Abstract
Background
Autosomal dominant hyper-IgE syndrome (AD-HIES) is caused by mutations in signal transducer and activator of transcription 3 (STAT3). We describe 2 subjects in whom somatic mosaicism was associated with intermediate phenotypes.
Objective
Somatic mosaics might shed light on the pathogenesis of dominant STAT3 mutations and the mechanisms behind the immunologic and nonimmunologic features of the disease.
Methods
Clinical evaluations were conducted. Mutant STAT3 was amplified from different tissues and sequenced, and the percentage of mosaicism in various cell types was calculated. Flow cytometry was performed to determine percentages of IL-171 cells, IL-221 cells, or both. Suction blisters were induced in 1 subject, and exudate fluid was analyzed for whether emigrating neutrophils were STAT3 mutant or wild-type; neutrophils from peripheral blood were simultaneously examined.
Results
The 2 subjects with STAT3 somatic mosaicism had intermediate phenotypes and were found to have preserved TH17 cell compartments and apparently normal CD8 cells. However, they still had infections, including mucocutaneous candidiasis. The percentage of STAT3 mutant neutrophils migrating into blisters at 16 hours was the same as in peripheral blood, suggesting normal chemotaxis.
Conclusion
STAT3 mosaicism accounts for a milder phenotype and allows for further investigation into the pathogenesis of AD-HIES. Despite having a preserved TH17 cell compartment, both subjects with mosaicism had chronic mucocutaneous candidiasis, suggesting that candidiasis in subjects with AD-HIES is not driven solely by low TH17 cell numbers. The percentage of STAT3 mutant neutrophils emigrating into a suction blister at 16 hours was the same as the percentage in peripheral blood, suggesting that early chemotaxis of STAT3 neutrophils is normal in vivo.
Keywords: Somatic mosaicism, autosomal dominant hyper-IgE syndrome, Job syndrome, signal transducer and activator of transcription 3
Autosomal dominant hyper-IgE syndrome (AD-HIES) is a rare primary immunodeficiency characterized by increased serum IgE levels, recurrent staphylococcal skin abscesses, lung infections with subsequent pneumatocele development, eczematoid rashes, and connective tissue and skeletal abnormalities.1,2 The diagnosis is confirmed by detection of dominant negative mutations in signal transducer and activator of transcription 3 (STAT3), which account for essentially all published AD-HIES cases.3–5
Shortly after detection of mutations in STAT3, it was determined that these patients have severely impaired production of IL-17–producing TH cells (TH17), leading to abnormal antimicrobial peptide upregulation, especially at epithelial surfaces.6–10 This TH17 defect was thought to explain the chronic mucocutaneous candidiasis (CMC) commonly seen in patients with AD-HIES and also found in patients with other defects affecting IL-17 production and response.11 Recently, other indirect effects on TH17 cells have been associated with CMC.12 Susceptibility to epithelial Staphylococcus aureus infections might also be affected by the integrity of the IL-17 pathway.11
Neutrophil chemotactic defects have long been proposed as contributing to the infectious manifestations of AD-HIES,13,14 but their demonstration has been inconsistent and controversial.15 Finally, diminished numbers of memory B lymphocytes16 and T lymphocytes likely contribute to infection susceptibility through impaired responses to encapsulated bacteria, such as Streptococcus pneumoniae, and reactivation of varicella zoster virus,17 respectively.
We describe 2 unrelated men who had multiple children with classic AD-HIES and STAT3 mutations who had somatic mosaicism for the STAT3 mutations found in their offspring. These somatic mosaic men had intermediate AD-HIES phenotypes.
METHODS
All patients, family members, and control subjects were seen and consented to institutional review board–approved protocols of the National Institutes of Health (NIH). Family J149 was also studied under an approved protocol at the University of California, San Francisco.
Clinical evaluation
History and physical examinations were performed for all patients, as well as routine laboratory studies and radiologic imaging.
