Whether or not the C terminus of MADS box proteins is relevant for their function as transcription factors and how floral homeotic complexes are specified remain open questions. This work shows that the floral homeotic B class MADS domain protein SEIRENE from California poppy requires its entire C-terminal domain for homeotic complex formation with specific C and E class proteins.
Abstract
The products of B class floral homeotic genes specify petal and stamen identity, and loss of B function results in homeotic conversions of petals into sepals and stamens into carpels. Here, we describe the molecular characterization of seirena-1 (sei-1), a mutant from the basal eudicot California poppy (Eschscholzia californica) that shows homeotic changes characteristic of floral homeotic B class mutants. SEI has been previously described as EScaGLO, one of four B class–related MADS box genes in California poppy. The C terminus of SEI, including the highly conserved PI motif, is truncated in sei-1 proteins. Nevertheless, like the wild-type SEI protein, the sei-1 mutant protein is able to bind CArG-boxes and can form homodimers, heterodimers, and several higher order complexes with other MADS domain proteins. However, unlike the wild type, the mutant protein is not able to mediate higher order complexes consisting of specific B, C, and putative E class related proteins likely involved in specifying stamen identity. Within the PI motif, five highly conserved N-terminal amino acids are specifically required for this interaction. Several families lack this short conserved sequence, including the Brassicaceae, and we propose an evolutionary scenario to explain these functional differences.
INTRODUCTION
The regular appearance of the angiosperm flower requires distinct floral homeotic gene functions acting in a combinatorial manner. The ABCE model of flower development explains how four different gene functions can specify organ identity of the four floral organ types. The action of the A function alone specifies the outer whorl sepals and has been found in Arabidopsis thaliana and close relatives. Concerted expression of class A and B governs petal organ identity; B and C function together to specify the stamens, and the C gene function alone is required for carpel identity. The E function acts throughout the flower and is required for the determination of all floral organs (Coen and Meyerowitz, 1991; Honma and Goto, 2001; Theissen and Saedler, 2001). The loss of the A, B, C, or E function leads to homeotic conversions of floral organs. For example, in Arabidopsis homeotic B class mutants such as ap3-3 and pi-1, petals are replaced by sepals and stamens are replaced by carpels (Bowman et al., 1989). The genetic factors constituting the ABCE classes have been identified mainly as floral homeotic MADS domain transcription factors. In Arabidopsis, the A class genes are APETALA1 (AP1) and AP2, the B class genes AP3 and PISTILLATA (PI); AGAMOUS (AG) carries out the C function and the E function is realized by the four largely redundantly acting genes SEPALLATA1 (SEP1) to SEP4 (Bowman et al., 1989, 1991b; Jack et al., 1992; Pelaz et al., 2001). There is evidence that the floral homeotic proteins form multimeric complexes to confer floral organ identity. For example, stamen organ identity is proposed to be governed by a protein complex consisting of AP3, PI, AG, and SEP proteins according to the floral quartet model (Honma and Goto, 2001; Theissen and Saedler, 2001).
AP3/PI-like genes have been identified from many representatives of diverse angiosperm lineages, but mutant analyses have so far been performed only in core eudicots such as Arabidopsis, snapdragon (Antirrhinum majus), petunia (Petunia hybrida), and Medicago truncatula and the monocot grasses rice (Oryza sativa) and maize (Zea mays); mutants are generally affected in petal and stamen organ identity, or in lodicules, organs likely to be homologous to petals (Schwarz-Sommer et al., 1992; Tröbner et al., 1992; Angenent et al., 1995; Bowman et al., 1999; Ambrose et al., 2000; Nagasawa et al., 2003; Benlloch et al., 2009). In line with this, expression of B class genes is relatively conserved in higher eudicots and grassy monocots and is found predominantly in developing stamens and petals or lodicules (Sommer et al., 1990; Bowman et al., 1991b; Ambrose et al., 2000; Nagasawa et al., 2003; Vandenbussche et al., 2004; Benlloch et al., 2009). Flowers of species outside the core eudicots often have a less well differentiated perianth; correspondingly, the B class genes show a higher degree of expression divergence and B class proteins show more variation in protein interaction partners (Kramer and Irish, 1999; Kim et al., 2005; Liu et al., 2010).
B class proteins, like MADS domain proteins generally, are able to bind to specific DNA sequences named CArG-boxes [for CCA/T-rich GG; consensus sequence 5′-CC(A/T)6GG-3′] only as homo- and heterodimers or in higher order complexes. Whereas AP3 and PI of Arabidopsis and DEFICIENS (DEF) and GLOBOSA (GLO) of snapdragon form obligate heterodimers, many B proteins from gymnosperms and early diverging eudicots, and monocots can also homodimerize (Goto and Meyerowitz, 1994; Winter et al., 2002; Wang et al., 2010). It has been hypothesized that homodimerization is the ancestral state since the GGM2 protein of the gymnosperm Gnetum gnemon forms homodimers (Winter et al., 2002). The obligate B protein heterodimers in snapdragon and Arabidopsis are required both for organ identity specification and to maintain B gene expression in an autoregulatory circuit (Schwarz-Sommer et al., 1992; Tröbner et al., 1992; Zachgo et al., 1995; Davies et al., 1996; Hill et al., 1998; Tilly et al., 1998; Manchado-Rojo et al., 2012). In addition to heterodimers of AP3/PI-like proteins, homodimers of AP3 orthologs have been found in basal eudicots like columbine (Aquilegia vulgaris) and opium poppy (Papaver somniferum) (Drea et al., 2007; Kramer et al., 2007), whereas homodimer formation of PI orthologs has been demonstrated so far for petaloid monocots like the tulip (Tulipa gesneriana) and the orchid Phalaenopsis equestris (Kanno et al., 2003; Tsai et al., 2008). However, a function could not be assigned to homodimers formed by either AP3 or PI orthologs.
The B class MADS domain proteins are of the MIKC type because they possess a characteristic domain structure composed of the MADS, intervening, keratin-like, and C-terminal domains. The N-terminally located MADS domain is highly conserved and responsible for DNA binding. It is followed by the weakly conserved I domain. The K domain contains three putative α helices, K1, K2, and K3, which mediate dimerization and the specification of protein–protein interactions (Jack, 2001). The K3 and C domains of the snapdragon MADS domain proteins DEF and GLO are required to mediate the assembly of protein multimeric complexes (Egea-Cortines et al., 1999).
The C-terminal domain of B class MADS domain proteins contains lineage-specific sequence motifs. A 16–amino acid–long PI motif is found in orthologs of PI. In AP3 orthologs, the eu-AP3 motif occurs in addition to a PI-derived motif (Kramer et al., 1998). The experimental evidence aimed at elucidating the function of these C-terminal motifs is contradictory. Overexpression of truncated versions of AP3 and PI lacking the C-terminal motifs were not able to rescue ap3 or pi mutant phenotypes of Arabidopsis, suggesting that the motifs are required for the B function (Lamb and Irish, 2003). However, another study showed the converse and suggested that the lack of complementation may have resulted from low expression of the complementing constructs in the earlier study (Piwarzyk et al., 2007). The PI motif is also dispensable for higher order complex formation, including those made up of PI, AP3, and SEP1 proteins when assayed with the yeast three-hybrid system (Piwarzyk et al., 2007). Moreover, pi mutants in Arabidopsis can be rescued with the atypical wild-type pea (Pisum sativum) Ps-PI protein that lacks the C-terminal domain, including the PI motif (Berbel et al., 2005). Furthermore, mutant and RNA interference analyses of another legume PI protein, Mt-PI from M. truncatula, which is similar in structure to Ps-PI, also supports the view that the C-terminal domain is not required for B class protein activity (Tzeng et al., 2004; Benlloch et al., 2009). Therefore, one wonders why the motifs within the C-terminal domain of class B proteins have been conserved for over 100 million years of evolution (Su et al., 2008).
Here, we describe the class B floral homeotic mutant seirena-1 (sei-1) in California poppy (Eschscholzia californica), a basal eudicot species from the order Ranunculales. The mutant phenotype results from insertion of a DNA fragment into SEI, the poppy ortholog of the Arabidopsis PI gene, resulting in truncation of the conserved C-terminal PI motif. The specificity of CArG-box binding of the sei-1 protein and its ability for dimeric protein interactions remain largely unchanged. However, the sei-1 protein is unable to participate in some higher order MADS protein interactions, including a BCE stamen identity combination. We hypothesize that the evolutionarily conserved PI motif is required for such higher order complex formation in many species but not in others, including Arabidopsis, which lack a short conserved subregion of the motif.
RESULTS
sei Shows Morphological Defects of a B Class Floral Homeotic Mutant
Screening the homozygous progeny of a fast neutron–irradiated mutant population of California poppy revealed the floral homeotic mutant sei, which is affected exclusively in floral organ formation. The mutant is named after the sirens of Greek mythology (σϵιρήνα) to indicate the replacement of male floral organs with female organs.
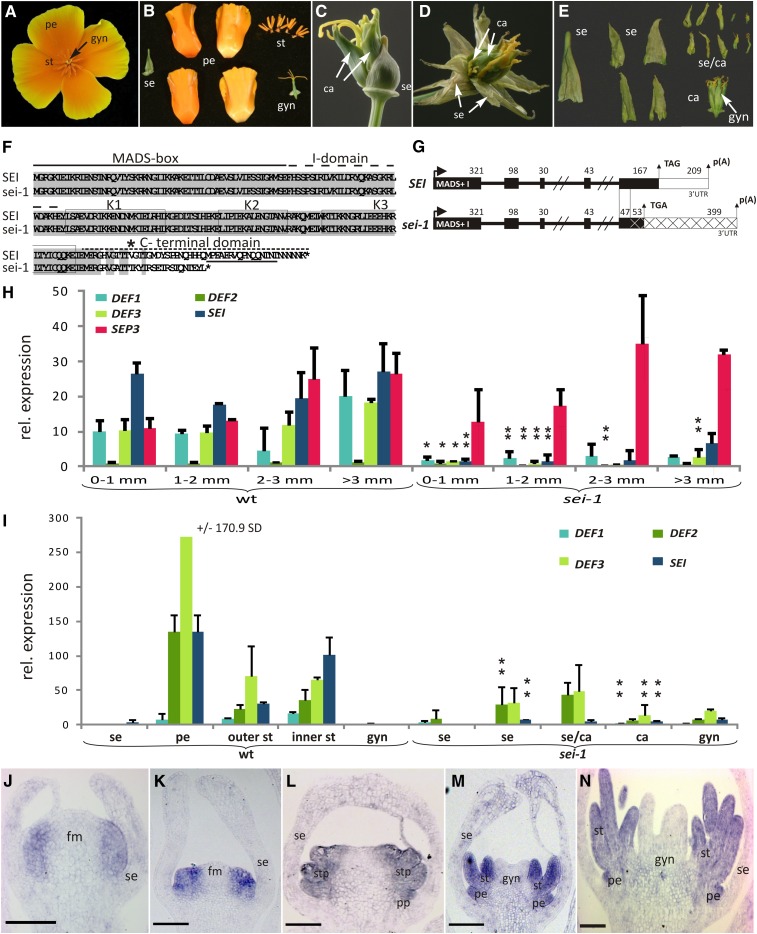
Wild-type flowers of California poppy are composed of a sepal in the first whorl that generally dehisces as a cap during bud opening, four orange petals arranged in two whorls, six to eight whorls of stamens, and two fused carpels constituting the gynoecium in the center of the flower (Figures 1A and 1B). sei-1 flowers (Figures 1C to 1E) show a sepal with wild-type morphology in the first whorl. The next two inner whorls comprise four sepal-like organs instead of petals, indicating a petal-to-sepal homeotic conversion. In the more central whorls, chimeric organs are produced that show a mix of sepal and carpel characters (Figure 1E). While the base of these organs is sepal like, they exhibit yellow stigmatic papillae on their apices (Figures 1C to 1E). Toward the center of the flower, the chimeric organs accumulate more carpel characteristics, including a carpel-specific surface structure (see Supplemental Figure 1 online), but are all unfused. The central whorl consists of a gynoecium of almost wild-type appearance but with incomplete carpel fusion (Figures 1D and 1E). The unfused or partially fused central carpels grow into tube-like structures that are open at the top and thus fail to develop seeds, presumably because the ovules dry prematurely. In spite of the homeotic organ conversions, the organ number in sei-1 mutants does not deviate significantly from the wild type (Figure 1E).
