Abstract
Perihypothalamic thyroid hormone signaling features prominently in the seasonal control of reproductive physiology. Triiodothyronine (T3) signaling stimulates gonadal development, and decrements in T3 signaling are associated with gonadal regression. Type 3 iodothyronine deiodinase (DIO3) converts the prohormone thyroxine (T4) into biologically inactive 3,3′,5′-triiodothyronine, and in long-day breeding Siberian hamsters exposure to long (LD) and short (SD) photoperiods, respectively, inhibit and stimulate hypothalamic dio3 mRNA expression. Reproductive responses to intermediate-duration photoperiods (IntD) occur in a history-dependent manner; IntDs are interpreted as inhibitory only when preceded by longer photoperiods. Because dio3 expression has only been evaluated under LD or SD photoperiods, it is not known whether hypothalamic dio3 encodes absolute photoperiod duration or the reproductive interpretation of photoperiod. Male Siberian hamsters with and without a prior history of LD were exposed to IntD photoperiods, and hypothalamic dio3 mRNA expression was measured 6 wk later. Hamsters with a LD photoperiod history exhibited gonadal regression in IntD and a marked upregulation of hypothalamic dio3 expression, whereas in hamsters without prior exposure to LD, gonadal responses to IntD were absent, and dio3 expression remained low. Patterns of deiodinase expression in hamsters maintained in chronic IntD photoperiods did not appear to reflect feedback effects of gonadal status. Hypothalamic expression of dio3 does not exclusively reflect ambient photoperiod, but rather the context-dependent reproductive interpretation of photoperiod. Neuroendocrine mechanisms that compare current and prior photoperiods, which permit detection of directional changes in day length, occur either upstream, or at the level, of hypothalamic dio3 expression.
Keywords: photoperiod, Siberian hamster, dio3, dio2, seasonality, reproduction
many temperate zone species use seasonal changes in day length as a cue to predict environmental conditions, allowing initiation and termination of reproductive behavior, such that offspring are born into favorable energetic conditions (6, 14, 44). In mammals, changes in day length (photoperiod) are translated into a neuroendocrine signal via nocturnal pineal melatonin secretion (2, 22, 23, 40). In long-day breeding Siberian hamsters (Phodopus sungorus), short day lengths (<12.5 h light/day) and the accompanying longer duration of nocturnal melatonin secretion predict winter and are sufficient to induce gonadal regression (14).
An emerging body of research suggests that thyroid hormone signaling figures prominently in photoperiodic signal transduction in birds (29, 46, 48, 50, 52) and mammals (1, 13, 19, 28, 31, 38, 46). Circulating thyroxine (T4) and triiodothyronine (T3) concentrations do not exhibit conspicuous changes in response to manipulations of photoperiod (3, 36), but the expression of the selenoproteins, iodothyronine deiodinase type II (DIO2) and type III (DIO3), in periventricular regions of the mediobasal hypothalamus changes markedly following changes in day length. DIO2 catabolizes the prohormone T4 into biologically active T3, whereas DIO3 converts T4 into the biologically inactive 3,3′,5′-triiodothyronine T3 (rT3). DIO3 also catabolizes T3 into T2; thus, DIO3 expression affords a potent mechanism to quench local T3 availability and diminish T3 signaling (25, 50). In adult and juvenile Siberian hamsters, exposure to short days (SDs) increases hypothalamic dio3 mRNA within 2 wk and 3 days, respectively (1, 38), and melatonin secretion is both necessary (1) and sufficient (38) for dio3 responses to photoperiod. Exogenous T3 treatments block reproductive responses to short days in Siberian hamsters (1, 13), and in Japanese quail, hypothalamic T3 treatments trigger retraction of glial processes, which otherwise ensheath the GnRH neurosecretory terminals (49), thus permitting increases in LH secretion (29, 52). Hypothalamic expression of dio2 mRNA, in contrast, is not consistently affected by photoperiod or melatonin in Siberian hamsters [(1, 38) but see Refs. 20 and 46]. In summary, DIO2- and DIO3-driven modulation of perihypothalamic T3 availability may provide a key molecular step in the control of seasonal reproductive transitions (51).
At present, several lines of evidence suggest DIO3 dominates the photoperiodic regulation of hypothalamic T4 catabolism in Siberian hamsters. Correlative measures point to close associations between elevated dio3 mRNA expression and reproductive inhibition. Nevertheless, absent direct manipulations of dio3 expression or activity, a causal role for dio3 in reproductive photoperiodism remains to be established. Convergent evidence is critical in this regard, but several issues remain unresolved regarding the role of hypothalamic dio3 expression in the mediation of reproductive photoperiodism.
First, it remains unknown whether upregulation of dio3 mRNA expression in short days causes reproductive inhibition in hamsters, or merely reflects a consequence of gonadal regression (e.g., as a result of decreases in gonadal hormone secretion). In adult hamsters, increases in dio3 emerge gradually in SD, coincident with and inversely paralleling gonadal regression (1). Ovariectomy decreases thyroid weight (18), and gonadectomy suppresses serum T4 in male and female rats (24), suggesting that gonadal hormone secretion is capable of modulating the HPG axis. Establishing independence of dio3 from gonadal status is essential to exclude the hypothesis that hypothalamic dio3 mRNA expression merely reflects a consequence of gonadal hormone secretion.
