Abstract
Objective
During coagulation, factor IX (FIX) is activated by two distinct mechanisms mediated by the active proteases of either factors VII (FVIIa) or XI (FXIa). Both coagulation factors may contribute to thrombosis; factor XI, however, plays only a limited role in the arrest of bleeding. Therefore, therapeutic targeting of FXI may produce an antithrombotic effect with relatively low hemostatic risk.
Approach and Results
We have reported that reducing FXI levels with FXI antisense oligonucleotides (ASOs) produces antithrombotic activity in mice, and that administration of FXI ASOs to primates decreases circulating FXI levels and activity in a dose- and time-dependent manner. Here we evaluated the relationship between FXI plasma levels and thrombogenicity in an established baboon model of thrombosis and hemostasis. In previous studies with this model, antibody-induced inhibition of FXI produced potent antithrombotic effects. In the present report, ASO-mediated reduction of FXI plasma levels by ≥50% resulted in a demonstrable and sustained antithrombotic effect without an increased risk of bleeding.
Conclusion
These results indicate that reducing FXI levels using ASOs is a promising alternative to direct FXI inhibition, and that targeting FXI may be potentially safer than conventional antithrombotic therapies that can markedly impair primary hemostasis.
Keywords: Thrombus Formation, Antisense Oligonucleotide, Factor XI, Platelets, Vascular Graft
Thrombin generation is required for hemostasis at sites of vascular injury. This process is typically initiated when extravascular and/or subendothelial proteins, including tissue factor (TF) and collagen, are exposed to flowing blood, triggering platelet activation and formation of the TF/FVIIa complex. TF/FVIIa, in turn, activates FIX and FX that catalyze the production of thrombin. Thrombin clots fibrinogen and activates platelets and FXIII, contributing to the formation of a stable hemostatic plug 1, 2. In contrast to hemostatic mechanisms that cause the arrest of bleeding, thrombosis that can restrict or block normal blood flow occurs within the lumen of blood vessels in response to vascular injury, altered blood flow patterns (e.g., stenoses) and/or predisposing functional abnormalities of platelets and coagulation pathways. The coagulation protein FXI plays a limited role compared to FVIIa in hemostasis 3-6. Humans lacking FXI have a relatively mild disorder characterized by excessive trauma-induced hemorrhage in tissues with high fibrinolytic activity 3. However, several recent studies in rodents and non-human primates suggest that FXI contributes significantly to thrombosis 7, 8. Furthermore, humans with increased levels of FXI are at an increased risk for venous thrombosis, myocardial infarction 9, 10, and stroke 9-11, whereas patients with severe FXI deficiency have a reduced incidence of ischemic stroke 12 and deep-vein thrombosis 13, 14. The observations that FXI plays a significant role in thrombosis, with only a modest contribution to hemostasis, makes it an attractive target for pharmacological anticoagulant therapy 3, 15, 16.
Several studies have supported the concept that inhibition of FXI might reduce thrombus formation without a significant risk of increased bleeding. FXI deficient mice are viable without obvious hemostatic abnormalities 7. FXI deficiency protects mice from experimental thrombosis, including ferric chloride (FeCl3)-induced vessel occlusion in both carotid artery 7, 17 and inferior vena cava thrombosis models 18. Antibodies that inhibit FXIa activity, or prevent FXI activation by FXII, potently inhibit experimental thrombosis in rodent, rabbit and primate models 8, 15, 16, 19. More recently, we described a novel therapeutic approach to targeting FXI in which antisense oligonucleotides (ASOs) were used to selectively inhibit FXI mRNA expression, leading to a corresponding reduction of plasma FXI protein and activity. Treatment of mice with FXI ASOs produced potent, dose-dependent antithrombotic effects in arterial and venous thrombosis models without increased bleeding 20. Furthermore, combining FXI inhibition with conventional anticoagulants and anti-platelet therapies (enoxaparin and clopidogrel) improved antithrombotic efficacy without an increase in bleeding tendency. These studies demonstrate that a significant antithrombotic benefit can be achieved through FXI inhibition, and that complete depletion of FXI is not required for this benefit in rodent models of thrombosis. The concept of selective inhibition of FXI with antisense technology has recently been extended to humans, where subcutaneous (weekly) administration of the FXI ASO ISIS-FXIRX resulted in dose-dependent reductions in circulating FXI protein and activity, with a corresponding elevation in activated partial thromboplastin time (aPTT), but without any effect on prothrombin times (PT) 21. Since a reduced risk of thrombosis in humans has only been demonstrated in patients with severe FXI deficiency, the present study in primates was undertaken to define FXI ASO dose-response relationships for levels of anticoagulation and antithrombotic activity that might ultimately be achieved in clinical applications in humans. Accordingly, we assessed the antithrombotic activity and safety of titrated inhibition of FXI in a well-characterized baboon model of thrombosis and hemostasis.
