Abstract
The increasing threat of antibiotic resistance in pathogenic bacteria and the dwindling supply of antibiotics available to combat these infections poses a significant threat to human health throughout the world. Antimicrobial peptides have long been touted as the next generation of antibiotics capable of filling the anti-infective void. Unfortunately, peptide based antibiotics have yet to realize their potential as novel pharmaceuticals, in spite of the immense number of known antimicrobial peptide sequences and our improved understanding of their antibacterial mechanism of action. Recently, the immunomodulatory properties of certain antimicrobial peptides have become appreciated. The ability of small synthetic peptides to protect against infection in vivo has demonstrated that modulation of the innate immune response is an effective strategy to further develop peptides as novel anti-infectives. This review focuses on the screening methods that have been employed to assess novel peptide sequences for their antibacterial and immunomodulatory properties. It will also examine how we have progressed in our ability to identify and optimize peptides with desired biological characteristics and enhanced therapeutic potential. In addition, the current challenges to the development of peptides as anti-infectives are examined and the strategies being used to overcome these issues are discussed.
Keywords: Antimicrobial peptides, immunomodulatory peptides, host defense peptides
Introduction
With the increasing emergence of antibiotic resistant pathogens1 and the dwindling supply of antibiotics capable of combating these infections, coupled with a reluctance from pharmaceutical companies to invest in infectious disease research2, the need for novel antibiotics has never been more urgent. Since their discovery in the 1980s, antimicrobial peptides (AMPs) have been lauded for their potential as novel antibiotics3,4. Their broad spectrum antimicrobial activity and selectivity for bacterial over eukaryotic cells make them attractive candidates for novel drugs compounds. Indeed, attempts have been made to harness this potential and a handful of peptides have been developed as novel pharmaceuticals and evaluated in clinical trials5. Countless more novel AMP sequences, with broad spectrum antibacterial activity, are reported in the literature on an almost daily basis. While antibiotics derived from naturally occurring AMPs have yet to supplant the most effective antibiotics on the market, significant advances have been made in the field of AMP research both in the identification of novel peptide sequences and in assessing their potential as anti-infectives.
More recently, the immunomodulatory properties of AMPs have also become appreciated and many of these peptides are now known to stimulate the immune system while suppressing the inflammatory response. Consequently, the term host defense peptide (HDP) is a better descriptor for these molecules as it encompasses both the direct antibacterial activity as well as their capacity for immunomodulation. As our understanding of the complex mechanisms underlying these immunomodulatory peptides has improved, it has become apparent that this represents a promising new route for expanding the therapeutic potential of HDPs6. This review will focus primarily on the strategies that have been developed or accessed in our research program to identify novel AMP sequences and how we have progressed in our understanding of the immunomodulatory properties of HDPs leading to the design of synthetic innate defence regulator peptides (IDRs) with desirable anti-infective and anti-inflammatory properties. For more comprehensive descriptions of the field of novel peptide design we refer the readers to overview references5–7. We will also address the current state of developing HDPs as antibiotics and immune modulators and discuss the challenges and strategies that are being used to optimize peptides for clinical use.
Antimicrobial Peptides
AMPs are ubiquitous throughout nature and the significant role they play in preventing and combating infectious pathogens is well established7. They play a major role in the immune defence mechanisms of insects and plants and are an important component of the innate immune response of animals, including crustaceans, mammals and humans. Traditionally, AMPs have been isolated from natural sources and their antimicrobial potency has been established in vitro. For instance, one of the first reported AMPs was magainin 2 isolated from the skin secretions of Xenopus laevis frogs, and the purified peptide had broad spectrum antimicrobial activity against an array of bacterial species8. Reports of novel AMP sequences isolated from natural sources are still commonplace in the literature. In fact, there are now over 2100 AMPs derived from natural sources listed in the Antimicrobial Peptide database9,10 and this number continues to grow.
AMPs are characterized as short peptide sequences typically between 12 and 50 residues in length11. There are exceptions to this as antimicrobial activity has been observed for synthetic peptides as short as 6 residues12 and some larger cationic proteins have direct antibacterial and immunomodulatory properties such as lysozyme13 and lactoferrin14. AMPs are typically rich in hydrophobic residues, including Leu, Ile, Val, Phe and Trp, and they usually have an excess of cationic amino acids which confers a net positive charge, on the order of +2 to +97. These properties allow AMPs, in the presence of phospholipid membranes, to adopt diverse amphipathic structures that can be separated into four broad structural classes: α-helical, β-sheet, extended conformation and looped peptides containing disulphide bridges7. The amphipathic nature of these structures is an important component of their mechanism of action against bacteria since amino acid changes that perturb amphipathicity reduce antimicrobial activity. Some AMPs have strong lytic effects on bacterial membranes resulting in direct killing of bacterial cells. Others interact with the cytoplasmic membrane to inhibit events dependent on this membrane including cell wall biosynthesis, energy generation and cell division. Alternatively, some peptides traverse the phospholipid bilayer and enter the bacterial cell where they ultimately interfere with intracellular processes by binding to DNA, RNA and certain proteins. A number of models have been described to explain the various mechanisms of action for AMPs and these have been discussed in detail in recent reviews5,15–17.
The classical approach to studying AMPs involves identifying and purifying the peptides from a natural source and then measuring the antibacterial potency of a highly pure sample in vitro. Some peptides are still identified in such a manner, such as three cysteine-rich peptides recently isolated from dandelions18. Unfortunately, purifying large quantities from a natural source is often impossible, and therefore synthetic peptides are made using solid phase peptide synthesis methods19, and are obtained at a high level of purity. Synthetic peptides can also be mutated at specific residues to examine the effect that this has on antimicrobial activity. If the peptide loses activity, then that residue is in some way necessary for the AMPs biological function. On the other hand, if the antibacterial potency improves, then this provides important information regarding the structural determinant of activity and elevates the potential of this peptide as a novel anti-infective. This iterative process has been successfully applied to a number of peptides including: indolicidin20, polyphemusin21 and bactenecin22.
Another strategy involves examining the antimicrobial activity of truncated versions of a larger peptide to see if activity is retained. An early study examined N-terminal truncations of magainin 2 and found that the first three residues of the native peptide could be removed without dramatically decreasing the activity while removing the Lys residue at position 4 dramatically decreased the potency of the amphibian peptide23. Such a strategy can be used to isolate the residues necessary for the bactericidal effect while reducing the costs required to synthesize longer peptides. For instance, a six residue fragment of bovine lactoferricin was shown to have equivalent antibacterial activity compared to the full length 25 residue lactoferricin B peptide24 and critically this hexamer is considerably less expensive to produce synthetically (although in our hands25 it is difficult to make peptides smaller than 8 amino acids with significant antimicrobial activity as measured by a modified CLSI method26). This strategy can also be employed to remove regions of peptides that have undesirable characteristics. For example, BMAP-18, a truncated version of the potent bovine myeloid antimicrobial peptide (BMAP-27), was shown to have antiparasitic activity against trypanosomatid parasites, but was significantly less toxic compared to the longer peptide27.
