Abstract
OBJECTIVE
To explore the relationships between lymphocyte and monocyte activation, inflammation, and subclinical vascular disease among HIV-1 infected patients on antiretroviral therapy.
METHODS
Baseline mean common carotid artery (CCA) intima-media thickness (IMT) and carotid plaque (IMT >1.5cm) were evaluated in the first 60 subjects enrolled in the Stopping Atherosclerosis and Treating Unhealthy bone with RosuvastatiN in HIV (SATURN-HIV) trial. All subjects were adults, on stable ART with evidence of heightened T-cell activation (CD8+CD38+HLA-DR+ ≥19%) or increased inflammation (high sensitivity C-reactive protein ≥2mg/L). All had fasting LDL-cholesterol ≤130mg/dL.
RESULTS
78% were men and 65% African-American. Median (IQR) age and CD4+ count were 47(43,52) years and 648(511, 857) cells/µL, respectively. All had HIV-1 RNA<400 cps/mL. Mean CCA-IMT was correlated with log-transformed CD8+CD38+HLA-DR+% (r=0.326, p=0.043), interleukin-6 (r=0.283, p=0.028), soluble vascular cell adhesion molecule (sVCAM, r=0.434, p=0.004), tumor necrosis factor-α receptor-I (TNFR-I, r=0.591, p=<0.0001) and fibrinogen (r=0.257, p=0.047). After adjustment for traditional CVD risk factors, the association with TNFR-I (p=0.007) and fibrinogen (p=0.033) remained significant. Subjects with plaque (n=22, 37%) were older [51(7.7) vs. 43(9.4) years, mean(SD), p=0.002], had higher CD8+CD38+HLA-DR+% [31(24, 41) vs. 23(20,29)%, median(IQR), p=0.046] and higher sVCAM [737(159) vs. 592(160) ng/mL, p=0.008] compared to those without plaque. Pro-inflammatory monocyte subsets and serum markers of monocyte activation (soluble CD163 and soluble CD14) were not associated with CCA-IMT or plaque.
CONCLUSIONS
Participants in SATURN-HIV have a high level of inflammation and immune activation that is associated with subclinical vascular disease despite low serum LDL-C.
Keywords: T-cell activation, Monocyte activation, Inflammation, Carotid intima-media thickness, Subclinical atherosclerosis
Introduction
Chronic HIV infection is associated with an elevated risk of atherosclerotic vascular disease, beginning with the early development of endothelial dysfunction and advancing more rapidly to sub-clinical atherosclerosis and in some cases life-threatening rupture of lipid-laden plaques(1, 2). The important drivers throughout this spectrum of vascular disease in HIV are complex and incompletely understood. Chronic inflammation, including lymphocyte and monocyte activation, may play an important role at each step.
Prior studies in HIV have separately associated sub-clinical atherosclerosis with serum markers of inflammation(3), generalized T-cell activation(4), cytomegalovirus-specific T-cell activation(5), and soluble CD163 (a marker of monocyte activation)(6); although the relative importance of these markers has not previously been compared in a virologically-suppressed population. Additionally, expression of the procoagulant tissue factor (TF) on the surface of monocytes is elevated in HIV-1 infected subjects compared to uninfected controls(7) and is highly prevalent in patients with acute coronary syndrome(8).
The primary aim of this study is to describe the cross-sectional associations of multiple markers of inflammation and immune activation with ultrasound measurements of carotid intima-media thickness and plaque among HIV-1 infected adults on stable antiretroviral therapy (ART).
Methods
Study design
This study is a cross-sectional analysis of the first 60 subjects enrolled in the Stopping Atherosclerosis and Treating Unhealthy bone with RosuvastatiN in HIV (SATURN-HIV) trial. SATURN-HIV is a randomized double-blind placebo-controlled trial designed to measure the effect of rosuvastatin 10mg daily on the progression of subclinical vascular disease. Enrollment began in March 2011.All subjects were ≥18 years of age, without diabetes or known coronary disease, and on stable ART with viral load <400 copies/mL. Additional entry criteria included either evidence of either heightened T-cell activation (CD8+CD38+HLA-DR+ ≥19%) or increased inflammation (high sensitivity C-reactive protein (hs-CRP ≥2mg/L), as well as LDL-cholesterol (LDL-C) ≤130mg/dL. Nineteen percent CD8+CD38+HLA-DR+ is the median level of CD8+ activation among patients successfully treated with ART and the 75th percentile of HIV-uninfected controls in our laboratory(9). Hs-CRP ≥2mg/L was the entry criterion of the JUPITER trial of rosuvastatin in HIV-uninfected adults(10). The study was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH).
