Abstract
Complex microbiota are being reported increasingly across a range of chronic infections, including those of the cystic fibrosis airways. Such diversity fits poorly into classical models of sterile tissue infections, which generally involve one species, and where microbe–outcome associations usually imply causality. It has been suggested that microbiota at sites of infection could represent pathogenic entities, analogous to individual species. We argue that our ability to identify causality in microbiota–disease associations is, however, inherently confounded. Although particular microbiota may be associated with clinical outcomes, niche characteristics at sites of infection will shape microbiota composition through exerting selective pressures. Here, we suggest that ecological theory can inform clinical understanding.
Keywords: microbiome, respiratory, ecological theory, disease causality
Chronic airway infections in an age of microbiota discovery
Many studies are detailing the bacterial species that inhabit sites in and on the human body [1–3]. One current research goal is to define the complex bacterial assemblages that colonise various sites within healthy individuals, such as the gut, skin, and oral cavity [4–6]. The resulting information, derived predominantly from the application of next generation sequencing (NGS) approaches, is being associated increasingly with a variety of conditions. This effort has naturally led to an interest in using the same study tools to better understand infection.
The infections that can develop in the lower airways in patients whose normal innate defences fail to function properly have been the subject of particular focus. Complex infective microbiota have now been described in conditions such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), bronchiectasis, and asthma [7–13]. Such findings appear at odds with traditional models of infection of sterile tissues, such as meningitis or sepsis, which generally involve one species of microbe and that are clearly associated with specific outcomes. Informed by these other (generally acute) diseases, many classical models of chronic airways infections identify individual microbial species that have been associated with specific clinical outcomes, labelling them as ‘pathogens’. In such chronic infection models, the observed microbe–outcome associations are therefore interpreted as representing causal relationships. Exactly how to incorporate the expanding cast of microbes identified by NGS approaches into classical models, and how to distinguish villains from disinterested spectators, is now a matter of considerable interest and controversy in chronic infection research.
Airway microbiota as a ‘pathogen’
Complex infective microbiota could, of course, contain within them individual species that are pathogens in the classical sense. Current practice in reporting the results of diagnostic microbiological testing for respiratory samples involves separating detected species into those believed to be clinically significant (even where there is no direct evidence to support this) and those species thought to be contaminants or bystanders. This distinction persists despite growing evidence that many of the ‘contaminants’ commonly infect the lower airways chronically and are pathogenic in other clinical contexts [14]. In addition, it is increasingly recognised that the properties of multispecies microbiota may be distinct from those of their constituent members, due in large part to interspecies interactions shaping behaviour [15–17]. Viewing a collective microbiota as a potential pathogenic entity in this way has led some clinicians to begin to examine whether particular microbiota cause or worsen disease, in a manner analogous to individual pathogens.
The clear clinical benefit that might be derived from identifying causal relationships between microbiota and pathogenesis is tantalising. Further, studies examining the lower airway microbiota associated with specific diseases, or at particular stages of disease (reviewed in [18]), suggest that such relationships may indeed exist [19,20]. However, it is questionable whether the application of classical medical microbiological logic in this way is appropriate when considering complex microbiota. Here, we argue that our ability to interpret such associations is inherently confounded by the impact of the selective properties of the infective environment. In short, although the presence of particular types of microbiota may be associated with certain clinical changes, the characteristics of the infective niche that a microbiota occupies can equally shape its microbial composition. As such, the relationship between microbiota and disease is best understood from an ecological perspective.
Host–microbe interactions: a two-way street
The classical clinical microbiology model, as described above, can be summarised as one where the characteristics of infective microbes determine the properties of the infective environment. For example, the introduction of Campylobacter jejuni into the human gastrointestinal tract can result in dysentery, a fundamental change in the physiochemical characteristics of the gut. The association between the presence of this pathogen and a specific clinical outcome have led to the identification of a causal relationship. However, such a model is the inverse of that which is commonly used when thinking about microbial systems generally. This alternative model can be summarised as one where the physiochemical characteristics of an environment define the microbes by which it is colonised, in accordance with the frequently quoted tenet put forth by microbiologist Lourens Baas Becking: ‘Everything is everywhere, but the environment selects’ [21]. In this model, the altered characteristics of a diseased tissue would facilitate colonisation by a subgroup of the microbes to which it is exposed. Although the presence of these microbes would then be associated with disease, the microbes would not in this case have initiated pathogenesis.
