Abstract
Neurotransmitter:sodium symporters (NSSs) play a critical role in signaling by reuptake of neurotransmitters. Eukaryotic NSSs are chloride-dependent, whereas prokaryotic NSS homologs like LeuT are chloride-independent but contain an acidic residue (Glu290 in LeuT) at a site where eukaryotic NSSs have a serine. The LeuT-E290S mutant displays chloride-dependent activity. We show that, in LeuT-E290S cocrystallized with bromide or chloride, the anion is coordinated by side chain hydroxyls from Tyr47, Ser290, and Thr254 and the side chain amide of Gln250. The bound anion and the nearby sodium ion in the Na1 site organize a connection between their coordinating residues and the extracellular gate of LeuT through a continuous H-bond network. The specific insights from the structures, combined with results from substrate binding studies and molecular dynamics simulations, reveal an anion-dependent occlusion mechanism for NSS and shed light on the functional role of chloride binding.
Keywords: membrane transport, X-ray crystallography, SLC6, antidepressant, psychostimulant
The neurotransmitter:sodium symporter (NSS) family includes both prokaryotic and eukaryotic proteins. NSS proteins [in humans, they are also referred to as the solute carrier 6 (SLC6) family] (1) perform the sodium- and chloride-dependent reuptake of neurotransmitters such as serotonin, dopamine, GABA, norepinephrine, and glycine (by NSS members named SERT, DAT, GAT, NET, and GlyT, respectively) from the synapse into the presynaptic neuron (2, 3). NSS activity is of key importance in the termination of neurotransmission, and these transporters have been implicated in the pathophysiology and treatment of neuropsychiatric disorders, including depression, attention deficit hyperactivity disorder, schizophrenia, epilepsy, and autism (4, 5). NSSs are also the primary targets for psychostimulants, including cocaine, amphetamine, and ecstasy (5).
The structure of a bacterial NSS LeuT, an amino acid transporter from Aquifex aeolicus (6), revealed two Na+ ions bound in in Na1 and Na2 sites and the substrate, leucine, bound to a centrally located binding site that is hereafter termed the primary or S1 binding site (7). This structure and subsequent structures (8–12) have served as templates for the exploration of NSS function in a structural context (7, 13–27). Computational studies combined with binding and flux experiments have led to proposing a second substrate (S2) site and a molecular mechanism of Na+-substrate symport that depends on the allosteric interaction of substrate molecules in the two high-affinity sites (7, 11, 18, 23, 27). Although the binding of a second substrate molecule in the S2 site has not been shown crystallographically, there is much data to support it, including findings that we describe here, although controversies about the interpretation of some experimental findings must be noted (6, 7, 18, 20, 27–29).
Mammalian members of the NSS family mediate the uptake of their cognate substrates in a Na+- and Cl−-dependent manner, but in their bacterial counterparts [e.g., TnaT (30), Tyt1 (31), LeuT (6), and MhsT (32); see below], transport is Cl−-independent. Notably, transport by the bacterial NSS is stimulated by a reverse proton gradient through a proton-antiport mechanism as demonstrated in Tyt1 and MhsT (20), and mutagenesis studies have shown that the dependence on Cl− or H+ is interchangeable between the mammalian and bacterial NSS proteins and depends on the charge of the amino acid side chains at positions 286 and 290 (LeuT numbering). Thus, in the mammalian transporters, polar residues in these positions have been proposed to form a Cl− binding site, and their replacement with a negatively charged residue produced Cl−-independent transporters, albeit with reduced activity (25, 33). In contrast, substitution of a negatively charged residue in the Cl−-independent bacterial transporters with serine, which is found at the aligned position in Cl−-dependent eukaryotic transporters including SERT, DAT, and GAT-1 (24), yields Cl−-dependent transporters. Thus, substrate binding by LeuT-E290S is Cl−-dependent. Although the slow transport rates of LeuT made it impossible to measure reliable Na+/substrate symport-coupled H+-antiport (24), similar substitutions render transport Cl−-dependent in the TnaT Na+/tryptophan transporter of Symbiobacterium thermophilum (33), the Tyt1 Na+/tyrosine transporter of Fusobacterium nucleatum (20), and the MhsT Na+/hydrophobic amino acid transporter of the alkaliphilic Bacillus halodurans (20).
The negative charge, provided by either Cl− or an acidic residue, has been proposed to be necessary for proper Na+ binding (18, 24, 34). Furthermore, whereas the negatively charged Cl− is released to the cytoplasm during transport, a glutamate/aspartate side chain must be protonated for the return step of the transport cycle, which leads to the Na+/substrate symport-coupled H+ antiport observed in the Cl−-independent NSS (20, 24). The mechanistic role that the Cl− and/or a negative charge near the substrate binding site plays in the transport process is explored further here in the context of crystallographic insight into the architecture of the Cl− site in NSS as well as the manner in which its structural properties support the function.