Study evaluations
At the initial screening visit, self-reported demographics and medical history were obtained along with a targeted physical exam. Blood was drawn after a 12-hour fast for glucose, lipoproteins, hs-CRP, and %CD8+ T cell activation. If enrollment criteria were met, subjects returned within 30 days for entry evaluations. At entry, a fasting blood draw was obtained for markers of inflammation. HIV-1 RNA level and CD4+ cell count were obtained as part of routine clinical care.
Soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble inter-cellular adhesion molecule-1 (sICAM-1), interleukin-6 (IL6), and soluble tumor necrosis factor-α receptor 1 (sTNFR-I) were determined by quantitative sandwich ELISAs (R&D Systems, Minneapolis, MN). Inter-assay variability ranged from 4.76%–8.77%, 3.43%–7.37%, 2.02%–15.36%, and 3.66%–5.77%, respectively. Hs-CRP and fibrinogen were determined by particle enhanced immunonepholometric assays on a BNII nephelometer (Siemens). Inter-assay variability ranged from 3.01%–6.46% and 3.42%–7.59%, respectively. D-dimer was determined by immuno-turbidometric assay on a STA-R Coagulation Analyzer (Diagnostica Stago). Inter-assay variability ranged from 1.54%–9.03%. Serum levels of soluble CD14 and soluble CD163 were measured using Quantikine ELISA kits (R&D Systems Minneapolis MN).
Flow Cytometry
Monocytes and T-cells were identified by size, granularity, and by expression of CD14 or CD3 and CD8, respectively. Cell surface molecule expression was monitored by staining cells with the following fluorochrome-labeled antibodies: anti-Tissue Factor fluorescein isothiocyanate (FITC) (American Diagnostica, Stamford, CT), anti-CD14 Pacific Blue, anti-CD16 phycoerythrin (PE), (BD Pharmingen, San Diego, CA). In order to assure that monocyte populations were not contaminated by lymphocytes, preliminary experiments using an exclusion-gate that included anti-CD3 FITC, anti-CD20 FITC, anti-CD56 FITC (BD Pharmingen) were performed.
T-cell activation was measured using anti-CD38 PE, anti-HLA-DR FITC, anti-CD3 Peridinin-chlorophyll-protein Complex (PerCP), anti-CD8 PerCP (BD Biosciences), and appropriate isotype control monoclonal antibodies.
Whole blood samples were incubated for 15 minutes on ice with FACS Lyse buffer (BD Biosciences) and then washed in wash buffer (phosphate buffered saline with 1% bovine serum albumin and 0.1% sodium azide). Cells were then stained for 30 minutes in the dark on ice and then washed in wash buffer, fixed in 1% formaldehyde. Monocytes were analyzed using a Miltenyi MACS Quant flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany) and MACS Quant software (version 2.21031.1, Miltenyi Biotec). T-lymphocytes were analyzed using an LSR II flow cytometer (Becton-Dickinson, San Jose, CA) and FACSDiva software version 6.1.1 (BD Biosciences, San Diego, CA).
Carotid Artery Ultrasound
An ultrasound scan of the carotid arteries was performed using a Philips iU22 ultrasound system with a L9-3 MHz linear array transducer according to American Society of Echocardiography guidelines(11). R-wave gated still frame images of the distal 1cm of the CCA far wall were obtained at 3 separate angles bilaterally (anterior, lateral, and posterior). CCA-IMT was measured offline by a single reader (CTL) using semi-automated edge detection software (Medical Imaging Applications LLC, Coralville, IA). The mean-mean CCA-IMT was used for analysis. Carotid plaque was defined as IMT >1.5cm or > 50% thicker than the adjacent vessel.
Statistical Analysis
Pearson correlation was used to examine the association of CD8+ activation, monocyte subsets, markers of monocyte activation, and serum markers of inflammation with mean CCA-IMT. This study had 80% power to detect a correlation of 0.35. Multivariable linear regression models were then constructed to examine the association of each of these factors with CCA-IMT after adjustment for age, sex, smoking, family history of myocardial infarction (MI), body-mass index (BMI), systolic blood pressure, fasting serum glucose and LDL-C concentrations. In a post-hoc analysis of 3 biomarkers of interest (IL-6, CD8+CD38+HLA-DR+, and sVCAM-1), the relationship with CCA-IMT was examined in models that adjusted for age alone. The t-test was used to compare subjects with and without plaque. For the initial analyses, activated T-cells and monocyte subsets were expressed as a percentage of the total cell population. In a post-hoc analysis, all tests were repeated using absolute numbers of monocytes.
