Abstract
Neural processes that direct an animal’s actions toward environmental goals are critical elements for understanding behavior. The hypothalamus is closely associated with motivated behaviors required for survival and reproduction. Intense feeding, drinking, aggressive, and sexual behaviors can be produced by a simple neuronal stimulus applied to discrete hypothalamic regions. What can these “evoked behaviors” teach us about the neural processes that determine behavioral intent and intensity? Small populations of neurons sufficient to evoke a complex motivated behavior may be used as entry points to identify circuits that energize and direct behavior to specific goals. Here, I review recent applications of molecular genetic, optogenetic, and pharmacogenetic approaches that overcome previous limitations for analyzing anatomically complex hypothalamic circuits and their interactions with the rest of the brain. These new tools have the potential to bridge the gaps between neurobiological and psychological thinking about the mechanisms of complex motivated behavior.
Man can do what he wills, but he cannot will what he wills. —Arthur Schopenhauer, On the Freedom of the Will [paraphrase].
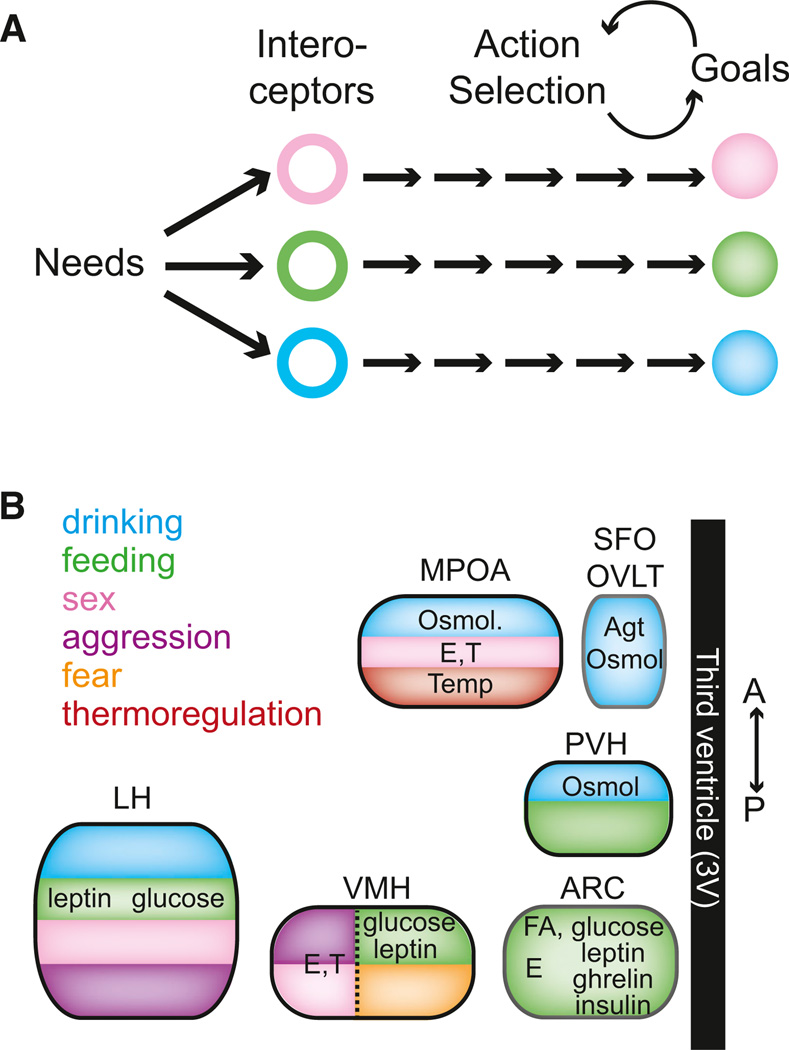
This statement reflects the view that actions can be consciously controlled but the needs and desires that give intent and intensity to behavior are imposed by unconscious processes. Most fundamental are the desires associated with basic behaviors required for survival and reproduction, such as hunger, thirst, and sex (Freud, 2001; Hull, 1943; LeDoux, 2012; Maslow, 1943). Specific actions involved in any one of these behaviors can be quite variable, both within and between species, but they are readily recognized as a means to a clearly defined end. For example, in hunger or thirst, the common element of these purposive behaviors is the capability of an animal to identify its need and to efficiently take steps to provide for it (Tolman, 1932). Because survival needs are essential for every organism, the corresponding behaviors and underlying neural processes are also under direct selective pressure. Therefore, neural circuits that mediate essential behaviors are expected to be, at least in part, under developmental control with some dedicated circuit components (Pfaff, 1999; Swanson, 2000; Thompson and Swanson, 2003). As such, genetically specified, interoceptive sensory neuron populations responsive to signals of physiological need are natural entry points that enable reproducible identification of these circuits and application of genetically encoded tools for manipulation of their function (Figure 1A).
Figure 1. Physiological Needs Influence Behavior through Discrete Hypothalamic Circuits.
(A) Circulating and neural signals of physiological needs are sensed by interoceptive cell types, which influence action selection to achieve goals that alleviate the needs.
(B) Discrete hypothalamic regions contain interoceptors for a variety of substances and also have been shown to evoke behavior with localized electrical or chemical perturbations. E, estrogen; T, testosterone; Agt, angiotensin; Osmol, osmolarity; Temp, temperature; ARC, arcuate nucleus; VMH, ventromedial hypothalamus; LH, lateral hypothalamus; PVH, paraventricular hypothalamus; SFO, subfornical organ; OVLT, organosum vaculosum of lamina terminalis; MPOA, medial preoptic area.
The hypothalamus is an ancient and conserved forebrain area (ventral diencephalon) (Tessmar-Raible et al., 2007; Venkatesh et al., 1997) that senses internal state and coordinates key physiological and behavioral responses for maintaining homeostatic balance (Simerly, 1994). This brain region contains diverse molecularly defined cell types (Lein et al., 2007; Markakis, 2002), which alter their electrical activity in response to physiological cues associated with survival needs. Remarkably, electrical and chemical manipulations in discrete hypothalamic areas that include need-sensing neurons produce complex, goal-directed behavioral motivations that are elicited de novo toward counteracting survival needs, even in fully replete animals (Figure 1B). Similarly, social behaviors such as mating and aggression, which are strongly influenced by hormonal state, can be evoked from hypothalamic regions containing interoceptive neurons responsive to peripherally produced sex steroid hormones. These are some of the most dramatic behavioral responses in neuroscience, and their wide-ranging implications for the relation of brain activity to behavior have been the subject of a large body of experimental investigation (not to mention inspiration for science fiction).
How can “evoked behaviors,” resulting from electrical activity manipulation of specific cell types, be used to access neural circuits underlying motivated behaviors associated with critical survival needs? This question is examined here primarily in the context of separate circuits controlling hunger and aggression behaviors with a narrow focus on recent advances using acute, cell type-specific manipulation of neuronal function. Methodological issues for analyzing molecularly defined circuits are emphasized, such as molecular genetics and new genetically encoded activity modulators, which have dramatically expanded the capability to probe the causal relationship of cell types and circuits to behavior. Moreover, the possibility is considered that these circuits permit investigation of motivational processes, ideally by identifying key interoceptive neurons as sensory channels into purposive behaviors.
Survival Needs Are Entry Points to Motivational Processes
Survival needs are primary motives that impose ground rules for behavior (Maslow, 1943; Tolman, 1932). Conditions of physiological deficit set priorities in an animal’s environment and direct its actions toward goals that will counteract the need—for example, a starved animal will tenaciously search for food. Although motivated behaviors are not exclusively associated with conditions of severe need, such primary motives are a starting point for understanding motivational processes and the underlying neural circuits. However, many survival needs develop with slowly varying deprivation or hormonal states that broadly affect brain and body (Cabanac, 1985; Schwartz et al., 2000; Steiner et al., 1981). Analysis of the associated neural circuits is complicated by the wide range of physiological processes engaged in these states, which simultaneously modulate an indeterminate number of complementary and partially redundant interoceptive circuits.
Isolating neural processes that influence behavioral direction and intensity is aided by rapid and selective control over a specific need-sensing system sufficient to control a motivated behavioral response. Different hypothalamic regions sense circulating hormones and metabolites important for hunger (Belgardt et al., 2009), thirst (Johnson and Thunhorst, 1997), and sexual behavior (Davidson, 1966). Some of these brain regions, when stimulated electrically or pharmacologically, rapidly induce in sated animals the corresponding behavioral responses associated with obtaining needs such as drinking (Grossman, 1960), eating (Stanley et al., 1993), and thermoregulation (Boulant and Dean, 1986), as well as basic social responses such as sexual activity (Davidson, 1966), aggression (Kruk et al., 1983), and fear/defensive (Canteras, 2002) behaviors (Figure 1B). Identification and manipulation of the underlying interoceptive neurons to produce “virtual” need states simplifies neural circuit analysis of these complex behaviors. These key interoceptive neurons provide well-defined entry points that enable the use of modern neuroscience techniques to step node-by-node and reveal the downstream circuits that elicit complex motivated behavioral responses.
