Abstract
Intraoperative fluorescence imaging especially near-infrared fluorescence (NIRF) imaging has the potential to revolutionize neurosurgery by providing high sensitivity and real-time image guidance to surgeons for defining gliomas margins. Fluorescence probes including targeted nanoprobes are expected to improve the specificity and selectivity for intraoperative fluorescence or NIRF tumor imaging. The main focus of this article is to provide a brief overview of intraoperative fluorescence imaging systems and probes including fluorescein sodium, 5-aminolevulinic acid, dye-containing nanoparticles, and targeted NIRF nanoprobes for their applications in image-guided resection of malignant gliomas. Moreover, photoacoustic imaging is a promising molecular imaging modality, and its potential applications for brain tumor imaging are also briefly discussed.
INTRODUCTION
Malignant gliomas are the most common and aggressive brain tumor in humans, accounting for 52% of all brain tumor cases.1,2 They are characterized by high proliferation and invasion into normal brain. Despite years of experience and refinement of treatments, the prognosis for gliomas patients remains poor, with a median survival time <14 months.3
Surgical resection plays a critical role in treatment of malignant gliomas. The key aspect for the prognosis of patients suffering from malignant gliomas is linked to the completeness of tumor removal.4,5 In current practice, intraoperative assessment of tumor-free margin is dependent on visual appearance and palpation of the tumor. As malignant gliomas are highly invasive, the distinction between tumor and normal brain is typically not obvious. Thus, the effectiveness of tumor resection in surgery is severely limited by poor visual contrast between neoplastic and normal tissue. Because residual tumor lesions cause local recurrence and increase the incidence of developing tumor metastasis, it is clear that novel approaches that allow defining tumor margins intra-operatively for complete removal of tumor lesions is critical for improving prognosis of glioma patients.
Recently, a wide variety of techniques have been explored in an effort to better visualize glioma margins. The techniques used include single-photon emission computed tomography combined with computed tomography (SPECT/CT) or positron emission tomography combined with computed tomography (PET/CT),6,7 preoperative magnetic resonance imaging (MRI),8,9 and preoperative magnetic resonance spectroscopy (MRS).10,11 Although, preoperative PET/CT and SPECT/CT may reveal anatomical details of organ orientation, and in some cases tumor location, they are often poorly translated into the surgical application.12 Preoperative MRI and MRS methods suffer from limited spatial resolution and incongruencies between the borders outlined in the preoperative MRI and the actual tumor borders during surgery because of brain shift.13
Many intraoperative imaging techniques thus have been applied to delineate tumor margins to improve surgical outcome, such as intraoperative ultrasound methods, intraoperative MRI (IMRI) scans and intraoperative fluorescent imaging for the resection of malignant gliomas.14–17 In these techniques, IMRI needs the significant upfront infrastructure costs and additional surgical time, which the average operating time using IMRI is 5.1 h and it is significantly longer than that in the conventional operation (3.4 h).18 Moreover, IMRI requires the administration of gadolinium (Gd) chelates, which may result in inaccuracies because of surgically induced false-positive contrast enhancement.19 Intraoperative ultrasound images are difficult to interpret, and various artifacts may affect the image quality during tumor removal.20
Intraoperative optical imaging using fluorescence especially near-infrared fluorescence (NIRF) is emerging as a promising noninvasive, real-time, and high-resolution modality for image-guided surgery. NIRF imaging displays properties of low absorption and relatively low autofluorescence from organisms and tissues in the NIR spectral range (700–1000 nm), which can minimize background interference and improve tissue depth penetration.21,22 In contrast to gross structural imaging, the advantage of molecular imaging is the ability to reveal the differences at molecular level between cancerous and normal tissues. To realize this ability, nanoparticles (NPs)-based NIRF molecular probes (nanoprobes) are generally modified with ligands that bind to specific molecular targets. A variety of targeting ligands have been developed for the modification and functionalization of nanoprobes, such as small molecules, peptides, proteins, antibodies, and affibody molecules.21,23,24 Targeted NIRF nanoprobes are expected to significantly improve the specificity and selectivity for tumor imaging. Considering there are many reviews on fluorescent NPs for targeted molecular imaging of tumors,23,25–29 in this short article, we focus on quickly emerging and highly important areas which are the development, current applications, and future prospects of NIRF imaging systems and fluorescent probes, including fluorescein sodium (FLS), 5-aminolevulinic acid, dye-containing NPs, and targeted NIRF nanoprobes, for guiding the surgery of malignant gliomas. Photoacoustic imaging (PAI) is a new rising molecular imaging modality and has been successfully applied for brain tumor imaging. It may be a very useful technique for image-guided resection of brain tumors in clinic, thus we briefly review some of recent progress in this field as well.
NIRF IMAGING AND PAI SYSTEMS
An Fluorescence imaging system usually consists of a light source, imaging chamber, and a digital camera. It operates on light with a defined bandwidth as a source of photons that encounters fluorescent molecule probes. Upon excitation, the probes emit signals with different spectral characteristics, that can be resolved with an emission filter and captured by a high-sensitivity charge-coupled device (CCD) camera.30 The light source in the NIRF imaging is a laser source with appropriate wavelengths to illuminate probes to produce signals in NIR spectrum window. For intraoperative NIRF systms, besides a filtered high-power xenon light source, a flexible light transmission system guiding the excitation light to a special endoscope or an operating microscope was used.31
The first imaging device for fluorescence endoscopy was introduced in the 1970s by Profio et al.32,33 The system is composed by a 200-W mercury vapor lamp and primary filter, a flexible fiberoptic bronchoscope with special violet-transmitting light conductor, secondary filter, and an image intensifier tube. Tests indicate that the system can detect a tumor 100-μm thick at the expected concentration of hematoporphyrin derivative (Hp-D). A similar approach was followed by Kato et al. using a filtered silicon-intensified target (SIT) camera to obtain the images for bronchoscopic localization of Hp-D-labeled early squamous cell carcinoma of the trachea and central bronchi.34 Later developments include a continuous-wave (CW) krypton-ion laser excitation source and rapid switching between the white-light illumination and fluorescence modes.35 In an initial clinical study conducted by Stummer et al.,36 an experimental system was used for the fluorescence diagnosis in neurosurgery. The main component is the D-light system which couples blue excitation light via a fluid light guide in the operation microscope. A long pass filter is integrated into the observation channel of the operation microscope to reduce the extrapolated autofluorescence. In white light mode, the long pass filter is switched out. The fluorescence detection can be realized by the observation channel of the operation microscope via the eye and simultaneously via a three-chip CCD camera.
Recent technological developments in light sources, high-sensitivity imaging detectors, high-performance spectrographs, and digital imaging/processing have enabled NIFR imaging a wide variety of medical applications, including intraoperative surgical guidance. Frangioni and colleagues, successfully develop a NIRF imaging system for use during human neurosurgery.37 They presented an operational prototype designed specifically for use during large animal surgery. Such a system can serve as a foundation for future clinical studies. In this system, the NIR light path is optically isolated from the visible (NIR-depleted) light path by use of separate excitation sources, a dichroic mirror, and barrier filters. All components of the system are under control by a computer. In 2009, Troyan et al. describe the successful clinical translation of a new NIRF imaging system (Fluorescence-Assisted Resection and Exploration, FLARE™) for image-guided oncologic surgery38 (Figure 1).
FIGURE 1 |.