These iterative approaches to evaluating and improving antimicrobial activity, while ultimately successful, are time consuming and large amounts of synthetic peptides are required to evaluate large numbers of derivatives with the observed increases in antimicrobial activity being oftentimes modest, at best. In fact, it is highly likely that many peptide sequences have been examined in this way and are underreported in the literature because the resulting peptides displayed reduced antibacterial potency. As a result, many researchers have developed methods to identify promising AMP sequences while decreasing the number of peptides that need to be produced synthetically to evaluate their in vitro activity.
Early efforts to screen large numbers of peptide sequences involved using combinatorial libraries of short peptides12. This method succeeded at identifying short peptide sequences with significant antimicrobial activity. Unfortunately, this method is not amenable to examining longer peptide sequences because the number of permutations and combinations of peptide sequences increases exponentially with the length of the polypeptide chain. With the increasing number of sequenced genomes available, other groups have used genomic approaches to search for novel peptide sequences in the DNA sequences of various organisms. Recent successes describe the identification of novel cathelicidin-like AMPs in pandas28 as well as monotremes and marsupials29. However, such a methodology is limited to scanning for molecules with homology or at least analogy to known AMP sequences and these peptides usually need to be optimized to enhance their therapeutic potential. More high-throughput approaches to examine large numbers of peptide sequences involve phage display30 and ribosome display31 with subsequent enrichment for peptides that bind to bacterial membranes. In these cases, researchers are limited by the choice of the immobilized interacting partner and the inherent technical complexity associated with these peptide display technologies. The current bottleneck in developing AMPs as promising pharmaceuticals lies in our ability to screen novel peptide sequences in a high-throughput fashion while synthesizing sufficient quantities of these peptides to evaluate their antimicrobial activity.
Recently, our lab described a method of peptide screening25 whereby peptides are synthesized on cellulose sheets and then their activity is screened against a luminescent strain of Pseudomonas aeruginosa constitutively expressing a luciferase gene cassette incorporated into the bacterial chromosome32. This method uses SPOT-synthesis33 and standard Fmoc (Fluorenyl methoxy carbonyl) chemistry to generate a series of cellulose tethered peptides with known sequences. The peptides are then cleaved from the cellulose sheets and their ability to inhibit growth of the P.aeruginosa strain is measured as inhibition of luminescence. This method was successfully used to generate a complete substitution library of Bac2a (RLARIVVIRVAR-NH2), a linear variant of the bovine peptide, bactenecin25. The single amino acid substitutions of Bac2a that resulted in increased activity were then combined to generate optimized 12-mer and 8-mer sequences with potent and broad spectrum antibacterial activities25. Peptide synthesis on cellulose sheets was also used to examine the sequence requirements of Bac2a34. In this case, 49 Bac2a derivatives were generated with scrambled amino acid sequences to examine if sequence specificity was required for the antimicrobial activity. Based on the luminescent P.aeruginosa killing assay, the peptides fell into 6 different activity classes varying from significantly more active than Bac2a, of equivalent activity to Bac2a or weak to no killing activity at the highest peptide concentration tested. This result supports the idea that AMPs lack a sequence-specific interaction to exert their bactericidal effect and represents a promising approach that can be used to generate novel candidate peptides. One of the most active scrambled peptides, Bac034, was further optimized through a complete substitution analysis and then combining the most active mutations to arrive at peptides with substantially better MIC values compared to Bac2a34, exactly as had been done previously for Bac2a itself25. It should be noted that any active AMP sequences identified using this technique still need to be synthesized in larger quantities to confirm the increased antimicrobial activity against other bacterial species as well as elucidate the mechanism of action and activity in animal infection models. However, the SPOT-synthesis technique of generating multiple peptide sequences on cellulose sheets is a relatively simple and inexpensive way to screen and identify large numbers of novel AMP sequences with potential pharmacological applications.
More recently, computer aided design of AMPs has been used to predict the antimicrobial activity of novel peptide sequences prior to synthesis. These methods rely on the chemo-informatic method of quantitative structure-activity relationship (QSAR) modelling to relate the measured antimicrobial activity to the structural characteristics associated with the equivalent peptide sequences, as defined through the use of dozens of physico-chemical “descriptors” (including inductive parameters such as contact energy between neighbouring amino acids that assess how the properties of amino acids change along the length of the peptide)35. Using a test set of peptides derived from Bac2a peptide, novel peptides with significant activity against P.aeruginosa were used to predict structure-activity relationships and test the validity of QSAR descriptors36,37. These developed QSAR descriptors were then used, along with pattern recognizing artificial neural networks, to predict the antimicrobial activity of a virtual library of 100,000 9 amino acid peptides38. A total of 200 peptides from this virtual screen were synthesized to validate the models generated by the QSAR descriptors. This approach proved remarkably accurate, since 94% of the peptides predicted to be better than Bac2a were actually found to be more active, while all of the peptides predicted to be worse than the linear bactenecin derivative had lower MIC values38. This strategy successfully identified optimized peptide sequences with antimicrobial activity more than ten-fold better than a peptide that showed efficacy in Phase III clinical trials and comparable to or better than conventional antibiotics against a broad spectrum of multidrug resistant “Superbugs”; the peptides were also active systemically in mouse infection models38. The use of such in silico methods has the potential to dramatically increase the number of candidate peptides with antimicrobial activity and is capable of predicting which peptides will be active in vitro. These methods save time and resources, by lowering the number of peptides that need to be synthesized (e.g. 100,000 peptides would cost $400,000 to synthesize on peptide arrays and at least $1.2 million for conventional synthesis) as well as decreasing the number of time-consuming MIC measurements that need to be performed.
Immunomodulatory Peptides
HDPs are important components of the immune response as evidenced by the fact that animals defective in production of the mouse cathelicidin CRAMP are more susceptible to infections39,40. However, researchers have critically re-examined the role that these biomolecules play in host defense against bacterial infections. Often, the reported MIC values for a given peptide are measured in minimal media or phosphate buffer but it is known that the antimicrobial activities of peptides are highly sensitive to salt concentrations and the presence of divalent cations, serum components and polyanionic glysoaminoglycans41. For example, the human cathelicidin peptide LL-37 has MICs against E.coli in the low μg/ml range under conditions of low ionic strength but these MIC values go up with increasing NaCl concentration42 and the antibacterial activity of LL-37 is virtually abolished when tested in tissue culture media41. Interestingly, under the latter more-physiological conditions, LL-37 exhibits a wide range of immunomodulatory properties in vitro and these activities can be recapitulated in animal models. For example, LL-37 is known to suppress pro-inflammatory cytokines in response to bacterial lipopolysaccharides and lipoteichoic acids43, prevents activation of macrophages by these bacterial components44, upregulates the production of chemokines and chemokine receptors44, promotes angiogenesis45 and wound healing46. These immunomodulatory properties are not limited to LL-37 since other peptides, such as mammalian defensins47,48, other cathelicidins49, and synthetic derivatives have immunomodulatory properties (For recent reviews, see 6,17,50).
