroenterology. 2003;124(suppl 1):A-783. [Google Scholar]
- 9.Poynard T, Schiff EG, Terg R, et al. Sustained virologic response (SVR) in the EPIC3 trial: week twelve virology predicts SVR in previous interferon/ribavirin treatment failures receiving PEG-Intron/Rebetol (PR) weight based dosing (WBD). Presented at the 40th annual meeting of the European Association for the Study of the Liver; April 13-17, 2005; Paris, France. Abstract 96. J Hepatol. 2005;42(suppl 2):40-41. [Google Scholar]
- 10.Sherman M, Yoshida EM, Deschenes M, et al. Peginterferon alfa-2a (40KD) plus ribavirin in chronic hepatitis C patients who failed previous interferon therapy. Gut. 2006;55:1631–1638. doi: 10.1136/gut.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taliani G, Gemignani G, Ferrari C, et al. Pegylated interferon alfa-2b plus ribavirin in the retreatment of interferon-ribavirin nonresponder patients. Gastroenterology. 2006;130:1098–1106. doi: 10.1053/j.gastro.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Teuber G, Kallinowski B, Niederau C, et al. Retreatment with pegylated interferon-alpha2b plus ribavirin in patients with chronic hepatitis C not responding to a previous antiviral treatment with standard interferons combined with ribavirin. Presented at Digestive Disease Week 2003; May 17-22, 2003; Orlando, Florida. Abstract 1216. Gastroenterology. 2003;124(suppl 1):A-699. [Google Scholar]
- 13.Gross J, Johnson S, Kwo P, Afdhal N, Flamm S, Therneau T. Double-dose peginterferon alfa-2b with weight-based ribavirin improves response for interferon/ribavirin non-responders with hepatitis C: final results of “RENEW.”. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases; November 11-15, 2005; San Francisco, California. Abstract 60. Hepatology. 2005;42(suppl 1):219A–220A. [Google Scholar]
- 14.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites [Erratum in N Engl J Med. 2004;351:1268] N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 15.Shiffman ML. Chronic hepatitis C: treatment of pegylated interferon/ribavirin nonresponders. Curr Gastroenterol Rep. 2006;8:46–52. doi: 10.1007/s11894-006-0063-z. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman ML. Retreatment of patients who do not respond to initial therapy for chronic hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S13–S16. doi: 10.3949/ccjm.71.suppl_3.s13. [DOI] [PubMed] [Google Scholar]
- 17.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(suppl 1):S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich-Rust M, Zeuzem S, Sarrazin C. Current therapy for hepatitis C. Int J Colorectal Dis. 2007;22:341–349. doi: 10.1007/s00384-005-0038-9. [DOI] [PubMed] [Google Scholar]
- 19.Crone C, Gabriel GM. Comprehensive review of hepatitis C for psychiatrists: risks, screening, diagnosis, treatment, and interferon-based therapy complications. J Psychiatr Pract. 2003;9:93–110. doi: 10.1097/00131746-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 20.De Filippi F, Colombo M. Treatment of chronic hepatitis C. J Biol Reggul Homeost Agents. 2003;17:133–137. [PubMed] [Google Scholar]
- 21.Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology. 1997;26:112S–121S. doi: 10.1002/hep.510260720. [DOI] [PubMed] [Google Scholar]
- 22.Zickmund SL, Bryce CL, Blasiole JA, et al. Majority of patients with hepatitis C express physical, mental, and social difficulties with antiviral treatment. Eur J Gastroenterol Hepatol. 2006;18:381–388. doi: 10.1097/00042737-200604000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 24.Aspinall RJ, Pockros PJ. The management of side effects during therapy for hepatitis C. Aliment Pharmacol Ther. 2004;20:917–929. doi: 10.1111/j.1365-2036.2004.02192.x. [DOI] [PubMed] [Google Scholar]
- 25.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 26.Mulhall BP, Younossi Z. Impact of adherence on the outcome of antiviral therapy for chronic hepatitis C. J Clin Gastroenterol. 2005;39:S23–S27. doi: 10.1097/01.mcg.0000145538.43865.72. [DOI] [PubMed] [Google Scholar]
- 27.Manns MP. Adherence to combination therapy: influence on sustained virologic response and economic impact. Gastroenterol Clin North Am. 2004;33:S11–S24. doi: 10.1016/j.gtc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Davis GL, Wong JB, McHutchison JG, et al. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 29.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 30.Zapater P, Lasso de la Vega MC, Horga JF, et al. Pharmacokinetic variations of acetaminophen according to liver dysfunction and portal hypertension status. Aliment Pharmacol Ther. 2004;20:29–36. doi: 10.1111/j.1365-2036.2004.02022.x. [DOI] [PubMed] [Google Scholar]
- 31.Collantes RS, Younossi ZM. The use of growth factors to manage the hematologic side effects of PEG-interferon alfa and ribavirin. J Clin Gastroenterol. 2005;39(1) suppl:S9–13. doi: 10.1097/01.mcg.0000142583.00102.45. [DOI] [PubMed] [Google Scholar]
- 32.Sulkowski M. Clinical Care Options-Hepatitis Annual Update 2003. Milford, MA: IMedOptions; 2003. Managing the hematologic complications of interferon/ribavirin [learning module] pp. 101–122. [Google Scholar]
- 33.Peck-Radosavljevic M, Wichlas M, Homoncik-Kraml M, et al. Rapid suppression of hematopoiesis by standard or pegylated interferon-alpha. Gastroenterology. 2002;123:141–151. doi: 10.1053/gast.2002.34175. [DOI] [PubMed] [Google Scholar]
- 34.Sulkowski MS, Wasserman R, Brooks L, et al. Changes in haemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J Viral Hepat. 2004;11:243–250. doi: 10.1111/j.1365-2893.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 35.Talal AH, Weisz K, Hau T, et al. A preliminary study of erythropoietin for anemia associated with ribavirin and interferon-alpha [letter] Am J Gastroenterol. 2001;96:2802–2804. doi: 10.1111/j.1572-0241.2001.04149.x. [DOI] [PubMed] [Google Scholar]
- 36.De Franceschi L, Fattovich G, Turrini F, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997–1004. doi: 10.1053/he.2000.5789. [DOI] [PubMed] [Google Scholar]
- 37.Dieterich DT. Hepatitis C virus and human immunodeficiency virus: clinical issues in coinfection. Am J Med. 1999;107:79S–84S. doi: 10.1016/s0002-9343(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 38.Sulkowski MS. Anemia in the treatment of hepatitis C virus infection. Clin Infect Dis. 2003;37(suppl 4):S315–S322. doi: 10.1086/376911. [DOI] [PubMed] [Google Scholar]
- 39.Jen JF, Glue P, Gupta S, et al. Population pharmacokinetic and pharm-acodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit. 2000;22:555–565. doi: 10.1097/00007691-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-a2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 41.Afdhal NH, Dieterich DT, Pockros PJ, et al. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–1311. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 42.Sulkowski MS. Management of the hematologic complications of hepatitis C therapy. Clin Liver Dis. 2005;9:601–616. doi: 10.1016/j.cld.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Hadziyannis SJ, Cheinquer H, Morgan T, et al. Peginterferon alfa-2a (40 kd) (Pegasys) in combination with ribavirin (RBV): efficacy and safety results from a phase III, randomized, double-blind, multicentre study examining effect of duration of treatment and RBV dose. Presented at the 37th annual meeting of the European Association for the Study of the Liver; April 15-21, 2002; Madrid, Spain. Abstract 536. J Hepatol. 2002;36(suppl 1):S3. [Google Scholar]