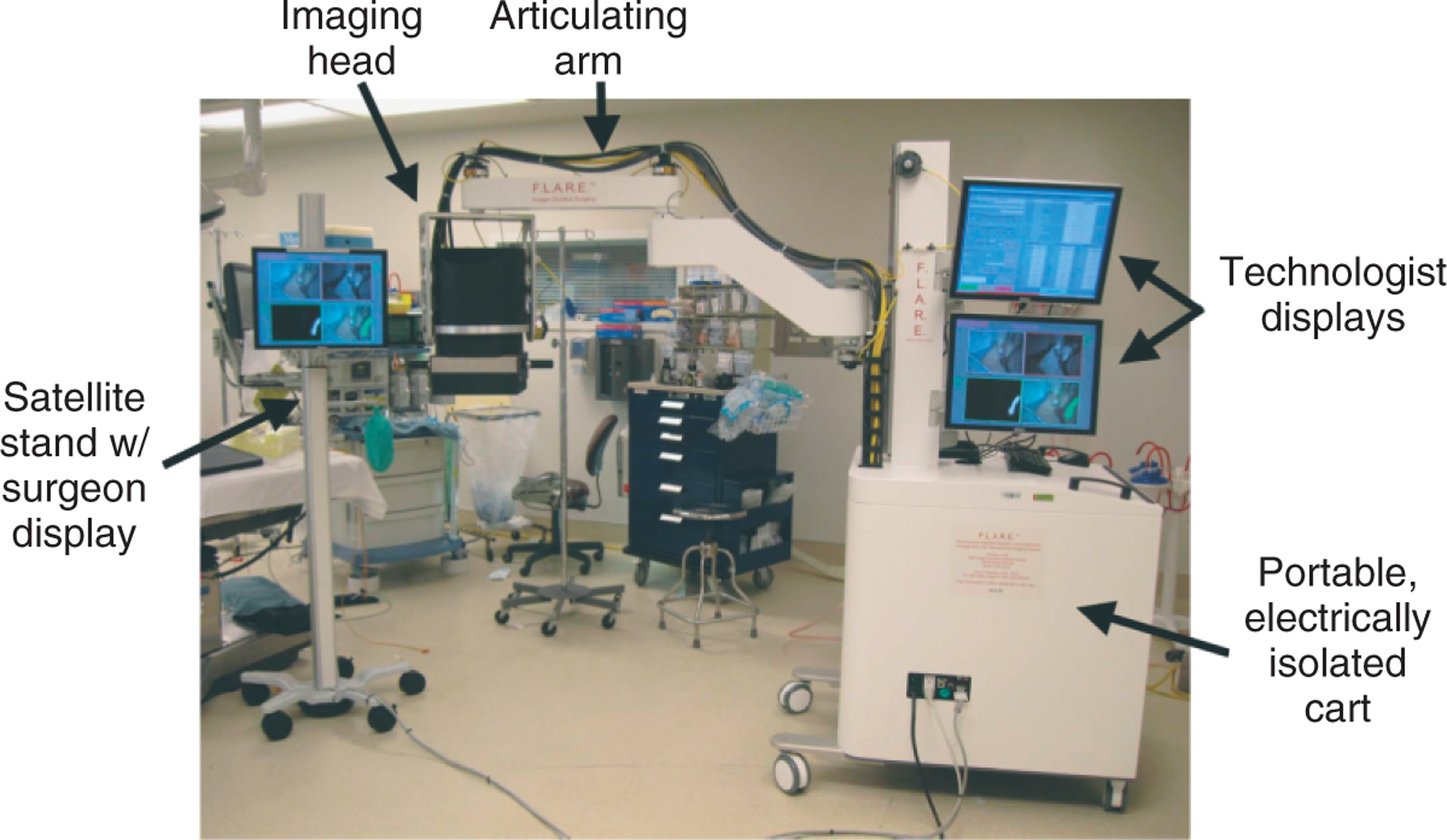
A portable near-infrared fluorescence (NIRF) imaging system and satellite monitor stand deployed in the operating room. (Reprinted with permission from Ref 38. Copyright 2009 PMC)
Bogaards et al. developed a system for quantitative fluorescence imaging and image-guided point fluorescence spectroscopy.39 They have used this system for fluorescence-guided gliomas resection to compare completeness of tumor resection and residual tumor volume after white light resection (WLR) versus additional ALA-PpIX [5-aminolevulinicacid (ALA)-induced protoporphyrin IX (PpIX)] fluorescence-guided resection. The residual tumor volumes were also quantified by histology of resected tissues and of the whole brain after resection. The tumor-to-normal ratios in tissues resected under white light as well as in tissues resected under fluorescence guidance were also determined.
To further detect a deep-seated brain tumor using intraoperative fluorescence imaging, Sun et al. developed an endoscopic fluorescence lifetime imaging microscopy (FLIM) system for the intraoperative diagnosis of glioblastoma multiforme (GBM).40 The clinically compatible FLIM prototype integrates a gated (down to 0.2 ns) intensifier imaging system with a fiber-bundle (fiber image guide of 0.5 mm diameter, 10,000 fibers with a gradient index lens objective 0.5 NA, and 4 mm field of view) to provide intraoperative access to the surgical field. This research demonstrates that fluorescence lifetime contrast between tumor and normal cortex can be consistently achieved and is independent of tissue illumination, irregular brain tissue surface, and presence of blood in the surgical field. With the rapid advancements on the developing NIRF systems and imaging probes, optical imaging is expected to gain more recognition in the medical community. NIRF imaging systems which are commercially available and suitable for the use in the brain tumor surgery suite will likely be realized in very near future.
PAI, an emerging powerful imaging modality using optical absorption and ultrasonic resolution, largely overcomes the depth and resolution limits of optical imaging. PAI involves the use of light pulses to excite molecular imaging agents, causing slight heat production and thermal expansion. This process further leads to the production of ultrasound waves, which can be recorded by an ultrasound transducer. A three-dimensional (3D) image can thus be generated to reveal the distribution of the imaging agents in living subjects.41,42 PAI systems can generally be categorized into two major types: photoacoustic microscopy (PAM) and photoacoustic tomography (PAT). In PAM, a focused ultrasonic transducer is mechanically scanned. The acoustic focusing yields lateral resolution while the acoustic time-of-flight provides axial resolution at each scanned position. In PAT, an array of unfocused ultrasonic transducers is used to detect photoacoustic waves in parallel and high frame rates (e.g. 50 Hz) thus can be achieved. An inverse reconstruction algorithm is required to reconstruct a tomographic image. PAT is highly sensitive to optical absorption, and a small change in optical absorption coefficient can be nicely converted to an equal fractional change in ultrasound signal.42 Recently, PAT has been successfully applied to the visualization of microvasculature of tumors43 and brain tumor margin.44,45
FLUORESCENT PROBES AND THEIR PRECLINICAL AND CLINICAL APPLICATIONS
In addition to an appropriate imaging system, fluorescent probes are also required to visualize specific anatomic structures intended to be resected (for example: tumor tissue) or to be spared (free-margin). Such probes contain a fluorescent moiety, which emits fluorescent especially NIRF light after being excited with a NIR light source; and depending on the application, a targeting ligand that directs the fluorophore to the structure under study. Visualization of the tissue is based on the signal of the contrast agent in the region of interest (ROI) relative to the background signal, known as signal-to-background ratio (SBR).46
Fluorescein Sodium
FLS is a synthetic fluorescent dye that gives a visual green emission (500–530 nm) following excitation at 465–490 nm. Although the emission is <700 nm, the green signal still provides high contrast in the surgical field. FLS is not a molecularly targeted probe and does not show high targeting specificity. When disruption of blood–brain barrier (BBB) happens, FLS can nicely stain tumors, but there are generally no differences in the staining intensity among the metastases of different types of primary cancer.47 Moreover, FLS, as a contrast agent under white light without a specific filter, is able to distinguish normal tissue from glioblastoma based on microscopic characteristics during surgery and able to improve the tumor tissue removal rate.48 The surgical procedure presented in the above study is a simple and readily available FLS-guided tumor resection method. It does not require any additional equipment other than a conventional surgical microscope and a higher dose of FLS (20 mg/kg) than that is generally used (8–10 mg/kg) in ophthalmoscopic examinations. Among patients who underwent the FLS-guided surgery, gross total resection (GTR) can be achieved in 100% of patients in whom all the visible yellow color (deeply and faintly stained regions) was resected during operation. Importantly, the most significant finding for distinguishing between the visible yellow-colored lesions (deeply and faintly) and the negatively stained regions is the existence of endothelial proliferation according to the histological findings. This study also demonstrates that the patients who underwent GTR show significantly better survival compared with those who did not undergo GTR. Importantly, it proves that the fluorescence-guided surgical procedure is simple, safe, useful, readily accomplished, and universally available for resection of glioma.
It should be noted that there are some limitations of the use of FLS. First, it could cause complications. Anaphylactoid reaction seems to be the most common side effects, and sometimes it is a serious side effect. For example, it was found that two patients who underwent FLS-guided brain tumor excisions experienced anaphylactic reactions with severe hypotension and bradycardia.49,50 Other side effects, including yellow stains of the skin, mucosa, and urine are transient, and they can disappear completely within 24 h postoperatively.48,51 Second, individual cells52 and the edematous brain, incised brain, and blood53 could also be visualized when FLS diffuses into the interstitial space51or the edematous brain tissue.53 Thus, FLS has low specificity for detecting tumor. To alleviate this drawback, human serum albumin (HSA)-conjugated FLS (HSA-FLS) was developed. Ichioka et al. reported that HSA-FLS has the advantages of specificity and persistence of fluorescence over FLS for the purpose of identifying glioma nodules.54
5-Aminolevulinic acid (5-ALA)
-Aminolevulinic acid (5-ALA) is a natural biochemical precursor of protoporphyrin IX (PpIX) in the heme biosynthesis pathway. Outside the mitochondria, a ring system with 4 pyrrol-rings is synthesized from 5-ALA molecules, yielding PpIX and finally into heme in the mitochondria via the addition of a ferrous ion to the center of the pyrrol-ring structure.55 PpIX is a potent photosensitizer and able to accumulate excessively when 5-ALA is given externally to cells. After 5-ALA administration, the abundantly produced PpIX cannot be rapidly converted into the end product heme by ferrochelatase and therefore accumulates intracellularly available as a sensitizer for a limited time. Limited availability of iron and decreased activity of the enzyme ferrochelatase, which is responsible for the conversion of PpIX into heme, have been shown to contribute to increased PpIX accumulation in tumor cells.56 Therefore, it has been demonstrated both in vitro and in vivo that excess 5-ALA provides selective and abundant accumulation of PpIX in malignant glioma, but only slight or no accumulation in normal brain.57,58 Although 5-ALA itself does not show fluorescence, PpIX is a highly fluorescent substance (emission at 635 nm) with maximum absorption at 440 nm. Thus, 5-ALA can be exploited intraoperatively to selectively mark and help to remove tumor cells.58
In an initial clinical study conducted by Stummer et al.,36 nine patients were administered orally with 10 mg 5-ALA/kg body weight at 3 h before the induction of anesthesia. Intraoperatively, tumor tissues can be determined with a prototype imaging system. Red porphyrin fluorescence is observed with a 455 nm long-pass filter after excitation with violet-blue (375–440 nm) xenon light. Normal brain tissue displays no porphyrin fluorescence, whereas tumor tissue is distinguished by bright red fluorescence. A total of 89 tissue samples were taken from the transition zone between tumor and apparently normal tissue, which a sensitivity of 85% and a specificity of 100% were achieved. In 2000, Stummer et al. reported another prospective study on 52 consecutive patients.59 A prototype imaging system composed by a modified surgical microscope and a three-chip color CCD camera optimized for red porphyrin fluorescence visualization was used to image the tumor. It has been found that tumor fluorescence is not homogenous. Rather, two clearly discernible fluorescence properties can usually be distinguished. Viable tumor tissue (the solid part of the tumor) shows a strong red fluorescence. The transition zone between the tumor and normal tissue (infiltration zone) exhibits a pink-looking fluorescence, which is encountered between solidly fluorescence tumor and nonfluorescence blue brain tissue. This fluorescence is called ‘vague’. The mean survival time of the patients in this study is 79.7 ± 7.6 weeks. Impressively, patients with no residual fluorescent tissue left survive for more than 100 weeks on average, compared with only 51 weeks for those with residual fluorescence.