Materials and methods
Materials and methods Online Supplement (unedited) at: Link (http://atvb.ahajournals.org..............?)
Results
Inhibition of FXI with aXIMab: Effects on thrombosis in the baboon model
Previously, a monoclonal antibody targeting FXI (aXIMab), given at a dose of 2mg/kg, was shown to reduce levels of plasma FXI activity in baboons by 99% 15. In order to estimate the minimum level of FXI inhibition necessary to achieve measurable anti-thrombotic activity in the baboon thrombosis model, aXIMab was administered intravenously to baboons at increasing doses that achieved graded levels of FXI inhibition. At the various levels of FXI which were observed after aXIMab treatment, propagated thrombus formation was assessed in each case as the accumulation of 111In-platelets within the 10 cm-long region of the AV shunt that was immediately distal to the 2 cm-long collagen-coated graft segment (designated the “tail” of the graft thrombus; Figure 1A). As shown in Figure 2A, 50% inhibition of FXI plasma activity resulted in ~50% inhibition of thrombus propagation after 60 minutes of blood flow. Measurable platelet accumulation was detected as early as 20 minutes after graft blood exposure, and a significant difference between the control and aXIMab treated groups was apparent by 30 minutes (Figure 2B). In the control group not given aXIMab, and the group in which aXIMab produced less than 40% inhibition of FXI, measurements of platelet accumulation in propagated thrombus were similar and averaged ~4 × 109 platelets deposited by 60 minutes (Figure 2B). Inhibition of FXI levels to 45%-75% of baseline values resulted in ≥ 50% inhibition of platelet deposition (~1.4 × 109 platelets deposited by 60 minutes), and greater than 80% FXI inhibition resulted in almost complete abolition of thrombosis (~0.2 × 109 platelets deposited by 60 minutes) compared to the control results (Figure 2B). These effects were specific for FXI as FXII activity was not affected when FXI levels were reduced with the aXIMab antibody (data not shown).
Figure 1.
Diagram of the baboon AV shunt thrombosis model showing interposition of a 2.0 cm-long, 4.0 mm i.d., thrombogenic vascular graft segment (A), and representative gamma camera images of an acutely developing thrombus within the thrombogenic segment, measured in a baboon with normal FXI levels (B). Circulating 111Indium-labeled platelets accumulate at sites of thrombus formation. Platelet deposition on the collagen segment (i.e., accumulated 111Indium-activity that is measurable above circulating background levels) appears within 10 minutes after restoring blood flow through the shunt. Images obtained at later times (30 min, 60 min) show propagation of the thrombus into the distal shunt tubing. Images were obtained in real time and acquired at 5 minute intervals. The blood flow rate was 100 mL/min (initial wall shear rate: 265/sec).
Figure 2.
An intravenous injection of aXIMab (doses ranging from 40-70 ug/kg) was given to 4 baboons and blood samples were collected into citrate anticoagulant. Inhibition of FXI plasma activity by ~50% produced ~50% inhibition of platelet deposition in propagating thrombus, as assessed from gamma camera images taken at the study endpoint, 60 mins after the intiation of blood flow (A), or by dynamic imaging of platelet deposition over the entire 0-60 min study interval (B).
Selective inhibition of baboon FXI with ASOs
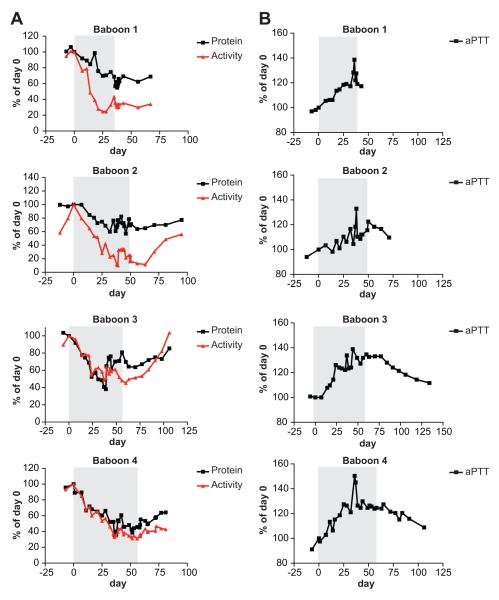
The cynomolgus monkey/human cross-reactive FXI ASOs contained two mismatched bases compared to the baboon FXI mRNA sequence, and would therefore not have been expected to be active in this species. These mismatches were converted to the baboon sequences, and three baboon-specific FXI ASOs were evaluated for in vivo activity in baboons. The most potent of these baboon-specific FXI ASOs was then used to characterize the relationship between FXI plasma levels and anti-thrombotic activity in the baboon thrombosis model. The ASO was administered to a cohort of 4 baboons at a dose of 25mg/kg, given three times per week. The dosing intervals in each case are indicated by the shaded regions in Figure 3. FXI protein and activity levels were measured over the course of each experiment. Following treatment, both FXI levels and FXI plasma activities were reduced in the 4 study animals, with similar kinetics. Inhibition of FXI plasma activity by 50% was achieved in all animals by day 25, reaching maximum inhibition (~70%) towards the end of each infusion period (Figure 3A). FXI protein levels were similarly reduced (~50%) during the infusions. After dosing was discontinued, both FXI protein and activity levels gradually increased over several months time. The prolonged reduction of FXI activity/protein is a function of the long tissue half life of ASO in liver. FXI ASO treatment in Cynomolgus monkey 22 produced a substantial reduction in FXI mRNA in liver following cessation of ASO dosing, which correlated with the long tissue half life of the ASO (~3 weeks) and with FXI antigen reduction and eventual recovery. Since baboons were not sacrificed in this study, we do not have data on RNA reduction. However, we expect results to be similar to our published results in Cynomolgus monkey.