Despite the anticipated advantages of using evoked behaviors for neural circuit analysis, the complex behaviors induced by brain activation and silencing remain mysterious processes. A brief consideration of the history of this approach is important to understand the challenges that have become apparent over the many decades in which this experimental paradigm has been used. Perhaps the most prominent examples are classic experiments involving electrical stimulation of the lateral hypo-thalamus, which, in rats, led to feeding (Delgado and Anand, 1953), drinking (Greer, 1955; Mogenson and Stevenson, 1967), predatory attack (Woodworth, 1971), gnawing (Miller, 1957; Valenstein et al., 1968), or sexual behaviors (Vaughan and Fisher, 1962). The magnitude of these behavioral responses was often modest upon initial exposure to brain stimulation but increased with successive stimulation sessions (Wise, 1969, 1974). The diversity of behavioral responses has been attributed to intermingled circuits controlling discrete behaviors (Coons et al., 1965; Wise et al., 1970) because electrical stimulation activates multiple neuronal cell types and axon projections passing through a region. However, these experiments have also been interpreted to show that electrical stimulation of the lateral hypothalamus intrinsically lacks behavioral specificity (Valenstein et al., 1968). Rats that showed lateral hypothalamus-mediated “stimulation-bound” feeding, drinking, or gnawing behaviors could be switched to one of the other behaviors by simply removing access to the initially preferred goal object (Valenstein et al., 1968). Behaviors evoked by electrical stimulation of the lateral hypothalamus were proposed to result from elevation of incentive salience attribution (Berridge and Valenstein, 1991). In this model, animals aroused by brain stimulation were said to reveal prepotent behaviors, which became progressively stamped in, such that their performance during electrical stimulation increased dramatically (in part due to activation of the medial forebrain bundle that passes through the lateral hypothalamus) (Berridge and Valenstein, 1991). It is important to note, though, that conversely to this view, pharmacological neuron manipulation in the hypothalamus and other regions has been reported to lead to more specific feeding and drinking behavioral responses (Grossman, 1960; Stratford et al., 1998). While additional investigation of the behavioral influence of the lateral hypothalamus is clearly warranted, important lessons from extensive past work are: (1) the potential for stimulation in some brain regions to nonspecifically increase complex behaviors that may not always involve processes associated with genesis of the natural behavioral response, (2) the resultant appeal of starting circuit analysis with need-sensing neurons that are necessary and sufficient for a counter-regulatory behavioral response, and (3) the requirement for techniques to selectively manipulate interoceptive neuronal cell types and their downstream circuits.
Molecularly Defined Neural Circuit Analysis
In the hypothalamus, as in other brain regions, intermingled cell populations have different, sometimes opposite functions. To gain discrete access to these populations, cell types can be defined by specific patterns of gene expression, such as ion channels, neuromodulators, and transcription factors. Consequently, approaches for investigating brain function are becoming increasingly granular, with gene expression markers for molecularly defined cell types distinguishing intermingled circuit nodes (Atasoy et al., 2008; Brown and Hestrin, 2009; Cardin et al., 2009; Kravitz et al., 2010; Lein et al., 2004, 2007; Luo et al., 2008; Tsai et al., 2009). The promoter elements from marker genes are repurposed to drive expression of transgenes for neuron actuators. Most notably, this involves Cre recombinase, which can be used to selectively switch on gene expression for Cre-dependent viral vectors (Atasoy et al., 2008; Betley and Sternson, 2011; Kuhlman and Huang, 2008; Saunders et al., 2012; Tsai et al., 2009) or actuator mouse lines (Madisen et al., 2010, 2012) that produce fluorescent proteins and synaptic tracers as well as optogenetic and pharmacogenetic tools for activating and silencing neurons (Fenno et al., 2011; Luo et al., 2008; Shapiro et al., 2012). These genetically encoded tools are driving rapid advances for understanding the causal relationships of activity in neuronal cell types to circuit function and animal behavior.
Although a large body of work has shown exquisite patterns of axonal projections between brain regions (Chamberlin et al., 1998; Swanson, 2000; Thompson and Swanson, 2003), mapping the axon connectivity between molecularly defined cell types is in early stages. Fluorescent protein expression from Cre-dependent viral vectors (Atasoy et al., 2008; Kuhlman and Huang, 2008) permits cell type-specific hypothalamic projections to be mapped (Atasoy et al., 2008; Gautron et al., 2010; Leshan et al., 2009; Louis et al., 2010). Synaptic connectivity between hypothalamic cell types has been probed by genetic techniques such as cell type-specific expression and anterograde transfer of wheat germ agglutinin or barley lectin for identifying cells downstream of molecularly defined leptin receptor-expressing neurons (Louis et al., 2010) and tetanus toxin subunit expression to provide a map of afferent inputs to orexin/hypocretin neurons (Sakurai et al., 2005). In addition, viral tools for anterograde (Yamagata and Sanes, 2012; Beier et al., 2011; Lo and Anderson, 2011) and retrograde (DeFalco et al., 2001; Wickersham et al., 2007) labeling of connected cell types are also gaining widespread usage for comprehensively mapping upstream and downstream circuits from molecularly defined cell types (Watabe-Uchida et al., 2012; Yoon et al., 2005). Yet another method, called GRASP, identifies connections between cell types by reconstitution of split-GFP across synaptic junctions (Feinberg et al., 2008; Kim et al., 2012; Yamagata and Sanes, 2012).
These anatomical approaches reveal wiring diagrams, but they lack key information about signs and strengths of the connections. Channelrhodopsin-assisted circuit mapping lever-ages the efficient distribution of channelrhodopsin-2 into axonal processes, which allows the local and long-range connectivity between two molecularly defined cell types to be examined using synaptic physiology (Petreanu et al., 2007). In this method, a photoexcitable presynaptic neuron population (or even the cut axons from a cell type) is activated during electrophysiological recording from a potential postsynaptic cell type, often identified by cell type-specific expression of a fluorescent marker protein. The presence of a short-latency synaptic response demonstrates connectivity between the populations. Channelrhodopsin-assisted circuit mapping experiments measure the connection probability between cell types, their functional synaptic properties, and the influence of the connection on the activity of postsynaptic cell types. Overall, the complementation of axon projection anatomy with functional synaptic connectivity analysis provides a rigorous framework for investigating pathways within the hypothalamus (Figure 2A) (Atasoy et al., 2008, 2012; Kong et al., 2012; Schöne et al., 2012) and other brain regions (Arenkiel et al., 2007; Callaway and Katz, 1993; English et al., 2012; Frick et al., 2008; Haubensak et al., 2010; Luo et al., 2008; Mao et al., 2011; Shepherd and Svoboda, 2005; Stuber et al., 2011; Tye et al., 2011).
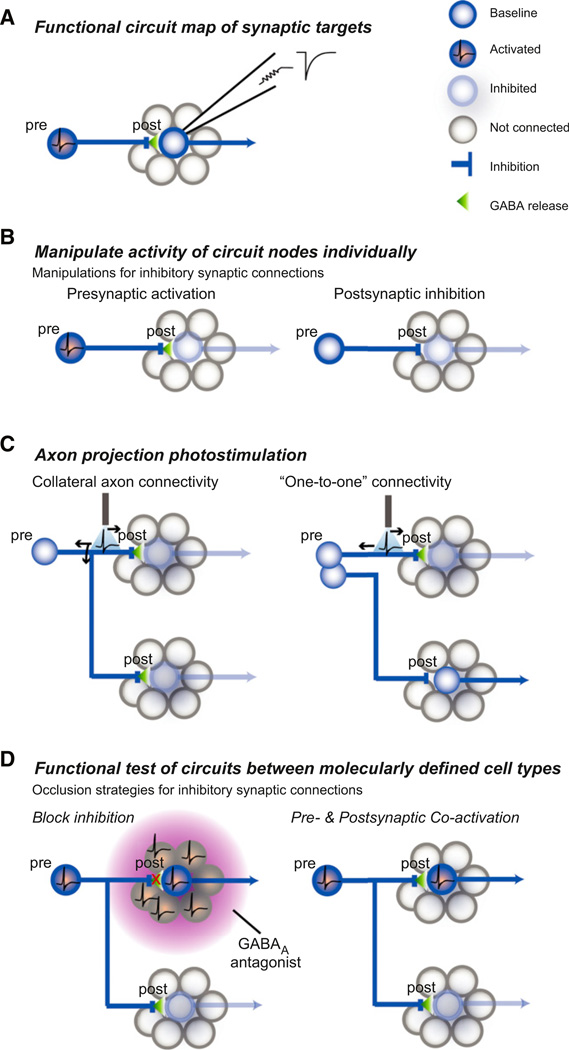
Figure 2. Functional Circuit Mapping and Behavioral Evaluation of Cell Type-Specific Circuit Connections.
(A) For molecularly defined neurons, channelrhodopsin-assisted circuit mapping is used to determine postsynaptic targets as well as their functional synaptic properties. Upper right: schematic postsynaptic current.
(B) For an example inhibitory circuit, cell type-specific neuron activation and silencing techniques are used to test whether activation of the presynaptic population or inhibition of the postsynaptic population are each sufficient for the behavioral response.
(C) Photostimulation of axon projections from a cell type (inhibitory) over a specific target region is performed while monitoring the behavioral response. The axon anatomy of a given molecularly defined cell type is often not known and may involve each cell within a molecularly defined type projecting to multiple target regions (collateral axon connectivity) or each projection target arising from a single subpopulation of the molecularly defined cell class (“one-to-one” connectivity). For axons with collateral connectivity, a back propagating action potential can activate axon collaterals projecting to other regions; thus, additional experiments are required to prove the behavioral consequence of activating a specific projection.
(D) Test of the functional necessity of specific circuit connections. Left: the behavioral role of identified inhibitory connections has typically been tested by blocking inhibition regionally with reagents that are not cell type-specific to the identified postsynaptic neuron. Thus, all neurons in the region, regardless of connectivity, are released from inhibition. Right: cell type-specific occlusion of synaptic inhibition by simultaneously activating presynaptic neurons (or their axons) and a molecularly defined postsynaptic neuron population. Because the presynaptic neuron also projects to other populations, this is a test for the behavioral necessity of this specific circuit connection while all other targets are receiving evoked synaptic input. Figure modified from Atasoy et al. (2012).