A further layer of complexity exists. Where particular microbes are able to colonise a niche due to disease, their presence may exacerbate symptoms of the pre-existing condition. This exacerbation would, in turn, further affect the niche characteristics, thus influencing the mix of microbes able to occupy it. In this way, a circular relationship can become established, with the composition of infective microbiota and disease progression proceeding hand-in-hand. Importantly, in such a model, our ability to determine which elements are causes of pathogenesis, and which are effects, is greatly limited.
Ecology and causality
Unlike traditional, single species infection models, causality with respect to infective microbiota cannot be determined by examination of the role played by each individual species in isolation. The complexity of the relationship between microbiota and disease therefore presents a clinical challenge. To interpret the roles and composition of microbiota infecting a specific tissue, it is important to consider the process by which they assemble. Here, niche–microbe interaction models that have been developed to study the microbiota in healthy hosts and environmental niches are useful.
In principle, the processes that drive microbiota assembly are the same, regardless of whether the niche being colonised is environmental, commensal, or infective. Current opinion suggests that these processes can range between ‘neutral’ (see Glossary), in which species are ecologically equivalent and interactions between species and the environment are not relevant to community assembly [22–24], and ‘deterministic’ (niche-related) processes, in which community assembly is guided by selective forces exerted by the local environment and competitive microbes [22]. The extent to which each of these processes contributes to the assembly of a microbiota will be determined by the characteristics of a given niche, which, in turn, may change over time.
To illustrate these processes in an infective context, we can take the chronic lung infections that characterise CF airways disease as an example. The lower airways are exposed to countless cells of diverse microbial species inhaled from the environment and upper airway. Airway defence systems apparently clear these microbes rapidly in healthy individuals, with little evidence of substantial growth. However, in individuals with CF, dysfunctional airway clearance, combined with the presence of substantial volumes of nutrient-rich secretions, present an opportunity for bacterial colonisation.
Were they to be driven by a neutral process of colonisation, the bacterial composition of lower airway secretions in these individuals would be expected to reflect only the relative abundances of the microbes to which they are exposed, with differences between individuals being stochastic or influenced by lifestyle, and hence exposure. However, despite culture-independent techniques revealing bacterial diversity far beyond the small group of species previously thought to infect the CF lower airways [25–27], the number of species observed is still smaller than that to which the airways are likely to be exposed [10]. Further, the relative abundance of microbes detected in the airways is inconsistent with a purely neutral process, with some species that are relatively rare in the wider environment commonly becoming numerically dominant within the airways [9]. A probable explanation for these observations is a strong deterministic involvement in airway microbiota assembly. Here, the ability of microbes entering the lower airways to thrive in that environment is key. Different bacterial species are better suited to growth under particular physiochemical conditions, with the lower airways having their own set of environmental characteristics. Together, these factors mean that whereas some bacterial species will be unable to exploit the lower airways as a growth environment, others will flourish. That the airways frequently contain a phylogenetically diverse array of species, as opposed to overgrowth by a single species, is probably the result of the heterogeneous nature of the airway environment, containing many carbon and nitrogen sources, as well as gradients of important physiochemical characteristics, such as oxygen tension and temperature.
