Abstract
Alpha‐fetoprotein is a well‐known tumor marker in the screening and follow‐up of hepatocellular carcinoma. In Taiwanese society, a high prevalence of hepatitis and hepatoma and elevation of alpha‐fetoprotein associated with liver function impairment usually suggested clinics undertake further examination for liver or genital tumor. We report the case of 45‐year‐old man who was found to have an alpha‐fetoprotein‐producing esophageal adenocarcinoma with an initial presentation of liver function impairment and rapid elevation of alpha‐fetoprotein. Esophageal cancer was diagnosed via endoscope and a biopsy proved the presence of adenocarcinoma. A small endoscopic biopsy specimen failed to identify the alpha‐fetoprotein positive tumor cell. Esophagectomy was performed and histopathological study of surgical specimen revealed grade II adenocarcinoma with regional metastatic lymphadenopathy. Immunohistochemical study was focal positive for alpha‐fetoprotein. Serum alpha‐fetoprotein declined transiently after esophagectomy and fluctuation of alpha‐fetoprotein level was noted during the treatment with adjuvant chemotherapy. Finally, 19 months after the operation, the patient died due to multiple organ metastases with multiple organ failure. Thus, a small specimen for upper endoscopy may not be sufficient in the presence of alpha‐fetoprotein‐producing adenocarcinoma. Monitoring of serum alpha‐fetoprotein may be useful in the evaluation and follow‐up of esophageal alpha‐fetoprotein‐producing adenocarcinoma.
Keywords: Adenocarcinoma, Alpha‐fetoprotein, Esophageal cancer
Introduction
In Taiwan, an area with high prevalence of hepatocellular carcinoma, elevation of alpha‐fetoprotein (AFP) in cases with abnormal level of liver enzymes usually advocate clinicians to do more examinations for liver or genital tumors. Increasing serum AFP that is associated with malignant neoplasm is well‐known in cases of hepatocellular carcinoma, yolk sac tumor and other malignancies including carcinoma of the stomach, gallbladder, lung, urinary tract, pancreas and genital organs [[1], [2], [3], [4], [5], [6], [7], [8]]; however, there are few reports of esophageal cancer expressing AFP. This case reports a rare AFP ‐producing adenocarcinoma and we briefly review the published case series.
Case report
A 45‐year‐old man had a history of poliomyelitis with lower leg muscular atrophy, essential hypertension, hyperlipidemia and fatty liver. He had taken regular medication prescribed by local clinics for hypertension for 4 years. He received a health work‐up and liver function impairment was evident. He was referred to our department for further evaluation of hepatitis. The patient suffered no discomfort except for dysphagia with solid food, which had been noted in past month. Neither body weight loss nor abdominal pain was mentioned. There were no obvious physical findings except for muscular atrophy and scoliosis due to poliomyelitis.
Our initial examination showed alanine aminotransferase 194 IU/L (normal range,10–40 IU/L), aspartate aminotransferase 99 IU/L (normal range, 10–42 IU/L), serum hepatitis B surface antigen negative, anti‐hepatitis C virus negative, antinuclear antibody negative and AFP 28.6 IU/ml (normal range, <9.0 IU/ml). Abdominal ultrasound study showed fatty liver and the tentative diagnosis was non‐alcoholic steatosis hepatitis.
Due to the unusual elevation of AFP, we checked his AFP level 2 months later and it had increased to 171.8 IU/ml. Further serum biochemical study showed hemoglobin 12.2gm/dl (normal range, 14–17.5 gm/dl), albumin 4.71 gm/dl (normal range, 3.5–5.0 gm/dl), alkaline phosphatase 88 IU/L (normal range, 32–92 IU/L), r‐glutamyl transpeptidase 49 IU/L(normal range,7–64 IU/L) and stool routine occult blood positive. Serum tumor marker studies showed squamous cell carcinoma 0.4 ng/ml (normal range, <1.5 ng/ml), carbohydrate‐19‐9 antigens 71.5 U/ml (normal range, <37 U/ml) and carcinoembryonic antigen 1.13 ng/ml (normal range, <5 ng/ml).