A separate but related issue is the nature of the photoperiod information represented by hypothalamic dio3 mRNA expression. To date, dio3 responses to photoperiod have only been evaluated under categorically long or short day lengths, which are unlikely to be the relevant environmental triggers for photoperiodic seasonal changes in reproductive physiology in nature (34). Rather, intermediate-duration photoperiods (12.5–14 h light/day) occurring during late summer initiate somatic and gonadal regression, such that the ''short-day'' phenotype is achieved many weeks in advance of the actual appearance of very short days (e.g., 8L; 15, 16). Because intermediate day lengths occur twice per year, presaging very different environmental conditions, reproductive responses to intermediate photoperiods are dependent on an individual's photoperiod history (8, 21, 43). Intermediate day lengths (e.g., 13.5L) are interpreted as relatively short (and induce gonadal regression) if preceded by ≥2 wk of longer (15L) day lengths (36).
Photoperiod history-dependent responses to intermediate day lengths provide a useful model to assess the role of DIO3 in the CNS representation of melatonin-mediated seasonal time information. Hypothalamic dio3 expression may faithfully reflect absolute day length (hypothesis 1); alternatively, dio3 expression may reflect the interpretation of day length information (hypothesis 2). Hamsters with a long-day photoperiod history exhibit gonadal regression in 13.5L, whereas those never exposed to longer days maintain fully developed gonads (35, 36). If hypothesis 1 is true, then dio3 expression should be comparable in all hamsters exposed to 13.5L, regardless of photoperiod history, and independent of their gonadal responses. This outcome would challenge the primacy of dio3 signaling in the processing of DL information by the reproductive system. Alternatively, if hypothesis 2 is true, then dio3 expression should be greater in hamsters exhibiting photoperiod history-induced gonadal regression in 13.5L (i.e., interpreting intermediate day lengths as a shorter day) relative to hamsters in 13.5L without a photoperiod history. This outcome would be consistent with the emerging role of dio3 as a critical mediator of seasonal time in the CNS and would provide an important and novel insight into the nature of the information represented by hypothalamic dio3 expression.
METHODS
Animals.
Siberian hamsters (Phodopus sungorus) were derived from a long-day photoperiod (15L:9D; lights off at 1800 CST; “LD”) breeding colony maintained at the University of Chicago. Hamsters were housed in polypropylene cages, with food (Harlan Teklad, Madison, WI) and filtered tap water provided ad libitum; cotton nesting material was also available in the cage. Ambient temperature and relative humidity were held constant at 19 ± 2°C and 53 ± 10%, respectively. All procedures conformed to the U.S. Department of Agriculture Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Long and intermediate photoperiod breeding colony.
At 3–5 mo of age, male and female hamsters from the LD colony were transferred to an intermediate-duration photoperiod (13.5L:10.5D; lights off at 1800 CST; “IntD”) and housed as breeding pairs (compare Ref. 32). At the same time, additional males and females from the LD colony were paired but remained in LD. Offspring from these LD and IntD breeding colonies were weaned at 18 days of age and reared in their natal day length until photoperiod manipulations began as described below.
Photoperiod manipulations and photoperiod history induction.
On week 0, adult (2–4 mo of age) male hamsters from the LD (n = 40) and IntD (n = 24) colonies were anesthetized with 3% methoxyflurane vapors, and reproductive status was assessed by measurement of the length and width of the left testis with analog calipers (± 0.1 mm). The product of testis width squared times testis length provides a measure of estimated testis volume (ETV) that is highly correlated (R>0.9) with testis weight and reproductive function (16). Hamsters that exhibited complete gonadal development in LD or IntD (ETV>500) were designated as “LD+” (n = 36) and “IntD+” (n = 16) hamsters, indicative of a reproductively stimulated phenotype in their respective photoperiods (32). IntD hamsters with week 0 ETV < 500 were designated as “IntD-” (n = 8). On week 0, LD+ hamsters were transferred into an intermediate photoperiod (LD-IntD group, n = 9) or remained in LD (LD-LD group, n = 6). IntD+ hamsters remained housed in IntD (IntD-IntD group, n = 16). In addition, a control group of LD+ hamsters was transferred to a 12L:12D photoperiod (LD-SD group, n = 21) on week 0. LD-SD hamsters were identified as reproductively nonresponsive to short photoperiods (37) based on testis weights at autopsy on week 6: hamsters with paired testis weights >400 mg (= 4 SD below the LD-LD mean) were designated as nonresponders and were removed from the main analysis (n = 7). Photoperiod history-dependent responses to IntD wane after 10–15 wk (36); thus, the principal data in this study were collected after 6 wk of final photoperiod treatments to capture the peak of the reproductive response to photoperiod.
Tissue collection.
After 6 wk of the respective photoperiod treatments, hamsters were euthanized via rapid decapitation at the midpoint of the light phase, and the whole hypothalamus was rapidly extracted, frozen in powdered dry ice, and stored at −70°C until RNA was extracted (see RNA extraction and qRT-PCR). The anatomical boundaries for hypothalamus dissection were the optic chiasm at the anterior border, the mammillary bodies at the posterior border, and laterally at the hypothalamic sulci. Extracted tissue was cut dorsally 3–4 mm from the ventral surface. Testes and seminal vesicles were dissected at autopsy and weighed.