We can find support for the involvement of such deterministic processes in shaping human-associated microbial communities among empirical data from animal models. For example, when gut microbiota are transplanted from conventionally raised mice to germ-free zebrafish and vice versa, the community composition changes to resemble the normal gut microbial community of the recipient host [28]. Such findings suggest that differences in microbiota composition that are observed in the lower airways of individuals with different conditions result from competitive exclusion, with those species best able to grow under the condition-specific, selective pressures coming to dominate the microbiota. In support of this concept, the airways of genetically engineered pigs with CF exhibit pathology similar to that in humans with CF, and yet the airways of pigs contain diverse microbiota that differ from that found in humans [29].
At early stages of chronic airway infections such as CF, the physicochemical differences in the airways between patients may be relatively minor. Accordingly, the divergence in the colonising microbiota in different disease groups, and in healthy individuals, may be slight. However, as diseases progress, physiochemical differences may become more marked, and divergence in disease-specific microbiota more pronounced. Probable contributors to this deterministic process include the immune response, rheology of respiratory secretions, and the development of bronchiectasis, as well as the impact of changes in treatment that follow clinical worsening, such as increased use of steroids and mucolytics, as well as the type and intensity of antibiotic exposure. The impact of these changes is reflected in the decreasing species diversity of the CF airway microbiota observed with advancing age [20,30], which is also associated with higher antibiotic burden [31]. The microbiota found in lungs from people with end-stage CF disease are particularly simple, often with only Pseudomonas aeruginosa or other individual species commonly detected [32]. Although P. aeruginosa is commonly the target of broad-spectrum antibiotics, this bacterium has high intrinsic resistance to many antimicrobials [33] and is capable of several adaptations that increase its tolerance of antibiotics [34,35], all of which may allow it to persist where other co-colonising species cannot.
Selective pressures, therefore, are likely to change with each stage of disease. Airway microbiota in this scenario would not only be determined by underlying disease but also by level of severity, a hypothesis supported by recent empirical observations. For example, just as the prevalence of specific recognised bacterial pathogens, such as Haemophilus influenzae and P. aeruginosa, tend to change with CF lung disease progression [7], so too does the overall composition of the infective microbiota [9,19,30,31,36,37].
In addition to neutral and deterministic processes influencing microbiota composition, a set of processes that can be broadly termed ‘historical’ probably play significant roles. Bacteria alter the characteristics of the niche in which they grow. They can do so by altering factors such as nutrient availability, pH, and oxygen tension, by influencing the behaviours of other colonising species (such as pathogenicity [38]), or by directly interacting with the host (e.g., by damaging airway epithelia [39] or triggering inflammation [40]). Microbial activity will therefore influence the deterministic processes that select for subsequent members of the microbiota. Thus, infection by one species can indirectly dictate microbiota composition. Such microbial community succession could be a further contributory factor to the predominance of P. aeruginosa associated with late, severe CF lung disease.
Microbiota dynamics and clinical events
It is important to remember that microbiota assembly is not a unidirectional process leading to equilibrium or maturity. Rather, the pressures affecting microbiota composition are dynamic. Understanding the drivers of microbial community stability, as defined by resistance (insensitivity to disturbance) and resilience (the rate of recovery after disturbance), is fundamental for predicting the way in which a microbiota will respond to perturbation [41]. For example, when challenged by intravenous antibiotic therapy, substantial shifts in CF airway microbiota composition are observed [36,42,43]. However, these communities tend to return to their pretreatment state following cessation of antibiotics, with long-term microbiota composition showing surprising stability [9,31,36]. The diversity of constituent species (both in terms of richness and evenness), their individual behaviours, and their interrelationships all influence the resistance and resilience of a microbiota [41], as well as the likelihood of incorporating a new species [44].