Ex vivo functional circuit mapping experiments provide a bridge to in vivo experiments that test the behavioral influence of connections between individual circuit nodes (Figure 2B). The capacity to evoke a complex behavior by manipulating activity in a molecularly defined cell type offers the opportunity to use a powerful repertoire of circuit mapping and perturbation techniques for examining the role of downstream circuit elements in mediating that behavioral response. Axonal projection fields from specific cell types can be locally activated or silenced in discrete target brain regions while monitoring the behavioral response (Figure 2C) (Atasoy et al., 2012; Stuber et al., 2011; Tye et al., 2011). Activating axon projections has caveats, such as the back propagation of action potentials that can excite collateral axon projections to other brain regions (Figure 2C). Behavioral responses that result from local axonal activation should be followed up by a corresponding manipulation of the postsynaptic targets to test the sufficiency and the necessity of the circuit interaction for the behavioral response, for example, by local pharmacological blockade of the released neurotransmitter in the target region under investigation (Figure 2D). For connections between two molecularly defined cell types, pharmacological blockade may be nonspecific for a particular cell type. In this case, postsynaptic activation or silencing (depending on the sign of the mapped synaptic interaction) can be used to demonstrate sufficiency of the downstream cell type to recapitulate the response associated with the presynaptic cell type (Figure 2B). To test the necessity of a molecularly defined circuit interaction, the presynaptic neuron can be manipulated simultaneously with the postsynaptic neuron while monitoring behavior: for an excitatory neuron sufficient to evoke the behavior, this means simultaneously silencing a postsynaptic partner; for an inhibitory neuron, the postsynaptic population would be simultaneously activated (Figure 2D). This approach is analogous to epistasis analysis (Avery and Wasserman, 1992), which has been used in cell biology to establish the functional significance of large scale protein-protein interaction networks (Collins et al., 2007). Overall, the framework of rapidly evoked behaviors mediated by manipulation of a single neuron population facilitates reverse-engineering circuit manipulations in neural systems that are regulated by slowly varying physiological parameters. Due to new tools for manipulating neuron function, coupled with availability of mouse lines with cell type-selective Cre recombinase expression in interoceptive neurons as well as their upsteam and downstream circuit nodes, neural circuits in the hypothalamus are increasingly accessible for analysis in the context of their interesting behavioral and physiological roles.
Circuits for Feeding Behavior
In animals, nutrient intake is essential for life and requires food seeking and consumption behaviors. These are mediated by the neural processes that underlie Walter Cannon’s “wisdom of the body” and Curt Richter’s “behavior regulators,” through which homeostatic processes influence both physiology and behavior (Cannon, 1939; Richter, 1943). In light of the necessity of feeding behavior, it is expected that such circuits are, in part, under developmental control with some “hard-wired” components that can be reproducibly identified in the brain.
A network of hormone- and nutrient-sensing neurons in the brain monitors energetic state (Gao and Horvath, 2007). These interoceptive sensory neurons modulate their electrical activity in response to fluctuations in the levels of hormones, metabolites, and neural signals of organismal nutrition. Nutrient- (Burdakov et al., 2005; Oomura et al., 1964, 1975; Wang et al., 2006) and hormone-(Cowley et al., 2001, 2003a, 2003b) sensing properties have been measured in recordings of neuron electrical activity. However, the direct contribution of specific energy-sensing neurons to the onset of behaviors associated with energy deficit have been obscured by (1) the slow timescale (hours to days) over which energy deficits unfold, (2) the diverse range of cellular and physiological processes affected by energy deficit across brain and body, and (3) the presence of multiple, partially redundant circuits throughout the brain that contain energy sensors. Together, these issues have severely limited analysis of the causal relationships between the components of deprivation-sensitive sensory systems, the downstream neural circuit processes, and their behavioral consequences.
Evidence of causality in multistep processes, such as circuit operations, requires localized perturbation within a system, and such evidence is strengthened by the contiguity of the resultant response. For exteroceptive sensory systems that respond rapidly to stimuli, neural and behavioral responses can be reliably correlated, and causal relationships can be defined with controlled perturbations to these systems. For example, retinal ganglion cells are well-defined, exclusive entry points into circuits for visual processing of distinct information channels separately influencing perception, movement, and circadian rhythms (Hattar et al., 2006; Kim et al., 2008, 2010). In contrast, circuits that respond to slowly varying parameters such as energetic state lack the temporal acuity and precisely circumscribed origin of action for probing the impact of natural stimuli on behavioral responses. A molecularly defined nutrient-sensing neuron population that is remotely controllable by an experimenter and is sufficient to rapidly influence food seeking and consumption behavior permits dissection of the processes underlying initiation and maintenance of hunger using approaches analogous to exteroceptive sensory systems such as vision. Indeed, such a neuron potentially constitutes a sensory neuron entry point into motivational circuits.
Starvation-Sensitive AGRP Neurons
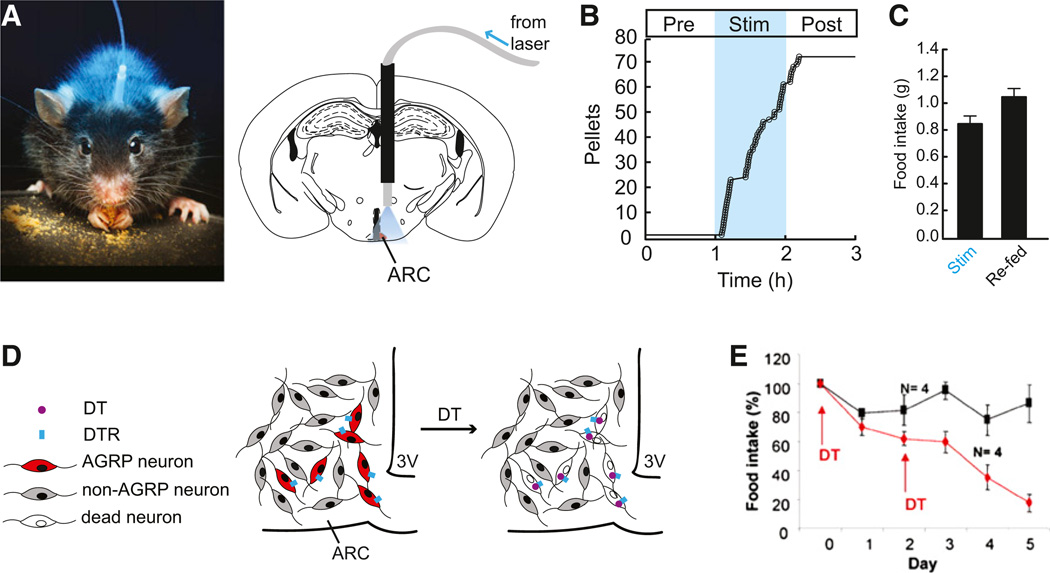
In the hypothalamic arcuate nucleus (ARC), the gene agouti-related protein (Agrp) defines an interoceptive neuron with properties that are suitable for investigating hunger processes. AGRP neurons are activated both directly and synaptically by circulating cues of energetic deficit, such as the gut-derived hormone ghrelin (Cowley et al., 2003b; van den Top et al., 2004; Yang et al., 2011), and they are inhibited by signals of energy surfeit such as glucose (Fioramonti et al., 2007), insulin (Könner et al., 2007), and the fat-derived hormone leptin (van den Top et al., 2004). These neurons release the neuromodulators AGRP and neuropeptide Y (NPY), which increase feeding when injected into the brain (Clark et al., 1984; Levine and Morley, 1984; Ollmann et al., 1997; Stanley and Leibowitz, 1984). Optogenetic (Aponte et al., 2011) and pharmacogenetic (Krashes et al., 2011) induction of electrical activity in AGRP neurons is sufficient to rapidly evoke voracious feeding behavior, even at times when mice normally do not eat (Figures 3A–3C). The influence of AGRP neuron activity on behavior also leads to a dramatic increase in the motivation to work for food (Atasoy et al., 2012; Krashes et al., 2011). In contrast, acute genetic ablation of AGRP neurons in adult mice resulted in pronounced aphagia and starvation (Figures 3D and 3E), even when palatable liquid food was placed in the animal’s mouth (Luquet et al., 2005; Wu et al., 2008a). Suppressing electrical activity in AGRP neurons also reduced feeding (Krashes et al., 2011). Together, these experiments indicate a key role for just a few hundred interoceptive AGRP neurons as sufficient and, in part, necessary for orchestrating the motivational and consummatory processes underlying feeding behavior.
Figure 3. AGRP Neurons Are Necessary and Sufficient for Feeding.
(A) Configuration for optogenetic activation of AGRP neurons in a behaving mouse. ARC, arcuate nucleus. Photo from Igor Siwanowicz.
(B) Photostimulation-evoked feeding from mice engineered to express channelrhodopsin in AGRP neurons (Aponte et al., 2011).
(C) Food intake from AGRP neuron photo-stimulation in ad libitum fed mice is similar to re-feeding 24 hr food-deprived mice (Aponte et al., 2011).
(D) Scheme for diphtheria toxin receptor-mediated acute AGRP neuron ablation.
(E) AGRP neuron ablation leads to aphagia (Luquet et al., 2005).