Locomotor activity monitoring.
Locomotor activity was recorded in the home cage continuously from week 4 until week 6 using passive infrared motion detectors (Quest PIR, Electronics Line UK, Redditch, UK) positioned 22 cm above the cage floor. Activity triggered closure of an electronic relay, recorded by a computer running ClockLab software (Actimetrics, Evanston, IL; Dr. David Ferster, Northwestern University). Cumulative activity counts were collected at 6-min intervals.
Activity analyses.
Daily activity onsets and offsets were determined automatically by ClockLab software, which defines an onset/offset as the time at which there is a maximum difference between the preceding and following 6-h windows in the number of bins in which the number of counts exceeds 20% of all nonzero bins. In a minority of cases, daily onsets were not identifiable automatically and were instead identified through visual inspection of the actograms by an experimenter who was blind to treatment groups. The duration of nocturnal locomotor activity (α) was calculated on a daily basis as the time elapsed between activity onset and offset (10); mean α over the last 10 days of the experiment (week 4.5 through week 6) provided a measure of entrainment of the locomotor activity rhythm.
RNA extraction and qRT-PCR.
Total RNA was isolated using RNeasy (Qiagen, Germantown, MD), and a cDNA library was constructed using MMLV RT enzyme (Invitrogen, Carlsbad, CA). qPCR was conducted on 1 μl of hypothalamic cDNA. Primers and probes were synthesized using PrimerExpress software, with probes labeled with 6-FAM and MGB (nonfluorescent quencher) at the 5′ and 3′ ends, respectively: dio2 forward 5′-ACCACCACCTTCCTTTGCAA-3′, dio2 reverse 5′-GCGGAAGGCTGGCAGTT-3′, dio2 probe 5′-AAGCAGAGTGCCCAGGA-3′; dio3 forward 5′-GTGCATCCGCAAGCATTTC-3′, dio3 reverse 5′-ACTTCAGGCTCGGGATGGT-3′, dio3 probe 5′-TGCGCCGTCGCCA-3′. Amplicons generated by these primers have been subjected to direct sequencing and are >90% similar to published sequences for mouse and rat dio2 and dio3 (30); dio2 and dio3 mRNA sequences have been entered in the GenBank database (dio2: EU812319; dio3: EU812320). A TaqMan 18S Ribosomal RNA primer and probe set (labeled with VIC; Applied Biosystems, Foster City, CA) was used as the control gene for quantification. Amplification was performed on an ABI 7900HT Sequencing Detection System using Taqman Universal PCR Master Mix. The universal two-step qRT-PCR cycling conditions used were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative gene expression of individual samples run in duplicate was calculated by comparison to a standard curve consisting of serial dilutions of pooled P. sungorus hypothalamic cDNA (1:1, 1:10, 1:100, 1:1,000, 1:10,000) followed by normalization to 18S rRNA gene expression.
Statistics.
Organ mass, activity, and gene expression data were evaluated by ANOVA and post hoc pairwise comparisons were performed conducted using two-tailed t-tests where permitted by a significant F statistic; differences were considered significant if P < 0.05. Linear regression analyses were used to evaluate relations between gonad and seminal vesicle weights and hypothalamic gene expression (StatView 5.0, SAS Institute, Cary, NC).
RESULTS
Reproductive measures.
LD-SD hamsters that failed to exhibit gonadal regression in SD were identified as short-day nonresponders (SD-NR; n = 7), and data from these animals were analyzed separately from those of LD-SD hamsters. Photoperiod treatments significantly affected testis and seminal vesicle sizes (testes: F = 41.9, P < 0.001; seminal vesicles: F = 12.7, P < 0.001; Fig. 1A). On week 6, hamsters housed in LD had significantly larger testes relative to hamsters housed in SD (LD-LD vs. LD-SD: P < 0.001). In IntD, hamsters previously housed in LD (LD-IntD) exhibited significant gonadal regression (LD-IntD vs. LD-LD: P < 0.001; LD-IntD vs. IntD-IntD: P < 0.005), whereas IntD hamsters without prior LD exposure (IntD-IntD) did not (IntD-IntD vs. LD-LD: P > 0.60; Fig. 1B). A similar pattern of differences was observed in measures of seminal vesicle weight (IntD-IntD vs. LD-LD: P > 0.70; all other comparisons: P < 0.01; Fig. 1B). Testis and seminal vesicle weights of SD-NR hamsters were substantially larger than those of LD-SD hamsters (P < 0.001, both comparisons), but also significantly smaller than those of LD-LD hamsters (P < 0.05, both comparisons; Fig. 1, A and B).
Fig. 1.
