Abstract
OBJECTIVE
Cervical cancer is the most common gynaecological cancer in developing countries. Visual Inspection with Acetic Acid (VIA) was introduced to screen for cervical premalignant lesions in developing countries due to the inability of many countries to implement high quality cytologic services. We sought to compare VIA performance among different health workers in Nigeria.
METHODS
In a population-based project, seven health workers working who had been screening women with VIA for about two years at local government health centers in rural Nigeria were retrained in a two-week program using the IARC training manual. Women from a rural village who had never had cervical cancer screening were recruited into the study. Each woman had cervical cancer screening by VIA, liquid-based cytology and oncogenic human Papillomavirus (HPV) DNA testing.
RESULTS
Despite similar participant characteristics, across all age groups, providers had wide ranges of VIA results; 0–21% suspect cancer and 0–25% VIA positive. VIA was insensitive compared to a combination of cytology and HPV testing.
CONCLUSION
In our study, VIA was not reproducible nor was it sensitive compared to cytology and HPV testing.
Keywords: Cervical cancer, VIA, Liquid-based cytology, HPV DNA, Health workers
INTRODUCTION
Cervical cancer is the third most common cancer in women, and the seventh overall, with an estimated 530,000 new cases in 2008 1. More than 85% of the global burden occurs in developing countries, where it accounts for 13% of all female cancers 1,2. Cervical cancer is a public health problem in developing countries and has enormous social and economic impacts on the populace since it often affects women who are within the reproductive age groups 3, 4. It is the most common female genital cancer in many developing countries; the highest burden of the disease has been reported in Asian, Latin American and African countries 5–6. In Nigeria, the age specific incidence increases from 18.8 per 100,000 in women aged 20–24 years to 373.8 per 100,000 years in women aged 60–64 years 7, 8, 9.
The incidence is not only higher in developing countries; survival too is poorer when compared to developed countries 1. This is due to both advanced clinical stages at presentation and to the fact that many patients do not receive or complete prescribed courses of treatment, due to deficiencies in treatment availability, accessibilities and affordability 2.
Developed countries have succeeded in reducing the incidence and mortality from cervical cancer through screening programmes for the detection of pre-malignant lesions and adequate treatment of detected cases 10. In contrast, Pap smear as a method of screening for cervical cancer has been practiced in many developing countries for several years, but the incidence and mortality from cervical cancer has not decreased; recent evidence indicates that it may even be increasing 11. This is partly because screening facilities in developing countries for cervical premalignant lesions are few and are mostly concentrated in urban areas whereas majority of the population resides in underserved rural areas. Even at these urban centres, there is an absence of high-quality cytological services 12, 13.
The Pap smear has been difficult to implement in many developing countries because it is laboratory-based and requires highly trained personnel to prepare, stain, and read the slides. In addition, this method of screening requires that the patients make several visits to the health centre before treatment can be given, first to collect the results, then for ancillary investigations like colposcopy-directed biopsy.
Moreover, a major problem with screening for cervical premalignant lesions in developing countries is that there is poor compliance rate with treatment. Many patients simply do not come back for treatment or do not complete their prescribed treatment. Reasons for this may be due to the economic hardships that many of the women face daily. An important factor is that cervical premalignant lesions rarely are symptomatic; thus, it is very difficult to convince these women to come for treatment of screen-detected lesions in the absence of symptoms.
Due to the above-mentioned reasons, several alternatives have been proposed for developing countries. Among these is direct Visualization of the Cervix using Acetic Acid (VIA) 14. This involves the application of 5% dilute acetic acid to the cervix and observation for color changes (whitening). The main attractions of this technique are that unlike conventional cytology, the results of the test are obtained almost immediately; it is relatively cheaper and most of the equipment can be sourced locally. It has been proposed that to minimize the problems of compliance with treatment; women screened with VIA in developing countries should be offered treatment at the same clinic visit, the so-called ‘see and treat’ approach 15.
VIA is controversial because of concerns over its reproducibility and accuracy 15–21. There is no consensus over its optimal role in cervical cancer prevention; thus, realistic evaluations remain important. In this study we evaluated the variability between seven health workers trained to perform VIA in rural Nigeria. The results of VIA were compared to cytology and HPV DNA assays performed by collaborators in the United States.