PCR amplification and sequencing
PBMCs were isolated from whole blood by means of Ficoll-Hypaque gradient centrifugation and lysed either in STAT 60 (Tel-Test, Friendswood, Tex) followed by RNA isolation or with the PureGene DNA Isolation kit cell lysis solution (Qiagen, Valencia, Calif) for DNA extraction. Amplification of either full-length cDNA or STAT3 exons 12 to 14 (Family J017) or exon 15 (J149) was as previously reported.3 Sequencing was performed with Big Dye Terminators version 3.1 (Applied Biosystems, Foster City, Calif).
Determination of percentage of mosaicism
Determination of the percentage of mutation-positive cells was performed as previously reported.17,18 DNA from various cell types was amplified by using custom genotyping primers/probe sets (Applied Biosystems) that differentially detect the mutant and wild-type (WT) alleles. Calculation of the percentage of mosaicism was performed by using the DD cycle threshold (DDCt) method (DCt was the difference between mutant and WT Ct values). DNA from the heterozygous children was used as the normalizer to account for any difference in probe affinity, as follows:
Because the mutant allele is represented 1 time in each mutant cell and the WT allele is represented twice in the normal cells, the DDCt value was divided by 2 as a correction factor. Therefore the final quantitation becomes 22(DDCt/2). Results were validated in subject J017.3 by means of cloning and sequencing of PCR fragments (TOPO TA cloning kit; Invitrogen, Carlsbad, Calif).
Stimulation and intracellular cytokine staining
Viably frozen PBMCs were thawed, washed with PBS, and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FCS (Gemini Bio-Products, West Sacramento, Calif), 100 U/mL penicillin G, 100 mg/mL streptomycin, and 29.2 mg/mL L-glutamine (Life Technologies, Carlsbad, Calif). Cells were stimulated with 20 ng/mL phorbol 12-myristate 13-acetate and 1 mmol/L ionomycin (Calbiochem, San Diego, Calif) for 16 hours at 378C in brefeldin A (Sigma, St Louis, Mo). Cells were stained with Live/Dead Fixable Aqua viability dye (Invitrogen) and then fixed with 4% paraformaldehyde and permeabilized with a saponin-based buffer.19 The following surface and intracellular markers were used for staining: CD3 Quantum-dot 705 (Invitrogen), CD4 phycoerythrin (PE) Cy7, CD8 allophycocyanin H7, IFN-g fluorescein isothiocyanate (all from BD Biosciences, San Jose, Calif), CD27 PE-Cy5, CD45RO Texas Red PE (TRPE; both from Beckman Coulter, Fullerton, Calif), IL-22 allophycocyanin (R&D Systems, Minneapolis, Minn), and IL-17 Alexa Fluor 647 (eBioscience, San Diego, Calif).
Flow cytometry
Flow cytometry was performed as previously reported.17 Events were collected on an LSR Fortessa with Diva 6.1.3 software (BD Biosciences) and analyzed with Flow Jo 9.4 software (TreeStar, Ashland, Ore). All plots were gated on singlet lymphocytes that were Aqua negative (live). All values used for analyzing proportionate responses were background subtracted.
Blister chamber technique (family J017)
Suction blisters were induced on the forearms of subject J017.3, essentially as previously described.20 After removal of the blister roof, the wound was bathed in 70% autologous heat-treated serum for 16 hours before collection of exudates. Emigrating neutrophils were studied to determine mutant and WT neutrophil percentages.
RESULTS
Case reports
Subject J017.3 (Fig 1, A). A 52-year-old white man had pustular dermatitis as an infant, followed by eczematous dermatitis and recurrent skin boils from age 7 to 20 years. He also had recurrent pneumonias from age 9 months to 15 years, requiring chest tube placement on 1 occasion. Chronic onychomycosis was treated on several occasions with oral antifungal agents. Oral candidiasis was diagnosed by means of culture. His main complaint as an adult was dysphagia, with esophageal food impaction on several occasions requiring the Heimlich maneuver. He had hypertension and chronic hepatitis C, the latter of which had been unsuccessfully treated with IFN-a and ribavirin. Clinical features are outlined in Table I.