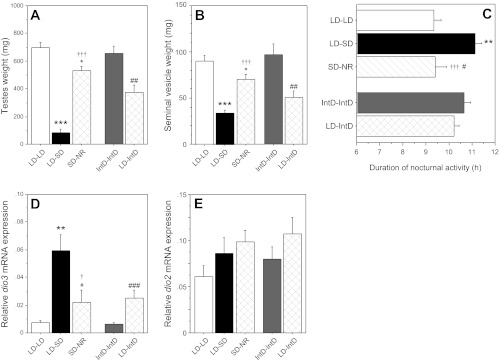
Values are expressed as means ± SE. A: paired testis weights. B: paired seminal vesicle weights. C: duration of nocturnal locomotor activity. Hypothalamic (D) dio3 and (E) dio2 mRNA expression of adult male Siberian hamsters after 6 wk of exposure to long- (LD), intermediate- (IntD), or short-duration (SD) photoperiods is shown. Group abbreviations indicate photoperiod treatments prior to and following experimental week 0. LD-LD (n = 6) and LD-SD (n = 14) hamsters were reared in a LD photoperiod (15L:9D) and subsequently exposed to LD and SD (12L:12D) photoperiods, respectively. LD-IntD (n = 9) and IntD-IntD (n = 16) were reared in LD and IntD photoperiods, respectively, and on week 0 were transferred to an IntD (13.5L:10.5D) photoperiod. SD-NR designates hamsters (n = 7) that failed to exhibit gonadal regression in SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. LD-LD value. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. IntD-IntD value. †P < 0.05, †††P < 0.001 vs. LD-SD value.
Nocturnal activity.
Photoperiod significantly affected the duration of nocturnal locomotor activity (F = 5.58, P < 0.005; Fig. 1C). Nocturnal α was significantly shorter in LD vs. all other photoperiods (SD: P < 0.005; IntD: P < 0.05, both comparisons) but did not differ between groups housed in IntD (LD-IntD vs. IntD-IntD: P > 0.20). Mean α of SD-NR hamsters was significantly shorter in duration than that of photoresponsive hamsters (LD-SD group) in the SD photoperiod (P < 0. 001) and did not differ from that of LD-LD hamsters (P > 0.90; Fig. 1C).
Hypothalamic gene expression.
Photoperiod treatments significantly affected hypothalamic dio3 mRNA expression (F = 11.4, P < 0.001; Fig. 1D). dio3 expression was significantly higher in LD-SD relative to LD-LD hamsters (P = 0.01). In IntD hamsters with no prior exposure to LD (IntD-IntD group), dio3 mRNA was relatively low (P < 0.001 vs. LD-SD) and did not differ from LD-LD values (P > 0.50). In contrast, dio3 expression was significantly, though not maximally, elevated in IntD hamsters previously housed in LD (LD-IntD group: P < 0.001 vs. IntD-IntD, P < 0.05 vs. LD-SD, P < 0.05 vs. LD-LD). dio3 expression among SD-NR hamsters was similar to that of LD-LD hamsters (P > 0.15) and significantly lower than that of hamsters that were reproductively responsive to SD (LD-SD group; P = 0.05; Fig. 1D). Photoperiod treatments did not significantly affect hypothalamic dio2 mRNA expression (F = 0.91, P > 0.40; Fig. 1E).
Categorical and correlative post hoc measures of gene expression in IntD hamsters.
Hamsters exhibited diverse reproductive responses to prolonged exposure to IntD. To evaluate the effect of reproductive condition on hypothalamic deiodinase expression, dio2 and dio3 expression on week 6 was compared among hamsters with small testes that were classified as IntD- on week 0, together with hamsters that were classified as IntD+ on week 0 and subsequently assigned to the IntD-IntD treatment group. Two analyses (categorical and correlational) were performed.
First, hamsters were classified as having undeveloped, moderately developed, or fully developed testes based on paired testis weights on week 6. Values <1 SD and >1 SD from the mean were classified as undeveloped (n = 5) and fully developed (n = 8), respectively; values within 1 SD of the mean were designated as moderately developed (n = 19; Fig. 2A). Reproductive classification significantly affected seminal vesicle weight (F = 60.7, P < 0.001; Fig. 2B), but hypothalamic dio3 and dio2 mRNA expression was comparable across all three reproductive categories (dio3: F = 0.40, P > 0.60, Fig. 2C; dio2: F = 0.80, P > 0.40, Fig. 2D).
Fig. 2.
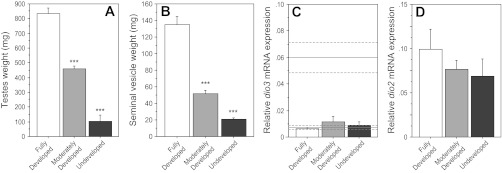
Values are expressed as means ± se. A: paired testis weights. B: paired seminal vesicle weights. Hypothalamic dio3 (C) and dio2 (D) mRNA expression of adult male Siberian hamsters gestated and reared in an intermediate-duration (IntD) photoperiod. Hamsters were classified as having undeveloped (n = 5), moderately developed (n = 19), or fully developed (n = 8) testes based on paired testis weights at autopsy (see results for criteria). C: horizontal lines indicate means ± se hypothalamic dio3 mRNA expression in LD-LD and LD-SD groups. ***P < 0.001 vs. all other groups.
Second, separate linear regression analyses were conducted using testis weight and seminal vesicle weight as predictor variables, against dio3 mRNA or dio2 mRNA as dependent variables. Neither testis weight (dio3: R2 = 0.05, P > 0.20, Fig. 3A; dio2: R2 = 0.07, P > 0.10, Fig. 3B) nor seminal vesicle weight (dio3: R2 = 0.04, P > 0.20, Fig. 3C; dio2: R2=0.08, P > 0.10, Fig. 3D) predicted hypothalamic deiodinase expression.
Fig. 3.