One of the greatest obstacles in the development of immunomodulatory peptides as therapeutics is identifying how the peptides interact with and stimulate the cells of the immune system. There is evidence that immunomodulatory peptides target multiple receptors and processes within cells, depending on both the cell type and the amino acid sequence of the peptide. For instance, LL-37 indirectly stimulates the P2X(7) receptor in human embryonic kidney cells51 and transactivates epidermal growth factor receptor in epithelial cells52, interacts with formyl peptide receptor-like 1 in many cell types53 and enhances TLR3 signalling in response to viral dsRNA54. Most HDPs share characteristics with cell penetrating peptides and they can translocate into eukaryotic cells, which appears to be necessary for many of their activities. For example, biotinylated LL-37 is actively internalized into epithelial cells through endocytosis and accumulates in the perinuclear region of the cell55. Once inside the cell, these peptides are free to bind to intracellular targets, such as LL-37 binding to GAPDH 56 or synthetic IDR-1 peptide (see below) binding to sequestosome-1/p6257 leading to signal transduction (e.g. through p38 mitogen-activated protein kinase [MAPK]) and chemokine induction.
Ultimately, HDPs affect multiple signalling pathways within a cell. A systems approach was used to examine the effect of LL-37 on the immune response of CD14+ monocytes. In total, 475 differentially expressed genes were detected by microarray analyses, and linked to the involvement of several signalling pathways in the activities of LL-3758. Some of these pathways including the MAPKs, p38, JNK, extracellular signal-regulated kinase-1/2 (ERK1/2), as well as Src-family kinases and PI3 kinases58. Evidently, the interactions between immunomodulatory peptides and cells of the immune system are complex and the response of the immune system to the stimulation of a peptide depends on the sequence of the peptide, the receptors that it interacts with, the cell type, and the other endogenous and pathogen related signals present.
Similar to the approaches used in optimizing the antimicrobial activity of peptides, iterative approaches have been used to try and understand the mechanisms of immune cell stimulation. For example, truncated versions of LL-37 were tested for their ability to induce IL-8 production in keratinocytes and the response to the peptide was different depending on whether the peptide was shortened from the N- or C- terminus59. The endotoxin neutralizing capacity of a truncated 18-mer of LL-37 was also optimized through amino acid substitution to generate a peptide that protected mice against endotoxin shock60.
Natural HDPs with inherent immunomodulatory activity have served as templates to generate synthetic IDR peptides with a remarkable ability to modulate the immune response in cell cultures and in vivo61–63. The potential of immunomodulatory peptides as novel therapeutics was first illustrated by the peptide IDR-1 (KSRIVPAIPVSLL-NH2)61. IDR-1 was generated from Bac2a by designing a sequence with two internal Pro residues that was incompatible with antimicrobial activity. It was screened for its ability to enhance chemokine induction and suppress LPS-stimulated pro-inflammatory cytokines such as TNF-α in human peripheral blood mononuclear cells and for efficacy in mouse infection models. As expected, IDR-1 displayed absolutely no direct antimicrobial activity, even in buffer, but protected mice from methicillin-resistant S.aureus, vancomycin resistant Enterococcus and Salmonella infections and influenced several signalling pathways in human monocytes leading to the production of certain immune cell-recruiting chemokines and suppression of inflammatory responses (as confirmed in the animal model studies)61. The discovery of IDR-1 and its effectiveness in preventing and treating infections provided the important discovery demonstrating that modulation of the innate immune response provides an effective strategy to combat antibiotic resistant infections as an effective complement to current therapeutic options.
Compared to the better-described structure-activity relationships concerning the direct antibacterial activity of AMPs, relatively little is known regarding the structural and sequence requirements underlying the immunomodulatory properties of HDPs. This is likely due to the multiple targets with which HDPs interact to eliciting cellular responses and the different requirements for peptide uptake into cells, making it complicated to isolate specific structural characteristics responsible for the stimulation or suppression of a specific signalling pathway; in addition the assay systems are more labour intensive making high throughput analyses difficult. Despite this, a synthetic library approach using QSAR methodology was recently undertaken to iteratively examine the effect of point substitutions, scrambling and deletion variants of Bac2a and how these affected the immune stimulating properties of the resulting peptides. Using this methodology, IDR-1002 (VQRWLIVWRIRK-NH2) was identified as a much stronger inducer of chemokines production than IDR-1 and was able to more effectively protect mice from invasive S.aureus infection63. It was found that IDR-1002 both induces chemokines63 and enhances monocyte migration towards chemokines on fibronectin64 suggesting that optimizing peptides that modulate chemokine production and immune cell migration is a promising avenue for the generation of peptides with improved in vivo protective properties. In addition, the benefits of immunomodulatory peptides may extend beyond their anti-infective activities as IDR-1002 has demonstrated potential as a treatment option to control chronic inflammation in arthritis65 and may incorporated into microparticle vaccine formulations to improve the immune response to vaccines66,67.
Another promising immunomodulatory peptide was identified from the above-described Bac2a screen. IDR-1018 (VRLIVAVRIWRR-NH2), has modest antibacterial activity but showed considerable promise as a novel immunomodulatory peptide by strongly inducing MCP-1 and MCP-3 chemokine expression and suppressing the LPS induced production of TNF-α in peripheral blood mononuclear cells62. More recently, it has been shown that IDR-1018 modulates the differentiation of human macrophages68, promotes wound healing69, protects against invasive S.aureus infections of mice and shows promise as an adjunctive treatment for malaria70 and protects against lung infections and pneumonia caused by multi-drug resistant strains, cf. IDR-100271. Structural studies were performed on IDR-1018 to better understand the structure-activity relationships responsible for its immunomodulatory properties. IDR-1018 was unstructured in phosphate buffer, adopted an α-helical conformation in DPC micelles and formed a predominantly β-turn structure in the presence of SDS micelles and anionic vesicles62. This structural plasticity, depending on the nature of the environment, indicates that the structural requirements for immunomodulatory and antibacterial activity are complex and that modest alterations in the sequence of the peptide can have dramatic impacts on the biological activity of a peptide. Intriguingly our preliminary QSAR studies have indicated that the descriptors for antimicrobial and immunomodulatory activity do not strongly overlap indicating that they are independently structurally determined (Jenssen and Hancock, unpublished). These studies provide an important first step in furthering our appreciation of the structural aspects that govern the activities of IDRs and as our understanding of these features improves, it can be applied to future QSAR studies to generate novel IDR sequences with optimized immunomodulatory characteristics.