All statistical tests were two-sided with a 0.05 significance level. Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
Results
Median (IQR) age was 47(43,52) years. 78% were men and 65% African-American. Median BMI was 27(24,31) kg/m2LDL-C was 102(86,114) mg/dL, CD4+ count was 648(511, 857) cells/µL, and ART duration was 104(50, 149) months. 58% were current smokers. 35% had a family history of MI, 45% were on protease inhibitors. Twenty-nine (48%) participants screened in with an hs-CRP ≥2mg/L; 31(52%) participants screened in with CD8+ activation ≥19%. Twelve participants (20%) had both hs-CRP ≥2mg/L and CD8+ activation ≥19%. Median hs-CRP of all participants was 2.45(0.78,4.55) mg/L. Among the 39 total participants who were tested for T-cell activation, median CD8+CD38+HLA-DR+ was 27(21,35)%. Median CCA-IMT among all participants was 647(628, 732) µm.
The associations of traditional risk factors, HIV-related factors, markers of inflammation and immune activation with mean CCA-IMT are displayed in Table 1. A statistically significant correlation was observed between CD8+ T-cell activation and CCA-IMT; although, the association did not remain statistically significant after adjustment for traditional risk factors. Significant correlations were also observed in the expected direction with age, sex and family history of MI as well as several serum markers of inflammation (IL-6, sVCAM-1, sTNFR-I, and fibrinogen). Age, family history of MI, sTNFR-I, and fibrinogen remained independently associated with CCA-IMT after adjustment for traditional risk factors. In a post-hoc analysis of CD8+CD38+HLA-DR+, IL-6 and sVCAM-1, adjustment for age alone resulted in significant attenuation of the relationship between the 3 biomarkers and CCA-IMT (all p>0.15). Soluble CD14, soluble CD163, monocyte subsets and TF expression, and HIV-related factors were not associated with CCA-IMT in these analyses.
Table 1.
Associations of traditional risk factors, HIV-related factors, inflammation, and immune activation with mean common carotid artery intima-media thickness.
| Traditional risk factors | |||
|---|---|---|---|
| Pearson Correlation* | Multivariable linear regression§ |
||
| r | P-value | Adjusted P-value | |
| Age | 0.444 | <0.001 | 0.003 |
| Male sex | 0.292 | 0.023 | 0.616 |
| Current smoking | 0.022 | 0.868 | 0.885 |
| Body mass index | −0.284 | 0.028 | 0.302 |
| Systolic blood pressure | 0.044 | 0.738 | 0.651 |
| Fasting glucose | −0.107 | 0.415 | 0.333 |
| Low-density lipoprotein | −0.019 | 0.887 | 0.328 |
| Family history of MI | 0.260 | 0.049 | 0.013 |
| HIV-related factors | |||
| Years since HIV diagnosis | 0.299 | 0.061 | 0.851 |
| Current CD4+ count | −0.164 | 0.214 | 0.826 |
| Nadir CD4+ count | −0.038 | 0.785 | 0.795 |
| Current PI use | 0.130 | 0.323 | 0.781 |
| Duration of PI use | 0.061 | 0.749 | 0.645 |
| Inflammation markers | |||
| Interleukin-6 | 0.283 | 0.028 | 0.411 |
| sICAM-1 | −0.027 | 0.836 | 0.904 |
| D-Dimer | 0.087 | 0.509 | 0.218 |
| sVCAM-1 | 0.434 | 0.004 | 0.677 |
| sTNFR-I | 0.591 | <0.001 | 0.007 |
| Fibrinogen | 0.257 | 0.047 | 0.033 |
| hs-CRP | 0.170 | 0.195 | 0.624 |
| Lymphocyte activation | |||
| log CD8+CD38+HLA-DR+ | 0.326 | 0.043 | 0.657 |
| Monocyte activation | |||
| log soluble CD14 | 0.163 | 0.214 | 0.631 |
| log soluble CD163 | 0.132 | 0.315 | 0.485 |
| CD14+CD16− | −0.063 | 0.647 | 0.829 |
| CD14+CD16+ | 0.080 | 0.564 | 0.876 |
| CD14dimCD16+ | −0.025 | 0.858 | 0.813 |