Figure 3.
FXI protein and activity measurements. Four baboons were given FXI ASO subcutaneously, 3 times per week, at a dose of 25 mg/kg. After dosing for various lengths of time (shaded areas, days 39, 49, 60, 53), FXI plasma protein levels and activity (A) and aPTT levels (B) were measured. Inhibition of FXI plasma activity by 50% was achieved in all animals by day 25, and reached maximum inhibition (~80%) by day 35.
ASO inhibition of FXI activity correlated with effects on a functional coagulation parameter, the aPTT. The aPTT measurements increased over the course of ASO treatment, and corresponded well with the decrease in FXI protein and activity levels (Figure 3B). When ASO administration was discontinued, elevated aPTT values gradually returned towards normal levels within several months. As expected for an inhibitor of the intrinsic pathway, no changes in PT values were observed following administration of FXI ASOs (data not shown).
Protection of FXI ASO treated baboons from collagen-initiated thrombus propagation
Since administration of FXI ASOs resulted in time-dependent reductions in both FXI protein and FXI activity in the blood of treated baboons (Figure 3A), the capacity of ASO-mediated inhibition of FXI to reduce thrombus formation was subsequently evaluated in the baboon thrombosis model. In this model, it has been shown that thrombus that forms on the collagen-coated graft segment is platelet-rich and relatively insensitive to inhibition by conventional anticoagulants such as heparin. While potent coagulation protease inhibitors such as PPACK and hirudin can block thrombus formation on collagen in this model, these and other inhibitors of thrombin activity, or thrombin formation, can also produce severe bleeding 23. Consistent with these findings, little effect on thrombus accumulation on the collagen-coated grafts was seen following either administration of aXIMab at a dose that inhibited plasma FXI activity by <80%, or administration of FXI ASOs that produced marked reductions in plasma FXI levels (Figure 4A). When the 60 minute endpoint results (Figure 4A) were combined with results taken in six additional control animals, the levels of platelet accumulation in the treated group (2.77 ± 0.11 × 109 platelets deposited at 60 minutes) were not different vs. the results found in the control group (2.67 ± 0.24 × 109 platelets deposited, p > 0.5).
Figure 4.
Effect of FXI ASO-mediated inhibition on thrombus formation in individual study animals. Platelet deposition was quantitated within the components of the thrombogenic device that was inserted into AV shunts in baboons, including (A) the proximal, 2 cm-long, collagen-coated graft segment (designated the “head” of thrombus in Figure 1), and (B) the 10 cm-long shunt segment containing thrombus that propagated immediately distal to the collagen segment (designated the “tail” of thrombus in Figure 1). FXI depletion with FXI ASO had little effect on thrombus formation on the collagen segment (panel A). In contrast, propagated thrombus, which is dependent on upstream thrombin generation, was reduced by ASO depletion of FXI (panel B).