Judging from the examples presented for AMPs and synthetic IDR peptides, it appears that we are at a point where we can reasonably identify and screen peptides for their direct antibacterial activity and immunomodulatory properties. Our understanding of the characteristics that contribute to direct antibacterial activity is quite extensive owing to years of research from many research groups that correlates the AMP sequence and structure to its potency, although the complexity of descriptors means that no simple relationship between structure and activity can be drawn. Nevertheless we can use combinations of these descriptors as inputs for QSAR modelling to potentially test hundreds of thousands of sequences in silico and predict novel peptides with excellent therapeutic potential as antibacterial agents. The structural requirements underlying immunomodulation are comparatively poorly understood, owing to the complexity of the cellular response to the presence of an immunomodulatory peptide. However, initial semi-random and iterative design studies have successfully generated synthetic IDRs with excellent in vivo activity, emphasizing that such an approach is a viable method for generating novel immunomodulatory peptides. As mentioned earlier, the immunomodulatory activities of novel synthetic IDRs are difficult to predict due to the many different responses that can occur depending on the cell type. As a result, specific tests that correlate with a desired immune response, such as increased chemokines release from peripheral blood mononuclear cells61 or anti-inflammatory activity reducing LPS-stimulated TNFα production62, can be used to screen and asses the immunomodulatory activity in vitro. Ultimately, since the innate immune response is inherently complex and dependent on other underlying stimuli (e.g. from the infection itself), the immunomodulatory activity of each new IDR peptide needs to be confirmed in vivo. This is typically labour-intensive and involves significant cost6. Regardless, efforts are currently underway to understand which cellular responses are the best predictors of immunomodulatory activity and improved QSAR modeling of synthetic IDR peptide using updated descriptors should generate novel sequences with applications towards improving human and animal health.
Therapeutic Applications of Peptides – Successes and Challenges
Despite the very substantial number of AMPs that have been identified and their recognized potential as antibacterials and immunomodulators, a relatively small number of peptides have made it to clinical trials. Examples of peptides at their most advanced stages of clinical development and their targeted clinical applications are shown in Table 1. Many of the antimicrobial HDPs are being considered for topical application either because of systemic toxicity or lability to proteases in the blood. Peptides are rapidly metabolized within the body and it has been suggested that high doses of peptide are required to achieve the desired antibiotic effect in vivo, which is much easier to achieve through topical application. It is worth mentioning that the protective effects of IDR-1 were observed in mice with intravenous, intraperitoneal or subcutaneous administration and when the peptide was administered either 48 hours prior to or 6 hours after infection61, suggesting that the immunomodulation induced by peptides continues even after the peptide is cleared from circulation. Regardless, several strategies have been used to overcome these issues associated with peptide stability and toxicity.
Table 1.
Examples of host defence peptide in clinical trials. Table adapted from Acafan et al.17 and Fjell et al.5
| Peptide | Sequence | Company | Application | Progress | Reference |
|---|---|---|---|---|---|
|
| |||||
| Pexiganan acetate | GIGKFLKKAKKFGKAFVKILKK-NH2 | Access Pharmaceuticals | Topical antibiotic | Phase III | NCT00563433 & NCT00563394 |
|
| |||||
| Omiganan (MX226/MBI-226) | ILRWPWWPWRRK-NH2 | Migenix/BioWest Therapeutics | Prevent catheter infections, topical antiseptic, severe acne and rosacea | Phase IIIb/II | NCT00027248 & NCT00231153 |
|
| |||||
| hLF1–11 | GRRRRSVQWCA-NH2 | AM-Pharma | Bacteraemia and fungal infections in immunocompromised haematopoetic stem cell transplant recipients | Phase I/II | NCT00509938 |
|
| |||||
| Iseganan (IB-367) | RGGLCYCRGRFCVCVGR-NH2 | Ardea Biosciences | Oral mucositis | Phase III | NCT00022373 |
|
| |||||
| PAC-113 | AKRHHGYKRKFH-NH2 | Pacgen Biopharmaceuticals | Oral candidiasis | Phase IIb | NCT00659971 |
|
| |||||
| IMX942 | 5 amino acid peptide derived from IDR-1 | Inimex | Nosocomial infections, neutropenia | Phase II | http://www.inimexpharma.com/prod_tech_profile.html |
|
| |||||
| OP-145 | IGKEFKRIVERIKRFLRELVRPLR-NH2 | OctoPlus; Leiden University | Chronic middle ear infections | Phase I/II | ISRCTN84220089 |
|
| |||||
| Plectasin (variant NZ2114 in development) | GFGC1NGPWDEDDMQC2HNHC3KSIKGYKGGYC1AKGGFVC2KC3Y | Novozymes | Broad spectrum antibiotic | Pre-clinical | http://www.novozymes.com/en/news/news-archive/Pages/45873.aspx |
The most obvious obstacle to the administration of AMPs as therapeutics is their inherent susceptibility to proteolytic degradation. If administered orally, the peptides will encounter the hydrolytic activities of pepsin, trypsin and chymotrypsin as they travel through the digestive tract. Alternatively, if administered systemically by IV, they can be degraded by proteases present in the blood or taken up by cells and rapidly distributed throughout the body. Additionally, some bacterial species are also known to produce proteases that inactivate certain AMPs72,73 leading to their enhanced survival in the presence of peptide. Consequently, while no formal pharmacokinetic studies have been published to date, peptides are likely to have an inherent short half life in vivo and several strategies have been employed to improve the proteolytic stability of peptide based drugs74.
One simple strategy to block proteolytic degradation involves acetylation of the N-terminus to block the activity of aminopeptidases75, although this does remove one positive charge, which might impact on activity. Peptide cyclization, through a disulphide bridge or joining the backbone at the N- and C- terminus, has also been shown to improve serum stability of short synthetic AMPs76. A popular strategy to improve proteolytic stability of peptides is to incorporate non-natural D-isomers of amino acids, altering the stereochemistry of the peptide backbone and inhibiting susceptibility to proteases. The D-enantiomer of a peptide, which is a mirror image of the native L-peptide, often retains the antimicrobial activity of the native sequence because the interactions with the bacterial membrane are not dependent on interactions with a specific receptor77,78. Interestingly, it was recently reported that a protease resistant D-enantiomer of peptide M33 (KKIRVRLSA) was more active against Gram-positive pathogens than the L-amino acid enantiomer79, indicating that D-isomers of AMPs might be further optimized beyond the simple conversion of the peptide sequence from the natural L-form. A similar approach uses the retro-inverso (RI) D-isoform of a peptide, in which the peptide is synthesized with the opposite N- to C- sequence using only D-enantiomers. Such an approach maintains the spatial orientation of the amino acid side chains found in the native peptide after folding, while protecting the backbone from proteolytic degradation. This strategy was recently used to generate protease resistant D- and RI- forms of bovine myeloid antimicrobial peptide 28 (BMAP28) that retained much of the antimicrobial activity of the parent peptide. Interestingly, the D and RI forms of BMAP28 also retained their immunomodulatory properties but the RI peptide was significantly less toxic towards epithelial cells and monocytes80. The immunomodulatory properties of D-peptides have not been examined in detail, but their immense potential is highlighted in the observation that D-LL-37 is a more potent stimulator of IL-8 in keratinocytes compared the natural L-isoform of LL-3759. These studies clearly demonstrate that peptide enantiomers have the potential to give rise to protease resistant HDPs with desirable immunomodulatory properties.
