Abstract
In the title compound, C36H28O4, the 1-naphthoyl groups at the 1- and 8-positions of the central 2,7-diethoxynaphthalene ring system are aligned almost antiparallel and make a dihedral angle of 76.59 (4)°. The dihedral angles between the central 2,7-diethoxynaphthalene ring system and the terminal naphthalene ring systems are 86.48 (4) and 83.97 (4)°. In the crystal, C—H⋯π interactions between the central naphthalene ring systems and the naphthoyl groups are observed along the a axis, with the molecules forming a columnar structure. The columns are linked into chains parallel to the b axis by C—H⋯O interactions.
Related literature
For electrophilic aroylation of naphthalene derivatives, see: Okamoto & Yonezawa (2009 ▶); Okamoto et al. (2011 ▶). For the structures of closely related compounds, see: Nakaema et al. (2008 ▶); Tsumuki et al. (2011 ▶); Sakamoto et al. (2012 ▶); Isogai et al. (2013 ▶); Tsumuki et al. (2013 ▶); Yoshiwaka et al. (2013 ▶).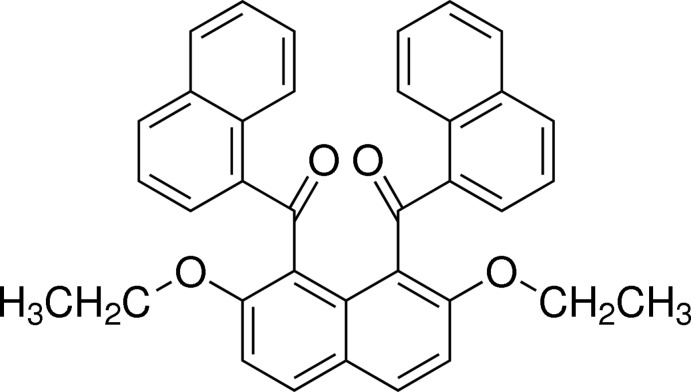
Experimental
Crystal data
C36H28O4
M r = 524.58
Triclinic,

a = 8.76532 (16) Å
b = 11.4266 (2) Å
c = 14.1972 (3) Å
α = 99.080 (1)°
β = 99.036 (1)°
γ = 104.277 (1)°
V = 1331.94 (4) Å3
Z = 2
Cu Kα radiation
μ = 0.67 mm−1
T = 193 K
0.60 × 0.40 × 0.20 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Absorption correction: numerical (NUMABS; Higashi, 1999 ▶) T min = 0.689, T max = 0.877
24143 measured reflections
4800 independent reflections
4142 reflections with I > 2σ(I)
R int = 0.043
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.106
S = 1.07
4800 reflections
364 parameters
H-atom parameters constrained
Δρmax = 0.20 e Å−3
Δρmin = −0.16 e Å−3
Data collection: PROCESS-AUTO (Rigaku, 1998 ▶); cell refinement: PROCESS-AUTO; data reduction: PROCESS-AUTO; program(s) used to solve structure: Il Milione (Burla et al., 2007 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPIII (Burnett & Johnson, 1996 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813005710/pk2467sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813005710/pk2467Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813005710/pk2467Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg4 and Cg6 are the centroids of the C16–C21 and C27–C32 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯Cg4i | 0.95 | 2.77 | 3.5662 (15) | 142 |
| C7—H7⋯Cg6i | 0.95 | 2.76 | 3.5662 (16) | 143 |
| C30—H30⋯O2ii | 0.95 | 2.53 | 3.3289 (19) | 142 |
| C34—H34A⋯O1iii | 0.98 | 2.47 | 3.423 (2) | 163 |
| C35—H35B⋯O2iv | 0.99 | 2.59 | 3.5476 (17) | 163 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Acknowledgments
The authors express their gratitude to Professor Keiichi Noguchi, Instrumentation Analysis Center, Tokyo University of Agriculture & Technology, for technical advice. This work was partially supported by an Iron and Steel Institute of Japan (ISIJ) Research Promotion Grant.
supplementary crystallographic information
Comment
In the course of our study on selective electrophilic aromatic aroylation of the naphthalene ring core, 1,8-diaroylnaphthalene compounds have proved to be formed regioselectively by the aid of a suitable acidic mediator (Okamoto & Yonezawa, 2009, Okamoto et al., 2011). Recently, we have reported the crystal structures of several 1,8-diaroylated naphthalene analogues exemplified by 1,8-dibenzoyl-2,7-dimethoxynaphthalene (Nakaema et al., 2008) and [2,7-dimethoxy-8-(2-naphthoyl)naphthalen-1-yl](naphthalen-2-yl)methanone (Tsumuki et al., 2011). Furthermore, crystal structures of 1,8-diaroylnaphthalene analogues bearing various alkoxy and aryloxy groups at the 2,7-positions such as 1,8-dibenzoylnaphthalene-2,7-diyl dibenzoate (Sakamoto et al., 2012) and [8-(4-phenoxybenzoyl)-2,7-bis(propan-2-yloxy)naphthalen-1-yl](4-phenoxyphenyl)methanone (Yoshiwaka et al., 2013) have been also revealed. Some 1,8-diaroylnaphthalene compounds bearing the ethoxy group, {2,7-diethoxy-8-[(naphthalen-2-yl)-carbonyl]naphthalen-1-yl}(naphthalen-2-yl)methanone (Tsumuki et al., 2013) and (8-benzoyl-2,7-diethoxynaphthalen-1-yl)(phenyl)methanone (Isogai et al., 2013), are stabilized by the molecular packing of C—H···O interactions between the aroyl groups. As a part of our ongoing studies on the molecular structures of these kinds of homologous molecules, the X-ray crystal structure of the title compound, the 2,7-diethoxynaphthalene bearing α-naphthoyl groups at the 1,8-positions, is reported on herein.
