Abstract
The aim of this study is to investigate the influence of macrophages on osteoblast performance and differentiation. In this regard, we studied the secretion of growth factors including bone morphogenetic proteins (BMPs) from before and after activation of macrophages. We also evaluated osteogenic markers in the co-culture of macrophages and osteoblsats (OBs). The macrophages were seeded on microparticles (MPs) based on chitosan (CS). Two types of MPs were fabricated including CS MPs and 10% calcium phosphate (CaHPO4) incorporated CS MPs. Macrophage seeded on MPs was activated using lipopolysacharide (LPS). The expression of BMP-2, BMP-6, BMP-7, and transforming growth-β) from macrophages seeded and cultured on hybrid MPs before and after activation of LPS at pre-determined times was quantified using a quantitative reverse transcription-polymerase chain reaction (RT-PCR). All of the above growth factors were expressed from MP-macrophage cultures before LPS activation. Ostesogenic markers such as alkaline phosphatase (ALP), osteocalcin (OCN) and collagen I (COL-I) in the cultures of MP-OB-macrophage were quantified using a quantitative RT-PCR at day 2, 4, and 7. We found an elevation of gene expression of ALP and COL-1 in the co-cultures of OB-macrophage on MPs compared to OB on MP cultures. These data suggest that macrophages enhance expression of osteogenic markers in OBs, and demonstrate the importance of the role of macrophages in bone regeneration.
Keywords: macrophages, osteoblasts, growth factors, bone morphogenetic protein, osteogenic markers, mesenchymal stem cells
INTRODUCTION
Bone healing and regeneration requires osteoinductive signals like bone morphogenetic proteins (BMPs). Champagne et al. showed that BMPs were expressed in macrophages, both before and after activation.1 Macrophages are normally activated using lipopolysacharide (LPS) or macrophage chemotactic peptide-1 (MCP-1). Some studies have shown that in addition to the scavenger role2 macrophages also play an important role in chemotaxis of different cell types towards the defect site.3 Lescaudron et al. showed an increase in muscle regeneration when the implant material contained blood borne macrophages.4 It has also been studied that macrophages play an important role in wound healing process.5,6 Macrophages are cells that are recruited in the early stages of wound healing. Human monocytes in culture can be differentiated into different cell types including macrophages, dendritic cells, and osteoclasts.7 The osteoblast-osteoclast interaction is very important in bone homeostasis and bone healing.
It has been studied that macrophage cell lines produce BMPs, which induce mesenchymal stem cells (MSCs) to differentiate into osteoblasts (OBs).8 BMPs are highly osteoinductive and promote bone healing.9,10 These studies indicated that BMP-2 plays a significant role in the intramembranous bone formation. However, what signals the macrophages to produce BMPs is not yet clearly understood. Activated macrophages secrete numerous enzymes, plasma proteins, cytokines and chemokines, and growth factors.1,11–13 Among them are several factors that have been associated with the bone repair, such as interleukins (ILs), tumor necrosis factor alpha (TNF-α), transforming growth factor beata (TGF-β), platelet-derived growth factor (PDGF), endothelial growth factor (EGF), and vascular endothelial growth factor (VEGF). Dagtekin et al. has studied the material based expression of angiogenic markers by macrophages.14
Macrophages are either classically activated (M1), or alternatively activated (M2).5,15,16 M1 activation is mediated by the priming stimulus interferon-γ, followed by a microbial trigger (e.g. LPS). Activated M1 Macrophages secrete pro-inflammatory cytokines. The M2 designation includes essentially all other types of Macrophages including wound healing Macrophages (M2a) and regulatory Macrophages (M2b).
The effects of hydroxyapatite or hydroxyapatite (HA)/tricalcium phosphate (TCP) particles derived from either sintering at several different temperatures or from plasma-spray coatings were examined using in vitro human monocyte/macrophages (M/M) culture system.17 The HA/TCP particles dried at 110°C were the most biologically active, stimulating significant release of IL-1β, IL-6, TNF-α, and prostaglandin E2 (PGE2). HA/TCP particles from plasma-spray coatings also did not release any proinflammatory products.
The present study focuses on the expression of the various growth factors in macrophages when seeded on chitosan (CS) based microparticles (MPs). CS is one of the most widely used natural polymer that is obtained by deacetylation of chitin. It is a co-polymer of glucosamine and N-acetyl glucosamine. The tissue compatibility of CS could be attributed to its structural similarity with glycosaminoglycan in the extracellular matrix.18 CS has been extensively used in drug and gene delivery.19,20 CS scaffold has been used in bone regeneration applications because of its osteoconductive and antimicrobial properties.21,22 Studies have shown that incorporation of calcium phosphates (CaHPO4) have increased the mechanical strength of the polymer23 and rendered the polymer with good osteoconductive properties. Natural bone contains calcium and phosphate, therefore, we decided to include CaHPO4 into the MPs. Hence, we have formulated one type of MPs to incorporate 10% CaHPO4, the other type of MPs did not contain CaHPO4. Our previous studies have indicated bone regeneration both in vitro and in vivo, when MSCs were seeded on the CS based MPs.24,25
Osteogenesis is initiated in bone defects caused due to tumor resections, trauma or congenital defects.26 The influence of macrophages on the osteogenic potential of OBs seeded MPs has not been studied. Even though CS based materials have been used in bone tissue engineering, the idea of co-culturing macrophages and OBs on the CS MPs is relatively new. In this study we investigated the expression of BMP-2, BMP-6, BMP-7, and TGF-β in macrophages seeded on hybrid MPs before and after LPS activation. These factors are extremely important to regenerate or heal the bone defects. Co-culturing macrophages with OBs provides us with a tool to understand the role of macrophages in regulating bone formation.