MATERIALS AND METHODS
Seven health workers working at local government health centres in a rural setting in Nigeria who had been screening women for cervical cancer with VIA for about two years were retrained for two weeks on how to perform VIA using the IARC training manual 22. The trained health workers were involved in a population-based project, in which 1282 women who had never had cervical cancer screening in a rural village (Irun) in Western Nigeria were recruited. One health worker examined each women with the following procedures detailed in a previous publication23. First, a liquid-based cytology (LBC), (ThinPrep, Hologic) specimen was collected. After collecting the cervical specimens for the LBC, VIA was done with 5% freshly prepared dilute acetic acid. The resultant colour changes were observed one minute after the application of the acetic acid. The results of the VIA were recorded as; suspect cancer, positive (for acetic white lesion at the squamo-columnar junction), negative (No acetowhite lesion) or squamo-columnar junction not visible. The liquid-based cytology was done and read in the United States and the residual fluid was tested for oncogenic and possibly oncogenic types of HPV-DNA by MY09-MY11-based PCR with dot-blot genotyping of the PCR product. In this analysis, the oncogenic types were judged to include HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.
To examine the consistency of VIA performance across patient characteristics and providers, VIA results were stratified by patient age, concurrent cytology and HPV results, and health worker who conducted the VIA exam. To assess statistical significance of findings, Chi-square and Anova tests were calculated for categorical and ordinal variables, respectively. Within age groups, VIA results by health worker were plotted and Fishers’ exact test of significance was calculated for to compare VIA positivity by health worker. For all statistical tests of comparison, suspicious cancer and positive results were combined and women with an SCJ not visible were excluded since the result is neither positive nor negative.
As an indicator of VIA accuracy, VIA results were compared to concurrent cytology and HPV results, by means of the following risk classification created from the cytology and HPV results. Women were classified as “high-risk” if both the results of the LBC was high grade cytology and any oncogenic HPV was detected; else as “increased risk” if either the LBC was abnormal cytology or if any of the oncogenic HPV were detected; else as “low risk” if the LBC was read as normal cytology and the sample was negative for HR HPV. First, within each risk category, the proportion with abnormal VIA result was calculated and compared using an ANOVA test of significance to approximate sensitivity and specificity. Then, for each given VIA result, the woman’s the risk of precancer was calculated to approximate VIA’s predictive value. Results were stratified by age and ANOVA tests of significance were calculated.
Of note, as laboratory results became available, we eventually referred to colposcopy all women with abnormal cytology and/or HPV positivity, along with a random sample of women screening negative. These referrals were often delayed due to the time lag between screening visit and laboratory results. Perhaps partly due to this time lag, the participation rate in colposcopy was low (68.3%) and we judged it best to use the well-validated cytology and HPV results as reference comparators for calculations of test accuracy.
RESULTS
Complete results were available for 1163 women (90.7%) of the 1282 women screened. Table 1 presents women’s age, concurrent screening results and health worker providing her VIA exam. VIA results are stratified by individual and exam characteristics. Women age 50 or older were more likely to have an exam where the SCJ was not visualized (26.4% vs. 73.6% in women 15–29, P<.01). Women with a cytologic abnormality or infected with an oncogenic HPV genotypes were more likely to test VIA abnormal (positive or suspicious cancer). Nonetheless, health workers varied significantly in their VIA diagnoses as providers judged between 57.0% to 90.4% of VIA results as normal.
Table 1.
Women’s risk of VIA abnormality, given age, concurrent cytology and HPV test results and VIA provider.