Results
To establish the connection between the architecture of the binding sites for the amino acid substrate, Na+ and Cl− ions, and the functional properties of LeuT (both WT and the Cl−-dependent LeuT-E290S mutant), we probed the ion dependence of the binding kinetics in both constructs. We found that replacement of Glu at position 290 with Ser increased the dissociation constant (Kd) for Leu binding from about 50 nM (determined for LeuT-WT) (5, 6) by more than one order of magnitude (0.79 ± 0.05 µM) (Fig. 1A). Nevertheless, LeuT-E290S maintains functional S1 and S2 sites as detected by a binding stoichiometry of 1.86 ± 0.09 molecules of Leu per protein molecule. Saturation binding of Leu to LeuT-WT was well-fit by a single-site hyperbolic function, consistent with similar affinities for the S1 and S2 sites (18), whereas 3H-Leu binding to LeuT-E290S exhibited a Hill coefficient of 2.04 ± 0.2, consistent with an alteration in the allosteric connectivity of the two sites.
Fig. 1.
Leu binding kinetics of LeuT-E290S. (A) The S2 site in LeuT-E290S is intact. Saturation binding of 3H-Leu (149 Ci/mmol) was performed with 25 ng LeuT-E290S in the presence of 250 mM NaCl by means of the SPA. Data from two independent experiments were subjected to one-site binding global fitting with variable Hill slope, yielding a molar binding stoichiometry of 1.86 ± 0.09 Leu:LeuT-E290S with a Kd of 0.79 ± 0.05 µM. (B) Leu binding by LeuT-E290S is dependent on Cl−. Binding of 3H-Leu by 25 ng LeuT-WT (100 nM 3H-Leu) or LeuT-E290S (1 µM 3H-Leu) was measured in the presence of 250 mM NaCl (solid bars) or Na-gluconate (open bars) with the SPA. Data were normalized with respect to the activity measured in the presence of NaCl. Error bars represent SEM of triplicate determinations.
Dependence of Leu Binding on Cl− or Br−.
Binding of 1 µM 3H-Leu to LeuT-WT was similar in the presence or absence of Cl− (replacement of 250 mM NaCl with Na-gluconate) (Fig. 1B), but in LeuT-E290S, the binding of Leu was reduced by ∼85% in the absence of Cl− (Fig. 1B). To determine the dependence of LeuT-E290S on Cl− or Br−, we measured binding of 1 μM 3H-Leu in the presence of saturating concentrations of Na+ (800 mM Na-gluconate) and increasing concentrations of Cl− or Br− (Fig. 2A). Binding of 3H-Leu was found to be dependent on the concentration of Cl− and Br−, with similar EC50 values of 160.0 ± 29.8 and 192.3 ± 4.9 mM, respectively.
Fig. 2.
Ion dependence of LeuT-E290S activity. (A) Binding of 1 µM 3H-Leu in 800 mM Na-gluconate in the presence of increasing concentrations of choline-chloride (■) or KBr (▽) yielded EC50 of 160 ± 29.8 and 192 ± 49.2 mM, respectively. (B) Anion dependence of the binding of 22Na+ by LeuT-WT or LeuT-E290S. Binding of 2 µM 22Na+ (19.1 Ci/L) was measured in the presence of increasing concentrations of choline-chloride or KBr. 22Na+ binding by LeuT-WT did not reveal any dependence on the Cl− (●) or Br− (○) concentration, whereas fitting of Cl−-dependent 22Na+ binding of LeuT-E290S yielded an EC50 of 156.6 ± 29.1 mM for Cl− (■) and an EC50 for Br− of 122.2 ± 20.3 mM (▽). (C) Binding of 2 µM 22Na+ by LeuT-E290S was measured in 0–800 mM unlabeled Na-gluconate in the presence of saturating Cl− (■) or Br− (▽). Fitting the data to a single binding site equation revealed an EC50 for Na+ of 58.4 ± 7.4 mM in the presence of Cl− and 80.1 ± 10.4 mM in the presence of Br−. (D) Na+ and Cl− dependence of Leu binding by LeuT-E290S; 1 µM 3H-Leu binding was measured in the presence of increasing concentrations of Na+ and Cl− (■) or Na-gluconate in the presence of 800 mM choline-chloride (□). Error bars represent SEM of triplicate determinations. Kinetic constants were obtained by using nonlinear algorithms in SigmaPlot and are expressed as mean ± SD of the fit.
Dependence of Na+ Binding on Cl− or Br−.
To obtain a direct readout of the effect of Cl− or Br− on Na+ binding by LeuT, we measured binding of 22Na+ in the presence of increasing concentrations of Cl− and Br− (Fig. 2B). Whereas binding of 2 µM 22Na+ by LeuT-WT was not dependent on the presence of Cl− or Br−, binding of 22Na+ to LeuT-E290S was, with similar EC50 values of 156.6 ± 29.8 and 122.2 ± 20.3 mM for Cl− and Br−, respectively.
To determine the EC50 of Na+ binding to LeuT-E290S in the absence of Leu, 2 μM 22Na+ binding was measured in the presence of increasing concentrations of Na-gluconate in either 800 mM Cl− or 800 mM Br−. The EC50 of Na+ binding was about 58–80 mM in the presence of saturating Cl− or Br− (Fig. 2C), similar to results obtained for Na+-dependent 3H-Leu binding as shown below.
Dependence of Leu Binding on Na+.