Figure 1.
The sei-1 Phenotype and Expression Analysis of Class B Genes in California Poppy.
(A) Wild-type flower.
(B) Wild-type floral organs.
(C) sei-1 flower showing homeotic conversions of petals into sepals and stamens into carpels.
(D) sei-1 flower with ectopic sepals peeled away.
(E) Overview of the sei-1 floral organs. The arrow indicates the central gynoecium.
(F) Amino acid alignment of the deduced wild-type SEI and the mutant sei-1 proteins. Regions of sequence identity are highlighted in gray; the MADS, I, and C domains, and the proposed amphipathic helices of the K domain are indicated by boxes; and the conserved C-terminal PI motif is underlined. The start position of the protein sequence change caused by the genomic DNA insert is marked by an asterisk.
(G) Organization of the SEI genomic locus in the wild-type and sei-1 mutant plants. Protein-coding portions are shown as black boxes, 3′UTR as white boxes, and insertion of unknown genomic DNA of California poppy in the sei-1 locus is marked with crosses. The numbers above the exons indicate exon length. The start codon is indicated by a horizontal arrow and the stop codon and polyadenylation (pA) site by vertical arrows.
(H) Quantitative PCR on floral buds of all developmental stages (Becker et al., 2005) in wild-type (wt) and sei-1 plants. Wild-type buds of 0 to 1 mm diameter develop through stages 1 to 5 when carpels initiate, 1- to 2-mm buds include stages 6 and 7 when male sporogenic tissue and ovules arise, 2- to 3-mm buds (stage 8) includes stamens with pollen, and after 3-mm buds pass through stage 9 and female meiosis. B class genes and the putative E class gene SEP3 are color coded. Asterisks indicate significant reduction in expression as inferred by analysis of variance (*P < 0.05; **P < 0.01). Error bars show sd, and expression was normalized with ACTIN2 and GAPDH.
(I) Expression analysis by quantitative RT-PCR in mature floral organs (stage 10) indicating the relative expression levels of DEF1, DEF2, DEF3, and SEI in the wild type (left) and sei-1 (right). ca, organs with only carpel-like characteristics; gyn, central gynoecium; se, organs with only sepal-like characteristics; se/ca, organs with a mix of sepal and carpel characteristics. Asterisks above the bars indicate a significant decrease of expression in sei-1 when compared with wild-type expression. sei-1 sepals were compared with wild-type petals, and sei-1 carpels were compared with wild-type inner and outer stamens.
(J) to (N) In situ hybridization pattern of SEI in longitudinal sections of buds of stage 2 (J), stage 3 (K), stage 4 (L), early stage 5 (M), and late stage 5 (N). Stages are according to Becker et al. (2005). fm, floral meristem; gyn, central gynoecium; pe, petals; pp, petal primordia; se, sepals; st, stamens; stp, stamen primordia. Bars = 100 µm.
Siblings of the sei-1 mutant line in the heterozygous F1 generation (heterozygous for sei-1) were intercrossed to analyze the mode of inheritance. Ninety-two F2 flowering plants were observed and 22 exhibited the sei phenotype (χ2 = 0.056, P = 0.8129, df = 1), indicating that the sei phenotype is caused by a mutation at a single locus. Heterozygous sei-1/SEI plants showed a phenotype not deviating from the wild type, except for <5% of plants in which one, or at most two, stamens developed into slightly petal-like organs, indicating that the sei-1 mutant allele is recessive, albeit slightly incomplete.
SEI Encodes the California Poppy GLO Protein
At least four putative B class genes exist in the California poppy genome: DEF1, DEF2, GLO (Zahn et al., 2005), and DEF3. Phylogeny reconstructions (see Supplemental Figure 2 online) based on a large data set comprising many putative B class genes from the Ranunculales (see Supplemental Data Set 1) show that the PI-like gene SEI forms a well-supported clade with the gene Sc-PI from the related Papaveraceae species Sanguinaria canadensis but is only distantly related to the two GLO-like genes from opium poppy (see Supplemental Figure 2 online). Of the AP3-like genes, California poppy DEF2 and DEF3 are very closely related paralogs (presumably recently duplicated) and are closely related to AP3-1 of opium poppy. California poppy DEF1 is most likely the ortholog of AP3-2 of opium poppy, and both genes fall into an orphan group of Ranunculales genes that does not form a well-supported clade. None of the poppy family AP3-like genes cluster within the other three groups of AP3-like genes observed from the related Ranunculaceae family (Kramer et al., 2003).
Investigations of the coding sequences of B class genes in the sei-1 mutant revealed changes in the transcript and protein sequence of California poppy GLO, termed SEI from now on (Figures 1F and 1G). Sequencing 3′ rapid amplification of cDNA ends (RACE) PCR products revealed that the mutant plant’s sei-1 transcripts include an altered nucleotide sequence starting at position 539 that encode a premature stop codon at nucleotide position 590. As a consequence, the sei-1 mutant protein contains 17 changed amino acids and is 22 amino acids shorter than the wild-type SEI (Figure 1F). Moreover, the highly conserved PI motif found in the vast majority of angiosperm PI orthologs (see Supplemental Figure 3) is absent in sei-1 (Figure 1F). Analyses of the genomic locus of sei-1 revealed no sequence deviation compared with the wild-type SEI locus in the first four exons, four introns, and 612 bp of sequence upstream of the start codon except for two amino acids in the C-terminal domain that differ most likely due to the generally high genetic diversity between the individual plants (Figure 1G). However, the mutant plants have a DNA fragment originating from an unknown locus inserted into exon 5 after nucleotide 47. In addition to the premature stop codon, this sequence apparently introduces several subsequent polyadenylation signals into the sei-1 transcripts (Figure 1G; see Supplemental Figure 4A online, fragment a). A second transcript was sequenced as well showing no difference to the first sei-1 transcript in the coding region but differing in the 3′untranslated region (UTR) (see Supplemental Figure 4A online, fragment b).
To determine if the sei-1 mutation has affected the level of transcription, expression levels of SEI and sei-1 were quantified by quantitative PCR in buds at stage 4 (<0.5 mm), when petal and stamen identity is likely to be established (see Supplemental Figure 4B online). In mutant buds, expression of sei-1 is reduced to 6.8% ± 2.0% (mean ± sd) of the homozygous SEI wild-type level. This may be due, at least in part, to reduced autoactivation known to occur for B function genes. To test this, SEI and sei-1 expression levels were compared in heterozygous buds. SEI expression is reduced to 15.6% ± 1.8% of wild-type levels, consistent with some reduction of autoactivation when only one copy of SEI is present. However, expression of the sei-1 allele is significantly less than this, 2.5% ± 1.0%. Even so, sei-1 transcripts still do accumulate, and their translation into significant amounts of sei-1 truncated protein seems likely.
To obtain confirmatory evidence that the sei phenotype is associated with this gene, virus-induced gene silencing (VIGS) was employed to transiently downregulate SEI gene expression in California poppy (see Supplemental Figure 5A online). The three first formed flowers of 62 plants treated with SEI-VIGS were analyzed morphologically (see Supplemental Figures 5A and 5B online). Of the 186 flowers scored, 38 flowers showed a strong phenotype, 81 a more mild disruption, and 67 were wild type. The strong phenotype was characterized by partial homeotic conversion of petals into sepals, such that they were sepaloid in size and shape but retained orange coloration except for a broad green stripe in the center. Organs that developed in the position of outer stamen whorls showed the morphology of petaloid organs, albeit with reduced size. In place of inner whorl stamens, single, unfused carpel-like organs developed (see Supplemental Figure 5B online). RT-PCR was performed on floral tissue at anthesis and significantly reduced expression of SEI was shown in the VIGS-treated plants (see Supplemental Figure 5C online). The VIGS results demonstrate that a very strong reduction in SEI expression is sufficient to induce homeotic changes closely similar to those observed in the sei-1 plants, albeit to a lesser extent, and thus corroborate the genetic basis of the sei phenotype.
Expression of all AP3-Like and PI-Like Genes Is Decreased in the sei-1 Mutant
Expression analysis of the four putative class B floral homeotic genes of California poppy was performed by quantitative RT-PCR in wild-type and mutant floral buds throughout their development (Figure 1H). In the wild type, putative B class genes and the putative E class gene AGL9, here renamed SEP3, are expressed throughout flower developmental stages 1 to 9 (stages from Becker et al., 2005), with their expression slightly increasing in later stages. Only DEF2 expression, the close paralog of DEF3, is almost undetectable throughout early flower development and but is expressed in petals and stamens at anthesis (Figures 1H and 1I). In the sei-1 plants, B class gene expression is generally strongly reduced in the four floral sizes tested, particularly in 1- to 2-mm buds (stages 6 and 7, when stamen morphogenesis is occurring in the wild type). In contrast with the B class genes, expression of SEP3 is unchanged in floral buds at all stages when compared with the wild type (Figure 1H). This is consistent with loss of B function resulting in reduced autoactivation of all B function genes but not E function genes.
In mature floral organs of the wild type 1 d before anthesis (stage 10), all of the class B genes are expressed at significant levels in petals and stamens (Figure 1I). In petals, DEF2, DEF3, and SEI are expressed strongly, but DEF1 only weakly. In inner (central) and outer (lateral) stamens, the expression of B genes is generally lower than in petals, with DEF1 being the most weakly expressed gene. No expression of B class genes is detected in the wild-type sepals or gynoecia. In mature sei-1 floral organs, expression of all four putative B class genes is strongly reduced, with sei-1 transcripts being the most compromised. Expression of DEF2 and DEF3 occurs but is reduced in the homeotic sepals and in the inner stamen whorls which now develop as carpelloid organs.