MATERIALS AND METHODS
Materials
CS (85% deacetylated), tripolyphosphate, cotton seed oil, span 85, and hexane were purchased from Sigma-Aldrich. Acetone was purchased from Fisher Scientific. The biological reagents for cell culture were purchased from Invitrogen.
Fabrication of microparticles
CS based MPs were fabricated using our scale-up procedures as previously described.26,27 Briefly, CS solution (1.5% w/v) was made by dissolving 0.75 g CS in 50 ml 1% acetic acid. The CS solution was filtered through nylon mesh to remove any particulate matter. CS solution (25 ml) was then mixed with equal volume of acetone and added drop wise into 600 ml cottonseed oil, mixed with 4 ml surfactant (span 85). After 14 h 64% (w/w) of TPP was added to the reaction mix. The CS MPs were purified using hexane followed by vacuum filtration. Next, the CS MPs were thoroughly washed with deionized water and lyophilized. Morphology of MPs was analyzed using a scanning electron microscope (SEM).
Isolation of MSCs from bone marrow of mice to derive osteoblasts
MSCs were isolated from the bone marrow harvested from C57BL/6 mice (Charles River, Jackson, MA) as previously described.24,29,30 The bone marrow from the femur and tibia of mice was flushed using phosphate buffered saline (PBS). The marrow was pipetted a couple of times to break down clumps. The resulting cell suspension was centrifuged and the PBS was aspirated off. The cell pellet was suspended in 1X alpha minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin-streptomycin. The cells were then plated in T-75 flasks and incubated at 37°C. The cells were cultured for 7 to 10 days before passaging them for in vitro studies.
Isolation of macrophages from bone marrow of mice
Macrophages were isolated using the methods published previously.31 Briefly, bone marrow from the femur of C57 BL/6 mice was flushed with 10 ml RPMI media containing 10% FBS and 30% L929 conditioned medium (macrophage expansion medium; see below) using a 27 G needle and 5 ml syringe. The cell pellets were dispersed by gently pipetting the suspension using the syringe. The cells were added to sterile petri dishes and incubated at 37°C for 4 days. The media was replaced on day 4 and re-incubated until day 6, when the macrophages were recovered by gentle scraping, counted, and used for in vitro studies.
Identify macrophage phenotype
To assess the phenotype of these resting macrophages, the day 6 cells were stained on ice with FITC-conjugated antibodies against CD11b, CD11c, and F4/80 (BD Biosciences-Pharmingen) and the percentages of positive cells were determined by flow cytometry (FACS Caliber; BD Biosciences) and Cellquest software analyses (BD Biosciences).
Preparation of L929 supernatants to culture macrophages
Primary murine macrophages from bone marrow are expanded in vitro either by using L929 supernatants or recombinant macrophages–colony stimulating factor (rM-CSF), or by thioglycolate elicitation from the peritoneum.31 This study utilized expansion of macrophages from murine bone marrow using L929 supernatants, as this method has been shown to consistently produce high yields of pure macrophage cultures with properties that are similar to those produced using rM-CSF, which is more expensive.32,33 L929 supernatants were obtained using previously established protocols.31 Briefly L929 cells were cultured in 5 ml RPMI supplemented with 10% FBS, using a T-25 flask. When the cells reached 75% confluence the cells were trypsinized and replated in a T-75 flask. When the cells were approximately 75% confluent the cells were trypsinized and plated in T-125 flasks. After 7 and 14 days in culture, the conditioned culture medium (L929 supernatant) was recovered from the T-125 flask, sterile filtered and frozen at −20°C. These supernatants were used to prepare the macrophage expansion medium, which contains 58 ml RPMI, 10 ml horse serum, 0.5 ml Glutamax (Life Technologies), 1 ml pen-strep (100×), and 100 μl β-mercaptoethanol, and 30 ml of L929 supernatant.
Macrophage attachment and proliferation
The MPs (25 mg) were placed in a 24-well and macrophages suspension with a cell density of 100,000 cells/ml in 90% RPMI+ 10% FBS was added to the well. The well plate was incubated in 5% CO2/95 air incubator at 37°C. After 24 h, medium was removed and the MPs-cell constructs transferred to another well before adding the live-dead cell assay (Molecular Probes). This transfer has been done to avoid any macrophages attached to the surface of the well. After treating the MP-cell constructs with live/dead assay the images were taken using a fluorescent microscope (Olympus).
Protein expression from macrophages
Macrophages were incubated in the wells containing 1 μg/ml LPS in RPMI supplemented with 10% FBS. The culture medium was changed in every three days. Supernatants were collected on day 1, 4, 7, and 10 after activation and frozen in aliquots. Enzyme-Linked Immuno-Sorbent Assay (ELISA) was performed using the mouse TGF-β1 kit (R&D Systems) and the procedure was carried out as directed in the instructions manual. The plate was read on a microplate reader at 450nm with wavelength correction of 570 nm. From the known concentrations of the standards and the OD values obtained, a standard curve was generated. The average concentration of each sample was determined using a standard graph.
