Abstract
In order to minimize complications on the skeleton and to prevent extraskeletal calcifications, the specific aims of the management of Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD) are to maintain blood levels of serum calcium and phosphorus as close to the normal range as possible, thereby maintaining serum PTH at levels appropriate for stage of CKD, preventing hyperplasia of the parathyroid glands, avoiding the development of extra-skeletal calcifications, and preventing or reversing the accumulation of toxic substances such as aluminum and β2-microglobulin. In order to limit cardiovascular calcification, daily intake of elemental calcium, including from dietary sources and from phosphate binders, should not exceed twice the daily recommended intake for age and should not exceed 2.5 g/day. Calcium-free phosphate binders such as sevelamer hydrochloride and sevelamer carbonate are safe and effective alternatives to calcium-based binders and their use widens the margin of safety for active vitamin D sterol therapy. Vitamin D deficiency is highly prevalent across the spectrum of CKD and replacement therapy is recommended in vitamin D deficient and insufficient individuals. Therapy with active vitamin D sterols is recommended after correction of vitamin D deficiency state and should be titrated based on target PTH levels across the spectrum of CKD. Although the use of calcimimetics drugs have proven to effectively control the biochemical features of secondary hyperparathyroidism, there is very limited experience with the use of such agent in pediatric patients and mainly during the first years of life. Studies are needed to further define the role of such agents in the treatment of pediatric CKDMBD.
Keywords: PTH, bone histomorphometry, FGF23, vitamin D
Introduction
“Chronic Kidney Disease Mineral and Bone Disorder” (“CKD-MBD”) is a systemic disorder of mineral and bone metabolism due to CKD that is manifested by either one or a combination of the following: abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism; abnormalities in bone histology, linear growth, or strength; or vascular or other soft tissue calcification. “Renal osteodystrophy” is the specific term used to describe the bone pathology that occurs as a complication of CKD and is therefore one aspect of CKD-MBD. These terms, newly defined by the Kidney Disease: Improving Global Outcomes (KDIGO) working group, link crucial aspects of end-organ damage, namely increased mortality from extra-skeletal tissue and vascular calcifications and skeletal morbidity from renal osteodystrophy[1], to disordered mineral metabolism. Indeed, a growing body of evidence demonstrates that cardiovascular calcifications accompany CKD, that cardiovascular disease is the leading cause of mortality in patients with CKD, and that therapies designed to treat the skeletal consequences of CKD affect the progression of vascular pathology.
In order to minimize complications on the skeleton and to prevent extraskeletal calcifications, the specific aims of the management of CKD-MBD are to maintain blood levels of serum calcium and phosphorus as close to the normal range as possible, thereby maintaining serum PTH at levels appropriate for stage of CKD, preventing hyperplasia of the parathyroid glands, avoiding the development of extra-skeletal calcifications, and preventing or reversing the accumulation of toxic substances such as aluminum and β2-microglobulin. The goal of this review is to summarize the impact of currently utilized treatments for the management of CKD-MBD, namely phosphate binders, active vitamin D analogues and calcimimetics, on mineral metabolism, bone disease, and cardiovascular disease in pediatric CKD patients.
Phosphate-Binding Agents
Phosphate binders are Federal Drug Administration (FDA) approved for patients treated with maintenance dialysis and calcium-containing salts are used worldwide for the control of hyperphosphatemia and also serve as a source of supplemental calcium. Several calcium salts are commercially available, including calcium carbonate, calcium acetate, and calcium citrate. Calcium carbonate is the most commonly used compound and studies in adults and children have shown its efficacy in controlling serum phosphorous levels [2]. The recommended dose is proportional to the phosphorous content of the meal and adjusted to achieve acceptable serum levels of calcium and phosphorous. However, large doses of calcium carbonate may lead to hypercalcemia, particularly in patients treated with vitamin D or those with adynamic bone[3 ]. Hypercalcemia is usually reversible with reductions in the dose of oral calcium salts, dose of vitamin D sterol, and dialysate calcium concentrations. In addition, higher doses of calcium-based binders have been associated with greater degree of vascular calcifications[4] and vascular stiffness and recent data demonstrate that patients with CKD 2-4 are in positive calcium balance when they are given 2 g/day of calcium supplementation [5]. To avoid the development and progression of cardiovascular calcifications, it is currently recommended that daily intake of elemental calcium, including from dietary sources and from phosphate binders, should not exceed twice the daily recommended intake for age and should not exceed 2.5 g/day[6]. To limit the vascular calcification risks associated with the use of calcium salts and the bone and neurologic toxicity associated with aluminum hydroxide, alternative phosphate binders have been developed. Once such calcium-free phosphate binder is sevelamer hydrochloride (RenaGelR), a hydrogel of cross-linked polyallylamine. This agent effectively binds phosphorus without inducing hypercalcemia in adult and pediatric patients treated with dialysis [7-8]. In contrast to the increase in vascular calcification observed during calcium-containing binder therapy, sevelamer also halts the progression of these lesions in adult CKD patients[9-10]. In addition to its effects on serum phosphorous levels, sevelamer has been shown to decrease concentrations of total serum cholesterol and low-density lipoprotein cholesterol and to increase high-density lipoprotein levels[11]. These effects may offer additional benefits in reducing cardiovascular complications in patients with end stage renal disease. Acidosis may occur in some patients treated with sevelamer hydrochloride; thus, a new form, sevelamer carbonate, has been introduced. This compound is as effective as sevelamer hydrochloride in binding phosphate while preventing acidosis[12].
