Abstract
Research indicates that very short or long durations of sleep and inefficient sleep, are associated with higher total cholesterol and risk of type 2 diabetes and hypertension. This study tested the hypothesis that inefficient sleep or short/long sleep durations are associated with an elevated prevalence of type 2 diabetes, dyslipidemia, and hypertension in a community-dwelling sample of elderly Alzheimer’s caregivers. Participants were 126 caregivers for spouses with Alzheimer’s disease who underwent in-home sleep assessment by wrist actigraphy for 72 consecutive hours. Sleep data were averaged across the 3 days/nights; nighttime sleep and daytime napping were computed. Morning fasting blood samples were collected to determine measures of blood lipids and glucose. The average of three resting blood pressure measurements was used to estimate mean resting blood pressure. Logistic regression models including covariates related to sleep and metabolic regulation indicated that nighttime sleep duration, percent sleep at night, and daytime naps were not significantly associated with odds of having diabetes (OR, 0.92; 95%CI, 0.56–1.53; OR, 0.93; 95%CI, 0.83–1.03; OR, 1.75; 95%CI, 0.74–4.11, respectively), dyslipidemia (OR, 0.83; 95%CI, 0.57–1.20; OR, 0.99; 95%CI, 0.92–1.07; OR, 0.64; 95%CI: 0.33–1.24, respectively), or hypertension (OR, 0.97; 95%CI, 0.62–1.52; OR, 1.02; 95%CI, 0.93–1.11; OR, 1.10; 95%CI, 0.44–2.74, respectively). When categorical and combined sleep parameters were examined, there were no significant associations with any of the metabolic conditions (all p>0.05). The current study suggests that in an elderly sample of Alzheimer’s caregivers, nighttime sleep duration, nighttime sleep efficiency and daytime naps are not significantly associated with prevalent type 2 diabetes, dyslipidemia, or hypertension. As several of the associations demonstrated clinically relevant magnitudes of the associations, larger studies to more fully test these hypotheses are warranted.
Keywords: Sleep, type 2 diabetes, hypertension, dyslipidemia, caregivers
1.1 Introduction
Caregivers are at increased risk for coronary heart disease (CHD), which could be partially due to the effect that inadequate sleep has on numerous physiologic processes, including metabolic regulation (McCurry, Logsdon, Teri, & Vitiello, 2007; Newman et al., 2000; Rowe, McCrae, Campbell, Benito, & Cheng, 2008; Schulz & Beach, 1999; von Känel et al., 2008; Wolk, Gami, Garcia-Touchard, & Somers, 2005). Compared to non-caregivers, sleep has been shown to be shorter and more disturbed in Alzheimer’s caregivers, presumably due to challenges associated with caregiving (McKibbin et al., 2005; Rowe et al., 2008). Although the underlying mechanisms of the adverse effects of insufficient sleep on glucose metabolism, blood pressure, and lipid-lipoprotein levels are not completely understood, numerous pathways have been proposed. These include: 1) insufficient nighttime sleep, whether caused by disrupted sleep or by inadequate sleep duration, may lead to increased caloric consumption and weight gain by mediating fluctuations in neuropeptide levels that regulate appetite (e.g. leptin and ghrelin) (Grandner, Patel, Gehrman, Perlis, & Pack, 2010); 2) insufficient sleep may increase sympathetic nervous system activity, which in turn may increase blood pressure (Kato et al., 2000; Tochikubo, Ikeda, Miyajima, & Ishii, 1996); 3) insufficient sleep may lead to decreased cerebral glucose utilization, which may increase risk of insulin resistance (Thomas et al., 2000); and 4) insufficient sleep leads to tiredness, which results in reduced physical activity and potential weight gain (Leproult, Copinschi, Buxton, & Van Cauter, 1997; K Spiegel, Leproult, & Van Cauter, 1999). These physiologic perturbations may lead to increased blood pressure, heightened cholesterol and triglyceride levels, and impaired insulin sensitivity and glucose tolerance; which in turn increase risk of hypertension, dyslipidemia, and type 2 diabetes.
Insufficient nighttime sleep and napping have been associated with the development of type 2 diabetes (Chao et al., 2011; Chaput, Després, Bouchard, Astrup, & Tremblay, 2009; J. C. M. Lam & Ip, 2010; Schmid et al., 2011; Karine Spiegel, Tasali, Penev, & Van Cauter, 2004). For example, the National Institutes of Health - AARP Diet and Health Study – a prospective study on older adults – reported that short self-reported nighttime sleep (<5 hours) and longer daytime napping (≥1 hour) were each associated with increased risk of type 2 diabetes 4–9 years later (Xu et al., 2010). Evidence regarding associations of sleep with blood pressure and risk of hypertension in older adults varies (Buxton & Marcelli, 2010; Gottlieb et al., 2006; Kim & Jo, 2010; Stranges et al., 2010; Vgontzas, Liao, Bixler, Chrousos, & Vela-Bueno, 2009). For example, Rod et al. reported that sleep disturbances were associated with an increased risk of developing hypertension during 19 years of follow-up (Rod et al., 2011). Conversely, analyses from a cross-sectional study indicated that self-reported nighttime sleep duration was not significantly associated with risk of hypertension among adults aged ≥65 years (Kim & Jo, 2010). The few studies that have examined associations between sleep and serum lipids and lipoproteins have also yielded conflicting results (Chaput et al., 2007; Ekstedt, Akerstedt, & Söderström, 2004; van den Berg et al., 2008). A study that objectively measured one night of sleep in young adults (mean age of 30 years) indicated that reduced sleep efficiency was associated with higher total cholesterol (Ekstedt et al., 2004). Conversely, a study that used actigraphy to quantify sleep in 983 elderly participants with a mean age of 69 years, reported that nighttime sleep duration and efficiency (defined as less fragmented sleep) were positively associated with total cholesterol and total/HDL cholesterol (Van den Berg et al., 2008). Researchers have argued that relationships of sleep with blood pressure and serum lipid-lipoprotein concentrations become less evident with age (Chaput et al., 2007; Kim & Jo, 2010; Knutson, 2010; Lima-Costa, Peixoto, & Rocha, 2008; van den Berg et al., 2007).