AGRP neuron manipulation experiments highlight the value of acute perturbations for probing the relationships of neuron function to behavior. AGRP neuron ablation was performed by injecting diphtheria toxin (DT) into adult mice engineered to cell type-selectively express the diphtheria toxin receptor (AgrpDTR mice), which resulted in loss of feeding behavior over 6 days (Luquet et al., 2005; Wu et al., 2008b). In contrast, slow or chronic approaches for AGRP neuron loss-of-function failed to strongly affect feeding behavior, including genetic knockout of the orexigenic neuromodulators and neurotransmitters released by AGRP neurons (such as NPY, AGRP, and GABA) (Erickson et al., 1996; Qian et al., 2002; Tong et al., 2008), slow-onset adult AGRP neuron degeneration (Xu et al., 2005), or even rapid AGRP neuron ablation in neonates (Luquet et al., 2005). Results from these different experimental tactics illustrate the importance of investigating neural circuits with acute manipulations and highlight the pitfalls of using long-term disruption of function due to the onset of compensatory mechanisms.
Acute gain-of-function methods enabled the systematic examination of the psychophysical relationship of electrical activity to the magnitude, dynamics, and selectivity of AGRP neuron-evoked feeding. For AGRP neuron activation, the magnitude of evoked feeding was nearly as great as for mice that had been food deprived for 24 hr, even on the very first exposure to AGRP neuron stimulation (Figure 3C) (Aponte et al., 2011; Krashes et al., 2011). A subsequent photostimulation trial showed similar response magnitude (Aponte et al., 2011). This is in contrast to feeding evoked by electrical stimulation of the lateral hypothalamus, which gradually increased consumption from a modest response to voracious eating with successive exposure to stimulation (Wise, 1969, 1974). In addition, AGRP neuron-evoked feeding was always selective for food in the presence of water (Aponte et al., 2011), unlike the observations with lateral hypothalamic electrical stimulation (Valenstein et al., 1968). Moreover, AGRP neuron activation experiments showed that the magnitude of food consumption was sensitive to both the number of photoexcitable neurons and the photostimulation frequency (Aponte et al., 2011). Interestingly, intermediate levels of either of these parameters led to lower magnitude of the behavioral responses (Aponte et al., 2011). Thus, cell type-specific AGRP neuron activation behaves as a “gas pedal” for feeding, meaning that the magnitude of the behavioral response is sensitive to the level of AGRP neuron activation. The capacity to cell type-specifically probe a continuum of neuron activity→behavior relationships is a broadly useful advance that is offered by the availability of new tools for precisely manipulating neuron function.
The sufficiency of AGRP neurons to rapidly recapitulate a hunger state, the necessity of AGRP neurons to maintain feeding behavior, as well as their well-established need-sensing capabilities highlights the attractiveness for these neurons as an entry point to downstream neural circuits influencing the motivation to seek and consume food. Axon projections of AGRP neurons provide a partial blueprint for this circuitry, which can be used to identify brain regions involved in the physiological and motivational processes coordinating feeding behavior. Two substantially different approaches have been pursued to understand the neural circuits that underlie the effects of AGRP neuron ablation and activation. These studies are reviewed in detail because they provide an indication of types of approaches that can be used to further investigate these and other molecularly defined hypothalamic circuits that regulate complex behaviors.
Anorexia Circuits
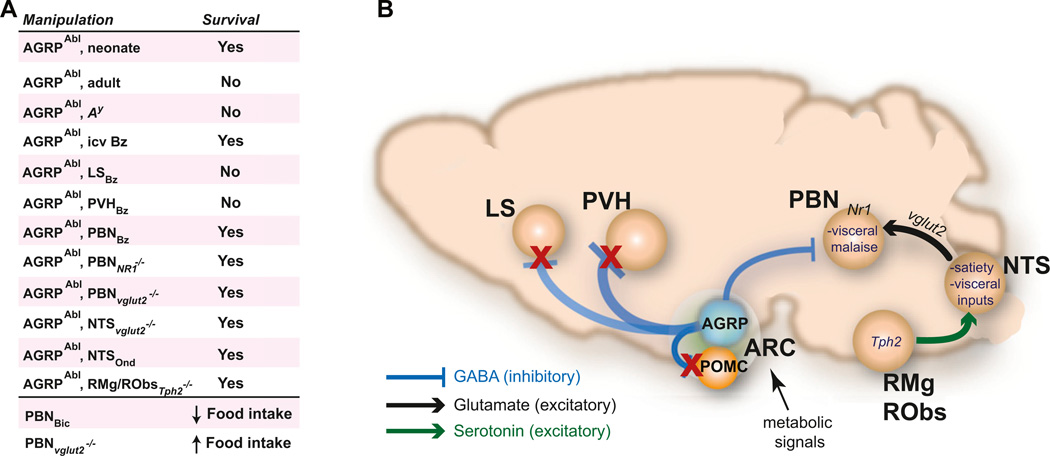
Use of acute AGRP neuron ablation allowed circuits mediating the resulting aphagia to be delineated by a functional circuit mapping approach coupled with identification of neuronal perturbations that could rescue feeding (Figure 4). Shortly after the loss of inhibitory AGRP neurons, inspection of eight brain regions that are normally innervated by AGRP axons showed elevated expression of Fos, a histochemical marker of neuronal activation (Wu et al., 2008b, 2009b). Food intake could be rescued by brain infusion of the benzodiazepine bretazenil, a GABA potentiator, and this treatment most strongly suppressed Fos expression in the lateral septum and the para-brachial nucleus (PBN). Chronic intracranial administration of bretazenil, targeted to both regions, showed that infusion into the PBN, but not the septum (or several other regions), was sufficient to rescue feeding (Wu et al., 2009b), presumably by compensating for the loss of AGRP neuron axons projecting from the hypothalamus to the PBN.
Figure 4. AGRP Neuron Axon Projections Regulate a Hindbrain Circuit that Mediates Aphagia.
(A) Summary of neuron and pharmacological manipulations individually and together that probe epistatic relationships for their effect on survival (resulting from rescue of feeding). Abl, ablation; Bz, bretazanil; Ond, ondansetron; icv, intracerebroventricular; LS, lateral septum.
(B) AGRP neurons project local and long-range axons to inhibit POMC, PVH, LS, and PBN neurons along with other targets. ARCAGRP/PBN connections are required to suppress visceral malaise, which is mediated in the PBN by glutamatergic excitatory drive from the nucleus of the solitary tract (NTS) that is regulated by serotonergic inputs.
The PBN is a hindbrain relay nucleus that receives visceral and taste information from the periphery. A hindbrain circuit that regulates PBN activity and sensitivity to acute AGRP neuron ablation was revealed by using a series of genetic manipulations involving viral delivery of Cre recombinase to hindbrain nuclei in AgrpDTR mice engineered with a series of floxed genes (Wu et al., 2012). Recombinant adeno-associated virus (rAAV) was used for targeted delivery of Cre recombinase to excise floxed Grin1 (an NMDA receptor subunit) in the PBN, which prevented starvation in mice after the loss of inhibitory input after AGRP neuron ablation. This manipulation disables NMDA receptor signaling and probably reduces PBN neuron excitability in response to glutamatergic synaptic input. Ascending excitatory glutamatergic inputs to the PBN largely originate in the nucleus of the solitary tract (NTS) (Herbert et al., 1990; Jhamandas and Harris, 1992; Tokita et al., 2009), the primary entry point of visceral information to the brain. Deletion of the vesicular glutamate transporter-2 gene (Slc17a6) in the NTS by rAAV-Cre, which disables glutamate loading into synaptic vesicles and reduces the ability of NTS neurons to excite PBN neurons, also rescued eating in mice with AGRP neuron ablation (Wu et al., 2012).
Because mice with AGRP neuron ablation do not even consume food when it is placed in their mouth (Wu et al., 2008a), the loss of AGRP neurons was proposed to result in “visceral malaise” or severe nausea (Wu et al., 2009b). Based on this hypothesis, after AGRP neuron ablation, mice were treated with the antiemetic ondansetron, which also rescued feeding behavior when delivered to the fourth cerebral ventricle in the hindbrain (Wu et al., 2012). Ondansetron is a serotonin 3 receptor antagonist, and the NTS is innervated with serotonin-containing axons (Thor and Helke, 1987). Serotonin is not produced in the NTS, and to determine the origin of the serotonergic innervation of the NTS, a canine adenoviral (CAV-2) vector was used based on its property that it is taken up by axon projections and transported back to the soma (Peltékian et al., 2002). CAV-2 expressing Cre recombinase was targeted to the NTS of mice engineered with floxed Tph2, an enzyme necessary for serotonin biosynthesis. These mice showed loss of serotonergic innervation in the NTS and loss of Tph2 in two of three major regions that contain serotonin neurons: raphe magnus and raphe obscurus (but not the dorsal raphe nucleus). Importantly, AGRP neuron ablation in these mice did not lead to aphagia, indicating that serotonergic projections from these regions to the NTS contribute to activating the NTS and consequently increasing excitatory drive in the PBN, which leads to aphagia when the balancing inhibitory drive from AGRP neurons is lost after neuron ablation (Wu et al., 2012).
This analysis of aphagia after AGRP neuron ablation used an elegant combination of pharmacological and viral tools to map a hindbrain circuit regulated by inhibitory AGRP projections (Figure 4). The acute onset of AGRP neuron ablation was essential for a functional circuit mapping approach that tested epistatic interactions between brain regions. The behavioral readout was simply survival, which facilitated the large number of viral and pharmacological manipulations in multiple brain regions (Figure 4A). The picture that emerges is a requirement for descending AGRP neuron projections to restrain circuits that, when overactivated, can lead to “visceral malaise” or extreme nausea. Based on the capacity for compensation (Luquet et al., 2005; Wu et al., 2009b), AGRP neurons are not strictly necessary for feeding behavior, but under normal circumstances the activity of these neurons is required for feeding in mice. This suggests that the malaise circuit plays a role in restraining eating, a process that is under control of hormone- and nutrient-sensitive AGRP neurons. Moreover, it has also been shown that the PBN can have bidirectional influence over feeding behavior: potentiating GABA signaling in the PBN with benzodiazepines (Higgs and Cooper, 1996) or suppression of PBN excitatory efferents by local ablation of Slc17a6 (Wu et al., 2012) both increase feeding even when AGRP neurons are left intact. However, the timescale of these experiments does not address whether this circuit projection is responsible for the rapid upregulation of feeding behavior associated with cell type-selective AGRP neuron activation. For this, gain-of-function circuit mapping techniques were required.
