Abstract
The uPAR·uPA protein-protein interaction (PPI) is involved in signaling and proteolytic events that promote tumor invasion and metastasis. A previous study had identified 4 (IPR-803) from computational screening of a commercial chemical library and shown that the compound inhibited uPAR·uPA PPI in competition biochemical assays and invasion cellular studies. Here, we synthesize 4 to evaluate in vivo pharmacokinetic (PK) and efficacy studies in a murine breast cancer metastasis model. First, we show, using fluorescence polarization and saturation transfer difference (STD) NMR, that 4 binds directly to uPAR with sub-micromolar affinity of 0.2 μM. We show that 4 blocks invasion of breast MDA-MB-231, and inhibits matrix metalloproteinase (MMP) breakdown of the extracellular matrix (ECM). Derivatives of 4 also inhibited MMP activity and blocked invasion in a concentration-dependent manner. 4 also impaired MDA-MB-231 cell adhesion and migration. Extensive in vivo PK studies in NOD-SCID mice revealed a half-life of nearly 5 hours and peak concentration of 5 μM. Similar levels of the inhibitor were detected in tumor tissue up to 10 hours. Female NSG mice inoculated with highly malignant TMD-MDA-MB-231 in their mammary fat pads showed that 4 impaired metastasis to the lungs with only four of the treated mice showing severe or marked metastasis compared to ten for the untreated mice. Compound 4 is a promising template for the development of compounds with enhanced PK parameters and greater efficacy.
INTRODUCTION
Cancer is a set of over 100 diseases that share a number of characteristics. Hanahan and Weinberg proposed several essential events as hallmarks of cancer,1 including the ability of malignant cells to invade tissue and spread to distal sites (metastasis). Studies have shown that protein interactions of the urokinase receptor (uPAR) are involved in the complex processes that promote invasion and metastasis. uPAR has been implicated in nearly every step of cancer metastasis including cell migration,2, 3 adhesion,4, 5 angiogenesis,6, 7 and invasion.5, 8–10 This has led to attempts to inhibit its protein interactions. Most efforts to date have been confined to the use of biologics consisting of either fusion proteins11–13 or peptides.14–17 Recently, we have reported the first small organic molecules that inhibit the protein interaction of uPAR with its highest affinity ligand, the urokinase-type plasminogen activator (uPA).18 11 (IPR-456) was identified using structure-based computational screening.18 A derivative of 11, namely 4 (IPR-803), emerged from a substructure search of commercial chemical libraries and showed higher potency in blocking breast cancer invasion in cellular studies. A structure-activity study provided valuable insight into the functional groups required to effectively inhibit the uPAR•uPA interaction.19 The effect of these compounds on lung cancer metastasis in cell culture-based studies was investigated in the same study. The results showed that 4 and other compounds impaired invasion, migration and adhesion in a panel of non-small cell lung cancer (NSCLC) cell lines.19
In an effort to develop uPAR antagonists with in vivo efficacy, we focus on breast cancer metastasis. We synthesize 4, for the first time, and assess it in a breast-to-lung orthotopic model. Previously, competition studies of 4 using surface plasmon resonance (SPR) established that it inhibited uPAATF binding to uPAR.18 Here, we probe the direct binding of the compound to uPAR by performing binding studies using fluorescence polarization and saturation transfer difference (STD) NMR. These studies afforded, for the first time, an equilibrium constant for binding to uPAR. Prior to performing in vivo studies, the effects of 4 on breast MDA-MB-231 invasion, migration and adhesion were explored. Extensive pharmacokinetic (PK) studies were carried out in SCID mice to generate PK parameters and measure the levels of the compound in plasma and tumor tissue. Finally, NSG mice were implanted with TMD-MDA-MB-231 (TMD-231) breast cancer cells in the mammary fat pads and dosed with 4 on a daily basis to assess its effect on breast cancer metastasis to the lungs.
MATERIALS AND METHODS
Fluorescence polarization
Varying concentrations of suPAR277 protein were titrated against intrinsically fluorescent 4 at a final concentration of 1 μM in 1 x PBS with 0.01% Triton X-100. The inhibitor-protein solution was incubated for 15 min. at room temperature. Polarized fluorescence intensities were measured using EnVision® Multilabel Plate Readers (PerkinElmer, Waltham, MA) with excitation and emission wavelengths of 531 and 595 nm, respectively.
STD NMR
To further characterize and confirm the binding of 4 to uPAR, saturation transfer difference (STD) nuclear magnetic resonance (NMR) was used.20 Selective pulses were applied at 0.8 ppm to irradiate the protein methyl groups (where no ligand peaks appear) while the off-resonance frequency was positioned at 30 ppm. Saturation was carried out with a total 2 sec pulse train composed of a repeated 50 ms gauss shaped pulse and 0.1 ms inter-pulse delay. The STD experiment of the small molecule with protein was acquired with 4,096 scans. The same experiment was collected on small molecule alone using exactly the same acquisition parameters except with 1,024 scans. To ensure that we only observed magnetization from the small molecule, the protein (uPAR) was added at a concentration of 50–100 fold less than the small molecule. 4 was dissolved in deuterated DMSO at a concentration of 50 mM; it was then diluted in phosphate buffer at a concentration of 0.7 mM for the NMR experiment. The compound was not fully soluble at 0.7 mM and thus the ratio of small molecule to protein may not be exactly 50 fold. NMR experiments were acquired at 298 K on a VNMRS 800 MHz NMR spectrometer operating at a magnetic field strength of 18.8 T and equipped with a cold probe (Agilent Technologies, Santa Clara CA). Shigemi NMR tubes were used for the NMR experiments. Water suppression was done using Varian water sculpting pulse sequence.
Cell culture
MDA-MB-231 and TMD-231 were cultured in Dulbecco’s Modified Eagle Medium (Cellgro, Manassas, VA) supplemented with 10% FBS and 1% penicillin/streptomycin in 5% CO2 atmosphere at 37°C.
Reagents
Biotinylated anti-human uPAR antibody (BAF807) was purchased from R&D Systems (Minneapolis, MN). Rabbit anti-human uPA antibody 389 was purchased from American Diagnostica Inc. (Stamford, CT).
Western blot analysis for MAPK signaling
Six well plates were coated with 30 ng μl−1 of fibronectin at 4 °C overnight. 1.5 × 106 serum starved MDA-MB-231 cells were plated onto each well in the presence of 1% DMSO (control) or 50 μM of 4 or IPR-69, a previously described positive control for 30 minutes as previously described.19 Total cell lysates were prepared in standard RIPA extraction buffer containing protease and phosphatase inhibitors (Sigma). 30 μg of protein was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane (Amersham, Arlington Heights, IL) and then blocked for 1 hour at room temperature in PBS-1% fish gelatin buffer. The membrane was immunoprobed with phospho-p44/42 MAPK (Thr202/Tyr204) rabbit monoclonal antibody (1:2000) and p44/42 MAPK mouse monoclonal antibody (1:2000) at 4°C overnight. Then, the membrane was incubated with IRDye 800-conjugated goat anti-mouse IgG (Rockland) and Alexa Fluor 680 goat anti-rabbit IgG (Invitrogen) as secondary antibodies (1:10,000). Bands were detected using Li-Cor Odyssey Imaging System (Li-Cor, Lincoln, NE).