- 44.Dieterich DT, Wasserman R, Brau N, et al. Once-weekly epoetin alfa improves anemia and facilitates maintenance of ribavirin dosing in hepatitis C virus-infected patients receiving ribavirin plus interferon alfa. Am J Gastroenterol. 2003;98:2491–2499. doi: 10.1111/j.1572-0241.2003.08700.x. [DOI] [PubMed] [Google Scholar]
- 45.Soza A, Everhart JE, Ghany MG, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology. 2002;36:1273–1279. doi: 10.1053/jhep.2002.36502. [DOI] [PubMed] [Google Scholar]
- 46.Morstyn G, Foote MA, Walker T, Molineux G. Filgrastim (r-metHuG-CSF) in the 21st century: SD/01. Acta Haematol. 2001;105:151–155. doi: 10.1159/000046557. [DOI] [PubMed] [Google Scholar]
- 47.Peck-Radosavljevic M, Wichlas M, Pidlich J, et al. Blunted thrombopoietin response to interferon alfa-induced thrombocytopenia during treatment for hepatitis C. Hepatology. 1998;28:1424–1429. doi: 10.1002/hep.510280535. [DOI] [PubMed] [Google Scholar]
- 48.McHutchison JG, Afdhal NH, Dusheiko G, et al. Eltrombopag, an oral platelet growth factor, facilitates initiation of interferon therapy in subjects with HCV associated thrombocytopenia: results from a phase II placebo controlled, double-blind, dose-ranging study. Abstract LB3. Hepatology. 2006;44(4) suppl 1:692A–693A. [Google Scholar]
- 49.Fried MW, Shiffman M, Sterling RK, et al. A multicenter, randomized trial of daily high-dose interferon-alfa 2b for the treatment of chronic hepatitis C: pretreatment stratification by viral burden and genotype. Am J Gastroenterol. 2000;95:3225–3229. doi: 10.1111/j.1572-0241.2000.03433.x. [DOI] [PubMed] [Google Scholar]
- 50.Bernstein D, Kleinman L, Barker CM, et al. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 51.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontana RJ. Neuropsychiatric toxicity of antiviral treatment in chronic hepatitis C. Dig Dis. 2000;18:107–116. doi: 10.1159/000051384. [DOI] [PubMed] [Google Scholar]
- 53.Forton DM, Thomas HC, Murphy CA, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433–439. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- 54.Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–446. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- 55.Kraus MR, Schafer A, Csef H, et al. Compliance with therapy in patients with chronic hepatitis C: associations with psychiatric symptoms, interpersonal problems, and mode of acquisition. Dig Dis Sci. 2001;46:2060–2065. doi: 10.1023/a:1011973823032. [DOI] [PubMed] [Google Scholar]
- 56.Poynard T, Cacoub P, Ratziu V, et al. Fatigue in patients with chronic hepatitis C. J Viral Hepat. 2002;9:295–303. doi: 10.1046/j.1365-2893.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- 57.Schiff ER, Ozden N. Hepatitis C and alcohol. Alcohol Res Health. 2003;27:232–239. [PMC free article] [PubMed] [Google Scholar]
- 58.Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207–1211. doi: 10.1053/jhep.2000.7880. [DOI] [PubMed] [Google Scholar]
- 59.Hauser P. Neuropsychiatric side effects of HCV therapy and their treatment: focus on IFN alpha-induced depression. Gastroenterol Clin North Am. 2004;33:S35–S50. doi: 10.1016/j.gtc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 60.McDonald EM, Mann AH, Thomas HC. Interferons as mediators of psychiatric morbidity. An investigation in a trial of recombinant alpha-interferon in hepatitis-B carriers. Lancet. 1987;2:1175–1178. doi: 10.1016/s0140-6736(87)91319-5. [DOI] [PubMed] [Google Scholar]
- 61.Renault PF, Hoofnagle JH, Park Y, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147:1577–1580. [PubMed] [Google Scholar]
- 62.Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316–2326. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 63.Gohier B, Goeb JL, Rannou-Dubas K, et al. Hepatitis C, alpha interferon, anxiety and depression disorders: a prospective study of 71 patients. World J Biol Psychiatry. 2003;4:115–118. doi: 10.1080/15622970310029904. [DOI] [PubMed] [Google Scholar]
- 64.Raison CL, Broadwell SD, Borisov AS, et al. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav Immun. 2005;19:23–27. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai W, Khaoustov VI, Xie Q, et al. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 67.Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 68.Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 69.Strite D, Valentine AD, Meyers CA. Manic episodes in two patients treated with interferon alpha. J Neuropsychiatry Clin Neurosci. 1997;9:273–276. doi: 10.1176/jnp.9.2.273. [DOI] [PubMed] [Google Scholar]
- 70.Onyike CU, Bonner JO, Lyketsos CG, Treisman GJ. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161:429–435. doi: 10.1176/appi.ajp.161.3.429. [DOI] [PubMed] [Google Scholar]
- 71.Gould RA, Ball S, Kaspi SP, et al. Prevalence and correlates of anger attacks: a two site study. J Affect Disord. 1996;20;39:31–38. doi: 10.1016/0165-0327(96)00017-1. [DOI] [PubMed] [Google Scholar]
- 72.Constant A, Castera L, Dantzer R, et al. Mood alterations during interfer-on-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 73.Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S27–S32. doi: 10.3949/ccjm.71.suppl_3.s27. [DOI] [PubMed] [Google Scholar]
- 74.Castera L, Constant A, Henry C, et al. Impact on adherence and sustained virological response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2006;24:1223–1230. doi: 10.1111/j.1365-2036.2006.03107.x. [DOI] [PubMed] [Google Scholar]
- 75.Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of inter-feron-alpha-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091–1099. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- 76.Asnis GM, De La Garza R., 2nd. Interferon-induced depression: strategies in treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:808–818. doi: 10.1016/j.pnpbp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Schramm TM, Lawford BR, Macdonald GA, Cooksley WG. Sertraline treatment of interferon-alfa-induced depressive disorder. Med J Aust. 2000;173:359–361. doi: 10.5694/j.1326-5377.2000.tb125687.x. [DOI] [PubMed] [Google Scholar]
- 78.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 79.Pariante CM, Landau S, Carpiniello B. Interferon-alfa-induced adverse effects in patients with a psychiatric diagnosis. N Engl J Med. 2002;347:148–149. doi: 10.1056/NEJM200207113470221. [DOI] [PubMed] [Google Scholar]
- 80.Edwards JG, Anderson I. Systematic review and guide to selection of selective serotonin reuptake inhibitors. Drugs. 1999;57:507–533. doi: 10.2165/00003495-199957040-00005. [DOI] [PubMed] [Google Scholar]
- 81.Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124:1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 82.Veterans Health Administration. Washington, DC: Department of Veterans Affairs; 2002. Treatment recommendations for patients with chronic hepatitis C. Version 3.0; pp. 1–38. [Google Scholar]
- 83.Levine CD, Ghalib RH. Assessment of the patient who failed treatment for chronic hepatitis C. Gastroenterol Nurs. 2005;28(3) suppl:S24–S30. doi: 10.1097/00001610-200505001-00005. [DOI] [PubMed] [Google Scholar]
- 84.Cornberg M, Hadem J, Herrmann E, et al. Treatment with daily consensus interferon (CIFN) plus ribavirin in non-responder patients with chronic hepatitis C: a randomized open-label pilot study. J Hepatol. 2006;44:291–301. doi: 10.1016/j.jhep.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 85.da Silva LC, Bassit L, Ono-Nita SK, et al. High rate of sustained response to consensus interferon plus ribavirin in chronic hepatitis C patients resistant to alpha-interferon and ribavirin: a pilot study. J Gastroenterol. 2002;37:732–736. doi: 10.1007/s005350200119. [DOI] [PubMed] [Google Scholar]