In situ hybridization was performed with a SEI-specific probe to obtain information on its expression pattern with a high temporal and spatial resolution in buds when primordia are arising (i.e., up to 1 mm in diameter) (Figures 1J to 1N). In stage 2 flowers when only sepal primordia have appeared, expression of SEI is restricted to the periphery of the floral meristem (Figure 1J). In buds of stage 3 when the petal primordia are initiated, SEI is expressed in the petal and stamen anlagen (Figure 1K). In stage 4, when the stamen primordia appear, SEI is restricted to petal and stamen primordia (Figures 1L and 1M). In early and late stage 5 (Figures 1M and 1N, respectively) when the gynoecium primordium emerges and the carpel walls elongate, SEI is expressed evenly throughout the developing stamens and the petal primordia. Thus, SEI expression is specifically associated with the anlagen and young primordia of petals and stamens, organs whose identity is changed in sei mutant flowers.
In summary, SEI is expressed specifically in petals and stamens throughout their development. The California poppy DEF genes, which are also putative B function genes differ in their expression patterns, with DEF1 involved in early flower development, DEF2 later, and DEF3, the close ortholog of DEF2, in both. Expression of all putative B function genes is reduced in sei-1 mutants.
Expression of C Class Genes Is Reduced in sei-1 Mutants
Unlike Arabidopsis class B mutants, in which the stamen whorl is replaced by organs with carpel identity, the sei-1 mutant does not show homeotic organs with one identity. Rather, stamens occur in multiple whorls in California poppy, and a smooth transition from outer organs with sepal identity to inner organs with carpel identity is observed in sei-1 mutants instead.
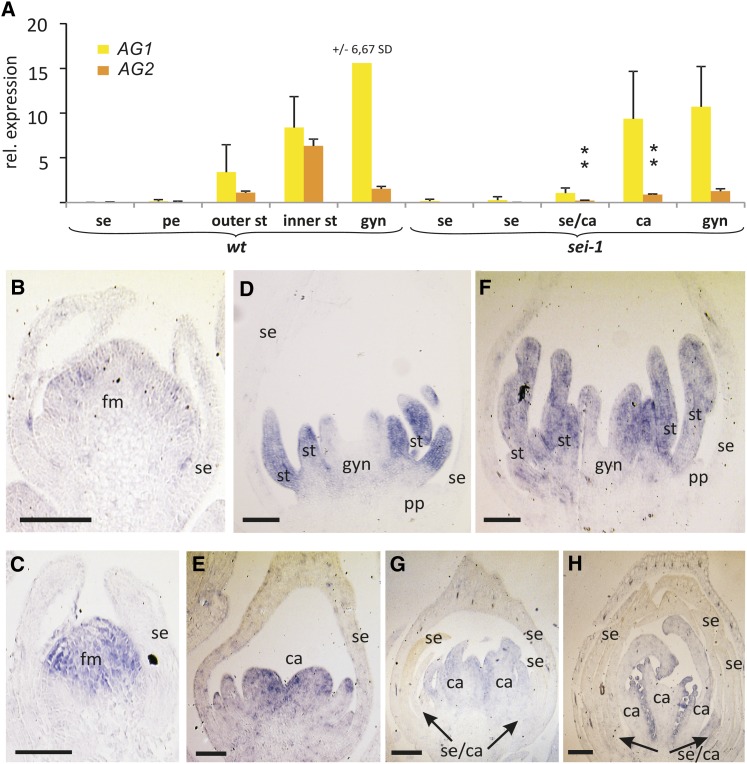
As class C genes are required for conferring stamen identity, which the sei-1 mutant is variably unable to maintain, class C gene expression was analyzed in wild-type and mutant floral organs at anthesis. AG1 and AG2 are the two known C class genes in California poppy (Zahn et al., 2005; Yellina et al., 2010). Both are expressed in mature stamens (Figure 2A), and the levels are higher in inner stamens compared with outer stamens. Both are also expressed in the gynoecium, with AG1 expression being markedly higher. In sei-1 mutants, expression of the AG2 gene is significantly reduced in organs in the whorls occupied by stamens in the wild type (Figure 2A). This occurs for both the sepal/carpel organs occupying the outer positions and in the carpel-like organs in the inner whorls. Expression of AG1 is less affected, so it may be regulated differently from AG2.
Figure 2.
Expression of C Class Genes in the Wild Type and sei-1 Mutants.
(A) Quantitative RT-PCR of AG1 and AG2 in floral organs at anthesis in wild-type (wt) and sei-1 plants. Error bars depict sd. Abbreviations are as in Figure 1.
(B), (D), and (F) In situ hybridization of combined AG1/2 transcripts in wild-type buds at stage 3 (B), early stage 5 (D), and late stage 5 (F).
(C), (E), (G), and (H) AG1/2 in situ hybridization in floral buds of sei-1 plants at stage 3 (C), early stage 5 (E), late stage 5 (G), and stage 7 (H). In (G) and (H), arrows indicate the presence of mosaic organs composed of sepal and carpel-like (se/ca) characteristics. ca, carpels; fm, floral meristem; gyn, central gynoecium; pp, petal primordia; se, sepals; se/ca, sepal-like/carpel-like mosaic organs; st, stamens. Bars = 100 µm.
In situ hybridization analysis of the two California poppy AG genes in developing wild-type and mutant flowers was examined next to reveal when changes in expression first appear. As the two genes are closely similar, a probe that does not discriminate between them had to be used. In the wild type, AG1/2 expression is present in the flower meristem at stages 3 and 4 (Figure 2B), in developing stamen primordia from early stage 5 (Figure 2D), and the gynoecium primordium at later stage 5 (Figure 2F) (Yellina et al., 2010). In the sei-1 mutant, early expression is detected in the floral meristem as in the wild type (Figure 2C). As primordia arise at positions occupied by stamens in the wild type, the more centrally located primordia show stronger AG1/2 expression at early stage 5 (Figure 2E) and especially later in stage 5 (Figure 2G). Overall, this expression seems to be weaker than at the equivalent stage in the wild type (Figure 2F). Later, as carpels start to differentiate, expression becomes patchy perhaps associated with mosaic organ identity (Figure 2H). The conclusion is that expression of the C function genes is weaker in mutant organs in outer stamen whorl positions from early in their development.
The Wild-Type SEI and Mutant sei-1 Proteins Are Capable of Similar Dimeric Interactions and CArG-Box Binding
The sei-1 mutant protein lacks the conserved PI motif at its C terminus, and the role of this in the formation of MADS multimers was investigated. First, dimeric interactions were examined. Two parallel approaches were adopted, yeast two-hybrid (Y2H) interaction tests (see Supplemental Figure 6 online) and tests of the recombination of split yellow fluorescent protein (YFP), bimolecular fluorescence complementation (BiFC) (see Supplemental Figure 7 online). Some combinations were further examined for their ability to interact with each other and a CArG-box by electrophoretic mobility shift assays (EMSAs) (see Supplemental Figure 8 online).
Homodimerization of SEI and of sei-1 were each clearly demonstrated by BiFC (Table 1) although not by Y2H (Table 1). However, gel shift assays also revealed SEI and sei-1 homomultimerization as well as their specific binding to a sequence carrying a CArG-box (see Supplemental Figure 8 online). Also, SEI and sei-1 were each able to heterodimerize with the three other putative B function California poppy MADS proteins, DEF1, DEF2, and DEF3. This was revealed by both Y2H and BiFC (Table 1, Figure 3A) as well as with EMSA for the DEF1 and DEF2 proteins (see Supplemental Figure 8 online). The two forms, SEI and sei-1, also interacted equally well with the C function protein AG1, at least by BiFC, although neither associated with the putative E function protein SEP3 (Table 1). Other tests of dimeric interactions not involving SEI or sei-1 revealed that the other three DEF class B proteins, the two AG class C proteins, and SEP3 (putative class E protein) can each homodimerize and that they can heterodimerize in most combinations, based on positive interactions in at least one of the three test methods used (see Supplemental Tables 1 and 2 and Supplemental Figures 6 to 8 online).
Table 1. Summary of Bimolecular Interactions of SEI and sei-1 with Class B, C, and E Homeotic Proteins of California Poppy.
| Partner | Y2Ha | BiFCb | ||
|---|---|---|---|---|
| SEI | sei-1 | SEI | sei-1 | |
| SEI or sei-1 | − | − | +++ | +++ |
| DEF1 | +++ | +++ | +++ | +++ |
| DEF2 | +++ | +++ | +++ | +++ |
| DEF3 | + | + | +++ | +++ |
| AG1 | − | − | +++ | +++ |
| AG2 | − | − | ND | ND |
| SEP3 | − | − | − | − |
+++, Strong interaction; +, weak interaction; −, no detectable interaction; ND, not determined.
Y2H results are illustrated in Figure 3A and Supplemental Figure 6 online.
BiFC results are illustrated in Supplemental Figure 7 online.
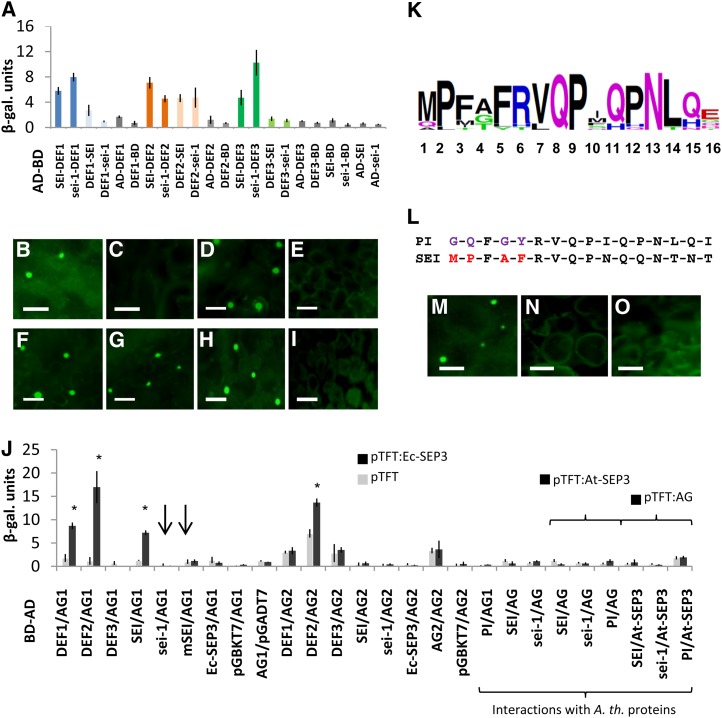
Figure 3.
Analysis of Interactions of B, C, and E Class Proteins Formed in Planta and in Yeast.
(A) B class protein dimerization in Y2H tests quantified with the β-Gal assay.
