Abstract
Phenylketonuria (PKU) is an autosomal recessive inherited metabolic disorder caused by a complete or near-complete deficiency of the liver enzyme phenylalanine hydroxylase (PAH), which converts the amino acid phenylalanine to tyrosine, leading to the increase of blood and tissue concentration of phenylalanine to toxic levels. PKU is not life threatening but is treated through lifelong dietary management. If untreated, it can lead to severe learning disability, brain function abnormalities, behavioural and neurological problems. The non-life threatening nature of PKU has until now caused some debate on whether to licence its detection by preimplantation genetic diagnosis (PGD). We report the first successful live birth in the UK following single cell embryo biopsy and PGD for the detection of two different mutations in the (PAH) gene. This case highlights both an important scientific development as well as the ethical challenge in offering couples who carry PKU this new reproductive option when starting their family.
Introduction
Phenylketonuria (PKU) is an inherited metabolic disorder that is transmitted as an autosomal recessive trait. Individuals with homozygous mutations in the phenylalanine hydroxylase (PAH) gene lack the enzyme PAH that is essential for the breakdown of the amino acid phenylalanine. Accumulation of phenylalanine in the body can cause damage to the central nervous system and subsequently cognitive and behavioural abnormalities and mental retardation in both children and adults (Mitchell et al. 2011; Enns et al. 2010; Feillet et al. 2010a).
The incidence of PKU varies in different populations, with a prevalence of 1 in 10,000 births in Europe and the United States (Dobrowolski et al. 2011; Blau et al. 2010; Hardelid et al. 2007). Its prevalence is higher in northern Europeans and it has an incidence of 1 in 4,500 in the Irish (Blau et al. 2010; Magee et al. 2002).
Treatment consists of a diet low in phenylalanine starting shortly after birth and continued into adulthood (‘diet for life’) (Feillet et al. 2010a). If the disorder is untreated it can result in severe mental retardation by the end of the first year of life. Screening of newborns immediately after birth is standard practice in most developed countries (van Spronsen 2010).
Couples who both carry mutations are faced with difficult decisions when considering starting or enlarging their family. Some couples will accept a one in four chance of an affected pregnancy. Others will choose to conceive naturally and then undergo more conventional invasive prenatal diagnoses such as chorionic villus sampling at around 11 weeks or amniocentesis at 16 weeks. These procedures carry around a 1% risk of pregnancy loss. If a pregnancy is diagnosed as affected by PKU couples will then face the option of either terminating the pregnancy or continuing the pregnancy knowing the child will need lifelong management. Preimplantation genetic diagnosis represents a further reproductive option for these couples. Embryos are produced by the in vitro fertilisation (IVF) process and can be analysed for the presence of the affected genes. Embryos free of the disease can then be transplanted back into the uterus. This allows the couple the reassurance from the very beginning of pregnancy that the child will be unaffected by PKU and enables them to avoid invasive prenatal diagnosis. PGD was first performed in our unit over 20 years ago (Handyside et al. 1989). During this time PGD for lethal disorders has become more widely accepted, but there remains some debate about the application of this technology to screen for more chronic disorders where effective management is available.
In the United Kingdom licences to perform PGD for any disease are granted by the UK Human Fertilisation and Embryology Authority (HFEA). Our case was the first case of PGD for PKU performed in the United Kingdom and a licence was granted by the HFEA following formal application.
Materials and Methods
A couple presented to our clinic for PGD after their first child was diagnosed with PKU. The mother was 31 years old and a carrier of a mutation in the PAH gene (c.1241 A > G). The father was a carrier of a different mutation in the PAH gene (c.194 T > C). This presented a one in four risk of an affected pregnancy. The couple were offered the option of conventional prenatal screening but declined and were keen on the option of PGD. A PGD workup was performed initially on the parents’ DNA and then tested on single cells. For direct detection of the mutations carried by the couple, primers were designed using the Primer3 software (http://frodo.wi.mit.edu/primer3) with reference to the human phenylalanine hydroxylase gene sequence (Genbank: NG_008690.1). The following primers for DNA amplification and minisequencing were designed:
c.194 T > C_F (5'-ACCCTCCCCATTCTCTCTTC-3'),
c.194 T > C_R (5'-AGGCAGGCTACGTTTATCCA-3'),
c.194 T > C_Minisequencing_F (5'-TGATGTAAACCTGACCCACA-3'),
c.1241A > G_F (5'-GTGGTTTTGGTCTTAGGAACTTTG-3'),
c.1241A > G_R (5'-ATCTTAAGCTGCTGGGTATTGT-3'),
c.1241A > G_Minisequencing_F (5'-TCGGCCCTTCTCAGTTCGCT-3').