Immunofluorescence imaging
MDA-MB-231 cells were grown on laminin- and poly-d-lysine-coated glass coverslips, exposed to 100 μM of 4 or 100 μM of 11 for 30 min at 37 °C, washed with PBS, fixed with 4% paraformaldehyde in PBS for 20 min, and permeabilized with 1% Triton X-100 in PBS for 10 min. Non-specific binding was blocked with 5% normal goat serum, 5% BSA, 0.01% Triton X-100 in PBS at RT for 1 h. The cells were incubated overnight at 4 °C with rabbit polyclonal antihuman uPA antibody 389 (dilution of 1:50 in 1% BSA/PBS). After 3-fold washing with PBS, the cells were incubated with anti-rabbit IgG conjugated with Alexa 594 Texas Red (Invitrogen Grand Island, NY), at a dilution of 1:500 in 1% BSA/PBS at RT for 1 h in the dark. Images were acquired using a Nikon swept-field confocal microscope.
Invasion assay
Invasion transwell chambers were purchased from BD biosciences (San Jose, CA).21 The undersurface of the inserts was coated with 30 ng μl−1 of fibronectin at 4 °C overnight. The filters were equilibrated with 0.5 mL of serum-free medium for 2 h at 37°C. After 4 h of serum starvation, cells were harvested and 5 × 104 cells in 500 μl medium containing 0.1% FBS and the indicated compounds or 1% DMSO control were plated onto the upper chamber of a transwell filter. 500 μL of 10% FBS medium containing the same amount of compounds or DMSO control was added to the lower chamber. After a 16 h incubation at 37°C in 5% CO2, the filters were fixed and the number of cells that had invaded was determined as described below in the migration assay.
Transwell migration assay
24-well transwell plates (Costar, Corning, NY) were coated with 30 ng μL−1 of fibronectin at 4°C overnight for migration assays as described previously.18, 19 After 4 h of serum starvation, 5 × 104 cells in 250 μL of 0.1% FBS medium containing the indicated compounds or 1% DMSO control were added to the upper chamber and incubated at 37 °C for 16 h. 500 μL of 10% FBS medium containing the same amount of compounds or 1% DMSO control was simultaneously added to the lower chamber. Non-migrated cells on the top of the transwell were scrapped off with a cotton swab, and the cells that migrated through the filter were fixed in methanol for 30 min at room temperature and stained with Hematoxylin Stain (Fisher Scientific) for 1 h at room temperature. The filters were washed with water 3 times. Filters were air-dried, and the number of migrated cells was counted in ten separate fields and averaged across two independent experiments with each concentration in triplicate.
Adhesion assay
96-well plates were coated with 15 ng μl−1 fibronectin (Sigma-Aldrich, St. Louis, Missouri) at 4 °C overnight, then blocked with 2% BSA in PBS for 1 h, as described previously.14, 18, 19, 22, 23 After starving with serum-free medium for 4 h, MDA-MB-231 cells (2.5 × 105 cells ml−1) were trypsinized and suspended in 100 μl of 0.1% FBS DMEM medium with various concentrations of compounds or DMSO control at 37°C for 90 min. Medium was then carefully suctioned out from each well. Each well was washed three times with PBS. The wells were washed, and the number of adherent cells was quantified by MTT assay at 570 nm and 630 nm. Results are representative of three independent experiments with each concentration in quadruplicate. Similar results were obtained following harvesting the cells with trypsin or 2 mM EDTA for the control compound IPR-69 (data not shown).
Cell viability assay
104 MDA-MB-231 cells were plated overnight in 100 μl/well of 96 well plates. Cells were treated with DMSO (control) or compounds at different concentrations for 3 days. Viable cells were quantified by MTT assay at absorbance of 570 nm with 630 nm for reference background as previously described.19
Gelatin zymography
MDA-MB-231 cells were treated with compounds in serum free medium for 24 h. The conditioned medium was collected and concentrated by Amicon Ultra centrifugal filter units (Millipore, #UFC 501024), and proteins were normalized and electrophoresed on 7.5% sodium dodecyl sulfate (SDS) polyacrylamide gels containing 1 mg/mL gelatin. After electrophoresis, the gel was washed twice in 2.5% Triton X-100 for 30 min at room temperature and incubated in buffer that contained 50 mM Tris-HCl (pH 7.6), 200 mM NaCl, 10 mM CaCl2, and 0.02% Brij 35 at 37 °C for 36 h. Then, the gels were stained with 0.05% Coomassie brilliant blue (CBB) and de-stained with 30% methanol in 10% acetic acid. Areas of gelatinolytic degradation appeared as transparent bands on the blue stained background of the gel. Data were quantified using Li-Cor Odyssey Imaging System (Li-Cor, Lincoln, NE).
Cloning, expression and purification of uPAR
suPAR was obtained by a one-step purification process as previously described.24
Western blot analysis
Total cell lysates were prepared in standard RIPA extraction buffer containing protease and phosphatase inhibitors (Sigma). Thirty μg of protein was separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Amersham, Arlington Heights, IL). The membranes were immunoprobed with uPAR (10G7), uPA (H-140) or Actin (C-2) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. Next, membranes were incubated with IRDye 800-conjugated goat anti-mouse IgG (Rockland) and Alexa Fluor 680 goat anti-rabbit IgG (Invitrogen) as secondary antibodies. Bands were detected using Li-Cor Odyssey Imaging System (Li-Cor, Lincoln, NE).
Apoptosis Assay
MDA-MB-231 cells were treated with indicated concentrations of 4 or 1% DMSO control for 24 hours and flow cytometry analysis was performed as described previously. 19
Tissue preparation
All lung tissues were collected and fixed in 10% neutral buffered formalin within 30 minutes of removal during surgery. The tissues were fixed overnight in neutral buffered formalin and then transferred to 70 percent ethanol prior to processing to a paraffin block. The slides are then baked overnight at 59 °C in an oven before staining with H&E.
Slide evaluation
Three investigators examined the slides by light microscopy to examine for metastatic carcinoma in the lungs. Each lung lobe was analyzed for tumor cells and large tumor metastases. The number of lung metastases were counted in each lobe, and the size of the tumor foci were scored on a scale of 0 to 3+ (0, no staining; 1, minimal: tumor foci composed of cells from 2 to 25; 2, moderate: tumor foci composed of cells from 25 to 150; 3, strong tumor foci composed of cells from 150 to 600 cells, and 4: marked: large coalescing tumor foci with 600 plus tumor cells). Each lobe was counted in its entirety.
Synthesis
All chemicals were purchased from commercially available sources and used as received. Column chromatography was carried out with silica gel (25–63μ). High-Res Mass Spectra were measured on an Agilent 6520 Accurate Mass Q-TOF instrument. 1H NMR was recorded in CDCl3 or DMSO on a Bruker 500 MHz spectrometer. RP-LCMS was carried out on a Agilent 1100 LC/MSD fitted with an Eclipse XBD-C18 (4.6 × 150 mm) column eluting at 1.0 ml/min employing a gradient of (acetonitrile:methanol):water (each containing 5 mM NH4OAc) from 70% to 100% acetonitrile:methanol over 15 min and holding at 100% acetonitrile:methanol for 2 min. Chemical shifts are reported in ppm using either residual CHCl3 or DMSO as internal references. Unless otherwise stated, the following procedures were modified or adopted from the literature.