Gene expression studies
Macrophage Study
Expression of BMP-2, BMP-6, BMP-7 and TGF-β, in macrophages was analyzed individually. MPs (60 mg) from each formulation were weighed separately into each well in triplicates. Macrophages (200,000) were seeded on the MPs in each well. Two sets of studies were performed in parallel. In one set, macrophages were cultured using an osteogenic medium. Another set involving similar seeding density was cultured in osteogenic medium containing 1 μg/ml LPS. At day 0, 1, 2 and 7, the expression of BMP-2, BMP-6, BMP 7, and TGF-β, were studied in both sets, using a quantitative reverse transcription polymerase chain reaction (RT-PCR). Table 1 shows the list of sequences of the primers used in this study.
Table 1.
List of primer sequences
| Gene | Primer Sequence | No. of base pairs |
|---|---|---|
|
| ||
| TGF-Beta | ||
| Forward | 5′ CACCAATGAAGCGTTCCAAGCCAT3′ | 24 |
| Reverse | 5′ TTTGTGGCCATGATGTCGGTTTCG3′ | 24 |
| BMP-2 | ||
| Forward | 5′AAGCAGAGACCCAAGTTCCCAGAA3′ | 24 |
| Reverse | 5′ATCCTGGTCCCAGCAGTCTTCAAT3′ | 24 |
| BMP-6 | ||
| Forward | 5′AGACCTGGGATGGCAGG3′ | 17 |
| Reverse | 5′ACCATCCCGCTTCGCTGTGC3′ | 20 |
| BMP-7 | ||
| Forward | 5′AAAGAACCAAGAGGCCCTGAGGAT3′ | 24 |
| Reverse | 5′AGCCTTCAGGTGCAATGATCCAGT3′ | 24 |
| ALP | ||
| Forward | 5′GTGCCAGAGAAAGAGAGAGAC3′ | 21 |
| Reverse | 5′ GACGCCCATACCATCTCC3′ | 18 |
| Osteocalcin | ||
| Forward | 5′GAGTCTGACAAAGCCTTCA3′ | 19 |
| Reverse | 5′AGCCATACTGGTCTGATAG3′ | 19 |
| Collagen I | ||
| Forward | 5′ACTGTCCCAACCCCCAAAG3′ | 19 |
| Reverse | 5′CGTATTCTTCCGGGCAGAAA3 | 20 |
| Beta-Actin | ||
| Forward | 5′ TTGCTGACAGGATGCAGAAGGAGA3′ | 24 |
| Reverse | 5′ ACTCCTGCTTGCTGATCCACATCT3′ | 24 |
Osteoblast Study
CS MPs or CS-10% CaHPO4 (60 mg) was weighed separately into each well in 24 well plates. MSCs were pre differentiated by culturing in osteogenic medium, containing α-MEM, 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 50 μg/ml L-ascorbic acid, 10 nm dexamethasone, 10 mM β-glycerol phosphate, for 7 days prior to seeding on MPs. After this culture period, the OBs were trypsinized and 200,000 osteoblasts/well were seeded on the MPs. Triplicates were used for statistical purpose. OBs seeded in wells without MPs were treated as negative controls. The cells were cultured in osteogenic medium at 0, 1, 2, and 7 days. At these time points the expression of TGF-β, BMP-2, BMP-6, BMP-7, alkaline phosphatase (ALP), collagen I (COLL-I), and osteocalcin (OCN) by the OBs were studied using quantitative real time RT-PCR. Table 1 lists the sequences of the various primers used in this study.
Co-culture of macrophages and OBs
MSCs were isolated from C57/BL6 mice and cultured for 10 days in α-MEM. After 10 days the cells were cultured in osteogenic medium for 7 days to allow osteogenic differentiation of the MSCs. The OBs were trypsinized and seeded onto 60 mg MPs, in triplicates, using 24 well plates. OBs (175,000) were seeded on either CS or CS-10% CaHPO4 MPs, in each well, and cultured using an osteogenic medium. The OBs were allowed to attach to the MPs for 2 days. Macrophages (25,000) were seeded onto the MPs/OBs culture at day 3. After 2 days the osteogenic medium was replaced with osteogenic medium containing 1 μg/ml LPS. The cells were cultured in medium containing LPS for 2 days, before being replaced with just osteogenic medium (day 0). The expression levels of TGF-β, BMP-2, BMP-6, BMP-7, ALP, COLL-I and OCN at 2, 4, and 7 days were analyzed using a quantitative real time RT-PCR.
Analysis by quantitative RT-PCR
At each time point, mRNA was isolated from the cells using a Qiagen RNeasy mini kit. The cells attached to the MPs were lysed by adding the RLT buffer directly to the 24 well plates. The supernatants were removed and mRNA was isolated according to the kit protocol. mRNA concentration at each time point was calculated from optical density (OD) 260 value. The mRNA isolated from the 1, 2 and 7 day time points was diluted to match the concentration of day 0 time point. Template RNA (4 μl) was mixed with 3 μl oligodT, in a 0.6 ml microtube. The tubes were incubated at 70°C for 5 min, and quickly chilled on ice. The mastermix (15 μl) containing nuclease free water, MMLV buffer, dNTP and reverse transcriptase (9:4:1:1 ratio) was added to each tube and incubated at room temperature for 5 min. The tubes were then placed at 42°C for 1 h, followed by 70°C for 15 min.