- 86.Kaiser S, Hass H, Gregor M. Successful retreatment of peginterferon nonresponder patients with chronic hepatitis C with high dose consensus inter-feron induction therapy. Presented at Digestive Disease Week 2004; May 15-20, 2004; New Orleans, Louisiana. Abstract 125. Gastroenterology. 2004;126(suppl 2):A-668. [Google Scholar]
- 87.Chen K, Seraphin P, Murphy L, Shah N. Consensus interferon and ribavirin in patients with chronic hepatitis C who were nonresponders to prior therapy with either interferon alfa and ribavirin or pegylated interferon and ribavirin. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases; November 11-15, 2005; San Francisco, California. Abstract 1203. Hepatology. 2005;42(suppl 1):670A. [Google Scholar]
- 88.Bacon B, Regev A, Ghalib R, et al. Use of daily interferon alfacon-1 (Infergen®, CIFN) plus ribavirin (RBV) in patients infected with hepatitis C (HCV) who are nonresponders to previous pegylated interferon plus RBV therapy: 24-week data from the DIRECT trial. Presented at the 57th annual meeting of the American Association for the Study of Liver Diseases; October 27-31, 2006; Boston, Massachusetts. Abstract LB18. Hepatology. 2006;44(suppl 1):698A. [Google Scholar]
- 89.Bacon BR, McHutchison JG. Treatment issues with chronic hepatitis C: special populations and pharmacy strategies. Am J Manag Care. 2005;11:S296–S306. [PubMed] [Google Scholar]
- 90.Miglioresi L, Bacosi M, Russo F, et al. Consensus interferon versus interferon-alpha 2b plus ribavirin in patients with relapsing HCV infection. Hepatol Res. 2003;27:253–259. doi: 10.1016/s1386-6346(03)00269-9. [DOI] [PubMed] [Google Scholar]
Consensus Interferon: Practical Management of Treatment Failures in Chronic Hepatitis C
Abstract: Association-sponsored guidelines for the treatment of chronic hepatitis C are essential to standardize therapy and provide an excellent guide to physicians. Because these guidelines are updated only periodically, however, they cannot always incorporate the full range of available treatment options. Independently developed guidelines may close these gaps by incorporating more up-to-date treatment regimens. Options available for the re-treatment of patients who failed earlier interferon-based therapy include re-treatment with a different pegylated interferon at a higher dose or as induction therapy, longer-duration therapy, maintenance therapy, a watch-and-wait strategy, or re-treatment with consensus interferon and ribavirin. The goal of all of these re-treatment options is to decrease the burden of hepatitis C virus-related morbidity and to eradicate the virus. Consensus interferon, a synthetic agent used to treat chronic hepatitis C, has been shown in some studies to increase rates of sustained virologic response in patients who failed previous therapy when compared to conventional therapy. The inclusion of consensus interferon into the current treatment guidelines would, therefore, provide another effective treatment option for patients who have failed the current standard of care.
Infection with the hepatitis C virus (HCV) is the main cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma in many parts of the world.1 The current clinical guidelines for managing chronic hepatitis C published by the National Institutes of Health (NIH), American Gastroenterological Association (AGA), and the American Association for the Study of Liver Disease (AASLD) recommend the use of pegylated interferon alfa (PEG-IFN) combined with ribavirin (RBV) in treatment-naive patients infected with HCV. Whereas this standard-of-care regimen represents a marked advance over past regimens, 40-50% of patients still fail to achieve sustained virologic response (SVR).2 After treatment failure due to nonresponse with standard interferon (IFN) and RBV, patients are typically considered for re-treatment with PEG-IFN and RBV, although no treatment is currently approved for this indication. SVR rates using this strategy in nonresponding patients have ranged between 5% and 23%.3–13
Treatment guidelines published by professional medical associations are essential to standardize hepatitis C therapy and are traditionally reliable and well regarded by physicians. They are updated only periodically, however, and cannot always incorporate the full range of therapeutic options. Independently developed guidelines, based on clinical experience and accumulating evidence, fill in gaps left by association guidelines14 and can include emerging treatment options that have shown promise in trials and clinical practice. For example, Hoefs and Aulakh review re-treatment strategies for special populations—including patients with previous nonresponse to IFN therapy, cirrhosis, steatosis, liver transplant, HIV infection, or African-American race—and discuss the use of stronger therapies as well as newer treatment paradigms that include agents such as consensus interferon (CIFN).14
Treatment recommendations should ideally reflect the most current and effective treatment options for patients with chronic hepatitis C and should provide strategies easily applicable in practice. This article reports recent developments in the management of treatment failure in chronic hepatitis C and suggests a new treatment algorithm that incorporates treatment strategies and regimens not yet included in the current association-sponsored guidelines.
Overview of Association-sponsored Treatment Guidelines
Clinicians currently rely on three important association-sponsored guidelines on the diagnosis and management of HCV. First, the NIH Consensus Development Conference Statement, which was prepared by a nonfederal, nonadvocate panel of experts and published in 2002 as an update of the 1997 NIH Consensus Development guidelines.3 Second, recommendations for HCV treatment prepared for the AASLD Practice Guidelines Committee were fully endorsed by the AASLD, the Infectious Diseases Society of America, and the American College of Gastroenterology and published in 2004.4 Finally, a literature review and recommendations for HCV management prepared for the AGA Clinical Practice and Economics Committee were approved by the Committee and the AGA Governing Board in 2005 and published in 2006.6
The three major guidelines agree on therapy for treatment-naive patients, and all recommend a combination regimen of PEG-IFN plus RBV in weight-based doses.3,4,6 The guidelines all state that SVR rates have remained low (≤20%) for most patients re-treated with PEG-IFN plus RBV after failure of initial therapy with a standard IFN-based regimen, but they make only limited recommendations about specific regimens for re-treating nonresponders and relapsers.3,4,6 In practice, although efficacy in these patients remains uncertain, re-treatment with PEG-IFN and RBV is generally pursued.3,4,6
The NIH guidelines suggest that re-treatment decisions be based on multiple factors, including previous type of response; prior therapy and difference in potency of new therapy; severity of underlying liver disease; factors predictive of response, including viral genotype; and tolerance of and adherence to prior therapy.3 Re-treatment with PEG-IFN and RBV is not recommended in patients with nonresponse to a prior PEG-IFN-based regimen.4 AAlthough both the NIH and AAASLD guidelines mention maintenance therapy with PEG-IFN-based therapy as a possible re-treatment option, they stop short of recommending this option in nonresponders pending the results of ongoing studies.3,4 Other re-treatment strategies, such as switching between IFNs or observation, are not described in the current guidelines.