An esophagogram was arranged and showed a polypoid filling defect in the distal esophagus with central depression. Mild contour irregularity and rigidity was also noted at the esophagus–cardiac junction (Fig. 1). Upper endoscope revealed a polypoid lesion in the lower esophagus at a distance of 36 cm from the incisor just above the esophageal–cardiac junction with easy fragility and mild lumen narrowing (Fig. 2). Endoscopic biopsy was performed and histological examination showed a grade II adenocarcinoma. However, endoscope biopsy specimen was too small to show immunohistochemical study of AFP positive (Data not shown). Abdomen computed tomography revealed low esophageal invasion to adventitia and extension to the adjacent gastric cardiac portion. Metastatic lymphadenopathy in the para‐aortic region and perigastric region was also noted. Image staging therefore classes the adenocarcinoma as being stage IV (T3N1M1a), see Fig. 3.
Figure 1.
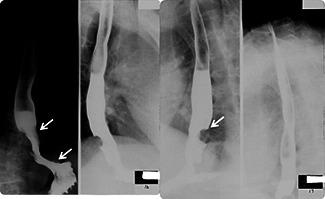
Barium esophagogram showed a polypoid filling defect in the distal esophagus and central depression was noted (arrow). Mild contour irregularity and rigidity was also identified at the esophagus–cardiac junction.
Figure 2.
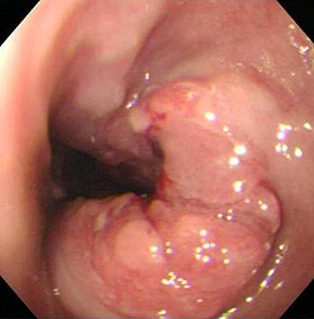
Upper endoscopy showed a polypoid lesion with fragility in the lower third of the esophagus. Mild lumen narrowing was also noted.
Figure 3.
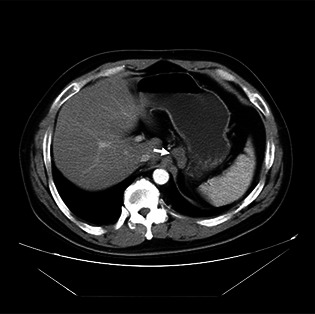
Abdominal computed tomography revealed annular wall thickening with a blurred adventitia margin in the lower esophagus and the soft tissue mass lesion was downward to the cardiac portion. Metastatic lymphadenopathy was noted (arrow).
Following patient request and a fine performance status, the patient received esophagectomy, lymphadenectomy and partial gastrectomy with reconstruction. The tumor was 4 × 3 × 1.5 cm in size. The tumor bulk was located 0.2 cm above the gastroesophageal junction. It extended to the adjacent stomach and deep into the adventitia of the esophagus. Microscopic examination showed grade II adenocarcinoma with adventitia invasion. Lymphovascular invasion and perineural invasion were also noted. Immunohistochemical study was focal positive for AFP stain (Fig. 4). Metastatic lymphadenopathy with adenocarcinoma was also noted. Surgical staging was stage IV (T3N1M1a). Serial examinations of AFP showed rapid elevation of AFP before the operation and an abrupt decrease after tumor resection (see Fig. 5).
Figure 4.
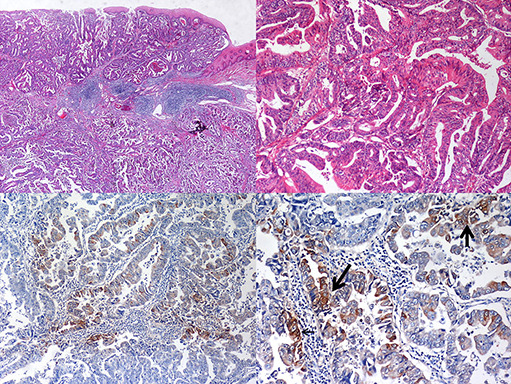
Histological appearance of the surgical specimen from the esophageal caner showed tubular adenocarcinoma. Immunochemistry stain with alpha‐fetoprotein was focal positive with alpha‐fetoprotein in cytoplasm (arrows).
Figure 5.