Lanthanum carbonate, another calcium-free phosphate binder, is also effective in controlling serum phosphorus levels without inducing hypercalcemia, adynamic bone disease, or osteomalacia [13-14]; however, lanthanum is a heavy metal which accumulates in different tissues in animals with normal renal and reduced kidney function [15]. Lanthanum also accumulates in the bone of dialysis patients where its presence persists despite discontinuation for as long as 2 years [16]; thus, further long-term studies are therefore needed to confirm the absence of toxicity before this agent is recommended for widespread use in pediatric patients. Magnesium carbonate also lowers serum phosphorous levels; however, large doses may result in diarrhea, limiting the use of this compound as a single agent and magnesium-free dialysate solutions should be used in those treated with dialysis [17]. Iron compounds, such as stabilized polynuclear iron hydroxide and ferric polymaltose complex, have also been shown to be effective phosphate binders in short-term studies in adults with CKD [18].
Since fibroblast growth factor 23 (FGF23) levels have been linked to cardiovascular morbidity and mortality in patients with all stages of CKD, recent trials have investigated the efficacy of phosphate-binding agents in lowering FGF23 values in patients with pre-dialysis CKD. Short term studies have demonstrated a simultaneous decrease in serum FGF23 and PTH levels and in urinary phosphate excretion with the administration of phosphate binders in normophosphatemic pre-dialysis CKD patients[19-21]. Some data suggest that reductions in FGF23 may only be observed during treatment with calcium-free phosphate binders[19-21] while others question the ability of any binders to lower serum FGF23 values[22]; however, the number of patients included in each group of each of these trials has been relatively small; thus the long-term effects of phosphate binders on FGF23 levels remains to be evaluated in larger cohorts. Current guidelines do not currently advocate the use of phosphate binders in normophosphatemic pre-dialysis CKD patients and, given the variation in normal ranges for serum phosphate that occurs during childhood, as well as age-dependent variations in FGF23 concentrations[23], age appropriate serum phosphorus levels should be taken into consideration whenever treating any child with phosphate binder medication.
Vitamin D Therapy
Assessment and Treatment of 25(OH)vitamin D Deficiency
Although active vitamin D sterol therapy is the mainstay of therapy for controlling serum PTH levels [6], assessment of 25(OH)D status and replacement therapy, if needed, may have implications on the treatment of secondary hyperparathyroidism [24]. Indeed, current recommendations suggest that 25(OH)vitamin D (25(OH)D) therapy should be administered to pediatric pre-dialysis CKD patients with 25(OH)D deficiency and insufficiency before active vitamin D sterols are initiated[6]. In addition to providing a substrate for the formation of calcitriol, thus indirectly suppressing PTH levels, Ritter et al. identified that 25(OH)vitamin D continues to directly suppress PTH synthesis even when parathyroid gland 1-alpha hydroxylase is inhibited, thus demonstrating a direct effect of 25(OH)vitamin D on PTH synthesis, independent of 1,25(OH)2vitamin D [25]. Moreover, a recent placebo-controlled randomized trial demonstrated that ergocalciferol delays the onset of secondary hyperparathyroidism in pediatric patients with pre-dialysis CKD [24]. 25(OH)vitamin D likely also has a direct effect on bone biology, independent of its effects on mineral metabolism; indeed, Priemel et al. demonstrated in a cohort of 675 deceased adults with previously normal renal function that pathologic mineralization defects could occur when serum 25(OH) D levels were below 30 ng/mL [26]and, more than two decades ago, Langman et al. demonstrated that treatment with 25(OH)D led to complete resolution of osteomalacia and increased growth velocity in children with pre-dialysis CKD [27]. The potential extra-skeletal effects of 25(OH)D are also important in the pediatric CKD population. 25(OH) D deficiency has been associated with higher prevalence of chronic heart failure, atherosclerosis, endothelial dysfunction, and cardiovascular mortality in adult dialysis patients[28] [29]. In a retrospective analysis, Saab et al. demonstrated that therapy with vitamin D2 therapy was associated with the need for lower doses of active vitamin D sterol and erythropoietin in dialysis patients [30], potentially by decreasing the degree of marrow fibrosis or by exerting local effect effects on the bone marrow. In addition to increasing 25(OH)D and 1,25(OH)2vitamin D levels, therapy with 25(OH)vitamin D increases FGF23 levels in healthy subjects [31]. The mechanisms involved in the increased release of FGF23 during ergocalciferol therapy have yet to be defined, as have the potential consequences associated with elevated FGF23 levels such as cardiovascular complications, deterioration of kidney function and mortality, occurring as a result of ergocalciferol therapy [32-35].