The intragenic STR-3 and VNTR-13 polymorphic markers were also used in the PGD protocol (Verlinsky et al. 1999). The STR-3 forward primer was 5'-fluorescently labelled with 6-carboxyfluorescein (6- FAM), while the VNTR-13 forward primer was labelled with 6-carboxyhexafluorescein (HEX).
The female patient then underwent a cycle of IVF. A long day 21 suppression protocol was used with a gonadotropin releasing hormone (GnRH) agonist. After 10 days of stimulation with 150 IU of recombinant follicle stimulating hormone (FSH), oocyte release was triggered with 0.25 mg of recombinant human chorionic gonadotropin (hCG). This was followed by ultrasound-guided transvaginal oocyte retrieval, performed under sedation. Sixteen oocytes were retrieved, of which fifteen were suitable for intracytoplasmic sperm injection (ICSI). Thirteen oocytes were fertilised normally and were cultured to day 3. On day 3 of development, 12 embryos had at least five blastomeres and were suitable for biopsy. A hole was performed in the zona pellucida using laser (SaturnTM, Research Instruments) and a single blastomere was removed from each embryo using Humagen biopsy micropipettes. Each blastomere was washed in NWB (non-stick washing buffer) (Reprogenetics UK, Oxford, UK) then placed in a sterile 0.2 mL PCR tube in 1 μL of clean NWB and placed on ice to be sent to Reprogenetics UK Ltd for genetic analysis. Following the biopsy the embryos were cultured individually to day 5 in numerically labelled 4-well dishes to prevent cross-contamination and to allow the identification of each embryo.
Biopsied cells were lysed and amplified using the SurePlex DNA Amplification System (Rubicon, USA) according to manufacturer’s instructions. Subsequently, each of the four loci (two mutation sites and two polymorphic markers) was amplified in singleplex reactions. For the amplification of the sequences encompassing the two mutations the HotMaster Taq DNA polymerase kit (5 Prime, UK) was used. Reaction mixtures contained PCR grade water, 1x HotMaster Taq Buffer (with 25 mM Mg2+), dNTPs (200 μM each), 0.8 μM each primer, 0.6 units HotMaster Taq DNA polymerase and 1 μl of SurePlex amplified product for a final volume of 15 μL. Thermal cycling consisted of an initial denaturation step of 96°C for 1 min, followed by 45 cycles of 94°C for 15 s, 54.5°C for 15 s, and 65°C for 45 s, then a final extension step of 65°C for 2 min. For amplification of the microsatellite markers the Expand Long Template PCR System was used (Roche Diagnostics Ltd., UK). Reaction mix contained PCR grade water, 1x Expand Long Template Buffer 3 (with 27.5 mM MgCl2 and detergents), dNTPs (350 μM each), 2 μM each primer, 1.5 units Expand Long Template enzyme mix and 1 μL of SurePlex amplified product for a final volume of 15 μL. Thermal cycling was carried out for 45 cycles as described by Kakourou et al. 2010 (annealing temperature was 54.5°C). After amplification of the sequences involving the two mutations, the amplified products were cleaned using EXOSAP-IT (Affymetrix, UK) and minisequencing was carried out using the SNaPshot Multiplex kit (Applied Biosystems, UK) according to manufacturer’s instructions. Analysis of the minisequencing products and amplified microsatellite products was accomplished through capillary electrophoresis by using a 3130 genetic analyser (Applied Biosystems). Analysis of the data was carried out using GeneMapper v4.0 software (Applied Biosystems).