| Total | VIA Result (row %) | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Column % | SCJ Not visible | Suspicious Cancer | Positive | Negative | ||||||
| N | % | N | % | N | % | N | % | ||||
| TOTAL | 1163 | 100.0 | 142 | 12.2 | 80 | 6.9 | 81 | 7.0 | 860 | 74.0 | |
|
| |||||||||||
| Age | |||||||||||
| 15–29 | 238 | 20.5 | 0 | 0.0 | 23 | 9.6 | 18 | 7.6 | 197 | 82.8 | |
| 30–49 | 422 | 36.3 | 9 | 2.1 | 27 | 6.4 | 28 | 6.6 | 358 | 84.8 | |
| 50–95 | 503 | 43.3 | 133 | 26.4 | 30 | 6.0 | 35 | 7.0 | 305 | 60.6 | .56a |
| Cytology | |||||||||||
| Normal | 1037 | 90.2 | 132 | 12.7 | 64 | 6.2 | 69 | 6.7 | 772 | 74.5 | |
| ASCUS/LSIL | 80 | 7.0 | 4 | 5.0 | 10 | 12.5 | 4 | 5.0 | 62 | 77.5 | |
| HSIL/Cancer | 33 | 2.9 | 5 | 15.2 | 2 | 6.1 | 8 | 24.2 | 18 | 54.6 | .008a |
| Oncogenic HPV Status | |||||||||||
| Negative | 977 | 15.9 | 117 | 12.0 | 64 | 6.6 | 63 | 6.5 | 733 | 75.0 | |
| Positive | 184 | 84.1 | 25 | 13.6 | 16 | 8.7 | 18 | 9.8 | 125 | 67.9 | .036b |
| Health Worker | |||||||||||
| A | 212 | 18.3 | 43 | 20.3 | 26 | 12.3 | 11 | 5.2 | 132 | 62.3 | |
| B | 207 | 17.9 | 47 | 22.7 | 18 | 8.7 | 24 | 11.6 | 118 | 57.0 | |
| C | 191 | 16.5 | 2 | 1.1 | 14 | 7.3 | 17 | 8.9 | 158 | 82.7 | |
| D | 99 | 8.6 | 12 | 12.1 | 7 | 7.1 | 4 | 4.0 | 76 | 76.8 | |
| E | 237 | 20.5 | 14 | 5.9 | 14 | 5.9 | 11 | 4.6 | 198 | 83.5 | |
| F | 75 | 6.5 | 21 | 28.0 | 0 | 0 | 4 | 5.3 | 50 | 66.7 | |
| G | 136 | 11.8 | 3 | 2.2 | 1 | 0.7 | 9 | 6.6 | 123 | 90.4 | .001b |
Anova p-value. For tests of significance, VIA results of positive and suspicious cancer are combined and excludes women with “SCJ not visible.”
Chi-square p-value. For tests of significance, VIA results of positive and suspicious cancer are combined and excludes women with “SCJ not visible.”
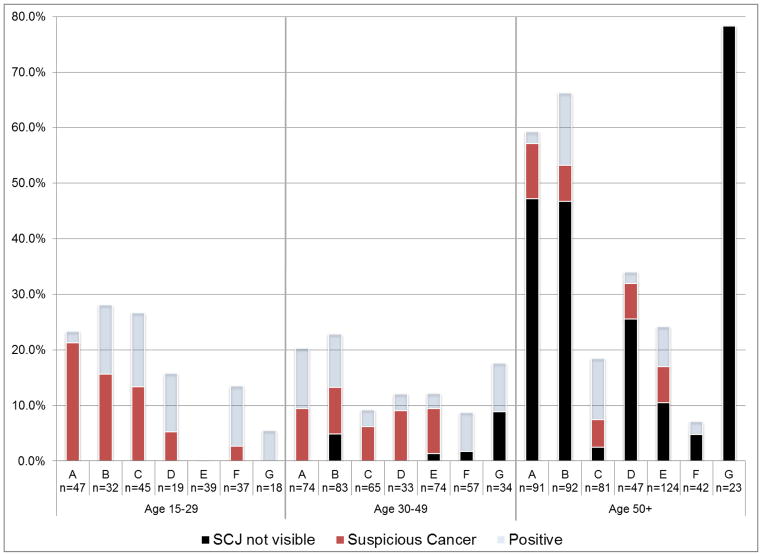
As shown in Figure 1, depending on the health worker, the SCJ was not visualized in 3–78% of women above 50 years. Therefore, the assessment of SCJ visibility was not reproducible. In addition, the proportion of women judged to have suspicious cancer also varied considerably within age groups.
Figure 1.
Percent of VIA examinations with inadequate or abnormal results, by health worker, by age group
Note: SCJ = squamocolumnar junction. N indicates number of VIA examinations for each provider given patient age. Fisher’s exact test of significance: P<.01 for age 15–29, P=.21 for age 30–49, and P<.01 for age 50–99. For tests of significance, VIA results of positive and suspicious cancer are combined and excludes women with “SCJ not visible.”
Table 2 presents each woman’s risk of cervical precancer as determined by her cytology and HPV results and the VIA results within each risk category. Overall, a women’s risk of precancer was associated with her VIA result (P=.02) yet the sensitivity was low with many missed cases. Specifically, of 18 women with a visible SCJ and considered high risk, only 6 (33.3%) had positive or suspect cancer results on VIA testing. Specificity was better as after excluding women with non-visible SCJ, 85.6% of the 815 women categorized as low risk for cervical cancer (HPV-negative and cytology-negative), were VIA-negative.