In contrast, the formation of fibrin-rich thrombus that propagates downstream from the collagen-graft segment (i.e., the thrombus “tail” in Figure 1) is triggered by upstream thrombin generation and is sensitively inhibited by anticoagulants such as heparin 16, low molecular weight heparin (enoxaparin, 1 mg/kg IV), or by a saturating dose of aXIMab (≥ 0.1mg/kg IV) that inhibits >99% of FXI procoagulant activity 15. In the present study, inhibition of FXI activity by >80% using aXIMab produced near complete inhibition of propagated thrombus (Figure 2). In accord with this finding, FXI ASO treatment also reduced platelet accumulation in propagated thrombus significantly (Figure 4B). As shown in Figure 5, when the endpoint results for the four treated animals (Figure 4B) were combined with the results from the six additional control studies, propagated thrombus accumulation after 60 minutes averaged 2.40 ± 0.33 × 109 platelets deposited in the ASO-treated animals, a value that was reduced by 41% vs. the control group results (4.06 ± 0.83 × 109 platelets deposited; p = 0.037 vs. the ASO-treated group). Interestingly, while a clear reduction of propagated thrombus formation was observed in three baboons (Figure 4B), little effect was seen in the fourth study animal (baboon #1). This result is most likely explained by the unusually low control values for platelet deposition on both collagen-induced and propagated thrombus, as discussed subsequently. Nonetheless, the overall results document a significant benefit of ASO therapy for inhibition of propagated thrombus formation, and are consistent with earlier studies, and the present results with the anti-FXI monoclonal antibody (aXIMab), showing that propagated thrombus (i.e., venous-type, fibrin-rich thrombus) is sensitively inhibited by anticoagulants, while collagen-induced thrombus (i.e., platelet-rich, arterial-type thrombus) is resistant to inhibition by anticoagulants (but may be inhibited by antiplatelet agents).
Figure 5.

Effect of ASO-mediated inhibition of FXI on thrombus formation in baboons. After 60 minutes of blood exposure, platelet accumulation in propagated thrombus in the four ASO-treated animals averaged 2.40 ± 0.33 × 109 platelets deposited (n = 18; Figure 4B), a value that was reduced significantly (by 41%; p = 0.037) vs. the control group results (4.06 ± 0.83 × 109 platelets deposited; n = 10). The control values included measurements in the four study animals that were taken before ASO treatment (data in Figure 4B), plus results in six additional untreated control animals.
The measurements of platelet deposition in propagated thrombus, taken over a wide range of FXI levels, showed a good correlation between antithrombotic efficacy and reduced FXI levels (Figure 6A). These data suggest that titrated inhibition of FXI can achieve graded and potent antithrombotic activity in a non-human primates, and that the minimally efficacious level of FXI inhibition is approximately 50% vs. baseline values as shown by results with both the FXI antibody (Figure 2) and FXI ASO (Figure 6A).
Figure 6.
Samples taken from FXI ASO treated animals were evaluated for their ability to generate thrombin upon stimulation with small amounts of TF. As plasma levels of FXI were reduced by ASO treatment, thrombus propagation (A), and peak thrombin generation (PTG) levels (B) were also reduced. When FXI plasma levels were inhibited by > 50%, thrombin generation was virtually abolished.
Reduced thrombin generation in FXI ASO treated baboons
Conceptually, FXI inhibition could reduce thrombus formation in vivo by limiting thrombin production (and thrombin-mediated platelet activation) through the FXI-dependent amplification pathway 15, and/or by increasing thrombolysis by limiting FXI-dependent TAFI activation 24. Previously, we showed that the antithrombotic activity of aXIMab was associated with potent inhibition of TAT (Thrombin-Antithrombin complex) generation and fibrin accumulation, but not with reduced D-dimer levels, suggesting that the antithrombotic effects of FXI inhibition were principally due to reduced platelet activation and fibrin formation 15. To assess the effects on thrombin generation of ASO-mediated reduction of FXI in baboons, an ex vivo thrombin generation assay (TGA) was used. Samples taken from FXI ASO-treated animals were evaluated for their ability to generate thrombin upon stimulation with small amounts of TF (Figure 6B). In order to exclude interference due to contact system activation, samples were pretreated with a ten-fold molar excess of CTI (corn trypsin inhibitor) with respect to FXII levels. At normal levels of FXI, thrombin generation (TPG) reached a peak of ~35nM. After 2 weeks of treatment with ASO, FXI plasma levels were reduced by 30%, and there was a significant delay in thrombin generation as well as a dramatic reduction in TPG (60-75%). When FXI plasma levels were reduced to <50% of normal, thrombin generation was virtually abolished (Figure 6C). Thus, when limiting amounts of tissue factor are used to trigger clotting, thrombin generation is sensitive to the plasma levels of FXI. These findings indicate that the potent antithrombotic effect in baboons of FXI depletion by ASO is mediated through an initial inhibition of thrombin generation, with subsequent reductions in platelet activation and fibrin mesh formation. In addition, these data demonstrate that even partial inhibition of FXI (~50%) can have a dramatic impact on thrombin generation in primates.