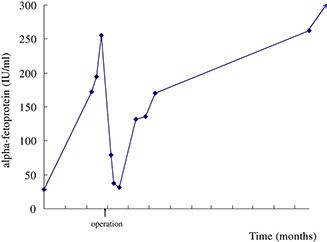
The alpha‐fetoprotein level dropped immediately after esophagectomy (arrow). It increased again with tumor recurrence.
In the following 8 months, the patient received 16 courses of adjuvant chemotherapy with cisplatin plus high‐dose 5‐fluorouracil. The image study with positron emission tomography, however, revealed multiple intensive foci in lymph nodes in the subclavian and middle thoracic paraesophageal region. As the patient appeared to be suffering from a relapse, he received a further nine courses of chemotherapy with oxaliplatin plus high‐dose 5‐fluorouracil and radiotherapy in the following 6 months. Despite this, peritoneal carcinomatosis with massive ascites, liver metastasis and right pleural metastasis were encountered in the following image study. AFP had increased to a level of 12267.0 ng/ml 14 months after the operation. Although the patient received another four courses of irinotecan plus carboplatin, the disease progressed rapidly. Nineteen months after the initial operation, the patient expired due to multiple organ failure.
Discussion
AFP is the fetal globulin that is synthesized by the fetal liver and yolk sac from the sixth week of gestation [9]. AFP has a molecular weight of 70,000 Daltons, consisting of a single peptide chain and containing about 4% carbohydrate. After birth, the level of AFP gradually decreases to a normal adult range of 10–20 IU/ml within a few weeks or months [9].
AFP is a useful tumor marker for hepatocellular carcinoma and yolk sac tumor. Elevation of serum AFP has been reported in patients with other malignancies including stomach, gallbladder, lung, urinary tract, pancreas and genital organ cancers. McIntire et al. reported that elevation of serum AFP had been observed in gastrointestinal neoplasm: 15% of 95 patients with gastric carcinoma, 3% of 191 patients with colorectal carcinoma, 24% of 45 patients with pancreatic carcinoma, 25% of eight patients with biliary tract carcinoma and 70% of 73 patients with hepatocellular carcinoma had elevated serum AFP [10]. Esophageal cancer and small intestine neoplasm had the lowest percentage of elevated AFP [10]. Overall, AFP was not a good parameter for the screening of gastrointestinal neoplasm or metastatic liver diseases [11].
Most esophageal adenocarcinomas were located over the lower third of the esophagus and were related to Barrett's esophagus. Few case reports have noted AFP‐producing esophageal cancer. Some cases of AFP‐producing esophageal adenocarcinoma were derived from Barrett's esophagus and they could also be regarded as closely resembling AFP‐producing gastric carcinomas.
Some carcinomas with high levels of serum AFP contained cells resembling a hepatic cell classified as hepatoid adenocarcinoma. In comparison with hepatoid adenocarcinoma, AFP‐producing carcinomas with no histologic characteristics of hepatocellular carcinomas are termed AFP‐producing adenocarcinomas [12].
Primary gastrointestinal hepatoid adenocarcinoma is very rare and cases mostly arise from the gastric epithelium. This neoplasm has a poor prognosis, readily metastasizes to the liver, and is frequently found at an advanced stage of disease. To the best of our knowledge, there have been only nine reported cases of AFP‐producing esophageal adenocarcinoma in the English literature. Five of these were esophageal hepatoid adenocarcinomas [[13], [14], [15], [16], [17]] and the others were designated AFP‐producing adenocarcinomas [[18], [19], [20], [21]] (summarized in Table 1). Similar to hepatoid carcinoma in other anatomical sites, hepatoid adenocarcinoma in the esophagus frequently metastasizes in the liver and usually pursues a poor clinical course. Most cases were treated with adjuvant chemotherapy with cisplatin plus 5‐fluorouracil as first‐line. The results of this, however, were mostly disappointing. Chiba et al. reported a case of esophageal hepatoid adenocarcinoma with multiple liver metastases that was treated with a combination of paclitaxel and cisplatin as the second regimen. The initial response was partially effective; however, the patient died due to gastrointestinal bleeding 14 months after the initial operation [15]. In contrast, non‐hepatoid adenocarcinoma may not be related to Barrett's esophagus and the clinical outcomes may differ [20]. However, the small number of cases reported to date makes it difficult to identify any definite trends.