Kajimoto et al. compared 5-ALA to FLS in guiding resection of malignant gliomas.53 The results show that the high-contrast ratios of tumor to normal brain fluorescence up to 5.6 in FLS and 160 in 5-ALA can be observed. Moreover, although the fluorescence intensity derived from 5-ALA is not stronger than that of FLS, fluorescence derived from 5-ALA is not detected in the surrounding brain tissue. Therefore, the specificity of the fluorescence derived from 5-ALA is higher than that of FLS (Figures 2 and 3). Finally, 5-ALA for fluorescence-guided surgery in malignant glioma was approved for all patients older than 18 years with no known porphyria, hepatopathy, or renal insufficiency.60 But patients should be protected against direct sun light within the following day to prevent sun burns caused by the elevated PpIX in the patient’s skin.61 Elevated liver enzymes can also be found the following days.
FIGURE 2 |.
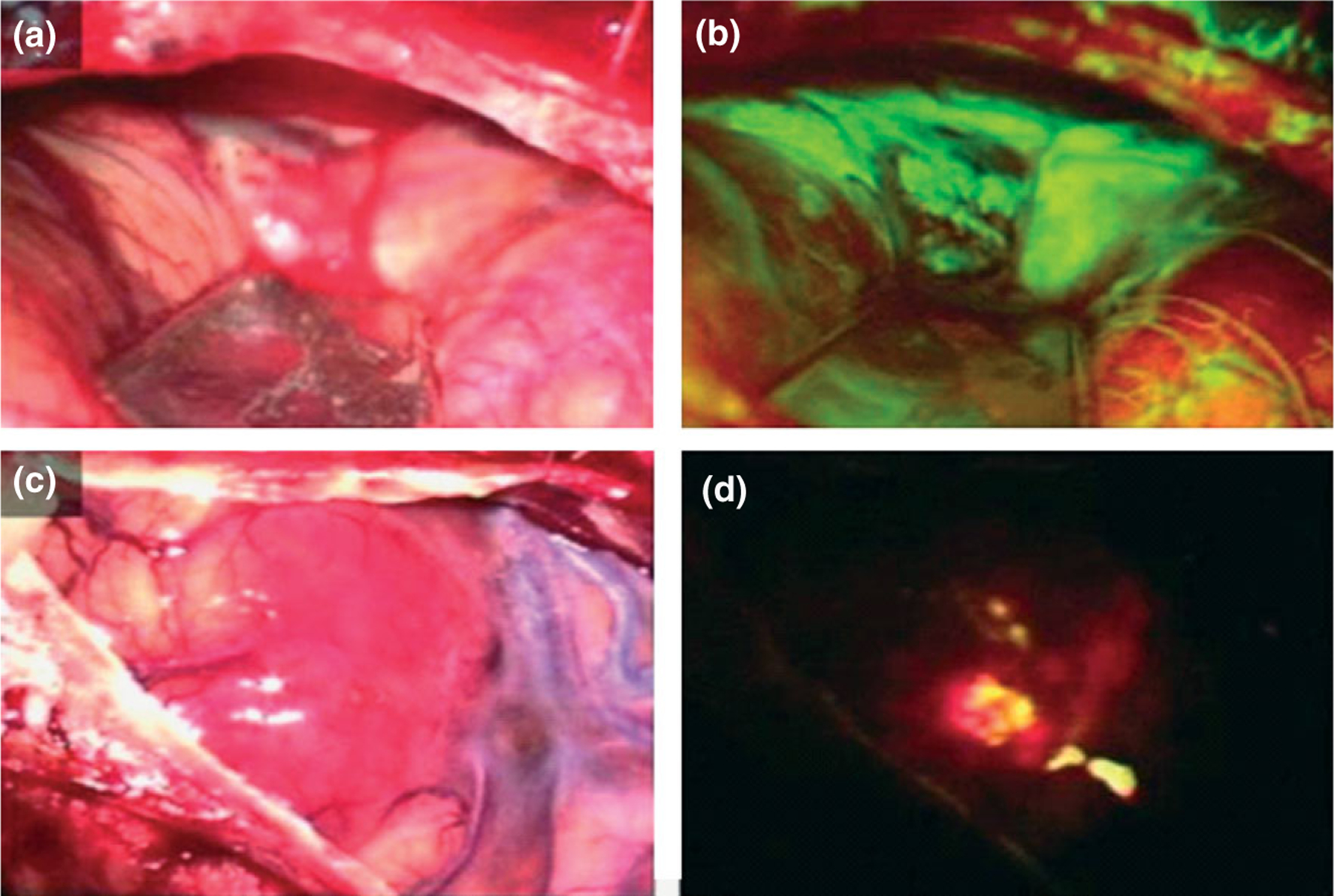
Intraoperative photographs demonstrating tumor in a patient viewed under conventional white light (a, b) and excitation light illumination (b, d). Metastatic brain tumor of the cerebellum (a) showed strong green fluorescence using fluorescein sodium (b). Glioblastoma multiforme of the temporal lobe (c) showed red fluorescence using 5-ALA (d). (Reprinted with permission from Ref 53. Copyright 2003 Elsevier BV)
FIGURE 3 |.
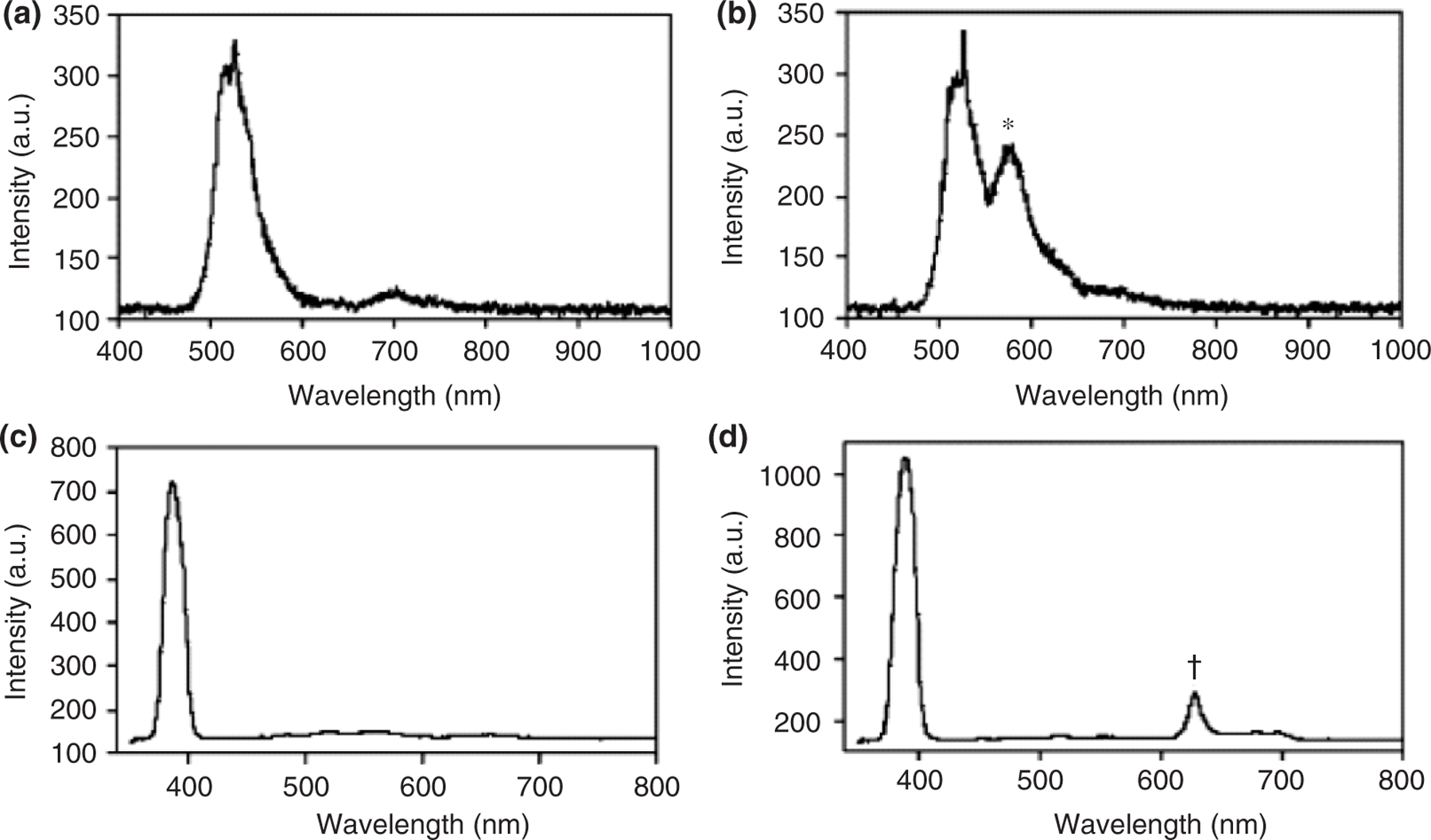
The spectra of tumor and normal brain. The spectrum of the normal brain showed only reflected excitation light from blue light (a) and violet blue light (c). The spectrum of tumor part contained fluorescence component of fluorescein sodium (b) with peak at 585 nm (*) and PpIX with peak at 635 nm (†). (Reprinted with permission from Ref 53. Copyright 2003 Elsevier BV)
Nanoprobes
NPs offer exciting and abundant opportunities for discovering new materials and tools for molecular imaging because of their intense and stable output, large payload delivery, multimodal signaling capacity, strong target binding ability via multiple ligands, and tunable biodistribution profiles.62 NP-based molecular probes, namely nanoprobes, could target tumors either through the enhanced permeability and retention (EPR) effect of the tumor microvasculature or by the specific binding with tumor-associated biomarkers, such as tumor cell receptors, tumor extracellular matrix, and enzymes. A variety of nanoprobes have been prepared, evaluated, and applied in various imaging modalities, including superparamagnetic iron oxide (SPIO) and ultrasmall SPIO (USPIO) for MRI,63 gold (Au) NPs, or single-wall carbon nanotubes (SWNTs) for PAI,41,64 and fluorescent NPs for in vivo fluorescence imaging.65 In recent years, many efforts have been made to develop novel NIRF nanoprobes for in vivo molecular imaging to overcome some of the limitations of conventional NIR organic dyes, such as poor hydrophilicity and photostability, low quantum yield and detection sensitivity.