| CD14+CD16−TF+ | −0.037 | 0.786 | 0.529 |
| CD14+CD16+TF+ | 0.053 | 0.699 | 0.454 |
| CD14dimCD16+TF+ | 0.050 | 0.716 | 0.833 |
Pearson correlation was used to test for univariable associations
Multivariable linear regression was used to test for associations after adjustment for all traditional risk factors listed in the table
r, correlation coefficient; MI, myocardial infarction; PI, protease inhibitor; sICAM-1, soluble intercellular adhesion molecule; sVCAM-1, soluble vascular cell adhesion molecule; sTNFR-I, tumor necrosis factor-á receptor I; hs-CRP, high-sensitivity C-reactive protein; TF, tissue factor
More than a third of the participants (22 of 60, 37%) had evidence of atherosclerotic plaque formation in one or more carotid vessel. Consistent with the CCA-IMT findings, participants with plaque had higher CD8+ activation but similar proportions of monocyte subsets and monocyte tissue factor expression (Figure 1). Those with plaque were also older [51(7.7) vs. 43(9.4) years, mean (SD), p=0.002] and had higher serum concentration of sVCAM [737(159) vs. 592(160) ng/mL, p=0.008] compared to those without plaque. No statistically significant differences in other serum markers of inflammation were observed. A repeated analysis using absolute numbers of each of the monocyte subsets confirmed a lack of association with either CCA-IMT or carotid plaque (data not shown).
Figure 1.
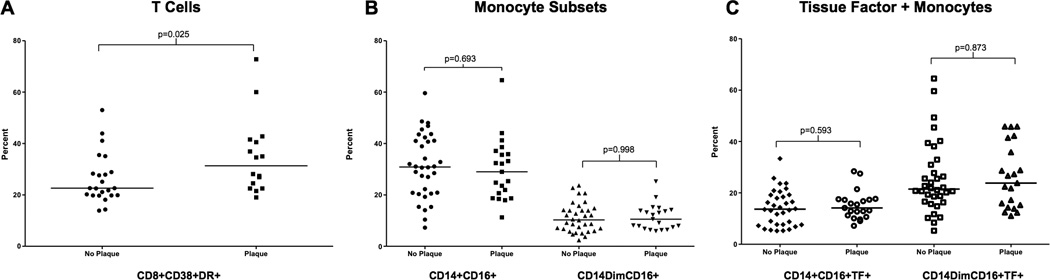
Immune activation in participants with plaque compared to those without plaque. A Proportions of T-cell activation as measured by %CD8+CD38+DR+. B Proportions of two pro-inflammatory monocyte subsets, CD14+CD16+ and CD14dimCD16+. C Proportions of these two monocyte subsets that express tissue factor on the cell surface. Similar results were seen for a third monocyte subset, CD14+CD16-. T-tests were used to test for statistical significance.
Discussion
CD8+ T-cell activation and inflammation, but not monocyte activation or TF expression, appear to be associated with subclinical vascular disease in virologically suppressed HIV-1 infected patients with high levels of baseline inflammation and immune activation. The moderate associations with some biomarkers appear to be independent of traditional risk factors; although, this study may not have adequate power to show independent relationships with other markers of inflammation. Our findings confirm a previous study linking carotid IMT with inflammation(3) and are consistent with data from the Women’s Interagency HIV Study (WIHS) that associated CD8+ activation with carotid plaque in a mixed population of ART treated and untreated participants(4). In contrast to the WIHS cohort, we additionally describe an association between CD8+ activation and CCA-IMT and extend these findings to a mostly male population.
Although both carotid plaque and IMT provide independent predictive value for cardiovascular events(12, 13), each reflects a different pathophysiology and distinct stage of atherogenesis. IMT thickening may occur with vascular remodeling in the absence of atherosclerosis(14); however, there is evidence that IMT above a certain level more likely represents early atherosclerotic plaque(15). Our findings suggest that CD8+ activation and inflammation are linked to both vascular remodeling and plaque. This association is partly related to aging, although the relationship with sTNFR-I and fibrinogen appears to be independent of several traditional CVD risk factors including age.