A New Treatment Algorithm for Chronic Hepatitis C
Multiple studies have shown SVR rates up to 23% after re-treatment with PEG-IFN plus RBV in patients with nonresponse to IFN monotherapy or IFN plus RBV.3,5–13 However, a new algorithm incorporating other re-treatment strategies after treatment failure has the potential to increase SVR rates (Figure 1). In the proposed algorithm, patients who fail initial therapy with PEG-IFN and RBV or any IFN-based regimen may be re-treated with any of the following regimens: CIFN plus RBV, high-dose or induction PEG-IFN plus RBV, longer-duration therapy, IFN maintenance therapy, or a watch-and-wait strategy.5,7–13,15–20
Figure 1.
New treatment algorithm illustrating initial treatment for patients infected with hepatitis C virus (HCV) genotypes 1, 4, 6, or 7 and incorporating newer re-treatment strategies after treatment failure with pegylated interferon (PEG-IFN) and ribavirin (RBV).
- CIFN
- consensus interferon.
CIFN and RBV
Re-treatment with CIFN and RBV has been shown to be effective in patients who failed earlier IFN-based therapy in a randomized clinical trial21 and in several open-label trials.22–25 CIFN is a non-naturally occurring recombinant type 1 IFN containing 166 amino acid residues that was bio-optimized by scanning the sequences of several IFN alfa nonallelic subtypes and assigning the most frequently observed amino acid in each corresponding position.26,27 CIFN was shown to have higher biologic activity than naturally occurring type 1 IFNs.26 Compared with IFN alfa-2a and IFN alfa-2b on a mass basis, CIFN exhibits higher antiviral, antiproliferative, and natural-killer-cell activation activity.26,28 The higher activity of CIFN could result from its demonstrated greater affinity for an array of type 1 IFN receptors.26,28
CIFN Efficacy in Nonresponders
Re-treatment with CIFN and RBV has produced SVR rates as high as 36% in nonresponders in both randomized and open-label studies.22,23,25 In an open-label pilot study in 14 patients who did not respond to IFN plus RBV therapy, 4 of the 11 patients (36%) re-treated with CIFN plus RBV who completed 24 weeks of follow-up achieved SVR.23 AAn open-label, randomized, pilot study in 77 nonresponders (90% genotype 1) to IFN or IFN plus RBV compared re-treatment with daily CIFN 18 µg daily as induction therapy for 8 weeks followed by 9 µg daily for 40 weeks with 9 µg daily for the full 48 weeks; both regimens included RBV 1,000–1,200 mg daily. Results showed an overall SVR rate of 30%. SVR in nonresponders to IFN plus RBV was 22%.22 In a larger prospective study of CIFN in nonresponders, 79 patients (80% genotype 1) who failed IFN alfa-2a monotherapy or PEG-IFN plus RBV combination therapy were administered CIFN at a starting dose of 15 µg daily plus RBV in weight-based doses.24 SVR was noted in 50% of these patients at 72 weeks of treatment. It is important to note, however, that the data presented above are from pilot studies or have not yet been published in peer-reviewed journals, so caution should be taken in interpreting the results.
The DIRECT trial (Daily-Dose Consensus Interferon and Ribavirin: Efficacy of Combined Therapy) is a phase III, open-label, multicenter study investigating the use of daily CIFN in previous nonresponders to PEG-IFN and RBV therapy. Treatment in this trial has ended, and patients are in the follow-up period at the time of this writing. In this trial, 343 patients who did not respond to earlier treatment were randomized to receive either CIFN 9 µg daily (n=171) or CIFN 15 µg daily (n=172) plus RBV 1.0-1.2 g daily.29 Of these patients, 67% had a high viral load (≥850,000 IU/mL), 20% were African American, and 77% had evidence of bridging fibrosis or cirrhosis on biopsy. In addition, 92% and 96% of the patients in the 9 µg and 15 µg groups, respectively, were infected with HCV genotype 1. The mean washout period from the end of previous therapy was 485 days for the CIFN 9 µg group and 535 days for the CIFN 15 µg group.
At 24 weeks, 14% and 20% of patients in the 9 µg and 15 µg groups, respectively, showed viral response.29 The highest viral response rate at 24 weeks occurred in the CIFN 15 µg group among those who adhered to at least 80% of their study dose (30%). Viral response was lower in patients with higher fibrosis scores. Of patients receiving CIFN 9 µg, 19% of those with fibrosis scores of F0–F2, 16% with scores of F3, and 8% with scores of F4 showed no detectable virus at 24 weeks and achieved end-oftreatment response. For patients in the 15 µg group, 28% with fibrosis scores of F0-F2, 19% with scores of F3, and 6% with scores of F4 achieved end-of-treatment response.
Adverse events were generally dose-related and included fatigue (40% and 45% for the 9 µg and 15 µg groups, respectively), neutropenia (36% and 45%), leukopenia (23% and 36%), insomnia (22% and 20%), and headache (19% and 20%).
Further analysis of the high percentage of patients with advanced liver disease and the high number of patients with a prolonged washout period in the DIRECT trial is warranted. Of note, the use of growth factors to support anemia or neutropenia was not allowed in the study and the RBV dose was adjusted to 600 mg daily for patients with anemia.
CIFN Efficacy in Relapsers
Patients who relapse generally respond well to re-treatment, although the risk of subsequent relapse remains, especially if the same regimen is used.4,30 However, studies of re-treatment confirm the efficacy of CIFN in patients who relapsed after prior successful treatment. In a randomized clinical trial in 103 patients with chronic HCV who did not respond to or who relapsed after IFN monotherapy or IFN plus RBV, patients were re-treated with high-dose CIFN induction therapy (27 µg/day during Weeks 1–2, 18 µg/day during Weeks 3-12, and 9 µg/day during Weeks 13–24, followed by 9 µg TIW for 24 weeks) plus RBV. Relapsers showed an SVR rate of 70% (n=34).31 Another randomized trial in 112 patients with relapsing HCV infection demonstrated an SVR rate of 58% among patients re-treated with 24 weeks of CIFN 9 µg daily and an SVR rate of 29% among patients retreated with IFN alfa-2b and RBV (P<.03).21
Tables 1 and 2 present the results of studies investigating SVR after re-treatment with PEG-IFN plus RBV or CIFN plus RBV in patients who did not respond to or relapsed after IFN monotherapy or IFN plus RBV combination therapy.