The DNA samples were amplified using lightcycler quantitative RT-PCR (Roche Diagnostics). Mastermix was prepared by mixing 4.75 μl of water, 0.9 μl of enzyme diluent, 1 μl of dNTP, 1 μl of 10X buffer, 0.2 μl of primers, 0.5 μl of syber green, and 0.1 μl of taq polymerase, for each sample. Mastermix (8 μl) was mixed with 2 μl of the template in a 20 μl light-cycler capillary. Beta-actin was used as the house keeping gene. The quantitative values of gene expression were calculated using the delta-delta method and the expression of the various genes were normalized to the control groups (No MPs group).
Statistical analysis
All experiments were performed in triplicates, unless and otherwise mentioned. The data in the graphs were reported as mean ± Standard Deviation. Minitab version 15 software was used to do statistical analysis. Two way ANOVA was performed using Tukey’s post-hoc test and p<0.05 was considered statistically significant.
RESULTS
Microparticle morphology
We prepared the CS MPs and CS-10%CaHPO4 using previously described scale-up method developed in our laboratory.27 The size and shape of MPs were examined using a SEM, and confirmed the spherical shape of both types of MPs. Figure 1 shows the SEM images of CS (A) and CS/10% CaHPO4 MPs (B). The both types of MPs were in a diameter range of 30–100 μm range.
Figure 1.
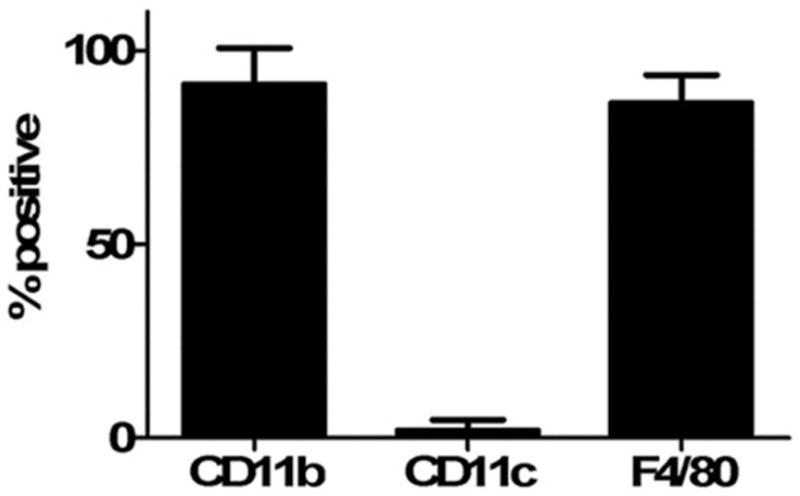
Identify macrophage phenotype
Macrophages were analyzed for the surface antigen expression such as CD11b, F4/80, and CD11c using a flow cytometry (Fig. 2). The phenotype is consistent with primary murine macrophages and is similar to the phenotypes obtained by murine macrophages expanded using MCSF or by thioglycollate elicitation from the peritoneum.31
Figure 2.
Macrophages attachment and spreading on CS MPs
We examined the macrophages attachment and spreading on CS MPs after 24 h culture period after treating with live/dead assay (Fig. 3). This result is an agreement with our previous results which has shown MSC/OB attachment and proliferation on CS based MPs.24,25
Figure 3.

Protein expression-macrophages
Figure 4 shows the TGF-β expression from the macrophages before and after activation on day 1, 4, 7, and 10 after measuring the TGF-β amounts in samples with ELISA. Significant TGF-β expression was observed LPS activated samples and control samples at day 10 (p<0.05).
Figure 4.
Gene expression-Macrophages
Before activation of macrophages
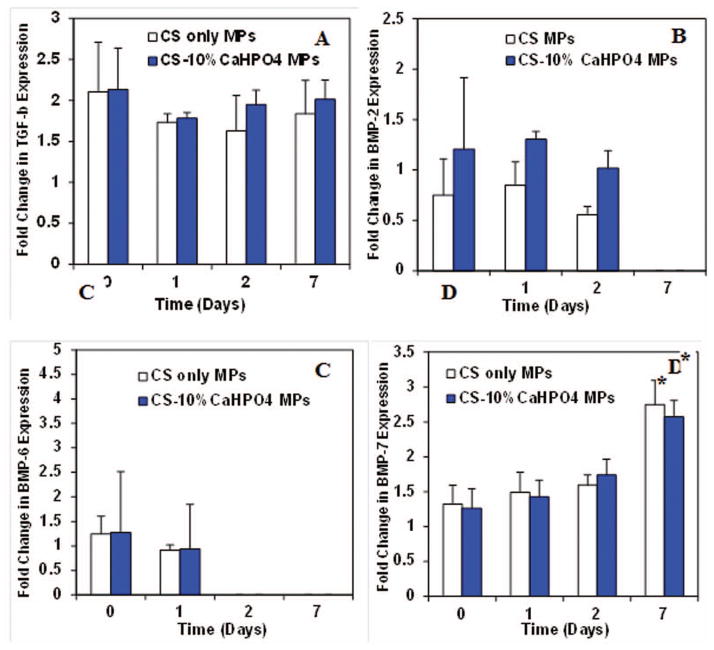
We examined the gene expression of TGF-β, BMP-2, BMP-6, and BMP-7 before LPS activation of macrophages (Fig. 5). The cells were seeded on two types of MPs and cultured in osteogenic medium. The gene expression data was normalized to the controls and plotted as a fold change over time. TGF-β was expressed at a constant rate by macrophages before activation on days 0, 1, 2 and 7 (Fig. 5A). No significant difference of TGF-β expression was observed among the time points or between the two types of MPs. Similarly, BMP-2 expression was seen at a constant rate at days 0, 1 and 2 and then dropped at day 7 (Fig. 5B). Similar to TGF-β, no significant difference was observed for BMP-2 expression among the time points and MP groups. BMP-6 was expressed only at day 0 and 1 exhibiting no significant difference between the times and MP groups (Fig. 5C). The expression of BMP-7 was constant at early time points, but the expression increased significantly at day 7 (p<0.05) (Fig. 5D). No significant difference in expression was observed between the different MP groups.