Hunger Circuits
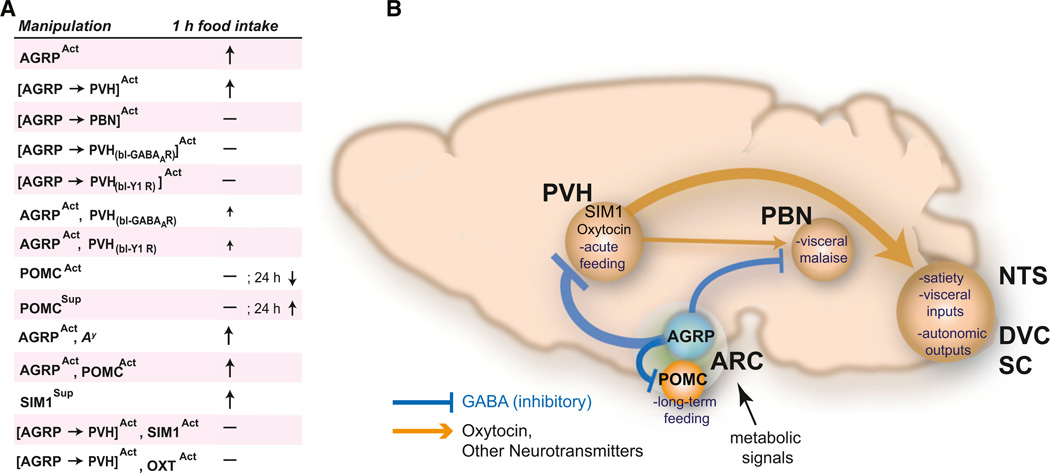
For complex food seeking and consumption behaviors, the capacity to rapidly activate feeding can be used as a simple readout for brain perturbations at successive circuit nodes. This approach was applied in a study (Atasoy et al., 2012) that focused on three brain areas that receive AGRP neuron axonal inputs and have been reported to influence feeding behavior: Proopiomelanocortin (Pomc)-expressing neurons in the ARC, PBN neurons in the hindbrain, and paraventricular hypothalamus (PVH) neurons. To address which of these brain regions and cell types contributed to the rapidly evoked feeding from AGRP neuron activation, Atasoy et al. (2012) used optogenetic techniques to determine the connectivity and synaptic properties downstream of AGRP neurons. The behavioral consequences of these mapped synaptic connections were then established by perturbing electrical activity in presynaptic AGRP neurons and a variety of postsynaptic cell types (Figure 5).
Figure 5. Distinct AGRP Neuron Axon Projections Regulate Different Aspects of Feeding Behavior.
(A) Summary of cell type-specific activity manipulations to probe epistatic relationships for their effect on feeding behavior. Act, activation; Sup, suppression; bl, pharmacological blockade.
(B) AGRP neurons project local and long-range axons to inhibit POMC, PVH, and PBN neurons along with other targets. ARCAGRP→ARCPOMC connections influence long-term regulation of food intake and other aspects of energy homeostasis. ARCAGRP→PVH and ARCAGRP→PVHOXT connections regulate acute food intake. PVH neurons in turn communicate with brainstem satiety centers (NTS, nucleus of the solitary tract; DVC, dorsal vagal complex; SC, spinal cord). Figure modified from Atasoy et al. (2012).
AGRP neurons are intermingled in the ARC with POMC neurons, a population that suppresses food intake via melanocortin receptor activation (Aponte et al., 2011; Fan et al., 1997; Huszar et al., 1997). In light of functional opposition between these neurons, their interaction has been implicated as a critical control point for feeding behavior (Cowley et al., 2001; Tong et al., 2008). Using channelrhodopsin-assisted circuit mapping, ARCAGRP→ARCPOMC connections were mapped, revealing that all POMC neurons examined received AGRP neuron synaptic input and that connections were strongly inhibitory due to GABA release (Atasoy et al., 2012). These direct circuit mapping observations were consistent with prior work showing that ablation of AGRP neurons or deletion of the vesicular GABA transporter gene in AGRP neurons both lead to dramatic reduction of spontaneous GABA-mediated inhibitory synaptic currents in POMC neurons (Tong et al., 2008; Wu et al., 2008a). However, multiple lines of evidence indicate that the ARCAGRP→ARCPOMC circuit does not mediate acute AGRP neuron-evoked feeding behavior. Manipulation of AGRP neuron function under constitutive melanocortin receptor blockade did not suppress the evoked feeding response during AGRP neuron photostimulation (Aponte et al., 2011) or rescue aphagia after AGRP neuron ablation (Wu et al., 2008a). This indicated that melanocortin receptor inhibition was not a necessary component of acute AGRP neuron-evoked feeding. In addition, POMC neuron inhibition was found to be insufficient to acutely evoke feeding behavior, but longer-term (24 hr) silencing did increase feeding (Atasoy et al., 2012). Furthermore, ARCAGRP→ARCPOMC circuit inhibition was not necessary for evoked eating, based on simultaneous optogenetic coactivation of POMC neurons with AGRP neurons (cf. Figure 2D), which did not suppress feeding (Atasoy et al., 2012). These studies indicate that POMC neurons and their regulation by AGRP neurons are relevant for longer-term regulation of energy homeostasis and feeding, and it is AGRP axon projections outside of the ARC that are responsible for acutely evoked feeding.
The functional contributions of long-range axon projections from AGRP neurons were examined by cell type-specific axon photostimulation in two downstream circuit nodes previously shown to regulate feeding behavior, the PVH and the PBN (cf. Figure 2C). The results showed a dissociation of the behavioral response between the two regions, where photostimulation of AGRP axons projecting to the PVH but not the PBN of the same animals led to evoked feeding similar to somatic AGRP neuron activation in the ARC (Atasoy et al., 2012). What does this mean in light of observations that benzodiazepines in the PBN rescue feeding after AGRP neuron ablation, while PVH delivery of benzodiazepines does not (Wu et al., 2009b)? It likely reflects very different processes involved in the role of AGRP neurons to suppress nausea circuits and their role in activating hunger circuits. This is supported by observations that, in animals with AGRP neurons intact, benzodiazepines in the PBN preferentially increase intake of liquid and palatable foods but not the standard rodent chow pellets (De Oliveira et al., 2011) that were used in AGRP neuron activation experiments (Aponte et al., 2011). In addition, the PVH sends projections to the PBN (Tokita et al., 2009), but manipulations that suppress PVH electrical activity in AGRP neuron-ablated mice (such as benzodiazepine delivery) may not be able to overcome PBN activation resulting from loss of AGRP neuron inhibitory input. Moreover, the antiemetic ondansetron rescues feeding in AGRP neuron-ablated mice, but it does not induce voracious eating when AGRP neurons are intact. Taken together, it does not appear that evoked feeding from AGRP neuron activation is simply the opposite of the process that leads to aphagia after AGRP neuron ablation.
Additional analysis of the ARCAGRP→PVH projection using optogenetic circuit mapping (cf. Figure 2A) established functional synaptic connectivity and revealed an unexpected property of AGRP neuron synaptic connections, which was a prolonged asynchronous release component (Atasoy et al., 2012). This property led to strong GABA-mediated inhibition that persisted over hundreds of milliseconds, a timescale usually associated with neuromodulation but, in AGRP neurons, mediated by fast synaptic neurotransmission. In behaving animals, pharmacological blockade of GABAA-R or NPY1R during ARCAGRP→PVH axon stimulation (cf. Figure 2D) strongly attenuated evoked eating, demonstrating that GABA and NPY signaling are necessary for AGRP neuron-evoked feeding (Atasoy et al., 2012). The sufficiency of the inhibitory ARCAGRP→PVH circuit operation to evoke eating was tested with direct, cell type-specific pharmacogenetic inhibition of PVH neurons, using the Sim1-Cre transgenic mouse line (cf. Figure 2B). Sim1 gene expression marks many PVH neurons (Michaud et al., 1998), which can be selectively transduced with a targeted viral injection in the area around the PVH. PVHSIM1 neuron silencing with two different pharmacogenetic methods recapitulated the behavioral effects of activating the presynaptic inhibitory AGRP neuron population (Atasoy et al., 2012). Similar to this, whole-brain SIM1 neuron ablation using diphtheria toxin led to long-term overeating (Xi et al., 2012). Moreover, just as activation of AGRP neurons increased the motivation to work for food pellets, motivation for food was increased to a similar level by SIM1 neuron silencing in the PVH, a brain region not previously associated with upregulating instrumental responses (Atasoy et al., 2012). Together, these experiments demonstrate that the behavioral consequences of molecularly defined circuits can be recapitulated in a node downstream to a need-sensing cell type, raising the possibility of following the neural representation of energetic needs and their motivational consequences across multiple circuit nodes in the brain.