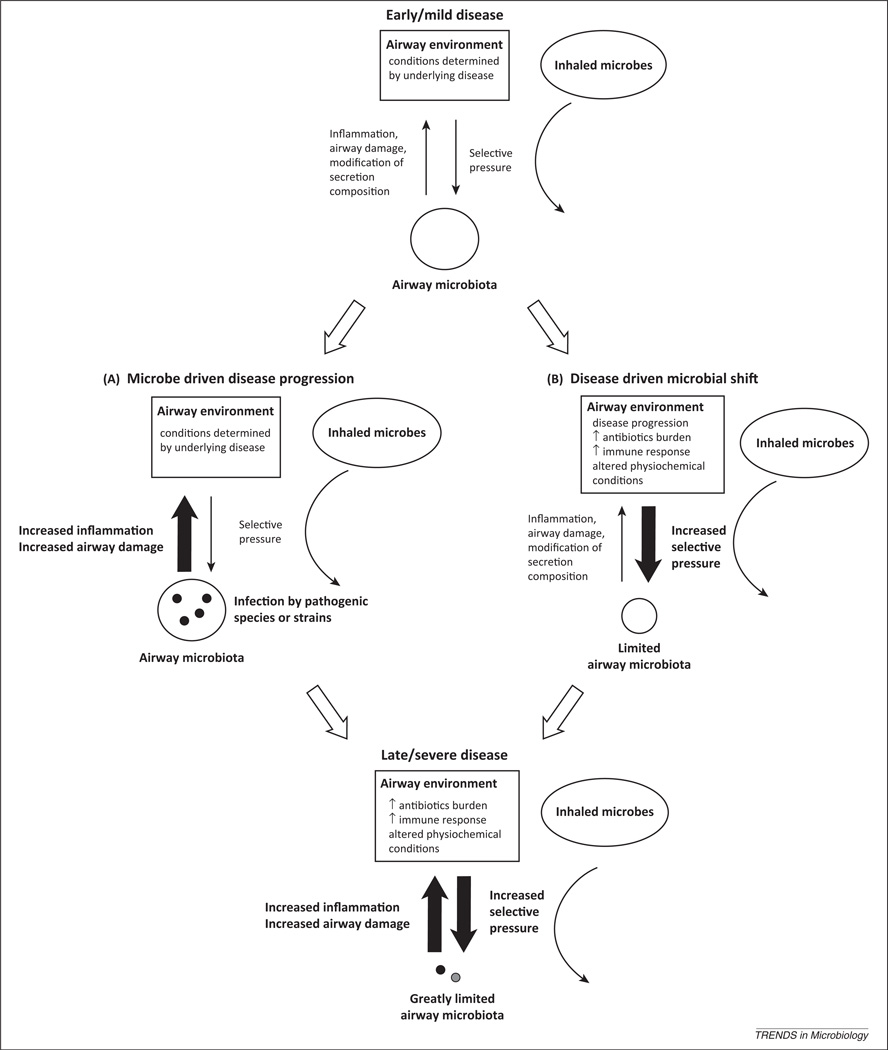
Such ecological models can therefore inform not only our understanding of microbiota dynamics and their relationship with disease but also those of individual pathogens. In adult CF lung disease, P. aeruginosa infection is associated with a poor clinical outcome [45]. From a ‘classical’ perspective, the independent association of P. aeruginosa with CF lung function can be interpreted as indicating that specific species drive lung disease progression, with the failure of antibiotic treatment to halt its increasing predominance resulting in increasing disease severity. By contrast, from a ‘selective’ and ecological perspective, the progression of disease reflects an airway environment increasingly suitable for the growth of such pathogens, leading to expansion of their populations. Crucially, these two models are not exclusive. Specific pathogens may play causal roles in the progression of infective disease. As underlying lung disease worsens, the characteristics of the airways change. In turn, the selective pressures on airway microbes shift, potentially altering the airway microbiota. At the same time, infective microbiota can themselves influence disease, both directly and through interactions with major pathogens (Figure 1).
Figure 1.
A proposed model of the relationship between the selective properties of the airway environment on microbiota membership and the ability of airway infection to promote damage. In early disease, the lower airway environment contains mild selective pressures and is colonised by a diverse array of bacterial species. Disease progression is contributed to by two separate processes: (A) members of the airway microbiota contribute to disease, either directly through exhibiting pathogenic traits, or indirectly, by augmenting the pathogenic behaviour of other species. (B) Increasing disease severity results in changes in the selective pressures on the infective microbiota, including levels of immune response and antibiotic exposure. These two scenarios are inherently linked, with changes in the airway environment, and the characteristics of the microbiota that infect it, proceeding together. We suggest that such a process could result in both the succession of bacterial species associated with different disease stages and the severely limited microbiota diversity associated with end-stage cystic fibrosis lung disease.