Figure 5.
After activation of macrophages
When the macrophages were activated using LPS, the TGF-β expression significantly increased at day 7 with respect to day 0 (p<0.05) (Fig. 6A). This is also a significant expression compared to the expression at day 7 before activation of macrophages (p<0.05). The LPS activation did not have a significant effect at day 0, 1, and 2 on TGF-β expression. Significant expression of TGF-β was not observed between the two MP groups. BMP-2 in activated macrophages was expressed at a constant rate at day 0, 1, and 2 (Fig. 6B). However, the expression significantly increased at day 7. Also, increased BMP-2 expression was seen for activated macrophages at day 7 compared to no expression of BMP-2 at day 7 before activation. LPS activation of macrophages completely abolished the expression of BMP-6 and BMP-7.
Figure 6.
Gene expression analysis-OBs
Expression of growth factors-OBs
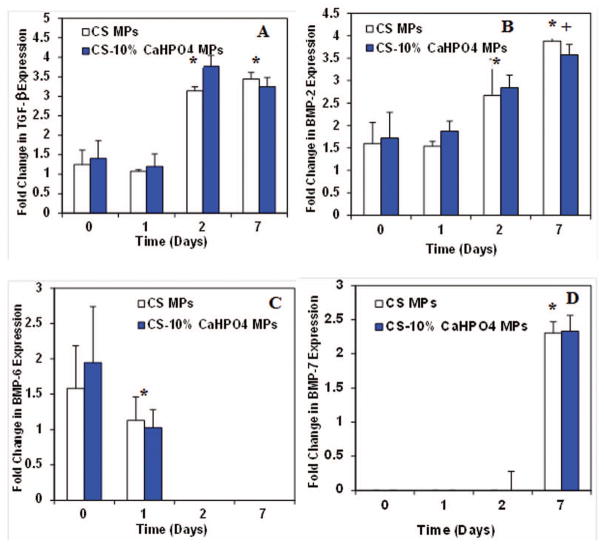
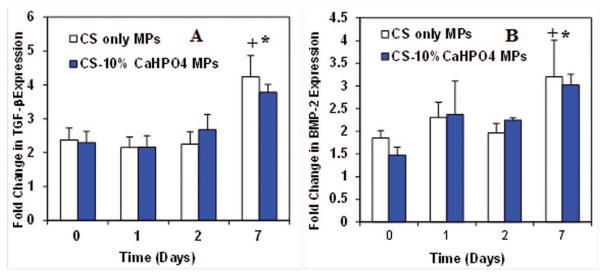
We analyzed the gene expression of TGF-β, BMP-2, BMP-6, and BMP-7 from the OB seeded CS and CS-10% CaHPO4 MP cultures, separately (Fig. 7). The TGF-β expression in OB seeded CS and CS-10% CaHPO4 MP cultures significantly increased at day 2 and day 7 compared to day 0 and day 1 (Fig. 7A). However, no significant difference in TGF-β expression was observed between day 0 and day 1. The BMP-2 expression significantly increased at day 2 for both types of cultures (Fig. 7B). Similar to TGF-β expression, no significant difference in BMP-2 expression was observed between day 0 and day 1. The BMP-2 expression for both cultures showed the significant increase at day 7 compared to day 2 expression. BMP-6 expression appeared between 1.5 and 2 fold change for both cultures at day 0. Next BMP-6 expression was significantly decreased at day 1 and no expression was seen at day 2 and 7 (Fig. 7C). High level of BMP-7 expression was noticed at day 7 with zero expression at earlier time points (Fig. 7D). No significant difference in TGF-β, BMP-2, BMP-6, and BMP-7 expressions were observed between the CS only MPs and the CS-10% CaHPO4 incorporated MPs at all time points.
Figure 7.
Expression of osteogenic markers-OBs
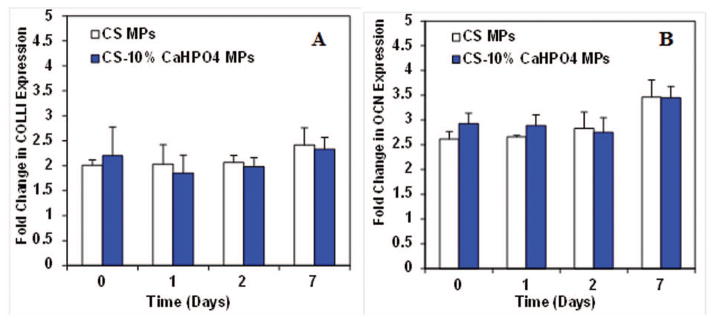
In addition to growth factor expression, we also analyzed osteogenic markers such as COLL-I, and OCN for OB seeded CS and CS-10% CaHPO4 MP separate cultures (Fig. 8). COLL-I was expressed at all time points at a constant rate with approximately 2 fold change (Fig. 8A). Similarly, OCN also expressed at a constant rate at days 0, 1, 2, and 7 (Fig. 8B) for both cultures. No significant difference in osteogenic markers was observed between the CS only MPs and the CS-10% CaHPO4 incorporated MPs at all time points.