Table 1.
Sustained Virologic Response (SVR) Rates after Re-treatment With Pegylated Interferon (PEG-IFN) and Ribavirin (RBV) in Nonresponders and Relapsers to Previous Interferon (IFN)-based Therapy
| Study | Study Group | Study Regimen | SVR Rate |
|---|---|---|---|
| Taliani et al.12 | 141 patients with nonresponse to IFN + RBV |
|
20% |
| Sherman et al.11 | 312 patients with either nonresponse or relapse after IFN monotherapy or IFN + RBV |
|
Nonresponders:
23% Relapsers: 41% |
| Jacobson et al.8 | 321 patients with nonresponse to IFN monotherapy or IFN + RBV or relapse after IFN + RBV |
|
Regimen 1:
18% Regimen 2: 13% |
| Krawitt et al.47 | 182 patients with nonresponse or relapse after IFN or IFN + RBV |
|
Nonresponders:
20% Relapsers: 55% |
| Poynard et al.10 | 575 patients with nonresponse or relapse after IFN + RBV |
|
21% |
| Gaglio et al.7 | 454 patients with nonresponse to IFN monotherapy or IFN + RBV |
|
Regimen 1:
17% Regimen 2: 20% |
| Lawitz et al.9 | 460 patients with nonresponse to IFN monotherapy, nonresponse to IFN + RBV, or relapse after IFN + RBV |
|
IFN monotherapy nonresponders: Induction: 14% Fixed: 13% IFN/RBV nonresponders: Induction: 10% Fixed: 5% IFN/RBV relapsers: Induction: 20% Fixed: 26% |
| Teuber et al.13 | 240 patients with nonresponse to IFN + RBV |
|
6.3% |
Table 2.
Sustained Virologic Response (SVR) after Re-treatment With Consensus Interferon (CIFN)/Ribavirin (RBV) in Nonresponders and Relapsers to Interferon (IFN)-based Therapy
| Study | Study Group | Study Regimen | SVR Rate |
|---|---|---|---|
| Cornberg et al.22 | 77 patients with nonresponse to IFN monotherapy or IFN/RBV (90% genotype 1) |
|
Regimen 1:
32% Regimen 2: 28% |
| Böcher et al.31 | 103 patients with nonresponse to IFN monotherapy or IFN/RBV (69 nonresponders, 34 relapsers) |
|
Regimen
1: Nonresponders: 26% Relapsers: 70% Regimen 2: Nonresponders: 26% Relapsers: 38% |
| Miglioresi et al.21 | 112 patients with relapse after IFN monotherapy |
|
Regimen 1:
29% Regimen 2: 58% |
| Barbaro and Barbarini25 | 24 patients with nonresponse or relapse after IFN/RBV (12 nonresponders, 12 relapsers) |
|
Nonresponders:
33% Relapsers: 42% |
| da Silva et al.23 | 14 patients with nonresponse or viral breakthrough after IFN/RBV |
|
36% |
| Kaiser et al.48 | 81 patients with relapse after PEG-IFN/RBV |
|
Regimen 1:
69% Regimen 2: 44% |
| Chen et al.24 | 76 patients with nonresponse to IFN + RBV or PEG-IFN + RBV |
|
50% |
- PEG-IFN
pegylated interferon
- HCV
hepatitis C virus.
The Impact of Type of Response
The type of response to standard therapy has important implications for re-treatment with CIFN. Leevy and associates, in an analysis of CIFN in prior nonresponders, showed that the efficacy of CIFN is lower in prior complete nonresponders than in prior partial responders.32 In a retrospective review of 137 consecutive patients, a partial response (at least a 0.5 log decline) to prior therapy with PEG-IFN at 12 weeks was an independent predictor of SVR during re-treatment with CIFN (P<.001).32 These data suggest that patients who might benefit most from re-treatment with CIFN are partial responders after prior PEG-IFN-based therapy. Therefore, the timing of CIFN use in clinical practice may have important implications for patient outcomes. In our clinical practice, switching to CIFN in patients with an incomplete response (<2 log10 viral decline) to PEG-IFN-based therapy at 12 or 24 weeks is considered an appropriate time for patients who are willing to continue their therapy. This approach has two major advantages: it builds on the degree of viral response initiated by the PEG-IFN therapy, and it helps ameliorate the adverse effects seen in patients switching to CIFN because many of these patients may have developed tolerance to IFN by 12 or 24 weeks. Patients may also switch to CIFN at 48 weeks after a positive result on a quantitative viral assay. A washout period is not needed when treating patients in clinical practice, although it is mandatory in patients treated in clinical trials.32,33
Safety of CIFN
Adverse events, including hematologic complications, fatigue, flu-like syndrome, and neuropsychiatric symptoms, are common in patients undergoing IFN-based therapy.34 CIFN has been shown to have a safety profile similar to that of other IFNs.23,31,35 In general, adverse events with CIFN seem to be dose-related22 and reversible.36
Data from a randomized, controlled study in 75 patients with chronic hepatitis C treated with CIFN 9 µg, 3 µg, or placebo 3 times weekly for 24 weeks showed that patients receiving CIFN had significantly greater decreases in platelet counts and increases in serum thrombopoietin than did those receiving placebo at Weeks 12 and 24, respectively; these changes were more notable in patients receiving CIFN 9 µg than patients receiving CIFN 3 µg.36 Importantly, both platelet count and thrombopoietin level returned to baseline values after the discontinuation of CIFN therapy.36
High-dose or Induction IFN Therapy
Data support further study of intensive regimens in the treatment of HCV infection, including high-dose and induction IFN therapy.17,19,37,38 The use of high-dose therapy was investigated by Fried and colleagues in 104 treatment-naive patients given IFN alfa-2b 5 MU daily or 3 MU TIW for 12 weeks and continued for up to 24 weeks in the presence of viral response.19 SVR rates measured at 48 weeks were 14% and 4% for the high- and standard-dose regimens, respectively (P=.08). Although not significant, these results provided a proof of concept for high-dose therapy with conventional IFN.19
In the RENEW trial (Re-treatment of Nonresponders with Escalating Weight-Based Therapy), 704 patients with chronic hepatitis C who did not respond to IFN/RBV therapy had SVR rates of 17% with PEG-IFN alfa-2b 3.0 µg/kg weekly plus RBV 1,200-1,500 µg/kg daily and 12% with PEG-IFN alfa-2b 1.5 µg/kg weekly plus RBV 1,200–1,500 µg/kg daily after 48 weeks of re-treatment (P=.03).37 Similar results were found in TARGET (Therapeutic Arthritis Research and Gastrointestinal Event Trial) in 285 patients with chronic hepatitis C who did not respond to previous therapy with IFN plus RBV.38 Patients in this trial showed an SVR rate of 18% with double-dose PEG-IFN alfa-2b (3.0 µg/kg/wk) plus RBV (1,300 ± 200 µg/kg/day) at the 24-week follow-up.38 These studies provide support for high-dose PEG-IFN in previous nonresponders.