In examining the Na+ dependence of Leu binding to LeuT-E290S, we observed that the Na+ concentration required for half-maximum binding of 1 µM 3H-Leu was significantly different when the assay was performed in saturating Cl− compared with titrating the Cl− along with Na+ (as NaCl) (Fig. 2D). Such an effect was not observed for LeuT-WT; rather, the EC50 of the Na+ stimulation effect on Leu binding to LeuT-WT was ∼8 mM, regardless of whether the assay was performed with NaCl or Na-gluconate (Fig. S1). In the assay performed with NaCl, the EC50 of Na+ stimulation effect on 3H-Leu binding to LeuT-E290S was 205 ± 30.3 mM, but when the assay was performed in the presence of saturating (800 mM) choline-chloride, the EC50 was 31.2 ± 7.1 mM (Fig. 2D), suggesting that Na+ binding depends on the presence of bound Cl− (see below).
Structures of LeuT-E290S with Bound Br− or Cl−.
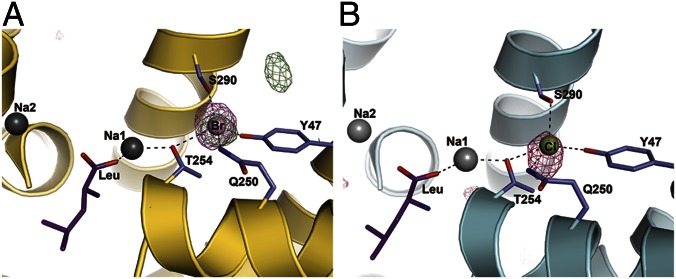
To investigate the Cl− site at atomic detail, we determined a structure of the LeuT-E290S mutant using a P21 crystal form obtained in the presence of 0.6 M Br− and octylglycoside and diffracting at 3.0 Å resolution. The asymmetric unit consists of a recurring dimer of LeuT, and with the exception of residues 132–134 and 472–476 in chain A and residues 132–134 in chain B, which are not ordered, the final structure is complete and refined to R/Rfree of 20.4/25.9% using the high-resolution LeuT structure (Protein Data Bank ID code 2A65) (6) for initial structure determination (Table S1 and Fig. S2). Unbiased, positive Fobs-Fcalc difference density exceeding a 6σ-contour level was observed at the putative Cl− binding site, indicating the presence of a bound Br− (Fig. 3A). Furthermore, the presence of Br− in the Cl− site in this structure was revealed from an experimental isomorphous difference map derived from the Br− crystal form and a previously published structure of the LeuT-E290S mutant in a similar P21 crystal form (Fig. 3A and Fig. S3), in which the anion at the Cl− binding sites could not be identified unequivocally (18). The strongest positive peak in this map, at a contour level of 5.8σ, was located at the Cl− site, representing the electron density difference of bound Br− and (partial) Cl− (18e or more). At the wavelength used for data collection, the anomalous scattering of bromine is unfortunately very low (<0.5e); thus, the anomalous difference Fourier map is featureless. Data collection at or above the absorption edge for Bromine yielded diffraction data of inferior quality for anomalous difference Fourier analysis. Consistent with previously obtained LeuT-WT structures (6, 8–10, 18), the structure features one molecule of Leu bound to the S1 site in an identical pose with Na+ in the Na1 and Na2 sites.
Fig. 3.
The substrate and ion binding sites in the two structures. (A) The substrate and ion binding sites in the LeuT-E290S-Br− structure. Na+ ions are shown as gray spheres, and Br− ions are shown in light pink. The bound Leu is shown as purple sticks, whereas the Br− coordinating residues are depicted as slate sticks. A simulated annealing omit map at a 4.5σ contour level obtained from refined coordinates in the absence of the Br− ion is shown as a pink mesh, confirming the position of the bound Br− ion. An isomorphous difference map between LeuT-E290S-Br− and 3GJC (18) is displayed at 4.0σ contour level (green mesh). The strongest positive peak is found at the position of Br− in the Cl− site in chain B. The adjacent peak is caused by a side chain shift in Phe31 (Fig. S3). (B) The substrate and ion binding sites in the LeuT-E290S-Cl− structure. The Na+ ions, the substrate, and coordinating residues are depicted as in A. Cl− is shown as a green sphere. The pink mesh depicts a simulated annealing omit map at a 3.0σ contour level produced from the refined coordinates in the absence of the Cl− ion.
In addition to the Br−-bound P21 structure of LeuT-E290S, we also obtained a 3.35-Å resolution structure of the same protein in a Cl−-containing buffer in the C2 crystal form characteristic of LeuT-WT (6) (Fig. 3B). Again, the unbiased Fobs-Fcalc map obtained after molecular replacement shows a clear positive peak exceeding 4σ and overlapping with the Br− site in the P21 structure. Unfortunately, multiple attempts to improve the resolution of the Cl−-bound C2 form of LeuT-E290S failed, because these experiments most often resulted in another P21 form reported earlier, in which Cl− and Na+ in the Na1 site could not be identified unequivocally (18). The available data for the Cl−-bound C2 form do not allow us to interrogate the site in any significant detail; however, it displays an occupied anion site, presumably Cl−, that seems to be identical to the Br−-bound site (Fig. 3), and the Br−- and Cl−-bound structures of LeuT-E290S are overall very similar, with an rmsd of 0.24 Å for all backbone atoms. We will, therefore, refer to their common anion binding site as the chloride site (Cl− site) in keeping with the physiological context.
Architecture of the Cl− Site.