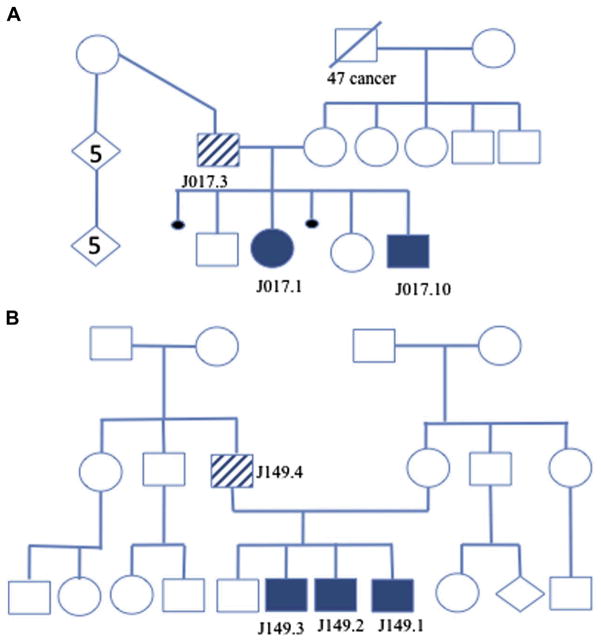
FIG 1.
A, Pedigree of family J017. B, Pedigree of family J149. Open symbols represent unaffected subjects, whereas solid symbols indicate STAT3 mutation, and striped symbols reflect the mosaic subjects. Small circles represent miscarriages. The diamond-shaped symbol indicates sex is unknown, and the number in the shape indicates the number of offspring.
TABLE I.
The NIH HIES scoring system includes both immunologic and nonimmunologic features of the disease
| Characteristics | Family J017
|
Family J149
|
|||||
|---|---|---|---|---|---|---|---|
| Subject J017.3 (52 y) | Subject J017.1 (28 y) | Subject J07.10 (15 y) | Subject J149.4 (34 y) | Subject J149.3 (11 y) | Subject J149.2 (9 y) | Subject J149.1 (2 y) | |
| IgE (IU/mL) | 10 (2,100) | 10 (69,760) | 10 (20,900) | 10 (4,828) | 10 (21,664) | 10 (29,448) | 10 (16,180) |
|
| |||||||
| Other infections | 4 (hepatitis C) | 4 (Pneumocystis jirovecii) | 0 | 0 | 0 | 0 | 0 |
|
| |||||||
| Eosinophilia (eosinophils/mL) | 3 (745) | 6 (1,140) | 6 (2,309) | 0 (140) | 6 (1,200) | 6 (1,300) | 6 (1,560) |
|
| |||||||
| Boils | 8 | 8 | 0 | 8 | 8 | 8 | 8 |
|
| |||||||
| Eczema | 4 | 4 | 4 | 1 | 4 | 4 | 4 |
|
| |||||||
| Candidiasis | 2 | 1 | 1 | 2 | 2 | 2 | 0 |
|
| |||||||
| Newborn rash | 0 | 4 | 4 | 0 | 4 | 4 | 4 |
|
| |||||||
| Sinusitis/otitis | 0 | 4 | 4 | 4 | 4 | 4 | 4 |
|
| |||||||
| Pneumonia | 8 | 8 | 8 | 6 | 4 | 2 | 0 |
|
| |||||||
| Parenchymal lung findings | 0 | 0 | 8 | 6 | 0 | 0 | 0 |
|
| |||||||
| Retained primary teeth | 0 | 8 | 8 | 0 | 4 | 4 | 0 |
|
| |||||||
| Scoliosis | 2 (128) | 2 (138) | 0 | 2 (138) | 0 | 2 (128) | 0 |
|
| |||||||
| Fractures | 0 | 4 | 4 | 0 | 4 | 8 | 8 |
|
| |||||||
| Hyperextensibility | 0 | 4 | 4 | NE | 4 | 4 | NE |
|
| |||||||
| Characteristic facies | 0 | 5 | 2 | 0 | 0 | 0 | 0 |
|
| |||||||
| Palate abnormalities | 0 | 2 | 2 | 0 | NE | NE | NE |
|
| |||||||
| Nasal width | 0 | 3 | 1 | NE | NE | NE | NE |
|
| |||||||
| Congenital anomalies | 0 | 5 (hypoplastic and fixed 1st and 2nd rib) | 0 | 5 (3rd finger bilaterally with PIP flexion deformity) | 5 (partial craniosynostosis) | 0 | 0 |
| Add on for young age | 0 | 0 | 0 | 0 | 0 | 0 | 3* |
| Total score | 41 | 82 | 66 | 44 | 59 | 66 | 47 |
Both mosaic subjects’ clinical characteristics are shown in the table, along with those of their affected children, with total scores in the bottom row.