Challenges, opportunities, and potential pitfalls
Traditional models of infection have been extremely helpful in approaching a great many infections, largely those caused by a single pathogen. However, understanding the interrelationships between the infective niche, microbiota composition, and the host are fundamental in unravelling the role of infective bacterial assemblages in pathogenesis. Although gaining such insight will take time, it is important to recognise that any measurable, reliable indicator of disease state represents a potentially useful disease biomarker, even in the absence of mechanistic clarity. By reflecting niche characteristics, microbiota composition could be a means by which the severity and extent of disease progression can be assessed. Further, such detailed analysis could provide a means to identify patients who are susceptible to infection by particular pathogens, to act as prognostic indicators, or to direct treatment.
Recently, our ability to characterise complex microbiota, both in health and disease, has increased vastly. The extent to which such technological advances can improve our understanding of infections, and inform the way in which we treat them, is dependent on our ability to interpret these data accurately. Here, a failure to integrate insights from studies of other microbiological systems into clinical conceptual frameworks would not only be an opportunity missed but could also potentially result in a misinterpretation of recent findings.
Glossary
- Competitive exclusion
a proposition that states that two species competing for the same resources cannot coexist if other ecological factors are constant. When one species has even a slight advantage over another, it will dominate in the long term.
- Deterministic processes
ecological processes that involve nonrandom, niche-based mechanisms.
- Historical processes
ecological processes constrained by historical events that are often random.
- Neutral processes
a process in which species are ecologically equivalent, with stochastic factors, such as chance colonisation or random extinction shaping communities.
- Perturbation (or disturbance)
a causal event that results in a discrete change in the physical or chemical environment that has anticipated effects on a community.
- Resilience
the rate at which a microbial community returns to its original composition after being disturbed.
- Resistance
the degree to which a community withstands change in response to perturbation.
- Selection
a process in which organisms possessing certain characteristics that make them better adjusted to an environment tend to survive, reproduce, and increase in abundance or frequency.
- Stability
the tendency of a community to return to a condition after perturbation.
- Succession
the gradual and orderly process of change in a microbial community brought about by the progressive replacement of its members.
References
- 1.Koren O, et al. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput. Biol. 2013;9:e1002863. doi: 10.1371/journal.pcbi.1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013;14:R1. doi: 10.1186/gb-2013-14-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Annu. Rev. Pathol. 2012;7:99–122. doi: 10.1146/annurev-pathol-011811-132421. [DOI] [PubMed] [Google Scholar]
- 4.Rajilić-Stojanović M, et al. Long-term monitoring of the human intestinal microbiota composition. Environ. Microbiol. 2013;15:1146–1159. doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- 5.Oh J, et al. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012;4:77. doi: 10.1186/gm378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiao WW, et al. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics. 2012;13:345. doi: 10.1186/1471-2164-13-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blainey PC, et al. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Sci. Transl. Med. 2012;4:153ra130. doi: 10.1126/scitranslmed.3004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers GB, et al. Reducing bias in bacterial community analysis of lower respiratory infections. ISME J. 2013;7:697–706. doi: 10.1038/ismej.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stressmann AF, et al. Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the cystic fibrosis lung are stable and resilient. Thorax. 2012;67:867–873. doi: 10.1136/thoraxjnl-2011-200932. [DOI] [PubMed] [Google Scholar]