Data on objectively measured sleep and metabolic risk factors in older adults are limited and somewhat conflicting (Eckel, Grundy, & Zimmet, 2005; Hasler et al., 2004; Vasudevan & Ballantyne, 2005; Wilson, D’Agostino, Parise, Sullivan, & Meigs, 2005). Research examining these associations in elderly dementia caregivers is even more limited, yet this group generally experiences frequent sleep difficulties, thereby potentially providing greater opportunity for poor sleep to impact health (Kring et al., 2010). The purpose of this cross-sectional analysis was to examine whether sleep duration and efficiency were associated with prevalent diabetes, hypertension, and dyslipidemia among 126 community-dwelling elderly spousal Alzheimer’s caregivers who participated in the Alzheimer Caregiver Coping Study.
2.1 Methods
2.1.1 The Alzheimer Caregiver Coping Study
The Alzheimer Caregiver Coping Study was conducted at the University of California, San Diego (UCSD) to examine the relationships between physiological and psychological stress markers, and health risk factors in spousal Alzheimer’s caregivers. Data were collected between 2007 and 2010.
Participants were 126 community-dwelling men and women over the age of 55 years who were married, living with, and providing continuous in-home care to a spouse diagnosed with Alzheimer’s disease. Caregivers were recruited via referrals from the UCSD Alzheimer’s Disease Research Center, community Alzheimer caregiver support groups, local agencies serving caregivers, recommendations from other participants enrolled in the study, flyers, media advertisements, and senior health fairs. Participants provided written informed consent, and the study was approved by the UCSD Institutional Review Board.
Caregivers were excluded if they had a current diagnosis or treatment for a life-threatening or terminal medical condition that required ongoing care (i.e., advanced CVD, Parkinson’s disease, and/or a severe psychiatric disorder), extreme hypertension (>200/120 mm Hg), current or recent (within the past 5 years) treatment for cancer, organ transplantation requiring anti-rejection medication, or use of corticosteroids, β-blocking, and/or anticoagulant medication.
2.2 Measures
All measurements and assessments, other than objective measures of sleep, were administered between 9:00AM and 11:00AM in participants’ homes by trained research personnel.
2.2.1 Sociodemographics, Medical Data, and Past Health History
The research assistant administered a semi-structured interview that gathered information on sociodemographics, medical history, health behaviors (e.g. smoking status) and hospitalizations. History of cardiovascular disease was defined as previous heart attack, heart failure, angina, heart disease, stroke, or transient ischemic attack. Caregivers were asked if they had ever been told by a physician that they had type 2 diabetes, hypertension, dyslipidemia, myocardial infarction or stroke, with answers coded yes/no. Information about cardiovascular medication (i.e., aspirin, angiotensin-converting enzyme inhibitors, and statins) and antidepressant use (i.e., atypicals, selective serotonin reuptake inhibitors, tricyclics, etc.) was obtained via participant report and confirmed by examination of the medication containers.
Smoking status was categorized as current/former smoker vs. never smoker and also measured in terms of years of smoking. Alcohol consumption was quantified in terms of the number of alcoholic drinks consumed per week. Data on height and weight were obtained by participant report, and body mass index (BMI) was calculated as the ratio of self-reported weight in kilograms to height in meters squared. Participants with a BMI > 30 were classified as obese.
2.2.2. Psychological Questionnaires
The semi-structured interview included questionnaires that evaluated duration of caregiving, psychological distress, perceived stress, depressive symptom severity, and anxiety and depressive symptoms. Duration of caregiving was determined by reported time in years that had elapsed since the spouse was diagnosed with Alzheimer’s disease. The 10-item short form of the Center for Epidemiologic Studies Depression Scale (CESD-10) was used to assess depressive symptoms (Andresen, Malmgren, Carter, & Patrick, 1994; HAMILTON, 1959, 1960). Levels of life stress were assessed with the 4-item Role Overload Scale, a self-report 4-point Likert scale ranging from l=not at all to 4=completely, which measured the extent to which participants felt overwhelmed by everyday tasks. Lower role overload and CESD-10 scores indicate lower stress and depressive symptoms, respectively. Reliability (Cronbach’s alpha) for the 4-item Role Overload Scale has ranged from 0.71 to 0.77 in different samples of elderly caregivers (Pearlin, Mullan, Semple, & Skaff, 1990), however, in our sample, the alpha coefficient is 0.82. Cronbach’s alpha for the CESD-10 in our sample of caregivers is 0.52.
2.2.3 Objective Sleep Measure
Objective sleep/wake activity was measured with the Actiwatch-Light® (Mini Mitter Co., Inc, a Respironics, Inc. Co., Bend, OR), which was worn for 72 consecutive hours (three consecutive 24-hour periods) on participants’ non-dominant wrist. While the ideal recording time for an actigraph is generally seven days, due to potential participant burden, the minimum of three days suggested by the American Academy of Sleep Medicine practice parameters for actigraphy was used (Ancoli-Israel et al., 2003). Actigraphy has been validated in and recommended for use among elderly populations and has been compared favorably with polysomnography (PSG), which is deemed the gold standard for sleep assessment (Ancoli-Israel et al., 2003, p. -; Blackwell et al., 2008). Additionally, three days of actigraphy measurement, which has been implemented in previous trials (Trento et al., 2008; van den Berg et al., 2007, 2008), corresponds well to PSG in studies with older participants (Ancoli-Israel et al., 2003; Blackwell et al., 2008). The Actiwatch-Light® uses a piezoelectric linear accelerometer (sensitivity <0.01 g-force) with a sampling rate of 32Hz to measure and record wrist movement. Movement, which was measured as the number of accelerations per minute, was captured via internal motion sensors in the watch. Calculating wrist activity over time allowed for an objective measure of duration and disruption of sleep. The recorded actigraphy data were analyzed using Actiware® sleep and activity monitoring software (version 5, by Mini Mitter|Respironics/Philips). Sleep diaries completed by participants estimated times the actigraphy watch was removed, bedtimes, and wake times, and were used for editing the actigraphy data.