In a recent multicenter Phase III trial, 5-ALA was used for fluorescence-guided surgery. This fluorescent staining enables the surgeons to perform complete resections in 65% of the patients, as compared with only 36% without administration of 5-ALA.66 But the contrast between the tumor tissue and surrounding brain tissue is relatively low, especially in the edematous brain. To improve the visualization of malignant glioma tissue during surgery and thus achieve a higher rate of complete resections as defined by the lack of gadolinium (Gd)-enhancing tumor tissue in postoperative MRI scans, Kremer et al. prepared HSA conjugated 5-ALA (HSA-ALA).67 Intraoperative fluorescence staining using HSA-ALA renders fluorescence guidance during surgery and results in a very high rate (69%) of complete tumor resections. The agreement between fluorescence and histopathology in tumor samples and samples of the tumor border was 83.3%.
However, preoperative diagnostic images are not correlated well with intraoperative pathology using these probes. Thus, it has sparked new research geared toward the development of multimodal nanoprobes that can be detected with both MRI and intraoperative optical devices. Kircher et al. explored a multimodal (NIRF and magnetic) NPs as a preoperative MRI contrast agent and intraoperative optical probe.68 They reported that the multimodal nanoprobe termed Cy5.5-CLIO permits the preoperative visualization of brain tumors by serving as an MRI contrast agent and affords an intraoperative discrimination of tumors from brain tissue because of its NIR fluorescence. The ability to track the same probe by both preoperative MRI and intraoperative optical imaging offers a new approach to visualize and accurate resection of tumors.
Currently, the major limitations of the multimodal probes are their low specificity and limited internalization by glioma cells, because of their absence of tumor targeting marker. Recent developments of new NIR fluorophores and nanomaterials have led to a revolution in fluorescence molecular imaging because considerable enhancement in specificity and sensitivity for tumor imaging can be achieved by using targeted contrast agents.69,70 Veiseh et al. reported the fabrication of a multifunctional nanoprobe capable of targeting glioma cells as well as detectable by both MRI and fluorescence imaging.71,72 The nanoprobe is comprised of an iron oxide NP coated with biocompatible polyethylene glycol (PEG)-grafted chitosan copolymer, to which a tumor targeting agent chlorotoxin and a NIR fluorophore are conjugated. Such a nanoprobe could potentially be used to image resections of glioma brain tumors in real time and to correlate the preoperative diagnostic images with intraoperative pathology at cellular-level resolution (Figures 4 and 5). Yan et al. developed a novel two-order targeted imaging strategy, which visualizes brain tumor by up-regulating the BBB permeability and receptor targeting specificity of nanoprobes. In this strategy, PAMAM-G5 dendrimer is modified with MRI/optical imaging agents, tumor vasculature targeting cyclic [RGDyK] peptides, and BBB-permeable Angiopep-2 peptides to prepare a targeted nanoprobe.73 This nanoprobe can successfully visualize orthotropic brain tumor xenografts with high sensitivity and target to background signal ratio in vivo. Moreover, the multimodal nanoprobe not only demonstrates the feasibility to preoperatively localize brain tumors by both MRI and optical imaging but also provides a potential solution to delineate the malignant tumor with uncompromised BBB (Figures 6 and 7).
FIGURE 4 |.
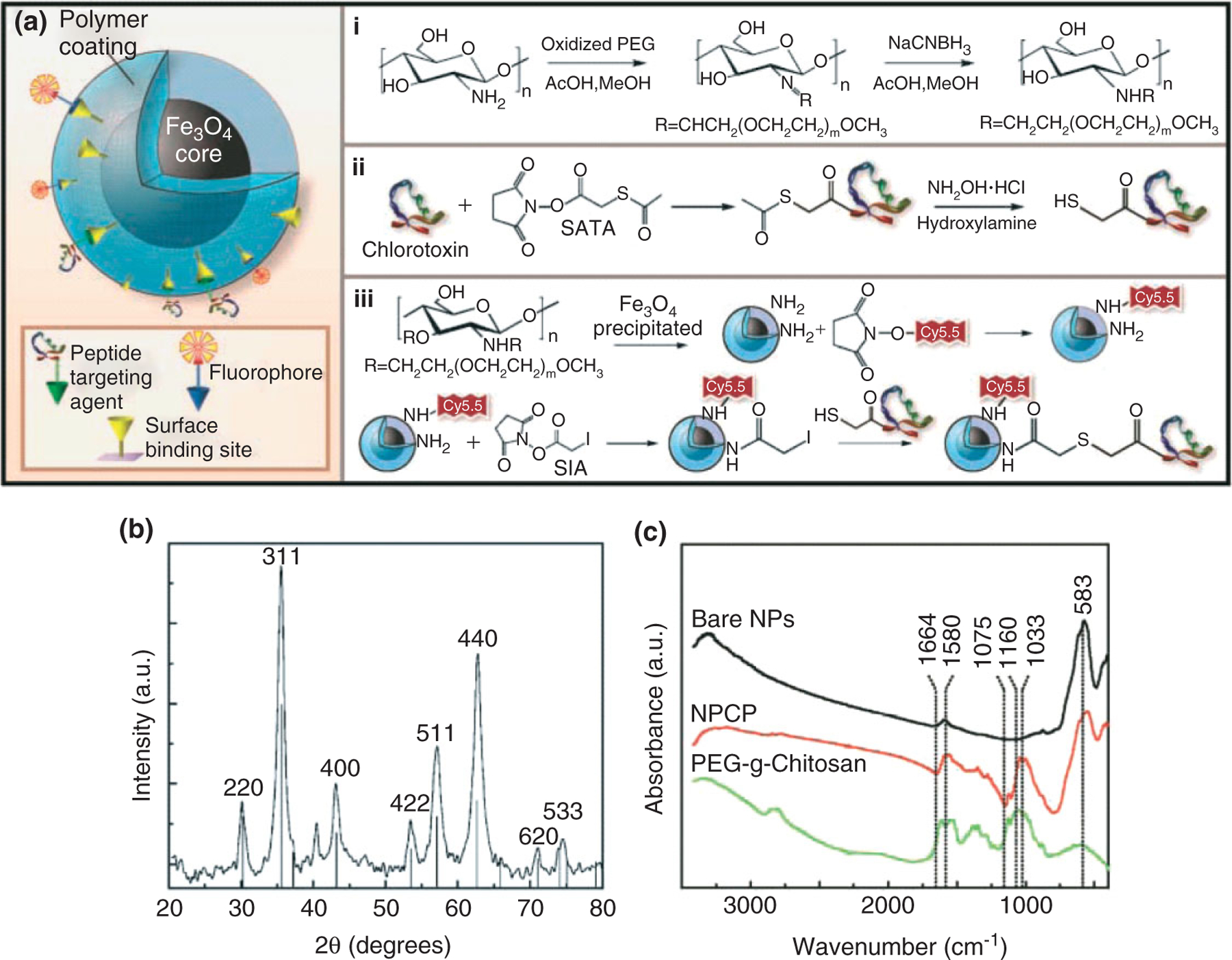
Synthesis and characterization of NPCP-Cy5.5-CTX nanoprobes. (a) Nanoprobe structure and chemical reaction schematic for the syntheses of (i) polyethylene glycol (PEG)-grafted chitosan, (ii) sulfhydryl functionalization of CTX, and (iii) CTX and Cy5.5 conjugation to NPCP. (b) X-ray diffraction pattern of NPCP confirming the magnetite (Fe3O4) crystalline structure of the nanoprobe. (c) Fourier-transformed IR spectra of bare iron oxide nanoparticle, PEGylated chitosan, and NPCP, confirming the successful immobilization of PEGylated chitosan on the surface of the nanoparticles. (Reprinted with permission from Ref 72. Copyright 2009 American Association for Cancer Research)
FIGURE 5 |.