Results
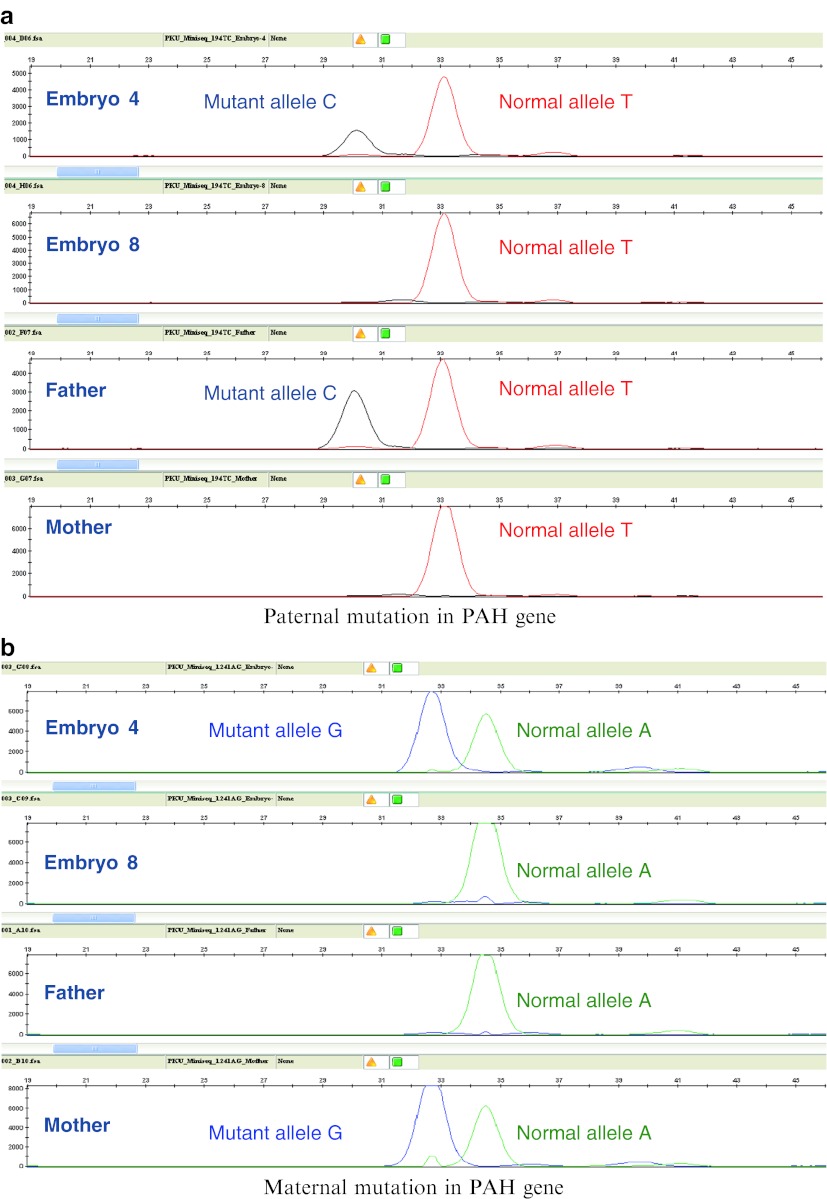
Embryo status was based upon interrogation of each of the mutation sites in the PAH gene and also analysis of the inheritance of alleles from two intragenic polymorphisms. The combination of direct mutation detection and linkage analysis provides multiple opportunities to detect affected embryos, a diagnostic redundancy that is critical for accurate analysis of single cells. Following genetic analysis, three embryos were found to be free of both parental mutations and therefore unaffected, four were found to be carriers of the maternal mutation, four were affected with PKU (both mutations detected) and one embryo failed to yield any result (Fig. 1). All embryos were cultured to the blastocyst stage and one hatching unaffected blastocyst of grade 5Cc (Gardner blastocyst grading system) (Gardner et al. 2000) was transferred on day 5 of development, using a soft embryo transfer catheter (Rocket® Embryon® SOFT ET catheter) under ultrasound guidance. This resulted in a clinical pregnancy and subsequently the live birth of a healthy baby boy, as confirmed by post-natal testing. The remaining two unaffected blastocysts and three of the four carriers were cryopreserved.
Fig. 1.
Results of PGD showing (a) the paternal mutation and (b) the maternal mutation. Embryo 4 was affected (both mutations present) and embryo 8 was unaffected (no mutations present)
Discussion
In the UK, fertility clinics require the approval of the HFEA prior to performing PGD for a genetic condition. The HFEA must therefore agree that a particular genetic condition is sufficiently serious to warrant using PGD. Once a condition is approved it is added to the list of HFEA licenced PGD conditions and approval on an individual basis is no longer required.
The HFEA Code of Practice – Guidance on embryo testing states that: Preimplantation genetic diagnosis (PGD) can be carried out for a heritable condition only in two circumstances: a) where there is a particular risk that the embryo to be tested may have a genetic, mitochondrial or chromosomal abnormality, and the Authority is satisfied that a person with the abnormality will have or develop a serious disability, illness or medical condition, or b) where there is a particular risk that any resulting child will have or develop a gender related serious disability, illness or medical condition. It further states that the centre performing the PGD should consider several factors when deciding if PGD is appropriate or not, one of which is the availability of effective therapy, now and in the future (HFEA Code of Practice 2011).