Figure 8.
Gene expression analysis of co-cultures
Expression of growth factors - co-cultures
We co-cultured macrophages and OBs with a ratio of 1:8 on CS MPs and CS-10% CaHPO4 in an osteogenic medium containing LPS. We examined the TGF-β, BMP-2, BMP-6, and BMP-7 expressions from both of the co-cultures and compared the expressions to that of MP-OB cultures (Fig. 9). TGF-β expression was observed at each time for both cultures. Almost 2 fold increase in TGF-β expression was seen at day 7 for both cultures. However, no significant difference was seen in TGF-β expression at day 2 and day 4 (Fig. 9A). The TGF-β expression from both co-cultures at day 2 and day 7 was significantly lower than the expression from MP-OB cultures. BMP-2 was expressed at a constant rate at day 2 and day 4 for both co-cultures (Fig. 9B). However, more than 2 fold increase of BMP-2 from day 2 was seen at day 7. The expression of BMP-2 at day 7 was significantly higher compared to MP-OB cultures.
Figure 9.
No BMP-6 expression was seen in the co culture studies. This was similar to that of MP-OB cultures and activated macrophage-MP cultures. Although BMP-7 expression was not seen at day 2, the expression was up regulated at day 4 and remained at a constant rate until day 7 (Fig. 9C). BMP-7 expression at day 7 was significantly lower compared to the MP-OB cultures. No significant difference in TGF-β, BMP-2, and BMP-6 expression was seen between the two types of MP co-cultures.
Expression of osteogenic markers – Co-cultures
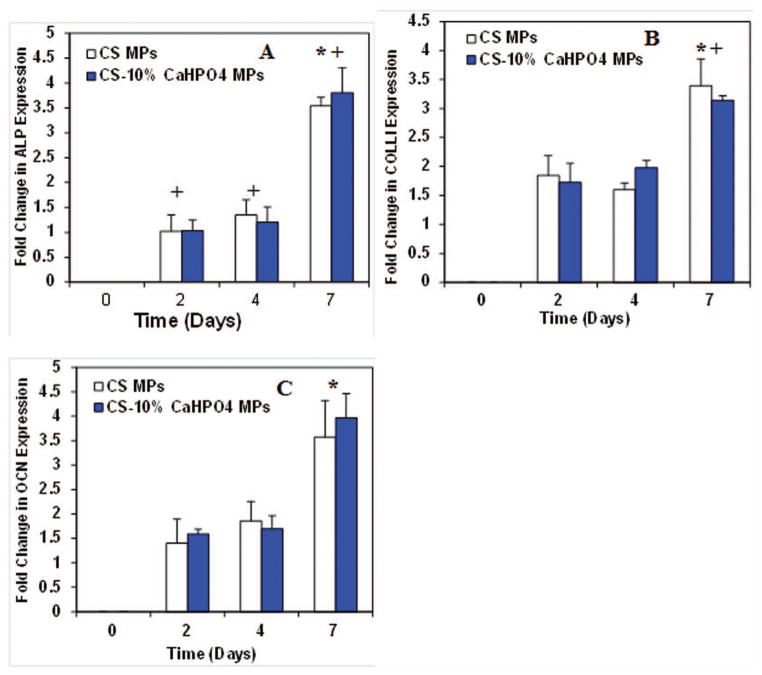
Similar to growth factors expression from co-cultures, the expression of ALP, COL-I, and OCN in co-cultures has been compared to the MP-OB cultures. Co-cultures showed ALP expression at days 2, 4, and 7 (Fig. 10A). Although no significant difference was seen at initial time points the expression was up regulated at day 7. Also, the expression of ALP at day 2, 4, and 7 was significantly higher when compared to MP-OB cultures.
Figure 10.
COLL-I expression in co-cultures showed no difference in expression at early time points, day 2 and day 4 (Fig. 10B). However, the expression was up regulated at day 7. The expression at day 7 was significantly higher than that of MP-OB cultures. The expression of OCN in co-cultures was seen at a constant rate at day 2 and day 4, but then increased significantly at day 7 (Fig. 10C). No significant difference in OCN expression was seen between co-cultures and MP-OB cultures at day 7. No significant difference in expression of ALP, COLLI, and OCN was seen between the two types of MP groups.
DISCUSSION
Bone trauma initiates a response from the tissue which mainly includes inflammation, repair, and remodeling.34,35 Although the mechanical and histological aspects of these stages have been well characterized the biochemical aspects need to be explored. It is currently unknown how macrophages play a role in bone healing. It is possible that the inflammatory response created at the fracture site may signal the macrophages to produce BMPs that eventually lead to bone healing.
In this unique study we tested the ability of secretion of growth factors related to bone healing from activated and non-activated macrophages. Our previous studies have shown that the rodent MSCs can be seeded onto our spherical shape hybrid MPs which can be used as a bone tissue construct to regenerate the bone.24,25 In this study we found that macrophages were also able to attach and spread on the hybrid MPs (Fig. 3).