Although optimal ranges of 25(OH)vitamin D remain controversial, with levels above 20 ng/ml defined as sufficient by the Institute of Medicine [36], current KDOQI guidelines suggest that values of 25(OH)vitamin D be maintained above 30 ng/ml [6]. Upper limits for 25(OH)D have not been defined by KDOQI, although European guidelines cite optimal effects on mineral metabolism at values between 20 and 50 ng/ml [37]. By the current KDOQI definition, data in children with CKD have documented a 20-75% prevalence of 25(OH) D deficiency/insufficiency [38-42]. Vitamin D deficiency is categorized as: 1) severe deficiency, defined as a serum level less than 5 ng/ml, 2) mild deficiency, equivalent to serum concentrations of 5 to 15 ng/ml, and 3) vitamin D insufficiency with levels between 16 and 30 ng/ml [6]. According to the opinion-based KDOQI guidelines, levels of less than 5 ng/ml should be treated with either ergocalciferol or cholecalciferol therapy administered at doses of 50,000 IU orally, once a week, for 12 weeks, then 50,000 IU orally once a month for a total of 6 months. Alternatively, 500,000 IU may be given as a single intramuscular dose. Levels between 5 and 15 ng/ml should be treated with 50,000 IU of ergocaciferol or cholecalciferol orally once a week for four weeks followed by 50,000 IU orally once a month for a total of 6 months. Levels of 15 to 30 ng/ml should be treated with 50,000 IU of ergocalciferol or cholecalciferol orally once a month for 6 months. Serum 25(OH)D levels should be rechecked after completion of the 6 month course of therapy[6].
Therapy with Active Vitamin D Sterols
Active vitamin D sterols act through a variety of pathways to decrease PTH production—by increasing calcium absorption in the gut and kidney, by binding to the CaSR, by increasing skeletal sensitivity to PTH and by altering prepro PTH transcription. Vitamin D sterols may also suppress PTH indirectly, through increasing osteocytic FGF23 production [43] which, in turn, suppresses parathyroid gland PTH expression[44-45]. Calcitriol and alfacalcidol have been effective in decreasing PTH levels and preventing osteitis fibrosis cystica for decades [46-47]. Beneficial effects of calcitriol, including improved survival in hemodialysis patients [48-50], amelioration of cardiac hypertrophy in animals[51], improved cardiac systolic function in hemodialysis patients[52], reductions in proteinuria, fibrosis, and podocyte hypertrophy in sub-totally nephrectomized rats [53] and decreased proteinuria in patients with pre-dialysis CKD patients [54] have been demonstrated. However, since treatment with these sterols in combination with calcium-based binders often results in hypercalcemia and hyperphosphatemia which contributes to the development of soft tissue calcification [55] newer vitamin D analogues were developed to minimize intestinal calcium and phosphorus absorption, while suppressing PTH. Three of these active vitamin D analogues are used in patients with chronic kidney disease: 22-oxacalcitrol in Japan and 19-nor-1,25-dihydroxyvitamin D2 (paricalcitol) and 1α -hydroxyvitamin D2 (doxercalciferol) in the United States. Although some benefits to overall survival and to bone health have been attributed to these newer analogues, they are considerably more expensive than is calcitriol, a factor that must be considered in their wide-spread utilization.
19-nor-1α ,25(OH)2D2 (paricalcitol), is effective in controlling serum PTH levels in adults across the spectrum of CKD [56] and in children treated with dialysis[57]. The long-term consequences of therapy with paricalcitol in conjunction with the use of calcium containing binders on vascular calcification and cardiovascular complications remain to be determined; however, in a large cohort of patients undergoing hemodialysis, higher survival rates were observed in dialyzed patients treated with paricalcitol when compared to those receiving calcitriol [48]. The effects of paricalcitol, relative to calcitriol, on the skeletal lesions of secondary hyperparathyroidism remain unknown.
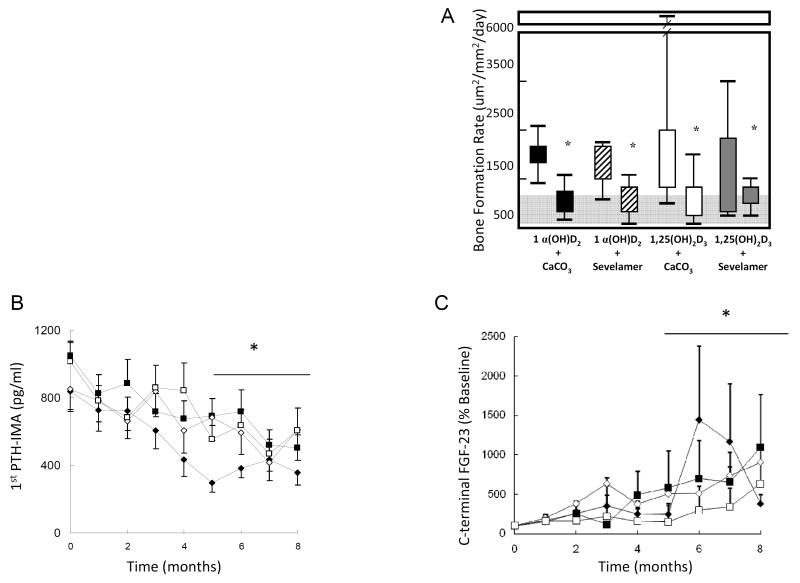
1α (OH)-vitamin D2 (1α D2, doxercalciferol) is a pro-hormone that undergoes 25-hydroxylation in the liver to form its active metabolite. In rats, this agent is equipotent to 1α (OH)-vitamin D3 in intestinal calcium absorption and bone calcium mobilization [58]. Similar to paricalcitol, beneficial survival effects were also found with doxercalciferol over the use of calcitriol [50]. In a head-to-head trial of calcitriol versus doxercalciferol, when used in combination with either calcium carbonate or sevelamer hydrochloride as phosphate binder, on the control of the biochemical and skeletal lesions of secondary hyperparathyroidism in pediatric peritoneal dialysis patients, serum PTH (determined by both 1st and 2nd generation assays), phosphorus, and bone formation rate were controlled equivalently irrespective of treatment group. PTH levels, as measured by the 1st generation Nichols immunometric assay, ranging between 300-600 pg/ml (Figure 1) were associated with normal