Treatment with FXI ASO does not prolong the bleeding time in baboons
Previously it was demonstrated that reducing functional FXI levels with aXIMab did not prolong the bleeding time in baboons, while pretreatment with a single dose of aspirin nearly doubled the bleeding time 15. Various other anti-platelet agents and anticoagulants have also been demonstrated to increase the bleeding time in baboons 25. When the safety of FXI ASOs with respect to bleeding was investigated in cynomolgus monkeys, the bleeding time in both naïve and FXI ASO-treated animals was ~2 min 22. In an enoxaparin treated group (2 mg/kg) the bleeding time was ~10 min 22. In the present study, the safety of FXI inhibition with ASO treatment was also measured using the same method (duration of bleeding following a standardized skin incision). The results are given in Figure 7. Multiple bleeding time measurements were taken in each of the 4 baboons studied, beginning at least 2 weeks after administration of the ASOs, at which time the levels of FXI antigen and activity were reduced and aPTT measurements were prolonged (Figure 3). Bleeding time measurements in the control group and in the four ASO-treated animals were similar (~4 min; Figure 7). Overall, 25 measurements were performed in nine naïve baboons and averaged 3.9 ± 0.3 min. In the four ASO-treated animals, a total of 21 measurements were performed after at least two weeks of ASO administration and averaged 4.0 ± 0.3 min (p > 0.5 vs. controls).
Figure 7.
FXI ASO treatment did not impair hemostasis in baboons. A total of 25 bleeding time measurements were performed in 9 untreated baboons, and averaged (3.9 ± 0.3 minutes). Twenty-one measurements were taken in 4 treated baboons beginning at least two weeks after FXI ASO administration had begun and averaged 4.0 ± 0.3 min (p > 0.5 vs. controls). Average bleeding time measurements in each of the four ASO treatment animals were also similar (~4 minutes).
Discussion
In mouse models of thrombosis, antithrombotic activity was observed when plasma FXI levels were reduced by ~80% or more 20. In cynomolgus monkeys, a 25-30% reduction in plasma FXI activity by ASOs resulted in significant elevations of the aPTT (by 10-17%), suggesting that even modest reductions in FXI may be therapeutically relevant 22. Since appropriate cynomolgus monkey models were not available to test this hypothesis, a well-characterized baboon model of thrombosis and hemostasis was employed. Previously, significant antithrombotic activity was demonstrated with the FXI monoclonal antibody aXIMab; however, the single dose level of aXIMab that was evaluated produced nearly complete elimination of FXI activity from plasma, making it difficult to ascertain the antithrombotic threshold for FXI inhibition 15. Therefore, the current study was performed to determine the minimum level of plasma FXI inhibition necessary to document an antithrombotic effect in primates, thereby helping guide dose selection for possible FXI inhibition therapies in humans. Dose titration of aXIMab demonstrated that a reduction of ~50% in FXI protein levels resulted in an ~50% inhibition of fibrin-rich thrombus that propagated distal to a collagen-coated segment of vascular graft, while > 80% reduction of FXI nearly abolished propagated thrombus formation. Similarly, following ASO-mediated inhibition of FXI, a reasonably good correlation was found between reduced FXI plasma levels and inhibition of propagated thrombus formation (Figure 6A). Similar to the results with aXIMab, ASO administrations that reduced FXI levels by ~50% produced 40-50% inhibition of thrombus propagation. Since the ASO dose regimen used here (25 mg/kg, 3 times/week) reduced FXI levels by ~50%, it is likely that even greater greater inhibition of FXI-dependent thrombus propagation (>50%) could be achieved by ASO regimens that would further reduce FXI levels, a possibility consistent with an earlier study in which near complete inhibition of FXI activity by aXIMab produced potent antithrombotic effects 15.
Antisense technology was employed to selectively reduce the level of the plasma coagulation protein factor XI and to evaluate this strategy for the treatment of thromboembolic disease. Potent second generation ASOs (2′-Methoxy Ethyl modified, MOE Gapmer ASOs, for review see 26) were used in the study. Antisense technology takes advantage of base-pair hybridization of the oligonucleotide with its complementary sequence in the target mRNA 20. Binding results in the selective and catalytic degradation of the targeted mRNA by a mechanism involving the nuclease RNAse H 26-28 and leads to a corresponding selective reduction in target protein level [20-27].
Second generation ASOs are an attractive drug class to target coagulation factors for several reasons. The technology allows for the rapid identification of highly selective inhibitors based on the linear sequence information of the targeted mRNA sequence, which are well characterized for coagulation factors. Due to prolonged tissue elimination half-lives, second generation ASOs can be administered by relatively infrequent subcutaneous injections (once weekly or even less often), regimens that are convenient for patients 28. Compared to other anticoagulant modalities, including small molecule inhibitors and the natural product anticoagulants such as warfarin, antisense inhibitors offer a high degree of target selectivity, which should confer an additional measure of safety vs. less selective pharmacologic agents. Finally, FXI is synthesized primarily in the liver, which is one of the most sensitive tissues for targeting with ASO therapy 20, 27, 29.