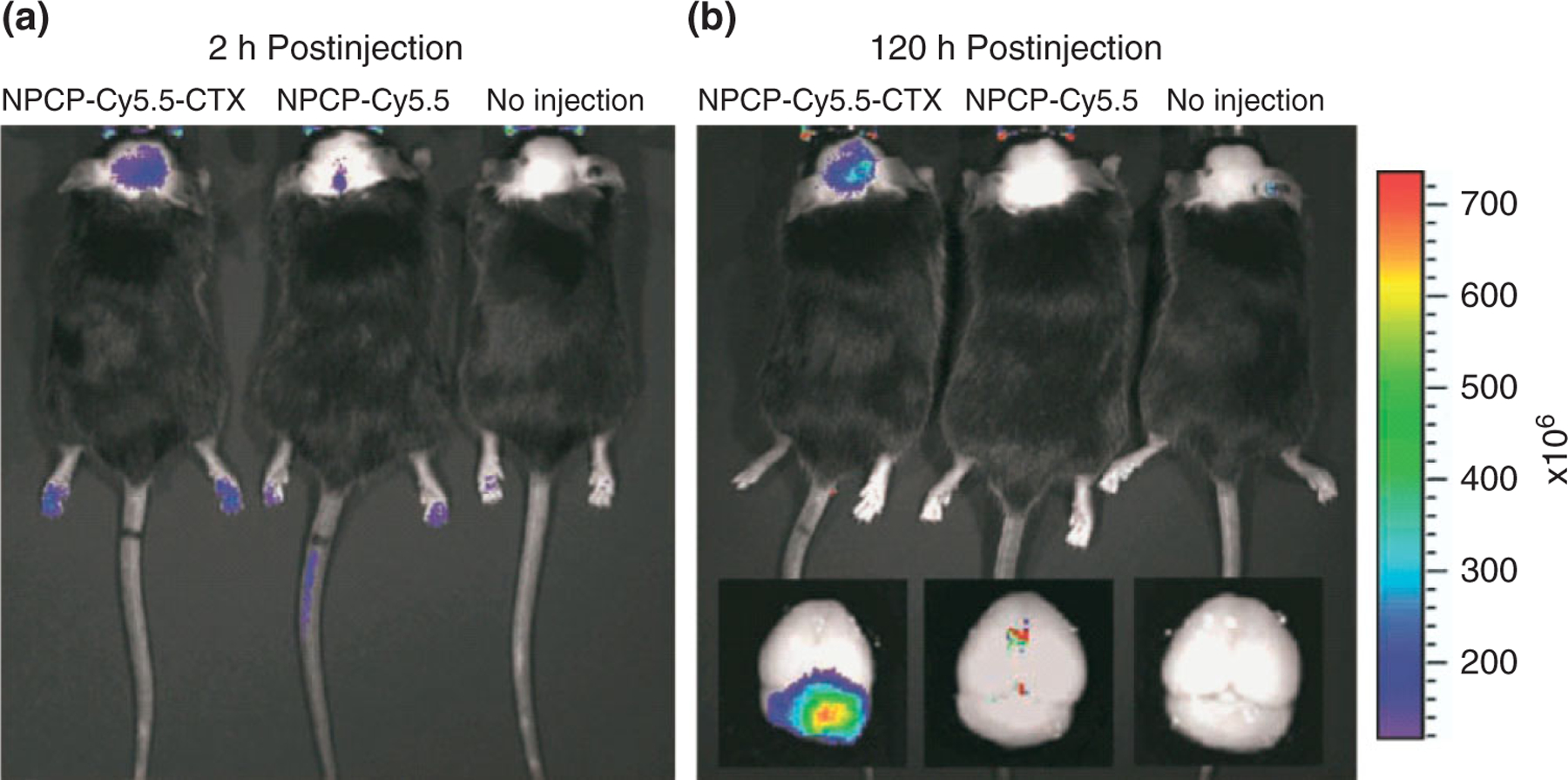
In vivo near-infrared fluorescence (NIRF) imaging of autochthonous medulloblastoma tumors in genetically engineered ND2:SmoA1 mice. (a and b) Fluorescence imaging of medulloblastoma tumors in ND2:SmoA1 mice injected with either NPCP-Cy5.5-CTX or NPCP-Cy5.5, or receiving no injection (left to right). Images were acquired at 2 h (a) and 120 h (b) postinjection. Ex vivo fluorescence images of mice brains from the same mice following necropsy (inset, b). The spectrum gradient bar (right) corresponds to the fluorescence intensity (p/s/cm2/sr) of the images. (Reprinted with permission from Ref 72. Copyright 2009 American Association for Cancer Research)
FIGURE 6 |.
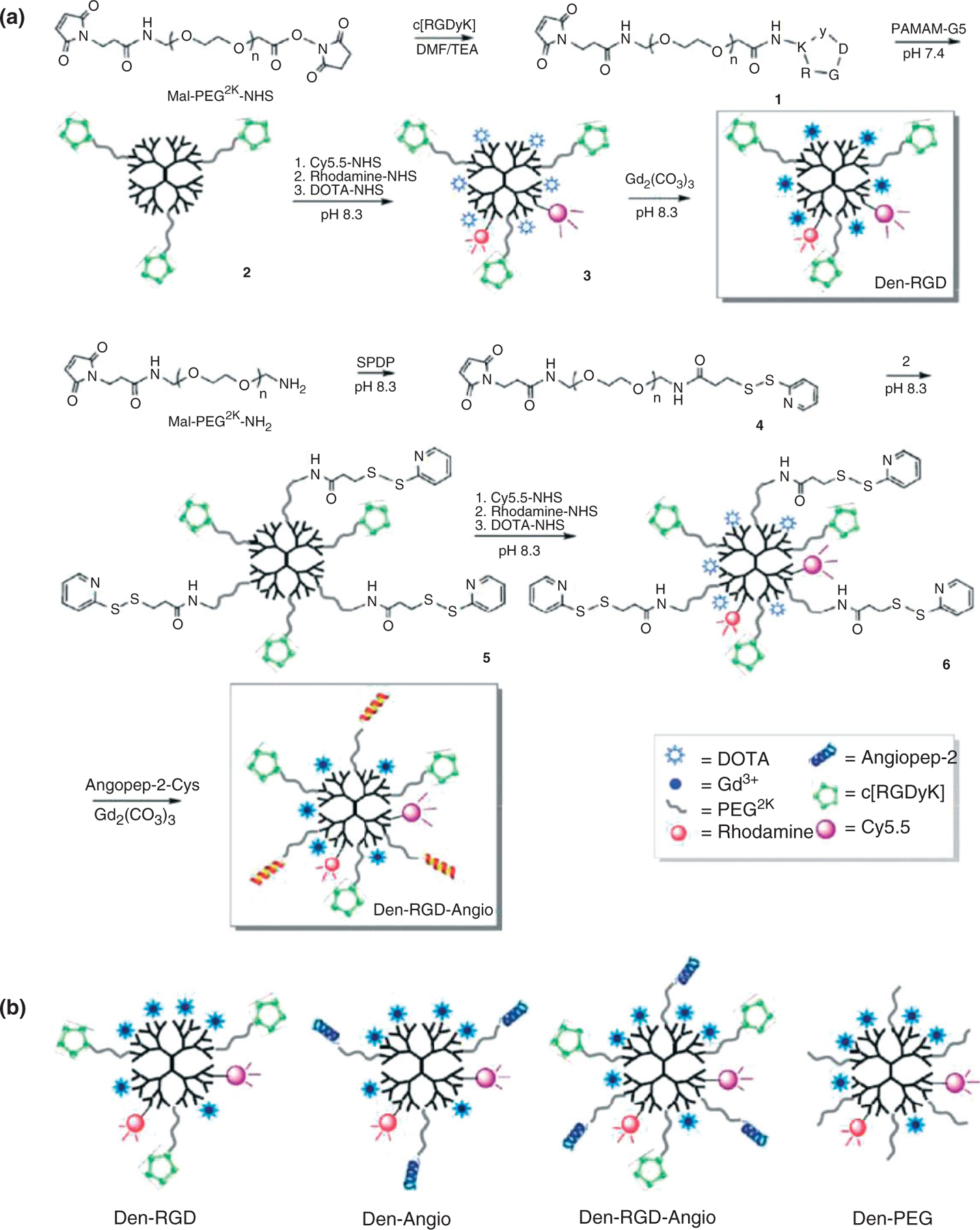
(a) Synthetic steps of nanoprobes Den-RGD and Den-RGD-Angio. (b) Schematics of the targeted and control nanoprobes. (Reprinted with permission from Ref 73. Copyright 2011 American Chemical Society)
FIGURE 7 |.