rates of bone turnover at the end of this trial; these PTH values consistent with those recommended by both KDOQI and KDIGO[59] for pediatric dialysis patients. Despite the beneficial effects on the control of secondary hyperparathyroidism, a mineralization defect persisted in the vast majority of patients [60]. The etiology behind the persistent defects in skeletal mineralization is incompletely defined, but the prevalence of 25(OH)D deficiency in the study population may have contributed[27] and the potential effect of increased osteocytic FGF23 expression on these persistent mineralization defects remains to be defined . Interestingly, doxercalciferol had a greater inhibitory effect on osteoclastogenesis than did calcitriol, independent of type of phosphate binder[60]. Serum calcium levels rose in patients receiving calcium carbonate as a phosphate binder, irrespective of the type of vitamin D sterol; this increase in calcium was not observed in patients treated with sevelamer hydrochloride, also regardless of whether calcitriol or doxercalciferol was prescribed [60]. Overall, serum calcium levels were 0.63 + 0.22 mg/dl higher in patients who were treated with calcium carbonate, an increase which carries with it a relative risk for increasing vascular calcification that is equivalent to an extra 2.5 year of hemodialysis[61-62]. The avoidance of hypercalcemia and the maintenance of serum calcium levels within the lower end of the physiologic range during sevelamer therapy thus widens the margin of safety for the use of vitamin D analogues in the treatment of secondary hyperparathyroidism.
Figure 1.
(a) Changes in bone formation rate (BFR) during treatment with active vitamin D sterols and phosphate binders, (b) plasma parathyroid hormone (PTH) levels throughout the course of the study by treatment group and c) plasma C-terminal fibroblast growth factor 23 (FGF-23) levels throughout the course of the study by treatment group. Treatment groups: 1) 1αD2 + CaCO3 (closed diamonds), 2) 1αD2 + sevelamer (open diamonds), 3) calcitriol + CaCO3 (closed squares) and 4) calcitriol + sevelamer (open squares). Asterix indicates p<0.01 from baseline.
Many beneficial effects of vitamin D sterol therapy, including affects on cardiac hypertrophy [51] and systolic function [52] as well as vitamin D-mediated reductions in proteinuria, fibrosis, and podocyte hypertrophy [53-54], appear to be mediated by suppression of the renin-angiotensin system (RAS) and in vitro studies have demonstrated that calcitriol, paricalcitol, and doxercalciferol all suppress the RAS to a similar degree [63]. However, active vitamin D sterols also increase FGF23 values. Indeed, plasma FGF23 levels are markedly elevated in pediatric dialysis patients [60, 64], and, although studies have demonstrated that treatment with sevelamer decreases FGF23 levels[65], values increase during vitamin D sterol therapy, despite control of serum phosphate concentrations and irrespective of phosphate binder used[60]. Although higher FGF23 levels have been associated with improvements in skeletal mineralization in the pediatric CKD population [64, 66], elevated levels have been also shown to play a role in cardiovascular morbidity and mortality [33-34, 67-68]. Thus, the clinical consequences of the paradox between the associations of elevated FGF23 levels with mortality and the survival beneficial effects of therapy with active vitamin D sterols that raise FGF23 levels remains to be defined.
Circulating PTH levels are used to guide vitamin D sterol therapy, but target PTH levels for children treated with dialysis remain controversial. PTH values of 2-3 times the upper limit of normal [37] and between 100-300 pg/ml have been recommended from data collected through the IPPN registry encompassing 75 pediatric dialysis centers from 27 countries around the world [69]. These levels were associated with decreased skeletal complications such as bone deformities, bone pain and radiological findings of renal osteodystrophy, however, no correlations between PTH values and growth were identified in this analysis. Unfortunately, data on PTH concentrations from this registry were comprised of values obtained by different, local, un-standardized assays. Previous analyses which have identified a 30-50% variation in PTH levels when identical samples are measured by different commercial assays [70], thus calling into question the validity of such findings. Indeed, current KDIGO recommendations which suggest that PTH values between 2-9 times the upper limit of normal [59] are consistent with indices of bone turnover within the normal range [60] in pediatric dialysis patients. These recommendations take the wide variation in assay results into consideration and reflect the difficulty in interpreting PTH values in patients treated with maintenance dialysis. Moreover, recent data from pediatric CKD patients across the spectrum of GFR suggested that higher PTH levels are required as renal function diminishes in order to maintain normal rates of bone remodeling (Table 1) [71].
Table 1.