Two models have been proposed for the Cl− site architecture (24, 25, 33, 35) based on mutagenesis and molecular modeling. Both models agree that Cl− is coordinated by residues aligned with positions 47, 254, and 290 in LeuT. Either Gln250 (24, 35) or Asn286 (25, 33) has been proposed as the fourth coordination ligand. In agreement with the former proposal (24, 35), we find that Cl− is coordinated by side chain hydroxyls from Tyr47, Ser290, and Thr254 and the side chain amide of Gln250 (Fig. 3 and Fig. S4). Asn286 does not directly coordinate the anion, but it is in close proximity and makes an H-bond with the Cl−-coordinating Thr254 (Fig. S3). In our molecular dynamics (MD) simulations, we found that such close proximity is retained, and Asn286 can even sporadically come into coordinating range (Fig. S5). During the dynamics simulation trajectory, the Asn286-Cl− distance seems to be inversely correlated with the H-bond distance of Asn286-Thr254 (correlation coefficient of −0.58). Interestingly, in similar MD simulations of our DAT and SERT homology models based on the LeuT structure, the residues aligned to Asn286 of LeuT, Asn353 of DAT, and Asn368 of SERT coordinate the Cl− directly as a fifth coordination ligand (Fig. S6). Indeed, when Asn368 in SERT was mutated to Asp (25), a loss of Cl− dependence was observed, but this mutant also exhibited greatly reduced transport activity. Thus, the introduction of a negatively charged side chain in proximity to the Cl− site likely hinders anion binding while partially compensating for it at the same time. In TnaT, mutations of Gln228 (the equivalent of Gln250 in LeuT) were generally inactivating (33), thus obscuring the importance of this residue for engineered Cl− binding.
Network of Interactions Connects the Binding Sites.
In the LeuT-E290S structure, the Na+ bound at the Na1 site is only 5.4 Å from the bound Cl−; the two ions are connected by the hydroxyl group of Thr254 and positioned in close proximity to the S1 binding site. To explore the mechanistic role of the bound Cl− in what seems to be an intricate network of interactions between the Na1 and S1 sites, we carried out comparative MD simulations of the LeuT-E290S structure in the presence or absence of Cl−. The MD results for these constructs were also compared with corresponding MD simulations of LeuT-WT, in which Glu290 was either negatively charged or neutralized by protonation, to enable a general perspective on the role of the negative charge.
With MD simulations of the LeuT-E290S mutant, it was possible to explore the hypothetical construct containing substrate and ions in the S1, Na1, and Na2 sites, even in the absence of Cl−. Under these conditions, the two Cl−-coordinating residues, Gln250 and Ser290, are seen to separate gradually and reconfigure the interaction network, thereby allowing water molecules into the site (Fig. 4 A, C, and E). Notably, we observe the same water penetration sequence in the parallel simulation of LeuT-WT with a protonated (uncharged) Glu290. Because the direct interaction between Gln250 and Glu290 is weakened without the negative charge on Glu290, the side chain of Gln250 rotates away gradually and exposes Glu290 to the solvent (Fig. 4 B, D, and F). In the absence of the charge-mediated organization of the site, water penetration into this region is seen in the simulations to result in the reorientation of the Tyr47 side chain in both the E290S construct and WT. This conformational reorganization of the network of interactions between the residues in positions 290-47-250 is propagated into the tertiary structure of the protein and affects the conserved interface between TM2 and TM6a. As discussed further below, this sensitivity of the TM2-TM6a interface to the presence of Cl− suggests a mechanistic role in the function of the transporter.
Fig. 4.
The impact of a negative charge on the interaction network near the Na1 and S1 binding sites revealed by MD simulations. A and B show the evolution of the distances between the closest heavy atoms of the residues at positions 250 and 290 in (A) the E290S mutant and (B) the WT in the course of the MD trajectories; the corresponding constructs are depicted in C–F. The curves in A (E290S mutant) are from simulations carried out in the presence or absence of Cl− (lines in cyan and orange, respectively), and the curves in B are for the corresponding constructs of the deprotonated (cyan) or protonated (orange) Glu290 in WT. Note that, in A, the dotted curves are from simulations with the n-octyl-β-glucopyranoside bound in the S2 site and found to reflect a similar trend as seen in the absence of n-octyl-β-glucopyranoside (solid curves). (C) In the E290S mutant, a negative charge provided by the bound Cl− ion is key in maintaining the interaction network near the Na1 and S1 binding sites. (D) In the WT, this function is fulfilled by the deprotonated Glu290. In the absence of such a negative charge, water molecules are seen to penetrate to this region in both the WT and the E290S mutant, interacting directly with the residue at position 290 as well as with Asn286 and Tyr47. This water entry results in an equivalent rotation of the Gln250 side chain in both (E) the E290S mutant and (F) the WT.