(B) to (I) TriFC experiments showing multimeric complex formation of three B, C, and/or E class proteins that differ between SEI and sei-1 interactions. The two partial YFP fusion constructs and the silent partner are as follows: (B) AG1:YFPN-DEF2:YFPC-SEI, (C) AG1:YFPN-DEF2:YFPC-sei-1, (D) SEP3:YFPN-SEI:YFPC-DEF1, (E) SEP3:YFPN-sei-1:YFPC-DEF1, (F) SEP3:YFPN-SEI:YFPC-DEF2, (G) SEP3:YFPN-sei-1:YFPC-DEF2, (H) AG1:YFPN-SEP3:YFPC-SEI, and (I) AG1:YFPN-SEP3:YFPC-sei-1. The bar length is 50 µm.
(J) Y3H analysis of multimer formation of three B, C, and E class proteins. The light-gray columns show interaction strength of the proteins expressed from BD and AD vectors together with the empty ternary vector pTFT1. The black columns show interaction strength when pTFT1 contains the coding sequence as given in the figure. Error bars depict the sd of replicates. Asterisks above the columns indicate significant differences (analysis of variance test; P < 0.05) in reporter gene activation between empty pTFT1 and pTFT1-EcSEP3 interactions, indicating formation of higher order complexes. Arrows indicate absence of multimer formation in cases where SEI was substituted by sei-1 or mSEI proteins.
(K) Sequence logo representation of the PI motif from selected PI-like proteins across angiosperms listed in Supplemental Figure 3 online. Numbers refer to the positions of the amino acid within the PI motif alignment.
(L) The sequence of the PI motifs of Arabidopsis PI and poppy SEI are shown, and the differing N-terminal amino acids are marked in red and purple.
(M) to (O) TriFC multimeric protein interactions of AG1, Ec-SEP3, and the modified SEI protein showing that the five N-terminal amino acids of the PI motif are required for the interaction: (M) AG1:YFPN-SEP3:YFPC-SEI, (N) AG1:YFPN-SEP3:YFPC-sei-1, and (O) AG1:YFPN-SEP3:YFPC-mSEI. The bar length is 50 µm.
The overall conclusion is that loss of the PI motif in the sei-1 protein has not compromised its ability to interact with itself or pairwise with other B, C, or E proteins. Also, both SEI and sei-1 can bind specifically to a CArG-box sequence.
The sei-1 Protein Is Unable to Form Specific Higher Order Protein Complexes
As floral homeotic function very likely requires the formation of higher order protein complexes (Honma and Goto, 2001; Theissen and Saedler, 2001), multimeric interactions of the B, C, and E class proteins of California poppy were analyzed using a modified BiFC approach and quantitative yeast three-hybrid (Y3H) analysis.
A modified BiFC procedure, termed trimolecular fluorescence complementation (TriFC), was performed with all protein combinations that did not form dimers in the BiFC assay. The two constructs used for BiFC were coinjected with a third construct encoding an untagged MADS protein to test if this allowed the two tagged molecules to associate (Figures 3B to 3I; see Supplemental Figure 9 online). In three independent experiments, a total of 12 complexes were consistently observed to form in planta dependent on the addition of the third partner (Table 2). Significantly, three of the complexes were unable to form in the TriFC assay when the mutated protein sei-1 was added instead of SEI (Table 2). One of these involved SEI or sei-1 as the silent partner added to a combination of California poppy AG1 and DEF2 (Figures 3B and 3C) in a BBC complex. The second was a BBE multimer of SEP3 and SEI or sei-1 plus the silent partner DEF1 (Figures 3D and 3E), although when DEF2 was used as the silent partner instead, it did not interact differentially with SEI and sei-1 (Figures 3F and 3G). The third differential response came in the BCE association of AG1 and SEP3 where the addition of SEI but not sei-1 led to multimer association (Figures 3H and 3I).
Table 2. Molecular Interactions between Three California Poppy B, C, and/or E Proteins Tested by TriFC Using Two Tagged Proteins and a Third Untagged (Silent) Version.
| Combination | YFP N | YFP C | Silent Partner | Interaction |
|---|---|---|---|---|
| BBC | ||||
| SEI/DEF1/AG1 | AG1 | DEF1 | SEI | − |
| AG1 | DEF1 | sei-1 | − | |
| SEI/DEF2/AG1 | AG1 | DEF2 | SEI | +++a |
| AG1 | DEF2 | sei-1 | −a | |
| SEI/DEF3/AG1 | AG1 | DEF3 | SEI | +++ |
| AG1 | DEF3 | sei-1 | +++ | |
| AG1 | DEF3 | mSEI | +++ | |
| DEF3 | AG1 | SEI | − | |
| DEF3 | AG1 | sei-1 | − | |
| BBE | ||||
| SEI/DEF1/SEP3 | SEP3 | SEI | DEF1 | +++a |
| SEP3 | sei-1 | DEF1 | −a | |
| SEP3 | DEF1 | SEI | − | |
| SEP3 | DEF1 | sei-1 | − | |
| SEI | SEP3 | DEF1 | − | |
| sei-1 | SEP3 | DEF1 | − | |
| SEI/DEF2/SEP3 | SEP3 | SEI | DEF2 | +++ |
| SEP3 | sei-1 | DEF2 | +++ | |
| DEF2 | SEP3 | SEI | +++ | |
| DEF2 | SEP3 | sei-1 | +++ | |
| SEI/DEF3/SEP3 | SEP3 | DEF3 | SEI | +++ |
| SEP3 | DEF3 | sei-1 | +++ | |
| DEF3 | SEP3 | SEI | − | |
| DEF3 | SEP3 | sei-1 | − | |
| BCE | ||||
| SEI/AG1/SEP3 | AG1 | SEP3 | SEI | +++a |
| AG1 | SEP3 | sei-1 | −a | |
| AG1 | SEP3 | mSEI | −a |
Interactions that differ between SEI and sei-1.
Some of the interactions are illustrated in Figures 3B to 3I, 3M to 3O, and Supplemental Figure 9 online. +++, Strong interaction; −, no interaction.
Not surprisingly, we found that the ability for higher order complex formation is dependent on the three-dimensional orientation of the proteins in the complex (Table 2). For example, a BBC multimeric complex is only formed by AG1 and DEF3 with untagged SEI or sei-1 if AG1 is bound to the N-terminal half of YFP and DEF3 to the C-terminal half. The same specificity occurs for the BBE combination of untagged SEI or sei-1 with SEP3 and DEF3. Thus, the absence of association of the three MADS proteins in one specific orientation does not demonstrate that the combination cannot occur.
It seems likely that BCE complexes are involved in stamen development, and because sei-1 was unable to support one such complex in the TriFC tests, this and a series of other BCE multimeric complexes were tested using the Y3H system (Figure 3J, Table 3). Significantly, the same three proteins involved in the TriFC BCE interaction that differed between SEI and sei-1 (Figures 3H and 3I, Table 2) also differed when tested by Y3H. In this case, BD-SEI and AD-AG1 interacted when pTFT-SEP3 was also present, but BD-sei-1 did not (Figure 3J, left arrow). In passing, the other C function protein, AG2, did not show any interaction with either SEI or sei-1. However, the other B function proteins, DEF1, DEF3, and especially DEF2, also interacted in many of the tests with either of the C function proteins AG1 or AG2 and the putative E function protein SEP3 (Table 3, Figure 3J).
Table 3. Molecular Interactions between Three California Poppy B, C, and E Proteins and the Arabidopsis PI Protein Tested by the Y3H Method.
| Combination | BD | AD | pTFT | Interaction |
|---|---|---|---|---|
| BCE | ||||
| SEI/AG1/SEP3 | SEI | AG1 | SEP3 | +++a |
| sei-1 | AG1 | SEP3 | −a | |
| mSEI | AG1 | SEP3 | −a | |
| PI | AG1 | SEP3 | − | |
| SEI/AG2/SEP3 | SEI | AG2 | SEP3 | − |
| sei-1 | AG2 | SEP3 | − | |
| DEF/AG1/SEP | DEF1 | AG1 | SEP3 | +++ |
| DEF2 | AG1 | SEP3 | +++ | |
| DEF3 | AG1 | SEP3 | − | |
| DEF/AG2/SEP | DEF1 | AG2 | SEP3 | − |
| DEF2 | AG2 | SEP3 | +++ | |
| DEF3 | AG2 | SEP3 | − |
Interactions that differ between SEI and sei-1. Interactions are illustrated in Figure 3J. +++, Strong interaction; −, no interaction.
Thus, differences between SEI and sei-1 in the establishment of a specific B, C, and E class protein complex were revealed by both the TriFC and Y3H assays. This suggests that the formation of this complex, involving SEI, AG1, and SEP3, requires the C-terminal domain of SEI. This complex is most likely required for stamen identity, and the failure of its formation may account for the homeotic conversions of stamens into carpels in the sei-1 mutant.
The Arabidopsis PI Motif Is Unable to Replace the California Poppy PI Motif in SEI
As the C-terminal domain of PI orthologous proteins contains the PI motif, we investigated the sequence and function of this highly conserved stretch of amino acids. First, an alignment of 37 PI orthologous proteins from all major groups of angiosperms was produced (see Supplemental Figure 3 online); second, a sequence logo representing the conserved amino acids within the PI motif was generated (Figure 3K). The most highly conserved amino acids are Pro at position 2, the three amino acids Val, Glu, and Pro, spanning positions 7 to 9 in the central region, and the two residues Asn and Leu near the C-terminal end at positions 13 and 14.
The alignment shows that different species of angiosperms exhibit lineage-specific changes within the PI motif, apparently and most dramatically in the Brassicaceae family, where the PI protein of Arabidopsis, for example, significantly differs at the N-terminal end, with a two amino acid deletion followed by FGY in the first five positions instead of the consensus sequence MPFAF (see Supplemental Figure 3 online). The SEI protein includes the highly conserved N-terminal part shared by all except the Brassicaceae, although it does differ in the last three amino acids of the otherwise highly conserved C-terminal part, a feature it shares with the opium poppy PI proteins (Figures 3K and 3L; see Supplemental Figure 3 online).
The Arabidopsis PI motif has been shown to be unnecessary for interaction with other MADS proteins, so the difference between SEI and sei-1 might involve these specific residues rather than the loss of the full PI motif in sei-1. To investigate this, the five amino acids preceding the highly conserved C-terminal part of the PI motif were replaced by those from Arabidopsis, including FGY and two N-terminal amino acids outside of the PI motif. Multimer formation was tested with the modified TriFC procedure. This resulted in the failure of the modified SEI (mSEI) to form multimers with California poppy SEP3 and AG1, very similar to what has been observed for sei-1 (Figures 3M to 3O, Table 2). In addition, the same modified mSEI sequence was tested in a Y3H test with AG1 and SEP3, and again multimerization was almost abolished (Figure 3J, right arrow, Table 3). Moreover, the full Arabidopsis PI sequence was unable to replace SEI in its interaction with California poppy AG1 and SEP3 (Figure 3J, Table 3).