Incorporation of unnatural amino acids into peptide sequences also provides improved metabolic stability and increases the range of physicochemical properties that can be used to optimize peptides as antibacterial agents. Because of the importance of positive charge to the antimicrobial activity, several conservative substitutions can be made for the cationic residues, Lys and Arg, that change the length of the side chain but preserve the positively charged amino or guanidino group. For instance, replacement of one arginine residue in the apidaecin 1b analog, Api88, by L-ornithine or L-homoarginine increased the peptide stability in serum without dramatically affecting the activity against E.coli81. Tryptophan residues are also considered important residues for determining the antimicrobial activity of AMPs82 and are another amino acid that is commonly substituted to modulate the activity of peptides. The Trp residues in peptide P-113 were replaced with β-naphthylalanine and β-(4,4′-biphenyl)alanine resulting in peptides that retained their potency at physiological salt concentrations83. Other groups have optimized for simple characteristics of AMPs, such as cationicity and amphipathicity, and applied these traits to the design and synthesis of novel peptides containing unnatural amino acids. A recent report describes the screening of 36 sequences that incorporate tetrahydroisoquinolinecarboxylic acid (Tic) and octahydroindolecarboxylic acid (Oic) residues and the resulting peptides were found to have MICs as low as 6.25μg/ml against the clinically relevant ESKAPE pathogens84. While the reported activities were relatively modest, as our understanding of the use of non-natural amino acids improves, they can be used to make test sets of peptides and then used in computational QSAR studies to optimize the antimicrobial potency of AMPs containing non-natural amino acids. This would expand the tool box for synthesizing AMPs from the 20 naturally occurring amino acids to an almost endless supply of amino acid derivatives that are only limited by the organic chemistry required to generate them.
Peptidomimetics are polymeric molecules that mimic peptides but have altered backbone structures to improve peptide stability while maintaining the biological properties of the parent peptides (for recent reviews see 85,86). The principle behind using peptidomimetics is to preserve the spatial orientation of the side chain residues while altering the peptide backbone to make it impervious to the activity of proteases. Peptidomimetics are used in a variety of biological applications and many examples of peptidomimetics based on AMP sequences have been described including: β-peptides87, peptoids88 and oligoacyllysines89. Peptidomimetics have not been widely studied for their immunomodulatory activities but it is conceivable that many of the immunomodulatory properties seen in IDR peptides could be engendered in mimetics provided that they are still able to translocate into cells and/or interact with above-described cellular receptors that influence the immunomodulatory response. Several peptidomimetics based on HDPs as well as non-natural amino acid substituted peptides are in various stages of clinical development5 demonstrating that these are viable approaches for harnessing the therapeutic potential of HDPs while addressing the issue of stability in vivo.
Various drug delivery systems have been designed to improve the stability of peptide based drugs, improve their bioavailability in vivo and target them to specific sites within the body. Since AMPs are known to interact with biological membranes, lipid based formulations are a logical extension of this to improve the biological properties of peptides90. An interesting example used melittin-loaded perfluorocarbon nanoemulsion particles to target the cytotoxic peptide to tumor cells while blocking the extremely hemolytic activity of the melittin peptide91. In addition to liposomal formulations, other nanoparticles have been examined as potential AMP carriers including: dendridite polymers, solid core nanoparticles, carbon nanotubes and DNA cages92. PEGylation, the process of covalently adding polyethylene glycol chains to polypeptides, is another relatively common practice used to improve stability bioavailability of protein and peptide drugs93. Indeed, PEGylated versions of synthetic AMPs have been shown to retain their antimicrobial activity while improving their biocompatibility and protease stability94,95. However, care needs to be taken when covalently attaching large PEG moieties to peptides as these may negatively impact the interactions between the peptide and bacterial cells, resulting in lowered antimicrobial activity96.
Another obstacle to the development of peptides as pharmaceuticals is the relatively high cost associated with generating synthetic peptides on a large scale7. Recombinant expression of peptides could be used to generate large quantities of peptides with low materials costs. However, there are drawbacks to using bacterial heterologous expression of AMPs. Firstly, the overexpressed peptide is often toxic to the bacterial cell as these molecules have inherent antibacterial activity. This can be blocked by expressing the peptide bound to a large (anionic) fusion protein which masks the toxic effects of the peptide inside the cell. A recent method, appropriate for large scale and cost-effective production of HDPs, successfully produced seven different recombinant HDPs as SUMO (Small Ubiquitin-like Modifier protein) fusions, including LL-37 and IDR-197. However, it should be pointed out that no AMPs currently being evaluated in clinical trials are made recombinantly, although plectasin that has been developed pre-clinically is indeed made recombinantly. Additionally, when using recombinant methods to generate peptides, one must neutralize the carboxyl terminus chemically and one is limited to using the 20 naturally occurring amino acids as building blocks, precluding the incorporation of non-natural amino acids or peptidomimetics to improve peptide activity and stability.
The potential of HDPs as novel antibacterial treatment options for human pathogens continues to drive much of the research into these natural molecules. Interestingly, their use may extend beyond topical and systemic antibiotics and their potential applications in other areas are currently being evaluated. For instance, biofilms are multicellular communities of bacteria that grow on surfaces with enhanced resistance to antibiotics and disinfectants, making them difficult to eradicate98,99. Increasing evidence demonstrates a clear link between biofilms and a negative impact on human health100 and it is estimated that as many as 80% of infections in the body are due to bacteria in biofilms99. Specific HDPs have been shown to inhibit the formation of biofilms, even at peptide concentrations below the MIC for planktonic bacteria101,102, suggesting that HDPs may be useful anti-biofilm agents. Intriguingly such peptides have broad spectrum anti-biofilm activity and this activity appears completely independent of activity against free swimming (planktonic) bacteria101. Additionally, tethering of AMPs to surfaces has generated non-toxic antimicrobial and anti-biofilm surfaces for use in implant devices103. It appears that the mechanism of bacterial killing by tethered peptides may be different from that of peptides in solution104, since a largely independent series of peptide descriptors define optimized tethered peptide sequences.