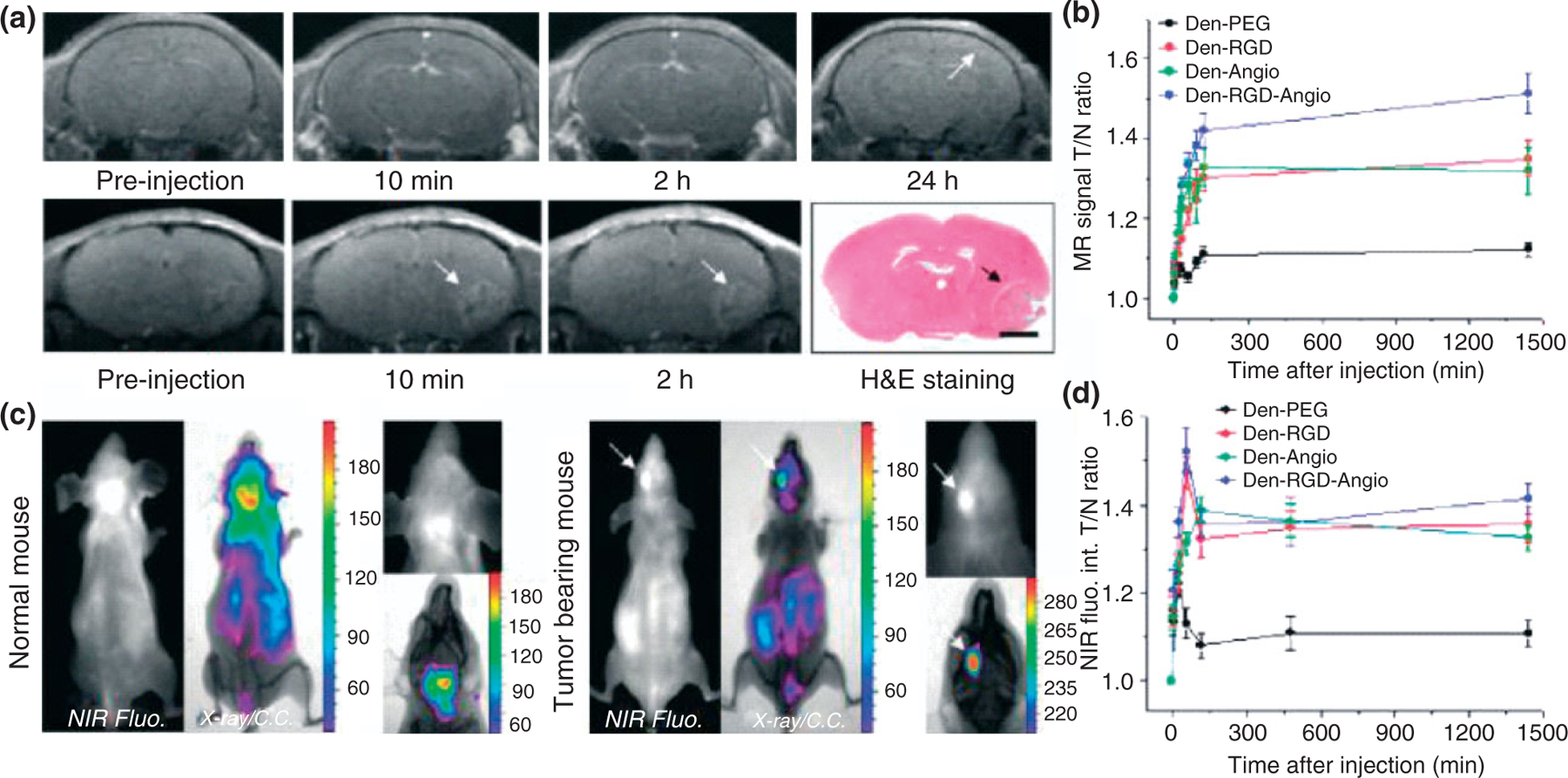
Den-RGD-Angio demonstrated high BBB permeability and T/N ratio in vivo. (a) Representative T1-weighted magnetic resonance (MR) images of normal mouse brain (upper panel, arrow points to the cortex) and tumor-bearing brain (lower panel, arrows point to the tumor) before and at selected time PI of Den-RGD-Angio (0.05 mmol/kg [Gd3+], iv). Histological H&E staining verified the tumor boundary in MRI (bar, 2.0 mm). (b) In vivo time-dependent MR signal associated T/N ratio before and PI of nanoprobe (n = 4). Points present mean values and bars show the maximum and minimum values (data range). (c) Representative near-infrared fluorescence (NIRF) and X-ray/color-coded NIRF images of the normal mouse (left panel) and brain tumor-bearing mouse (right panel) at 24 h PI of Den-RGD-Angio (5.0 nmol/mouse based on dendrimer). (d) In vivo time-dependent NIR fluorescence T/N ratio (n = 3). Points present mean values and bars show the data range. (Reprinted with permission from Ref 73. Copyright 2011 American Chemical Society)
PAI allows relatively deeper tissues imaging (up to 5 cm) with higher spatial resolution compared to most optical techniques, and it is capable of detecting optical contrast agents with high sensitivity and specificity.41,44 Metal nanocomposites such as gold NPs and carbon nanotubes have been used for PAI. The large optical absorption cross sections of NPs result excellent contrast for PAI. Kircher et al.44 recently explored a unique triple-modality MRI-PAI-Raman imaging NP (named as MPR NP). It can accurately help to delineate the margins of brain tumors in living mice both preoperatively and intraoperatively. In another study, by taking advantage of excellent biocompatibility of gold NPs, Lu et al. synthesized a PAI probe (c(KRGDf)-PEGHAuNS) targeting to integrins that are overexpressed in glioma on the basis of hollow gold nanospheres (HAuNS).45 Such a nanoprobe could potentially be used for image-guided brain tumor surgery with PAI.
CONCLUSIONS AND PERSPECTIVES
Fluorescence-guided surgery for malignant gliomas has been intensively explored over a relatively short time. This technique has shown to improve intraoperative identification and demarcation of gliomas. It could potentially be a useful tool to increase the completeness of resection of malignant gliomas. More importantly, NPs especially nanoprobes specifically targeting to tumor biomarkers are expected to improve intraoperative imaging. Various molecular probes have been developed to image receptors which play important roles in tumor biology, including αvβ3-integrin receptor, epidermal growth factor receptor (EGFR), and vascular endothelial growth receptor (VEGFR). The enzyme activation of targeting NIRF probes, which is mediated mainly by tumor-associated proteases, including cathepsins71 and matrix metalloproteinases,72 may also improve specificity of surgical guidance for malignant gliomas.
Multimodal imaging incorporates two or more components to give multifunctional capabilities, and it has quickly risen up as an attractive approach for providing accurate and comprehensive diagnosis with both anatomical and functional information. Considering advantages of multimodalities, attempts for the combination of NIRF imaging with other modalities have been developed. Moreover, by the multiple imaging modalities, preoperative diagnostic imaging can be correlated with intraoperative pathology. Therefore, much effort have been made to actively develop novel multimodal nanoprobes that can be detected with both MRI or nuclear imaging (SPECT or PET) and intraoperative fluorescent devices.71,72 Furthermore, it is possible to attach a fluorophore, magnetic NP, or radioactive label with many targeted ligands such as peptides, proteins, and/or antibodies, thereby realizing targeted multimodal nanoprobes. Of particular interest would be multimodal nanoprobes for the most common biomarkers in brain tumor. For example, for glioma, wherein multimodal probes have been developed preclinically for biomarkers such as VEGF,74 EGFR,75 and integrins.76,77 Nevertheless, for all these new probes currently under development, their clinical translations still require enormous research to overcome many scientific technological, and regulation hurdles.
Development of new imaging systems can offer new prospects for image-guided surgery. To date, most imaging systems are limited to the image resolution, sensitivity, and imaging of limited target for each scan. To improve the resolution and sensitivity, Yang et al. developed an optical intraoperative detection system (a multispectral fluorescence imaging system, MFI), which can be combined with preoperative or intraoperative CT or MRI.78 The system provides complementary information to radiological imaging methods. For example, intraoperative CT or MRI is limited by shifts in the brain tissue due to retraction, resection, and cerebrospinal fluid drainage, and intra-operative real-time fluorescence imaging/spectroscopy can reduce these artifacts. Hence, the advantages are their very high resolution and sensitivity in detecting minimal residual disease. Moreover, quantification in optical imaging remains challenging. As tissue absorbance and probe concentration at the site of the tumor can vary among patients, and the contrast of probe properties between tumor and surrounding tissue is important and also the basis of clinical decision making,46 current research is focused on new methods to improve signal quantification ability such as using spatial frequency domain imaging and time domain imaging.79,80 For example, Valdés et al. established a spectrally resolved quantitative fluorescence approach to accurately discriminate normal from neoplastic brain tissue.81 Recently, with the improvements in multimodal probe development, multispectral imaging systems are under active developing. For example, a simultaneous PET and 3D fluorescence optical tomography (FOT) imaging was designed and constructed.82 In this way, multimodality imaging probes can be used in the same multimodal imaging instrument, potential providing more specific imaging of the area of interest. Lastly, Brouwer et al. reported an image navigation system for surgical guidance based on pre-operative and intraoperatively acquired imaging data with multimodal probes that are both radioactive and fluorescent.83 This technique can help extend the use of fluorescence-guided surgery to deeper structures and improve the accuracy of fluorescence-guided surgery even further. Interestingly, new instrumentation, intraoperative PAI device is currently under development. This device is designed for easy intraoperative navigation and could enable real-time imaging and has the potential to be conveniently used to guide surgical resection of tumors.84
In summary, it is clear that fluorescence image-guided surgery is a promising new technique that may improve surgical accuracy in the field neurological tumor. Currently, 5-ALA and FLS have received the FDA approval and are used for the identification of tumor locations and surgical tumor margins in clinic. However, the major limitations of these fluorescent markers are their low specificity and limited uptakes by glioma cells. Recent development of new targeted fluorescent nanoprobes has led to a revolution in fluorescence molecular imaging because these probes can considerably enhance their specificity and sensitivity for tumor imaging. The current evidence also suggests that there are many hurdles to overcome for advancing the targeted fluorescent nanoprobes into clinical applications. Overall, there are tremendous chances and challenges in this highly dynamic and relatively new rising field. Development of NP-based targeted fluorescent probes is a promising approach for real-time fluorescence imaging during surgery.