Similarly, we tested if the poppy proteins AG1 and SEP3 could be replaced by AG and SEP3 of Arabidopsis in their differential interaction with SEI or sei-1. Again, no interactions could be observed when any Arabidopsis protein replaced an California poppy protein in a multimeric complex comprising B, C, and E proteins (Figure 3J), suggesting that the B, C, and E proteins in California poppy and Arabidopsis have coevolved since the plant lineages separated producing lineage-specific interaction motifs required for complexes.
Overall, our results indicate that the first five amino acids of the PI motif are required for multimerization of the poppy B, C, and E proteins and that this homeotic complex is required to confer stamen identity.
DISCUSSION
The sei-1 Mutant Is a B Class Floral Homeotic Mutant
We characterized a floral homeotic mutant, sei-1, in a basal eudicot, California poppy. SEI was shown to be a B function gene of the PI/GLO clade. Only one PI/GLO gene has been identified in the California poppy genome, although there are three known AP3/DEF paralogs, one of which is identified here. The sei-1 mutant flower generates sepals in place of petals, and the multiple stamen whorls are variably changed to sepal-carpel mosaics, with progressively increasing carpelloidy toward the center of the flower. The sei-1 mutant results from a DNA insertion near the C-terminal end of SEI, and the deduced sei-1 mutant protein lacks the conserved PI domain present in this region in most PI/GLO proteins. The main novelty of our study lies in the finding that the PI motif is required for several specific multimeric associations of MADS proteins.
The PI Motif of SEI Is Required for Specific BCE MADS Protein Interactions Likely to Control Stamen Identity
To deduce a possible role of the PI motif, we first compared dimeric interactions of SEI and sei-1 with other MADS domain proteins using a combination of Y2H and BiFC methods (Table 1), but no differences were detected. Also, both forms could bind specifically to a CArG sequence generally recognized by MADS domain proteins.
We then examined higher order interactions using a TriFC procedure in planta (Table 2). In some cases, interactions were also examined by the Y3H procedure (Table 3). Using both methods, we showed that the SEI protein could interact simultaneously with the California poppy C function protein AG1 and the putative E function protein SEP3 but that this interaction did not occur when the sei-1 protein was present instead. Evidence for a role of the PI motif in promoting this interaction was strengthened by changing four of the five amino acid residues at the N-terminal end of the wild-type SEI protein to match those of the divergent PI motif structure in Arabidopsis. Even this slight change was sufficient to prevent the multimeric association.
A multimeric combination of B, C, and E MADS proteins is likely to be required to confer stamen identity, consistent with this being a function of the SEI protein. The other California poppy B function proteins, DEF1, 2, and 3, can dimerize with SEI, and it may be that they are normally involved in a quaternary interaction with SEI, AG1, and SEP3. Certainly, DEF1 and DEF2 at least can each also form a three way interaction with AG1 and SEP3 in yeast (Table 3). The fact that loss of SEI function alone reveals a typical B function mutant phenotype suggests that the DEF proteins cannot function redundantly in place of SEI, so a quaternary combination may be the main functional unit in vivo.
Although E function is thought to be involved in stamen identity, higher order multimeric combinations of B and C function proteins in the absence of SEP3 were also examined by the TriFC method (i.e., BBC combinations). One of these, involving the B function proteins SEI and DEF2 together with the C function AG1, also required SEI rather than sei-1 for its formation (Table 2), providing further evidence for the importance of the PI motif in higher order combinations. DEF2 is expressed in mature petals and stamens but only weakly in newly developing buds, and parallel tests using DEF1 (expressed specifically in early buds) and DEF3 (expressed throughout flower development) were examined. However, these yielded no differences between the behavior of SEI and sei-1. The experimental combinations tried here may have involved molecular constraints that prevented differences in interactions being detected. The same constraints may have masked the possible involvement of the second C function protein AG2, whose expression is more closely correlated with stamens than AG1, in a multimeric involvement with SEI or sei-1 and SEP3 (Table 3).
The PI Motif Is Also Required for a Specific BBE MADS Protein Interaction That May Control Petal Identity
Petal identity is defined by the action of B function without C function, and E function is also likely to be required no candidate A function partners are known in poppy. TriFC tests of interactions of SEI and sei-1 with DEF1-3 and SEP3 were also set up (i.e., BBE tests). Again, a difference was detected between SEI and sei-1 in one of these, in this case involving DEF1 and SEP3 partners (Table 2). Thus, petal identity, too, may depend on the PI motif in SEI promoting multimeric combinations of B, B, and E units. Interestingly, DEF1 expression occurs specifically in early developing buds when petal identity is likely to be conferred.
BBE Protein Complexes May Be Sufficient for Transcriptional Autoregulation of B Gene Expression
In the sei-1 mutant, the expression of the sei-1 gene as well as of the three DEF-like genes is severely reduced. This could be because organ identity of the petals and stamens, organs of strong B class gene expression in the wild type, cannot be established, so these organs are replaced by sepals and carpels. Also, the DEF-like genes could be activated by SEI. However, B gene expression is lost even in very young buds when the organ primordia are just arising (Figure 1H), so positive reinforcement of early B class gene expression by autoregulation may be disrupted.
It is relevant here that comparative expression analysis of SEI and sei-1 alleles in heterozygous plants shows that the level of sei-1 transcript is substantially reduced. It may be that the DNA insertion has resulted in compromised transcription. Alternatively, the aberrantly long 3′UTR may trigger specific RNA decay mechanisms leading to a shorter mRNA half-life (Kertész et al., 2006). However, sei-1 transcripts still accumulate to around 15% of the SEI transcript level in the heterozygote, indicating that significant translation does occur in planta, as it clearly can in yeast (Saccharomyces cerevisiae) and tobacco (Nicotiana benthamiana) leaves. Interestingly, the expression of the SEI allele is also significantly reduced in heterozygotes, although the plants are still able to produce wild type flowers. In heterozygotes, it may be that the sei-1 protein exerts a negative effect on complex formation affecting transcriptional auto-regulation of both alleles at the SEI locus.
Previous work has shown that several B-class proteins, such as AP3, actively maintain their expression by binding of AP3/PI-like heterodimers to CArG-box motifs in their own promoter (Tröbner et al., 1992; Hill et al., 1998; Honma and Goto, 2000). However, in Arabidopsis, the overexpression of AP3 and PI alone is not able to transform leaves into petals and is unable to activate the transcriptional autoregulatory loop outside of the flower, suggesting that the AP3-PI dimer alone is unable to regulate target genes. The concerted overexpression of AP3, PI, and SEP3 is able to convert leaves into petals and SEP3 additionally mediates higher order complexes. And while SEP3 can upregulate B gene expression in Arabidopsis, it is most likely also required for transcriptional autoregulation in Arabidopsis (Goto et al., 2001; Honma and Goto, 2001; Pelaz et al., 2001; Kaufmann et al., 2009).
Our data suggest that in California poppy, the transcriptional autoactivation of B genes is interrupted in sei-1 plants. Consistent with this, we have evidence that the SEI-DEF1-SEP3 (BBE) interaction is not supported by sei (see above), although the other two DEF proteins, DEF2 and DEF3, did not show a similar difference. Further investigation of the possible generality of BBE activation of B class gene expression is necessary.
C Class Gene Regulation by B Class Genes
The sei-1 mutant allowed us to characterize a B class mutant in a species with multiple stamen whorls. Figure 1D, 1E and 2 indicate that the transition in identity of organs in the position of the wild-type stamen whorl from sepal to carpel is correlated with a change in class C gene expression. As expression of AG2 at least is significantly reduced in sei-1, we propose that the AP3/PI-like genes are directly or indirectly required to maintain the full level of class C gene expression throughout all stamen whorls in the wild-type California poppy plants. This finding is in line with recent studies showing that the AP3-PI heterodimer binds to the AG genomic locus (Wuest et al., 2012) of Arabidopsis, but genetic evidence for activation of AG by AP3 and/or PI is lacking (Bowman et al., 1991a). C class gene expression is restricted to the central whorls by the A function genes, which include AP1 (Bowman et al., 1991a). However, the A function is thought to be Brassicaceae specific, and in the asterids petunia and snapdragon, C gene expression is excluded from the perianth by the action of microRNA genes BLIND and FISTULATA, respectively (Cartolano et al., 2007). Interestingly, recent experiments by Pabón-Mora et al. (2012) have revealed that expression of the opium poppy AG homolog and reproductive organ identity are expanded when AP1/FUL-like genes are downregulated by VIGS. However, downregulation of the AP1/FUL-like genes in California poppy had no effect on floral organ identity, indicating that spatial regulation of AG homolog expression is variable among angiosperms (Pabón-Mora et al., 2012).
The reinforcing effect of B class gene expression on C class genes might be specific to species that generate multiple stamen whorls and that might require a mechanism to maintain stamen identity throughout several whorls. However, silencing of PI in columbine, also a member of the Ranunculales and with multiple stamen whorls, results in organs with carpeloid identity in the position of the stamens and no sepal-like organs were observed (Kramer et al., 2007). Similarly, downregulation of either of the B genes in opium poppy did not result in sepal-like organs in whorls of wild-type stamens (Drea et al., 2007). Two scenarios are plausible to explain the evolution of this regulation of class C genes considering that recent comparative morphology analyses suggest that the ancestral angiosperm flower had more than two stamen whorls (or stamen series in species with spiral phyllotaxy) (Endress and Doyle, 2009). One possibility is that this regulatory module is the ancestral condition for angiosperms and the lineages leading to columbine and opium poppy have lost this regulatory module. The second scenario is that the regulatory module is specific to the lineage leading to California poppy and organ identity of outer stamens is assured with a different, unknown mechanism in other lineages.
The PI Motif Carries a More Rapidly Evolving Short Linear Interaction Motif
The sei-1 mutation has provided a tool to study the function of the C-terminal domain and to possibly understand the reason for the conflicting results on the function of the PI motif in an evolutionary context. Here, we have shown that while the PI motif is completely lost in sei-1 mutants, it is the first five residues that are required for a multimeric association of SEI with specific C and E proteins. When we converted four of these residues to those present in the Arabidopsis PI protein, the association was lost.