NE, Not evaluated; PIP, proximal interphalangeal joints.
Age points are assigned to children less than 5 years of age to compensate for clinical features too early to be seen. The point range is as follows: 7 points for children less than 1 year of age, 5 points for children 1 to 2 years of age, and 3 points for children 2 to 5 years of age.
Laboratory and imaging studies obtained in adulthood showed protective antibody titers after S pneumoniae, Haemophilus influenzae, tetanus, and diphtheria vaccinations. Lymphocyte phenotyping showed normal numbers of circulating B cells (209 CD201 cells) and T cells (2043 CD31 cells), both memory and naive cells, including normal numbers of central (CD271 CD451 RO1) and effector (CD272 CD451 RO1/2) memory T cells, as previously described.17 Chest computed tomography (CT) showed a calcified nodule but no pneumatoceles or bronchiectasis. Brain magnetic resonance imaging (MRI) showed no T2-weighted focal hyperintensities or Chiari I malformation. Heart CT showed dilation of the left anterior descending coronary artery, minimal right coronary artery tortuosity, and mild noncalcified atheromatous plaque. Bone mineral density of the lumbar spine was low (T score 5 21.3), with normal bone density of the hip and radius. Upper gastrointestinal endoscopy revealed grade 1 esophageal varices, ulcerations of the gastroesophageal junction, portal hypertensive gastropathy, and esophageal dysmotility, resulting in a trachealized appearance of the esophagus.
He has 4 children (Fig 1, A), 2 of whom have AD-HIES (subjects J017.1 and J017.10), with STAT3 mutation c.1145G>A resulting in p.R382Q. Using the published hyper-IgE syndrome (HIES) clinical scoring system that combines immunologic/infectious disease and nonimmunologic components of hyper-IgE syndrome (HIES), his score was 41, with a score of greater than 40 consistent with HIES (Table I). In contrast, his 2 affected children had significantly more features reflected by scores of 82 for subject J017.1 and 66 for subject J017.10. Fig 2 demonstrates the classic AD-HIES facial features (increased interalar distance, prominent forehead, facial asymmetry, and coarse skin) affecting subjects J017.1 and J017.10, whereas subject J017.3 is noticeably without such features.
FIG 2.

Members of family J017: subject J017.3 along with his 2 affected children, subjects J017.1 and J07.10. The classic AD-HIES facial features(broad nose, prominent brow, and facial asymmetry) are present in the children but absent in the father.
Family J149, subject 4 (J149.4; Fig 1, B). A 34-year-old Hispanic man had recurrent skin boils requiring incision and drainage, yearly bronchitis, recurrent sinusitis, and 3 pneumonias over his lifetime. He had chronic tinea pedis and onychomycosis. Clinical features are outlined in Table I.
Additional laboratory studies obtained in adulthood included protective titers (≥1.3 —g/mL) against S pneumoniae for 10 of 23 serotypes, as well as protective titers for H influenzae, tetanus, and diphtheria. Lymphocyte phenotype showed normal numbers of circulating B cells (222 CD201 cells), memory B cells (32 CD201CD271 cells), and T cells (2136 CD31 cells), both memory (789 CD41CD451RO1 cells; 107 CD81CD451RO1 cells) and naive (229 CD41CD451RA1 cells; 458 CD81CD451RA1 cells).
Brain MRI showed maxillary sinus hypoplasia with sinusitis and septal deviation but without brain T2 hyperintensities or Chiari I malformation. A dual-energy x-ray absorptiometry scan did not find osteopenia or osteoporosis. Chest CT showed right lung bronchiectasis. Heart MRI showed mild tortuosity of the right coronary artery without significant dilation.
He has 4 sons, 3 of whom have typical features of AD-HIES; subjects J149.1, J149.2, and J149.3 were positive for STAT3 c.1309C>T, causing H439Y. His HIES clinical scoring system score was 44. The HIES score for the youngest son, subject J149.1, was 47, and the older 2 sons, subjects J149.2 and J149.3, had scores of 66 and 59, respectively.