When the simulations are performed for these systems in the presence of all of the bound species (at the S1, Na1, Na2, and Cl− sites), the results bring to light the role of the coupling between the Na+ in the Na1 site and the negative charge provided by either the bound Cl− (for the E290S mutant) or the charged Glu290 (for WT) as also indicated by the crystal structures. Thus, the simulations support a model of a local network composed of Ser290, Tyr47, and Gln250, which connects to the extracellular gate formed by the side chains of Arg30 and Asp404 (Fig. S7). Together, this integrated network organizes a tightly packed and dehydrated form, which indicates a role for the negative charge in establishing an occluded state of LeuT in the presence of bound S1 substrate. Such a role of the negative charge near position 290 and the effect of the Gln250 side chain conformation on the interaction network were implicated in the functional mechanism of GAT-1 as a result of the S331E mutation (24). Thus, the mutation introduces a negative charge at the position aligned to the Glu290 of LeuT, rendering substrate transport independent of Cl−, albeit significantly impaired by the bulky side chain. Transport was rescued, however, by a second mutation, in which the residue aligned with Gln250 of LeuT (Gln291 in GAT-1) was replaced by one with a smaller side chain (35).
The functional importance of Gln250 is not only because of its direct coordination of negative charge (Cl− or Glu290) but also its effect on the organization of the local structure that becomes evident in the presence and absence of a negative charge in the Cl− site. Thus, we find in the simulations that, in the presence of the negative charge (Cl− or Glu290), Gln250 is one of two residues interacting alternatingly with Arg30 (the other is Asp404). In the absence of such a negative charge, the Gln250 side chain gains significant freedom of motion, and consequently, Arg30 is seen to interact preferentially with Asp404 (Fig. S8).
Conserved TM2-TM6a Interface Is Sensitive to Cl− Binding.
The conformational reorganization in the network of interactions involving the residues in positions 290-47-250 affects the tertiary structure of the protein at the conserved TM2-TM6a interface as discussed above. Thus, in LeuT-E290S, we find that perturbations caused by the presence or absence of Cl− near the Ser290-Tyr47-Gln250 interaction network propagate through rearrangements in a strip of hydrophobic and aromatic residues along the TM2-TM6a interface: the FxxPY motif in TM2 and the VWxxAxxQI/V motif in TM6a.
A similar impact of the negative charge is observed in the simulation of the WT model with a protonated Glu290, and it seems to be initiated by related rotations of the Gln250 and Tyr47 side chains. The propagation of these changes is facilitated by the flexibility introduced in the TM by Pro46. Notably, the TM2-TM6a interface residues involved in this propagation are all highly conserved in mammalian transporters (Fig. S6), suggesting that the dynamic organizing effect of a negative charge—the Cl− in eukaryotes and a corresponding negatively charged residue in prokaryotes—constitutes an important general component of the transport mechanism in the NSS family.
Discussion
The correlation between Cl−-dependent Na+/substrate symport by mammalian members of the NSS family and the H+ dependence of Na+/substrate symport in their bacterial counterparts has been shown in a variety of studies (18, 20, 24, 25, 33). The negative charge in the Cl− site, which in LeuT-WT is provided by a glutamic acid residue, must be neutralized during the transport cycle. In LeuT-E290S and other chloride-dependent NSS, this neutralization is achieved by release of the cotransported Cl− along with Na+ and the amino acid substrate, whereas in LeuT-WT, it requires the protonation of Glu290 from the intracellular milieu, producing the countertransport of a proton (20). Here, we provide direct structural evidence that, in LeuT, the Ser replacing Glu at position 290 is, indeed, involved in securing the negative charge by binding Cl− or Br−. Our Br−-bound crystal structure has a higher resolution than the Cl−-bound structure, but because the binding kinetics for these anions were comparable (Fig. 2), the Br−-bound LeuT-E290S structure represents a valid model for the architecture of the Cl− site. Furthermore, a lower-resolution structure of the Cl−-bound complex is consistent with the Br−-bound structure within the experimental error. In addressing the fundamental question of the role that the negative charge at the Cl− site has in the transport mechanism, we explored the role of the network of interactions in which the charge is involved. Thr254 connects to the carboxy group of Leu through Na+ in the Na1 site (Fig. 3). This connection would be absent in the monoamine transporters of the NSS family (DAT, SERT, and NET), because the substrates lack a carboxy group; however, in these transporters, an Asp residue near the bound neurotransmitter substrates can supply the negative charge, mimicking the carboxylic group of the bound amino acid substrates in bacterial transporters (6). The structural details suggest how a negative charge at the Cl− site becomes involved in the binding of Na+ in the Na1 site and the substrate in the S1 site. Indeed, results from the functional studies presented here clearly show that binding of both Na+ and Leu to LeuT-E290S is dependent on the presence of Cl− or Br− (Fig. 2 A and B).
Congruent evidence for the crucial role of the negative charge at the Cl− site for substrate and inhibitor binding can be found in functional studies of eukaryotic NSS mutants involving residues at or near the Cl− site in DAT, NET, GAT-1, and SERT (36–41). Bönisch et al. (40) found that a mutation of Ser354 in NET (aligned with Glu290 in LeuT) significantly reduces desipramine binding, whereas Tavoulari et al. (39) reported that the presence of Cl− improves binding to SERT of several widely used antidepressants and that mutation of key residues in the Cl− site that renders SERT Cl−-independent also removes the Cl− dependence of fluoxetine and imipramine binding. Thus, the notion that the absence of a negative charge at the Cl− site can affect substrate and inhibitor binding is supported by (i) the proximity of the Cl− site in our Cl−-dependent LeuT mutant to the S1 site, which is also proposed as the binding site for the majority of NSS inhibitors (42–46), and (ii) the observation that Na+ and Cl− have a common coordinating residue. The effects of that negative charge on binding emerge from a complex reorganization of the local structural architecture as summarized below.