Table 1.
Summary of hepatoid carcinoma and alpha‐fetoprotein (AFP)‐producing carcinoma.
| Ref. | Age | Sex | Tumor name | BE | Outcome | Metastasis | Reporter |
|---|---|---|---|---|---|---|---|
| [13] | 80 | F | Hepatoid | + | Died, 2m | Liver, lung | Motoyama, T |
| [14] | 44 | F | Hepatoid | + | Died, 4m | Liver | Tanigawa, H |
| [15] | 47 | M | Hepatoid | + | Died, 14m | Liver | Chiba, N |
| [16] | 56 | M | Hepatoid | ? | ?? | Liver | Atiq, M |
| [17] | 76 | M | Hepatoid | + | Alive at 2m* | — | Kuroda, N |
| [18] | 80 | M | AFP‐producing | ? | Died, 4m | Liver, lung, spleen, LN | Sawada, H |
| [19] | 59 | M | AFP‐producing | + | Died, 2m | Liver, LN | Shimakawa, T |
| [20] | 51 | M | AFP‐producing | − | Died, 67m | Pleura, LN | Kobayashi, N |
| [21] | 69 | M | AFP‐producing | ? | Alive at 6m# | — | Kawai, H |
*Alive at the follow‐up of 2 months; #alive at the follow‐up of 6 months. BE = Barrett's esophagus; LN = lymph node metastasis.
In our case, liver function impairment and increasing AFP caused confusion as to the origin of the site of malignancy. Upper endoscope biopsy failed to prove AFP‐positive this may be due to the small biopsy specimen taken. Focal positive of AFP‐producing adenocarcinoma showed the heterogeneous character of tumor growth and cell type. No obvious presentation of Barrett's esophagus was noted and histological examination of the specimen removed during the operation did not show intestinal metaplasia in the surrounding tissue, which is characteristic for Barrett's esophagus. We therefore believed that our patient had a case of AFP‐producing adenocarcinoma similar to the case reported by Kobayashi et al [20]. The clinical outcome appears to be better than that of esophageal hepatoid adenocarcinoma. As in this case with AFP‐producing adenomcarcinoma without coexisting Barret's esophagus, the levels of AFP could be used as therapeutic markers.
References
- [1]. Ciftci A.O., Bingol‐Kologlu M., Senocak M.E., Tanyel F.C., Buyukpamukcu M., Buyukpamukcu N.. Testicular tumors in children. J Pediatr Surg. 2001; 36: 1796–1801. [DOI] [PubMed] [Google Scholar]
- [2]. Huang H.Y., Ko S.F., Chuang J.H., Jeng Y.M., Sung M.T., Chen W.J.. Primary yolk sac tumor of the urachus. Arch Pathol Lab Med. 2002; 126: 1106–1109. [DOI] [PubMed] [Google Scholar]
- [3]. Lopez‐Beltran A., Luque R.J., Quintero A., Requena M.J., Montironi R.. Hepatoid adenocarcinoma of the urinary bladder. Virchows Arch. 2003; 442: 381–387. [DOI] [PubMed] [Google Scholar]
- [4]. Michielsen P.P., Francque S.M., van Dongen J.L.. Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol. 2005; 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Nakashima H., Nagafuchi K., Satoh H., Takeda K., Yamasaki T., Yonemasu H., et al. Hepatoid adenocarcinoma of the gallbladder. J Hepatobiliary Pancreat Surg. 2000; 7: 226–230. [DOI] [PubMed] [Google Scholar]
- [6]. Paner G.P., Thompson K.S., Reyes C.V.. Hepatoid carcinoma of the pancreas. Cancer. 2000; 88: 1582–1589. [DOI] [PubMed] [Google Scholar]
- [7]. Poulakis V., Witzsch U., de Vries R., Becht E., Altmannsberger H.M., Storkel S.. Alpha‐fetoprotein‐producing renal cell carcinoma. Urol Int. 2001; 67: 181–183. [DOI] [PubMed] [Google Scholar]