PKU is a chronic genetic condition that requires life-long management in order to prevent the occurrence of learning and behavioural disability and mental retardation. The condition is not life threatening, albeit it requires chronic treatment. The life-long requirement of treatment and the implications this may have on the affected individual’s life, were the determining factors in approving PKU for PGD in the UK.
The licensing of PGD for PKU in the UK represents a major ethical shift in the application of PGD for chronic treatable conditions. This was evident during our process of applying for HFEA approval for testing for this condition. The first application was rejected on the grounds that PKU is a treatable non-life-threatening condition and that differences in outcomes exist between those whose condition is detected at birth and those whose condition is detected at a later stage causing more serious consequences. The HFEA also required more evidence of the effect of the condition on the quality of life of affected individuals and their families. Our application was reviewed and approved following correspondence from the National Society of Phenylketonuria in the UK (www.nspku.org), a patient support organisation which is a member of Genetic Alliance UK (www.geneticalliance.org.uk), a national charity supporting those affected by genetic disorders and their families. This correspondence outlined the adverse effects of PKU on the quality of life of patients and their caregivers, especially in childhood when a strict diet is required until the age of 10 years, and in adolescence when the diet compliance proves to be more difficult. In adulthood, self-management of the condition becomes quite challenging and poses great difficulties both psychologically and socially (Feillet et al. 2010a; Macdonald et al. 2010). The cost, inconvenience, and sometimes difficult access to PKU diet increase the difficulty of adhering to it. Although there is still debate over whether adult PKU patients need a strict phenylalanine-restricted diet and despite the fact that there are some adults with PKU who do not follow a restricted diet and lead normal lives, many studies have advocated following a restricted diet in adulthood as it has been shown that high phenylalanine levels in adults can have adverse effects on mood, sustained attention, concentration, and cognitive ability (Feillet et al. 2010b; Ten Hoedt et al. 2011; Macdonald et al. 2010; Moyle et al. 2007; Simon et al. 2008). Restricted diet is also crucial in female adults with PKU planning for a pregnancy, as maternal PKU has been shown to cause fetal abnormalities including microcephaly, congenital heart disease, and mental retardation (Prick et al. 2012). Moreover, it has been shown in a systemic literature review by Enns et al. (2010) that even with early detection and management of PKU through diet, the neurocognitive, psychosocial, quality of life, growth, nutrition, and bone pathology outcomes in these patients were suboptimal. Individuals with PKU require lifelong monthly blood tests to ensure that blood phenylalanine levels are not elevated above the recommended values ranging from 120 to 360 μM/L up to 12 years of age and from 120 to 900 μM/L after the age of 12, with national guidelines varying from one country to another (Ahring et al. 2011; Anastasoaie et al. 2008; Enns et al. 2010; van Spronsen 2010). Moreover, fluctuation in blood phenylalanine levels and not just the absolute values is equally important and can also affect cognitive functioning in PKU patients (Anastasoaie et al. 2008; Feillet et al. 2010b).
In addition to a diet low in phenylalanine which is present in all protein-rich foods, dietary supplementation, such as protein-free formulas and protein substitutes, is also required in combination with the diet (Feillet et al. 2010b; Macdonald et al. 2010). Other non-traditional therapies are also being used such as large neutral amino acids (LNAA) which have shown to be beneficial in maintaining low phenylalanine levels in the body without the need of dietary control (van Spronsen et al. 2010) and other therapies that are still under experimentation such as gene therapy, enzyme replacement therapy and cell transplantation (Mitchell et al. 2011; van Spronsen and Enns 2010).
The licence committee were satisfied that our revised application provided clarification on the likelihood of PKU being detected in the newborn: there is a neonatal screening programme in the UK to test all newborns at 6–14 days, but it has been shown that there are regional variations in the timing of this test and the time of starting treatment, with about 8% of newborns with severe PKU still not treated by 20 days after birth (Smith et al. 1991). After studying our revised application and correspondence from the NSPKU, the licensing committee concluded that even if the condition is detected at birth and is treated immediately, the lifelong dietary regime and medication in the child can be seriously intrusive and socially and psychologically invasive and damaging. It can be physically unpleasant, emotionally difficult, and disruptive of family life and can seriously adversely affect the quality of family and social relationships. Moreover, there is still the possibility that the affected individual will develop neurological problems or have seizures or mental retardation, and even when detected early, there is a risk of the condition not being treated sufficiently early so that in these cases more serious effects can manifest. In making this decision the HFEA licence committee authorised our centre to perform embryo testing for PKU and agreed that PKU should be added to the publicly available list of PGD conditions, allowing all licenced PGD centres in the UK to test for this condition.