The Cl− site is in the vicinity of the innermost part of the extracellular gate formed by Arg30 and Asp404 (Fig. 5A), and it provides additional clues for the functional implications of the negative charge. In the current structures as well as the previously published structure of LeuT-E290S (18), Arg30 and Asp404 form a salt bridge closing the extracellular gate (Fig. 5A), whereas in the LeuT-WT structure (6), the side chain of Arg30 faces Gln250 and hydrogen bonds with it, leaving the gate open (Fig. 5B). In turn, Gln250 in the WT, outward-occluded LeuT structure (Protein Data Bank ID code 2A65) interacts with the carboxyl group of Glu290 (or with Br−/Cl− in the LeuT-E290S structures). Combining these structural data, one can infer a coherent sequence of molecular interactions that couples the substrate and ions to the movement of the extracellular gate. An H-bond network can be traced from the substrate to the coordinated Na+ in the Na1 site and then on to Thr254, the Cl− through Gln250, and Arg30, which can, through a simple hinge movement, interact with either Asp404 or Gln250 to close or open the gate, respectively. The integrity of this interaction network connecting the substrate with the extracellular gate depends on the presence of a negative charge at the Cl− site, and this negative charge can be provided by either Cl− or the side chain of the corresponding amino acid (Glu or Asp). Thus, in the structure presented here and presumably, also in the Cl−-dependent eukaryotic transporters, the ion acts as a bridge connecting the substrate with the extracellular gate.
Fig. 5.
The network of interactions between the substrate and the extracellular gate. (A) Ligand binding sites and the extracellular gate in the new structure. The dashed green lines show hydrogen bonds, which are part of the substrate-Cl−-gate bridge, of which Cl− (or the negatively charged Glu290) is an integral part. The gate is closed, and Arg30 does not interact with Gln250. Ligands are shown as spheres in Inset. (B) Ligand binding sites and the extracellular gate in the WT outward-occluded LeuT structure (Protein Data Bank ID code 2A65) (6). Here, the negative charge of Glu290 effectively replaces Cl− and keeps the substrate-Cl−-gate bridge intact. The gate is open, and Arg30 hydrogen bonds to Gln250.
We note that, unlike the Na+ in the Na1 site, the one in the Na2 site does not interact with the Cl− (or charged E290). However, the Na2 site is conserved in several transporters belonging to very different transporter families, including the Na+/galactose cotransporter (vSGLT) from the sodium-solute symporter (SSS) family (47), the sodium-benzylhydantoin transporter (Mhp1) from the nucleobase-cation-symport-1 (NCS1) family (48, 49), and the betaine transporter from the betaine-choline-carnitine-transporter family (50, 51). In the H+-coupled arginine/agmatine transporter (AdiC) (52) and aminoacid transporter (ApcT) (53), the architectural role of the Na2 site identified in the Na+-coupled transporters is conserved through a lysine residue that can change its protonation state and lose the charge (54), reminiscent of the change in protonation state of the negatively charged residue at the Cl− site in bacterial NSS transporters. In the E290S construct presented here, we were unable to measure 22Na+ binding in the absence of Cl−, reflecting the primacy of Cl− binding. This inference is substantiated in our results showing that Na+ stimulates Leu binding much better under Cl− saturating conditions than when both Na+ and Cl− are titrated together (Fig. 2B), indicating that a negative charge at the Cl− site stimulates binding of both the cations and the substrate. Furthermore, Na+ binds in the absence of Leu nearly as well as in the presence of Leu (Fig. 2C), suggesting that Leu is not essential for Na+ binding, consistent with the sequence in which at least one of two Na+ binds before Leu. This scheme is supported by the recent structure of LeuT-WT (12) in the open-to-out nonoccluded conformation with a negatively charged Glu290, two bound Na+, and no Leu.
The dynamics of the functional mechanism inferred from the structural details that we studied are also revealed by a focus on the effect of the negative charge. These MD simulations show that, in both the LeuT-WT and the E290S construct, the Cl− site in the absence of the negative charge favors an open and extensively hydrated structure, which was seen from a comparison of Fig. 4 C and D with Fig. 4 E and F. This comparison further shows that, in the absence of a negative charge at the Cl− site, the side chain of Gln250, which can be reached by Arg30 when it is not closing the extracellular gate with Asp404, has turned (as indicated by the arrow) and is now out of reach for Arg30 (Fig. 4).
After all of the ions and ligands are bound, MD simulations indicate that the coupling between the substrate in S1, Na+ at the Na1 site, and the negative charge provided either by the bound Cl− (for the E290S mutant) or the charged Glu290 (for WT) organizes a local network composed of Ser290, Tyr47, and Gln250 in a tightly packed and dehydrated form, establishing an occluded state of LeuT. Furthermore, this reorganization around the negative charge is found in the simulations to propagate along the conserved TM2-TM6a interface, taking advantage of the structural flexibility produced by the Prokink at position 46 in TM2. This reorganization coincides with the substantial rearrangements of TM2 and TM6a observed in comparing the recently published structures of LeuT in the outward- and inward-open conformations (12). The fact that this propagated structural rearrangement depends on the reorganizing effect of the negative charge on the local architecture is underscored by the finding that the calculated pKa of Glu290 changes from 5.4 in the outward-open structure to 7.6 in the inward-open structure (Materials and Methods); the change is consistent with the side chain being negatively charged in the outward-open state. Because Glu290 participates in a functionally significant transition, it is readily protonated from the intracellular milieu to facilitate return from the inward- to the outward-open state. In the Cl−-dependent NSS, the charge changes are accomplished by loading Cl− from the extracellular milieu and releasing it to the inside of the cell along with the other substrates.