Biochemical parameters across the spectrum of chronic kidney disease (CKD). Bone turnover was normal in the majority of subjects with CKD stage 2 and 3 while rates were above the normal range in 29% of patients with CKD stage 4[71]. Biochemical values from pediatric dialysis patients with normal rates of bone formation are displayed for reference[76].
|
CKD 2 (n=14) |
CKD 3 (n=24) |
CKD 4/5 (n=14) |
CKD 5D with normal BFR/BS^ (n=62) |
|
|---|---|---|---|---|
| Ca (mg/dl) | 9.5±0.1 | 9.2±0.1 | 9.3±0.2 | 9.3±0.1 |
| P (mg/dl) | 4.7±0.2 | 4.7±0.2 | 6.1 ±0.3‡# | 6.0±0.2 |
| 25(OH)vitamin D (ug/ml) |
31.2±2.6 | 25.2±1.7 | 32.6±3.6 | 15.8±1.2 |
| 1,25(OH)2vitamin D (pg/ml) |
39.5±3.8 | 34.5±2.7 | 26.6±5.0 | N/A |
| PTH (pg/ml)* | 52 (48,87) | 92 (46,142)‡ | 125 (88,366)‡# | 326 (113,524) |
| FGF-23 (RU/ml)* | 181 (101,291)‡ | 197 (120,300)‡ | 344 (255,742)‡ | 2771(1027, 10636)# ‡ |
| BFR/BS^ (μm3/μm2/d) |
24.9±4.2 | 39.9±7.3 | 64.2±15.0# | 12.5±1.5 |
PTH – parathyroid hormone, FGF-23 – fibroblast growth factor 23, BFR/BS – bone formation rate/bone surface
expressed as median (IQ range)
p<0.05 above the normal range
p<0.05 from CKD stages 2 and 3
normal range: 8.0-73.4 μm3/μm2/d
Furthermore, the mode of vitamin D sterol administration may alter interpretation of PTH levels. In the past, we have demonstrated that the administration of intermittent oral doses of calcitriol with calcium-based binders effectively corrects many of the skeletal histological manifestations of secondary hyperparathyroidism [3]; unfortunately, overcorrection was common, leading to adynamic renal osteodystrophy, growth failure [72-74]and vascular calcification [75] even, at times, in the context of markedly elevated PTH concentrations. Careful titration of vitamin D sterols based on concurrent determinations of serum calcium, phosphorus, alkaline phosphatase, and PTH levels is able to avoid the development of adynamic bone; thus, the concurrent utilization of multiple biomarkers is likely the best method of controlling the skeletal lesions of secondary hyperparathyroidism [76] [64] while preventing the side effects associated with therapy.
Calcimimetics
Cinacalcet, an allosteric activator of the calcium sensing receptor, is now available for the treatment of secondary hyperparathyroidism. This small organic molecule reduces serum PTH levels and has also been shown to decrease FGF23, serum calcium, phosphorus and the calcium-phosphorous ion product in adult patients treated with maintenance dialysis, regardless of the specific phosphate binding agent [77-78]. Experiments in uremic rats have demonstrated that calcimimetics are able to halt the progression of parathyroid cell hyperplasia [77]; the antiproliferative effect of this agent shows promise for use of the molecule as a “medical parathyroidectomy” [79]. These agents may play a role in reversing the process of vascular calcification[78] and in lowering FGF-23 levels [80]; however, when cinacalcet in addition to low dose vitamin D was prospectively compared to flexible doses of vitamin D sterols, the Agatston calcification scores did not differ between both groups after one year of therapy in hemodialysis patients receiving calcium-based phosphate binders [81]. Furthermore, recent data demonstrate that cardiovascular complications and mortality are not prevented by calcimimetic therapy in hemodialysis patients [82], Calcimimetics may also have a direct effect on bone biology; indeed, studies in animals suggest that these agents may increase osteoclast number and activity [83], thus increasing bone erosion. In a relative small cohort of dialysis patients, skeletal indices of bone turnover were controlled with combined cinacalcet and vitamin D therapy but a similar degree of improvement was observed in the placebo group treated with vitamin D. It is interesting to note that indices of mineralization remained within the normal range with cinacalcet [84]. Currently there is very limited experience with the use of such compound in pediatric patients with CKD [85-86]. Furthermore, precocious puberty has been described in a child with CKD after initiation of treatment with cinacalcet and lanthanum carbonate [87]. The effects of cinacalcet on longitudinal growth are not known and, since the calcium-sensing receptor is expressed in the growth plate, caution with wide use of this agent is recommended until clinical trials can be performed.
Summary
Calcium-based binders are still widely utilized but, in order to limit cardiovascular calcification, the daily intake of elemental calcium, from dietary sources and from phosphate binders, should not exceed twice the daily recommended intake for age and should not exceed 2.5 g/day. Sevelamer hydrochloride and sevelamer carbonate are safe and effective alternatives to calcium-based binders and their use widens the margin of safety for active vitamin D sterol therapy [60]. Lanthanum carbonate controls serum phosphorus levels in adult patients with CKD but has not been utilized in pediatric patients due to the potential risk for tissue accumulation. Vitamin D deficiency is highly prevalent across the spectrum of CKD and replacement therapy is recommended in vitamin D deficient and insufficient individuals. Therapy with active vitamin D sterols is recommended after correction of vitamin D deficiency state and should be titrated based on target PTH levels across the spectrum of CKD. Therapy with active vitamin D sterols is associated with increased circulating FGF23 values and the long-term consequences of these levels remain to be evaluated. The recommendations for patients treated with dialysis are still controversial between the Pediatric European Guidelines and KDIGO, but current data based on bone histomorphometry support KDIGO recommendations. Although the use of calcimimetics drugs have proven to effectively control the biochemical features of secondary hyperparathyroidism, there is very limited experience with the use of such agent in pediatric patients and mainly during the first years of life. Studies are needed to further define the role of such agents in the treatment of pediatric CKD-MBD.