3,5-dibromo-6H-anthra[1,9-cd]isoxazol-6-one (1)
Sodium nitrite (993 mg, 14.4 mmol) was added with stirring to conc. H2SO4 (25 mL) at 30–40°C over 10 min then stirred for an additional 30 min. Next 1-amino-2,4-dibromoanthraquinone (5.0 g, 13.1 mmol) was added over 15 min and the mixture was stirred overnight (16h) at 50–55°C. The heated solution was poured directly over ice and the resulting yellow precipitate was filtered, washed with cold water, and a 1:1 mixture of ethanol-ether. The moist anthraquinonediazonium hydrogensulfate was added to a solution of NaN3 (1.37 g, 21.0 mmol) in water (25 mL) and stirred overnight (16h). The light orange solid was filtered off and washed with water followed by a 9:1 mixture of acetone-water. The moist azide was suspended in toluene (40 mL) and heated to 70°C with stirring. Water and acetone were slowly distilled (using a Dean-Stark apparatus) over a 12 h period. The yellow-orange crystals were filtered and washed with methanol to give 3.78 g (76%) of 3,5-dibromo-6H-anthra[1,9-cd]isoxazol-6-one.25 1H NMR (500 MHz, CDCl3) δ 8.44 (d, J = 7.8 Hz, 1H-C5), 8.07 (d, J = 7.4 Hz, 1H-C8), 7.97 (s, 1H), 7.80 (t, J = 7.0 Hz, 1H-C7), 7.71 (t, J = 7.4 Hz, 1H-C6); 13CNMR (126 MHz, CDCl3) δ 179.9, 153.6, 141.8, 134.0, 131.9, 131.4, 130.2, 125.0, 123.5, 123.5, 122.8, 121.6, 115.6. HRMS calc’d for C14H5Br2NO2 (M+H)+ 379.8742, found 379.8740.
methyl 3-((3-bromo-6-oxo-6H-anthra[1,9-cd]isoxazol-5-yl)amino)benzoate (2)
To a solution of methyl 3-aminobenzoate (481 mg, 3.18 mmol) in nitrobenzene (3 mL), anhydrous AlCl3 (353 mg, 2.65 mmol) was added with vigorous stirring. After five minutes, 1 was added and the reaction mixture stirred for 3 h at ambient temperature. The reaction mixture was poured into an ice-water slurry that precipitated a reddish solid which was filtered off. The reddish solid was recrystallized from toluene to give 144 mg (61%) of 2.26 1H NMR (500 MHz, CDCl3) δ 11.40 (s, 1H-NH), 8.55 (d, J = 8 Hz, 1H-C5), 8.15 (d, J = 8 Hz, 1H-C8), 8.07-8.01 (m, 2H), 7.81 (t, J = 7.5 Hz, 1H-C7), 7.72-7.66 (m, 2H), 7.63-7.53 (m, 2H), 3.97 (s, 3H-OMe). 13CNMR (126 MHz, CDCl3) δ 181.0, 166.0, 157.9, 151.2, 148.7, 137.5, 132.7, 132.5, 132.2, 130.2, 129.3, 128.7, 128.6, 128.0, 126.5, 125.4, 123.5, 122.5, 119.9, 117.1, 101.9, 52.5. HRMS calc’d for C22H13Br N2O4 (M+H)+ 449.0135, found 449.0141.
methyl 3-((3-(azepan-1-yl)-6-oxo-6H-anthra[1,9-cd]isoxazol-5-yl)amino)benzoate (3)
Hexamethyleneimine (62 μL, 0.55 mmol) and 2 (100 mg, 0.22 mmol) were dissolved in acetonitrile (4 mL).. The reaction solution was heated to 70–80 °C. As determined by TLC the reaction was complete after 3 h afterwards the reaction mixture was cooled to ambient temperature then to −15 °C for 1 h. The reddish solid was filtered off and washed with cold acetonitrile to give 52 mg (50%) of 3.27 1H NMR (500 MHz, CDCl3) δ 11.94 (s, 1H-NH), 8.63 (d, J = 8 Hz, 1H-C5), 8.19-8.14 (m, 2H), 7.91 (d, J = 7 Hz, 1H, H para to amino group), 7.73 (t, J = 7 Hz, 1H-C7), 7.64 (t, J = 8 Hz, 1H-C6), 7.58-7.50 (m, 2H), 6.18 (s, 1H), 4.51-3.40 (vbr s, 4H), 3.94 (s, 3H-OMe), 1.91 (br s, 4H), 1.65 (br s, 4H). 13C NMR (126 MHz, CDCl3) δ 176.0, 166.3, 154.7, 153.6, 148.0, 146.7, 139.1, 133.5, 131.4, 130.6, 129.7, 127.9, 127.7, 126.2, 124.2, 121.8, 118.9, 96.2, 91.9, 52.3, 26.6, 18.9. HRMS calc’d for C28H25N3O4 (M−H)− 466.1775, found 466.1772.
3-((3-(azepan-1-yl)-6-oxo-6H-anthra[1,9-cd]isoxazol-5-yl)amino)benzoic acid (4)
3 (433 mg, 0.93 mmol) was hydrolyzed in a methanol (4 mL) and 2M aq. NaOH (2 mL) solution at 80°C with stirring overnight. The residual methanol was removed in vacuo, and the resulting residue was acidified to pH 2. The precipitate was filtered off and washed with cold water to give 4 (314 mg, 74%) as a reddish-brown solid. 1H NMR (500 MHz, DMSO) δ 11.79 (s, 1H-NH), 8.44 (app d, J = 8 Hz, 1H-C5), 8.13 (app d, J = 8 Hz, 1H-C8), 8.01 (s, 1H), 7.84-7.78 (m, 2H), 7.72-7.67 (m, 2H), 7.64-7.59 (m, 1H), 6.12 (s, 1H), 4.51-3.97 (br s, 4H), 1.80 (br s, 4H), 1.55 (br s, 4H); 13CNMR (126 MHz, DMSO) δ 174.7, 166.8, 153.6, 152.8, 147.6, 146.0, 138.5, 132.9, 132.4, 131.2, 130.2, 128.3, 127.5, 127.4, 126.0, 123.4, 121.8, 118.5, 99.5, 95.0, 91.7, 56.0, 26.0, 18.6. HRMS calc’d for C27H22N3O4 (M−H)− 452.1610, found 452.1601.
RESULTS
Synthesis of 4
Despite its availability in a commercial database, the synthesis of 4 has not been reported. To enable cellular and in vivo studies of the compound, we conceived a synthesis route that successfully led to the preparation of the compound in gram-scale (Scheme 1). It consisted of first preparing 3,5-dibromo-6H-anthra[1,9-cd]isoxazol-6-one by the diazotation of 1-amino-2,4-dibromoanthraquinone. This was accomplished by adding sodium nitrite to a solution of the amine in concentrated sulfuric acid to give the 1-anthraquinonediazonium hydrogen sulfate, which was subsequently converted to the 1-azidoanthraquinone by reacting with an aqueous solution of sodium azide. The azidoanthraquinone was azeotropically refluxed in toluene to provide, by evolution of nitrogen, the required isoxazole ring in 1.25 Arylamination of 1 at the 5-position was accomplished with anhydrous AlCl3 in nitrobenzene at ambient temperature to give a moderate yield of the 6-arylamino substituted.26 This occurs by an increase in electrophilicity at the 4-bromo position via conjugation with the keto group after its complexation with AlCl3. A second amination was carried out at the 3-position using refluxing acetonitrile to give a moderate yield of 3.27 Next, hydrolysis of the benzoate ester 3 to the carboxylic acid 4 occurred under conditions of heating an aqueous solution of sodium hydroxide and methanol.