Materials and Methods
LeuT-E290S was expressed in Escherichia coli C41(DE3) cells. Datasets from crystals were collected at the European Synchrotron Radiation Facility as well as at MaxLab using a 0.9395-Å wavelength for the Br− dataset where bromine displays an insignificant anomalous scattering. Phasing was carried out by molecular replacement using PHASER (55) with the WT structure (6) as a model. The structures were refined with PHENIX (56). Radiotracer binding studies were performed by means of the scintillation proximity assay (SPA) using copper-coated YSi SPA beads to capture the His-tagged LeuT variants (7, 32). All experiments were repeated at least in duplicate. Figures represent typical experiments, and unless otherwise noted, errors represent the SEM of triplicate determinations. Nonlinear regression fitting of the data was performed in Prism 4 or SigmaPlot (version 10). Based on our established simulation protocols and molecular system, the MD simulations of LeuT were carried out with Not (just) Another Molecular Dynamics Program (NAMD) (57) as described previously (7). Detailed materials and methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Fernanda Delmondes de Carvalho and MiEstrella Miller-Cruz for the preparation of membrane vesicles, Maike Bublitz and Jesper Karlsen for assistance with crystallographic procedures, and Anna Marie Nielsen for technical assistance. This work was supported in part by National Institutes of Health Grants DA023694 (to L.S.), DA12408 (to H.W.), U54GM087519 (to H.W. and J.A.J.), DA17293 (to J.A.J.), and DA022413 (to J.A.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4HMK and 4HOD).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221279110/-/DCSupplemental.
References
- 1.Hediger MA, et al. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447(5):465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 2.Rudnick G. In: Mechanisms of Biogenic Amine Neurotransmitter Transporters. Reith MEA, editor. Totowa, NJ: Humana Press; 2002. pp. 25–52. [Google Scholar]
- 3.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: Structure, regulation and function. Nat Rev Neurosci. 2003;4(1):13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK. LeuT: A prokaryotic stepping stone on the way to a eukaryotic neurotransmitter transporter structure. Channels (Austin) 2008;2(5):380–389. doi: 10.4161/chan.2.5.6904. [DOI] [PubMed] [Google Scholar]
- 5.Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: Molecular function of important drug targets. Trends Pharmacol Sci. 2006;27(7):375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437(7056):215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 7.Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter—inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30(6):667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448(7156):952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science. 2007;317(5843):1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322(5908):1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z, et al. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16(6):652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481(7382):469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravna AW, Jaronczyk M, Sylte I. A homology model of SERT based on the LeuT(Aa) template. Bioorg Med Chem Lett. 2006;16(21):5594–5597. doi: 10.1016/j.bmcl.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70(5):1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- 15.Caplan DA, Subbotina JO, Noskov SY. Molecular mechanism of ion-ion and ion-substrate coupling in the Na+-dependent leucine transporter LeuT. Biophys J. 2008;95(10):4613–4621. doi: 10.1529/biophysj.108.139741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noskov SY. Molecular mechanism of substrate specificity in the bacterial neutral amino acid transporter LeuT. Proteins. 2008;73(4):851–863. doi: 10.1002/prot.22108. [DOI] [PubMed] [Google Scholar]
- 17.Noskov SY, Roux B. Control of ion selectivity in LeuT: Two Na+ binding sites with two different mechanisms. J Mol Biol. 2008;377(3):804–818. doi: 10.1016/j.jmb.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quick M, et al. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc Natl Acad Sci USA. 2009;106(14):5563–5568. doi: 10.1073/pnas.0811322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claxton DP, et al. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17(7):822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, et al. Substrate-dependent proton antiport in neurotransmitter:sodium symporters. Nat Chem Biol. 2010;6(2):109–116. doi: 10.1038/nchembio.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, Noskov SY. The role of local hydration and hydrogen-bonding dynamics in ion and solute release from ion-coupled secondary transporters. Biochemistry. 2011;50(11):1848–1856. doi: 10.1021/bi101454f. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, et al. Single-molecule dynamics of gating in a neurotransmitter transporter homologue. Nature. 2010;465(7295):188–193. doi: 10.1038/nature09057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, et al. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474(7349):109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zomot E, et al. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449(7163):726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]