The molecular structure of the title molecule is illustrated in Fig.1. The two terminal naphthoyl groups are oriented in opposite directions and are twisted away from the central 2,7-diethoxynaphthalene unit. The carbonyl moieties deviate slightly from the attached naphthalene rings. The dihedral angle between the two naphthalene rings of the terminal naphthoyl groups (C12–C21 and C23–C32) is 76.59 (4)°. The dihedral angles between the terminal naphthalene rings and the central naphthalene ring (C1–C10) are 86.48 (4) and 83.97 (4)°. The torsion angles between the carbonyl moieties and the central naphthalene ring are -60.91 (16)° (C10—C1—C11—O1) and -65.50 (17)° (C10—C9—C22—O2), and those between the carbonyl moieties and the terminal naphthalene rings are -47.50 (17)° (O1—C11—C12—C21) and -46.38 (17)° (O2—C22—C23—C32).
In the molecular packing, C—H···π interactions between the central naphthalene rings and the naphthoyl groups are observed along the a axis, and form columnar structures (Fig. 2, 3 and Table 1). Each column is linked into chains along the b axis by C—H···O interactions (Fig. 4 and Table 1).
Experimental
To a solution of 1-naphthoyl chloride (630 mg, 3.3 mmol) and TiCl4 (1.88 g, 9.9 mmol) in CH2Cl2 (2.5 ml), 2,7-diethoxynaphthalene (220 mg, 1.0 mmol) was added. The reaction mixture was stirred at r.t. for 3 h, then poured into ice-cold water (20 ml). The aqueous layer was extracted with CHCl3 (20 ml × 3). The combined organic extracts were washed with 2 M aqueous NaOH (25 ml × 3) followed by washing with brine (25 ml × 3). The organic layer was dried over anhydrous MgSO4. The solvent was removed under reduced pressure to give a cake (yield 95%). The crude product was purified by recrystallization from chloroform (isolated yield 60%). Colorless platelet single crystals suitable for X-ray diffraction were obtained by repeated crystallization from chloroform.
1H NMR δ (500 MHz, CDCl3): 0.57 (6H, broad), 3.78 (4H, broad), 7.13 (2H, d, J = 9.0 Hz), 7.27–7.33 (6H, m), 7.71–7.83 (6H, m), 7.91 (2H, d, J = 9.0 Hz), 8.15 (2H, broad) p.p.m.; 13C NMR δ (125 MHz, CDCl3): 14.12, 64.99, 112.73, 124.29, 124.70,125.53, 125.65, 126.45, 127.26, 127.88, 130.31, 130.52, 130.85, 132.33, 132.39,133.61, 137.15, 156.87, 199.49 p.p.m.; IR (KBr): 1658, 1607, 1512, 1471, 1275 cm-1; HRMS (m/z): [M+H]+ calcd. for C36H29O4, 525.2066, found, 525.2032.
Refinement
All the H atoms were located in a difference Fourier map and were subsequently refined as riding atoms: C—H = 0.95 (aromatic), 0.98 (methyl) and 0.99 (methylene) Å, with Uiso(H) = 1.2 Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound, with displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
The arrangement of the molecules in the crystal structure, viewed down the a axis.
Fig. 3.
A partial view of the crystal packing of the title compound, showing the intermolecular C—H···π interactions. Cg4 and Cg6 are centroid of the C16–C21 and C27–C32 (see Table 1 for details; symmetry codes: (i) 1 + x, y, z).
Fig. 4.
A partial view of the crystal packing of the title compound, showing the intermolecular C—H···O interactions (see Table 1 for details; symmetry codes: (ii) 1 - x, 2 - y, 2 - z; (iii) - x, - 1 - y, - 1 - z (iv); - x, 2 - y, 2 - z).