Previously, PGD for PKU was performed following polar body biopsy as the mutation which was being detected was maternal (Verlinsky et al. 2001). As only the polar body from maternal oocytes was analysed no paternal input could be assessed. This case represents the first use of PGD in the UK for the diagnosis of PKU and, more specifically, the first reported case of PGD for PKU following embryo biopsy for the detection of two different mutations in the PAH gene, one maternal and the other paternal.
Assisted conception techniques often involve the transfer of multiple embryos into the uterus in an attempt to maximise success. This has led to an epidemic of multiple pregnancies. Our couple were already caring for a child affected by PKU and so a mutual decision was made to electively transfer one embryo to try and avoid the possibility of caring for both an affected child and the burden of a multiple pregnancy.
Whilst this case adds to the scientific literature for PGD and gives additional reproductive choices for couples who carry the PKU mutations, it is the ethical considerations that may have more impact. This case highlights a tension between personal reproductive choices that couples have and an interest from the state as represented by the regulatory body. Who is best placed to make these decisions? Having reversed their initial refusal to grant a licence, it is likely this could herald a change in how the HFEA regulator might view applications for other PGD cases for other non-life threatening conditions.
Acknowledgements
The authors would like to thank Anastasia Mania (IVF Hammersmith) for her invaluable role during the licence application to the HFEA.
DW is supported by the NIHR Biomedical Research Centre Oxford.
Synopsis
We report the first successful live birth in the UK following single cell embryo biopsy and PGD for the detection of two different mutations in the PAH gene.
Conflict of Interest
Dagan Wells is a recipient of research grants from the Oxford NIHR Biomedical Research Centre, Gema Diagnostics and from Merck Serono. He has also received honoraria for lectures at educational meetings.
Footnotes
Competing interests: None declared
References
- Ahring A, Belanger QA, Dokoupil K, Gokmen-Ozel H, Lammardo AM, MacDonald A, Motzfeldt K, Nowacka N, Robert M, van Rijn M. Blood phenylalanine control in phenylketonuria: a survey of 10 European centres. Eur J Clin Nutr. 2011;65:275–278. doi: 10.1038/ejcn.2010.258. [DOI] [PubMed] [Google Scholar]
- Anastasoaie V, Kurzius L, Forbes P, Waisbren S. Stability of blood phenylalanine levels and IQ in children with phenylketonuria. Mol Genet Metab. 2008;95:17–20. doi: 10.1016/j.ymgme.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376:1417–1427. doi: 10.1016/S0140-6736(10)60961-0. [DOI] [PubMed] [Google Scholar]
- Dobrowolski SF, Heintz C, Miller T, Ellingson C, Ellingson C, Ozer I, Gokcay G, Baykal T, Thony B, Demirkol M, Blau N. Molecular genetics and impact of residual in vitro phenylalanine hydroxylase activity on tetrahydrobioprotein responsiveness in Turkish PKU populations. Mol Genet Metab. 2011;102:116–121. doi: 10.1016/j.ymgme.2010.11.158. [DOI] [PubMed] [Google Scholar]
- Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab. 2010;101:99–109. doi: 10.1016/j.ymgme.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Feillet F, MacDonald A, Hartung D, Burton B. Outcomes beyond phenylalanine: an international perspective. Molec Genet Metab. 2010;99:S79–S85. doi: 10.1016/j.ymgme.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Feillet F, van Spronsen FJ, MacDonald A, Trefz FK, Demirkol M, Giovannini M, Belanger-Quintana A, Blau N. Challenges and pitfalls in the management of phenylketonuria. Pediatrics. 2010;126:333–341. doi: 10.1542/peds.2009-3584. [DOI] [PubMed] [Google Scholar]
- Gardner D, Lane M, Stevens J, et al. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- Handyside AH, Pattinson JK, Penketh RJ, Delhanty JD, Winston RM, Tuddenham EG. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1989;18:347–349. doi: 10.1016/S0140-6736(89)91723-6. [DOI] [PubMed] [Google Scholar]