One of the interesting finding of this study was that macrophages seeded on MPs expressed BMPs and TGF-β and before and after LPS activation. BMP-2, BMP-6, and BMP-7 and TGF-β, were expressed from the macrophages seeded on MPs before LPS activation (Fig. 5). While BMP-7 and TGF-β were expressed in all the time points, BMP-2 and BMP-6 were expressed in early time points before LPS activation (Fig. 5). Significantly higher level of BMP-2 and TGF-β were expressed at day 7 for activated macrophages seeded on MPs. The increase in BMP-2 expression in LPS activated macrophages is an important factor to mediate the bone regeneration in bone defects (Fig. 6B). Our results have shown that the expression of growth factors from macrophages was not dependent on the MP group.
Previous studies demonstrated up regulation on TGF-β in LPS activated macrophages.5 Our results also confirm that up regulation of TGF-β at day 10 compared to initial time punts (Fig. 4). Blocking of TGF-β expression in macrophages significantly reduced the alkaline phosphatase (ALP) expression in MSCs.36 Another study showed BMP-2 and BMP-6 expression in macrophages, both before and after activation.1 However, LPS activation completely abolished BMP-2 expression in macrophages and completely down regulated ALP expression.9 Our results have shown the up regulation of BMP-2 (Fig. 6B), deviation from this study which could be due to the interaction of the macrophages with the MPs. BMP-2 is a key regulator of bone morphogenesis and other organ systems during embryonic development.37–39 The extrinsic addition of growth factors to aid osteogenic differentiation has been documented previously.40,41
A recent study6 has shown that a discrete population of resident macrophages, OsteoMacs, was intercalated throughout murine and human osteal tissues. They found that removal of OsteoMacs from calvarial cultures significantly decreased osteocalcin mRNA induction and osteoblast mineralization in vitro. In addition, macrophages were required for efficient osteoblast mineralization in response to the physiological remodeling stimulus, elevated extracellular calcium.
Co-culture of macrophage-OB on MPs showed expression of ALP, COLL-I, and OCN at all times assessed (Fig. 10A, B, C). ALP, early marker for osteogenesis23 and COLL-1 levels were significantly increased at day 7 compared to the expression levels obtained from the osteoblasts only samples. OCN is a late marker specific to bone mineral matrix,42 thus it was not surprising to see any increase of OCN in the co-cultures. However, macrophages did not adversely affect the OCN levels in the co-cultures. These results suggest that co-culture of OBs and activated macrophages on MPs drives towards osteogenesis.
CONCLUSIONS
In this unique study, we investigated the gene expression of BMP-2, BMP-6, BMP-7 and TGF-β from macrophages-MP cultures before activation. When macrophages were activated by LPS, the gene expression of BMP-2 and TGF-β was upregulated. However, the gene expression of BMP-6 and BMP-7 was not observed with activated macrophages-MP cultures. This study revealed that macrophages have an ability to secrete the growth factors that are critical for bone healing while macrophages-MPs are in a tissue engineering setting. We also found that macrophages can be seeded and co-cultured onto bone tissue engineering constructs with OBs. MP/macrophage/OB cultures expressed upregulated markers for bone formation such as ALP and COL-I. These cultures also have shown gene expression of OCN. This investigation suggests that the macrophages have a great potential to enhance bone formation.
Acknowledgments
This work is supported by National Science Foundation (NSF) grant #0652024 and National Institute of Health (NIH) grant #DE019508.
References
- 1.Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoconductive signals that include bone morphogenic protein-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- 2.Casstella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 3.Cantini M, Carraro U. Macrophage released factor stimulates selectively myogenic cells in primary muscle culture. J Neutropathol Exp Neurol. 1995;54:121–128. doi: 10.1097/00005072-199501000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D. Blood borne macrophages are essential for triggering muscle regeneration following muscle transplant. Neuromuscular disorder. 1999;3:211–220. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 5.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181(2):1232–44. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 7.Heinemann DE, Siggelkow H, Ponce LM, Viereck V, Wiese KG, Peters JH. Alkaline phosphatase expression during monocyte differentiation. Overlapping markers as a link between monocytic cells, dendritic cells, osteoclasts and osteoblasts. Immunobiology. 2000;202:68–81. doi: 10.1016/S0171-2985(00)80054-6. [DOI] [PubMed] [Google Scholar]
- 8.Baur ST, Mai JJ, Dymecki SM. Combinatorial signaling through BMP receptor IB and GDF5: shaping of distal mouse linmb and the genetics of distal limb diversity. Development. 2000;127:605–619. doi: 10.1242/dev.127.3.605. [DOI] [PubMed] [Google Scholar]
- 9.Engstrand T, Daluiski A, Melhus H, Lyons KM. Transient production of bone morphogenic protein 2 by allagenic transplanted cells induces bone formation. Hum Gene Ther. 2000;11:205–210. doi: 10.1089/10430340050016274. [DOI] [PubMed] [Google Scholar]
- 10.Dean DB, Watson JT, Moed BR, Zhang Z. Role of bone morphogenetic proteins and their antagonists in healing of bone fracture. Front Biosci. 2009;14:2878–88. doi: 10.2741/3419. [DOI] [PubMed] [Google Scholar]