- 10.Erb-Downward JR, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sze MA, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012;185:1073–1080. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YJ, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011;127:372–381. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tunney MM, et al. The lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. AmJ. Respir. Crit. Care Med. 2013 doi: 10.1164/rccm.201210-1937OC. http://dx.doi.org/10.1164/rccm.201210-1937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers GB, et al. Use of 16S rRNA gene profiling by terminal restriction fragment length polymorphism analysis to compare bacterial communities in sputum and mouthwash samples from patients with cystic fibrosis. J. Clin. Microbiol. 2006;44:2601–2604. doi: 10.1128/JCM.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faust K, et al. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012;8:e1002606. doi: 10.1371/journal.pcbi.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trosvik P, et al. Convergent temporal dynamics of the human infant gut microbiota. ISME J. 2010;4:151–158. doi: 10.1038/ismej.2009.96. [DOI] [PubMed] [Google Scholar]
- 17.Korgaonkar A, et al. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc. Natl. Acad. Sci. U.S.A. 2013;110:1059–1064. doi: 10.1073/pnas.1214550110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han MK, et al. Significance of the microbiome in obstructive lung disease. Thorax. 2012;67:456–463. doi: 10.1136/thoraxjnl-2011-201183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson RL, et al. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 20.Klepac-Ceraj V, et al. Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics and Pseudomonas aeruginosa. Environ. Microbiol. 2010;12:1293–1303. doi: 10.1111/j.1462-2920.2010.02173.x. [DOI] [PubMed] [Google Scholar]
- 21.de Wit R, Bouvier T. ‘Everything is everywhere but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ. Microbiol. 2006;8:755–758. doi: 10.1111/j.1462-2920.2006.01017.x. [DOI] [PubMed] [Google Scholar]
- 22.Cavender-Bares J, et al. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 23.Emerson BC, Gillespie RG. Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol. Evol. 2008;23:619–630. doi: 10.1016/j.tree.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol. 2011;65:411–429. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 25.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willner D, et al. Spatial distribution of microbial communities in the cystic fibrosis lung. ISME J. 2012;6:471–474. doi: 10.1038/ismej.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JK, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawls JF, et al. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox MJ, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS ONE. 2010;5:e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc. Natl. Acad. Sci. U.S.A. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goddard AF, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc. Natl. Acad. Sci. U.S.A. 2012;109:13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poole K. Outer membranes and efflux: the path to multidrug resistance in Gram-negative bacteria. Curr. Pharm. Biotechnol. 2002;3:77–98. doi: 10.2174/1389201023378454. [DOI] [PubMed] [Google Scholar]
- 34.Amini S, et al. Fitness landscape of antibiotic tolerance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2011;7:e1002298. doi: 10.1371/journal.ppat.1002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver A. Mutators in cystic fibrosis chronic lung infection: prevalence, mechanisms, and consequences for antimicrobial therapy. Int. J. Med. Microbiol. 2010;300:563–572. doi: 10.1016/j.ijmm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Fodor AA, et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS ONE. 2012;7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Gast CJ, et al. Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J. 2011;5:780–791. doi: 10.1038/ismej.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibley CD, et al. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008;4:e1000184. doi: 10.1371/journal.ppat.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King P. Pathogenesis of bronchiectasis. Paediatr. Respir. Rev. 2011;12:104–110. doi: 10.1016/j.prrv.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Essilfie AT, et al. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax. 2012;67:588–599. doi: 10.1136/thoraxjnl-2011-200160. [DOI] [PubMed] [Google Scholar]
- 41.Shade A, et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012;3:417. doi: 10.3389/fmicb.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniels TWV, et al. Impact of antibiotic treatment for pulmonary exacerbations on bacterial diversity in cystic fibrosis. J. Cyst. Fibros. 2013;12:22–28. doi: 10.1016/j.jcf.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Tunney MM, et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax. 2011;66:579–584. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- 44.Britton RA, Young VB. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012;20:313–319. doi: 10.1016/j.tim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzsimmons SC. Cystic Fibrosis Foundation; 1997. Cystic Fibrosis Foundation Patient Data Registry Annual Data Report 1996. [Google Scholar]