Table 2.
VIA result given women’s risk for cervical precancer as determined by cytology and HPV resultsa
| Total | Risk for cervical precancer (column %) | |||||||
|---|---|---|---|---|---|---|---|---|
| # | Column % | High-risk | Increased risk | Low risk | ||||
| # | % | # | % | # | % | |||
| Total (row %) | 1163 | 100.0 | 21 | 1.8 | 214 | 18.4 | 928 | 79.8 |
|
| ||||||||
| VIA Result | ||||||||
| Suspect Cancer | 80 | 6.9 | 1 | 4.8 | 20 | 9.4 | 59 | 6.4 |
| Positive | 81 | 7.0 | 5 | 23.8 | 18 | 8.4 | 58 | 6.3 |
| Negative | 860 | 74.0 | 12 | 57.1 | 150 | 70.1 | 698 | 75.2 |
| SCJ not visible | 142 | 12.2 | 3 | 14.3 | 26 | 12.2 | 113 | 12.2 |
Note: Anova P=.02. For tests of significance, VIA results of positive and suspicious cancer are combined and excludes women with “SCJ not visible.”
Women were classified as “high-risk” if both the results of the liquid-based cytology (LBC) was high grade cytology and any oncogenic HPV was detected; else as “increased risk” if either the LBC was abnormal cytology or if any of the oncogenic HPV were detected; else as “low risk” if the LBC was read as normal cytology and the sample was negative for HR HPV.
Table 3 shows the predictive value of VIA results stratified by age. Among women age <30 years, the overall risk of a cervical precancer was so low (0.8%), that the positive predictive value of a VIA-positive or suspicious cancer result was 0%. The positive predictive value of abnormal VIA was highest in women age 30–49 years where 10.7% of 28 women with a VIA-positive result were at high risk of cervical precancer. The negative predictive values were highest in older women as 81.6% of VIA-negative women age 30–49 and 83.4% of VIA-negative women age 50–95 had concurrently negative HPV and cytology results
Table 3.
Proportion of women at risk for cervical precancer given VIA result, by age group
| Total | Risk for cervical precancer%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| High-risk | Increased risk | Low risk | |||||||
| # | Column % | # | % | # | % | # | % | p-valuea | |
| Age 15–29 | |||||||||
|
| |||||||||
| Suspect Cancer | 23 | 9.7 | 0 | 0.0 | 9 | 39.1 | 14 | 60.9 | |
| Positive | 18 | 7.6 | 0 | 0.0 | 6 | 33.3 | 12 | 66.7 | |
| Negative | 197 | 82.8 | 2 | 1.0 | 47 | 23.9 | 148 | 75.1 | |
| SCJ not visible | 0 | 0.0 | |||||||
|
| |||||||||
| Total (Row %) | 238 | 100.0 | 2 | 0.8 | 62 | 26.1 | 174 | 73.1 | 0.21 |
|
| |||||||||
| Age 30–49 | |||||||||
|
| |||||||||
| Suspect Cancer | 27 | 6.4 | 1 | 3.7 | 3 | 11.1 | 23 | 85.2 | |
| Positive | 28 | 6.6 | 3 | 10.7 | 3 | 10.7 | 22 | 78.6 | |
| Negative | 358 | 84.8 | 5 | 1.4 | 61 | 17.0 | 293 | 81.6 | |
| SCJ not visible | 9 | 2.1 | 0 | 0.0 | 0 | 0.0 | 9 | 100.0 | |
|
| |||||||||
| Total (Row %) | 422 | 100.0 | 9 | 2.1 | 67 | 15.9 | 346 | 82.0 | 0.01 |
|
| |||||||||
| Age 50–95 | |||||||||
|
| |||||||||
| Suspect Cancer | 30 | 6.0 | 0 | 0.0 | 8 | 26.7 | 22 | 73.3 | |
| Positive | 35 | 7.0 | 2 | 5.7 | 9 | 25.7 | 24 | 68.6 | |
| Negative | 305 | 60.6 | 5 | 1.6 | 48 | 15.0 | 267 | 83.4 | |
| SCJ not visible | 133 | 26.4 | 3 | 2.3 | 26 | 19.6 | 104 | 78.2 | |
|
| |||||||||
| Total (Row %) | 503 | 100.0 | 10 | 2.0 | 85 | 16.9 | 408 | 81.1 | 0.03 |
Anova p-values. For tests of significance, VIA results of positive and suspicious cancer are combined and excludes women with “SCJ not visible.”