Scheme 1. Reagents and Conditions.
a) NaNO2/H2SO4, NaN3, toluene, reflux; b) AlCl3, nitrobenzene, r.t.; c) acetonitrile, 80°C; d) aq. NaOH/MeOH 80°C; e) NaH, THF, r.t
Direct binding and inhibition studies
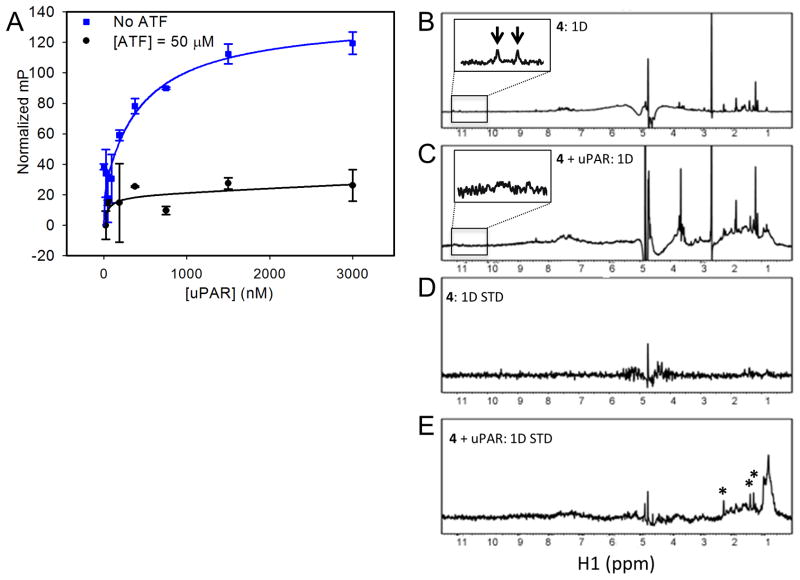
In a previous study, we had reported the use of SPR to show that 4 inhibited uPA binding to uPAR in a concentration-dependent manner.18 Here, we sought to provide evidence that 4 inhibited the protein interaction by direct binding to uPAR. We also use this opportunity to determine a binding constant for the compound. The red-shifted fluorescence of 4 was exploited to measure its direct binding to uPAR using fluorescence polarization (FP).18 When exposed to a larger binding partner, a small molecule is expected to adopt a slower tumbling rate leading to an increase in light polarization. Increasing the concentration of uPAR in the presence of 4 at a fixed concentration of 1 μM led to a corresponding increase in fluorescence polarization (Fig. 1A). This confirmed the direct binding of 4 to uPAR. A fit of the data resulted in a dissociation constant KD of 0.19 μM. When the titration of 4 with uPAR was repeated in the presence of excess uPAATF at 50 μM concentration, no increase in FP was detected (Fig. 1A). This suggests that 4 and uPAATF compete for the same site on the receptor.
Figure 1. Biochemical characterization of 4.
(A) Inhibition of uPAR•uPAATF by 4 using a microtiter-based ELISA; (B–E) STD NMR experiments were used to probe direct binding of 4 to uPAR; (B) 1D NMR spectrum of 4 only, the NH peaks of 4 highlighted by arrows in the inset, which shows region inside the box magnified, are broadened in the presence of uPAR as shown in the inset of the spectrum below; (C) 1D NMR spectrum of 4 in the presence of uPAR; (D) STD NMR spectrum of 4 only showing no ligand peaks arising; (E) STD NMR spectrum of 4 in the presence of uPAR where peaks highlighted with asterisks represent peaks arising from binding of 4 to uPAR. Saturation of the protein peaks was set at 0.8 ppm.
To further confirm the binding of 4 to uPAR and gain additional insight into the recognition process, we resorted to the use of saturation transfer difference (STD) NMR. STD NMR is an entirely ligand-based method, such that measurement of the binding of a small molecule to a protein is done by monitoring the transfer of magnetization by fast chemical exchange. First, a standard 1D spectrum of 4 was collected alone (Fig. 1B) and in the presence of uPAR (Fig. 1C). Comparison of the two spectra reveals that two NH peaks for the compound alone (inset of Fig. 1B). These peaks disappear when uPAR is added (inset of Fig. 1C). This observation is evidence that 4 binds to uPAR. STD spectra for 4 alone (Fig. 1D) and in the presence of uPAR (Fig. 1E) were subsequently collected. In the presence of uPAR, there are distinctive peaks that are attributed to 4 (Fig. 1E). These peaks occur only when spin diffusion signal (originating from the protein) is transferred to the small molecule. This indicates that the small molecule is binding. In addition, comparison of Fig. 1D and 1E shows distinct peaks for 4, further confirming binding of the compound to uPAR.
Effect of 4 on uPAR•uPA binding in cells
Immunofluorescence confocal microscopy was used to probe the effect of 4 on the uPAR•uPA interaction at the cell surface of breast MDA-MB-231 cancer cells. Endogenous uPA in a breast cancer cell line MDA-MB-231 was immunostained with a selective antibody and subsequently visualized by staining with a fluorescently conjugated secondary antibody. Representative deconvolved confocal immunofluorescence images are presented in Fig. 2A and Fig. 2B. To get a quantitative estimate of the effect of 4 on the uPA level, we quantified its surface staining intensity (Fig. 2C). The pixel immunodensities for uPA were determined using Nikon elements software. In the absence of any compound (DMSO; vehicle control), uPA was detected in intracellular clusters as well as on surface membranes of the MDA-MB-231 cells (Fig. 2A, arrows). In the presence of 4, added 30 min prior to immunostaining, there was a significant reduction of uPA staining at the cell surface (Fig. 2C). Surface staining was reduced by ~95% and ~80% at 100 μM of 4 and at 100 μM of 11, respectively. These results suggest that 4 can effectively prevent uPA from binding to uPAR, supporting the findings from our biochemical assays that 4 acts as a direct inhibitor of the PPI.
Figure 2. Immunofluorescence imaging to probe inhibition in cells.
Representative deconvolved confocal immunofluorescence images of MDA-MB-231 cells incubated with DMSO (control, A) or 100 μM of 4 (B) and visualized by staining with a polyclonal antibody against uPA (red). Arrows indicate examples of two cells with clear surface staining of uPA; (C) Quantification of the surface expression levels of uPA in MDA-MB-231 cells exposed to the indicated conditions. 100 μM of the previously reported 11 was used a positive control.18, 19 The pixel immunodensity of the surface uPA compared to the total staining of uPA was calculated. Only cells where the cell membrane was clearly discernible were included in the analyses. Each value represents the mean ± SE from 17 to 38 cells taken from six different fields from two independent samples.
Effect of 4 on invasion, migration and adhesion of MDA-MB-231 cells
Previously, we had shown that siRNA knockdown of uPAR in MDA-MB-231 cells resulted in more than 60 percent impairment of cell invasion.18 We had also shown that 4, which was purchased from a commercial library, inhibited MDA-MB-231 invasion using the same Boyden chamber apparatus. Here, we confirm that our synthesized compound showed similar inhibition of invasion as the purchased compound reported earlier (Fig. S1).18 To gain further insight into the mechanism by which these compounds inhibit invasion, we study the effect of the compound on matrix metalloproteinase (MMP) activity using gelatin zymography. MMPs are well-known proteases that degrade components of the ECM, such as collagen, laminin, fibronectin and vitronectin. Activation of MMPs is promoted by uPA binding to uPAR. We found that 4 effectively inhibited matrix metalloproteinase (MMP-9) mediated degradation of gelatin in a concentration-dependent manner (Fig. 3A). At 25 μM nearly 40 percent inhibition is achieved. Cell viability studies using MTT were carried out on 4 to evaluate its effects on MDA-MB-231 cell growth. A concentration-dependent curve was constructed over a period of 3 days. It resulted in an IC50 of 58 μM for 4 (Fig. 3B). Since the invasion studies are carried out over a significantly shorter time, and given the more than 90 percent blockage of invasion that is observed for 4 at 50 μM, it is therefore expected that most of the inhibition of cell invasion is unlikely due to cytotoxicity of the compound.