Mosaicism detection (family J017)
Amplification and sequencing of the full-length STAT3 cDNA demonstrated a heterozygous G-A transition at c.1145 in subjects J017.1 and J017.10, resulting in R382Q, a recognized hotspot.3,5 The tracings clearly show 2 distinct alleles of equal height in subject J017.10. In contrast, subject J017.3 had a decreased WT G peak height with a small mutant A peak not seen in the control sequence. These tracings suggested that in subject J017.3 a portion of his cells were WT, as well as a small fraction containing the family c.1145G>A mutation (Fig 3, A).
FIG 3.

Family J017: STAT3 sequence for a control subject, subject J017.10, and subject J017.3. A, cDNA sequencing (c.1145 G>A). The control shows only the G allele in the boxed region. Subject J017.10 demonstrates 2 alleles with WT and mutant bases at similar heights, roughly half the height of the control subject. Subject J017.3 has decreased WT 1145G compared with control values and a small 1145G>A peak seen near the baseline. The arrow points to the mutant green A peak. B, Genomic DNA sequencing. The common C/G SNP found within intron 13 shows 2 separate and equally abundant alleles in subject J017.3, whereas only the G allele is seen in the control subject.
A single nucleotide polymorphism (SNP) within the amplicon was used to confirm the mosaicism of subject J017.3 and the presence of 3 alleles (2 WT and 1 mutant). Subjects J017.10 and J017.3 have a common SNP (c.1233144G>C) in intron 13, 132 bases 39 from the mutation. Subject J017.3 is heterozygous, with equal peak heights for the SNP indicating equally expressed heterozygous alleles, whereas subject J017.10 is homozygous G for the SNP (Fig 3, B). The amplicon was cloned, and individual colonies were sequenced. There were 3 species of clones present from the blood of subject J017.3. The first, c.1145G(wt) c.1233144C, is the WT allele, and the second and third clones are from the second allele, c.1145G(wt) c.1233144G without the mutation and c.1145A (mut) c.1233144G, which the affected children inherited.
The sequence tracings and clones confirm that subject J017.3 has 3 alleles present in his PBMCs.
Mosaic detection (family J149)
Amplification and sequencing of genomic STAT3 demonstrated heterozygous c.1309C>T in the 3 affected sons of subject J149.4, resulting in p.H437Y (Fig 4). The mutant peak is significantly smaller in the tracing from subject J149.4.
FIG 4.
Family J149: STAT3 cDNA sequencing. The c.1309C>T sequence for the healthy son and subjects J149.1, J149.2, J149.3, and J149.4 is shown. The STAT3 c.1309C>T mutation is absent in the healthy son (homozygous C) but easily appreciated in the heterozygous affected sons J149.1, J149.2, and J149.3 (heterozygous C and T). Subject J149.4 has a diminished WT 1309C allele compared with the control subject and a small 1309T peak near the baseline. The arrow is pointing to the mutant red T peak.
Determination of percentage of mosaicism
Subject J017.3. Analysis of real-time PCR probes specific to either mutant or WT alleles allowed us to determine the relative abundance of each allele within subject J017.3’s PBMCs. Adjusting for the presence of 2 WT alleles in his normal cells, the percentage of mosaicism was calculated to be 33%. This was in accord with individual clones isolated from his PBMCs, in which 5 of 30 clones were mutation positive. Expecting one half of all clones from mutant cells to be WT, the 5 mutant clones detected represent 33% mosaicism within the PBMCs. Examining clones from granulocytes only, we obtained 6 mutant clones of 36 total, again representing 33% mosaicism.
The hematopoietic lineages examined (neutrophils, T cells, natural killer cells, and monocytes) all appeared to have similar levels of mosaicism (Fig 5). Two EBV-transformed B-cell lines made several years apart both showed mosaicism for STAT3 mutation, even after more than 3 months in active culture (data not shown).
FIG 5.
Family J017. Genomic DNA sequences for subject J017.3 in T cells, natural killer cells, monocytes, B cells, eosinophils, and neutrophils all have a mosaic pattern at c.1145G>A and are heterozygous for the SNP at c.1233144C>G.