The role of T-cells and the adaptive immune response in atherosclerosis has long been recognized. Although mouse models suggest CD4+ cells may be more atherogenic than CD8+ cells within plaques, circulating CD8+ cells may also promote atherosclerosis in response to activation by intracellular infections such as cytomegalovirus or Chlamydia pneumoniae(16). Kaplan et al have demonstrated that in HIV, CD8+ activation may be more predictive of carotid plaque(4) while CD4+ activation is more closely associated with carotid stiffness, another measure of subclinical vascular disease(17).
Blood monocytes and tissue macrophages are also well-described mediators of atherosclerosis. In humans, pro-inflammatory CD14+CD16+ monocytes produce unique cytokine profiles in response to Toll-like receptor ligands such as bacterial lipopolysaccharide (LPS), whereasCD14dimCD16+ cells typically serve an anti-inflammatory function as they “patrol” the endothelium, but become pro-inflammatory in response to viral infections(18). Soluble markers of monocyte activation such as sCD14 and sCD163 have been linked to mortality(19) and coronary disease(6) in HIV. Surprisingly, neither proportions of inflammatory monocytes nor sCD14 or sCD163 were associated with CCA-IMT or plaque in our study. Monocyte TF expression also did not correlate with subclinical atherosclerosis, but may still be an important driver of cardiovascular risk and thrombotic plaque rupture. Our group has demonstrated that the profile of TF expression on monocyte subsets in HIV is similar to uninfected patients with acute coronary syndrome(20).
The small sample size is a notable limitation of our study. Specifically, in regards to monocyte activation, our study may not have adequate power to detect a small correlation of CIMT with sCD14 and sCD163; however, the nearly absent observed correlations between CIMT and the monocyte subsets are unlikely to significantly change with a larger sample size. As in all cross-sectional studies, we cannot prove causality or rule out residual confounding. These observations were made in a select population of virologically controlled patients on ART with favorable lipid profiles and may not be generalized to all patients with HIV infection. Finally, because of the number of statistical tests performed, there is a risk of type I error.
In conclusion, participants in SATURN-HIV have a high level of baseline inflammation and immune activation that is associated with subclinical vascular disease despite low serum LDL-C. We anticipate that longitudinal follow-up of this trial will help clarify the detrimental effects of chronic immune-activation and inflammation on vascular disease in this population.
Acknowledgements
The project described was supported by an award from the National Institutes of Health NR012642 (to GAM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. Additional technical support was provided by the CWRU/UH Center for AIDS Research (NIH Grant Number P30 AI36219). The study is registered with clinicaltrials.gov, number NCT01218802.
References
- 1.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies. Heart. 2009 Nov;95(22):1826–1835. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 2.Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004 Jan 27;109(3):316–319. doi: 10.1161/01.CIR.0000114520.38748.AA. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC, Rizk N, O'Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009 Oct 1;49(7):1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict sub-clinical carotid artery disease in HIV-infected women. J Infect Dis. 2011 Feb 15;203(4):452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. Aids. 2006 Nov 28;20(18):2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 6.Burdo TH, Lo J, Abbara S, Wei J, Delelys ME, Preffer F, et al. Soluble CD163, a Novel Marker of Activated Macrophages, Is Elevated and Associated With Noncalcified Coronary Plaque in HIV-Infected Patients. J Infect Dis. 2011 Oct;204(8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010 Jan 14;115(2):161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leatham EW, Bath PM, Tooze JA, Camm AJ. Increased monocyte tissue factor expression in coronary disease. Br Heart J. 1995 Jan;73(1):10–13. doi: 10.1136/hrt.73.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011 Oct 15;204(8):1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 11.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008 Feb;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89-90. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999 Jan 7;340(1):14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 13.Wyman RA, Mays ME, McBride PE, Stein JH. Ultrasound-detected carotid plaque as a predictor of cardiovascular events. Vasc Med. 2006 May;11(2):123–130. doi: 10.1191/1358863x06vm666ra. [DOI] [PubMed] [Google Scholar]
- 14.Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W, Jr, Richardson M, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1992 Jan;85(1):391–405. doi: 10.1161/01.cir.85.1.391. [DOI] [PubMed] [Google Scholar]
- 15.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke. 1997 Dec;28(12):2442–2447. doi: 10.1161/01.str.28.12.2442. [DOI] [PubMed] [Google Scholar]
- 16.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010 Jan;134(1):33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011 Jul;217(1):207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010 Sep 24;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011 Mar 15;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndromes. Blood. 2012 Oct 11; doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]