Crystal data
| C36H28O4 | Z = 2 |
| Mr = 524.58 | F(000) = 552 |
| Triclinic, P1 | Dx = 1.308 Mg m−3 |
| Hall symbol: -P 1 | Melting point = 506.6–508.4 K |
| a = 8.76532 (16) Å | Cu Kα radiation, λ = 1.54187 Å |
| b = 11.4266 (2) Å | Cell parameters from 20940 reflections |
| c = 14.1972 (3) Å | θ = 3.2–68.2° |
| α = 99.080 (1)° | µ = 0.67 mm−1 |
| β = 99.036 (1)° | T = 193 K |
| γ = 104.277 (1)° | Platelet, colorless |
| V = 1331.94 (4) Å3 | 0.60 × 0.40 × 0.20 mm |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 4800 independent reflections |
| Radiation source: rotating anode | 4142 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.043 |
| Detector resolution: 10.000 pixels mm-1 | θmax = 68.2°, θmin = 3.2° |
| ω scans | h = −10→10 |
| Absorption correction: numerical (NUMABS; Higashi, 1999) | k = −13→13 |
| Tmin = 0.689, Tmax = 0.877 | l = −17→17 |
| 24143 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.037 | H-atom parameters constrained |
| wR(F2) = 0.106 | w = 1/[σ2(Fo2) + (0.058P)2 + 0.2087P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max = 0.001 |
| 4800 reflections | Δρmax = 0.20 e Å−3 |
| 364 parameters | Δρmin = −0.16 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0072 (5) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.08739 (10) | 0.64809 (8) | 0.66867 (6) | 0.0379 (2) | |
| O2 | 0.19599 (10) | 0.86929 (8) | 0.83185 (6) | 0.0388 (2) | |
| O3 | −0.20151 (10) | 0.67148 (9) | 0.48478 (6) | 0.0419 (2) | |
| O4 | −0.01872 (10) | 0.83966 (9) | 1.00812 (6) | 0.0429 (2) | |
| C1 | −0.13726 (14) | 0.73032 (10) | 0.65387 (9) | 0.0312 (3) | |
| C2 | −0.25357 (15) | 0.70050 (11) | 0.56859 (9) | 0.0345 (3) | |
| C3 | −0.41501 (15) | 0.69700 (12) | 0.56983 (10) | 0.0399 (3) | |
| H3 | −0.4924 | 0.6770 | 0.5106 | 0.048* | |
| C4 | −0.45851 (15) | 0.72264 (12) | 0.65675 (10) | 0.0407 (3) | |
| H4 | −0.5671 | 0.7211 | 0.6576 | 0.049* | |
| C5 | −0.34659 (14) | 0.75145 (11) | 0.74590 (9) | 0.0360 (3) | |
| C6 | −0.39763 (15) | 0.77691 (13) | 0.83424 (10) | 0.0429 (3) | |
| H6 | −0.5073 | 0.7742 | 0.8325 | 0.052* | |
| C7 | −0.29423 (16) | 0.80531 (13) | 0.92212 (10) | 0.0430 (3) | |
| H7 | −0.3305 | 0.8231 | 0.9810 | 0.052* | |
| C8 | −0.13216 (15) | 0.80769 (12) | 0.92383 (9) | 0.0364 (3) | |
| C9 | −0.07595 (14) | 0.78095 (11) | 0.83942 (9) | 0.0313 (3) | |
| C10 | −0.18165 (14) | 0.75431 (10) | 0.74623 (9) | 0.0313 (3) | |
| C11 | 0.02752 (14) | 0.72402 (10) | 0.63903 (8) | 0.0306 (3) | |
| C12 | 0.11099 (14) | 0.81261 (11) | 0.58353 (9) | 0.0327 (3) | |
| C13 | 0.11822 (17) | 0.93448 (12) | 0.61063 (10) | 0.0440 (3) | |
| H13 | 0.0676 | 0.9596 | 0.6617 | 0.053* | |
| C14 | 0.1996 (2) | 1.02299 (13) | 0.56390 (13) | 0.0560 (4) | |
| H14 | 0.2063 | 1.1076 | 0.5848 | 0.067* | |
| C15 | 0.26881 (18) | 0.98776 (14) | 0.48875 (12) | 0.0530 (4) | |
| H15 | 0.3246 | 1.0484 | 0.4581 | 0.064* | |
| C16 | 0.25873 (15) | 0.86244 (12) | 0.45584 (9) | 0.0402 (3) | |
| C17 | 0.31914 (16) | 0.82289 (15) | 0.37281 (10) | 0.0489 (4) | |
| H17 | 0.3743 | 0.8826 | 0.3412 | 0.059* | |
| C18 | 0.29954 (16) | 0.70146 (15) | 0.33786 (10) | 0.0481 (4) | |