DISCUSSION
In a population-based study in rural Nigeria, we observed high variability between nurses performing VIA. The results of VIA were only weakly associated with risk of cervical cancer as measured by cervical cytology and oncogenic HPV DNA testing. We conclude that VIA as performed in this project was not an acceptable cervical screening method.
VIA was introduced as an alternative testing method for cervical pre-malignant lesions in low-resource countries, which often have high incidence and mortality from cervical cancer. These countries typically lack the manpower and resources for cytology-based cervical cancer screening program. One of the advantages of VIA is that the results are obtained instantly. Other advantages are that it is a relatively inexpensive method and can be performed by most categories of health workers and does not require an elaborate laboratory set up unlike cytology-based screening. Virtually all equipment needed for its performance can be sourced locally. Due to the fact that the results of VIA are obtained immediately, it is theoretically suited for a “see and treat” approach, paired with cryotherapy of screen-positive women, especially in a setting where women may be lost to follow up. However, if it not reproducible or accurate, it cannot be recommended for a see and treat strategy.
In a comparison study of VIA among physicians and nurses, there was moderate agreement between the doctors and the nurses with the nurses having more VIA positive cases than doctors24. In a similar study by Bhavana et al, 25 VIA as performed by nurses had lower sensitivity and specificity compared to doctors.
An important aspect of the present study was that VIA was performed by the exact class of health workers who are most likely to perform the VIA were it to be instituted widely in Nigeria, due to a shortage of physicians especially in the rural areas. The nurses were formally trained and had previous experience. Thus, the variability of the results demands notice.
Reasons for the different interpretations of the results of VIA may stem from the fact that it is a technique of pattern recognition of macroscopic changes that only partly reflect underlying, causal interactions of HPV and the cervical cells. The inherent variability of the appearance of the cervix may have led to different subjective interpretations of the results among different health workers. Most strongly, the results of VIA are based on the observation of aceto-white colour at the squamo-columnar junction. Apart from cervical premalignant lesions, there are many benign lesions of the female genital tract that could give aceto-white colour appearance; examples are inflammation, leukoplakia and cervical condyloma 22. This may account for 15% of low-risk (cytology negative and oncogenic HPV negative) women testing VIA positive in our population.
Conclusions
Our data imply that the results of VIA are poorly reproducible even among a uniform category of trained professional health workers. Our study also revealed that VIA is not accurate enough for the detection of cervical premalignant lesions, if the reference is taken to be a combination of cytology and HPV testing. A major limitation of our project is that we were not able to bring back all women for a thorough colposcopic biopsy procedure, which would have permitted a better reference standard of disease. However, importantly, most of the women with positive VIA results, even those with suspect cancer, had no indication of cervical neoplasia according to cytology and HPV testing. In developing countries where the highest number of cervical cancer is found, we believe that the use of VIA especially if employed in a screen and treat manner may lead to under- and over-treatment with adverse health consequences.
Contributor Information
Julia C. Gage, Email: gagej@mail.nih.gov.
Akinfolarin C. Adepiti, Email: akinfolarindepiti@yahoo.co.uk.
Nicolas Wentzensen, Email: wentzenn@mail.nih.gov.
Claire Eklund, Email: CEklund@WIHRI.org.
Mary Reilly, Email: MReilly@WIHRI.org.
Martha Hutchinson, Email: mhutchin@earthlink.net.
Robert D. Burk, Email: robert.burk@einstein.yu.edu.