- 25.Forrest LR, Tavoulari S, Zhang YW, Rudnick G, Honig B. Identification of a chloride ion binding site in Na+/Cl−-dependent transporters. Proc Natl Acad Sci USA. 2007;104(31):12761–12766. doi: 10.1073/pnas.0705600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forrest LR, et al. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci USA. 2008;105(30):10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quick M, Shi L, Zehnpfennig B, Weinstein H, Javitch JA. Experimental conditions can obscure the second high-affinity site in LeuT. Nat Struct Mol Biol. 2012;19(2):207–211. doi: 10.1038/nsmb.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piscitelli CL, Krishnamurthy H, Gouaux E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature. 2010;468(7327):1129–1132. doi: 10.1038/nature09581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Gouaux E. Substrate binds in the S1 site of the F253A mutant of LeuT, a neurotransmitter sodium symporter homologue. EMBO Rep. 2012;13(9):861–866. doi: 10.1038/embor.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Androutsellis-Theotokis A, et al. Characterization of a functional bacterial homologue of sodium-dependent neurotransmitter transporters. J Biol Chem. 2003;278(15):12703–12709. doi: 10.1074/jbc.M206563200. [DOI] [PubMed] [Google Scholar]
- 31.Quick M, et al. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter:sodium symporter from Fusobacterium nucleatum. J Biol Chem. 2006;281(36):26444–26454. doi: 10.1074/jbc.M602438200. [DOI] [PubMed] [Google Scholar]
- 32.Quick M, Javitch JA. Monitoring the function of membrane transport proteins in detergent-solubilized form. Proc Natl Acad Sci USA. 2007;104(9):3603–3608. doi: 10.1073/pnas.0609573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavoulari S, Rizwan AN, Forrest LR, Rudnick G. Reconstructing a chloride-binding site in a bacterial neurotransmitter transporter homologue. J Biol Chem. 2011;286(4):2834–2842. doi: 10.1074/jbc.M110.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, et al. Ion-controlled conformational dynamics in the outward-open transition from an occluded state of LeuT. Biophys J. 2012;103(5):878–888. doi: 10.1016/j.bpj.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Yona A, Bendahan A, Kanner BI. A glutamine residue conserved in the neurotransmitter:sodium:symporters is essential for the interaction of chloride with the GABA transporter GAT-1. J Biol Chem. 2011;286(4):2826–2833. doi: 10.1074/jbc.M110.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Z, Wang W, Kopajtic T, Revay RS, Uhl GR. Dopamine transporter: Transmembrane phenylalanine mutations can selectively influence dopamine uptake and cocaine analog recognition. Mol Pharmacol. 1999;56(2):434–447. doi: 10.1124/mol.56.2.434. [DOI] [PubMed] [Google Scholar]
- 37.Danek Burgess KS, Justice JB., Jr Effects of serine mutations in transmembrane domain 7 of the human norepinephrine transporter on substrate binding and transport. J Neurochem. 1999;73(2):656–664. doi: 10.1046/j.1471-4159.1999.0730656.x. [DOI] [PubMed] [Google Scholar]
- 38.Mari SA, et al. Role of the conserved glutamine 291 in the rat gamma-aminobutyric acid transporter rGAT-1. Cell Mol Life Sci. 2006;63(1):100–111. doi: 10.1007/s00018-005-5512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavoulari S, Forrest LR, Rudnick G. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J Neurosci. 2009;29(30):9635–9643. doi: 10.1523/JNEUROSCI.0440-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bönisch H, Runkel F, Roubert C, Giros B, Brüss M. The human desipramine-sensitive noradrenaline transporter and the importance of defined amino acids for its function. J Auton Pharmacol. 1999;19(6):327–333. doi: 10.1111/j.1365-2680.1999.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 41.Kitayama S, et al. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc Natl Acad Sci USA. 1992;89(16):7782–7785. doi: 10.1073/pnas.89.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henry LK, Adkins EM, Han Q, Blakely RD. Serotonin and cocaine-sensitive inactivation of human serotonin transporters by methanethiosulfonates targeted to transmembrane domain I. J Biol Chem. 2003;278(39):37052–37063. doi: 10.1074/jbc.M305514200. [DOI] [PubMed] [Google Scholar]
- 43.Henry LK, et al. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2006;281(4):2012–2023. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- 44.Beuming T, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11(7):780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bisgaard H, et al. The binding sites for benztropines and dopamine in the dopamine transporter overlap. Neuropharmacology. 2011;60(1):182–190. doi: 10.1016/j.neuropharm.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plenge P, et al. Steric hindrance mutagenesis in the conserved extracellular vestibule impedes allosteric binding of antidepressants to the serotonin transporter. J Biol Chem. 2012;287(47):39316–39326. doi: 10.1074/jbc.M112.371765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faham S, et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321(5890):810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weyand S, et al. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322(5902):709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyand S, et al. The alternating access mechanism of transport as observed in the sodium-hydantoin transporter Mhp1. J Synchrotron Radiat. 2011;18(1):20–23. doi: 10.1107/S0909049510032449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature. 2009;458(7234):47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 51.Perez C, et al. Substrate specificity and ion coupling in the Na+/betaine symporter BetP. EMBO J. 2011;30(7):1221–1229. doi: 10.1038/emboj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao X, et al. Structure and mechanism of an amino acid antiporter. Science. 2009;324(5934):1565–1568. doi: 10.1126/science.1173654. [DOI] [PubMed] [Google Scholar]
- 53.Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325(5943):1010–1014. doi: 10.1126/science.1176088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi L, Weinstein H. Conformational rearrangements to the intracellular open states of the LeuT and ApcT transporters are modulated by common mechanisms. Biophys J. 2010;99(12):L103–L105. doi: 10.1016/j.bpj.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen VB, et al. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 1):12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.