| H18 | 0.3374 | 0.6766 | 0.2809 | 0.058* | |
| C19 | 0.22326 (15) | 0.61257 (13) | 0.38595 (9) | 0.0427 (3) | |
| H19 | 0.2106 | 0.5277 | 0.3615 | 0.051* | |
| C20 | 0.16714 (14) | 0.64674 (11) | 0.46737 (9) | 0.0351 (3) | |
| H20 | 0.1180 | 0.5853 | 0.4997 | 0.042* | |
| C21 | 0.18097 (13) | 0.77222 (11) | 0.50452 (8) | 0.0325 (3) | |
| C22 | 0.10078 (14) | 0.78898 (10) | 0.85622 (8) | 0.0307 (3) | |
| C23 | 0.15239 (14) | 0.69517 (11) | 0.90702 (8) | 0.0321 (3) | |
| C24 | 0.06855 (16) | 0.57328 (11) | 0.87330 (9) | 0.0385 (3) | |
| H24 | −0.0238 | 0.5518 | 0.8222 | 0.046* | |
| C25 | 0.11646 (19) | 0.47971 (13) | 0.91269 (11) | 0.0484 (3) | |
| H25 | 0.0583 | 0.3957 | 0.8873 | 0.058* | |
| C26 | 0.2463 (2) | 0.50928 (14) | 0.98733 (11) | 0.0515 (4) | |
| H26 | 0.2797 | 0.4455 | 1.0128 | 0.062* | |
| C27 | 0.33248 (16) | 0.63426 (14) | 1.02763 (9) | 0.0426 (3) | |
| C28 | 0.46076 (18) | 0.66683 (18) | 1.10997 (11) | 0.0558 (4) | |
| H28 | 0.4948 | 0.6036 | 1.1360 | 0.067* | |
| C29 | 0.53555 (17) | 0.78660 (18) | 1.15211 (10) | 0.0583 (4) | |
| H29 | 0.6198 | 0.8066 | 1.2080 | 0.070* | |
| C30 | 0.48893 (16) | 0.88165 (16) | 1.11340 (10) | 0.0518 (4) | |
| H30 | 0.5410 | 0.9654 | 1.1437 | 0.062* | |
| C31 | 0.36913 (15) | 0.85385 (13) | 1.03242 (9) | 0.0408 (3) | |
| H31 | 0.3408 | 0.9188 | 1.0061 | 0.049* | |
| C32 | 0.28633 (14) | 0.72967 (12) | 0.98702 (8) | 0.0351 (3) | |
| C33 | −0.31667 (16) | 0.62668 (13) | 0.39438 (9) | 0.0426 (3) | |
| H33A | −0.3755 | 0.6882 | 0.3820 | 0.051* | |
| H33B | −0.3954 | 0.5489 | 0.3965 | 0.051* | |
| C34 | −0.2252 (2) | 0.60500 (16) | 0.31584 (10) | 0.0560 (4) | |
| H34A | −0.1664 | 0.5449 | 0.3294 | 0.067* | |
| H34B | −0.1490 | 0.6829 | 0.3138 | 0.067* | |
| H34C | −0.3005 | 0.5730 | 0.2529 | 0.067* | |
| C35 | −0.06724 (16) | 0.86450 (12) | 1.09918 (9) | 0.0395 (3) | |
| H35A | −0.1508 | 0.7922 | 1.1063 | 0.047* | |
| H35B | −0.1118 | 0.9364 | 1.1028 | 0.047* | |
| C36 | 0.07978 (18) | 0.89118 (14) | 1.17804 (10) | 0.0472 (3) | |
| H36A | 0.1255 | 0.8209 | 1.1717 | 0.057* | |
| H36B | 0.0504 | 0.9048 | 1.2419 | 0.057* | |
| H36C | 0.1595 | 0.9652 | 1.1721 | 0.057* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0401 (5) | 0.0440 (5) | 0.0379 (5) | 0.0200 (4) | 0.0118 (4) | 0.0157 (4) |
| O2 | 0.0334 (5) | 0.0402 (5) | 0.0419 (5) | 0.0070 (4) | 0.0067 (4) | 0.0117 (4) |
| O3 | 0.0345 (5) | 0.0570 (6) | 0.0308 (5) | 0.0128 (4) | 0.0012 (4) | 0.0042 (4) |
| O4 | 0.0364 (5) | 0.0645 (6) | 0.0302 (5) | 0.0211 (4) | 0.0071 (4) | 0.0055 (4) |
| C1 | 0.0292 (6) | 0.0307 (6) | 0.0343 (6) | 0.0085 (5) | 0.0052 (5) | 0.0093 (5) |
| C2 | 0.0336 (6) | 0.0352 (6) | 0.0346 (6) | 0.0094 (5) | 0.0049 (5) | 0.0094 (5) |
| C3 | 0.0307 (6) | 0.0474 (7) | 0.0384 (7) | 0.0080 (5) | −0.0011 (5) | 0.0119 (6) |
| C4 | 0.0268 (6) | 0.0497 (8) | 0.0474 (7) | 0.0108 (5) | 0.0055 (5) | 0.0169 (6) |
| C5 | 0.0287 (6) | 0.0412 (7) | 0.0405 (7) | 0.0112 (5) | 0.0070 (5) | 0.0132 (5) |
| C6 | 0.0296 (6) | 0.0597 (8) | 0.0467 (8) | 0.0186 (6) | 0.0125 (5) | 0.0177 (6) |