Figure 3. Invasion, migration and adhesion studies.
(A) Matrix metalloproteinase (MMP-9) activity using gelatin zymography. Results are representative of three independent experiments; (B) Effect of 4 on MDA-MB-231 cell viability as assessed by MTT assay; (C) Cell migration studies with the Boyden chamber apparatus to characterize the role of 4 in chemotaxis-mediated cell migration; Error bars represent means +/− S.D. Representative experimental cells from control and in the presence of 4 were photographed (x 400) to illustrate the effect of 4 on migration, as shown below; (D) Effect of 4 on MDA-MB-231 cell attachment to fibronectin; Error bars represent means +/− S.D. IPR-69 was used as a positive control.19
For cells to effectively metastasize, they must acquire additional phenotypes, such as adhesion and migration. The Boyden chamber apparatus without the Matrigel layer can be used to assess the effect of 4 on cell migration (Fig. 3C). At 40 μM, nearly 30 percent inhibition of MDA-MB-231 migration is observed, and at 80 μM nearly 70 percent inhibition is measured. The effect of 4 on adhesion was studied using MDA-MB-231 cells that were added to wells pre-coated with fibronectin as we have done previously.18 In the presence of 4, a concentration-dependent impairment of cell adhesion is observed with an IC50 of approximately 30 μM (Fig. 3D). The effect on adhesion, albeit weaker than those observed in invasion, may be attributed to the effect of the compound on other interactions mediated by uPAR.
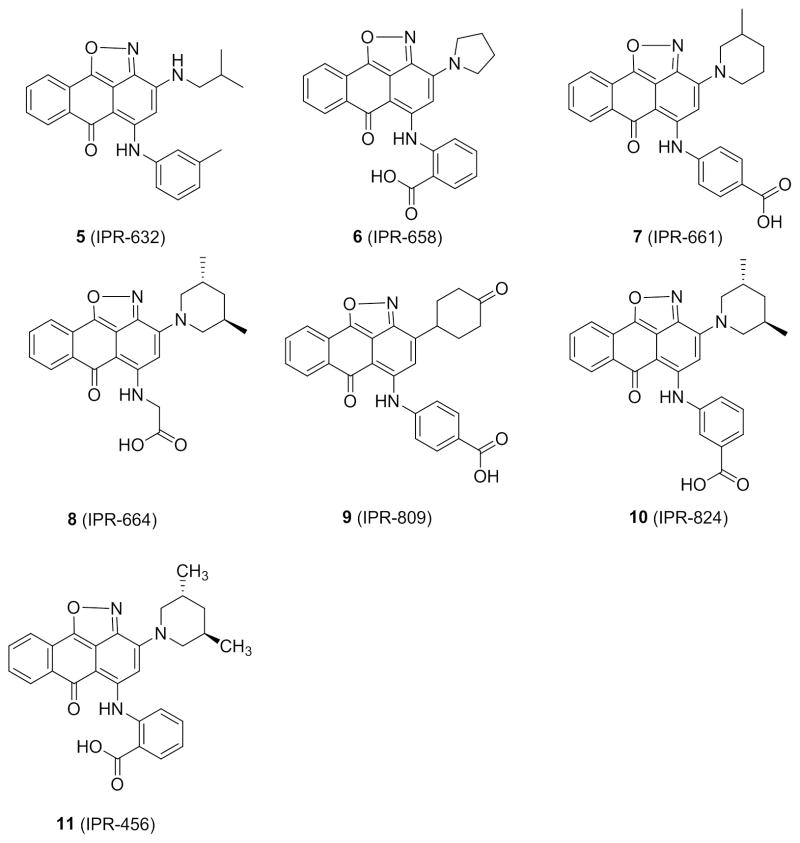
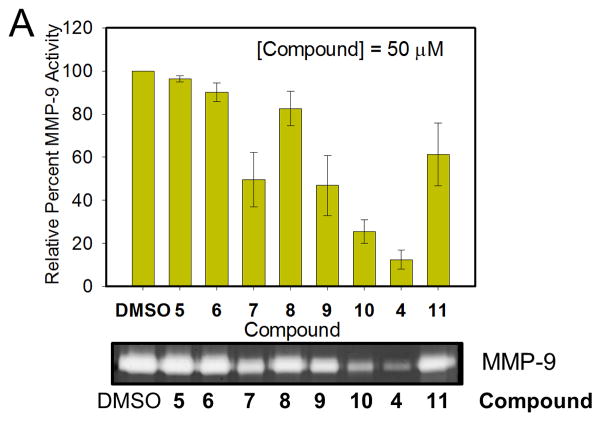
A limited structure-activity study is conducted on six derivatives of 4 (Scheme 2). Their effect on ECM degradation was determined with gelatin zymography (Fig. 4). The binding affinity of these compounds to uPAR was recently assessed in a comprehensive structure-activity (SAR) study.28 A range of anti-invasion activity was observed for the compounds. At a concentration of 50 μM, 4 showed highest potency with nearly 90% inhibition of gelatinase activity. The compound was more active that the parent 11, which showed about 40% inhibition. 5 (IPR-632) revealed no inhibition, consistent with findings from a previous study that the compound did not inhibit the tight uPAR•uPA interaction.28 6 (IPR-658) and 8 (IPR-664) inhibit the uPAR•uPA more weakly,28 consistent with their reduced efficacy in blocking ECM degradation (Fig. 4). 10 (IPR-824) is more potent than 7 (IPR-661) consistent with our previous observation that m-carboxylate is more effective than p-carboxylate in blocking binding of uPA to uPAR. Compound 9 (IPR-809) also inhibited gelatinase activity despite its significantly weaker inhibition of uPAR•uPA.28 We had previously argued that the combination of a para-carboxylate on the benzoic acid moiety with a piperidinone may have contributed to its weaker activity and potential off-target effects. Invasion studies using the Boyden chamber apparatus confirmed the gelatin zymography results. For example, compound 7 inhibited MDA-MB-231 cell invasion in a concentration-dependent manner with an IC50 of approximately 30 μM (Fig. S2A). Cell viability studies using MTT over a period of three days revealed an IC50 of 33 μM for 7 (Fig. S2B).
Scheme 2.
Structure of derivatives of 4.
Figure 4. Invasion and cytotoxicity studies of 4 and its derivatives.
(A) Gelatin zymogram (below) showing the effects of 4 derivatives at 50 μM concentration on matrix metalloproteinase (MMP-9) activity as quantified above. Error bars represent means +/− S.D.
To determine whether 4 causes apoptosis, a flow cytometry analysis was performed with Annexin V-FITC and PI staining. The level of apoptosis and necrosis in MDA-MB-231 cells was assessed after exposure to increasing concentrations of 4 for 24 hours as a percentage of Annexin V-positive/PI-negative cells (apoptotic) and Annexin V-positive/PI-positive cells (necrotic) respectively (Fig. S3). Control DMSO treated cells showed 3 percent apoptotic and 6 percent necrotic cells (Fig. S3A). At concentrations of 1 (Fig. S3B), 10 (Fig. S3C), 25 (Fig. S3D) and 50 μM (Fig. S3E), 4 showed 3, 2, 2 and 0 percent apoptotic cells and 7, 5, 6 and 3 percent necrotic cells, respectively. These results indicate that 4 does not have a significant effect on apoptosis or necrosis.
We also tested the effects of 4 on MAPK signaling following plating of serum starved cells onto fibronectin-coated culture plates for 30 minutes in the presence of 1% DMSO control, 50 μM of 4 or 50 μM of IPR-69, a previously described compound that was used as a positive control. Western immunoblotting shows that as compared to DMSO control, IPR-69 significantly impaired MAPK phosphorylation as previously shown.19 4 also showed inhibition of MAPK phosphorylation, as compared to DMSO control but the effect was weaker than that IPR-69 (Fig. S4).