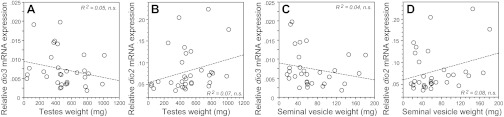
Linear regression between paired testis weight (A and B) and paired seminal vesicle weight (C and D) and hypothalamic dio3 and dio2 mRNA expression in adult male Siberian hamsters (n = 32) gestated and reared in an intermediate-duration (IntD) photoperiod.
DISCUSSION
Six weeks of exposure to SD increased hypothalamic dio3 mRNA expression and induced gonadal regression in male Siberian hamsters. This outcome is consistent with several prior reports documenting DIO3-mediated catabolism of T4 as a critical step in the inhibition of gonadotrophin signaling in long-day breeding rodents (1, 38, 46). Intermediate-duration (IntD) photoperiods elicited gonadal regression in hamsters that were previously housed in longer day lengths (LD-IntD group) but did not inhibit reproductive physiology among hamsters lacking a LD photoperiod history (IntD-IntD group). In hamsters exhibiting gonadal regression in IntD, hypothalamic dio3 expression was increased ∼4-fold relative to hamsters that retained developed testes in IntD. These data indicate that intermediate day lengths are capable of eliciting dio3 responses in the brain and do so in a manner that is entirely dependent on the individual's photoperiod history.
Changes in photoperiod regulate hypothalamic T3 concentrations via iodothyronine deiodinase-mediated T4 catabolism (51). In Siberian hamsters, short days increase dio3 mRNA within 2 wk after the initiation of photoperiod treatments (1), and this effect is abolished by pinealectomy (1). Perihypothalamic expression of dio2 mRNA, in contrast, is not consistently affected by photoperiod in Siberian hamsters (1, 20, 38, 46). In Syrian hamsters, photoperiodic regulation of hypothalamic T3 signaling is likely mediated by DIO2. Long day lengths rapidly increase ependymal layer dio2 mRNA, and melatonin treatments that mimic SDs inhibit dio2 expression (51). Taken together, the available data suggest that in Siberian hamsters dio3 expression is the major determinant of photoperiodic changes in hypothalamic T3 concentrations, whereas in Syrian hamsters, this is accomplished by dio2. The present data confirm and extend such observations. Neither LD-SD nor LD-IntD hamsters exhibited decreases in dio2 expression, despite clear inhibitory reproductive responses to the respective photoperiod treatments.
By virtue of its ability to quench T3 signaling, hypothalamic dio3 has been suggested to be a melatonin-mediated gatekeeping mechanism for seasonal changes in physiology (1); however, whether hypothalamic dio3 expression reflects absolute photoperiod (hypothesis 1), as opposed to the reproductive interpretation of photoperiod (hypothesis 2), has remained unexamined. Patterns of dio3 expression in the present report suggest the latter.
Entrainment of circadian locomotor activity served as a proxy for nocturnal melatonin secretion. Although prenatally acquired photoperiod history alters the duration of melatonin secretion under intermediate day lengths (42), marked differences in paradigms (specifically, age, prenatal/postnatal paradigm, and the magnitude of photoperiod change), preclude direct extrapolation of such data toward the present work. In a prior report, the amplitude of nocturnal melatonin was transiently higher among hamsters transferred from LD to IntD, relative to that of hamsters transferred from SD to IntD (30); however, the overall duration of elevated (>60 pg/ml) serum melatonin was comparable in both groups, consistent with abundant data indicating that the amplitude of the nocturnal melatonin signal is largely irrelevant toward the reproductive interpretation of the signal (4). In the present report, patterns of entrainment (α duration) were comparable in LD-IntD and IntD-IntD hamsters. A common circadian oscillator mediates entrainment of locomotor activity and melatonin secretion rhythms (5, 9); thus, we infer that nocturnal melatonin was likewise comparable in LD-IntD and IntD-IntD groups. But despite similar circadian responses to IntD, levels of dio3 expression were substantially higher in LD-IntD hamsters (Fig. 1D). Thus, unlike melatonin secretion, hypothalamic expression of dio3 is not a faithful reflection of ambient photoperiod, but rather, reflects the interpretation of directional change in photoperiod.
No study has directly manipulated DIO3 in a seasonal context; thus the necessity of photoperiodic changes in dio3 expression for inducing the SD reproductive phenotype has yet to be established. Likewise, absent direct administration or targeted upregulation of DIO3, it remains unknown whether hypothalamic dio3 responses to SD are sufficient to induce gonadal regression. Thus, the hypothesis that photoperiod-driven changes in dio3 occur absent changes in the reproductive axis remains unexamined. Bearing on this issue is the absence of evidence excluding changes in dio3 expression as a consequence, rather than a cause, of gonadal regression. In frogs [Silurana (Xenopus) tropicalis], liver dio2 and dio3 expression is highly responsive to manipulations of sex steroid levels during development (7), suggesting the potential for gonadal hormones to directly influence brain deiodinase expression. In the present study, not all hamsters housed in invariant IntDs exhibited full gonadal development. Heterogeneous reproductive responses to IntD afforded the opportunity to investigate, absent any changes in photoperiod, whether gonadal status predicted brain deiodinase expression. A categorical evaluation (Fig. 2) indicated that hamsters in very different reproductive states (e.g., fully developed vs. undeveloped) exhibited comparable levels of hypothalamic dio3 and dio2 mRNA expression; regression analyses (Fig. 3) likewise indicated that neither testis size, nor seminal vesicle mass (which is highly responsive to circulating testosterone concentrations; Ref. 41) predicted hypothalamic dio3 or dio2 expression. Rather, across all reproductive phenotypes in IntD, dio3 expression remained low and LD-like. Thus, whereas decreases in photoperiod are sufficient to trigger a marked upregulation of hypothalamic dio3 mRNA, photoperiod-independent (i.e., in a fixed IntD) variation in testis size alone does not incur constitutive changes in dio3 expression. These data support the conjecture that brain dio3 expression does not merely reflect effects of gonadal regression, and are consistent with a mechanism in which DIO3-initiated decreases in T3-signaling inhibit gonadotrophin activity (49).