The following sleep parameters were averaged across the three consecutive 24-hour time periods and selected a priori: 1) total nighttime sleep duration (hours per night spent sleeping from reported bedtime to final uptime); 2) total hours of daytime sleep (nap time), with naps defined as no activity for a minimum of 10 minutes; and 3) percent of time asleep at night between initial sleep onset and final awakening, which was used to denote sleep efficiency.
2.2.4 Outcomes – Type 2 Diabetes, Dyslipidemia and Hypertension
All participant blood samples were collected within one week of the initial visit in a fasting state between 9:00AM and 11:00AM to decrease the impact of diurnal fluctuations, and the first blood draw took place after 10 minutes of rest in the supine position. Total cholesterol, high density lipoproteins, and glucose were measured at the clinical chemistry laboratories at the UCSD Medical Center. Dyslipidemia was defined either by total-to-HDL ratio >5, or by self-report of current use of prescription cholesterol-lowering medications. Type 2 diabetes was defined either by self-report of physician-diagnosed diabetes and current use of antiglycemic medication, or a fasting blood glucose ≥126 mg/dL, as classified by the American Diabetes Association (“Diagnosis and classification of diabetes mellitus,” 2009).
Resting blood pressure was measured three times (after 5, 10, and 25 minutes of rest in the supine position) using a non-invasive Microlife BP monitor, model #3AC1-1PC. The average of the three measurements was used to create a composite resting blood pressure estimate. Hypertension was defined either by self-report of physician-diagnosed hypertension and current use of a prescription antihypertensive, or as a resting diastolic blood pressure or systolic blood pressure ≥90 or ≥ 140 mmHg, respectively.
2.3 Statistical Analysis
Associations between continuous sleep measures, key covariates (e.g. physical activity), and metabolic markers/conditions were first examined using Pearson r and t-tests, which identified potential confounders. Univariate analyses were performed to examine sleep parameters and covariates by type 2 diabetes, hypertension, and dyslipidemia status. First, the sleep variables were treated as continuous. To test for other non-linear associations or thresholds, sleep parameters were also modeled categorically by tertiles (nighttime sleep duration tertiles: <6.8, 6.8–7.8, and ≥7.8 hours; daytime sleep duration tertiles: <21, 21–56, and ≥56 minutes; and percent sleep at night tertiles: <85.9%, 85.9–90.2%, and ≥90.2%) and by combining sleep parameters into three new variables: 1) nighttime sleep duration with daytime sleep duration; 2) nighttime sleep duration with percent sleep at night; and 3) daytime sleep duration with percent sleep at night. In these secondary analyses, daytime sleep duration was reported in minutes for ease of interpretation, and in analyses with combined sleep parameters, reference groups were chosen based on current viewpoints about clinically significant cut points of these sleep parameters in elderly adults. Specifically, 7–8 hours of sleep at night, ≥85% sleep at night, and <30 minutes of daytime sleep are generally considered the normal cut-offs for elderly adults, and were therefore used as the reference groups and/or cut points (Blackwell et al., 2008; Stone et al., 2009).
Three multivariable logistic regression models were used to determine whether sleep parameters were associated with prevalent type 2 diabetes, dyslipidemia, and hypertension (separately). To prevent over-fitting in the multiple regression models, covariates were restricted to age, gender, those variables showing significant (p<0.10) univariate correlations with the outcome variables (metabolic markers/conditions), and variables selected a-priori likely to be related to metabolic regulation and sleep. These models were created to examine changes in the sleep-metabolic marker relationships as related groups of covariates were added. Covariates adjusted for in the first model (Model 1) included age and gender. The second multivariate model (Model 2) included the covariates from Model 1 plus physical activity, alcohol consumption, smoking status, and BMI. In the third model (Model 3), covariates from Model 2 were included plus Role overload and depression (CESD-10) scores. An alpha level of p<0.05 (2-tailed) was used to indicate statistical significance, and all statistical analyses were conducted using SPSS version 16.0 statistical package.
3.1 Results
3.1.1 Participant Characteristics
Demographic and health characteristics for the sample of elderly Alzheimer’s caregivers are presented in Table 1. Caregivers were a mean age of 74 years, primarily women (71%), Caucasian (92%), slightly overweight, and had been providing care for an average of 4.3 years. The majority of caregivers had hypertension (81%) and dyslipidemia (62%), while 19% were found to have Type 2 diabetes, and 20% had a history of cardiovascular disease. On average, caregivers slept 7 hours and 20 minutes and were asleep for 87% of the night, and spent 48 minutes napping.
Table 1.
Characteristics of a sample of elderly Alzheimer’s caregivers (N=126) in a study of the associations of sleep with type 2 diabetes, dyslipidemia, and hypertension
| Age (years), mean (SD) | 74.16 (7.98) |
| Female, n (%) | 89 (71.20) |
| Ethnicity, n (%) | |
| Caucasian | 115 (92.00) |
| Non-Caucasian | 10 (8.00) |
| Education (years), mean (SD) | 15.15 (3.05) |
| Body mass index (kg/m2), mean (SD) | 26.49 (4.71) |
| Systolic blood pressure (mmHg), mean (SD) | 134.3 (15.3) |
| Diastolic blood pressure (mmHg), mean (SD) | 75.8 (8.6) |
| Blood glucose (mg/dL), mean (SD) | 105.1 (43.7) |
| History of cardiovascular disease, n (%)a | 25 (19.84) |
| Duration of caregiving (years), mean (SD) | 4.33 (3.38) |
| Health Behaviors | |
| Ever smoker, n (%) | 58 (46.03) |
| Alcohol consumption (drinks/week), mean (SD) | 1.40 (1.46) |
| Meets CDC physical activity recommendation, n (%)b | 42 (33.30) |
| Psychological Variables | |
| Role overload score, mean (SD) | 5.18 (3.15) |
| CESD-10 score, mean(SD) | 8.78 (5.81) |
| Medication Use | |
| Current use of antidepressants, n (%) | 32 (25.40) |
| Current use of cholesterol-lowering medication, n (%) | 57 (45.20) |
| Current use of high blood pressure medication, n (%) | 76 (60.30) |
| Current use of diabetes medication, n (%) | 15 (11.90) |
| Objective Sleep Variables | |
| Nighttime sleep duration (hours), mean (SD) | 7.32 (1.12) |
| Daytime sleep duration (hours), mean (SD) | 0.79 (0.67) |
| Percent (%) sleep at night, mean (SD) | 87.31 (5.35) |
| Metabolic Markers | |
| Hypertension, n (%) | 99 (80.50) |
| Dyslipidemia, n (%) | 74 (61.70) |
| Type 2 Diabetes, n (%) | 23 (19.17) |
Includes heart attack, heart failure, angina, heart disease, and stroke or transient ischemic attack.