Studies incorporating the use of induction dosing— high-dose IFN during the early weeks of treatment followed by either high or standard dosing—have also shown promise in treating patients who failed earlier treatment.14 Efficacy analysis of 856 patients after 12 weeks of induction therapy in the ongoing REPEAT trial (Re-treatment with Pegasys® in Patients Not Responding to Prior Peginterferon alfa-2b/Ribavirin Combination Therapy) showed that re-treatment with PEG-IFN alfa-2a results in higher rates of early virologic response in patients who failed PEG-IFN alfa-2b.17 Patients in this analysis had prior nonresponse to 12 weeks or more of therapy with PEG-IFN alfa-2b plus RBV and had HCV RNA levels that remained positive throughout treatment. They were randomized to receive 1 of 2 dosages of PEG-IFN alfa-2a plus RBV 1,000-1,200 mg daily: (1) 360 µg weekly for 12 weeks (induction dose) followed by 180 µg weekly for 60 weeks (longer duration) or 36 weeks (standard duration) or (2) 180 µg weekly for 72 weeks or 48 weeks.17 At 12 weeks, 274 of 430 patients (64%) in the former group had achieved at least a 2 log10 decrease in HCV RNA levels or nonquantifiable HCV RNA levels, compared to 199 of 426 patients (47%) in the latter group.17 Although these results are promising, data from the 48- and 72-week phases of the study are needed.
High-dose induction therapy with CIFN may also be a useful strategy for re-treatment. Preliminary data from a small single-center pilot study suggest that 39-44% of patients with previous nonresponse to an IFN-based combination regimen could achieve SVR using an induction approach with CIFN, although more research is needed.39
Longer-duration Treatment
Two clinical studies explored the use of longer-duration therapy—extending the course of therapy to as long as 72 weeks—as a possible strategy to improve SVR rates in patients with chronic hepatitis C.20,40 In the first study, 455 treatment-naive patients infected with HCV genotype 1 received PEG-IFN alfa-2a 180 µg weekly plus RBV 800 mg daily and were randomized to 48 or 72 weeks of treatment preceding a 24-week follow-up.40 No significant differences between the 48- and 72-week groups were noted in either the end-of-treatment response rates (71% vs 63%) or SVR rates (53% vs 54%). However, patients who had positive HCV RNA levels at 12 weeks had significantly higher rates of SVR when treated for 72 weeks than those treated for 48 weeks (29% vs 17%, P=.04).40 The second study specifically examined the treatment of patients who did not respond to HCV therapy by 4 weeks.20 In this study, 326 treatment-naive patients with detectable HCV RNA levels at week 4 received PEG-IFN alfa-2a 180 µg weekly plus RBV 800 mg daily and were treated for either 72 or 48 weeks. Although the end-of-treatment response was the same for the two groups (61%), patients treated for 72 weeks had a significantly higher SVR rate than those treated for 48 weeks (45% vs 32%; P=.014). However, the rate of treatment discontinuation was also higher in the 72-week group (36% vs 18%; P=.0002), which raises questions about tolerability and adherence in a clinical setting.20 These data show an increased SVR rate with longer-duration therapy among slow responders but do not support extending treatment in patients who achieve rapid or early virologic response; therefore, further studies are needed. The ongoing REPEAT trial will evaluate the role of extended therapy in patients who failed a prior course of HCV therapy.17
Maintenance Therapy
The rationale of maintenance therapy with IFN for the management of chronic hepatitis C is not to achieve viral clearance, but rather to improve liver histologic evidence and delay or prevent the development of hepatic decompensation or hepatocellular carcinoma.41 In an early study, patients treated for 6 months using an IFN maintenance strategy showed decreases in serum alanine aminotransferase (ALT) and HCV RNA levels.42 After 30 months of treatment, the mean fibrosis score declined from 2.5 to 1.7, and 80% of patients showed histologic improvement (P<.03).
Three ongoing, prospective, multicenter trials investigating long-term low-dose PEG-IFN maintenance therapy to delay or prevent the progression of HCV should further elucidate the effectiveness of HCV maintenance therapy.41 In the CO-PILOT trial (Colchicine versus Peg-Intron Long-Term), the effectiveness of low-dose PEG-IFN alfa-2b (0.5 µg/kg/wk) is being compared with that of colchicine (0.6 mg twice daily) in 800 patients with advanced fibrosis who failed previous IFN-based therapies.43 Results of the planned 2-year interim analysis in 534 patients showed that PEG-IFN was more effective than colchicine at preventing bleeding from varices.43 The NIH-sponsored HALT-C trial (Hepatitis C Antiviral Long-term Treatment against Cirrhosis) is investigating the effect of PEG-IFN alfa-2a (90 µg/wk) versus placebo in 1,400 nonresponders to previous IFN therapy.15 Preliminary results in the first 604 enrolled patients showed that 210 of patients (35%) cleared the virus by 20 weeks and 109 (18%) went on to achieve SVR after 48 weeks of treatment and 24 weeks of follow-up; patients who did not clear the virus by 20 weeks entered a maintenance phase of the study and results are pending.5 Enrollment is ongoing in the United States and Europe for the EPIC trial (Efficacy of PEG-IFN in Chronic Hepatitis C), which will evaluate the benefit of PEG-IFN alfa-2b (0.5 µg/kg) versus placebo in 1,700 patients (700 with cirrhosis) who did not respond to previous PEG-IFN and RBV therapy.41
Patients who are most likely to benefit from maintenance therapy include those who did not maintain viral clearance to the most effective regimens and have significant underlying fibrosis or compensated cirrhosis.41,44 Given the goals of maintenance therapy, performing a liver biopsy for noncirrhotics every 2 years and screening for hepatocellular carcinoma and varices for cirrhotics is recommended.41 All patients undergoing maintenance therapy should be advised against alcohol intake and encouraged to maintain a healthy body mass index and avoid hepatotoxic medications.41
Difficult clinical issues surround the evaluation and follow-up of patients receiving maintenance therapy.41 The treatment period is open-ended and may continue for years.41 Also, the definition of successful maintenance therapy is controversial,41 leaving in question whether reduced viral load or normalized ALT levels are prerequisites of clinical benefit.41
Watch and Wait
Therapy discontinuation followed by a watch-and-wait strategy may be a reasonable option for many nonresponders, especially those with mild fibrosis.45 Most notably, this strategy avoids the adverse events associated with IFN-based therapy. In a study of 384 patients with cirrhosis, the 5-year estimated survival probability was 96% in patients treated with IFN and 95% in untreated patients, suggesting a slow disease progression and relatively long life expectancy in this patient population; nonetheless, 8% of the study group developed hepatocellular carcinoma, with an annual incidence of 1.4%.46 Conversely, it may be argued that antiviral therapy should be considered even in patients with mild chronic hepatitis C, as deferring treatment could increase the risk of adverse outcomes. Approximately 15ߝ20% of untreated patients with chronic hepatitis C and cirrhosis could be expected to develop either hepatic decompensation or hepatocellular carcinoma after 5 years without treatment.45 Additionally, a recent study in 106 untreated patients showed that liver fibrosis progressed within 5-10 years in two thirds of patients with initially mild HCV infection, and advanced fibrosis/cirrhosis developed in one-third of patients with an initial METAVIR score of F1.18
Conclusion
Treatment guidelines provide an excellent resource for clinicians to guide the management of chronic hepatitis C patients, yet they may become out of date as new clinical data appear. Clinically based guidelines may, therefore, close gaps left by the association guidelines by incorporating newer research and a wider array of available treatment options. Options available for the re-treatment of patients who failed earlier IFN-based therapy include re-treatment with a different IFN, high-dose or induction therapy, longer-duration therapy, maintenance therapy, observation, or re-treatment with CIFN plus RBV. CIFN has been shown to be efficacious, safe, and well tolerated for the treatment of select patients who have failed earlier IFN-based therapy. Although more investigation is needed, CIFN should be considered for inclusion in future treatment guidelines.