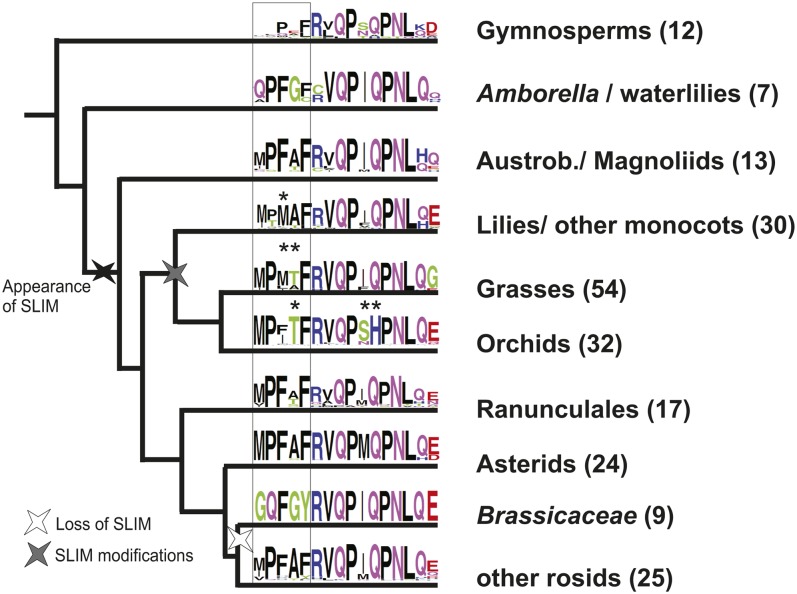
More generally, detailed sequence analysis of the PI motif reveals lineage-specific differences in the extremely conserved amino acid residues in the N-terminal part of the motif (Figure 4; see Supplemental Figure 3 online). Rosids like Populus trichocarpa or Carica papaya show the consensus residues, but the Brassicaceae have accumulated lineage-specific changes leading to the loss of a hydrophobic residue and a chain bending Pro as well as the gain of two flexible Gly residues, and the legumes have lost the PI motif altogether. The two amino acids lost in the Brassicaceae are conserved in PI orthologs of all other selected representatives of the magnoliids, monocots, basal eudicots, and asterids (see Supplemental Figure 3 online). Our sequence analysis further suggests that the N-terminal part of the PI motif is angiosperm specific and not present in gymnosperm AP3/PI-like genes.
Figure 4.
Hypothesis of PI Motif SLiM Evolution.
Schematic and highly simplified representation of the phylogeny of seed plants (based on Soltis et al., 2011). Lineage names are given, and the numbers of sequences used for PI motif representation are shown in parentheses. The black rectangle symbolizes the hypothesized SLiM in the PI motif. The SLiM postulated to be required for B-C-E multimer complex formation appeared before the divergence of the Magnoliidae ancestor from the rest of the angiosperm lineages and is indicated by a black star. The PI motif is conserved in sequence and position in representatives of the Magnoliidae, Monocotyledonae, Ranunculales, Asteridae, and many rosids but has been lost in Brassicaceae species shown by a white star, symbolizing the mutation of four amino acid residues. The gray star highlights lineages within the monocots with deviations from the SLiM consensus sequence with positions indicated by asterisks.
[See online article for color version of this figure.]
We hypothesize that the N-terminal part of the PI motif represents a short linear interaction motif (SLiM; Neduva and Russell, 2005). SLiMs are stretches of three to 10 amino acids that are often part of an otherwise unstructured region of the protein, and they play crucial roles in protein interaction networks. SLiMs show different degrees of sequence conservation; some positions are more tolerant to exchanges allowing for a high degree of evolutionary plasticity, but others are extremely conserved. In many examples, they have been shown to mediate specific protein interactions (Neduva and Russell, 2005; Diella et al., 2008; Wagner and Lynch, 2008).
The SLiM in the PI motif apparently evolved in the angiosperm lineage after the Amborellaceae and Nymphaeaceae ancestors diverged from the lineage that led to all other angiosperms, but before the magnoliids evolved (Figure 4). It was then maintained in at least one PI-like protein in the different angiosperm lineages, except that the lineage leading to the Brassicaceae, after their split from the Cleomaceae, lost the important residues for specifically mediating floral homeotic complexes. Independent from the loss of only a few conserved residues in the Brassicaceae, at least part of the Fabaceae lost the C-terminal domain completely. Major modifications of the PI motif seem to have also occurred in the monocot lineage where grasses and orchids in particular deviate from the consensus sequence of angiosperm PI motifs (Figure 4; see Supplemental Figure 3 online). Functional evidence from another monocot, Lilium longiflorum, also indicates that B function diversity is associated with SLiM variation (Chen et al., 2012).
The loss of a protein interaction SLiM that is crucial for establishing floral homeotic complexes required for stamen organ identity would ultimately lead to sterility of the affected plant. However, compensatory mutations in the other participating proteins, such as SEP-like or AG-like proteins, may be able to overcome this failure in protein complex formation and can be hypothesized to have arisen in the Fabaceae and Brassicaceae.
The PI motif has been conserved during evolution for tens of millions of years, but its functional importance has remained controversial. Our results suggest that several conserved residues in the N-terminal region are required for the specific higher order MADS protein interactions that are necessary to confer stamen identity, and possibly petal identity, in most flowering plants.
METHODS
California Poppy DEF3 Identification and Expression Analysis of AP3/PI-Like and AG-Like Genes
RNA was extracted from tissue samples of all floral whorls of California poppy (Eschscholzia californica) wild type and sei with the OLS Plant RNA isolation kit (OLS Life Sciences). Five hundred nanograms of total RNA with an oligo(dT) primer was used to synthesize first-strand cDNA using RevertAid H Minus first-strand cDNA synthesis kit (Fermentas). The California poppy DEF3 coding sequence was amplified with 3′RACE using the RACE-DEF3fw primer, which was derived from NG sequencing data (Wall et al., 2009), and AB07 rev, cloned into pGEM, and sequenced. All primer sequences are provided in Supplemental Table 3 online.
cDNA pools were diluted 1:25 or 1:50 for subsequent quantitative RT-PCR analysis, using ACTIN2 and GAPDH gene expression as reference genes. Relative target gene expression was measured, and analysis was done with the ΔΔCT (cycle threshold) method following normalization by geometric averaging of the two internal control genes (Vandesompele et al., 2002). Primer sequences for California poppy DEF1, DEF2, DEF3, and SEI as well as for California poppy SEP3, AG1, and AG2 are provided in Supplemental Table 3 online. Expression was measured with three technical replicates for each of the two biological replicates. In situ hybridization of SEI transcripts was performed on sections of floral buds of consecutive developmental stages with DIG-labeled probe encompassing nucleotides 509 of the SEI coding sequence to nucleotide 53 into its 3′UTR (198 nucleotides total length) as described earlier (Orashakova et al., 2009). The California poppy AG in situ hybridization experiments were performed as described by Yellina et al. (2010).
Phylogeny Reconstruction and PI Motif Analysis
Nucleotide sequences of AP3 and PI orthologs from Ranunculales, GLO and DEF of snapdragon (Antirrhinum majus), and PI and AP3 of Arabidopsis thaliana were gathered from the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov) and translated in silico with BioEdit (Hall, 1999). The amino acid sequences were aligned using ClustalW2 using default parameters (http://www.ebi.ac.uk/Tools/msa/clustalw2). Neighbor-joining analysis using observed distances and 1000 bootstrap replicates were employed on the protein alignment spanning amino acid position 25 of the conserved MADS domain to the end of the K domain with the program SEAVIEW 4 (Gouy et al., 2010). The GGM2 sequence of the gymnosperm Gnetum gnemon was used as an outgroup representative.
A collection of 37 PI amino acid sequences from all major angiosperm lineages was aligned using ClustalW2 (http://www.ebi.ac.uk/-Tools/msa/clustalw2) and used to build a sequence logo representation of the conservation of individual positions in the alignment using default parameters employing Weblogo 2.8.2 (http://weblogo.berkeley.edu) (Crooks et al., 2004).
Molecular Characterization of the sei-1 Mutant Locus
3′RACE
Coding sequences of California poppy DEF3 and SEI from wild-type and sei-1 mutants were PCR amplified from cDNA pools with gene-specific forward and the AB07 reverse primer. Amplified fragments were cloned using the pDRIVE cloning kit (Qiagen) and sequenced.
Rapid Amplification of Genomic Ends (RAGE)
Genomic DNA from wild-type and sei plants was isolated with the Peqlab Mini Gold kit (Peqlab). DNA was treated with the restriction enzymes BamHI, EcoRI, HindIII, XbaI, and XhoI and blunt-ended with T4 DNA polymerase, and the blunt-ended DNA was ligated to the RAGE adaptor (Siebert et al., 1995). Cloning of the SEI locus was performed in a nested PCR approach with the Phusion polymerase (Finnzymes). The cycling profile was as follows: 94°C for 25 s, 67°C for 3 min for 7 cycles, and 94°C for 25 s and 65°C for 3 min for 35 cycles. The secondary PCR was done with the following PCR conditions: 94°C for 25 s, 67°C for 3 min for five cycles and 94°C for 25 s and 65°C for 3 min for 20 cycles. The obtained genomic DNA fragments were sequenced.
VIGS
A fragment of the SEI cDNA (nucleotide positions 215 of the coding sequence to position 43 of the 3′UTR [489 nucleotides]) was amplified with primers containing restriction sites. SEI was digested with BamHI and XhoI and cloned into the equivalently digested pTRV2 vector to create pTRV2-SEI. Agrobacterium tumefaciens GV3101 was used to inoculate 3-week-old California poppy seedlings as described previously (Orashakova et al., 2009), and plants were grown under the conditions described earlier (Wege et al., 2007).
Protein Interaction Analysis
Y2H
California poppy DEF1, DEF2, DEF3, SEI, and sei-1 open reading frames (ORFs), all lacking their MADS box, were amplified from cDNA and cloned in frame into the yeast expression vectors pGADT7 and pGBKT7 (Clontech). Y2H analyses were performed as described previously (Erdmann et al., 2010). California poppy AG1ΔC lacking the C-terminal domain (ΔC) and AG2ΔM lacking only the MADS domain were assayed. The full-length SEP3 was cloned into both yeast expression vectors. The strength of interaction as observed in the Y2H experiments was classified in three categories: strong, when yeast growth on SD media (-Leu/-Trp/-His) with 3 mM 3-Amino-1,2,4-triazole at 30°C was observed in all dilutions, all yeast colonies were stained blue after the β-Gal assay, and the interaction was observed regardless of the vector the protein was expressed from; weak interactions show yeast growth in undiluted and 1:10 dilutions only, all colonies were stained blue after the β-Gal assay, and protein interactions were observed in at least one vector combination; and no interactions. The Y2H experiments were performed in at least three biological replicates.
Y3H
California poppy AG1ΔM and AG2ΔM ORFs were cloned into the pGADT7, the full-length AGL9 ORF was cloned into the ternary vector pTFT1 (Egea-Cortines et al., 1999), and the AP3/PI-like ORFs were used in pGBKT7 without their MADS box. The Arabidopsis ORFs of PI, AG, and SEP3 were cloned from cDNA and introduced into the corresponding vectors for Y3H analysis.
All tested combinations were cotransformed into AH109 yeast cells and selected on SD media lacking Leu, Trp, and adenine. To quantify the interaction of putative B class proteins with AG1, AG2, and SEP3, Y3H β-Gal liquid assays using ortho-Nitrophenyl-β-galactoside as substrate were employed (Miller, 1972). Three to six independent clones for every combination and three technical replicates for each clone were used to determine the β-Gal activity.