One potential issue with ASOs is their relatively slow onset of action that arises due to the need for accumulation in target tissues (liver). However, some clinical conditions requiring anticoagulant therapy are chronic, and therefore the onset of action is of less concern. In such cases, ASOs can potentially be self-administered at home by weekly to twice monthly subcutaneous injection. While less convenient than oral drug administration, for serious chronic diseases such a regimen appears quite acceptable 30. Several second generation ASOs are currently in clinical development and have demonstrated significant therapeutic activity and safety in multiple disease indications 20, 27, 28. With FXI ASO having a slow onset of action but being long acting, this drug may be ideal for prevention purposes, including prevention of stroke in patients with atrial fibrillation, and prevention of adverse cardiovascular events in patients with coronary artery disease. In addition, this drug may be useful for preventing clots in patients at risk for venous thromboembolic disease. Towards this end, the human FXI antisense drug, FXI ASO ISIS-FXIRx, has recently demonstrated robust activity in humans. FXI antigen and activity levels were safely reduced by >95% in a phase I study involving healthy volunteers, with no evidence of bleeding 21.
FXI is a component of the classic intrinsic pathway of blood coagulation, which has been shown to contribute to thrombin generation and clot formation in both arterial and venous models of thrombosis. We previously demonstrated that targeting FXI expression with ASO technology in mice resulted in a highly specific, dose-dependent reduction of FXI mRNA in the liver and FXI protein in plasma 20. In several venous and arterial thrombosis models, the ASOs exhibited anti-thrombotic effects that were comparable in magnitude to those of warfarin and enoxaparin. However, unlike treatment with warfarin and enoxaparin, FXI ASO treatment did not result in increased bleeding. Combining FXI ASOs with conventional anti-platelet drugs such as clopidogrel, or anticoagulants such as enoxaparin, might further enhance antithrombotic potency without potentiating the bleeding risk commonly associated with such drugs 20.
In baboons, a single administration of aXIMab led to rapid inhibition of plasma FXI levels (~40% by 60 minutes) and an increase in total FXI plasma protein levels circulating in complex with the antibody, reaching ~300% of control values by day 8. Recovery of FXI procoagulant activity to baseline levels was delayed for more than 3 weeks 15. FXI protein levels continued to decrease over the period of ASO dosing and after cessation of ASO dosing. FXI protein levels and activity subsequently recovered over time but never surpassed 100% of baseline levels. Thus, no rebound effect occurred following FXI inhibition by ASOs. Similarly, FXI reduction by ASOs in mice was not associated with a rebound increase in FXI protein or activity levels 20. The absence of a rebound of functional FXI in multiple species following FXI inhibition is important since it suggests that the risk of an induced prothrombotic state after ASO cessation is minimal. It is not clear at this time whether the elevated factor XI antigen levels seen in the antibody-treated baboons represents increased factor XI synthesis, or whether the clearance of antibody-associated FXI is prolonged vs. the normal plasma half-life of factor XI (~48 hours in humans).
Surprisingly, in one of four animals studied, ASO therapy did not reduce platelet thrombus propagation as expected (baboon #1; Figure 4B). In reviewing these data it was noted that platelet deposition onto the proximal collagen-coated graft segment in this study was unusually low (Figure 4A); in fact, the apparent effect of ASO in this animal was to increase platelet thrombus deposition on collagen. Such an effect seems highly unlikely both conceptually 4, and in view of the other results reported here. This finding is also not in accord with previous studies documenting that anticoagulants have invariably inhibited thrombosis in this model 23, 25, and that FXI inhibition by monoclonal antibodies (aXIMab) and FXI ASOs has consistently reduced thrombus formation in this and other thrombosis models 8, 15-20. Moreover, FXI levels were markedly reduced in this study animal to levels comparable to those seen in the other three ASO-treated animals. It is more likely, therefore, that the control data for baboon #1 (Figure 4A, 4B) were artifactually reduced. Indeed, in this animal platelet deposition on collagen averaged only 1.7 × 109 platelets, a value well below the other results reported here (Figure 4A), and elsewhere for this model (average range: 2.8-3.7 × 109 platelets deposited) 8, 15, 31. Since platelet deposition on collagen was reduced vs. expected and historical values, it is therefore likely that distal thrombus propagation, which is triggered by proximal thrombus formation on the collagen surface, was reduced as well. The reason for this aberrant finding is unclear, but may have been related to the paired experimental design, which necessitated for each ASO-treated animal that approximately 5 weeks elapse between the performance the control study (before ASO treatment) and the measurements of thrombus formation (after FXI reduction by ASO), during which time the animal’s hemostatic baseline may have changed. Despite this unusual finding, inclusion of all animal data in the final analysis documented a significant reduction (by 41%) in thrombus propagation by ASO therapy vs. the control results (p = 0.037). Moreover, since the data from baboon #1 must be considered questionable in terms of observed antithrombotic benefit, the overall benefit of ASO therapy reported here (average reduction of thrombus by 41%) should probably be considered a minimum, rather than maximum, estimate.