We then examined nonhematopoietic tissues to determine their extent of mosaicism. DNA amplified from upper-arm fibroblasts showed only the WT peak at c.1145 (Fig 6, A). DNA isolated from hair follicles showed the mutated allele in a mosaic pattern. A gastric biopsy specimen selected from a region without overt inflammation showed the mutant allele to be fully heterozygous. A benign rectal polyp biopsied during routine colonoscopy had a mosaic pattern. Lastly, DNA extracted from a mouthwash sample, which is typically composed predominantly of neutrophils, was mosaic (Fig 6, B), which is similar to the other hematopoietic cells.21
FIG 6.

A, Genomic DNA from various somatic tissues were amplified and sequenced from J017.4 exons 12 to 14 of STAT3. Fibroblasts demonstrated only the WT peak. DNA from the stomach biopsy specimen showed heterozygosity for the mutation. B, Oral, circulating, and migrating blister neutrophils contain mutant peaks at similar heights. The chromatograms suggest that the percentage of mosaicism is similar in sorted circulating neutrophils and migrating blister neutrophils. The mouthwash sample (neutrophils) also demonstrates a mosaic pattern.
Subject J149.4. Allele-specific TaqMan analysis was performed to quantitate the percentage of mosaicism in subject J149.4 (Fig 7). An affected son (first column) showed heterozygosity with equal levels of both alleles. DNA from subject J149.4’s whole blood, sorted cells, and buccal DNA demonstrated reduced allele presence for the mutant c.1309T allele compared with the c.1309C WT allele.
FIG 7.
TaqMan real-time PCR demonstrating the percentage of mutant cells for isolated lineages of subject J149.4. Calculating the percentage of cells with the mutant allele yielded 52% mosaicism in whole blood, 51% in T cells, 63% in B cells, 53% in natural killer (NK) cells, 55% in monocytes, 50% in granulocytes, and 60% in buccal DNA.
TH17 cell analysis
Flow cytometry was performed on PBMCs from healthy control subjects, subject J017.3, subject J149.3, subject J149.2, and subject J149.4 (Fig 8, A). Subject J017.3 had 1.35% total CD41 IL-171 cells similar to subject J149.4, who had 2.35%, whereas healthy control subjects had a mean of 1.98% (n 5 6). Two heterozygous children, subjects J149.3 and J149.2, had significantly fewer CD41IL-171 cells with 0.409% and 0.312, respectively. Similar results were found for CD41IL-221 cells in healthy control subjects (n 5 5), subject J017.3, and subject J149.4 (1.46%, 1.62%, and 0.906%, respectively), although the 2 heterozygous children, subjects J149.3 and J149.2, had very few CD41IL-221 cells (0.075% and 0.097%, respectively). Mean fluorescence intensity data for CD41IL-171 cells in a control subject, subject J017.3, and subject J149.4 (Fig 8, B) showed no difference in intensity (16685, 16373, and 17065, respectively).
FIG 8.
A, IL-17– and IL-22–producing cells from patients and control subjects. CD31CD41IL-171 and CD31CD41IL-221 cell percentages for control subjects and subjects J017.3, J149.3, J149.2, and J149.4 after stimulation with phorbol 12-myristate 13-acetate/ionomycin. The 2 mosaic subjects (subjects J017.3 and J149.4) have IL-171 and IL-221 cell numbers in the normal range, whereas cell numbers in the heterozygous children (subjects J149.3 and J149.2) are greatly diminished. B, Mean fluorescence intensity (MFI) in the control subject and the 2 mosaic subjects (subjects J017.3 and J149.4).
Blister analysis (subject J017.3)
Sorted, circulating, and migrating blister neutrophils contained similar levels of the mutant peak, suggesting that there was no preferential migration of normal or mutant neutrophils into inflamed skin at 16 hours of inflammation (Fig 6, B).