Conclusions
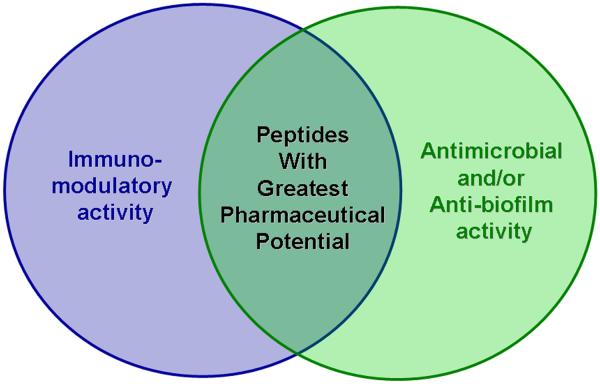
The increasing prevalence of antibiotic resistant pathogenic bacteria and the burden that this places on health systems throughout the world105,106 highlights the desperate need to develop novel antibiotic compounds. HDPs have long been touted for their potential to fill the current void in novel antibiotic discovery, but this potential has yet to be realized and only a handful of anti-infective peptides have entered clinical trials with no approved drugs to date. Much of the research thus far has focused on optimizing the direct antibacterial activity of HDPs but this strategy has yet to yield novel therapies and clinical trials have been largely limited to topical applications. Recently, the immunomodulatory properties of HDPs have garnered significant attention and many peptides are now known to stimulate the innate immune response while suppressing potentially harmful inflammation. Our group has developed several peptide screening techniques to evaluate the antibacterial and immunomodulatory properties of peptides in an attempt to quickly and effectively identify synthetic IDR peptides with therapeutic potential. Ideally, the peptides with the greatest pharmaceutical potential might be those that posses both immunomodulatory and antibacterial (or anti-biofilm) activities (Figure 1). Several strategies have also been developed to improve the stability of HDPs in vivo which should lead to better pharmacokinetic and pharmacodynamic profiles for HDP based drugs and this is a research area of great importance to the field. Evidently, more work is required to completely understand HDPs but the outlook for HDPs as novel antimicrobial and immunomodulatory therapeutics remains promising and we anticipate that in the near future their potential as anti-infectives will finally be realized.
Figure 1.
HDPs can have direct antimicrobial (or anti-biofilm) activity and/or immunomodulatory properties. Peptide sequences can be optimized for their direct antimicrobial activity, or they can be optimized for their ability to modulate the immune response. Those peptides that possess strong immunomodulatory properties and have potency in inhibiting biofilms or killing bacteria are likely to have the greatest potential to be developed as novel anti-infective drugs.
Acknowledgements
Funding for Hancock's peptide research is provided by the Canadian Institutes of Health Research (CIHR) and was previously supported by the Foundation for the National Institutes for Health through the Grand Challenges in Global Health Research program. EFH is supported by a postdoctoral fellowship from the CIHR. REWH holds a Canada Research Chair.
References
- 1.Levy SB, Marshall B. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 2.Overbye KM, Barrett JF. Drug Discov Today. 2005;10:45–52. doi: 10.1016/S1359-6446(04)03285-4. [DOI] [PubMed] [Google Scholar]
- 3.Hancock REW, Patrzykat A. Curr Drug Targets Infect Disord. 2002;2:79–83. doi: 10.2174/1568005024605855. [DOI] [PubMed] [Google Scholar]
- 4.Hancock REW, Lehrer R. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 5.Fjell CD, Hiss JA, Hancock REW, Schneider G. Nat Rev Drug Discov. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 6.Easton DM, Nijnik A, Mayer ML, Hancock REW. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hancock REW, Sahl HG. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 8.Zasloff M. Proc Natl Acad Sci U S A. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Li X, Wang Z. Nucleic Acids Res. 2009;37:D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Wang G. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock REW, Diamond G. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 12.Blondelle SE, Houghten RA. Trends Biotechnol. 1996;14:60–65. doi: 10.1016/0167-7799(96)80922-X. [DOI] [PubMed] [Google Scholar]
- 13.Masschalck B, Michiels CW. Crit Rev Microbiol. 2003;29:191–214. doi: 10.1080/713610448. [DOI] [PubMed] [Google Scholar]
- 14.Vogel HJ. Biochem Cell Biol. 2012;90:233–244. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen LT, Haney EF, Vogel HJ. Trends Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Hale JD, Hancock REW. Expert Rev Anti Infect Ther. 2007;5:951–959. doi: 10.1586/14787210.5.6.951. [DOI] [PubMed] [Google Scholar]
- 17.Afacan NJ, Yeung AT, Pena OM, Hancock REW. Curr Pharm Des. 2012;18:807–819. doi: 10.2174/138161212799277617. [DOI] [PubMed] [Google Scholar]
- 18.Astafieva AA, Rogozhin EA, Odintsova TI, Khadeeva NV, Grishin EV, Egorov Ts A. Peptides. 2012;36:266–271. doi: 10.1016/j.peptides.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Merrifield RB. J Am Chem Soc. 1963;85:2149–2154. [Google Scholar]
- 20.Falla TJ, Hancock REW. Antimicrob Agents Chemother. 1997;41:771–775. doi: 10.1128/aac.41.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Scott MG, Yan H, Mayer LD, Hancock REW. Biochemistry. 2000;39:14504–14514. doi: 10.1021/bi0011173. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Hancock REW. Antimicrob Agents Chemother. 1999;43:1274–1276. doi: 10.1128/aac.43.5.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zasloff M, Martin B, Chen HC. Proc Natl Acad Sci U S A. 1988;85:910–913. doi: 10.1073/pnas.85.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomita M, Takase M, Bellamy W, Shimamura S. Acta Paediatr Japon; Overseas edition. 1994;36:585–591. doi: 10.1111/j.1442-200x.1994.tb03250.x. [DOI] [PubMed] [Google Scholar]
- 25.Hilpert K, Volkmer-Engert R, Walter T, Hancock REW. Nat Biotechnol. 2005;23:1008–1012. doi: 10.1038/nbt1113. [DOI] [PubMed] [Google Scholar]
- 26.Wiegand I, Hilpert K, Hancock RE. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 27.Haines LR, Thomas JM, Jackson AM, Eyford BA, Razavi M, Watson CN, Gowen B, Hancock REW, Pearson TW. PLoS Negl Trop Dis. 2009;3:e373. doi: 10.1371/journal.pntd.0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X, Zhong J, Liu H, Liu C, Zhang K, Lai R. Gene. 2012;492:368–374. doi: 10.1016/j.gene.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Wong ES, Whitley JC, Li J, Stringer JM, Short KR, Renfree MB, Belov K, Cocks BG. PLoS One. 2011;6:e24030. doi: 10.1371/journal.pone.0024030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka T, Kokuryu Y, Matsunaga T. Appl Environ Microbiol. 2008;74:7600–7606. doi: 10.1128/AEM.00162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Q, Matsunaga S, Wen Z, Niimi S, Kumano M, Sakakibara Y, Machida S. J Pept Sci. 2006;12:643–652. doi: 10.1002/psc.774. [DOI] [PubMed] [Google Scholar]