Engages in ≥30 minutes of moderate intensity physical activity on 5 or more days of the week.
3.1.2 Continuous Sleep Parameters and Metabolic Conditions
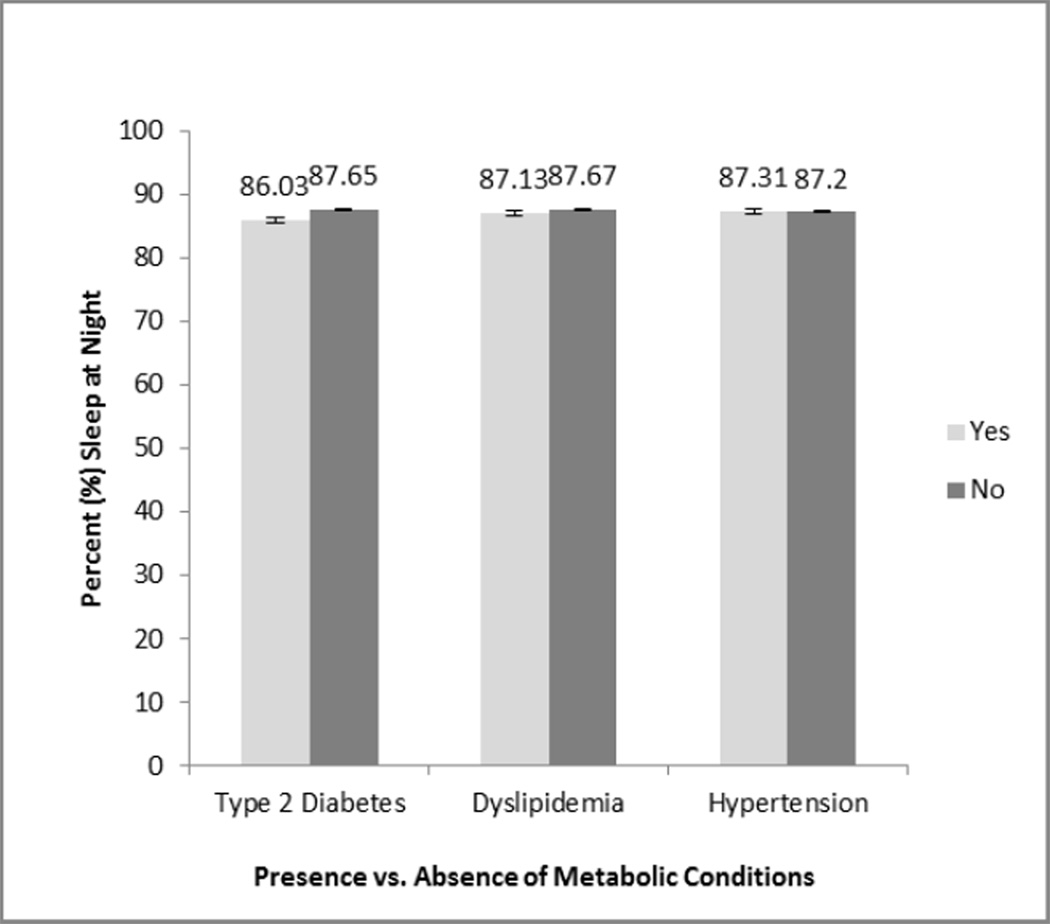
In unadjusted analyses, and compared to caregivers without diabetes, those with diabetes were significantly older, more likely to be Non-Caucasian, had higher BMI, were more likely to have a history of CVD, and had higher Role overload scores and more depressive symptoms on the CESD-10 (p<0.05 for all). Similarly, compared to caregivers without hypertension, those with hypertension were significantly older (p=0.01). Caregivers with dyslipidemia were not significantly different from those without dyslipidemia (table not shown). As shown in Figure 1–Figure 3, there were no significant differences in napping, nighttime sleep duration or nighttime sleep efficiency between those with or without diabetes, hypertension or dyslipidemia.
Figure 1.
Daytime sleep duration does not significantly differ by type 2 diabetes, dyslipidemia, or hypertension status in a sample of elderly Alzheimer's caregivers (N=126), unadjusted.
Figure 3.
Nighttime sleep efficiency does not significantly differ by type 2 diabetes, dyslipidemia, or hypertension status in a sample of elderly Alzheimer's caregivers (N=126), unadjusted.
Multiple adjusted logistic regression analyses were performed to assess the impact of each sleep parameter on the likelihood that caregivers would have diabetes, hypertension or dyslipidemia. Associations between sleep parameters and each of diabetes, hypertension and dyslipidemia were not statistically significant at the 5% level in any of the models (Table 2). Of note, all confidence intervals included “1”, yielding results consistent with the null hypothesis of no significant associations between sleep and diabetes, hypertension or dyslipidemia among our Alzheimer’s caregivers.
Table 2.