A possible alternate interpretation of the heterogeneous gonadal responses under chronic IntD photoperiods (IntD-IntD hamsters) is that they reflect different individuals interpreting the IntD photoperiod as categorically long or short. Such an interpretation challenges the hypothesis that dio3 expression reflects the neuroendocrine interpretation of ambient photoperiod. However, we view this as unlikely to be the case. Hamsters that exhibit gonadal inhibition in IntD following transfer from LD eventually initiate gonadal regrowth, typically within ∼4 mo, indicative of a dissipation of the LD photoperiod history (16, 36). In contrast, hamsters that exhibit gonadal inhibition in IntD in the absence of a LD photoperiod history (i.e., IntD-IntD hamsters) can sustain gonadal inhibition for 6–9 mo, without any evidence of regrowth (32). Because Siberian hamsters cannot indefinitely sustain gonadal regression in inhibitory photoperiods (35), we interpret the gonadal inhibition among some IntD-IntD hamsters not as a reflection of photoperiodic reproductive responses to IntD, but rather as indicative of nonphotic modulation of the reproductive neuroendocrine system (32).
As reported previously, Siberian hamsters exhibit diverse reproductive responses to intermediate photoperiods (32), which reflect enhanced sensitivity to nonphotic (social, energetic) cues; similar effects of nonphotic cues are not evident under categorically long or short photoperiods (32, 33). The mechanisms by which nonphotic cues modulate the reproductive system under IntD photoperiods have received considerable attention. Mild (10–20%) food restriction is sufficient to inhibit kisspeptin immunoreactivity in the anteroventral paraventricular nucleus (33), consistent with the hypothesis that kisspeptin activity is an important mediator of nutritional status on the GnRH system (11). Social “crowding” sufficient to inhibit reproductive physiology in IntDs does not elevate plasma cortisol (32), indicating that nonphotic social cues are unlikely to be mediated by hypothalamus-pituitary-adrenal axis activity. Notably, under IntD photoperiods, RF-amide related peptide-3 (RFRP-3; also referred to as gonadotrophin inhibitory hormone, GnIH; Ref. 26) is markedly upregulated in the dorsomedial nucleus of the hypothalamus (33), which may constitute a mechanism by which IntD photoperiods “gate” reproductive responsiveness to nonphotic cues (45). In the present study, dio3 expression was relatively low, and LD-like, among IntD-IntD hamsters, independent of reproductive condition. Relatively low dio3 expression, and the consequent increase in local T3-signaling, may yield a perihypothalamic neuroendocrine milieu permissive for the expression of nonphotic effects on the reproductive system, which are evidently mediated via alternative pathways (e.g., RFRP-3, kisspeptin) that impinge on the GnRH system.
An additional post hoc analysis indicated that dio3 expression was low and LD-like in hamsters that were reproductively nonresponsive to SD (SD-NR). Unlike SD nonresponders in other rodent species (e.g., voles and deer mice) Siberian hamsters do not exhibit the normal expansion of nocturnal melatonin secretion following transfer to SD (27, 39). Prepineal mechanisms (likely operating at the level of circadian entrainment to photoperiod; Ref. 12) result in SD-NR hamsters exhibiting a “compressed” nocturnal active phase and a marked compression of the duration of nocturnal melatonin secretion (37). Thus, although SD-NR hamsters were exposed to a SD photoperiod, they were likely generating a LD-like nightly melatonin signal. Consistent with this supposition is the observation that nocturnal α in SD-NR hamsters was comparable in duration to that of LD-LD hamsters (Fig. 1C). The present data, which indicate that the SD-like increase in dio3 expression fails to occur in SD-NR hamsters, is consistent with recent reports documenting the pineal dependence of photoperiodic changes in dio3 expression (1, 31). In addition, these data document for the first time that hypothalamic deiodinase expression fails to respond to changes in photoperiod in reproductively nonresponsive hamsters.