EMSAs were conducted as described previously (Melzer and Theissen, 2009) except that ∼400 ng of Poly(deoxyinosinic/deoxycytidylic) instead of salmon sperm DNA was used as nonspecific competitor for every binding reaction. Full-length coding sequences of DEF1, DEF2, SEI, sei1, AG1, and SEP3 were amplified and cloned into the in vitro translation vector pSPUTK. An SEI allele was used that differed in two amino acid positions C-terminal of the PI motif from the SEI sequences used for other interaction assays. This was assumed not to influence the DNA binding activity of SEI. Two microliters of in vitro–translated protein and ∼0.1 ng of labeled DNA probes were used per reaction. Cotranslation was performed when two proteins were assayed for heterodimer formation. The CArG-box encoded on the DNA probe was derived from the regulatory intron of AG from Arabidopsis. The sequence of the complete probe was 5′-AATTCGAAATTTAATTATATTCCAAATAAGGAAAGTATGGAACGTTGAATT-3′ (CArG-box is underlined). As a specificity control, a probe with the same nucleotide composition but in randomized order was used. The sequence of this probe was 5′-AATTCATAAAACGGCAAGGAGAATTATATTTTTATGATGAACATATGAATT-3′.
BiFC was performed according to Hu et al. (2002). Full-length sequences of California poppy DEF1, DEF2, DEF3, SEI, sei1, AG1, and SEP3 were cloned, with their native stop codon deleted, into the BiFC vectors pNBV-YC and pNBV-YN (Walter et al., 2004). All pNBV-YC and pNBV-YN vector constructs were verified by sequencing and subsequently cloned into the plant expression vector pMLBART by NotI digestion. All pMLBART constructs were transformed into the Agrobacterium strain GV3101. As a positive control, the vector constructs pSPYCE-35S/bzip63yc and pSPYNE-35S/bzip63yn were employed (Walter et al., 2004). Leaves of 4-week-old Nicotiana benthamiana plants were inoculated with mixtures of Agrobacterium strains carrying pMLBART-YN and pMLBART-YC constructs in different protein combinations and additionally an Agrobacterium strain harboring the p19 plasmid to suppress RNA silencing response in transformed plant cells. To detect higher order interactions (TriFC), a third coding sequence without a YFP fragment was expressed under the control of the 35S promoter from the pMLBART vector that was cotransformed into N. benthamiana. The YFP fluorescence signal demonstrating protein–protein interactions in living plant cells was observed 3 to 4 d after inoculation. The BiFC experiments were performed in at least three biological replicates.
Site directed mutagenesis was done according to Wang and Malcolm (1999), and two sets of primers (see Supplemental Table 3 online) were used to introduce multiple nucleotide substitutions simultaneously into the SEI ORF to change the N-terminal sequence of the PI motif of SEI into the N-terminal part of the PI motif of the Arabidopsis PI protein. The resulting ORF is mSEI in which the first five amino acids of the Arabidopsis PI motif replace the California poppy PI motif. A two-stage PCR was employed, and the resulting PCR products were digested with DpnI to remove nonmutated vector of the original PCR template. Mutated variants were sequenced and cloned into pMLBART as described above.
Accession Numbers
Sequence data from this article can be found in The Arabidopsis Information Resource or GenBank/EMBL databases under the following accession numbers: California poppy DEF1 (EF378697), DEF2 (EF378698), DEF3 (HE573239), SEI (EF378699), SEP3 (AY850180), AG1 (DQ088996), and AG2 (DQ088997); Arabidopsis PI (AT5G20240), AG (AT4G18960); and SEP3 (AT1G24260).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Scanning Electron Microscopy of Wild-Type Organs and sei-1 Carpel-Like Organs.
Supplemental Figure 2. Extended Phylogeny of Ranunculales AP3/PI-Like Proteins.
Supplemental Figure 3. Alignment of PI Protein Sequences and Their PI Motif.
Supplemental Figure 4. Amplification of the sei-1 Mutant Transcripts.
Supplemental Figure 5. SEI-VIGS Phenotypes and RT-PCR Expression Analysis of the SEI-VIGS–Treated Plants.
Supplemental Figure 6. Yeast Two-Hybrid Growth Assay of Floral Homeotic Proteins of California Poppy.
Supplemental Figure 7. Protein Dimerization Analysis by BiFC.
Supplemental Figure 8. DNA Binding of E. californica MADS Domain Proteins.
Supplemental Figure 9. Higher Order Protein Complex Formation Analyzed by TriFC.
Supplemental Table 1. Other Y2H Interactions Not Involving SEI or sei-1.
Supplemental Table 2. Other BiFC Interactions Not Involving SEI or sei-1.
Supplemental Table 3. A List of Oligonucleotide Primers Used in This Study.
Supplemental Data Set 1. Text File of Alignment Corresponding to Phylogenetic Analysis in Supplemental Figure 2.
Acknowledgments
We thank Robert Erdmann for help with the initial Y2H analysis, and Tezz Quon and Martin O’Brien for the thorough introduction to BiFC. Anna Fees cloned and sequenced the DEF3 gene. We thank Christian Gafert and Kenny Peuker for help with the EMSAs. We also thank Ralf Dringen for the use of his epifluorescence microscope for BiFC and TriFC observations as well as Angelika Trambacz and Werner Vogel for taking care of the poppy plants. This work was made possible by the Deutsche Forschungsgemeinschaft Grant BE 2547/6-1 and 6-2 and support of the University of Bremen to A.B.
AUTHOR CONTRIBUTIONS
M.L. performed all experiments except for the in situ hybridizations, some BiFC and TriFC, and EMSAs. S.O. performed the in situ hybridizations. S.L. performed parts of the BiFC and TriFC. R.M. performed the EMSAs. G.T., D.R.S., and A.B. analyzed data. A.B. and D.R.S. wrote the article with the help of all coauthors.
Glossary
- RACE
rapid amplification of cDNA ends
- UTR
untranslated region
- VIGS
virus-induced gene silencing
- Y2H
yeast two-hybrid
- YFP
yellow fluorescent protein
- BiFC
bimolecular fluorescence complementation
- EMSA
electrophoretic mobility shift assay
- Y3H
yeast three-hybrid
- TriFC
trimolecular fluorescence complementation
- SLiM
short linear interaction motif
- ORF
open reading frame
References
- Ambrose B.A., Lerner D.R., Ciceri P., Padilla C.M., Yanofsky M.F., Schmidt R.J. (2000). Molecular and genetic analyses of the silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol. Cell 5: 569–579 [DOI] [PubMed] [Google Scholar]
- Angenent G.C., Busscher M., Franken J., Dons H.J.M., van Tunen A.J. (1995). Functional interaction between the homeotic genes fbp1 and pMADS1 during petunia floral organogenesis. Plant Cell 7: 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A., Gleissberg S., Smyth D.R. (2005). Floral and vegetative morphogenesis in California poppy (Eschscholzia californica Cham.). Int. J. Plant Sci. 166: 537–555 [Google Scholar]
- Benlloch R., Roque E., Ferrándiz C., Cosson V., Caballero T., Penmetsa R.V., Beltrán J.P., Cañas L.A., Ratet P., Madueño F. (2009). Analysis of B function in legumes: PISTILLATA proteins do not require the PI motif for floral organ development in Medicago truncatula. Plant J. 60: 102–111 [DOI] [PubMed] [Google Scholar]
- Berbel A., Navarro C., Ferrándiz C., Cañas L.A., Beltrán J.P., Madueño F. (2005). Functional conservation of PISTILLATA activity in a pea homolog lacking the PI motif. Plant Physiol. 139: 174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J.L., Baum S.F., Eshed Y., Putterill J., Alvarez J. (1999). Molecular genetics of gynoecium development in Arabidopsis. Curr. Top. Dev. Biol. 45: 155–205 [DOI] [PubMed] [Google Scholar]
- Bowman J.L., Drews G.N., Meyerowitz E.M. (1991a). Expression of the Arabidopsis floral homeotic gene AGAMOUS is restricted to specific cell types late in flower development. Plant Cell 3: 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J.L., Smyth D.R., Meyerowitz E.M. (1989). Genes directing flower development in Arabidopsis. Plant Cell 1: 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J.L., Smyth D.R., Meyerowitz E.M. (1991b). Genetic interactions among floral homeotic genes of Arabidopsis. Development 112: 1–20 [DOI] [PubMed] [Google Scholar]
- Cartolano M., Castillo R., Efremova N., Kuckenberg M., Zethof J., Gerats T., Schwarz-Sommer Z., Vandenbussche M. (2007). A conserved microRNA module exerts homeotic control over Petunia hybrida and Antirrhinum majus floral organ identity. Nat. Genet. 39: 901–905 [DOI] [PubMed] [Google Scholar]
- Chen M.-K., Hsieh W.-P., Yang C.-H. (2012). Functional analysis reveals the possible role of the C-terminal sequences and PI motif in the function of lily (Lilium longiflorum) PISTILLATA (PI) orthologues. J. Exp. Bot. 63: 941–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen E.S., Meyerowitz E.M. (1991). The war of the whorls: Genetic interactions controlling flower development. Nature 353: 31–37 [DOI] [PubMed] [Google Scholar]
- Crooks G.E., Hon G., Chandonia J.-M., Brenner S.E. (2004). WebLogo: A sequence logo generator. Genome Res. 14: 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Egea-Cortines M., de Andrade Silva E., Saedler H., Sommer H. (1996). Multiple interactions amongst floral homeotic MADS box proteins. EMBO J. 15: 4330–4343 [PMC free article] [PubMed] [Google Scholar]
- Diella F., Haslam N., Chica C., Budd A., Michael S., Brown N.P., Trave G., Gibson T.J. (2008). Understanding eukaryotic linear motifs and their role in cell signaling and regulation. Front. Biosci. 13: 6580–6603 [DOI] [PubMed] [Google Scholar]
- Drea S., Hileman L.C., de Martino G., Irish V.F. (2007). Functional analyses of genetic pathways controlling petal specification in poppy. Development 134: 4157–4166 [DOI] [PubMed] [Google Scholar]
- Egea-Cortines M., Saedler H., Sommer H. (1999). Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J. 18: 5370–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress P.K., Doyle J.A. (2009). Reconstructing the ancestral angiosperm flower and its initial specializations. Am. J. Bot. 96: 22–66 [DOI] [PubMed] [Google Scholar]
- Erdmann R., Gramzow L., Melzer R., Theissen G., Becker A. (2010). GORDITA (AGL63) is a young paralog of the Arabidopsis thaliana B(sister) MADS box gene ABS (TT16) that has undergone neofunctionalization. Plant J. 63: 914–924 [DOI] [PubMed] [Google Scholar]
- Goto K., Kyozuka J., Bowman J.L. (2001). Turning floral organs into leaves, leaves into floral organs. Curr. Opin. Genet. Dev. 11: 449–456 [DOI] [PubMed] [Google Scholar]
- Goto K., Meyerowitz E.M. (1994). Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 8: 1548–1560 [DOI] [PubMed] [Google Scholar]