| C7 | 0.0386 (7) | 0.0600 (8) | 0.0387 (7) | 0.0223 (6) | 0.0144 (5) | 0.0144 (6) |
| C8 | 0.0337 (6) | 0.0440 (7) | 0.0345 (6) | 0.0150 (5) | 0.0059 (5) | 0.0108 (5) |
| C9 | 0.0301 (6) | 0.0331 (6) | 0.0337 (6) | 0.0122 (5) | 0.0073 (5) | 0.0091 (5) |
| C10 | 0.0292 (6) | 0.0313 (6) | 0.0353 (6) | 0.0099 (5) | 0.0059 (5) | 0.0104 (5) |
| C11 | 0.0311 (6) | 0.0336 (6) | 0.0257 (5) | 0.0091 (5) | 0.0033 (4) | 0.0041 (5) |
| C12 | 0.0274 (6) | 0.0352 (6) | 0.0343 (6) | 0.0083 (5) | 0.0016 (5) | 0.0088 (5) |
| C13 | 0.0447 (7) | 0.0376 (7) | 0.0500 (8) | 0.0126 (6) | 0.0082 (6) | 0.0093 (6) |
| C14 | 0.0584 (9) | 0.0336 (7) | 0.0743 (11) | 0.0085 (6) | 0.0098 (8) | 0.0168 (7) |
| C15 | 0.0466 (8) | 0.0470 (8) | 0.0667 (10) | 0.0039 (6) | 0.0119 (7) | 0.0298 (7) |
| C16 | 0.0292 (6) | 0.0499 (8) | 0.0415 (7) | 0.0067 (5) | 0.0026 (5) | 0.0206 (6) |
| C17 | 0.0341 (7) | 0.0743 (10) | 0.0429 (8) | 0.0104 (6) | 0.0093 (6) | 0.0312 (7) |
| C18 | 0.0372 (7) | 0.0768 (11) | 0.0331 (7) | 0.0175 (7) | 0.0084 (5) | 0.0155 (7) |
| C19 | 0.0342 (7) | 0.0552 (8) | 0.0363 (7) | 0.0126 (6) | 0.0048 (5) | 0.0044 (6) |
| C20 | 0.0283 (6) | 0.0410 (7) | 0.0340 (6) | 0.0067 (5) | 0.0042 (5) | 0.0084 (5) |
| C21 | 0.0233 (5) | 0.0415 (7) | 0.0317 (6) | 0.0071 (5) | 0.0006 (4) | 0.0128 (5) |
| C22 | 0.0300 (6) | 0.0348 (6) | 0.0265 (6) | 0.0098 (5) | 0.0049 (5) | 0.0034 (5) |
| C23 | 0.0303 (6) | 0.0391 (6) | 0.0311 (6) | 0.0136 (5) | 0.0102 (5) | 0.0089 (5) |
| C24 | 0.0417 (7) | 0.0396 (7) | 0.0356 (6) | 0.0119 (5) | 0.0100 (5) | 0.0086 (5) |
| C25 | 0.0640 (9) | 0.0384 (7) | 0.0488 (8) | 0.0193 (6) | 0.0170 (7) | 0.0132 (6) |
| C26 | 0.0662 (10) | 0.0569 (9) | 0.0508 (8) | 0.0367 (8) | 0.0221 (7) | 0.0259 (7) |
| C27 | 0.0406 (7) | 0.0661 (9) | 0.0345 (7) | 0.0275 (6) | 0.0158 (5) | 0.0210 (6) |
| C28 | 0.0453 (8) | 0.0967 (13) | 0.0415 (8) | 0.0351 (8) | 0.0136 (6) | 0.0318 (8) |
| C29 | 0.0339 (7) | 0.1093 (14) | 0.0337 (7) | 0.0193 (8) | 0.0065 (6) | 0.0219 (8) |
| C30 | 0.0329 (7) | 0.0773 (10) | 0.0369 (7) | 0.0036 (7) | 0.0087 (6) | 0.0046 (7) |
| C31 | 0.0311 (6) | 0.0548 (8) | 0.0358 (7) | 0.0099 (6) | 0.0092 (5) | 0.0079 (6) |
| C32 | 0.0309 (6) | 0.0505 (7) | 0.0303 (6) | 0.0171 (5) | 0.0125 (5) | 0.0116 (5) |
| C33 | 0.0426 (7) | 0.0432 (7) | 0.0364 (7) | 0.0109 (6) | −0.0042 (5) | 0.0059 (5) |
| C34 | 0.0647 (10) | 0.0738 (10) | 0.0340 (7) | 0.0399 (8) | −0.0017 (6) | 0.0034 (7) |
| C35 | 0.0448 (7) | 0.0451 (7) | 0.0345 (7) | 0.0209 (6) | 0.0129 (6) | 0.0074 (5) |
| C36 | 0.0519 (8) | 0.0569 (8) | 0.0339 (7) | 0.0207 (7) | 0.0083 (6) | 0.0042 (6) |
Geometric parameters (Å, º)
| O1—C11 | 1.2148 (14) | C18—H18 | 0.9500 |
| O2—C22 | 1.2131 (14) | C19—C20 | 1.3644 (18) |
| O3—C2 | 1.3617 (15) | C19—H19 | 0.9500 |
| O3—C33 | 1.4350 (14) | C20—C21 | 1.4157 (18) |
| O4—C8 | 1.3650 (15) | C20—H20 | 0.9500 |
| O4—C35 | 1.4315 (15) | C22—C23 | 1.5015 (16) |
| C1—C2 | 1.3901 (16) | C23—C24 | 1.3723 (17) |
| C1—C10 | 1.4301 (17) | C23—C32 | 1.4266 (16) |
| C1—C11 | 1.5093 (16) | C24—C25 | 1.4031 (18) |
| C2—C3 | 1.4088 (18) | C24—H24 | 0.9500 |