Hypothalamic dio3 responses do not require categorically short photoperiods to manifest, but rather are evident under intermediate photoperiods. Previous work has exclusively used very long (>14L) or very short (<12L) photoperiods to activate dio2 and dio3 responses, respectively. Although useful as an experimental manipulation, categorically long and short photoperiods are not the environmental stimuli that trigger reproductive responses in the majority of hamsters in nature (47); rather, in nature, hamsters initiate seasonal reproductive quiescence in response to intermediate-duration day lengths (16, 34). To initiate seasonally appropriate reproductive responses, hamsters use a rudimentary form of memory (photoperiodic memory or photoperiodic history) for longer photoperiods to disambiguate late-summer and early-spring IntD photoperiods: IntD photoperiods elicit gonadal regression only if preceded by longer photoperiods (36). In the present study, hamsters with a history of exposure to LD exhibited dio3 responses to IntD, whereas those without a LD photoperiod history did not. This outcome offers a novel insight into the nature of the information represented by hypothalamic dio3 expression. If dio3 faithfully mirrored absolute day length, then photoperiod history effects (i.e., comparisons between current and antecedent photoperiods) would be required to occur downstream of dio3 signaling; however, the present data indicate that dio3 expression reflects the reproductive interpretation of the IntD photoperiod, not absolute photoperiod, which suggests that comparisons between current and prior photoperiods occur upstream, or at the level, of dio3 expression.
Perspectives and Significance
The present data afford several novel insights into how photoperiod and photoperiod history effects manifest within the reproductive neuroendocrine system. Photoperiodic regulation of iodothyronine deiodinase expression in the mediobasal hypothalamus is emerging as an important mechanism for gating local T3 concentrations and thereby altering seasonal gonadotrophic activity (1, 31, 38, 46, 50, 52). Intermediate-duration photoperiods occur twice per year and elicit different reproductive responses depending on the photoperiods that precede them. The present work indicates that intermediate-duration photoperiods engage dio3 responses in a context-dependent manner: in hamsters with a LD history, IntD photoperiods elicit dio3 expression in a manner consistent with inhibiting the conversion of T4 into T3 (1, 25). Additionally, hypothalamic dio2 and dio3 responses do not appear to merely reflect gonadal status. Lastly, the use of IntD photoperiods as experimental stimuli permits disentangling the absolute duration of photoperiod from the reproductive interpretation of photoperiod and indicate that dio3 expression encodes the latter; dio3 expression may reflect the output of a comparison between current and prior photoperiod. Together, the data are consistent with an emerging role for dio3 as a key mediator of seasonal time information in the CNS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.K.-L. and B.J.P. conception and design of research; A.K.-L. performed experiments; A.K.-L. and B.J.P. analyzed data; A.K.-L. and B.J.P. interpreted results of experiments; A.K.-L. and B.J.P. prepared figures; A.K.-L. and B.J.P. drafted manuscript; A.K.-L. and B.J.P. edited and revised manuscript; A.K.-L. and B.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Priyesh Patel, Leah Pyter, and Dr. Betty Theriault for expert technical assistance. This work was supported by Grant AI-67406 from the National Institute of Allergy and Infectious Diseases and by a seed grant from the Institute for Mind and Biology.
REFERENCES
- 1.Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, Archer ZA, Mercer JG, Morgan PJ. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology 148: 3608–3617, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bartness TJ, Goldman BD. Peak duration of serum melatonin and short-day responses in adult Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 255: R812–R822, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, O'Jile JR. Effects of thyroxine on the photoperiodic control of energy balance and reproductive status in Siberian hamsters. Physiol Behav 52: 267–270, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res 15: 161–190, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Borjigin J, Zhang LS, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol 349: 12–19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms 16: 365–380, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Duarte-Guterman P, Langlois VS, Pauli BD, Trudeau VL. Expression and T3 regulation of thyroid hormone- and sex steroid-related genes during Silurana (Xenopus) tropicalis early development. Gen Comp Endocrinol 166: 428–435, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Duncan MJ, Goldman BD, Di Pinto MN, Stetson MH. Testicular function and pelage color have different critical day lengths in the Djungarian hamster, Phodopus sungorus sungorus. Endocrinology 16: 424–430, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A 174: 469–484, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Evans JA, Elliott JA, Gorman MR. Dim nighttime illumination accelerates adjustment to time zone travel in an animal model. Curr Biol 19: R156–R157, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol 254–255: 127–132, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Freeman DA, Goldman BD. Evidence that the circadian system mediates photoperiodic nonresponsiveness in Siberian hamsters: the effect of running wheel access on photoperiodic responsiveness. J Biol Rhythms 12: 100–109, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Freeman DA, Teubner BJ, Smith CD, Prendergast BJ. Exogenous T3 mimics long day lengths in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 292: R2368–R2372, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurements. J Biol Rhythms 16: 283–301, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Gorman MR, Lee TM. Daily novel wheel running reorganizes and splits hamster circadian activity rhythms. J Biol Rhythms 16: 541–551, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gorman MR, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in day length controls annual testicular and body weight rhythms. Biol Reprod 53: 116–125, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Gorman MR, Zucker I. Pattern of change in melatonin duration determines testicular responses in Siberian hamsters, Phodopus sungorus. Biol Reprod 56: 668–673, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Grunt JA, Cunningham RD. Long-term effects of adrenalectomy and gonadectomy on thyroid function in the rat. Acta Endocrinol 48: 556–560, 1965 [DOI] [PubMed] [Google Scholar]