Synaptic circuit mapping techniques can also be used to identify new connections between molecularly defined neurons. Using channelrhodopsin-assisted circuit mapping, oxytocin (OXT) neurons within the PVH were shown to be targeted by AGRP neuron axons (cf. Figure 2A) (Atasoy et al., 2012). The behavioral significance of this interaction between two molecularly defined neuron populations was probed with cell type-specific occlusion of the inhibitory circuit connection. Coactivation of AGRP neuron axons and PVHOXT neurons (cf. Figure 2D) suppressed AGRP neuron-evoked feeding (Atasoy et al., 2012), and this epistatic interaction is consistent with an important role for AGRP neuron-mediated suppression of PVHOXT neurons in feeding. OXT neurons are thought to regulate feeding through projections to the hindbrain where oxytocin signaling enhances hindbrain responses to circulating satiety signals (Blevins et al., 2003), and genetic disruption of synaptic release from OXT neurons (Zhang et al., 2011) or ablation of OXT receptor-expressing hindbrain neurons both lead to overeating (Baskin et al., 2010). Because PVHOXT neurons are selectively lost in Prader-Willi Syndrome (PWS) (Swaab et al., 1995) and also SIM1 haploinsufficiency (Kublaoui et al., 2006, 2008; Traurig et al., 2009), which are genetic conditions associated with insatiable appetite, the inhibitory interaction of AGRP neurons with PVHOXT neurons may be a useful model for these overeating behaviors.
These experiments also illustrate the selective interactions that are revealed through cell type-specific deconstruction of neural circuits. For example, although optogenetic PVHOXT neuron activation in mice completely suppressed AGRP neuron-evoked eating, PVHOXT activation did not significantly reduce food consumption after a 24 hr fast (Atasoy et al., 2012). Thus, while PVHOXT neuron suppression may be important for the feeding behavior induced by selectively activating the ARCAGRP→ PVH circuit, this is just a fragment of the circuitry involved in food deprivation-induced feeding. In food deprivation, other projections of AGRP neurons are activated along with complementary pathways that signal hunger in addition to AGRP neurons. Consistent with this, pharmacological blockade of ARCAGRP→PVH projections while stimulating AGRP neuron somata (and presumably all of the additional projections of AGRP neurons) was found to result in a significant increase in feeding, suggesting that the PVH is not the sole region responsible for AGRP neuron-evoked feeding (Atasoy et al., 2012).
Together, recent studies indicate that AGRP neurons can have distinct functions mediated by specific circuit connections. AGRP neurons can acutely evoke feeding, in part, by inhibiting the PVH, but not the PBN or POMC neurons in the ARC. POMC neurons appear to regulate longer-term feeding responses, and AGRP neuron projections targeting the PBN restrain visceral malaise that results from AGRP neuron ablation and consequent unopposed excitation from a serotonin-modulated hindbrain circuit. Thus, multiple mechanisms involved in hunger are dissociated into distinct behavioral modules by anatomically separate AGRP neuron axonal projections. These findings would not have been accessible without rapid, cell type-specific manipulation of AGRP neuron function, permitting circuit nodes with the most influence over feeding behavior to be identified for further analysis. These experiments provide a circuit framework that extends across molecular, synaptic, cellular, and behavioral functions and prioritizes circuit nodes for other methods of analysis, such as in vivo electrophysiology and imaging, guided by the blueprint from this sensory neuron entry point to hunger-related motivational processes.
Circuits for Aggressive and Sexual Behaviors
Aggression and sex are critical social behaviors associated with protecting territory and propagating the species, respectively. These behaviors appear to be linked because male mice tend to engage in aggressive interactions with male intruder mice, but this is suppressed for interactions with females, and sexual behavior is seen instead. Genetic disruption of pheromone signaling and physical ablation of neurons in the pheromone-sensing vomeronasal organ both lead to aberrant sexual and aggression behavior in which mice fail to show male-male aggression and engage in sexual behaviors toward both males and females (Kimchi et al., 2007; Stowers et al., 2002). Tracing studies have shown that pheromone and olfactory receptor neurons participate in a polysynaptic circuit with hypothalamic subregions (Boehm et al., 2005; Kevetter and Winans, 1981a, 1981b; Yoon et al., 2005). These hypothalamic areas contain discrete interoceptive neuron populations that sense circulating hormone levels, such as estrogen and testosterone, which strongly influence both sexual and aggressive behaviors (Davidson, 1966; Juntti et al., 2010; Musatov et al., 2006; Ogawa et al., 2000). A variety of studies involving brain lesion, electrical stimulation, or intracranial hormone injection point to the hypothalamus as necessary and sufficient for aggressive and sexual behaviors (Davidson, 1966; Edwards and Burge, 1971; Kruk et al., 1983; Pfaff and Sakuma, 1979). How hypothalamic subregions and their component neuronal cell types orchestrate these complex social behaviors remains poorly understood, and this question is well suited to investigation with new tools for cell type-specific neuron perturbations.
In the hypothalamus, aggressive and sexual behaviors have been recently examined using a combination of correlative electrical activity measurements and genetically encoded tools for manipulating neuron activity (Lin et al., 2011). This study did not start with a specific population of interoceptive sensory neurons but instead looked broadly to identify brain regions selectively activated during male aggressive attack behaviors. Compartment analysis of temporal activity by fluorescent in situ hybridization (catFISH) was used to determine the nuclear versus cytoplasmic localization of Fos transcript after mice received two successive exposures to sexual and/or fighting episodes (Lin et al., 2011). Based on the relationship between increased neuronal electrical activity and Fos expression as well as the temporal dynamics of Fos mRNA export from the nuclear compartment, this technique distinguishes between neurons that were activated during either behavioral episode. In a subset of brain regions, successive exposure to fighting-then-mating or mating-then-fighting led primarily to different neuronal subpopulations being activated as a result of these distinct behavioral episodes.
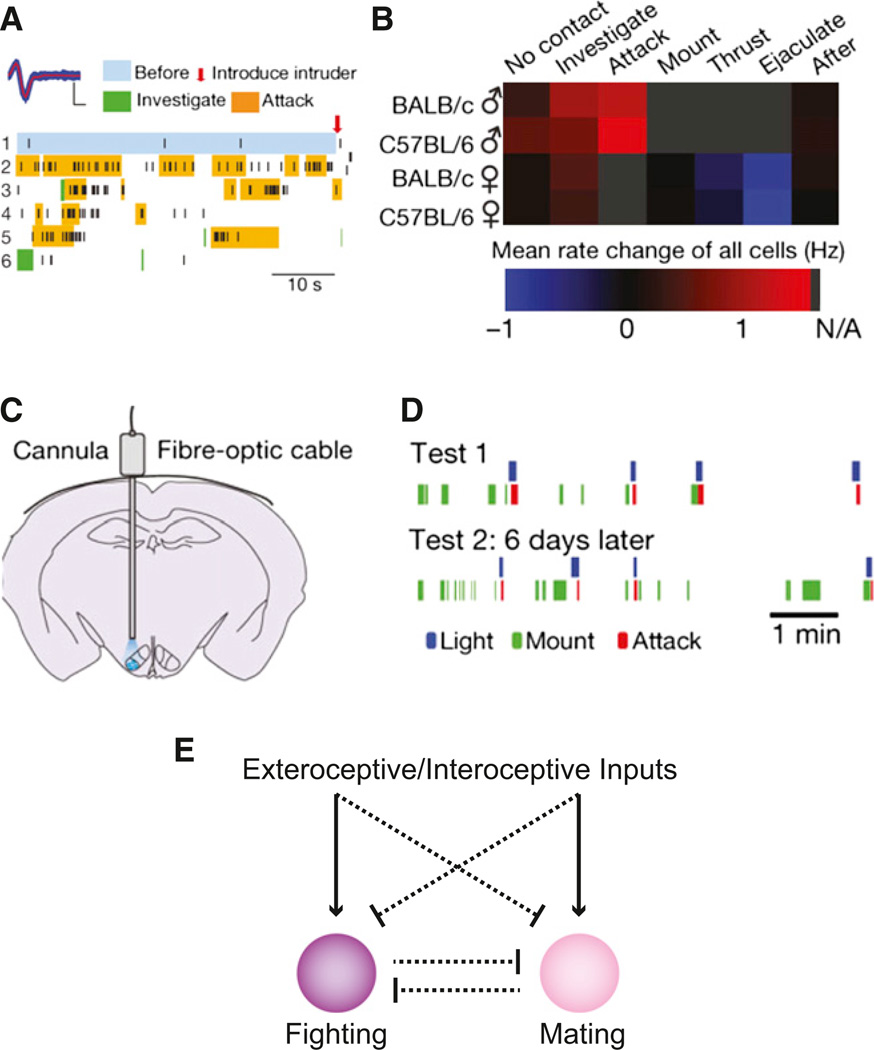
The ventolateral portion of the ventromedial hypothalamic nucleus (VMHvl) showed a high distribution of neurons expressing Fos after fighting that was also significantly greater than those neurons expressing Fos after mating. Subsequent in vivo electrophysiological recordings within the VMHvl (Figures 6A and 6B) revealed that 41% of VMHvl cells were significantly activated during a social encounter with a male intruder mouse (Lin et al., 2011), indicating a role in male-male interactions. The number of activated cells progressively increased as the behavioral sequence evolved across intruder introduction, investigation, and then attack. In contrast, exposure to a female mouse led predominantly to a reduction of activity in the VMHvl as exploratory social behavior gave way to sexual behaviors (Lin et al., 2011). This included the male-excited neurons, of which 78% showed reduction of activity in the presence of a female mouse. Thus, VMHvl contains neurons sensitive to aggressive behaviors that are suppressed by interactions with a female, especially sexual behavior.
Figure 6. An Aggression Locus in the Ventrolateral Subdivision of the Ventromedial Hypothalamus.
(A) In vivo electophysiological recordings in mice show electrical activity from neurons in the VMHvl during male-male social interactions (Lin et al., 2011). High levels of activity are observed during attack behaviors.
(B) Summary of mean firing rate changes across different social interactions between male and female mice (Lin et al., 2011).
(C) Configuration of optogenetic experiments for VMHvl photostimulation (Lin et al., 2011).