The safety of FXI ASO therapy has been evaluated in cynomolgus monkeys 22, and in a human phase 1 clinical trial as noted above 21. Reductions of FXI by more than 80% in cynomolgus monkeys did not cause increased bleeding after surgical or other mechanical injuries, including partial tail amputation and gum and skin laceration 22. Similarly, baboons treated with anti-FXI antibody or ASOs in the present study did not exhibit increased bleeding during surgical procedures for AV shunt placement or removal. Moreover, in healthy human volunteers, ISIS-FXIRX reduced FXI plasma activity by >90% with no reported drug-related bleeding 21. These findings are consistent with observations that spontaneous bleeding (with the exception of menorrhagia) is rare in patients with severe FXI deficiency. Although FXI inhibition does appear to be safe with respect to bleeding, the prolonged tissue half-life of antisense drugs makes it necessary to have strategies available that can reverse the effect of the ASO during a bleeding episode, or when surgery or other interventional cardiovascular procedures are required on an emergency basis. Since ASOs reduce circulating levels of FXI, and do not produce a direct inhibitory effect on the circulating protein, replacement with FXI concentrate rapidly reverses the anticoagulant effect of FXI ASO treatment in mice 20. Similarly, simple protein replacement using plasma should quickly reverse any hemostatic defect produced by reduced FXI levels following ASO therapy.
In summary, this study demonstrates that selective inhibition of FXI can be achieved in cynomolgus monkeys and in baboons, without an increased risk of bleeding. In baboons treated with both aXIMab and FXI ASOs, an antithrombotic effect was achieved with only modest reductions in plasma FXI levels. These results suggest that FXI ASOs represent an attractive therapeutic strategy based on their potentcy, selectivity, and favorable risk/benefit profile.
Supplementary Material
Significance.
FXI is a coagulation protein which may play a role in thrombosis with a more limited role in hemostasis. Finding new treatments to prevent thrombosis while limiting bleeding has important public health significance. This study demonstrates the successful use of antisense oligonucleotides to reduce FXI levels in a well-characterized baboon model of thrombosis and hemostasis. Our findings that reductions in FXI levels ≥ 50% results in a demonstrable and sustained antithrombotic effect without increasing the risk of bleeding will be useful for determining the minimal level of FXI inhibition necessary for clinical development of FXI targeting agents.
Acknowledgement
We gratefully acknowledge Tracy Reigle for her work on the manuscript figures.
Sources of Funding: Supplemental support was provided by NIH grants RO1HL095471 (SRH), RR000163 (to ONPRC) and R43HL106919 to Aronora, Inc. Authorship: BPM, JRC, AG and SRH designed the research. JRC, ARM, ASR, AG, DG, SRH and BPM contributed to writing the manuscript. UM, JRC, ASR, CZ, DG, AM, EIT performed research and analyzed data.
Footnotes
Disclosures: Ownership interest: JRC, ASR, CZ, DG, ARM and BPM are employees of ISIS Pharmaceuticals. UM, AM, DG, EIT, AG and SRH have no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 3.Asakai R, Chung DW, Davie EW, Seligsohn U. Factor xi deficiency in ashkenazi jews in israel. New England Journal of Medicine. 1991;325:153–158. doi: 10.1056/NEJM199107183250303. [DOI] [PubMed] [Google Scholar]
- 4.Gailani D, Renne T. The intrinsic pathway of coagulation: A target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 5.Renne T, Gailani D. Role of factor xii in hemostasis and thrombosis: Clinical implications. Expert Rev Cardiovasc Ther. 2007;5:733–741. doi: 10.1586/14779072.5.4.733. [DOI] [PubMed] [Google Scholar]
- 6.Girolami A, Candeo N, De Marinis GB, Bonamigo E, Girolami B. Comparative incidence of thrombosis in reported cases of deficiencies of factors of the contact phase of blood coagulation. J Thromb Thrombolysis. 2011;31:57–63. doi: 10.1007/s11239-010-0495-z. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Gailani D, Castellino FJ. Fxi is essential for thrombus formation following fecl3-induced injury of the carotid artery in the mouse. Thrombosis and Haemostasis. 2002;87:774–776. [PubMed] [Google Scholar]
- 8.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renne T, Gruber A, Gailani D. A role for factor xiia-mediated factor xi activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor xi as a risk factor for venous thrombosis. New England Journal of Medicine. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 10.Yang DT, Flanders MM, Kim H, Rodgers GM. Elevated factor xi activity levels are associated with an increased odds ratio for cerebrovascular events. American Journal of Clinical Pathology. 2006;126:411–415. doi: 10.1309/QC259F09UNMKVP0R. [DOI] [PubMed] [Google Scholar]