Odds ratios of type 2 diabetes, dyslipidemia, and hypertension by sleep parameters in a sample of elderly Alzheimer’s caregivers (N=126), adjusted for covariates
| Diabetes | Dyslipidemia | Hypertension | ||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Number with Condition (%) | 23 (19.2) | 74 (61.7) | 99 (80.5) | |
| Nighttime sleep duration (hours) | Model 1 | 0.96 (0.61, 1.49) | 0.85 (0.59, 1.22) | 0.96 (0.62, 1.49) |
| Model 2 | 0.92 (0.57, 1.49) | 0.85 (0.59, 1.23) | 0.96 (0.61, 1.52) | |
| Model 3 | 0.92 (0.56, 1.53) | 0.83 (0.57, 1.20) | 0.97 (0.62, 1.52) | |
| Daytime sleep duration (hours) | Model 1 | 1.80 (0.89, 3.64) | 0.75 (0.40, 1.39) | 1.19 (0.51,2.81) |
| Model 2 | 1.61 (0.75, 3.49) | 0.69 (0.36, 1.32) | 1.04 (0.42, 2.57) | |
| Model 3 | 1.75 (0.74,4.11) | 0.64 (0.33, 1.24) | 1.10 (0.44,2.74) | |
| Percent sleep at night (%) | Model 1 | 0.94 (0.86, 1.02) | 0.99 (0.92, 1.06) | 1.01 (0.93, 1.11) |
| Model 2 | 0.94 (0.85, 1.04) | 0.99 (0.92, 1.06) | 1.02 (0.93, 1.12) | |
| Model 3 | 0.93 (0.83, 1.03) | 0.99 (0.92, 1.07) | 1.02 (0.93, 1.11) | |
Model 1 – Adjusted for age and gender.
Model 2 – Adjusted for variables in Model 1 + physical activity, alcohol, smoking, and BMI.
Model 3 – Adjusted for variables in Model 2 + Role Overload and depression (CESD-10) scores.
As presented in Table 2, when adjusting for covariates in Model 3, for every additional hour of sleep obtained during the day, participants were 1.75 times more likely to have diabetes. For each 1% increase in time spent asleep during the night, caregivers were 7% less likely to have diabetes. For each additional hour of sleep obtained during the night, caregivers were 8% less likely to have diabetes. With regard to dyslipidemia, for every additional hour of sleep obtained during the day, caregivers were 36% less likely to have dyslipidemia. For every additional hour of sleep obtained at night, caregivers were 17% less likely to have dyslipidemia. For every 1% increase in time spent asleep at night, the likelihood of having ‘dyslipidemia decreased by 1%. With regard to hypertension, for every additional hour of sleep obtained at night, caregivers were 3% less likely to have hypertension. For every additional hour of sleep obtained during the day, caregivers were 10% more likely to have hypertension. Lastly, for every 1% increase in time spent asleep at night, the likelihood of having hypertension increased by 2%. None of these associations were statistically significant.
3.1.3 Categorical and Combined Sleep Parameters and Metabolic Conditions
Adjusting for age and gender in sleep-tertile analyses (table not shown), there were no clear trends in prevalence of diabetes, dyslipidemia, or hypertension across nighttime sleep duration tertiles (Ptrend=0.95, Ptrend=0.40, Ptrend=0.57, respectively), daytime sleep duration tertiles (Ptrend=0.27, Ptrend=0.12, Ptrend=0.40, respectively), or percent sleep at night tertiles (Ptrend=0.23, Ptrend=0.29, Ptrend=0.78, respectively). Additionally, prevalence of diabetes, dyslipidemia, and hypertension did not significantly differ by nighttime sleep duration tertile, daytime sleep duration tertile, or percent sleep at night tertile (p>0.05 for all).
Associations between the combined sleep variables (nighttime sleep duration with daytime sleep duration; nighttime sleep duration with percent sleep at night; and daytime sleep duration with percent sleep at night) and the three metabolic markers/conditions were examined using multinomial logistic regression. As shown in Table 3–Table 5, presence of diabete, dyslipidemia, and hypertension did not significantly differ by any of the combined sleep groups in any of the 3 adjusted models (all p>0.05).
Table 3.
Odds ratios of type 2 diabetes, dyslipidemia, and hypertension by combined sleep groups (nighttime sleep duration with percent sleep at night) in a sample of elderly Alzheimer’s caregivers (N=126), adjusted for covariates
| <7 or >8 hours & <85% sleep at night |
<7 or >8 hours & ≥85% sleep at night |
7–8 hours & <85% sleep at night |
7–8 hours & ≥85% sleep at night |
||
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | Reference | ||
| Number (%) | 18 (14.3) | 55 (43.7) | 12 (9.5) | 33 (26.2) | |
| Diabetes | Model 1 | 1.76 (0.36, 8.58) | 1.25 (0.38,4.15) | 1.06 (0.17,6.77) | -- |
| Model 2 | 1.47 (0.25, 8.54) | 1.11 (0.30,4.16) | 0.70 (0.10,5.05) | -- | |
| Model 3 | 1.07 (0.17,6.63) | 0.92 (0.23, 3.63) | 0.66 (0.09,5.17) | -- | |
| Dyslipidemia | Model 1 | 1.46 (0.37, 5.78) | 0.76 (0.30, 1.90) | 0.46 (0.11, 1.86) | -- |
| Model 2 | 1.54 (0.37,6.35) | 0.78 (0.30, 2.01) | 0.46 (0.11, 1.89) | -- | |
| Model 3 | 1.31 (0.30,5.66) | 0.70 (0.27, 1.84) | 0.46 (0.11,2.02) | -- | |
| Hypertension | Model 1 | 3.24 (0.56, 18.83) | 2.11 (0.66,6.69) | 0.33 (0.07, 1.50) | -- |
| Model 2 | 2.91 (0.48, 17.53) | 2.02 (0.60, 6.80) | 0.22 (0.04, 1.19) | -- | |
| Model 3 | 3.10 (0.51, 18.94) | 2.12 (0.62,7.21) | 0.26 (0.05, 1.37) | -- | |
Model 1 - Adjusted for age and gender.
Model 2 - Adjusted for variables in Model 1 + physical activity, alcohol, smoking, and BMI.
Model 3 - Adjusted for variables in Model 2 + Role Overload and depression (CESD-10) scores.
Table 5.