(D) Ethogram showing VMHvl photostimulation-evoked attack behavior (Lin et al., 2011).
(E) Schematic of functional interactions between antagonistic circuits that mediate fighting and mating. Circuits to support the proposed inhibitory interactions have not been identified. Inhibitory interactions may be at the level of inputs to circuits mediating fighting and mating or may result from antagonism between these circuits.
To test the causal relationship of VMHvl electrical activity and attack behaviors, increased electrical activity could be externally imposed by ChR2-mediated photostimulation of VMHvl, which was sufficient to evoke attack behaviors (Figures 6C and 6D) by male mice on other males and also under conditions in which attack normally would not occur such as toward females and even an inflated glove (Lin et al., 2011). Attack-related motor responses were not apparent if mice were photostimulated in the absence of an intruder and were rarely observed with motionless targets (inflated glove or anesthetized mouse), indicating an important influence of visual (motion) contextual cues on the evoked behavior. Based on post hoc analysis of ChR2-expression, this aggression locus appeared to be in the VMHvl, and ChR2 expression in the adjacent lateral hypothalamus and dorsomedial VMH did not induce attack behaviors. The VMHvl is a portion of a “hypothalamic attack area” that was mapped previously using electrical stimulation in the rat (Kruk et al., 1983; Lammers et al., 1988) and the cat (Hess, 1957). In the mouse, though, electrical stimulation, including in the VMHvl, did not evoke attack responses but instead induced locomotion or no clear behavior (Lin et al., 2011). The contrasting results using electrical and optogenetic stimulation illustrates the importance of stimulation methods with clearly delineated origin of action such as ChR2 and other genetically encoded neuronal activators, which are amenable to post hoc anatomical localization, as opposed to electrical stimulation, which can spread indeterminate distances and can also activate axons of passage from other brain regions.
There is also a relationship between ChR2-evoked male aggressive behavior and sexual behavior. Evoked aggression toward a female was suppressed during intromission and could be reinitiated after ejaculation (Lin et al., 2011). This is consistent with the natural activity pattern in VMHvl that shows a reduction of firing during intromission, but in this case the effect of photactivation of the VMHvl is also suppressed. While this might be attributable to a reduction in the excitability of ChR2-expressing neurons mediated by mating circuits (Figure 6E), it may also reflect modulation of activity downstream in the local or long-range circuitry. This inhibitory gating function is a fascinating feature uncovered by the combined use of in vivo electrophysiology and gain-of-function optogenetic behavioral manipulation.
Elevated VMHvl neuron activity also appears to be necessary for male-male aggression. Pharmacogenetic inhibition of VMHvl neurons showed a dramatic reduction in the duration of attack events. This effect was selective for aggression over sexual behavior as evidenced by the observation that mount duration and latency were not significantly affected by VMHvl silencing. This is further consistent with electrophysiological data showing that VMHvl activity is reduced during sexual behavior and highlights the necessity of VMHvl activity for attack behavior (Lin et al., 2011). Suppression of natural aggressive behavior by silencing the area associated with evoked behavior supports the conclusion that VMHvl circuitry is important for generating attack behavior.
Intermingled, antagonistic circuits for aggressive and sexual behaviors (Figure 6E) are evident based on the catFISH technique, in vivo electrophysiology, and optogenetic manipulations (Lin et al., 2011). A future challenge will be to gain discrete access to the component cell populations, so that circuit interactions in the VMHvl as well as in upstream and downstream targets can be investigated. Identification of sex- and aggression-specific hypothalamic cell types may be guided by circuit-tracing studies from exteroceptive pheromone and olfactory sensory systems, which play a key role for controlling these social interactions. Cellular entry points may also be derived from interoceptive neurons sensing sex steroid hormones (e.g., by hormone receptor expression) or neuron populations defined by gender-specific differences in gene expression that are seen in the VMHvl and influence aggression and sexual behaviors (Juntti et al., 2010; Kayasuga et al., 2007; Musatov et al., 2006; Ogawa et al., 2000; Wersinger et al., 2002; Wu et al., 2009a; Xu et al., 2012). Application of cell type-specific tools to these and other cell types is a next step for establishing the distinct functional properties of separate populations and then mapping their neural circuits to understand the upstream and downstream circuits controlling these complex behaviors.
Conclusions and Future Directions
Because hypothalamic circuits exert a profound influence on animal behavior, they are an entry point to basic processes that regulate behavioral intent and intensity. The first steps toward understanding these neural circuits and behaviors have been taken by gathering a parts list of neural components, the connections between cell types, and the means to manipulate groups of neurons independently with rapid temporal control. The emergence of new cell type-specific tools coupled with the rapid increase in Cre-driver mouse lines (Gong et al., 2007; Taniguchi et al., 2011) has unlocked the hypothalamus for indepth neural circuit analysis. Leveraging the capacity of hypothalamic circuits to rapidly evoke motivated behaviors will become an increasingly powerful approach to evaluate the relative functional contribution of individual circuit components.
A key question is how well do evoked behaviors inform us about the normal physiologic and behavioral role of the circuits under investigation? Evoked behavior and loss-of-behavior experiments contribute to our understanding by testing causal relationships between cell types or circuits and complex behavioral responses. The ability to externally control circuit elements is essential to reverse-engineer neural circuits controlling fundamental motivational processes. In this approach, the circuit is evaluated with respect to the necessity and sufficiency of molecularly defined circuit functions, typically with full gain-of-function and loss-of-function perturbations. The main limitation of this approach, which is also encountered in most other types of perturbation experiments, is that the endogenous activity levels in these circuits are often not known or cannot be recapitulated exactly with experimental operations. Neuron manipulation studies aim to test the capabilities of a system but may not precisely reflect specific activity patterns that are found in these circuits. Therefore, questions about the specificity of evoked behaviors must be addressed, for example, by using perturbations to suppress the natural behavior in sites shown to elicit the evoked behavior. Nevertheless, the advantage of new tools is that a range of activity levels can be imposed on the system to examine the relationship of different activity patterns in various numbers of neurons on a behavioral response. The end result is information about the relative capacity of circuits to influence behavior. Such circuit analysis approaches serve the purpose of prioritizing new circuits for their relative influence on behavior and for subsequent analysis using electrophysiology and imaging. The extension of cell type-specific imaging techniques or electrophysiological recording techniques to hypothalamic circuits deep in the brain within awake, behaving animals are challenging but also sorely needed to extend nonperturbative monitoring of cell types and circuits identified with perturbation approaches (Ghosh et al., 2011; Lima et al., 2009).
The most interesting questions about the role of hypothalamic circuits in behavior still remain to be addressed. How can a simple and arbitrary stimulus, which is applied to a small neuron population, direct behavioral intent and concurrently elevate behavioral intensity to levels associated with extreme need? These issues should be investigated by manipulation of circuit nodes and their projections under experimental conditions designed to probe their influence on motivational components of the behavioral response. The capability for AGRP neuron activity to induce food-seeking behaviors that involve trained actions such as nose poking or lever pressing is suggestive of a motivational process, but the influence of AGRP neurons on underlying processes such as reinforcement is not understood. Whether reinforcement mechanisms operate for VMHvl-evoked attack behavior is even less clear (Anderson, 2012). However, the access to distinct but intermingled circuits through molecularly defined cell types is the first step toward uncovering principles through which these complex behaviors are mediated. More generally, the relationship of survival needs to the higher-order motivations more commonly associated with the modern human experience will be far more tractable once a basic substrate is established on which progenitor-motivated behaviors are based. The implications of how our basic needs capture our “will” and thereby influence our behavior can impact our understanding of why we do what we do at levels spanning molecular mechanisms to psychological concepts.
ACKNOWLEDGMENTS
S.M.S.’s research is supported by the Howard Hughes Medical Institute.