- 19.Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanisms signals summer in a photoperiodic mammal. Curr Biol 18: 1147–1152, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Herwig A, Petri I, Barrett P. Hypothalamic gene expression rapidly changes in response to photoperiod in juvenile Siberian hamsters (Phodopus sungorus). J Neuroendocrinol 24: 991–998, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann K, Illnerová H. Photoperiod effects in the Djungarian hamster. Rate of testicular regression and extension of pineal melatonin pattern depend on the way of change from long to short photoperiods. Neuroendocrinology 43: 317–321, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Hoffman RA, Reiter RJ. Pineal gland: influence on gonads of male hamsters. Science 148: 1609–1611, 1965 [DOI] [PubMed] [Google Scholar]
- 23.Illnerová H. The suprachiasmatic nucleus and rhythmic pineal melatonin production. In: Suprachiasmatic Nucleus: The Mind's Clock, edited by Klein DC, Moore RY, Reppert SM. New York: Oxford University Press, 1991, p. 157–176 [Google Scholar]
- 24.Jeyaraj DA, Mani Maran RR, Aruldhas MM, Govindarajulu P. Progesterone induced modulations of serum hormonal profiles in adult male and female rats. Endocr Res 27: 223–232, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid 15: 883–897, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103: 2410–2415, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margraf RR, Zlomanczuk P, Liskin LA, Lynch GR. Circadian differences in neuronal activity of the suprachiasmatic nucleus in brain slices prepared from photo-responsive and photo-non-responsive Djungarian hamsters. Brain Res 22: 42–48, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Murphy M, Jethwa PH, Warner A, Barrett P, Nilaweera KN, Brameld JM, Ebling FJ. Effects of manipulating hypothalamic triiodothyronine concentrations on seasonal body weight and torpor cycles in Siberian hamsters. Endocrinology 153: 101–112, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T. Thyrotrophin in the pars tuberalis triggers photoperiodic responses. Nature 452: 317–322, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Niklowitz P, Lerchl A, Nieschlag E. Photoperiodic responses in Djungarian hamsters (Phodopus sungorus): importance of light history for pineal and serum melatonin profiles. Biol Reprod 51: 714–724, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA 105: 18238–18242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul MJ, Galang J, Schwartz WJ, Prendergast BJ. Intermediate-duration day lengths unmask reproductive responses to nonphotic environmental cues. Am J Physiol Regul Integr Comp Physiol 296: R1613–R1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul MJ, Pyter LM, Freeman DA, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. J Neuroendocrinol 21: 1007–1014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prendergast BJ. Internalization of seasonal time. Horm Behav 48: 503–511, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Prendergast BJ, Bilbo SD, Dhabhar FS, Nelson RJ. Effects of photoperiod history on immune responses to intermediate day lengths in Siberian hamsters (Phodopus sungorus). J Neuroimmunol 149: 31–39, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Prendergast BJ, Gorman MR, Zucker I. Establishment and persistence of photoperiodic memory in hamsters. Proc Natl Acad Sci USA 97: 5586–5591, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q Rev Biol 76: 293–325, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Prendergast BJ, Pyter LM, Kampf-Lassin A, Patel PN, Stevenson TJ. Rapid induction of hypothalamic iodothyronine deiodinase expression by photoperiod and melatonin in juvenile male and female Siberian hamsters (Phodopus sungorus). Endocrinology 154: 831–841, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puchalski W, Lynch GR. Characterization of circadian function in Djungarian hamsters insensitive to short day photoperiod. J Comp Physiol A 162: 309–316, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev 1: 109–131, 1980 [DOI] [PubMed] [Google Scholar]
- 41.Schindelmeiser J, Aumüller G, Enderle-Schmitt U, Bergmann M, Hoffmann K. Photoperiodic influence on the morphology and androgen receptor level of the ventral prostate gland and seminal vesicles of the Djungarian hamsters (Phodopus sungorus). Andrologia 20: 105–113, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Shaw D, Goldman BD. Gender differences in influence of prenatal photoperiods on postnatal pineal melatonin rhythms and serum prolactin and follicle-stimulating hormone in the Siberian hamster (Phodopus sungorus). Endocrinology 136: 4237–4246, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Stetson MH, Ray SL, Creyaufmiller N, Horton TH. Maternal transfer of photoperiodic information in Siberian hamsters. II The nature of the maternal signal, time of signal transfer, and the effect of the maternal signal on peripubertal reproductive development in the absence of photoperiodic input. Biol Reprod 40: 458–465, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Stevenson TJ, Ball GF. Information theory and the neuropeptidergic regulation of seasonal reproduction in mammals and birds. Proc Biol Sci 278: 2477–2485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 153: 373–385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, Ebihara S, Yoshimura T. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am J Physiol Regul Integr Comp Physiol 292: R568–R572, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770). Symp Zool Soc Lond 57: 167–188, 1987 [Google Scholar]
- 48.Wilson FE, Reinert BD. Thyroid hormone acts centrally to programme photostimulated male American tree sparrows for vernal and autumnal components of seasonality. J Neuroendocrinol 12: 87–95, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology 145: 4264–4267, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Yasuo S, Nakao N, Ohkura S, Iigo M, Hagiwara S, Goto A, Ando H, Yamamura T, Watanabe M, Watanabe T, Oda S, Maeda K, Lincoln GA, Okamura H, Ebihara S, Yoshimura T. The reciprocal switching of two thyroid hormone-activating and inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology 146: 2551–2554, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Yasuo S, Yoshimura T. Comparative analysis of the molecular basis of photoperiodic signal transduction in vertebrates. Integr Comp Biol 49: 507–518, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426: 178–181, 2003 [DOI] [PubMed] [Google Scholar]