Odds ratios of type 2 diabetes, dyslipidemia, and hypertension by combined nighttime and daytime sleep duration groups in a sample of elderly Alzheimer’s caregivers (N=126), adjusted for covariates
| <7 or >8 hours at night & ≥30 minutes of daytime sleep |
<7 or >8 hours at night & <30 minutes of daytime sleep |
7–8 hours at night & ≥30 minutes of daytime sleep |
7–8 hours at night & <30 minutes of daytime sleep |
||
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | Reference | ||
| Number (%) | 48 (38.1) | 25 (19.8) | 23 (18.3) | 22 (17.5) | |
| Diabetes | Model 1 | 2.13 (0.41, 11.12) | 1.57 (0.24, 10.07) | 1.75 (0.28, 10.99) | -- |
| Model 2 | 1.85 (0.31, 11.09) | 1.27 (0.17,9.32) | 1.31 (0.17,9.90) | -- | |
| Model 3 | 2.55 (0.38, 17.33) | 1.28 (0.16, 10.47) | 2.84 (0.30, 26.50) | -- | |
| Dyslipidemia | Model 1 | 0.60 (0.20, 1.86) | 0.91 (0.25, 3.34) | 0.46 (0.13, 1.68) | -- |
| Model 2 | 0.48 (0.15, 1.60) | 0.76 (0.20, 2.95) | 0.34 (0.09, 1.38) | -- | |
| Model 3 | 0.49 (0.14, 1.71) | 0.72 (0.18,2.94) | 0.46 (0.11, 1.98) | -- | |
| Hypertension | Model 1 | 2.42 (0.24, 7.92) | 1.22 (0.31,6.22) | 2.12 (0.48,9.32) | -- |
| Model 2 | 2.43 (0.70, 8.44) | 1.31 (0.27,6.33) | 2.23 (0.45, 11.00) | -- | |
| Model 3 | 2.40 (0.67, 8.62) | 1.31 (0.26,7.51) | 2.40 (0.44, 13.11) | -- | |
Model 1 – Adjusted for age and gender.
Model 2 – Adjusted for variables in Model 1 + physical activity, alcohol, smoking, and BMI.
Model 3 – Adjusted for variables in Model 2 + Role Overload and depression (CESD-10) scores.
4.1 Discussion
In this sample of community-dwelling elderly Alzheimer’s caregivers, we did not find significant associations of nighttime sleep duration, sleep efficiency at night, or daytime naps with prevalence of diabetes, dyslipidemia, or hypertension. Our secondary analyses with categorical and combined sleep parameters also failed to show significant associations. This may be due to the fact that the caregivers in our sample had less disturbed sleep than has been observed in previous studies, were not considered sleep deprived, and variance in the sleep variables was limited (Von Känel et al., 2006). For instance, our caregivers slept 7 hours and 20 minutes at night and were asleep for 87% of the time, while caregivers in a study by Dhruva et al. slept 6 hours and 45 minutes at night and were asleep 83% of the time based on actigraphy data (Dhruva et al., 2012). Access to additional resources (e.g. home help) could be one explanation for why caregivers in our sample had adequate sleep, as their average annual income was $dollar;71,020. The fact that the sleep of the caregivers in our sample was within normal limits may explain the lack of significant associations between the sleep and metabolic measures in our study.
Our null findings are inconsistent with most published studies reporting associations between nighttime sleep and diabetes (Cappuccio, D’Elia, Strazzullo, & Miller, 2010; Chao et al., 2011; Xu et al., 2010). For example, a meta-analysis of 10 prospective studies indicated that reported short nighttime sleep (≤5–6 hours), long nighttime sleep (>8–9 hours) and difficulty maintaining sleep (assessed by questionnaire) significantly predicted (OR: 1.28, 95%CI: 1.03–1.06; OR: 1.48, 95%CI: 1.13–1.96; and OR: 1.84, 95%CI: 1.39–2.43, respectively) incident diabetes (Cappuccio et al., 2010). In an attempt to compare our data with results from this metaanalysis, we ran exploratory analyses, which indicated that the prevalence of diabetes among caregivers who slept 6–8 hours at night did not significantly differ (OR: 0.83, 95%CI: 0.32–2.15) from the prevalence among caregivers who slept <6 or >8 hours at night. A different cross-sectional study on 70 participants with a mean age of 60 years that used wrist actigraphy for 3 consecutive days reported that reduced sleep efficiency, defined as percent sleep at night, was associated with the presence of diabetes (Trento et al., 2008). Alternatively, literature regarding daytime sleep in older adults is conflicting, as several studies have found that napping is associated with an increased risk of diabetes, while others reported that napping decreases risk of CVD (Bursztyn & Stessman, 2005; Campbell, Murphy, & Stauble, 2005; Campos & Siles, 2000; Naska, Oikonomou, Trichopoulou, Psaltopoulou, & Trichopoulos, 2007; Xu et al., 2010). For example, a cross-sectional study on community-dwelling adults over age 50 years found significant dose-response relationships of napping frequency and duration with increased prevalence of type 2 diabetes (K.-B. H. Lam et al., 2010).
Data are conflicting from the limited number of studies that investigated relationships between sleep and dyslipidemia in older adults (Chaput et al., 2007; Ekstedt et al., 2004; van den Berg et al., 2008). Our findings of no significant associations between the sleep parameters and dyslipidemia are consistent with those from a cross-sectional study on women over the age of 50 years that found that reported sleep duration was not significantly associated with plasma lipid-lipoprotein concentrations; the authors concluded that the relationship between sleep and serum lipid-lipoprotein concentrations becomes less evident with age (Chaput et al., 2007). Our findings are inconsistent with studies suggesting that short nighttime sleep is associated with dyslipidemia. For example, a study by Kaneita et al. found that men who reported sleeping 6–7 hours at night had higher LDL cholesterol levels compared with those who slept ≥8 hours (Kaneita, Uchiyama, Yoshiike, & Ohida, 2008).
None of the sleep parameters were significantly associated with hypertension in our sample of caregivers. These finding are supported by three known studies that reported no significant associations of sleep with blood pressure, and/or risk of hypertension among elderly adults, perhaps supporting the hypothesis that this relationship decreases with age (Kim & Jo, 2010; Knutson, 2010; Lima-Costa et al., 2008; van den Berg et al., 2007). For example, data from a cross-sectional study on participants over the age of 58 years indicated that sleep duration measured by actigraphy was not associated with hypertension (Van den Berg et al., 2007).