In vivo PK studies
Compound 4 was administered to mice via a single PO delivery at 200 mg/kg using a formulation of saline. Blood (20 μl) was taken from the mice at their tails at 1, 2, 4, 6, 8 and 10 hours (three time points from each of the 18 mice29) post injection. Plasma was separated from blood and 4 was quantified by internal standardization, protein precipitation, and HPLC-MS/MS (Fig. 5A). The lower limit of quantification was 100 ng/mL using 20 μL of plasma. The compound was detected in plasma reaching a maximum concentration (Cmax) of 5 μM after 1 hour (tmax) (Fig. 5C). The stability of the compound in plasma is reflected by its half-life (t1/2) that was estimated at 5 hours. To gain insight into its absorption following oral dosing, intravenous bioavailability of 4 was determined. The compound was injected intravenously (IV) into the tail vein. Blood samples were obtained at 5, 10, 20, 45 and 120 minutes (three mice per time point) post injection. Plasma was separated from blood and 4 was quantified by internal standardization, protein precipitation, and HPLC-MS/MS (Fig. 5B). Based on these data, the oral bioavailability was 4 percent. Despite the low bioavailability in plasma, HPLC-MS/MS quantification of 4 in the primary tumor at 1, 4, and 10 hour intervals detected the inhibitor at concentrations near 10 μM. The levels of the compound in tumor tissue remained high even after 10 hours.
Figure 5. In vivo PK parameters.
(A) PK analysis of 4 in female NOD/SCID mice (n = 3 per time point) dosed by oral gavage (200 mg/Kg); (B) Plasma levels of 4 as a result of intravenous injection; (C) PK parameters.
Effect on breast cancer metastasis
An evaluation of the efficacy of 4 on metatsasis was conducted in vivo using an orthotopic model that we have previously implemented.19 TMD-231 cells were inoculated into the mammary fat pads of female NSG mice. Like MDA-MB-231, TMD-231 overexpress uPAR as shown by immublotting analysis in Fig. 6A. Dosing was initiated at day 15 post implantation. Animals were randomized and treated with vehicle or with 4 by daily oral gavage at a dose of 200 mg/kg (n = 13). Tumor volumes were determined by caliper measurements on a twice weekly basis, and calculated according to the formula (α2 × β)/2, where α is the shorter and β is the longer of the two dimensions. The study was conducted over a period of 33 days. The primary tumor in both control and treated mice grew over the course of the study (Fig. 6B). Tumor volumes reached nearly 693 and 785 mm3 for treated and untreated mice, respectively. The small effect (~10%) on tumor size is fully consistent with previous studies that have shown that uPAR is not a lethal target. In addition, these studies further support our cellular studies that have found that 4 is not cytotoxic with IC50 of 58 μM. There was no statistical significance to the differences in body weight between treated and untreated, suggesting that the compounds may be well tolerated by the mice (Fig. 6C).
Figure 6. In vivo efficacy studies.
(A) Western immunoblot showing the expression levels of uPAR and uPA in MDA-MB-231 and TMD-231 cell lines; (B) Effect of 4 on TMD-231 tumor growth. TMD-231 cells were inoculated in the mammary fat pads of female NOD/SCID mice. Once the tumor volume reached 30 to 50 mm3, animals were randomized and treated with vehicle alone as control or 200 mg/kg of compound 4 three times a week for 5 weeks by oral gavage. Tumor volumes were determined by caliper measurements obtained weekly; (C) Body weight change upon completion of the study after 33 days; (D) Representative H&E staining images that illustrate metastasis in the lungs of animals; (E) Scoring system was used to quantify the level of metastasis in the lungs. The number of lung tumor nuclei/cells were counted in each lobe, and their size were scored on a scale of 0 to 4 (0, no staining; 1, minimal; 2, moderate; 3, strong; and 4: marked).
To assess the effect of 4 on metastasis, control and experimental animals were sacrificed and organs (lungs) were removed and evaluated for the presence of tumors. The analysis of tumor metastasis lesions showed that they ranged roughly from 2 to 25 cells, 25 to 150 cells, and about 150 to 600 cells. There were a few large clusters of coalescing tumor balls seen. A few vehicle control lungs had tumor metastases that were coalescing into large tumor balls (greater than 1000 cells). The metastases were characterized by very large bizarre cells, which had large undifferentiated nuclei with large nucleoli. These were easy to discern by routine H&E staining and count. The number and size of metastasis in two to five fields per sample were calculated. A score of 4+ was given to a sample with highest metastasis index and relative metastasis in other samples are calculated (i.e., 1+, 2+, 3+) by a sample-blinded pathologist (Fig. 6E). The extent of metastasis in treated versus untreated was significantly different. For example, 10 out of 13 untreated mice developed severe (score = 3) or marked (score = 4) metastasis to the lung. This is compared to only 4 out of 12 in the treated mice (2 severe and 2 marked). Among the treated mice, 4 developed moderate (score = 2) metastasis. Another four were scored at 1 or lower, showing mild metastasis to the lungs. Representative H&E staining images for control and treated mice are shown in Fig. 6D.
DISCUSSION
Recently, we reported the discovery of 11, a small molecule inhibits the tight PPI between uPAR and its ligand uPA.18 A derivative, namely 4, was also found to inhibit the PPI.28 A recent study showed that 4 inhibited invasion in a panel of non-small cell lung cancer (NSCLC) cells.28 Here, we extend on these studies and focus on probing uPAR with 4 in breast cancer metastasis and assess its efficacy in animal studies. The compound, which was previously purchased, is prepared in gram-scale to enable in vivo studies. In a departure from previous work that was confined to competition studies, direct binding studies are carried out using fluorescence polarization and STD NMR. These showed that the compound binds directly to uPAR with a sub-micromolar affinity of 0.2 μM, which is nearly an order of magnitude higher than previously-measured IC50 in competition assays. The nearly 10-fold difference between KD and IC50 is not unexpected. The binding interface of the uPAR•uPA PPI (estimated at 1,200 Å2) is significantly larger than the surface occupied by the small molecule. Hence, a higher concentration of the compound is required to shift the equilibrium towards dissociation of the PPI complex. STD NMR studies not only provided further confirmation that the compounds binds to uPAR directly, but also generated data that supports the computationally predicted binding mode of 4.
Compound 4 and its derivatives were used to probe the role of the uPAR·uPA interaction in breast cancer cell invasion and metastasis. Gelatin zymography revealed that MMP9 activity is impaired by 4, suggesting that blocking uPA binding to uPAR likely affects activation of uPA and the subsequent proteolytic cascade that has been shown to lead to MMP activation.30–32 The effects of 4 on cell adhesion (IC50 = 30 μM) and its weaker effect on migration (IC50 = 50 μM) may be attributed to inhibition of uPAR binding to integrins. It is well-known that uPAR engages integrins, which are responsible for attachment of cells to ECM components and migration.22, 33, 34 Although the integrin binding pocket is located away from the uPA binding site where 4 was targeted,33 studies have shown cooperativity between uPA and vitronectin binding.35 Extensive biophysical studies have shown that uPA binding to uPAR stabilizes the receptor and promotes binding to vitronectin.36, 37 Hence, blocking of uPA binding by 4 likely alters the dynamics of uPAR resulting in impaired vitronectin binding and therefore less interaction with integrins. However, recent FRET-based cellular studies have shown that the uPAR•integrin interaction can occur even in the absence of uPA.38 Since uPA is highly expressed in malignant cells, the effects on adhesion of our compounds could be attributed to destabilization of the uPAR-vitronectin complex. It is interesting that 4 inhibited signal transduction of the MAPK pathway, consistent with previous studies that have reported a role for uPAR in MAPK signaling.