REFERENCES
- Anderson DJ. Optogenetics, sex, and violence in the brain: implications for psychiatry. Biol. Psychiatry. 2012;71:1081–1089. doi: 10.1016/j.biopsych.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Wasserman S. Ordering gene function: the interpretation of epistasis in regulatory hierarchies. Trends Genet. 1992;8:312–316. doi: 10.1016/0168-9525(92)90263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, Simhan HN, Moralejo DH, Blevins JE. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology. 2010;151:4207–4213. doi: 10.1210/en.2010-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Saunders A, Oldenburg IA, Miyamichi K, Akhtar N, Luo L, Whelan SP, Sabatini B, Cepko CL. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc. Natl. Acad. Sci. USA. 2011;108:15414–15419. doi: 10.1073/pnas.1110854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Okamura T, Brüning JC. Hormone and glucose signalling in POMC and AgRP neurons. J. Physiol. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Valenstein ES. What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behav. Neurosci. 1991;105:3–14. doi: 10.1037//0735-7044.105.1.3. [DOI] [PubMed] [Google Scholar]
- Betley JN, Sternson SM. Adeno-associated viral vectors for mapping, monitoring, and manipulating neural circuits. Hum. Gene Ther. 2011;22:669–677. doi: 10.1089/hum.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu. Rev. Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Cell-type identity: a key to unlocking the function of neocortical circuits. Curr. Opin. Neurobiol. 2009;19:415–421. doi: 10.1016/j.conb.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M. Influence of food and water deprivation on the behavior of the white rat foraging in a hostile environment. Physiol. Behav. 1985;35:701–709. doi: 10.1016/0031-9384(85)90400-7. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. USA. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. The Wisdom of the Body. Second Edition. New York: W.W. Norton & Co.; 1939. [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol. Biochem. Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, Schuldiner M, Gebbia M, Recht J, Shales M, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- Coons EE, Levak M, Miller NE. Lateral hypothalamus: learning of food-seeking response motivated by electrical stimulation. Science. 1965;150:1320–1321. doi: 10.1126/science.150.3701.1320. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann. N Y Acad. Sci. 2003a;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003b;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Davidson JM. Activation of the male rat’s sexual behavior by intracerebral implantation of androgen. Endocrinology. 1966;79:783–794. doi: 10.1210/endo-79-4-783. [DOI] [PubMed] [Google Scholar]
- De Oliveira LB, Kimura EH, Callera JC, De Luca LA, Jr, Colombari DS, Menani JV. Baclofen into the lateral parabrachial nucleus induces hypertonic sodium chloride and sucrose intake in rats. Neuroscience. 2011;183:160–170. doi: 10.1016/j.neuroscience.2011.02.019. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am. J. Physiol. 1953;172:162–168. doi: 10.1152/ajplegacy.1952.172.1.162. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm. Behav. 1971;2:49–58. [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsáki G, Deisseroth K, Tepper JM, Koos T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat. Neurosci. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and proopio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- Freud S. Instincts and their vicissitudes. In: Strachey J, editor. In Standard Edition of the Complete Psychological Works of Sigmund Freud. London: Hogarth Press; 2001. pp. 111–140. [Google Scholar]
- Frick A, Feldmeyer D, Helmstaedter M, Sakmann B. Monosynaptic connections between pairs of L5A pyramidal neurons in columns of juvenile rat somatosensory cortex. Cereb. Cortex. 2008;18:397–406. doi: 10.1093/cercor/bhm074. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Gautron L, Lazarus M, Scott MM, Saper CB, Elmquist JK. Identifying the efferent projections of leptin-responsive neurons in the dorso-medial hypothalamus using a novel conditional tracing approach. J. Comp. Neurol. 2010;518:2090–2108. doi: 10.1002/cne.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nat. Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer MA. Suggestive evidence of a primary drinking center in hypothalamus of the rat. Proc. Soc. Exp. Biol. Med. 1955;89:59–62. doi: 10.3181/00379727-89-21716. [DOI] [PubMed] [Google Scholar]
- Grossman SP. Eating or drinking elicited by direct adrenergic or cholinergic stimulation of hypothalamus. Science. 1960;132:301–302. doi: 10.1126/science.132.3422.301. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hess WR. The Functional Organization of the Diencephalon. New York: Grune & Stratton; 1957. [Google Scholar]
- Higgs S, Cooper SJ. Hyperphagia induced by direct administration of midazolam into the parabrachial nucleus of the rat. Eur. J. Pharmacol. 1996;313:1–9. doi: 10.1016/0014-2999(96)00446-3. [DOI] [PubMed] [Google Scholar]
- Hull CL. Principles of behavior. New York: D. Appleton-Century Co.; 1943. [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Harris KH. Excitatory amino acids may mediate nucleus tractus solitarius input to rat parabrachial neurons. Am. J. Physiol. 1992;263:R324–R330. doi: 10.1152/ajpregu.1992.263.2.R324. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front. Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav. Brain Res. 2007;185:110–118. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J. Comp. Neurol. 1981a;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the “olfactory amygdala”. J. Comp. Neurol. 1981b;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J. Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhao T, Petralia RS, Yu Y, Peng H, Myers E, Magee JC. mGRASP enables mapping mammalian synaptic connectivity with light microscopy. Nat. Methods. 2012;9:96–102. doi: 10.1038/nmeth.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk MR, Van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AH. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Holder JL, Jr, Gemelli T, Zinn AR. Sim1 haploinsufficiency impairs melanocortin-mediated anorexia and activation of paraventricular nucleus neurons. Mol. Endocrinol. 2006;20:2483–2492. doi: 10.1210/me.2005-0483. [DOI] [PubMed] [Google Scholar]
- Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Huang ZJ. High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression. PLoS ONE. 2008;3:e2005. doi: 10.1371/journal.pone.0002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers JH, Kruk MR, Meelis W, van der Poel AM. Hypo-thalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988;449:311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J. Neurosci. 2004;24:3879–3889. doi: 10.1523/JNEUROSCI.4710-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J. Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Hromádka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L, Anderson DJ. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GW, Leinninger GM, Rhodes CJ, Myers MG., Jr Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J. Neurosci. 2010;30:11278–11287. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA. Development of the neuroendocrine hypothalamus. Front. Neuroendocrinol. 2002;23:257–291. doi: 10.1016/s0091-3022(02)00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow AH. A theory of human motivation. Psychol. Rev. 1943;50:370–396. [Google Scholar]
- Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12:3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NE. Experiments on motivation. Studies combining psychological, physiological, and pharmacological techniques. Science. 1957;126:1271–1278. doi: 10.1126/science.126.3286.1271. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Stevenson JA. Drinking induced by electrical stimulation of the lateral hypothalamus. Exp. Neurol. 1967;17:119–127. doi: 10.1016/0014-4886(67)90139-2. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl. Acad. Sci. USA. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc. Natl. Acad. Sci. USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal activities of the ventromedial and lateral hypothalamic areas of cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Nakamura T, Sugimori M, Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol. Behav. 1975;14:483–486. doi: 10.1016/0031-9384(75)90015-3. [DOI] [PubMed] [Google Scholar]
- Peltékian E, Garcia L, Danos O. Neurotropism and retrograde axonal transport of a canine adenoviral vector: a tool for targeting key structures undergoing neurodegenerative processes. Mol. Ther. 2002;5:25–32. doi: 10.1006/mthe.2001.0517. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Drive: Neurobiological and Molecular Mechanisms of Sexual Motivation. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J. Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP. Total self regulatory functions in animals and human beings. Harvey Lect. 1943;38:63–103. [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, et al. Input of orexin/ hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Saunders A, Johnson CA, Sabatini BL. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front Neural Circuits. 2012;6:47. doi: 10.3389/fncir.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J. Neurosci. 2012;32:12437–12443. doi: 10.1523/JNEUROSCI.0706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shapiro MG, Frazier SJ, Lester HA. Unparalleled control of neural activity using orthogonal pharmacogenetics. ACS Chem Neurosci. 2012;3:619–629. doi: 10.1021/cn300053q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG, Svoboda K. Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J. Neurosci. 2005;25:5670–5679. doi: 10.1523/JNEUROSCI.1173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Anatomical substrates of hypothalamic integration. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academic Press; 1994. pp. 353–376. [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Ha LH, Spears LC, Dee MG., 2nd Lateral hypothalamic injections of glutamate, kainic acid, D,L-alpha-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-D-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 1993;613:88–95. doi: 10.1016/0006-8993(93)90458-y. [DOI] [PubMed] [Google Scholar]
- Steiner M, Katz RJ, Baldrighi G, Carroll BJ. Motivated behavior and the estrous cycle in rats. Psychoneuroendocrinology. 1981;6:81–90. doi: 10.1016/0306-4530(81)90051-2. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav. Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J. Clin. Endocrinol. Metab. 1995;80:573–579. doi: 10.1210/jcem.80.2.7852523. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res. Brain Res. Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J. Comp. Neurol. 1987;265:275–293. doi: 10.1002/cne.902650210. [DOI] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience. 2009;161:475–488. doi: 10.1016/j.neuroscience.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman EC. Purposive Behavior in Animals and Men. New York: The Century Co.; 1932. [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traurig M, Mack J, Hanson RL, Ghoussaini M, Meyre D, Knowler WC, Kobes S, Froguel P, Bogardus C, Baier LJ. Common variation in SIM1 is reproducibly associated with BMI in Pima Indians. Diabetes. 2009;58:1682–1689. doi: 10.2337/db09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Modification of motivated behavior elicited by electrical stimulation of the hypothalamus. Science. 1968;159:1119–1121. doi: 10.1126/science.159.3819.1119. [DOI] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat. Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Vaughan E, Fisher AE. Male sexual behavior induced by intra-cranial electrical stimulation. Science. 1962;137:758–760. doi: 10.1126/science.137.3532.758-a. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Si-Hoe SL, Murphy D, Brenner S. Transgenic rats reveal functional conservation of regulatory controls between the Fugu isotocin and rat oxytocin genes. Proc. Natl. Acad. Sci. USA. 1997;94:12462–12466. doi: 10.1073/pnas.94.23.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J. Neurophysiol. 2006;95:1491–1498. doi: 10.1152/jn.00697.2005. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Plasticity of hypothalamic motivational systems. Science. 1969;165:929–930. doi: 10.1126/science.165.3896.929-a. [DOI] [PubMed] [Google Scholar]
- Wise RA. Lateral hypothalamic electrical stimulation: does it make animals ‘hungry’? Brain Res. 1974;67:187–209. doi: 10.1016/0006-8993(74)90272-8. [DOI] [PubMed] [Google Scholar]
- Wise RA, Devor MG, Milgram NW, Hoebel BG. Physiological control of hypothalamically elicited feeding and drinking. J. Comp. Physiol. Psychol. 1970;73:226–232. doi: 10.1037/h0030209. [DOI] [PubMed] [Google Scholar]
- Woodworth CH. Attack elicited in rats by electrical stimulation of the lateral hypothalamus. Physiol. Behav. 1971;6:345–353. doi: 10.1016/0031-9384(71)90166-1. [DOI] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc. Natl. Acad. Sci. USA. 2008a;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J. Neurosci. 2008b;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009a;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009b;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Gandhi N, Lai M, Kublaoui BM. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS ONE. 2012;7:e36453. doi: 10.1371/journal.pone.0036453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Transgenic strategy for identifying synaptic connections in mice by fluorescence complementation (GRASP) Front Mol Neurosci. 2012;5:18. doi: 10.3389/fnmol.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, Cai D. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69:523–535. doi: 10.1016/j.neuron.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]