References
- 1.Alberti A, Chemello L, Noventa F, et al. Therapy of hepatitis C: re-treatment with alpha interferon. Hepatology. 1997;26:137S–142S. doi: 10.1002/hep.510260724. [DOI] [PubMed] [Google Scholar]
- 2.Keeffe EB. Chronic hepatitis C: management of treatment failures. Clin Gastroenterol Hepatol. 2005;3:S102–S105. doi: 10.1016/s1542-3565(05)00698-1. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002—June 10-12, 2002. Hepatology. 2002;36(suppl 1):S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 4.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C [correction in Hepatology. 2004;40:269] Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 5.Shiffman ML, Di Bisceglie AM, Lindsay KL, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Gaglio P, Choi J, Zimmerman D, et al. Weight based ribavirin in combination with pegylated interferon alpha 2-b does not improve SVR in HCV infected patients who failed prior therapy: results in 454 patients [abstract 59]. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases, November 11-15, 2005, San Francisco, California. Hepatology. 2005;42(suppl 1):219A. [Google Scholar]
- 8.Jacobson IM, Gonzalez SA, Ahmed F, et al. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the re-treatment of chronic hepatitis C. Am J Gastroenterol. 2005;100:2453–2462. doi: 10.1111/j.1572-0241.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 9.Lawitz EJ, Bala NS, Becker S, et al. Pegylated interferon alfa 2b and ribavirin for hepatitis C patients who were nonresponders to previous therapy [abstract 1293]. Presented at Digestive Disease Week 2003, May 17-22, 2003, Orlando, Florida. Gastroenterology. 2003;124(suppl 1):A-783. [Google Scholar]
- 10.Poynard T, Schiff EG, Terg R, et al. Sustained virologic response (SVR) in the EPIC3 trial: week twelve virology predicts SVR in previous interferon/ribavirin treatment failures receiving PEG-Intron/Rebetol (PR) weight based dosing (WBD) [abstract 96]. Presented at the 40th annual meeting of the European Association for the Study of the Liver, April 13-17, 2005, Paris, France. J Hepatol. 2005;42(suppl 2):40–41. [Google Scholar]
- 11.Sherman M, Yoshida EM, Deschenes M, et al. Peginterferon alfa-2a (40KD) plus ribavirin in chronic hepatitis C patients who failed previous interferon therapy. Gut. 2006;55:1631–1638. doi: 10.1136/gut.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taliani G, Gemignani G, Ferrari C, et al. Pegylated interferon alfa-2b plus ribavirin in the re-treatment of interferon-ribavirin nonresponder patients. Gastroenterology. 2006;130:1098–1106. doi: 10.1053/j.gastro.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Teuber G, Kallinowski B, Niederau C, et al. Re-treatment with pegylated interferon-alpha2b plus ribavirin in patients with chronic hepatitis C not responding to a previous antiviral treatment with standard interferons combined with ribavirin [abstract 1216]. Presented at Digestive Disease Week 2003, May 17-22, 2003, Orlando, Florida. Gastroenterology. 2003;124(suppl 1):A-699. [Google Scholar]
- 14.Hoefs J, Aulakh VS. Treatment of chronic HCV infection in special populations. Int J Med Sci. 2006;3:69–74. doi: 10.7150/ijms.3.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee WM, Dienstag JL, Lindsay KL, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser S, Hass H, Gregor M. Successful re-treatment of peginterferon nonresponder patients with chronic hepatitis C with high dose consensus interferon induction therapy [abstract 125]. Presented at Digestive Disease Week 2004, May 15-20, 2004, New Orleans, Louisiana. Gastroenterology. 2004;126(suppl 2):A-668. [Google Scholar]
- 17.Marcellin P, Jensen D. Re-treatment with Pegasys in patients not responding to prior peginterferon alfa-2b/ribavirin (RBV) combination therapy—efficacy analysis of the 12-week induction period of the REPEAT study [abstract LB04]. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases, November 11-15, 2005, San Francisco, California. Hepatology. 2005;42(suppl 1):749A–750A. [Google Scholar]
- 18.Boccato S, Pistis R, Noventa F, et al. Fibrosis progression in initially mild chronic hepatitis C. J Viral Hepat. 2006;13:297–302. doi: 10.1111/j.1365-2893.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 19.Fried MW, Shiffman M, Sterling RK, et al. A multicenter, randomized trial of daily high-dose interferon-alfa 2b for the treatment of chronic hepatitis C: pre-treatment stratification by viral burden and genotype. Am J Gastroenterol. 2000;95:3225–3229. doi: 10.1111/j.1572-0241.2000.03433.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Tapias JM, Diago M, Escartin P, et al. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131:451–460. doi: 10.1053/j.gastro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Miglioresi L, Bacosi M, Russo F, et al. Consensus interferon versus interferonalpha 2b plus ribavirin in patients with relapsing HCV infection. Hepatol Res. 2003;27:253–259. doi: 10.1016/s1386-6346(03)00269-9. [DOI] [PubMed] [Google Scholar]
- 22.Cornberg M, Hadem J, Herrmann E, et al. Treatment with daily consensus interferon (CIFN) plus ribavirin in non-responder patients with chronic hepatitis C: a randomized open-label pilot study. J Hepatol. 2006;44:291–301. doi: 10.1016/j.jhep.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 23.da Silva LC, Bassit L, Ono-Nita SK, et al. High rate of sustained response to consensus interferon plus ribavirin in chronic hepatitis C patients resistant to alpha-interferon and ribavirin: a pilot study. J Gastroenterol. 2002;37:732–736. doi: 10.1007/s005350200119. [DOI] [PubMed] [Google Scholar]
- 24.Chen K, Seraphin P, Murphy L, Shah N. Consensus interferon and ribavirin in patients with chronic hepatitis C who were nonresponders to prior therapy with either interferon alfa and ribavirin or pegylated interferon and ribavirin [abstract 1203]. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases, November 11-15, 2005, San Francisco, California. Hepatology. 2005;42(suppl 1):670A. [Google Scholar]