| C3—C4 | 1.3576 (19) | C25—C26 | 1.361 (2) |
| C3—H3 | 0.9500 | C25—H25 | 0.9500 |
| C4—C5 | 1.4113 (17) | C26—C27 | 1.418 (2) |
| C4—H4 | 0.9500 | C26—H26 | 0.9500 |
| C5—C6 | 1.4081 (18) | C27—C28 | 1.419 (2) |
| C5—C10 | 1.4373 (17) | C27—C32 | 1.4239 (18) |
| C6—C7 | 1.3621 (19) | C28—C29 | 1.355 (2) |
| C6—H6 | 0.9500 | C28—H28 | 0.9500 |
| C7—C8 | 1.4104 (18) | C29—C30 | 1.409 (2) |
| C7—H7 | 0.9500 | C29—H29 | 0.9500 |
| C8—C9 | 1.3843 (17) | C30—C31 | 1.3661 (19) |
| C9—C10 | 1.4319 (16) | C30—H30 | 0.9500 |
| C9—C22 | 1.5073 (16) | C31—C32 | 1.4185 (19) |
| C11—C12 | 1.4994 (16) | C31—H31 | 0.9500 |
| C12—C13 | 1.3678 (18) | C33—C34 | 1.496 (2) |
| C12—C21 | 1.4283 (17) | C33—H33A | 0.9900 |
| C13—C14 | 1.406 (2) | C33—H33B | 0.9900 |
| C13—H13 | 0.9500 | C34—H34A | 0.9800 |
| C14—C15 | 1.363 (2) | C34—H34B | 0.9800 |
| C14—H14 | 0.9500 | C34—H34C | 0.9800 |
| C15—C16 | 1.411 (2) | C35—C36 | 1.5008 (19) |
| C15—H15 | 0.9500 | C35—H35A | 0.9900 |
| C16—C17 | 1.421 (2) | C35—H35B | 0.9900 |
| C16—C21 | 1.4251 (17) | C36—H36A | 0.9800 |
| C17—C18 | 1.356 (2) | C36—H36B | 0.9800 |
| C17—H17 | 0.9500 | C36—H36C | 0.9800 |
| C18—C19 | 1.404 (2) | ||
| C2—O3—C33 | 119.13 (10) | C21—C20—H20 | 119.5 |
| C8—O4—C35 | 118.97 (10) | C20—C21—C16 | 118.15 (12) |
| C2—C1—C10 | 119.84 (11) | C20—C21—C12 | 123.61 (10) |
| C2—C1—C11 | 114.61 (10) | C16—C21—C12 | 118.08 (11) |
| C10—C1—C11 | 125.36 (10) | O2—C22—C23 | 122.19 (10) |
| O3—C2—C1 | 115.41 (11) | O2—C22—C9 | 121.24 (10) |
| O3—C2—C3 | 122.72 (11) | C23—C22—C9 | 116.54 (10) |
| C1—C2—C3 | 121.84 (12) | C24—C23—C32 | 120.11 (11) |
| C4—C3—C2 | 119.11 (11) | C24—C23—C22 | 118.12 (11) |
| C4—C3—H3 | 120.4 | C32—C23—C22 | 121.76 (11) |
| C2—C3—H3 | 120.4 | C23—C24—C25 | 121.34 (13) |
| C3—C4—C5 | 121.73 (12) | C23—C24—H24 | 119.3 |
| C3—C4—H4 | 119.1 | C25—C24—H24 | 119.3 |
| C5—C4—H4 | 119.1 | C26—C25—C24 | 119.97 (13) |
| C6—C5—C4 | 119.63 (11) | C26—C25—H25 | 120.0 |
| C6—C5—C10 | 120.35 (11) | C24—C25—H25 | 120.0 |
| C4—C5—C10 | 120.01 (12) | C25—C26—C27 | 120.78 (12) |
| C7—C6—C5 | 121.85 (12) | C25—C26—H26 | 119.6 |
| C7—C6—H6 | 119.1 | C27—C26—H26 | 119.6 |
| C5—C6—H6 | 119.1 | C26—C27—C28 | 121.37 (13) |
| C6—C7—C8 | 118.56 (12) | C26—C27—C32 | 119.59 (12) |
| C6—C7—H7 | 120.7 | C28—C27—C32 | 119.00 (14) |
| C8—C7—H7 | 120.7 | C29—C28—C27 | 121.08 (14) |
| O4—C8—C9 | 115.18 (11) | C29—C28—H28 | 119.5 |
| O4—C8—C7 | 122.69 (11) | C27—C28—H28 | 119.5 |
| C9—C8—C7 | 122.11 (11) | C28—C29—C30 | 120.29 (13) |
| C8—C9—C10 | 120.16 (11) | C28—C29—H29 | 119.9 |
| C8—C9—C22 | 114.25 (10) | C30—C29—H29 | 119.9 |
| C10—C9—C22 | 125.53 (10) | C31—C30—C29 | 120.27 (15) |
| C1—C10—C9 | 125.67 (11) | C31—C30—H30 | 119.9 |
| C1—C10—C5 | 117.42 (11) | C29—C30—H30 | 119.9 |
| C9—C10—C5 | 116.91 (11) | C30—C31—C32 | 121.15 (14) |
| O1—C11—C12 | 121.76 (11) | C30—C31—H31 | 119.4 |
| O1—C11—C1 | 121.13 (10) | C32—C31—H31 | 119.4 |
| C12—C11—C1 | 117.07 (10) | C31—C32—C27 | 118.16 (12) |
| C13—C12—C21 | 120.45 (11) | C31—C32—C23 | 123.58 (11) |
| C13—C12—C11 | 117.95 (11) | C27—C32—C23 | 118.12 (12) |