It is important to note our study used three days/nights of actigraphy to objectively measure sleep, while previous studies that reported significant correlations between sleep and metabolic conditions often based their sleep measurements on one night of polysomnography or self-report (Buxton & Marcelli, 2010; Gottlieb et al., 2006; Kaneita et al., 2008; K.-B. H. Lam et al., 2010; Rod et al., 2011). One night of polysomnography may not be representative of habitual sleep, and self-reported sleep measures are less reliable than objective measurements (Gangwisch et al., 2006; Lauderdale, Knutson, Yan, Liu, & Rathouz, 2008; Patel et al., 2008; Vgontzas et al., 2009). Therefore, additional trials with non-invasive, objectively measured sleep are needed to help elucidate relationships between sleep and metabolic conditions in older adults.
This sample of caregivers had relatively undisturbed sleep, they were not considered sleep deprived, and there was limited variance in the sleep variables (Von Känel et al., 2006). Our results were consistent with the null hypothesis; however, non-rejection of the null is not necessarily confirmation of it. In particular, the small sample size may have limited the power of the study. For example, given the wide confidence interval for the association between napping and diabetes in model 3 (95%CI: 0.74–4.11), we may have been underpowered to detect an odds ratio as high as 4. Alternatively, the tight confidence interval for the association between percent sleep at night and dyslipidemia in model 3 (95%CI: 0.92–1.07) is consistent with the null hypothesis. Therefore, there may have been inadequate power to detect significant associations between napping and type 2 diabetes, as only 23 caregivers had diabetes, but adequate power to detect associations between percent sleep at night and dyslipidemia, as 74 caregivers had dyslipidemia. Nevertheless, the majority of the associations between the sleep parameters and metabolic conditions were in the hypothesized direction, and with a larger sample size some of the associations that were close to significance may in fact be significant. Lastly, since data on snoring and obstructive sleep apnea were unavailable, we were not able to adjust for this syndrome or distinguish between sleep loss due to spousal or environmental disturbances, or other pathological conditions that might disrupt sleep.
4.2 Conclusions
Limited physical activity, poor diet, smoking, and excessive alcohol consumption increase risk of type 2 diabetes, dyslipidemia, and hypertension; but insufficient sleep, a modifiable health behavior, could be an additional risk factor (Ayas et al., 2003; Vgontzas et al., 2009; von Känel et al., 2010; Xu et al., 2010). A more complete theoretical model of the etiology of type 2 diabetes, dyslipidemia, and hypertension in older adults that includes sleep ought to be considered in future studies. If the hypothesized relationships are found to exist, this model could offer valid, directionally-oriented physiologic mechanisms that link sleep to these metabolic conditions. Understanding these mechanisms that connect insufficient nighttime sleep and daytime naps to metabolic regulation has the potential to aid practitioners in the prevention and management of these prevalent and escalating chronic diseases.
Figure 2.
Nighttime sleep duration does not significantly differ by type 2 diabetes, dyslipidemia, or hypertension status in a sample of elderly Alzheimer's caregivers (N=126), unadjusted.
Table 4.
Odds ratios of type 2 diabetes, dyslipidemia, and hypertension by combined sleep groups (daytime sleep duration with percent sleep at night) in a sample of elderly Alzheimer’s caregivers (N=126), adjusted for covariates
| ≥30 minutes of daytime & <85% sleep at night |
≥30 minutes of daytime & ≥85% sleep at night |
<30 minutes of daytime & <85% sleep at night |
<30 minutes of daytime & ≥85% sleep at night |
||
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | Reference | ||
| Number (%) | 15 (12%) | 56 (44%) | 15 (12%) | 32 (25%) | |
| Diabetes | Model 1 | 2.19 (0.48,9.94) | 1.09 (0.33, 3.58) | 0.42 (0.04, 4.27) | -- |
| Model 2 | 1.72 (0.32,9.13) | 0.88 (0.24, 3.27) | 0.25 (0.02, 3.05) | -- | |
| Model 3 | 2.56 (0.43, 15.32) | 1.35 (0.33,5.58) | 0.22 (0.02, 2.94) | -- | |
| Dyslipidemia | Model 1 | 0.55 (0.14,2.13) | 0.60 (0.23, 1.55) | 1.06 (0.26, 4.40) | -- |
| Model 2 | 0.48 (0.12, 1.97) | 0.55 (0.21, 1.46) | 1.26 (0.27, 5.80) | -- | |
| Model 3 | 0.53 (0.12,2.31) | 0.63 (0.23, 1.74) | 1.25 (0.26, 6.02) | -- | |
| Hypertension | Model 1 | 0.71 (0.11,4.57) | 0.58 (0.17, 1.96) | 0.41 (0.09, 1.85) | -- |
| Model 2 | 0.55 (0.08, 3.80) | 0.52 (0.15, 1.81) | 0.31 (0.06, 1.53) | -- | |
| Model 3 | 0.58 (0.08,4.16) | 0.54 (0.14,2.03) | 0.31 (0.06, 1.60) | -- | |
Model 1 – Adjusted for age and gender.
Model 2 – Adjusted for variables in Model 1 + physical activity, alcohol, smoking, and BMI.
Model 3 – Adjusted for variables in Model 2 + Role Overload and depression (CESD-10) scores.
Acknowledgments
Funding
This research was supported by the National Institute on Ageing [AG15301 to I.G., AG08415 to S.A-I.]; and the National Institute of General Medical Sciences [T32GM084896 to M.H.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the authors report a conflict of interest.