- 11.Tsiridis E, Upadhyay N, Giannoudis PV. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 13.Lazarus JJ, Kay MA, McCarter AL, Wooten RM. Viable Borrelia burgdorferi enhances interleukin-10 production and suppresses activation of murine macrophages. Infect Immun. 2008;76(3):1153–62. doi: 10.1128/IAI.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagtekin G, Schiffer R, Klein B, Jahnen-Dechent W. Modulation of angiogenic functions in human macrophages by biomaterials. Biomaterials. 2003;24:3395–3401. doi: 10.1016/s0142-9612(03)00201-1. [DOI] [PubMed] [Google Scholar]
- 15.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 16.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9(4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada Y, Wang JT, Doppalapudi VA, Willis AA, Jasty M, Harris WH, Nagase M, Goldring SR. Differential effects of different forms of hydroxyapatite and hydroxyapatite/tricalcium phosphate particulates on human monocyte/macrophages in vitro. J Biomed Mater Res. 1996;31(1):19–26. doi: 10.1002/(SICI)1097-4636(199605)31:1<19::AID-JBM3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Bran G, Straeter J, Hormann K. Apoptosis in bone for tissue engineering. Archives of medical research. 2008;39:467–482. doi: 10.1016/j.arcmed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Kato Y, Onishi H, Machida Y. Application of chitin and chitosan derivatives in the pharmaceutical field. Curr Pharm Biotechnol. 2003;4:303–309. doi: 10.2174/1389201033489748. [DOI] [PubMed] [Google Scholar]
- 20.Portero A, Remuñán-López C, Criado MT, Alonso MJ. Reacetylated chitosan microspheres for controlled delivery of antimicrobial agents to the gastric mucosa. J Microencap. 2002;19:797–809. doi: 10.1080/0265204021000022761. [DOI] [PubMed] [Google Scholar]
- 21.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26(30):5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 23.Zhao F, Yin Y, Lu WW. Preparation and evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002;23:3227–3234. doi: 10.1016/s0142-9612(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 24.Jayasuriya AC, Bhat A. Mesenchymal stem cell function on hybrid organic/inorganic microparticles in vitro. J Tissue Eng Regen Med. 2010;4(5):340–348. doi: 10.1002/term.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhat A, Dreifke M, Gomez C, Kandimalla Y, Ebraheim NA, Jayasuriya AC. Evaluation of cross-linked chitosan microparticles for bone regeneration. J Tissue Eng Regen Med. 2010;4(7):532–542. doi: 10.1002/term.270. [DOI] [PubMed] [Google Scholar]
- 26.Greenwald SA, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg. 2001;83-A:98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 27.Jayasuriya AC, Bhat A. Optimization of scaled-up Chitosan Microparticles for Bone Regeneration. Biomed Mater. 2009;4(5):55006. doi: 10.1088/1748-6041/4/5/055006. [DOI] [PubMed] [Google Scholar]
- 28.Jayasuriya AC, Bhat A. Fabrication and characterization of novel hybrid organic/inorganic microparticles to apply in bone regeneration. J Biomed Mater Res A. 2010;92:1280–1288. doi: 10.1002/jbm.a.32623. [DOI] [PubMed] [Google Scholar]
- 29.Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10 (2):165–81. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- 30.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63(8):1059–69. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 31.Brown JP, Zachary JF, Teuscher C, Weis JJ, Wooten RM. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rama R, Sánchez J. Transferrin uptake by bone marrow macrophages is independent of the degree of iron saturation. Br J Haematol. 1992;82(2):455–459. doi: 10.1111/j.1365-2141.1992.tb06444.x. [DOI] [PubMed] [Google Scholar]
- 33.Gessl A, Boltz-Nitulescu G, Wiltschke C, Holzinger C, Nemet H, Pernerstorfer T, Förster O. Expression of a binding structure for sialic acid-containing glycoconjugates on rat bone marrow-derived macrophages and its modulation by IFN, TNF-alpha, and dexamethasone. J Immunol. 1989;142(12):4372–4377. [PubMed] [Google Scholar]
- 34.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14(2):179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huges FJ, Collyer J, Stanfield M, Godmann SA. The effect of bone morphogenic protein 2, 4 and 6 on differentiation of rat osteoblast cell in vitro. Endocrinology. 1995;136:2671–2677. doi: 10.1210/endo.136.6.7750491. [DOI] [PubMed] [Google Scholar]
- 37.De Biase P, Capanna R. Clinical applications of BMPs. Injury. 2005;36 (Suppl 3):S43–46. doi: 10.1016/j.injury.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman HJThM. Bone Morphogenetic Proteins. Development and Clinical Efficacy in the Treatment of Fractures and Bone Defects. J Bone Joint Surg Am. 2005;87:1367–1378. doi: 10.2106/JBJS.D.02585. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Zhang M. Calcium phosphate/chitosan composite scaffold for controlled in vitro antibiotic drug release. J Biomed Mater Res. 2002;62:378–386. doi: 10.1002/jbm.10312. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical application. J Bone Joint Surg Am. 2002;84:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Granjeiro JM, Oliveira RC, Bustos-Valenzuela JC, Sogayar MC, Taga R. Bone morphogenetic proteins: from structure to clinical use. Braz J Med Biol Res. 2005;38(10):1463–1473. doi: 10.1590/s0100-879x2005001000003. [DOI] [PubMed] [Google Scholar]
- 42.Ishaug-Riley SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]