| C21—C12—C11 | 121.60 (10) | O3—C33—C34 | 107.15 (11) |
| C12—C13—C14 | 120.83 (14) | O3—C33—H33A | 110.3 |
| C12—C13—H13 | 119.6 | C34—C33—H33A | 110.3 |
| C14—C13—H13 | 119.6 | O3—C33—H33B | 110.3 |
| C15—C14—C13 | 120.21 (14) | C34—C33—H33B | 110.3 |
| C15—C14—H14 | 119.9 | H33A—C33—H33B | 108.5 |
| C13—C14—H14 | 119.9 | C33—C34—H34A | 109.5 |
| C14—C15—C16 | 120.85 (12) | C33—C34—H34B | 109.5 |
| C14—C15—H15 | 119.6 | H34A—C34—H34B | 109.5 |
| C16—C15—H15 | 119.6 | C33—C34—H34C | 109.5 |
| C15—C16—C17 | 121.61 (12) | H34A—C34—H34C | 109.5 |
| C15—C16—C21 | 119.50 (13) | H34B—C34—H34C | 109.5 |
| C17—C16—C21 | 118.84 (13) | O4—C35—C36 | 107.01 (11) |
| C18—C17—C16 | 121.22 (12) | O4—C35—H35A | 110.3 |
| C18—C17—H17 | 119.4 | C36—C35—H35A | 110.3 |
| C16—C17—H17 | 119.4 | O4—C35—H35B | 110.3 |
| C17—C18—C19 | 119.91 (13) | C36—C35—H35B | 110.3 |
| C17—C18—H18 | 120.0 | H35A—C35—H35B | 108.6 |
| C19—C18—H18 | 120.0 | C35—C36—H36A | 109.5 |
| C20—C19—C18 | 120.76 (13) | C35—C36—H36B | 109.5 |
| C20—C19—H19 | 119.6 | H36A—C36—H36B | 109.5 |
| C18—C19—H19 | 119.6 | C35—C36—H36C | 109.5 |
| C19—C20—C21 | 121.07 (12) | H36A—C36—H36C | 109.5 |
| C19—C20—H20 | 119.5 | H36B—C36—H36C | 109.5 |
| C33—O3—C2—C1 | 173.31 (10) | C14—C15—C16—C21 | −2.7 (2) |
| C33—O3—C2—C3 | −4.73 (17) | C15—C16—C17—C18 | −175.60 (13) |
| C10—C1—C2—O3 | −175.91 (10) | C21—C16—C17—C18 | 2.06 (19) |
| C11—C1—C2—O3 | −0.66 (15) | C16—C17—C18—C19 | −2.2 (2) |
| C10—C1—C2—C3 | 2.16 (18) | C17—C18—C19—C20 | 0.5 (2) |
| C11—C1—C2—C3 | 177.41 (11) | C18—C19—C20—C21 | 1.36 (18) |
| O3—C2—C3—C4 | 177.47 (11) | C19—C20—C21—C16 | −1.48 (17) |
| C1—C2—C3—C4 | −0.46 (19) | C19—C20—C21—C12 | 173.70 (11) |
| C2—C3—C4—C5 | −0.7 (2) | C15—C16—C21—C20 | 177.51 (11) |
| C3—C4—C5—C6 | −179.75 (12) | C17—C16—C21—C20 | −0.20 (17) |
| C3—C4—C5—C10 | 0.06 (19) | C15—C16—C21—C12 | 2.06 (17) |
| C4—C5—C6—C7 | −179.93 (12) | C17—C16—C21—C12 | −175.65 (10) |
| C10—C5—C6—C7 | 0.3 (2) | C13—C12—C21—C20 | −174.76 (12) |
| C5—C6—C7—C8 | −0.7 (2) | C11—C12—C21—C20 | 5.09 (17) |
| C35—O4—C8—C9 | −177.76 (10) | C13—C12—C21—C16 | 0.42 (16) |
| C35—O4—C8—C7 | 3.98 (18) | C11—C12—C21—C16 | −179.73 (10) |
| C6—C7—C8—O4 | 177.47 (12) | C8—C9—C22—O2 | −111.50 (13) |
| C6—C7—C8—C9 | −0.7 (2) | C10—C9—C22—O2 | 65.60 (17) |
| O4—C8—C9—C10 | −175.82 (10) | C8—C9—C22—C23 | 66.74 (14) |
| C7—C8—C9—C10 | 2.45 (19) | C10—C9—C22—C23 | −116.16 (12) |
| O4—C8—C9—C22 | 1.45 (16) | O2—C22—C23—C24 | −132.46 (12) |
| C7—C8—C9—C22 | 179.73 (11) | C9—C22—C23—C24 | 49.32 (15) |
| C2—C1—C10—C9 | 177.29 (11) | O2—C22—C23—C32 | 46.38 (17) |
| C11—C1—C10—C9 | 2.59 (18) | C9—C22—C23—C32 | −131.84 (11) |
| C2—C1—C10—C5 | −2.66 (17) | C32—C23—C24—C25 | −3.29 (18) |
| C11—C1—C10—C5 | −177.36 (10) | C22—C23—C24—C25 | 175.57 (12) |
| C8—C9—C10—C1 | 177.30 (11) | C23—C24—C25—C26 | 1.5 (2) |
| C22—C9—C10—C1 | 0.36 (19) | C24—C25—C26—C27 | 1.4 (2) |
| C8—C9—C10—C5 | −2.76 (17) | C25—C26—C27—C28 | 175.37 (13) |
| C22—C9—C10—C5 | −179.70 (10) | C25—C26—C27—C32 | −2.4 (2) |
| C6—C5—C10—C1 | −178.61 (11) | C26—C27—C28—C29 | −175.54 (13) |