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American journal of preventive medicine. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes care. 2003;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Redline S, Ancoli-lsrael S, Schneider JL, Surovec S, Johnson NL, Stone KL. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31(2):283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursztyn M, Stessman J. The siesta and mortality: twelve years of prospective observations in 70-year-olds. Sleep. 2005;28(3):345–347. [PubMed] [Google Scholar]
- Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Social science & medicine (1982) 2010;71(5):1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. Journal of the American Geriatrics Society. 2005;53(1):48–53. doi: 10.1111/j.1532-5415.2005.53009.x. [DOI] [PubMed] [Google Scholar]
- Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population-based, case-control study in Costa Rica. International journal of epidemiology. 2000;29(3):429–437. [PubMed] [Google Scholar]
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C-Y, Wu J-S, Yang Y-C, Shih C-C, Wang R-H, Lu F-H, Chang C-J. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism: clinical and experimental. 2011;60(6):799–804. doi: 10.1016/j.metabol.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Chaput J-P, Després J-P, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep medicine. 2009;10(8):919–924. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Chaput J-P, Lord C, Aubertin-Leheudre M, Dionne IJ, Khalil A, Tremblay A. Is overweight/obesity associated with short sleep duration in older women? Aging clinical and experimental research. 2007;19(4):290–294. doi: 10.1007/BF03324704. [DOI] [PubMed] [Google Scholar]
- Dhruva A, Lee K, Paul SM, West C, Dunn L, Dodd M, Miaskowski C. Sleep-wake circadian activity rhythms and fatigue in family caregivers of oncology patients. Cancer nursing. 2012;35(1):70–81. doi: 10.1097/NCC.0b013e3182194a25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Ekstedt M, Akerstedt T, Söderström M. Microarousals during sleep are associated with increased levels of lipids, Cortisol, and blood pressure. Psychosomatic medicine. 2004;66(6):925–931. doi: 10.1097/01.psy.0000145821.25453.f7. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep medicine reviews. 2010;14(4):239–247. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON M. The assessment of anxiety states by rating. The British journal of medical psychology. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27(4):661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- Kaneita Y, Uchiyama M, Yoshiike N, Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31(5):645–652. doi: 10.1093/sleep/31.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35(5):1173–1175. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- Kim J, Jo I. Age-dependent association between sleep duration and hypertension in the adult Korean population. American journal of hypertension. 2010;23(12):1286–1291. doi: 10.1038/ajh.2010.166. [DOI] [PubMed] [Google Scholar]
- Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best practice & research. Clinical endocrinology & metabolism. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring SII, Brummett BH, Barefoot J, Garrett ME, Ashley-Koch AE, Boyle SH, Williams RB. Impact of psychological stress on the associations between apolipoprotein E variants and metabolic traits: findings in an American sample of caregivers and controls. Psychosomatic medicine. 2010;72(5):427–433. doi: 10.1097/PSY.0b013e3181de30ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JCM, Ip MSM. Sleep & the metabolic syndrome. The Indian journal of medical research. 2010;131:206–216. [PubMed] [Google Scholar]
- Lam K-BH, Jiang CQ, Thomas GN, Arora T, Zhang WS, Taheri S, Cheng KK. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep. 2010;33(3):402–407. doi: 10.1093/sleep/33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology (Cambridge, Mass.) 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of Cortisol levels the next evening. Sleep. 1997;20(10):865–870. [PubMed] [Google Scholar]
- Lima-Costa MF, Peixoto SV, Rocha FL. Usual sleep duration is not associated with hypertension in Brazilian elderly: The Bambui Health Aging Study (BHAS) Sleep medicine. 2008;9(7):806–807. doi: 10.1016/j.sleep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep medicine reviews. 2007;11(2):143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin CL, Ancoli-lsrael S, Dimsdale J, Archuleta C, Von Kanel R, Mills P, Grant I. Sleep in spousal caregivers of people with Alzheimer's disease. Sleep. 2005;28(10):1245–1250. doi: 10.1093/sleep/28.10.1245. [DOI] [PubMed] [Google Scholar]
- Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Archives of internal medicine. 2007;167(3):296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. Journal of the American Geriatrics Society. 2000;48(2):115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- Patel SR, Blackwell T, Redline S, Ancoli-lsrael S, Cauley JA, Hillier TA, Stone KL. The association between sleep duration and obesity in older adults. International journal of obesity (2005) 2008;32(12):1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. The Gerontologist. 1990;30(5):583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Rod NH, Vahtera J, Westerlund H, Kivimaki M, Zins M, Goldberg M, Lange T. Sleep disturbances and cause-specific mortality: Results from the GAZEL cohort study. American journal of epidemiology. 2011;173(3):300–309. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MA, McCrae CS, Campbell JM, Benito AP, Cheng J. Sleep pattern differences between older adult dementia caregivers and older adult noncaregivers using objective and subjective measures. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2008;4(4):362–369. [PMC free article] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34(3):371–377. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA: the journal of the American Medical Association. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel Karine, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of internal medicine. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Stone KL, Ewing SK, Ancoli-lsrael S, Ensrud KE, Redline S, Bauer DC, Cummings SR. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. Journal of the American Geriatrics Society. 2009;57(4):604–611. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, Trevisan M. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. Journal of hypertension. 2010;28(5):896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. Journal of sleep research. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27(6):1318–1324. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, Porta M. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta diabetologica. 2008;45(4):225–229. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- Van den Berg JF, Miedema HME, Tulen JHM, Neven AK, Hofman A, Witteman JCM, Tiemeier H. Long sleep duration is associated with serum cholesterol in the elderly: the Rotterdam Study. Psychosomatic medicine. 2008;70(9):1005–1011. doi: 10.1097/PSY.0b013e318186e656. [DOI] [PubMed] [Google Scholar]
- Van den Berg JF, Tulen JHM, Neven AK, Hofman A, Miedema HME, Witteman JCM, Tiemeier H. Sleep duration and hypertension are not associated in the elderly. Hypertension. 2007;50(3):585–589. doi: 10.1161/HYPERTENSIONAHA.107.092585. [DOI] [PubMed] [Google Scholar]
- Vasudevan AR, Ballantyne CM. Cardiometabolic risk assessment: an approach to the prevention of cardiovascular disease and diabetes mellitus. Clinical cornerstone. 2005;7(2-3):7–16. doi: 10.1016/s1098-3597(05)80063-8. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler E0, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Känel R, Ancoli-lsrael S, Dimsdale JE, Mills PJ, Mausbach BT, Ziegler MG, Grant I. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56(1):41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Känel R, Dimsdale JE, Ancoli-lsrael S, Mills PJ, Patterson TL, McKibbin CL, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer's disease. Journal of the American Geriatrics Society. 2006;54(3):431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Von Känel R, Mausbach BT, Patterson TL, Dimsdale JE, Aschbacher K, Mills PJ, Grant I. Increased Framingham Coronary Heart Disease Risk Score in dementia caregivers relative to non-caregiving controls. Gerontology. 2008;54(3):131–137. doi: 10.1159/000113649. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, D′Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- Wolk R, Garni AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Current problems in cardiology. 2005;30(12):625–662. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes care. 2010;33(1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]