Acknowledgements
This work was supported in part by USPHS grants DK-67563, DK-35423, DK-51081, DK-073039, and UL1 RR-033176 and funds from the Casey Lee Ball Foundation.
References
- 1.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Salusky IB, Coburn JW, Foley J, Nelson P, Fine RN. Effects of oral calcium carbonate on control of serum phosphorus and changes in plasma aluminum levels after discontinuation of aluminum-containing gels in children receiving dialysis. J Pediatr. 1986;108:767–770. doi: 10.1016/s0022-3476(86)81064-2. [DOI] [PubMed] [Google Scholar]
- 3.Salusky IB, Kuizon BD, Belin TR, Ramirez JA, Gales B, Segre GV, Goodman WG. Intermittent calcitriol therapy in secondary hyperparathyroidism: a comparison between oral and intraperitoneal administration. Kidney Int. 1998;54:907–914. doi: 10.1046/j.1523-1755.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- 4.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012;81:1116–1122. doi: 10.1038/ki.2011.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Kidney F. K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis. 2005;46:S1–121. [Google Scholar]
- 7.Bleyer AJ, Burke SK, Dillon M, Garrett B, Kant KS, Lynch D, Rahman SN, Schoenfeld P, Teitelbaum I, Zeig S, Slatopolsky E. A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis. 1999;33:694–701. doi: 10.1016/s0272-6386(99)70221-0. [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant. 1999;14:2907–2914. doi: 10.1093/ndt/14.12.2907. [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 10.Di Iorio B, Bellasi A, Russo D. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7:487–493. doi: 10.2215/CJN.03820411. [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Dillon M, Burke SK, Steg M, Bleyer AJ, Garrett BN, Domoto DT, Wilkes BM, Wombolt DG, Slatopolsky E. A randomized trial of sevelamer hydrochloride (RenaGel) with and without supplemental calcium. Strategies for the control of hyperphosphatemia and hyperparathyroidism in hemodialysis patients. Clin Nephrol. 1999;51:18–26. [PubMed] [Google Scholar]
- 12.Russo D, Battaglia Y, Buonanno E. Phosphorus and coronary calcification in predialysis patients. Kidney Int. 2010;78:818. doi: 10.1038/ki.2010.307. [DOI] [PubMed] [Google Scholar]
- 13.Hutchison AJ, Maes B, Vanwalleghem J, Asmus G, Mohamed E, Schmieder R, Backs W, Jamar R, Vosskuhler A. Long-term efficacy and tolerability of lanthanum carbonate: results from a 3-year study. Nephron Clin Pract. 2006;102:c61–c71. doi: 10.1159/000088932. [DOI] [PubMed] [Google Scholar]
- 14.Sprague SM, Abboud H, Qiu P, Dauphin M, Zhang P, Finn W. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol. 2009;4:178–185. doi: 10.2215/CJN.02830608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slatopolsky E, Liapis H, Finch J. Progressive accumulation of lanthanum in the liver of normal and uremic rats. Kidney Int. 2005;68:2809–2813. doi: 10.1111/j.1523-1755.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- 16.Spasovski GB, Sikole A, Gelev S, Masin-Spasovska J, Freemont T, Webster I, Gill M, Jones C, De Broe ME, D’Haese PC. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol Dial Transplant. 2006;21:2217–2224. doi: 10.1093/ndt/gfl146. [DOI] [PubMed] [Google Scholar]
- 17.O’Donovan R, Baldwin D, Hammer M, Moniz C, Parsons V. Substitution of aluminium salts by magnesium salts in control of dialysis hyperphosphataemia. Lancet. 1986;1:880–882. doi: 10.1016/s0140-6736(86)90987-6. [DOI] [PubMed] [Google Scholar]
- 18.Hergesell O, Ritz E. Stabilized polynuclear iron hydroxide is an efficient oral phosphate binder in uraemic patients. NephrolDialTransplant. 1999;14:863–867. doi: 10.1093/ndt/14.4.863. [DOI] [PubMed] [Google Scholar]
- 19.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Jüppner H, Wolf M. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira RB, Cancela AL, Graciolli FG, dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moyses RM. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A. Lanthanum carbonate reduces FGF23 in chronic kidney disease stage 3 patients. Nephrol Dial Transplant. 2011;266:2567–2571. doi: 10.1093/ndt/gfr144. [DOI] [PubMed] [Google Scholar]
- 22.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D. Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem. 2012;49:546–553. doi: 10.1258/acb.2012.011274. [DOI] [PubMed] [Google Scholar]
- 24.Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, Wells D, Aitkenhead H, Manickavasagar B, van’t Hoff W, Rees L. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clin J Am Soc Nephrol. 2012;7:216–223. doi: 10.2215/CJN.04760511. [DOI] [PubMed] [Google Scholar]
- 25.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–659. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- 26.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Puschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 27.Langman CB, Mazur AT, Baron R, Norman ME. 25-hydroxyvitamin D3 (calcifediol) therapy of juvenile renal osteodystrophy: beneficial effect on linear growth velocity. JPediatr. 1982;100:815–820. doi: 10.1016/s0022-3476(82)80602-1. [DOI] [PubMed] [Google Scholar]
- 28.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 29.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr., Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 30.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract. 2007;105:c132–c138. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 31.Burnett-Bowie SA, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS. Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol. 2012;7:624–631. doi: 10.2215/CJN.10030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 33.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institute of Medicine of The National Aacademies Dietary reference intakes for calcium and vitamin D. 2011 http://www.iom.edu/Reports/2010/Dietary-Reference-Intakesfor-Calcium-and-Vitamin-D.aspx.