| C4—C5—C10—C1 | 1.59 (17) | C32—C27—C28—C29 | 2.3 (2) |
| C6—C5—C10—C9 | 1.45 (17) | C27—C28—C29—C30 | −1.3 (2) |
| C4—C5—C10—C9 | −178.35 (11) | C28—C29—C30—C31 | −0.7 (2) |
| C2—C1—C11—O1 | −114.04 (12) | C29—C30—C31—C32 | 1.7 (2) |
| C10—C1—C11—O1 | 60.90 (16) | C30—C31—C32—C27 | −0.59 (18) |
| C2—C1—C11—C12 | 64.06 (14) | C30—C31—C32—C23 | 175.03 (11) |
| C10—C1—C11—C12 | −121.00 (12) | C26—C27—C32—C31 | 176.52 (12) |
| O1—C11—C12—C13 | −132.64 (13) | C28—C27—C32—C31 | −1.34 (17) |
| C1—C11—C12—C13 | 49.27 (15) | C26—C27—C32—C23 | 0.66 (18) |
| O1—C11—C12—C21 | 47.51 (16) | C28—C27—C32—C23 | −177.21 (11) |
| C1—C11—C12—C21 | −130.58 (11) | C24—C23—C32—C31 | −173.48 (12) |
| C21—C12—C13—C14 | −2.40 (19) | C22—C23—C32—C31 | 7.71 (17) |
| C11—C12—C13—C14 | 177.75 (12) | C24—C23—C32—C27 | 2.15 (17) |
| C12—C13—C14—C15 | 1.9 (2) | C22—C23—C32—C27 | −176.67 (11) |
| C13—C14—C15—C16 | 0.7 (2) | C2—O3—C33—C34 | 179.03 (11) |
| C14—C15—C16—C17 | 175.00 (14) | C8—O4—C35—C36 | 177.51 (11) |
Hydrogen-bond geometry (Å, º)
Cg4 and Cg6 are the centroids of the C16–C21 and C27–C32 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···Cg4i | 0.95 | 2.77 | 3.5662 (15) | 142 |
| C7—H7···Cg6i | 0.95 | 2.76 | 3.5662 (16) | 143 |
| C30—H30···O2ii | 0.95 | 2.53 | 3.3289 (19) | 142 |
| C34—H34A···O1iii | 0.98 | 2.47 | 3.423 (2) | 163 |
| C35—H35B···O2iv | 0.99 | 2.59 | 3.5476 (17) | 163 |
Symmetry codes: (i) x+1, y, z; (ii) −x+1, −y+2, −z+2; (iii) −x, −y+1, −z+1; (iv) −x, −y+2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PK2467).
References
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G., Siliqi, D. & Spagna, R. (2007). J. Appl. Cryst. 40, 609–613.
- Burnett, M. N. & &Johnson, C. K. (1996). ORTEPIII Report ORNL-6895. Oak Ridge National Laboratory. Tennessee, USA.
- Higashi, T. (1999). NUMABS Rigaku Corporation, Tokyo, Japan.
- Isogai, A., Tsumuki, T., Murohashi, S., Okamoto, A. & Yonezawa, N. (2013). Acta Cryst. E69, o71. [DOI] [PMC free article] [PubMed]
- Nakaema, K., Watanabe, S., Okamoto, A., Noguchi, K. & Yonezawa, N. (2008). Acta Cryst. E64, o807. [DOI] [PMC free article] [PubMed]
- Okamoto, A., Mitsui, R. & Yonezawa, N. (2011). Chem. Lett. 40, 1283–1284.
- Okamoto, A. & Yonezawa, N. (2009). Chem. Lett. 38, 914–915.
- Rigaku (1998). PROCESS-AUTO Rigaku Corporation, Tokyo, Japan.
- Sakamoto, R., Sasagawa, K., Hijikata, D., Okamoto, A. & Yonezawa, N. (2012). Acta Cryst. E68, o2454. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tsumuki, T., Hijikata, D., Okamoto, A., Oike, H. & Yonezawa, N. (2011). Acta Cryst. E67, o2095. [DOI] [PMC free article] [PubMed]
- Tsumuki, T., Okamoto, A., Oike, H. & Yonezawa, N. (2013). Acta Cryst. E69, o369. [DOI] [PMC free article] [PubMed]
- Yoshiwaka, S., Hijikata, D., Sasagawa, K., Okamoto, A. & Yonezawa, N. (2013). Acta Cryst. E69, o242. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813005710/pk2467sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813005710/pk2467Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813005710/pk2467Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report