- 25.Barbaro G, Barbarini G. Consensus interferon for chronic hepatitis C patients with genotype 1 who failed to respond to, or relapsed after, interferon alpha-2b and ribavirin in combination: an Italian pilot study. Eur J Gastroenterol Hepatol. 2002;14:477–483. doi: 10.1097/00042737-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Blatt LM, Davis JM, Klein SB, Taylor MW. The biologic activity and molecular characterization of a novel synthetic interferon-alpha species, consensus interferon. J Interferon Cytokine Res. 1996;16:489–499. doi: 10.1089/jir.1996.16.489. [DOI] [PubMed] [Google Scholar]
- 27.Costa Mesa, CA: Valeant Pharmaceuticals; 2003. Infergen (interferon alfacon-1) [package insert] [Google Scholar]
- 28.Ozes ON, Reiter Z, Klein S, et al. A comparison of interferon-Con1 with natural recombinant interferons-alpha: antiviral, antiproliferative, and natural killer-inducing activities. J Interferon Res. 1992;12:55–59. doi: 10.1089/jir.1992.12.55. [DOI] [PubMed] [Google Scholar]
- 29.Bacon B, Regev A, Ghalib R, et al. Use of daily interferon alfacon-1 (Infergen, CIFN) plus ribavirin (RBV) in patients infected with hepatitis C (HCV) who are nonresponders to previous pegylated interferon plus RBV therapy: 24-week data from the DIRECT trial [abstract LB18]. Presented at the 57th annual meeting of the American Association for the Study of Liver Diseases, October 27-31, 2006, Boston, Massachusetts. Hepatology. 2006;44(suppl 1):698A. [Google Scholar]
- 30.NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1–46. [PubMed] [Google Scholar]
- 31.Böcher WO, Schuchmann M, Link R, et al. Consensus interferon and ribavirin for patients with chronic hepatitis C and failure of previous interferon-alpha therapy. Liver Int. 2006;26:319–325. doi: 10.1111/j.1478-3231.2005.01239.x. [DOI] [PubMed] [Google Scholar]
- 32.Leevy C, Chalmers C, Blatt LM. Predictive model and sustained virologic response for PEG IFN-alfa-2 + weight-based ribavirin nonresponders re-treated with IFN alfacon-1 + weight-based ribavirin [abstract S1538]. Presented at Digestive Disease Week 2005, May 13-19, 2005, Chicago, Illinois. Gastroenterology. 2005;128(suppl 2):A-715. [Google Scholar]
- 33.Barnett K, Levine C. Hepatitis C treatment failure: when and how to switch to consensus interferon [educational resource]. Point-of-HCV-Care. [May 3, 2007]. Available at: http://www.point-of-hcv-care.com/
- 34.De Filippi F, Colombo M. Treatment of chronic hepatitis C. J Biol Reggul Homeost Agents. 2003;17:133–137. [PubMed] [Google Scholar]
- 35.Heathcote EJ, Keeffe EB, Lee SS, et al. Re-treatment of chronic hepatitis C with consensus interferon. Hepatology. 1998;27:1136–1143. doi: 10.1002/hep.510270431. [DOI] [PubMed] [Google Scholar]
- 36.Chu CW, Hwang SJ, Lu RH, et al. Clinical significance of the changes of platelet counts and serum thrombopoietin levels in chronic hepatitis C patients treated with different doses of consensus interferon. Hepatol Res. 2002;24:236–244. doi: 10.1016/s1386-6346(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 37.Gross J, Johnson S, Kwo P, et al. Double-dose peginterferon alfa-2b with weight-based ribavirin improves response for interferon/ribavirin non-responders with hepatitis C: final results of “RENEW” [abstract 60]. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases, November 11-15, 2005, San Francisco, California. Hepatology. 2005;42(suppl 1):219A–220A. [Google Scholar]
- 38.White C, Wentworth C, Malet P, et al. The TARGET Trial: final results using 3.0mg/kg pegylated interferon alfa 2-b (PEG;PEG-Intron) plus ribavirin (RBV;Rebetol) for chronic hepatitis C patients who were non-responders (NR) and relapsers (R) to previous therapy [abstract 1157]. Presented at the 56th annual meeting of the American Association for the Study of Liver Diseases, November 11-15, 2005, San Francisco, California. Hepatology. 2005;42(suppl 1):651A. [Google Scholar]
- 39.Kaiser S, Hass H, Gregor M. Successful re-treatment of chronic hepatitis C patients with a nonresponse to standard interferon/ribavirin using daily consensus interferon and ribavirin [abstract 173]. Presented at the 54th annual meeting of the American Association for the Study of Liver Diseases, October 29-November 2, 2004, Boston, Massachusetts. Hepatology. 2004;40(suppl 1):240A. [Google Scholar]
- 40.Berg T, von Wagner M, Nasser S, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–1097. doi: 10.1053/j.gastro.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Kelleher TB, Afdhal N. Maintenance therapy for chronic hepatitis C. Curr Gastroenterol Rep. 2005;7:50–53. doi: 10.1007/s11894-005-0066-1. [DOI] [PubMed] [Google Scholar]
- 42.Shiffman ML, Hofmann CM, Contos MJ, et al. A randomized, controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology. 1999;117:1164–1172. doi: 10.1016/s0016-5085(99)70402-6. [DOI] [PubMed] [Google Scholar]
- 43.Afdhal N, Freilich B, Levine R, et al. Colchicine versus PEGIntron long-term (COPILOT) trial: interim analysis of clinical outcomes at year 2 [abstract 171]. Presented at the 55th Annual Meeting of the American Association for the Study of Liver Diseases, October 29-November 2, 2004, Boston, Massachusetts. Hepatology. 2004;40(suppl 1):239A. [Google Scholar]
- 44.Levine CD, Ghalib RH. Assessment of the patient who failed treatment for chronic hepatitis C. Gastroenterol Nurs. 2005;28(3) suppl:S24–S30. doi: 10.1097/00001610-200505001-00005. [DOI] [PubMed] [Google Scholar]
- 45.Shiffman ML. Chronic hepatitis C: treatment of pegylated interferon/ribavirin nonresponders. Curr Gastroenterol Rep. 2006;8:46–52. doi: 10.1007/s11894-006-0063-z. [DOI] [PubMed] [Google Scholar]
- 46.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 47.Krawitt EL, Ashikaga T, Gordon SR, et al. Peginterferon alfa-2b and ribavirin for treatment-refractory chronic hepatitis C. J Hepatol. 2005;43:243–249. doi: 10.1016/j.jhep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Kaiser S, Hass HG, Bissinger L, Gregor M. Comparison of daily consensus interferon versus peginterferon alfa 2a extended therapy of 72 weeks for peginterferon /ribavirin relapse patients with chronic hepatitis C [abstract S1060]. Presented at Digestive Disease Week 2006, May 20-25, 2006, Los Angeles, California. Gastroenterology. 2006;130(suppl 2):A-784. [Google Scholar]
Contributor Information
Tarek Hassanein, University of California, San Diego.
Mitchell L. Shiffman, Virginia Commonwealth University.
Nizar N. Zein, Cleveland Clinic Foundation.