- Gouy M., Guindon S., Gascuel O. (2010). SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27: 221–224 [DOI] [PubMed] [Google Scholar]
- Hall T.A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41: 95–98 [Google Scholar]
- Hill T.A., Day C.D., Zondlo S.C., Thackeray A.G., Irish V.F. (1998). Discrete spatial and temporal cis-acting elements regulate transcription of the Arabidopsis floral homeotic gene APETALA3. Development 125: 1711–1721 [DOI] [PubMed] [Google Scholar]
- Honma T., Goto K. (2000). The Arabidopsis floral homeotic gene PISTILLATA is regulated by discrete cis-elements responsive to induction and maintenance signals. Development 127: 2021–2030 [DOI] [PubMed] [Google Scholar]
- Honma T., Goto K. (2001). Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409: 525–529 [DOI] [PubMed] [Google Scholar]
- Hu C.D., Chinenov Y., Kerppola T.K. (2002). Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9: 789–798 [DOI] [PubMed] [Google Scholar]
- Jack T. (2001). Plant development going MADS. Plant Mol. Biol. 46: 515–520 [DOI] [PubMed] [Google Scholar]
- Jack T., Brockman L.L., Meyerowitz E.M. (1992). The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68: 683–697 [DOI] [PubMed] [Google Scholar]
- Kanno A., Saeki H., Kameya T., Saedler H., Theissen G. (2003). Heterotopic expression of class B floral homeotic genes supports a modified ABC model for tulip (Tulipa gesneriana). Plant Mol. Biol. 52: 831–841 [DOI] [PubMed] [Google Scholar]
- Kaufmann K., Muiño J.M., Jauregui R., Airoldi C.A., Smaczniak C., Krajewski P., Angenent G.C. (2009). Target genes of the MADS transcription factor SEPALLATA3: Integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol. 7: e1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertész S., Kerényi Z., Mérai Z., Bartos I., Pálfy T., Barta E., Silhavy D. (2006). Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 34: 6147–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Koh J., Yoo M.-J., Kong H., Hu Y., Ma H., Soltis P.S., Soltis D.E. (2005). Expression of floral MADS-box genes in basal angiosperms: Implications for the evolution of floral regulators. Plant J. 43: 724–744 [DOI] [PubMed] [Google Scholar]
- Kramer E.M., Di Stilio V.S., Schluter P.M. (2003). Complex patterns of gene duplication in the APETALA3 and PISTILLATA lineages of the Ranunculaceae. Int. J. Plant Sci. 164: 1–11 [Google Scholar]
- Kramer E.M., Dorit R.L., Irish V.F. (1998). Molecular evolution of genes controlling petal and stamen development: Duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics 149: 765–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E.M., Holappa L., Gould B., Jaramillo M.A., Setnikov D., Santiago P.M. (2007). Elaboration of B gene function to include the identity of novel floral organs in the lower eudicot Aquilegia. Plant Cell 19: 750–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E.M., Irish V.F. (1999). Evolution of genetic mechanisms controlling petal development. Nature 399: 144–148 [DOI] [PubMed] [Google Scholar]
- Lamb R.S., Irish V.F. (2003). Functional divergence within the APETALA3/PISTILLATA floral homeotic gene lineages. Proc. Natl. Acad. Sci. USA 100: 6558–6563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.J., Zhang J.A., Zhang N., Shan H.Y., Su K.M., Zhang J.S., Meng Z., Kong H.Z., Chen Z.D. (2010). Interactions among proteins of floral MADS-box genes in basal eudicots: Implications for evolution of the regulatory network for flower development. Mol. Biol. Evol. 27: 1598–1611 [DOI] [PubMed] [Google Scholar]
- Manchado-Rojo, M., Delgado-Benarroch, L., Roca, M.J., Weiss, J., and Egea-Cortines, M. (2012). Quantitative levels of Deficiens and Globosa during late petal development show a complex transcriptional network topology of B function. Plant J. 72: 294–307. [DOI] [PubMed]
- Melzer R., Theissen G. (2009). Reconstitution of ‘floral quartets’ in vitro involving class B and class E floral homeotic proteins. Nucleic Acids Res. 37: 2723–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.H. (1972). Experiments in Molecular Genetics. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press). [Google Scholar]
- Nagasawa N., Miyoshi M., Sano Y., Satoh H., Hirano H., Sakai H., Nagato Y. (2003). SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development 130: 705–718 [DOI] [PubMed] [Google Scholar]
- Neduva V., Russell R.B. (2005). Linear motifs: Evolutionary interaction switches. FEBS Lett. 579: 3342–3345 [DOI] [PubMed] [Google Scholar]
- Orashakova S., Lange M., Lange S., Wege S., Becker A. (2009). The CRABS CLAW ortholog from California poppy (Eschscholzia californica, Papaveraceae), EcCRC, is involved in floral meristem termination, gynoecium differentiation and ovule initiation. Plant J. 58: 682–693 [DOI] [PubMed] [Google Scholar]
- Pabón-Mora N., Ambrose B.A., Litt A. (2012). Poppy APETALA1/FRUITFULL orthologs control flowering time, branching, perianth identity, and fruit development. Plant Physiol. 158: 1685–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S., Tapia-López R., Alvarez-Buylla E.R., Yanofsky M.F. (2001). Conversion of leaves into petals in Arabidopsis. Curr. Biol. 11: 182–184 [DOI] [PubMed] [Google Scholar]
- Piwarzyk E., Yang Y., Jack T. (2007). Conserved C-terminal motifs of the Arabidopsis proteins APETALA3 and PISTILLATA are dispensable for floral organ identity function. Plant Physiol. 145: 1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Hue I., Huijser P., Flor P.J., Hansen R., Tetens F., Lönnig W.E., Saedler H., Sommer H. (1992). Characterization of the Antirrhinum floral homeotic MADS-box gene deficiens: Evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J. 11: 251–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P.D., Chenchik A., Kellogg D.E., Lukyanov K.A., Lukyanov S.A. (1995). An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 23: 1087–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis D.E., et al. (2011). Angiosperm phylogeny: 17 genes, 640 taxa. Am. J. Bot. 98: 704–730 [DOI] [PubMed] [Google Scholar]
- Sommer H., Beltran J.P., Huijser P., Pape H., Lonnig W.E., Saedler H., Schwarzsommer Z. (1990). Deficiens, a Homeotic Gene Involved in the Control of Flower Morphogenesis in Antirrhinum-Majus - the Protein Shows Homology to Transcription Factors. Embo Journal 9: 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K.M., Zhao S.H., Shan H.Y., Kong H.Z., Lu W.L., Theissen G., Chen Z.D., Meng Z. (2008). The MIK region rather than the C-terminal domain of AP3-like class B floral homeotic proteins determines functional specificity in the development and evolution of petals. New Phytol. 178: 544–558 [DOI] [PubMed] [Google Scholar]
- Theissen G., Saedler H. (2001). Plant biology. Floral quartets. Nature 409: 469–471 [DOI] [PubMed] [Google Scholar]
- Tilly J.J., Allen D.W., Jack T. (1998). The CArG boxes in the promoter of the Arabidopsis floral organ identity gene APETALA3 mediate diverse regulatory effects. Development 125: 1647–1657 [DOI] [PubMed] [Google Scholar]
- Tröbner W., Ramirez L., Motte P., Hue I., Huijser P., Lönnig W.E., Saedler H., Sommer H., Schwarz-Sommer Z. (1992). GLOBOSA: A homeotic gene which interacts with DEFICIENS in the control of Antirrhinum floral organogenesis. EMBO J. 11: 4693–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W.-C., Pan Z.-J., Hsiao Y.-Y., Jeng M.-F., Wu T.-F., Chen W.-H., Chen H.-H. (2008). Interactions of B-class complex proteins involved in tepal development in Phalaenopsis orchid. Plant Cell Physiol. 49: 814–824 [DOI] [PubMed] [Google Scholar]
- Tzeng T.Y., Liu H.C., Yang C.H. (2004). The C-terminal sequence of LMADS1 is essential for the formation of homodimers for B function proteins. J. Biol. Chem. 279: 10747–10755 [DOI] [PubMed] [Google Scholar]
- Vandenbussche M., Zethof J., Royaert S., Weterings K., Gerats T. (2004). The duplicated B-class heterodimer model: whorl-specific effects and complex genetic interactions in Petunia hybrida flower development. Plant Cell 16: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., and Speleman, F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: 34.1–34.11. [DOI] [PMC free article] [PubMed]
- Wagner G.P., Lynch V.J. (2008). The gene regulatory logic of transcription factor evolution. Trends Ecol. Evol. (Amst.) 23: 377–385 [DOI] [PubMed] [Google Scholar]
- Wall P.K., et al. (2009). Comparison of next generation sequencing technologies for transcriptome characterization. BMC Genomics 10: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang W., Malcolm B.A. (1999). Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. Biotechniques 26: 680–682 [DOI] [PubMed] [Google Scholar]
- Wang Y.Q., Melzer R., Theissen G. (2010). Molecular interactions of orthologues of floral homeotic proteins from the gymnosperm Gnetum gnemon provide a clue to the evolutionary origin of ‘floral quartets’. Plant J. 64: 177–190 [DOI] [PubMed] [Google Scholar]
- Wege S., Scholz A., Gleissberg S., Becker A. (2007). Highly efficient virus-induced gene silencing (VIGS) in California poppy (Eschscholzia californica): An evaluation of VIGS as a strategy to obtain functional data from non-model plants. Ann. Bot. (Lond.) 100: 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K.U., Saedler H., Theissen G. (2002). On the origin of class B floral homeotic genes: Functional substitution and dominant inhibition in Arabidopsis by expression of an orthologue from the gymnosperm Gnetum. Plant J. 31: 457–475 [DOI] [PubMed] [Google Scholar]
- Wuest S.E., O’Maoileidigh D.S., Rae L., Kwasniewska K., Raganelli A., Hanczaryk K., Lohan A.J., Loftus B., Graciet E., Wellmer F. (2012). Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc. Natl. Acad. Sci. USA 109: 13452–13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellina A.L., Orashakova S., Lange S., Erdmann R., Leebens-Mack J., Becker A. (2010). Floral homeotic C function genes repress specific B function genes in the carpel whorl of the basal eudicot California poppy (Eschscholzia californica). Evodevo 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachgo S., Silva Ede.A., Motte P., Tröbner W., Saedler H., Schwarz-Sommer Z. (1995). Functional analysis of the Antirrhinum floral homeotic DEFICIENS gene in vivo and in vitro by using a temperature-sensitive mutant. Development 121: 2861–2875 [DOI] [PubMed] [Google Scholar]
- Zahn L.M., Leebens-Mack J., DePamphilis C.W., Ma H., Theissen G. (2005). To B or not to B a flower: The role of DEFICIENS and GLOBOSA orthologs in the evolution of the angiosperms. J. Hered. 96: 225–240 [DOI] [PubMed] [Google Scholar]