- 32.Lewenza S, Falsafi RK, Winsor G, Gooderham WJ, McPhee JB, Brinkman FS, Hancock REW. Genome Res. 2005;15:583–589. doi: 10.1101/gr.3513905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frank R. J Immunol Methods. 2002;267:13–26. doi: 10.1016/s0022-1759(02)00137-0. [DOI] [PubMed] [Google Scholar]
- 34.Hilpert K, Elliott MR, Volkmer-Engert R, Henklein P, Donini O, Zhou Q, Winkler DF, Hancock REW. Chem Biol. 2006;13:1101–1107. doi: 10.1016/j.chembiol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Cherkasov A. Int J Mol Sci. 2005;6:63–86. [Google Scholar]
- 36.Jenssen H, Fjell CD, Cherkasov A, Hancock REW. J Pept Sci. 2008;14:110–114. doi: 10.1002/psc.908. [DOI] [PubMed] [Google Scholar]
- 37.Jenssen H, Lejon T, Hilpert K, Fjell CD, Cherkasov A, Hancock REW. Chem Biol Drug Des. 2007;70:134–142. doi: 10.1111/j.1747-0285.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 38.Cherkasov A, Hilpert K, Jenssen H, Fjell CD, Waldbrook M, Mullaly SC, Volkmer R, Hancock REW. ACS Chem Biol. 2009;4:65–74. doi: 10.1021/cb800240j. [DOI] [PubMed] [Google Scholar]
- 39.Chromek M, Arvidsson I, Karpman D. PLoS One. 2012;7:e46476. doi: 10.1371/journal.pone.0046476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 41.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock REW. J Leukoc Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 42.Bals R, Wang X, Zasloff M, Wilson JM. Proc Natl Acad Sci U S A. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nell MJ, Tjabringa GS, Wafelman AR, Verrijk R, Hiemstra PS, Drijfhout JW, Grote JJ. Peptides. 2006;27:649–660. doi: 10.1016/j.peptides.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock REW. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 45.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandamme D, Landuyt B, Luyten W, Schoofs L. Cell Immunol. 2012;280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Semple F, Dorin JR. J Innate Immun. 2012;4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rehaume LM, Hancock REW. Crit Rev Immunol. 2008;28:185–200. doi: 10.1615/critrevimmunol.v28.i3.10. [DOI] [PubMed] [Google Scholar]
- 49.Yeung AT, Gellatly SL, Hancock REW. Cell Mol Life Sci. 2011;68:2161–2176. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi KY, Chow LN, Mookherjee N. J Innate Immun. 2012;4:361–370. doi: 10.1159/000336630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, Di Virgilio F, Zanetti M. J Biol Chem. 2008;283:30471–30481. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 53.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai Y, Adhikarakunnathu S, Bhardwaj K, Ranjith-Kumar CT, Wen Y, Jordan JL, Wu LH, Dragnea B, San Mateo L, Kao CC. PLoS One. 2011;6:e26632. doi: 10.1371/journal.pone.0026632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau YE, Rozek A, Scott MG, Goosney DL, Davidson DJ, Hancock REW. Infect Immun 2005. 73:583–591. doi: 10.1128/IAI.73.1.583-591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mookherjee N, Lippert DN, Hamill P, Falsafi R, Nijnik A, Kindrachuk J, Pistolic J, Gardy J, Miri P, Naseer M, Foster LJ, Hancock REW. J Immunol. 2009;183:2688–2696. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- 57.Yu HB, Kielczewska A, Rozek A, Takenaka S, Li Y, Thorson L, Hancock REW, Guarna MM, North JR, Foster LJ, Donini O, Finlay BB. J Biol Chem. 2009;284:36007–36011. doi: 10.1074/jbc.C109.073627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mookherjee N, Hamill P, Gardy J, Blimkie D, Falsafi R, Chikatamarla A, Arenillas DJ, Doria S, Kollmann TR, Hancock REW. Mol Biosyst. 2009;5:483–496. doi: 10.1039/b813787k. [DOI] [PubMed] [Google Scholar]
- 59.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DY, Gallo RL. J Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 60.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Tanaka S, Heumann D. Clin Diagn Lab Immunol. 2002;9:972–982. doi: 10.1128/CDLI.9.5.972-982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, Wang A, Lee K, Doria S, Hamill P, Yu JJ, Li Y, Donini O, Guarna MM, Finlay BB, North JR, Hancock REW. Nat Biotechnol. 2007;25:465–472. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- 62.Wieczorek M, Jenssen H, Kindrachuk J, Scott WR, Elliott M, Hilpert K, Cheng JT, Hancock REW, Straus SK. Chem Biol. 2010;17:970–980. doi: 10.1016/j.chembiol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Nijnik A, Madera L, Ma S, Waldbrook M, Elliott MR, Easton DM, Mayer ML, Mullaly SC, Kindrachuk J, Jenssen H, Hancock REW. J Immunol. 2010;184:2539–2550. doi: 10.4049/jimmunol.0901813. [DOI] [PubMed] [Google Scholar]
- 64.Madera L, Hancock REW. J Innate Immun. 2012;4:553–568. doi: 10.1159/000338648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner-Brannen E, Choi KY, Lippert DN, Cortens JP, Hancock REW, El-Gabalawy H, Mookherjee N. Arthritis Res Ther. 2011;13:R129. doi: 10.1186/ar3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garlapati S, Eng NF, Kiros TG, Kindrachuk J, Mutwiri GK, Hancock REW, Halperin SA, Potter AA, Babiuk LA, Gerdts V. Vaccine. 2011;29:6540–6548. doi: 10.1016/j.vaccine.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Garlapati S, Garg R, Brownlie R, Latimer L, Simko E, Hancock REW, Babiuk LA, Gerdts V, Potter A, van Drunen Littel-van den Hurk S. Vaccine. 2012;30:5206–5214. doi: 10.1016/j.vaccine.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Pena OM, Afacan N, Pistolic J, Chen C, Madera L, Falsafi R, Fjell CD, Hancock REW. PLoS One. 2013;8:e52449. doi: 10.1371/journal.pone.0052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinstraesser L, Hirsch T, Schulte M, Kueckelhaus M, Jacobsen F, Mersch EA, Stricker I, Afacan N, Jenssen H, Hancock REW, Kindrachuk J. PLoS One. 2012;7:e39373. doi: 10.1371/journal.pone.0039373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Achtman AH, Pilat S, Law CW, Lynn DJ, Janot L, Mayer ML, Ma S, Kindrachuk J, Finlay BB, Brinkman FS, Smyth GK, Hancock REW, Schofield L. Sci Transl Med. 2012;4:135ra164. doi: 10.1126/scitranslmed.3003515. [DOI] [PubMed] [Google Scholar]