- Hardelid P, Cortina-Borja M, Munro A, Jones H, Cleary M, Champion MP, Foo Y, Scriver CR, Dezateux C. The birth prevalence of PKU in Populations of European, South Asian and Sub-Saharan African ancestry living in south east England. Annals Hum Genet. 2007;71:1–7. doi: 10.1111/j.1469-1809.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- Human Fertilisation and Embryology Authority (HFEA) (2011) Code of Practice 8th Edition
- Kakourou G, Dhanjal S, Mamas T, Serhal P, Delhanty JD, Sengupta SB. Modification of the triplet repeat primed polymerase chain reaction method for detection of the CTG repeat expansion in myotonic dystrophy type 1: application in preimplantation genetic diagnosis. Fertil Steril. 2010;94:1674–1679. doi: 10.1016/j.fertnstert.2009.10.050. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Gokmen-Ozel H, van Rijn M, Burgard P. The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis. 2010;33:665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- Magee AC, Ryan K, Moore A, Trimble ER. Follow up of fetal outcome in cases of maternal phenylketonuria in Northern Ireland. Arch Dis Child Fetal Neonatal Ed. 2002;87:F141–F143. doi: 10.1136/fn.87.2.F141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JJ, Trakadis YJ, Scriver CR. Phenylalanine hydroxylase deficiency. Genet Med. 2011;13:697–707. doi: 10.1097/GIM.0b013e3182141b48. [DOI] [PubMed] [Google Scholar]
- Moyle JJ, Am F, Arthur M, Bynevelt M, Burnett JR. Meta-analysis of neuropsychological symptoms of adolescents and adults with PKU. Neuropsychol Rev. 2007;17:91–101. doi: 10.1007/s11065-007-9021-2. [DOI] [PubMed] [Google Scholar]
- Prick BW, Hop WC, Duvekot JJ. Maternal phenylketonuria and hyperphenylalaninemia in pregnancy: pregnancy complications and neonatal sequelae in untreated and treated pregnancies. Am J Clin Nutr. 2012;95:374–382. doi: 10.3945/ajcn.110.009456. [DOI] [PubMed] [Google Scholar]
- Simon E, Schwarz M, Roos J, Dragano N, Geraedts M, Siegrist J, Kamp G, Wendel U. Evaluation of quality of life and description of the sociodemographic state in adolescent and young adult patients with phenylketonuria (PKU) Health Qual Life Outcomes. 2008;6:25–31. doi: 10.1186/1477-7525-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I, Cook B, Beasley M. Review of neonatal screening programme for phenylketonuria. BMJ. 1991;303:333–335. doi: 10.1136/bmj.303.6798.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Hoedt AE, de Sonneville LMJ, Francois B, ter Horst NM, Janssen MCH, Rubio-Gozalbo ME, Wijburg FA, Hollak CEM, Bosch AM. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomised, double-blind, placebo-controlled, crossover trial. J Inherit Metab Dis. 2011;34:165–171. doi: 10.1007/s10545-010-9253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Spronsen FJ. Phenylketonuria: a 21st century perspective. Nat Rev Endocrinol. 2010;6:509–514. doi: 10.1038/nrendo.2010.125. [DOI] [PubMed] [Google Scholar]
- Van Spronsen FJ, Enns GM. Future treatment strategies in phenylketonuria. Mol Genet Metab. 2010;99:S90–S95. doi: 10.1016/j.ymgme.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Van Spronsen FJ, de Groot MJ, Hoeksma M, Reijngoud D, van Rijn M. Large neutral amino acids in the treatment of PKU: from theory to practice. J Inherit Metab Dis. 2010;33:671–676. doi: 10.1007/s10545-010-9216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinsky Y, Rechitsky S, Verlinsky O, Ivachnenko V, Lifchez A, Kaplan B, Moise J, Valle J, Borkowski A, Nefedova J, Goltsman E, Strom C, Kuliev A. Prepregnancy testing for single-gene disorders by polar body analysis. Genet Test. 1999;3:185–190. doi: 10.1089/gte.1999.3.185. [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Rechitsky S, Verlinsky O, Strom C, Kuliev A. Preimplantation testing for phenylketonuria. Fertil Steril. 2001;76:346–349. doi: 10.1016/S0015-0282(01)01912-4. [DOI] [PubMed] [Google Scholar]