- [8]. Yamanaka A., Enokibori T., Kato H., Okada Y.. A case of alpha‐fetoprotein producing lung cancer. Nihon Kyobu Shikkan Gakkai Zasshi. 1987; 25: 257–261 [in Japanese]. [PubMed] [Google Scholar]
- [9]. Knopfle G., Becker M., Rotthauwe H.W.. Alpha1‐fetoprotein: physiology, pathology and diagnosis especially in childhood (author's transl). Klin Padiatr. 1977; 189: 207–226[article in German]. [PubMed] [Google Scholar]
- [10]. McIntire K.R., Waldmann T.A., Moertel C.G., Go V.L.. Serum alpha‐fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975; 35: 991–996. [PubMed] [Google Scholar]
- [11]. Pardela M., Buntner B., Ostrowska Z., Wylezol M., Kozlowski A., Piecuch J., et al. Diagnostic value of the analysis of alpha fetoprotein levels in malignant neoplasms of the digestive system. Wiad Lek. 1992; 45: 593–596, [in Polish, with English abstract]. [PubMed] [Google Scholar]
- [12]. Aizawa K., Motoyama T., Suzuki S., Tanaka N., Yabusaki H., Tanaka S., et al. Different characteristics of hepatoid and non‐hepatoid alpha‐fetoprotein‐producing gastric carcinomas: an experimental study using xenografted tumors. Int J Cancer. 1994; 58: 430–435, [in English]. [DOI] [PubMed] [Google Scholar]
- [13]. Motoyama T., Higuchi M., Taguchi J.. Combined choriocarcinoma, hepatoid adenocarcinoma, small cell carcinoma and tubular adenocarcinoma in the oesophagus. Virchows Arch. 1995; 427: 451–454. [DOI] [PubMed] [Google Scholar]
- [14]. Tanigawa H., Kida Y., Kuwao S., Uesugi H., Ojima T., Kobayashi N., et al. Hepatoid adenocarcinoma in Barrett's esophagus associated with achalasia: first case report. Pathol Int. 2002; 52: 141–146. [DOI] [PubMed] [Google Scholar]
- [15]. Chiba N., Yoshioka T., Sakayori M., Yoshiki M., Miyazaki S., Akiyama S., et al. AFP‐producing hepatoid adenocarcinoma in association with Barrett's esophagus with multiple liver metastasis responding to paclitaxel/CDDP: a case report. Anticancer Res. 2005; 25: 2965–2968. [PubMed] [Google Scholar]
- [16]. Atiq M., Husain M., Dang S., Olden K.W., Malik A.H., Brown D.K.. Hepatoid esophageal carcinoma: a rare cause of elevated alpha fetoprotein. J Gastrointest Cancer. 2008; 39: 58–60. [DOI] [PubMed] [Google Scholar]
- [17]. Kuroda N, Onishi K, Lee GH. Combined tubular adenocarcinoma and hepatoid adenocarcinoma arising in Barrett esophagus. Ann Diagn Pathol;15:450–453. [DOI] [PubMed]
- [18]. Sawada H., Watanabe A., Yamada Y., Yano T., Nakano H., Konishi Y.. Alpha‐fetoprotein‐producing esophageal adenocarcinoma: report of a case. Surg Today. 1993; 23: 1103–1107. [DOI] [PubMed] [Google Scholar]
- [19]. Shimakawa T., Ogawa K., Naritaka Y., Wagatsuma Y., Katsube T., Hamaguchi K., et al. Alpha‐fetoprotein producing Barrett's esophageal adenocarcinoma: a case report. Anticancer Res. 1999; 19: 4369–4373. [PubMed] [Google Scholar]
- [20]. Kobayashi N., Ohbu M., Kuroyama S., Kikuchi S., Shimao H., Mitomi H., et al. Alpha‐Fetoprotein‐producing esophageal adenocarcinoma: report of a case. Surg Today. 2001; 31: 915–919. [DOI] [PubMed] [Google Scholar]
- [21]. Kawai H., Sekine S., Sanada T., Andoh T., Takechi Y., Okada S.. Alpha‐fetoprotein‐producing esophageal carcinoma: a case report. Anticancer Res. 2003; 23: 3837–3840. [PubMed] [Google Scholar]