ACKNOWLEDGMENTS
This research was partially supported by DOE Stanford Molecular Imaging Research and Training Program (DE-SC0008397), NCI of Cancer Nanotechnology Excellence Grant CCNE-TR U54 CA119367, and National Natural Science Foundation of China (810711 82) and Medical Innovation Foundation of Fujian, China (2009-CXB-46).
REFERENCES
- 1.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol 2008, 67:139 – 152. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N, Berger MS. Glioma extent of resection and its impact on patient out-come. Neurosurgery 2008, 62:753 – 764. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005, 352:987 – 996. [DOI] [PubMed] [Google Scholar]
- 4.Bucci MK, Maity A, Janss AJ, Belasco JB, Fisher MJ, Tochner ZA, Rorke L, Sutton LN, Phillips PC, Shu HK. Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem, malignant gliomas: results from a single center in the magnetic resonance imaging era. Cancer 2004, 101:817 – 824. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Changing paradigms-an update on the multidisciplinary management of malignant glioma. Oncologist 2006, 11:165 – 180. [DOI] [PubMed] [Google Scholar]
- 6.Chien CY, Chuang HC, Huang SH, Lin WC, Huang HY. A pilot study of segmental mandibulectomy with surgical navigation using fluorine-18 fluorodeoxyglucose positron-emission tomography/computed tomography. Laryngoscope 2012, 29:447 – 452. [DOI] [PubMed] [Google Scholar]
- 7.Bunschoten A, Buckle T, Visser NL, Kuil J, Yuan H, Josephson L, Vahrmeijer AL, van Leeuwen FW. Multimodal interventional molecular imaging of tumor margins and distant metastases by targeting αvβ3 integrin. Chembiochem 2012, 13:1039 – 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levman JE, Martel AL. A margin sharpness measurement for the diagnosis of breast cancer from magnetic resonance imaging examinations. Acad Radiol 2011, 18:1577 – 1581. [DOI] [PubMed] [Google Scholar]
- 9.Price SJ, Jena R, Burnet NG, Hutchinson PJ, Dean AF, Penã A, Pickard JD, Carpenter TA, Gillard JH. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 2006, 27:1969 – 1974. [PMC free article] [PubMed] [Google Scholar]
- 10.Berman JI, Berger MS, Chung SW, Nagarajan SS, Henry RG. Accuracy of diffusion tensor magnetic resonance imaging tractography assesed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg 2007, 107:488 – 494. [DOI] [PubMed] [Google Scholar]
- 11.Konukoglu E, Clatz O, Bondiau PY, Delingette H, Ayache N. Extrapolating tumor invasion margins for physiologically determined radiotherapy regions. Med Image Comput Comput Assist Interv 2006, 9:338 – 346. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berg NS, van Leeuwen FW, van der Poel HG. Fluorescence guidance in urologic surgery. Curr Opin Urol 2012, 22:109 – 120. [DOI] [PubMed] [Google Scholar]
- 13.Reinges MH, Nguyen HH, Krings T, Hütter BO, Rohde V, Gilsbach JM. Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien) 2004, 146:369 – 377. [DOI] [PubMed] [Google Scholar]
- 14.Chacko AG, Kumar NK, Chacko G, Athyal R, Rajshekhar V. Intraoperative ultrasound in determining the extent of resection of parenchymal braintumoursa comparative study with computed tomography and histopathology. Acta Neurochir (Wien) 2003, 145:743 – 748. [DOI] [PubMed] [Google Scholar]
- 15.Cengiz C, Keramettin A. Intraoperative ultrasonographic characteristics of malignant intracranial lesions. Neurol India 2005, 53:208 – 211. [DOI] [PubMed] [Google Scholar]
- 16.Kremer P, Tronnier V, Steiner HH, Metzner R, Ebinger F, Rating D, Hartmann M, Seitz A, Unterberg A, Wirtz CR. Intraoperative MRI for interventional neurosurgical procedures and tumor resection control in children. Childs Nerv Syst 2006, 22:674 – 678. [DOI] [PubMed] [Google Scholar]
- 17.Bozzao A, Romano A, Angelini A, D’Andrea G, Calabria LF, Coppola V, Mastronardi L, Fantozzi LM, Ferrante L. Identification of the pyramidal tract by neuronavigation based on intraoperative magneticresonance tractography: correlation with subcortical stimulation. Eur Radiol 2010, 20:2475 – 2481. [DOI] [PubMed] [Google Scholar]
- 18.Hirschberg H, Samset E, Hol PK, Tillung T, Lote K. Impact of intraoperative MRI on the surgical results for high-grade gliomas. Minim Invasive Neurosurg 2005, 48:77 – 84. [DOI] [PubMed] [Google Scholar]
- 19.Knauth M, Aras N, Wirtz CR, Dörfler A, Engelhorn T, Sartor K. Surgically induced intracranial contrast enhancement: potential source of diagnostic errorin intraoperative MR imaging. AJNR Am J Neuroradiol 1999, 20:1547 – 1553. [PMC free article] [PubMed] [Google Scholar]
- 20.Gerganov VM, Samii A, Akbarian A, Stieglitz L, Samii M, Fahlbusch R. Reliability of intraoperative high-resolution 2D ultrasound as an alternative to high-field strength MR imaging for tumor resection control: a prospective comparative study. J Neurosurg 2009, 111:512 – 519. [DOI] [PubMed] [Google Scholar]
- 21.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol 2010, 14:71 – 79. [DOI] [PubMed] [Google Scholar]
- 22.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003, 7:626 – 634. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Chen X, Cheng Z. Near-infrared quantum dots as optical probes for tumor imaging. Curr Top Med Chem 2010, 10:1147 – 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, Chen K, Miao Z, Ren G, Chen X, Gambhir SS, Cheng Z. Affibody-based nanoprobes for HER2-expressing cell and tumor imaging. Biomaterials 2011, 32:2141 – 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He X, Gao J, Gambhir SS, Cheng Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: status and challenges. Trends Mol Med 2010, 16:574 – 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Wang K, Cheng Z. In vivo near-infrared fluorescence imaging of cancer with nanoparticle-based probes. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010, 2:349 – 366. [DOI] [PubMed] [Google Scholar]
- 27.Hellebust A, Richards-Kortum R. Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (Lond) 2012, 7:429 – 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol 2007, 18:17 – 25. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Bloch S, Akers W, Achilefu S. Near-infrared molecular probes for in vivo imaging. Curr Protoc Cytom 2012, 12.27:1– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol 2003, 13:195 – 208. [DOI] [PubMed] [Google Scholar]
- 31.Ehrhardt A, Stepp H, Irion KM, Stunner W, Zaak D, Baumgartner R, Hofstetter A. A fluorescence detection of human malignancies using incoherent light systems. Med Laser Appl 2003, 18:27 – 35. [Google Scholar]
- 32.Doiron DR, Profio E, Vincent RG, Dougherty TJ. Fluorescence bronchoscopy for detection of lung cancer. Chest 1979, 76:27 – 32. [DOI] [PubMed] [Google Scholar]
- 33.Profio AE, Doiron DR. A feasibility study of the use of fluorescence bronchoscopy for localization of small lung tumours. Phys Med Biol 1977, 22:949 – 957. [DOI] [PubMed] [Google Scholar]
- 34.Kato H, Cortese DA. Early detection of lung cancer by means of hematoporphyrin derivative fluorescence and laser photoradiation. Clin Chest Med 1985, 6:237 – 253. [PubMed] [Google Scholar]
- 35.Profio AE, Doiron DR, Balchum OJ, Huth GC. Fluorescence bronchoscopy for localization of carcinomain situ. Med Phys 1983, 10:35 – 39. [DOI] [PubMed] [Google Scholar]
- 36.Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42:518 – 525. [DOI] [PubMed] [Google Scholar]
- 37.De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat 2003, 2:553 – 562. [DOI] [PubMed] [Google Scholar]
- 38.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, Ngo L, Khamene A, Azar F, Frangioni JV. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol 2009, 16:2943 – 2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogaards A, Varma A, Collens SP, Lin A, Giles A, Yang VX, Bilbao JM, Lilge LD, Muller PJ, Wilson BC. Increased brain tumor resection using fluorescence image guidance in a preclinical model. Lasers Surg Med 2004, 35:181 – 190. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Hatami N, Yee M, Phipps J, Elson DS, Gorin F, Schrot RJ, Marcu L. Fluorescence lifetime imaging microscopy for brain tumor image-guided surgery. J Biomed Opt 2010, 15:056022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol 2008, 3:557 – 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nat Photonics 2009, 3:503 – 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu W, Huang Q, Ku G, Wen X, Zhou M, Guzatov D, Brecht P, Su R, Oraevsky A, Wang LV, et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials 2010, 31:2617 – 2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, Pitter K, Huang R, Campos C, Habte F, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med 2012, 18:829 – 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu W, Melancon MP, Xiong C, Huang Q, Elliott A, Song S, Zhang R, Flores LG 2nd, Gelovani JG, Wang LV, et al. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res 2011, 71:6116 – 6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbeek FP, van der Vorst JR, Schaafsma BE, Hutteman M, Bonsing BA, van Leeuwen FW, Frangioni JV, van de Velde CJ, Swijnenburg RJ, Vahrmeijer AL. Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J Hepatobiliary Pancreat Sci 2012, 19:626 – 637. Doi: 10.1007/S00534-012-0534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okuda T, Kataoka K, Taneda M. Metastatic brain tumor surgery using fluorescein sodium: technical note. Minim Invasive Neurosurg 2007, 50:382 – 384. [DOI] [PubMed] [Google Scholar]