- 71.Rivas-Santiago B, Castañeda-Delgado JE, Rivas Santiago CE, Waldbrook M, González-Curiel I, Leon-Contreras JC, Enciso-Moreno A, del Villar V, Méndez-Ramos J, Hancock REW, Hernandez-Pando R. PLoS One. 2013 doi: 10.1371/journal.pone.0059119. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ulvatne H, Haukland HH, Samuelsen O, Kramer M, Vorland LH. J Antimicrob Chemother. 2002;50:461–467. doi: 10.1093/jac/dkf156. [DOI] [PubMed] [Google Scholar]
- 73.Schmidtchen A, Frick IM, Andersson E, Tapper H, Bjorck L. Mol Microbiol. 2002;46:157–168. doi: 10.1046/j.1365-2958.2002.03146.x. [DOI] [PubMed] [Google Scholar]
- 74.Gentilucci L, De Marco R, Cerisoli L. Curr Pharm Des. 2010;16:3185–3203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 75.Jayawardene DS, Dass C. Peptides. 1999;20:963–970. doi: 10.1016/S0196-9781(99)00089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen LT, Chau JK, Perry NA, de Boer L, Zaat SA, Vogel HJ. PLoSOne. 2010;5:e12684. doi: 10.1371/journal.pone.0012684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG, Merrifield RB. Proc Natl Acad Sci U S A. 1990;87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. FEBS Letters. 1990;274:151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- 79.Falciani C, Lozzi L, Pollini S, Luca V, Carnicelli V, Brunetti J, Lelli B, Bindi S, Scali S, Di Giulio A, Rossolini GM, Mangoni ML, Bracci L, Pini A. PLoS One. 2012;7:e46259. doi: 10.1371/journal.pone.0046259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kindrachuk J, Scruten E, Attah-Poku S, Bell K, Potter A, Babiuk LA, Griebel PJ, Napper S. Biopolymers. 2011;96:14–24. doi: 10.1002/bip.21441. [DOI] [PubMed] [Google Scholar]
- 81.Berthold N, Czihal P, Fritsche S, Sauer U, Schiffer G, Knappe D, Alber G, Hoffmann R. Antimicrob Agents Chemother. 2013;57:402–409. doi: 10.1128/AAC.01923-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan DI, Prenner EJ, Vogel HJ. Biochim Biophys Acta. 2006;1758:1184–1202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Yu HY, Tu CH, Yip BS, Chen HL, Cheng HT, Huang KC, Lo HJ, Cheng JW. Antimicrob Agents Chemother. 2011;55:4918–4921. doi: 10.1128/AAC.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hicks RP, Abercrombie JJ, Wong RK, Leung KP. Bioorg Med Chem. 2013;21:205–214. doi: 10.1016/j.bmc.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 85.Giuliani A, Rinaldi AC. Cell Mol Life Sci. 2011;68:2255–2266. doi: 10.1007/s00018-011-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rotem S, Mor A. Biochim Biophys Acta. 2009;1788:1582–1592. doi: 10.1016/j.bbamem.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 87.Raguse TL, Porter EA, Weisblum B, Gellman SH. J Am Chem Soc. 2002;124:12774–12785. doi: 10.1021/ja0270423. [DOI] [PubMed] [Google Scholar]
- 88.Jahnsen RD, Frimodt-Moller N, Franzyk H. J Med Chem. 2012;55:7253–7261. doi: 10.1021/jm300820a. [DOI] [PubMed] [Google Scholar]
- 89.Radzishevsky IS, Kovachi T, Porat Y, Ziserman L, Zaknoon F, Danino D, Mor A. Chem Biol. 2008;15:354–362. doi: 10.1016/j.chembiol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Li P, Nielsen HM, Mullertz A. Expert Opin Drug Deliv. 2012;9:1289–1304. doi: 10.1517/17425247.2012.717068. [DOI] [PubMed] [Google Scholar]
- 91.Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, Heuser JE, Arbeit JM, Wickline SA, Schlesinger PH. J Clin Invest. 2009;119:2830–2842. doi: 10.1172/JCI38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Urban P, Valle-Delgado JJ, Moles E, Marques J, Diez C, Fernandez-Busquets X. Curr Drug Targets. 2012;13:1158–1172. doi: 10.2174/138945012802002302. [DOI] [PubMed] [Google Scholar]
- 93.Harris JM, Chess RB. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 94.Morris CJ, Beck K, Fox MA, Ulaeto D, Clark GC, Gumbleton M. Antimicrob Agents Chemother. 2012;56:3298–3308. doi: 10.1128/AAC.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang G, Han B, Lin X, Wu X, Yan H. J Biochem. 2008;144:781–788. doi: 10.1093/jb/mvn134. [DOI] [PubMed] [Google Scholar]
- 96.Imura Y, Nishida M, Ogawa Y, Takakura Y, Matsuzaki K. Biochim Biophys Acta. 2007;1768:1160–1169. doi: 10.1016/j.bbamem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 97.Bommarius B, Jenssen H, Elliott M, Kindrachuk J, Pasupuleti M, Gieren H, Jaeger KE, Hancock REW, Kalman D. Peptides. 2010;31:1957–1965. doi: 10.1016/j.peptides.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fux CA, Costerton JW, Stewart PS, Stoodley P. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 99.Davies D. Nat Rev Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 100.Hoiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PO, Molin S, Givskov M, Tolker-Nielsen T, Bjarnsholt T. Int J Oral Sci. 2011;3:55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de la Fuente-Nunez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock REW. Antimicrob Agents Chemother. 2012;56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Overhage J, Campisano A, Bains M, Torfs EC;, Rehm BH, Hancock REW. Infect Immun. 2008;76:4176–4182. doi: 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JT, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock REW, Kizhakkedathu JN. Biomaterials. 2011;32:3899–3909. doi: 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 104.Gao G, Cheng JT, Kindrachuk J, Hancock REW, Straus SK, Kizhakkedathu JN. Chem Biol. 2012;19:199–209. doi: 10.1016/j.chembiol.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 105.Gyssens IC. Int J Antimicrob Agents. 2011;38(Suppl):11–20. doi: 10.1016/j.ijantimicag.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 106.Nathwani D. J Infect. 2009;59(Suppl 1):S40–50. doi: 10.1016/S0163-4453(09)60007-4. [DOI] [PubMed] [Google Scholar]