- 37.Klaus G, Watson A, Edefonti A, Fischbach M, Ronnholm K, Schaefer F, Simkova E, Stefanidis CJ, Strazdins V, Vande Walle J, Schroder C, Zurowska A, Ekim M. Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr Nephrol. 2006;21:151–159. doi: 10.1007/s00467-005-2082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali FN, Arguelles LM, Langman CB, Price HE. Vitamin D deficiency in children with chronic kidney disease: uncovering an epidemic. Pediatrics. 2009;123:791–796. doi: 10.1542/peds.2008-0634. [DOI] [PubMed] [Google Scholar]
- 39.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–1033. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Belostotsky V, Mughal MZ, Berry JL, Webb NJ. Vitamin D deficiency in children with renal disease. Arch Dis Child. 2008;93:959–962. doi: 10.1136/adc.2007.134866. [DOI] [PubMed] [Google Scholar]
- 41.Hari P, Gupta N, Hari S, Gulati A, Mahajan P, Bagga A. Vitamin D insufficiency and effect of cholecalciferol in children with chronic kidney disease. Pediatr Nephrol. 2010;25:2483–2488. doi: 10.1007/s00467-010-1639-2. [DOI] [PubMed] [Google Scholar]
- 42.Menon S, Valentini RP, Hidalgo G, Peschansky L, Mattoo TK. Vitamin D insufficiency and hyperparathyroidism in children with chronic kidney disease. Pediatr Nephrol. 2008;23:1831–1836. doi: 10.1007/s00467-008-0842-x. [DOI] [PubMed] [Google Scholar]
- 43.Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HA, Hsieh JC, Slater SA, Hsieh G, Kaczmarska M, Jurutka PW, Kolek OI, Ghishan FK, Haussler MR. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol. 2007;103:381–388. doi: 10.1016/j.jsbmb.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro O, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 46.Baker LR, Muir JW, Sharman VL, Abrams SM, Greenwood RN, Cattell WR, Goodwin FJ, Marsh FP, Adami S, Hately W. Controlled trial of calcitriol in hemodialysis patients. Clin Nephrol. 1986;26:185–191. [PubMed] [Google Scholar]
- 47.Kanis JA, Henderson RG, Heynen G, Ledingham JG, Russell RG, Smith R, Walton RJ. Renal osteodystrophy in nondialysed adolescents. Long-term treatment with 1alpha-hydroxycholecalciferol. Arch Dis Child. 1977;52:473–481. doi: 10.1136/adc.52.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl JMed. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 49.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA, Jr., Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 50.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 51.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh NP, Sahni V, Garg D, Nair M. Effect of pharmacological suppression of secondary hyperparathyroidism on cardiovascular hemodynamics in predialysis CKD patients: A preliminary observation. Hemodial Int. 2007;11:417–423. doi: 10.1111/j.1542-4758.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E. Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998;53:1696–1705. doi: 10.1046/j.1523-1755.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 55.Milliner DS, Zinsmeister AR, Lieberman E, Landing B. Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int. 1990;38:931–936. doi: 10.1038/ki.1990.293. [DOI] [PubMed] [Google Scholar]
- 56.Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, Fadem S, Levine B, Williams L, Andress DL, Sprague SM. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47:263–276. doi: 10.1053/j.ajkd.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Greenbaum LA, Benador N, Goldstein SL, Paredes A, Melnick JZ, Mattingly S, Amdahl M, Williams LA, Salusky IB. Intravenous paricalcitol for treatment of secondary hyperparathyroidism in children on hemodialysis. Am J Kidney Dis. 2007;49:814–823. doi: 10.1053/j.ajkd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Sjoden G, Smith C, Lindgren U, DeLuca HF. 1 alpha-Hydroxyvitamin D2 is less toxic than 1 alpha-hydroxyvitamin D3 in the rat. Proc Soc Exp Biol Med. 1985;178:432–436. doi: 10.3181/00379727-178-42028. [DOI] [PubMed] [Google Scholar]
- 59.Group KW. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Preventrion, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76:s1–s130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 60.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79:112–119. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 61.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 62.Martin KJ, Jüppner H, Sherrard DJ, Goodman WG, Kaplan MR, Nassar G, Campbell P, Curzi M, Charytan C, McCary LC, Guo MD, Turner SA, Bushinsky DA. First-and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int. 2005;68:1236–1243. doi: 10.1111/j.1523-1755.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 63.Nakane M, Ma J, Ruan X, Kroeger PE, Wu-Wong R. Mechanistic analysis of VDR-mediated renin suppression. Nephron Physiol. 2007;107:35–44. doi: 10.1159/000106792. [DOI] [PubMed] [Google Scholar]