- 48.Shinoda J, Yano H, Yoshimura S, Okumura A, Kaku Y, Iwama T, Sakai N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. J Neurosurg 2003, 99:597 – 603. [DOI] [PubMed] [Google Scholar]
- 49.Dilek O, Ihsan A, Tulay H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J Clin Neurosci 2011, 18:430 – 431. [DOI] [PubMed] [Google Scholar]
- 50.Tanahashi S, Lida H, Dohi S. An anaphylactoid reaction after administration of fluorescein sodium during neurosurgery. Anesth Analg 2006, 103:503. [DOI] [PubMed] [Google Scholar]
- 51.Okuda T, Kataoka K, Yabuuchi T, Yugami H, Kato A. Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium. J Clin Neurosci 2010, 17:118 – 1121. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen NQ, Biankin AV, Leong RW, Chang DK, Cosman PH, Delaney P, Kench JG, Merrett ND. Real time intraoperative confocal laser microscopy-guided surgery. Ann Surg 2009, 249:73. [DOI] [PubMed] [Google Scholar]
- 53.Kajimoto Y, Miyatake SI, Kuroiwa T . T Fiber-optic spectroscopic detection of neoplasm by intraoperative fluorescence labeling. Int Cong Series 2004, 1259:33 – 38. [Google Scholar]
- 54.Ichioka T, Miyatake S, Asai N, Kajimoto Y, Naka-gawa T, Hayashi H, Kuroiwa T. et al. Enhanced detection of malignant glioma xenograft by fluorescein-human serum albumin conjugate. J Neurooncol 2004, 67:47 – 52. [DOI] [PubMed] [Google Scholar]
- 55.Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci 2008, 7:283 – 289. [DOI] [PubMed] [Google Scholar]
- 56.Musiol R, Serda M, Polanski J. Prodrugs in photodynamic anticancer therapy. Curr Pharm Des 2011, 17:3548 – 3559. [DOI] [PubMed] [Google Scholar]
- 57.Hebeda KM, Saarnak AE, Olivo M, Sterenborg HJCM, Wolber JG. 5-aminolevulinic acid induced endogenous porphyrin fluorescence in 9L and C6 brain tumors and in the normal brain. Acta Neurochir (Wien) 1998, 140:503 – 513. [DOI] [PubMed] [Google Scholar]
- 58.Eléouet S, Rousset N, Carré J, Bourré L, Vonarx V, Lajat Y, Beijersbergen van Henegouwen GM, Patrice T. In vitro fluorescence, toxicity and phototoxicity induced by delta-aminolevulinic acid (ALA) or ALA-esters. Photochem Photobiol 2000, 71:447 – 454. [DOI] [PubMed] [Google Scholar]
- 59.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 2000, 93:1003 – 1013 [DOI] [PubMed] [Google Scholar]
- 60.Hefti M, Mehdorn HM, Albert I, Dorner L. Fluorescence-guided surgery for malignant glioma: a review on aminolevulinic acid induced protoporphyrin IX photodynamic diagnostic in brain tumors. Curr Med Imag Rev 2010, 6:254 – 258(5). [Google Scholar]
- 61.Divaris DX, Kennedy JC, Pottier RH. Phototoxic damage to sebaceous glands and hair follicles of mice after systemic administration of 5-aminolevulinic acid correlates with localized protoporphyrin IX fluorescence. Am J Pathol 1990, 136:891 – 897. [PMC free article] [PubMed] [Google Scholar]
- 62.Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc Chem Res 2011, 44:1050 – 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Artemov D, Mori N, Okollie B, Bhujwalla ZM. MR molecular imaging of the Her-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magn Reson Med 2003, 49:403 – 408. [DOI] [PubMed] [Google Scholar]
- 64.Yang X, Stein EW, Ashkenazi S, Wang LV. Nanoparticles for photoacoustic imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2009, 1:360 – 368. [DOI] [PubMed] [Google Scholar]
- 65.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled nearinfrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett 2006, 6:669 – 676. [DOI] [PubMed] [Google Scholar]
- 66.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006, 7:392 – 401. [DOI] [PubMed] [Google Scholar]
- 67.Kremer P, Fardanesh M, Ding R, Pritsch M, Zoubaa S, Frei E . Intraoperative fluorescence staining of malignant brain tumors using 5-aminofluorescein-labeled albumin. Neurosurgery 2009, 64:ons53 – 60. [DOI] [PubMed] [Google Scholar]
- 68.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res 2003, 63:8122 – 8125. [PubMed] [Google Scholar]
- 69.Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, et al. Artificially engineered magnetic nanoparticles for ultrasensitive molecular imaging. Nat Med 2007, 13:95 – 99. [DOI] [PubMed] [Google Scholar]
- 70.Yang L, Mao H, Cao Z, Wang YA, Peng X, Wang X, Sajja HK, Wang L, Duan H, Ni C, et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology 2009, 136:1514 – 1525.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett 2005, 5:1003 – 1008. [DOI] [PubMed] [Google Scholar]
- 72.Veiseh O, Sun C, Fang C, Bhattarai N, Gunn J, Kievit F, Du K, Pullar B, Lee D, Ellenbogen RG, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res 2009, 69:6200 – 6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan H, Wang L, Wang J, Weng X, Lei H, Wang X, Jiang L, Zhu J, Lu W, Wei X, et al. Two-order targeted brain tumor imaging by using an optical/paramagnetic nanoprobe across the blood brain barrier. ACS Nano 2012, 6:410 – 420. [DOI] [PubMed] [Google Scholar]
- 74.Towner RA, Smith N, Asano Y, He T, Doblas S, Saunders D, Silasi-Mansat R, Lupu F, Seeney CE. Molecular magnetic resonance imaging approaches used to aid in the understanding of angiogenesis in vivo: implications for tissue engineering. Tissue Eng Part A 2010, 16: 357 – 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, Ntziachristos V, Hollema H, Herek JL, Schröder CP, Kosterink JG, MN Lub-de Hoog, de Vries EG . Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factorand human epidermal growth factor receptor 2 targeting anti-bodies. J Nucl Med 2011, 52:1778 – 1785. [DOI] [PubMed] [Google Scholar]
- 76.Themelis G, Harlaar NJ, Kelder W, Bart J, Sarantopoulos A, van Dam GM, Ntziachristos V. Enhancing surgical vision by using real-time imaging of αvβ3-integrin targeted near-infrared fluorescent agent. Ann Surg Oncol 2011, 18:3506 – 3513. [DOI] [PubMed] [Google Scholar]
- 77.Hak S, Helgesen E, Hektoen HH, Huuse EM, Jarzyna PA, Mulder WJ, Haraldseth O, Davies Cde L. The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targetingstudied in vivo by dual modality imaging. ACS Nano 2012, 6:5648 – 5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang VX, Muller PJ, Herman P, Wilson BC. A multi-spectral fluorescence imaging system: design and initial clinical tests in intra-operative photofrin-photodynamic therapy of brain tumors. Lasers Surg Med 2003, 32:224 – 232. [DOI] [PubMed] [Google Scholar]
- 79.Kumar AT, Raymond SB, Bacskai BJ, Boas DA. Comparison of frequency-domain and time-domain fluorescence lifetime tomography. Opt Lett 2008, 33: 470 – 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazhar A, Dell S, Cuccia DJ, Gioux S, Durkin AJ, Frangioni JV, Tromberg BJ. Wavelength optimization for rapid chromophore mapping using spatial frequency domain imaging. J Biomed Opt 2010, 15:061716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valdés PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan X, Tosteson TD, Hartov A, Ji S, Erkmen K, et al. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg 2011, 115:11 – 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li C, Yang Y, Mitchell GS, Cherry SR. Simultaneous PET and multispectral 3-dimensional fluorescence optical tomography imaging system. J Nucl Med 2011, 52:1268 – 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brouwer OR, Buckle T, Bunschoten A, Kuil J, Vahrmeijer AL, Wendler T, Valdés-Olmos RA, van der Poel HG, van Leeuwen FW. Image navigation as a means to expand the boundaries of fluorescence-guided surgery. Phys Med Biol 2012, 57:3123 – 3136. [DOI] [PubMed] [Google Scholar]
- 84.Xi L, Grobmyer SR, Wu L, Chen R, Zhou G, Gutwein LG, Sun J, Liao W, Zhou Q, Xie H, et al. Evaluation of breast tumor margins in vivo with intraoperative photoacoustic imaging. Opt Express 2012, 20:8726 – 8731. [DOI] [PubMed] [Google Scholar]