DISCUSSION
We identified 2 men with somatic mosaicism for STAT3 mutation, both of whom were fathers of children with inherited AD-HIES. Both mosaic subjects, subjects J107.3 and J149.4, showed immunologic manifestations of the disease, although milder than those typically seen in fully affected patients at their ages. Although an HIES score of greater than 40 is consistent with this disease, the average AD-HIES score for patients followed at the NIH who are over 34 years is 76 (range, 58–100; n 5 22), demonstrating that age-matched heterozygous subjects have a more severe phenotype than these 2 mosaic men, whose scores were in the low 40s.
Patients with AD-HIES have impaired TH17 cell differentiation, which is hypothesized to underlie their susceptibility to CMC and potentially their staphylococcal epithelial infections. TH17 lymphocytes are responsible for secretion of the proinflammatory cytokines IL-17 and IL-22, which upregulate antimicrobial peptides.6,22,23 TH17 cells and their associated cytokines might also play a role in epithelial defense against S aureus.24,25 Surprisingly, both our mosaic subjects had normal TH17 cell numbers, which has also been reported in another STAT3 mosaic subject26 but still had mucocutaneous fungal infections. Non–T-cell factors might be responsible for CMC in these mosaic subjects, including abnormal responses in STAT3 mutant epithelial cells. This unexpected but robust observation seen in these 2 subjects suggests that the lack of TH17 cells in patients with AD-HIES is not sufficient as the sole cause for CMC in patients with AD-HIES. Therefore the resolution of CMC in subjects with STAT3 mutations who have undergone bone marrow transplantation might reflect hematopoietic components beyond the normalization of TH17 cells.27
Both subjects with mosaicism experienced recurrent boils and pneumonias, suggesting that TH17 cells do not explain the susceptibility to S aureus epithelial infections. Memory lymphocytes were preserved in both subjects, which might explain their protective antibody titers to vaccines, which are often variable in patients with AD-HIES.
On the basis of this blister study, we found no evidence for impaired early migration of STAT3-mutated neutrophils from the circulation into a site of inflammation, suggesting that there is not a clinically significant impairment of chemotaxis, at least in the subject studied at the time point examined.
Many of the somatic manifestations of AD-HIES are thought to be secondary to impaired tissue remodeling and healing. For instance, lung tissues heal abnormally after pneumonias, leading to pneumatoceles; skin becomes progressively coarse, leading to some of the distinct appearance; and the vascular aneurysms are similar to other disorders of impaired remodeling.28 The lack of bronchiectasis or pneumatocele formation in subject J017.3 might have been due to the limited number of pneumonias he had compared with other patients with AD-HIES or to preserved tissue remodeling. His coronary artery involvement was mild compared with that of fully mutant patients of his age. One of his most marked manifestations is dysphagia, which is reported frequently in patients with AD-HIES and is often associated with eosinophilic esophageal infiltration.29 The degree of mosaicism was most fully heterozygous on the stomach biopsy specimen, which is consistent with the idea that the involved endoderm might play a role in his dysmotility. The lack of characteristic delayed primary tooth deciduation in both mosaic subjects suggests adequate STAT3 signaling by IL-11.30
Somatic mosaic mutations pose a challenge for both clinical and molecular diagnosis. Mosaic phenotypes can have incomplete syndromic features, leading to their being overlooked and thus underreported. Molecularly, the small buried chromatographic peaks are typically not recognized by automated software and require an experienced reader with knowledge of the clinical phenotype. Mosaic subjects with STAT3 mutations and HIES might account for some presentations of incomplete phenotypes of STAT3 deficiency.
Key messages.
Somatic mosaicism can lead to a mild, incomplete phenotype of AD-HIES, which may not be clinically recognized.
The lack of TH17 cells in AD-HIES may not fully explain the susceptibility to mucocutaneous candidiasis and staphylococcal infections.
STAT3 mutated neutrophils migrate normally to an induced site of skin inflammation.
Acknowledgments
Supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and the Neutrophil Monitoring Laboratory, Applied/Developmental Research Directorate, SAIC-Frederick, Frederick, Maryland. J.M.P. acknowledges support from the National Center for Research Resources NCRR UCSF CTSI (UL1 RR024131) and the UCSF Jeffrey Modell Foundation Diagnostic Center for Primary Immunodeficiencies. The views expressed in this article are those of the authors and do not reflect the official policy of the US Government.