- 11.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors xi and xii. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 12.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor xi deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 13.Salomon O, Apter S, Shaham D, Hiller N, Bar-Ziv J, Itzchak Y, Gitel S, Rosenberg N, Strauss S, Kaufman N, Seligsohn U. Risk factors associated with postpartum ovarian vein thrombosis. Thrombosis and Haemostasis. 1999;82:1015–1019. [PubMed] [Google Scholar]
- 14.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor xi deficiency have a reduced incidence of deep-vein thrombosis. Thrombosis and Haemostasis. 2011;105:269–273. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- 15.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor xi. Blood. 2009;113:936–944. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruber A, Hanson SR. Factor xi-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor ix or factor xi deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Smith PL, Hsu MY, Gailani D, Schumacher WA, Ogletree ML, Seiffert DA. Effects of factor xi deficiency on ferric chloride-induced vena cava thrombosis in mice. J Thromb Haemost. 2006;4:1982–1988. doi: 10.1111/j.1538-7836.2006.02093.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita A, Nishihira K, Kitazawa T, Yoshihashi K, Soeda T, Esaki K, Imamura T, Hattori K, Asada Y. Factor xi contributes to thrombus propagation on injured neointima of the rabbit iliac artery. J Thromb Haemost. 2006;4:1496–1501. doi: 10.1111/j.1538-7836.2006.01973.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Lowenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, Black C, Revenko AS, Meijers JC, Stroes ES, Levi M, Monia BP. Inhibition of the intrinsic coagulation pathway factor xi by antisense oligonucleotides: A novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–4692. doi: 10.1182/blood-2010-04-277798. [DOI] [PubMed] [Google Scholar]
- 21.Que Liu ED, Claudette Bethune, Shuting Xia, John Grundy, Brett P, Monia, Richard Geary, Sanjay Bhanot. Isis-fxirx, a novel and specific antisense inhibitor of factor xi, significantly reduces fxi antigen and activity and increases aptt without causing bleeding in healthy volunteers. Blood. 2011;118:209. [Google Scholar]
- 22.Younis HS, Crosby J, Huh JI, Lee HS, Rime S, Monia B, Henry SP. Antisense inhibition of coagulation factor xi prolongs aptt without increased bleeding risk in cynomolgus monkeys. Blood. 2012 doi: 10.1182/blood-2011-10-387134. [DOI] [PubMed] [Google Scholar]
- 23.Runge MS, Harker LA, Bode C, Ruef J, Kelly AB, Marzec UM, Allen E, Caban R, Shaw SY, Haber E, Hanson SR. Enhanced thrombolytic and antithrombotic potency of a fibrin-targeted plasminogen activator in baboons. Circulation. 1996;94:1412–1422. doi: 10.1161/01.cir.94.6.1412. [DOI] [PubMed] [Google Scholar]
- 24.Stasko J, Hudecek J, Kubisz P. thrombin activatable fibrinolysis inhibitor (tafi) and its importance in the regulation of fibrinolysis. Vnitrni Lekarstvi. 2004;50:36–44. [PubMed] [Google Scholar]
- 25.Gruber A, Marzec UM, Bush L, Di Cera E, Fernandez JA, Berny MA, Tucker EI, McCarty OJ, Griffin JH, Hanson SR. Relative antithrombotic and antihemostatic effects of protein c activator versus low-molecular-weight heparin in primates. Blood. 2007;109:3733–3740. doi: 10.1182/blood-2006-07-035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geary RS, Watanabe TA, Truong L, Freier S, Lesnik EA, Sioufi NB, Sasmor H, Manoharan M, Levin AA. Pharmacokinetic properties of 2′-o-(2-methoxyethyl)-modified oligonucleotide analogs in rats. Journal of Pharmacology and Experimental Therapeutics. 2001;296:890–897. [PubMed] [Google Scholar]
- 27.Bennett CF, Swayze EE. Rna targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 28.Kastelein JJ, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, Chuang E, Graham MJ, Crooke RM. Potent reduction of apolipoprotein b and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein b. Circulation. 2006;114:1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 29.Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, Dean NM, Bhanot S, Monia BP. Specific inhibition of pten expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 30.Richard S, Geary RZY, Andrew Siwkowski, Arthur A. Levin. Pharmacokinetic/pharmacodynamic properties of phosphorothioate 2_-o-(2-methoxyethyl)-modified antisense oligonucleotides in animals and man. Antisense Drug Technology. (2nd ed) 2008:305–326. [Google Scholar]
- 31.Tucker EI, Marzec UM, Berny MA, Hurst S, Bunting S, McCarty OJ, Gruber A, Hanson SR. Safety and antithrombotic efficacy of moderate platelet count reduction by thrombopoietin inhibition in primates. Science translational medicine. 2010;2:37ra45. doi: 10.1126/scitranslmed.3000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.