The inhibition of the uPAR•uPA interaction and the effect of 4 on invasion prompted us to probe the compound for its effect on metastasis in vivo. When administered orally, 4 reached a concentration of 5 μM in plasma. It was encouraging that the levels of the compound in tumor tissue also reached a high concentration of up to 10 μM and was stable even after 10 hours. The accumulation of the compound in tumor ensures constant binding to uPAR and inhibition of uPA binding. The half-life of the compound in plasma is estimated at nearly 5 hours. The high levels of the compound in plasma and tumor tissue despite the low bioavailability of 4 percent suggests that small improvements in absorption could results in significant increases in plasma and tumor concentrations.
In vivo, compound 4 was tested using an orthotopic breast-to-lung metastasis model that we had previously implemented.19 TMD-231 cells are highly aggressive breast cancer cell lines derived from MDA-MB-231.39 Just like MDA-MB-231, TMD-231 cells overexpress uPAR. Analysis of the tumor growth reveals that 4 had little effect on tumor growth, in agreement with the weak cytotoxicity that was found in our proliferation assay study. The lack of cytotoxicity is consistent with the fact that uPAR is not required for normal biological function, as it is not even expressed in normal cells except during embryogenesis.40, 41 It is highly expressed during inflammation42, 43 or metastasis.44, 45 The confined effect of 4 on metastasis and the lack of cytotoxicity suggest selectivity. Compound 4 and its analogs are the first small molecules that have been identified to inhibit the uPAR•uPA PPI. These results are an excellent starting point, as 4 provides a lead molecule for the design and synthesis of derivatives with higher affinity and better PK properties.
Supplementary Material
Figure S1. Effect of 4 on MDA-MB-231 cell invasion. Boyden chamber apparatus (with Matrigel) is used to assess the effect of 4 on MDA-MB-231 invasion; Error bars represent means +/− S.D. Representative experimental cells from control and in the presence of 4 were photographed (x 200) to illustrate the effect of 4 on invasion, as shown below.
Figure S2. Effect of 7 on invasion. (A) Boyden chamber apparatus (with Matrigel) is used to assess the effect of 7 on MDA-MB-231 invasion; Error bars represent means +/− S.D. (B) Effect of 7 on MDA-MB-231 cell viability as assessed by MTT assay.
Figure S3. Flow cytometry analysis using Annexin V-FITC and PI staining. MDA-MB-231 cells were treated for 24 hours with (A) DMSO; (B) 1μM of 4; (C) 10 μM of 4; (D) 25 μM of 4 or (E) 50 μM of 4 and analyzed for apoptosis and necrosis by flow cytometry as quantified in (F).
Figure S4. MAPK signaling study. Western immunoblotting showing the effects of 4 or IPR-69 (positive control) at 50 μM concentration on phospho-p44/42 MAPK and total p44/42 MAPK in MDA-MB-231 cell lysates prepared as described in Materials and Methods.
Acknowledgments
The research was supported by the National Institutes of Health (CA135380) (SOM), the INGEN grant from the Lilly Endowment, Inc (SOM), by The Indiana University Melvin and Bren Simon Cancer Center Translational Research Acceleration Collaboration (ITRAC) (SOM), the Showalter Trust (SOM), by the Indiana University Biomedical Research Fund (SOM) and by the American Cancer Society Research Scholar Grant RSG-12-092-01-CDD (SOM). We acknowledge the analytical work performed by the Clinical Pharmacology Analytical Core laboratory, a core laboratory of the Indiana University Melvin and Bren Simon Cancer Center supported by the National Cancer Institute grant P30 CA082709.
ABBREVIATIONS
- uPAR
urokinase receptor
- uPA
urokinase-type plasminogen activator
- suPAR
soluble urokinase receptor
- uPAATF
Amino terminal fragment of uPA
- MMP
matrix metalloproteinase
- FP
fluorescence polarization
- NOD/SCID
non-obese diabetic severe combined immunodeficient
- PK
pharmacokinetic
- MDA-MB-231
MD Anderson Metastatic Breast
- FBS
fetal bovine serum
- DMEM
Dulbecco’s Modified Eagle Medium
- SDS
sodium dodecyl sulfate
- CBB
Coomassie brilliant blue
- BSA
bovine serum albumin
- DEAE
Diethylaminoethyl cellulose
- FITC
Fluorescein isothiocyanate
- TBST
Tris-Buffered Saline and Tween 20
- IB
immunoblot
- NMR
nuclear magnetic resonance
- DCM
dichloromethane
- HRMS
high-resolution mass spectrometry
- THF
tetrahydrofuran
- PDB
protein databank
- DMAP
4-Dimethylaminopyridine
- DCC
N,N′-Dicyclohexylcarbodiimide
- FAM
6-carboxyfluorescein
- HPLC
high-performance liquid chromatography
- GPI
Glycosylphosphatidylinositol
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TMD-MDA-MB-231
tumor-derived MDA-MB-231
- AUC
area under the curve
- AUMC
area under the moment curve
- LARC
laboratory animal resources center
- IACUC
Institutional animal care and use committee
- HPLC
high-performance liquid chromatography
- PPI
protein-protein interaction
- FN
fibronectin
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Cell. 2011;144:646. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Prager GW, Breuss JM, Steurer S, Olcaydu D, Mihaly J, Brunner PM, Stockinger H, Binder BR. Circ Res. 2004;94:1562. doi: 10.1161/01.RES.0000131498.36194.6b. [DOI] [PubMed] [Google Scholar]
- 3.Schiller HB, Szekeres A, Binder BR, Stockinger H, Leksa V. Mol Biol Cell. 2009;20:745. doi: 10.1091/mbc.E08-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. Int J Cancer. 1997;72:1. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Liang X, Yang X, Tang Y, Zhou H, Liu X, Xiao L, Gao J, Mao Z. Oral Oncol. 2008 doi: 10.1016/j.oraloncology.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Mignatti P, Rifkin DB. Enzyme & protein. 1996;49:117. doi: 10.1159/000468621. [DOI] [PubMed] [Google Scholar]
- 7.Rabbani SA, Mazar AP. Surgical oncology clinics of North America. 2001;10:393. [PubMed] [Google Scholar]
- 8.Kondraganti S, Gondi CS, McCutcheon I, Dinh DH, Gujrati M, Rao JS, Olivero WC. Int J Oncol. 2006;28:1353. [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian R, Gondi CS, Lakka SS, Jutla A, Rao JS. Int J of Oncol. 2006;28:831. [PMC free article] [PubMed] [Google Scholar]