Abbreviations used
- AD-HIES
Autosomal dominant hyper-IgE syndrome
- CMC
Chronic mucocutaneous candidiasis
- CT
Computed tomography
- Ct
Cycle threshold
- HIES
Hyper-IgE syndrome
- MRI
Magnetic resonance imaging
- NIH
National Institutes of Health
- PE
Phycoerythrin
- SNP
Single nucleotide polymorphism
- STAT3
Signal transducer and activator of transcription 3
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: J. M. Puck has been supported by one or more grants from the National Institutes of Health and the Jeffrey Modell Foundation. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Davis SD, Schaller J, Wedgwood RJ. Job’s syndrome. Recurrent, “cold” staphylococcal abscesses. Lancet. 1966;1:1013–5. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;49:59–70. [PubMed] [Google Scholar]
- 3.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 4.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections—an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 5.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 6.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, et al. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–55. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–50. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–7. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Veerdonk FL, Marijnissen RJ, Joosten LA, Kullberg BJ, Drenth JP, Netea MG, et al. Milder clinical hyperimmunoglobulin E syndrome phenotype is associated with partial interleukin-17 deficiency. Clin Exp Immunol. 2010;159:57–64. doi: 10.1111/j.1365-2249.2009.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donabedian H, Gallin JI. The hyperimmunoglobulin E recurrent-infection (Job’s) syndrome. A review of the NIH experience and the literature. Medicine (Baltimore) 1983;62:195–208. doi: 10.1097/00005792-198307000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Minegishi Y, Saito M. Molecular mechanisms of the immunological abnormalities in hyper-IgE syndrome. Ann N Y Acad Sci. 2011;1246:34–40. doi: 10.1111/j.1749-6632.2011.06280.x. [DOI] [PubMed] [Google Scholar]
- 15.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore) 2012;91:E1–19. doi: 10.1097/MD.0b013e31825f95b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–18. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KD, Hsu AP, Ryu MJ, Wang J, Gao X, Boyer ME, et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J Clin Invest. 2012;122:3692–704. doi: 10.1172/JCI61623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster B, Prussin C, Liu F, Whitmire JK, Whitton JL. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2007;Chapter 6(Unit 6.24) doi: 10.1002/0471142735.im0624s78. [DOI] [PubMed] [Google Scholar]
- 20.Kuhns DB, Decarlo E, Hawk DM, Gallin JI. Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J Clin Invest. 1992;89:1734–40. doi: 10.1172/JCI115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright DG, Meierovics AI, Foxley JM. Assessing the delivery of neutrophils to tissues in neutropenia. Blood. 1986;67:1023–30. [PubMed] [Google Scholar]
- 22.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolls JK, McCray PB, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–35. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 25.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger BD, Woellner C, Dueckers G, Sawalle-Belohradsky J, Hagl B, Anslinger K, et al. Challenges of genetic counseling in patients with autosomal dominant diseases, such as the hyper-IgE syndrome (STAT3-HIES) J Allergy Clin Immunol. 2012;130:1426–8. doi: 10.1016/j.jaci.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Goussetis E, Peristeri I, Kitra V, Traeger-Synodinos J, Theodosaki M, Psarra K, et al. Successful long-term immunologic reconstitution by allogeneic hematopoietic stem cell transplantation cures patients with autosomal dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2010;126:392–4. doi: 10.1016/j.jaci.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Sekhsaria V, Dodd LE, Hsu AP, Heimall JR, Freeman AF, Ding L, et al. Plasma metalloproteinase levels are dysregulated in signal transducer and activator of transcription 3 mutated hyper-IgE syndrome. J Allergy Clin Immunol. 2011;128:1124–7. doi: 10.1016/j.jaci.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heimall J, Arora M, Holland SM, Heller T, Freeman AF. Gastrointestinal manifestations of autosomal dominant hyper IgE syndrome. Presented in abstract form at the Clinical Immunology Society (CIS) Annual Meeting; Philadelphia. 2010. [Google Scholar]
- 30.Nieminen P, Morgan NV, Fenwick AL, Parmanen S, Veistinen L, Mikkola ML, et al. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet. 2011;89:67–81. doi: 10.1016/j.ajhg.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]