- 64.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Jüppner H, Salusky IB. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial. 2005;9:336–339. doi: 10.1111/j.1744-9987.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 66.Pereira RC, Jüppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivaths PR, Goldstein SL, Silverstein DM, Krishnamurthy R, Brewer ED. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26:945–951. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 69.Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, Zambrano P, Ahlenstiel T, Bakkaloglu SA, Spizzirri AP, Lopez L, Ozaltin F, Printza N, Hari P, Klaus G, Bak M, Vogel A, Ariceta G, Yap HK, Warady BA, Schaefer F. The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int. 2010;78:1295–1304. doi: 10.1038/ki.2010.316. [DOI] [PubMed] [Google Scholar]
- 70.Joly D, Drueke TB, Alberti C, Houillier P, Lawson-Body E, Martin KJ, Massart C, Moe SM, Monge M, Souberbielle JC. Variation in serum and plasma PTH levels in second-generation assays in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2008;51:987–995. doi: 10.1053/j.ajkd.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Wesseling-Perry K, Pereira RC, Tseng CH, Elashoff R, Zaritsky JJ, Yadin O, Sahney S, Gales B, Jüppner H, Salusky IB. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodman WG, Ramirez JA, Belin TR, Chon Y, Gales B, Segre GV, Salusky IB. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int. 1994;46:1160–1166. doi: 10.1038/ki.1994.380. [DOI] [PubMed] [Google Scholar]
- 73.Kuizon BD, Goodman WG, Jüppner H, Boechat I, Nelson P, Gales B, Salusky IB. Diminished linear growth during intermittent calcitriol therapy in children undergoing CCPD. Kidney Int. 1998;53:205–211. doi: 10.1046/j.1523-1755.1998.00724.x. [DOI] [PubMed] [Google Scholar]
- 74.Kuizon BD, Salusky IB. Intermittent calcitriol therapy and growth in children with chronic renal failure. Miner Electrolyte Metab. 1998;24:290–295. doi: 10.1159/000057384. [DOI] [PubMed] [Google Scholar]
- 75.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. JAmSocNephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 76.Bakkaloglu SA, Wesseling-Perry K, Pereira RC, Gales B, Wang HJ, Elashoff RM, Salusky IB. Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol. 2010;5:1860–1866. doi: 10.2215/CJN.01330210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wada M, Furuya Y, Sakiyama J, Kobayashi N, Miyata S, Ishii H, Nagano N. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest. 1997;100:2977–2983. doi: 10.1172/JCI119851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wada M, Nagano N. Control of parathyroid cell growth by calcimimetics. Nephrol Dial Transplant. 2003;18(Suppl 3):iii13–iii17. doi: 10.1093/ndt/gfg1004. [DOI] [PubMed] [Google Scholar]
- 79.Nakagawa K, Perez EC, Oh J, Santos F, Geldyyev A, Gross ML, Schaefer F, Schmitt CP. Cinacalcet does not affect longitudinal growth but increases body weight gain in experimental uraemia. Nephrol Dial Transplant. 2008;23:2761–2767. doi: 10.1093/ndt/gfn143. [DOI] [PubMed] [Google Scholar]
- 80.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5:110–116. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, Moustafa M, Goodman WG, Lopez N, Downey G, Dehmel B, Floege J. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- 82.Effect of Cinacalcet on Cardiovascular Disease in Patients Undergoing Dialysis. N Engl J Med. 2012 doi: 10.1056/NEJMoa1205624. DOI: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen-Yamamoto L, Bolivar I, Strugnell SA, Goltzman D. Comparison of Active Vitamin D Compounds and a Calcimimetic in Mineral Homeostasis. J Am Soc Nephrol. 2010;21:1713–1723. doi: 10.1681/ASN.2009050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malluche HH, Monier-Faugere MC, Wang G, Fraza OJ, Charytan C, Coburn JW, Coyne DW, Kaplan MR, Baker N, McCary LC, Turner SA, Goodman WG. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008;69:269–278. doi: 10.5414/cnp69269. [DOI] [PubMed] [Google Scholar]
- 85.Platt C, Inward C, McGraw M, Dudley J, Tizard J, Burren C, Saleem MA. Middle-term use of Cinacalcet in paediatric dialysis patients. Pediatr Nephrol. 2010;25:143–148. doi: 10.1007/s00467-009-1294-7. [DOI] [PubMed] [Google Scholar]
- 86.Padhi D, Langman CB, Fathallah-Shaykh S, Warady BA, Salusky IB, Lee E, Wang C, Posvar E. An open-label study to evaluate a single-dose of cinacalcet in pediatric dialysis subjects. Pediatr Nephrol. 2012;17:1953–1959. doi: 10.1007/s00467-012-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bacchetta J, Plotton I, Ranchin B, Vial T, Nicolino M, Morel Y, Cochat P. Precocious puberty and unlicensed paediatric drugs for severe hyperparathyroidism. Nephrol Dial Transplant. 2009;24:2595–2598. doi: 10.1093/ndt/gfp211. [DOI] [PubMed] [Google Scholar]