Mark Schiffman, Email: schiffmm@mail.nih.gov.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pract & Res Clin Obstet Gynaecol. 2006;20:207–222. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Safaeian S, Solomon S, Castle PE. Cervical cancer prevention – cervical screening: science in evolution. Obstet Gynecol Clin N Am. 2007;34:739–760. doi: 10.1016/j.ogc.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Chokunonga E, Levy LM, Basset MT, et al. Cancer incidence in the African population of Harare, Zimbabwe: Second results from the Cancer: Registry 1993–1995. Int J Cancer. 2000;85:54–55. doi: 10.1002/(sici)1097-0215(20000101)85:1<54::aid-ijc10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Koulibaby M, Kabba IS, Cisse A, et al. Cancer incidence in Conakry Guinea: First results from the Cancer Registry 1992–1995. Int J Cancer. 1997;6:39–45. doi: 10.1002/(sici)1097-0215(19970106)70:1<39::aid-ijc6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Adeniji KA. Analysis of the histopathological Pattern of Carcinoma of the Cervix in Ilorin Nigeria. Nig J Med. 2001;10:165–168. [PubMed] [Google Scholar]
- 8.Onwudiegwu U, Bako A, Ojewunmi A. Cervical Cancer—A neglected health tragedy. J Obstet Gynaecol. 1999;19:61–64. doi: 10.1080/01443619966001. [DOI] [PubMed] [Google Scholar]
- 9.Thomas J, Oyemakinde O, Izebvaye I. Current Concepts in Cervical Carcinogenesis and New Perspectives in Prevention. Archives of Ibadan Medicine. 2002 Apr;3:36–39. [Google Scholar]
- 10.Cannistra SA, Niloff JM. Cancer of the uterine cervix. N Engl J Med. 1996;334:1030–1038. doi: 10.1056/NEJM199604183341606. [DOI] [PubMed] [Google Scholar]
- 11.Ajenifuja KO, Onwudiegwu U, Solomon SO, et al. Trend in the presentation of cervical cancer in Nigeria. Saudi Med J. 2008;29:447–48. [PubMed] [Google Scholar]
- 12.Lazcano-Ponce EC, Moss S, Alonso de Ruiz P, et al. Cervical screening in developing countries: why is it ineffective? The case of Mexico. Arch Med Res. 1999;30:240–250. doi: 10.1016/s0188-0128(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 13.PATH. Planning Appropriate Cervical Cancer Prevention Programs. 2. Seattle: PATH; 2000. [Google Scholar]
- 14.Megevand E, Denny L, Dehaeck K, et al. Acetic acid visualization of the cervix: an alternative to cytologic screening. Obstet Gynecol. 1996;88:383–386. doi: 10.1016/0029-7844(96)00189-5. [DOI] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R, Rajkumar R, Esmy PO, et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738–743. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbyn M, Sankaranarayanan R, Muwonge R, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153–160. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 17.Pretorius RG, Bao YP, Belinson JL, et al. Inappropriate gold standard bias in cervical cancer screening studies. Int J Cancer. 2007;121:2218–2224. doi: 10.1002/ijc.22991. [DOI] [PubMed] [Google Scholar]
- 18.Cagle AJ, Hu SY, Sellors JW, et al. Use of an expanded gold standard to estimate the accuracy of colposcopy and visual inspection with acetic acid. In J Cancer. 2010;126:156–161. doi: 10.1002/ijc.24719. [DOI] [PubMed] [Google Scholar]
- 19.Denny L, Kuhn L, Pollack A, et al. Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer. 2000;89:826–833. doi: 10.1002/1097-0142(20000815)89:4<826::aid-cncr15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Belinson JL, Qiao YL, Pretorius R, et al. Shanxi province cervical cancer screening study: a cross-sectional comparative trial multiple techniques to detect cervical neoplasia. Gynecol Oncol. 2001;83:439–444. doi: 10.1006/gyno.2001.6370. [DOI] [PubMed] [Google Scholar]
- 21.Almonte M, Ferreccio C, Winkler JL, et al. Cervical screening by visual inspection, HPV testing, liquid-based and conventional cytology in Amazonian Peru. Int J Cancer. 2007;121:796–802. doi: 10.1002/ijc.22757. [DOI] [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer. A practical manual on visual screening for cervical neoplasia. Lyon, France: IARC; 2003. [Google Scholar]
- 23.Gage JC, Ajenifuja KO, Wentzensen NA, et al. The age-specific prevalence of human papillomavirus and cytologic abnormalities in rural Nigeria: Implications for screen-and-treat strategies. Int J Cancer. 2011;130:2111–7. doi: 10.1002/ijc.26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatla N, Mukhopadhyay A, Joshi S, et al. Visual inspection for cervical cancer Screening; Evaluation by doctor versus paramedical worker. Indian J Cancer. 2004;41:32–36. [PubMed] [Google Scholar]
- 25.Sherigar S, Dalal A, Durdi G, et al. Cervical Cancer Screening by Visual Inspection with Acetic Acid - Interobserver Variability between Nurse and Physician. Asian Pac J Cancer Prev. 2012;11:619–622. [PubMed] [Google Scholar]