- 10.Kunigal S, Lakka SS, Gondi CS, Estes N, Rao JS. Int J Cancer. 2007;121:2307. doi: 10.1002/ijc.22962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Crowley CW, Cohen RL, Lucas BK, Liu GH, Shuman MA, Levinson AD. Proc Natl Acad Sci U S A. 1993;90:5021. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignar DM, Andrews JL, Witherspoon SM, Leray JD, Clay WC, Kilpatrick K, Onori J, Kost T, Emerson DL. Clin Exp Metastas. 1998;16:9. doi: 10.1023/a:1006503816792. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Sugino D, She MY, Ohi H, Hirashima Y, Shinohara H, Fujie M, Shibata K, Terao T. Eur J of Biochem. 1998;253:817. doi: 10.1046/j.1432-1327.1998.2530817.x. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA. Science. 1996;273:1551. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y, Eble JA, Wang ZM, Kreidberg JA, Chapman HA. Mol Biol Cell. 2001;12:2975. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkenblit A, Matulonis UA, Kroener JF, Dezube BJ, Lam GN, Cuasay LC, Brunner N, Jones TR, Silverman MH, Gold MA. Gynecol Oncol. 2005;99:50. doi: 10.1016/j.ygyno.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Higazi AA, Arakelian A, Sachais BS, Cines D, Goldfarb RH, Jones TR, Kwaan H, Mazar AP, Rabbani SA. Faseb J. 2000;14:1400. doi: 10.1096/fj.14.10.1400. [DOI] [PubMed] [Google Scholar]
- 18.Khanna M, Wang F, Jo I, Knabe WE, Wilson SM, Li L, Bum-Erdene K, Li J, GWS, Khanna R, Meroueh SO. ACS chemical biology. 2011;6:1232. doi: 10.1021/cb200180m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Li J, Sinn AL, Knabe WE, Khanna M, Jo I, Silver JM, Oh K, Li L, Sandusky GE, Sledge GW, Nakshatri H, Jones DR, Pollok KE, Meroueh SO. Journal of medicinal chemistry. 2011;54:7193. doi: 10.1021/jm200782y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer B, Peters T. Angew Chem Int Ed Engl. 2003;42:864. doi: 10.1002/anie.200390233. [DOI] [PubMed] [Google Scholar]
- 21.Kunapuli P, Chitta KS, Cowell JK. Oncogene. 2003;22:3985. doi: 10.1038/sj.onc.1206584. [DOI] [PubMed] [Google Scholar]
- 22.Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Mol Biol Cell. 2001;12:2975. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. J Biol Chem. 1994;269:32380. [PubMed] [Google Scholar]
- 24.Jacobsen B, Gardsvoll H, Juhl Funch G, Ostergaard S, Barkholt V, Ploug M. Protein Expr Purif. 2007;52:286. doi: 10.1016/j.pep.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Sutter PW, CD J Heterocyclic Chem. 1982;19:997. [Google Scholar]
- 26.Nabar UTK, VR, Sunthankar SV. Indian J Chem. 1983;22B:812. [Google Scholar]
- 27.Gornostaev LMD, LV, Titova NG, Arnol’d EV, Lavrikova TI. Russ J Org Chem. 2006;42:1364. [Google Scholar]
- 28.Wang F, Eric Knabe W, Li L, Jo I, Mani T, Roehm H, Oh K, Li J, Khanna M, Meroueh SO. Bioorg Med Chem. 2012;20:4760. doi: 10.1016/j.bmc.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong S, Chen X, Zhu X, Dziegielewska B, Bachman KE, Ellenberger T, Ballin JD, Wilson GM, Tomkinson AE, MacKerell AD. Journal of medicinal chemistry. 2008;51:4553. doi: 10.1021/jm8001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarui T, Andronicos N, Czekay RP, Mazar AP, Bdeir K, Parry GC, Kuo A, Loskutoff DJ, Cines DB, Takada Y. J Biol Chem. 2003;278:29863. doi: 10.1074/jbc.M304694200. [DOI] [PubMed] [Google Scholar]
- 31.Bass R, Ellis V. Thromb Haemost. 2009;101:954. [PubMed] [Google Scholar]
- 32.Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. Cancer cell. 2002;1:445. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 33.Simon DI, Wei Y, Zhang L, Rao NK, Xu H, Chen ZP, Liu QM, Rosenberg S, Chapman HA. J Biol Chem. 2000;275:10228. doi: 10.1074/jbc.275.14.10228. [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Tang CH, Kim Y, Robillard L, Zhang F, Kugler MC, Chapman HA. J Biol Chem. 2007;282:3929. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- 35.Gardsvoll H, Jacobsen B, Kriegbaum MC, Behrendt N, Engelholm L, Ostergaard S, Ploug M. J Biol Chem. 2011;286:33544. doi: 10.1074/jbc.M111.220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mertens HD, Kjaergaard M, Mysling S, Gardsvoll H, Jorgensen TJ, Svergun DI, Ploug M. J Biol Chem. 2012;287:34304. doi: 10.1074/jbc.M112.398404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li YD, Yuan C, Bian CB, Chen LQ, Furie B, Furie BC, Cines DB, Huang MD. Science. 2006;311:656. doi: 10.1126/science.1121143. [DOI] [PubMed] [Google Scholar]
- 38.Tang ML, Vararattanavech A, Tan SM. J Biol Chem. 2008;283:25392. doi: 10.1074/jbc.M802311200. [DOI] [PubMed] [Google Scholar]
- 39.Patel JB, Appaiah HN, Burnett RM, Bhat-Nakshatri P, Wang G, Mehta R, Badve S, Thomson MJ, Hammond S, Steeg P, Liu Y, Nakshatri H. Oncogene. 2011;30:1290. doi: 10.1038/onc.2010.510. [DOI] [PubMed] [Google Scholar]
- 40.Bugge TH, Suh TT, Flick MJ, Daugherty CC, Romer J, Solberg H, Ellis V, Dano K, Degen JL. J Biol Chem. 1995;270:16886. doi: 10.1074/jbc.270.28.16886. [DOI] [PubMed] [Google Scholar]
- 41.Solberg H, Ploug M, Hoyer-Hansen G, Nielsen BS, Lund LR. J Histochem Cytochem. 2001;49:237. doi: 10.1177/002215540104900211. [DOI] [PubMed] [Google Scholar]
- 42.Plesner T, Ralfkiaer E, Wittrup M, Johnsen H, Pyke C, Pedersen TL, Hansen NE, Dano K. Am J Clin Pathol. 1994;102:835. doi: 10.1093/ajcp/102.6.835. [DOI] [PubMed] [Google Scholar]
- 43.Nykjaer A, Moller B, Todd RF, 3rd, Christensen T, Andreasen PA, Gliemann J, Petersen CM. J Immunol. 1994;152:505. [PubMed] [Google Scholar]
- 44.Jacobsen B, Ploug M. Curr Med Chem. 2008;15:2559. doi: 10.2174/092986708785909012. [DOI] [PubMed] [Google Scholar]
- 45.Smith HW, Marshall CJ. Nat Rev Mol Cell Biol. 2010;11:23. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of 4 on MDA-MB-231 cell invasion. Boyden chamber apparatus (with Matrigel) is used to assess the effect of 4 on MDA-MB-231 invasion; Error bars represent means +/− S.D. Representative experimental cells from control and in the presence of 4 were photographed (x 200) to illustrate the effect of 4 on invasion, as shown below.
Figure S2. Effect of 7 on invasion. (A) Boyden chamber apparatus (with Matrigel) is used to assess the effect of 7 on MDA-MB-231 invasion; Error bars represent means +/− S.D. (B) Effect of 7 on MDA-MB-231 cell viability as assessed by MTT assay.
Figure S3. Flow cytometry analysis using Annexin V-FITC and PI staining. MDA-MB-231 cells were treated for 24 hours with (A) DMSO; (B) 1μM of 4; (C) 10 μM of 4; (D) 25 μM of 4 or (E) 50 μM of 4 and analyzed for apoptosis and necrosis by flow cytometry as quantified in (F).
Figure S4. MAPK signaling study. Western immunoblotting showing the effects of 4 or IPR-69 (positive control) at 50 μM concentration on phospho-p44/42 MAPK and total p44/42 MAPK in MDA-MB-231 cell lysates prepared as described in Materials and Methods.