Abstract
For fluorescence-based single-molecule studies, photobleaching of the dye reporter often limits the time window over which individual molecules can be followed. As such, many strategies, for example using a cocktail of chemical reagents, have been developed to decrease the rate of photobleaching. Herein, we introduce a new and highly effective method to enhance the photostability of one of the commonly used fluorescent dyes, rhodamine 6G (R6G). We show that micron-sized polydimethylsiloxane (PDMS) wells, when the PDMS surface is properly treated, not only provide a confined environment for single-molecule detection, but can also significantly increase the survival time of individual R6G molecules before photobleaching. Moreover, our results suggest, consistent with several previous studies, that R6G photobleaching involves a radical state.
Keywords: Fluorescence, Photobleaching, Single molecule, Confinement, Fluorescence correlation spectroscopy
1. INTRODUCTION
Fluorescence based single-molecule techniques are extremely useful in mechanistic studies of chemical and biological reactions as well as conformational changes. This is because they offer high sensitivity and specificity. More importantly, they have the ability to unveil processes that evade detection by traditional ensemble measurements, due to averaging.1-3 In practice, however, single-molecule fluorescence measurements are often complicated and/or limited by nonradiative transitions that lead to the formation of long-lived non-fluorescent states, which cause fluorescence blinking4,5 and photobleaching, thus reducing the observation time window over which the process of interest can be followed. For this reason, various methods and strategies have been developed to reduce the probability of fluorescence intermittency and photobleaching.6-11 While the mechanism by which photobleaching occurs is not well understood and likely depends on the fluorophore, it is commonly believed that molecular oxygen plays an important role in mediating photoproduct formation.7 As such, in an effort to curb photobleaching many studies9-11 have sought to use oxygen scavengers. However, it has been shown that, depending on the photophysics of the fluorophore under consideration, addition of an oxygen scavenger could either decrease9-11 or increase12,13 the rate of photobleaching, or leave the rate mainly unaffected.14,15 More recently, Blanchard and coworkers6 have shown that it is possible to reduce the photobleaching rate of cyanine fluorophores up to 70 fold via a covalently-linked “protective” agent, such as cyclooctatetraene (COT), 4-nitrobenzyl alcohol (NBA), or 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox). Further studies indicated that COT, when covalently linked to Cy5, substantially reduces the lifetime of the Cy5 triplet state and that the degree of triplet state quenching correlates with the enhancement in photostability observed in single-molecule fluorescence measurements.16 On the other hand, NBA and Trolox do not quench the Cy5 triplet state and possible stabilization mechanisms of NBA and Trolox could involve passivation of reactive oxygen species and radicals, which can damage the fluorophore.16 Other methods to enhance the photostability of various fluorophores include the usage of a triplet state quencher17 or a microfluidic device.18 While these previous studies relied on different principles or strategies, they clearly demonstrated that effectively suppressing photobleaching is not a trivial task, and that development of new methods is warranted. Herein, we introduce an alternative and simple method that could be used to decrease the rate of photobleaching, based on confining one or few fluorescent molecules in micron-sized PDMS wells. Using a common fluorescent dye, R6G, as a testbed, we show that this method is very effective in reducing the probability of photobleaching, with the added advantage that no molecular quenchers are needed.
We chose PDMS for the following reasons: (1) it can be easily patterned through conventional molding techniques,19-22 (2) it can be easily integrated into an existing confocal microscope setup through adhesion with commonly used glass cover slides, (3) it is transparent over a wide spectral region, (4) it has a low affinity for hydrophilic molecules, (5) if needed, surface passivation with polymers or proteins to prevent any potential interactions between the biomolecules of interest and PDMS can be easily achieved, and (6) water molecules cannot diffuse through PDMS, thus greatly reducing evaporation when a glass cover slide is used to seal the PDMS wells.23
2. EXPERIMENTAL SECTION
2.1. Preparation of Micron-Sized PDMS Wells
A silicon wafer displaying arrays of regular cylinders with a diameter of 3 μm and a height of 5 μm was obtained from the laboratory of Professor Haim H. Bau at the School of Engineering and Applied Science of the University of Pennsylvania. This silicon mold was used as a master template to produce a series of identical PDMS sheets containing arrays of micron-sized wells. The liquid silicone elastomer PDMS base and the curing agent were purchased from Dow Corning Corporation (Midland, MI) and thoroughly mixed with a 10:1 (m/m) ratio. The mixture was degassed under vacuum for 1 hour and poured on top of the silicon mold. The PDMS was then cured at room temperature overnight. After being peeled from the silicon mold, the surface of the PDMS sheet, which has the featured micron-sized wells on it, was coated by either bovine serum albumin (BSA) or polyethylene glycol (PEG). Specifically, BSA coating was achieved by adding 100 μL of a BSA solution (80 mg/mL) on top of the target PDMS sheet and then covering with a glass cover slide. After 3 hours of incubation at room temperature, which allows BSA to bind to the PDMS surface, any unbound BSA molecules were then removed by washing the PDMS sheet with water (100 μL × 3 times). The PDMS sheet was then left uncovered to dry.
2.2. Oxygen Plasma Treatment and PEG Coating
PDMS sheets and glass slides were treated with oxygen plasma using a March PX-250 plasma cleaning system (March Plasma, Concord, CA) at 50 W, 660 mTorr for 120 s. After oxygen plasma treatment, PDMS sheets and glass slides were immediately treated with a solution which was prepared as follows: an ethanol/water (95/5, v/v) solution was made and the pH was adjusted to 5.3 with acetic acid, then 2% (by volume) 2-[methoxy(polyethyleneoxy)propyl]trimethoxysilane (PEG) (Gelest, Morrisville, PA) was added and allowed to sit for 5 minutes. PDMS sheets and cover slides were added to the solution and stirred for 2 minutes, then removed and rinsed vigorously with ethanol and allowed to dry for 24 hours. Glass cover slides were further rinsed with water and dried before use.
2.3. Sample Preparation
R6G (MW = 479) was purchased from Molecular Probes (Carlsbad, CA) and used as received. A stock solution of 1 μM R6G in water was made and the concentration was determined optically using the absorbance at 530 nm and a molar extinction coefficient of 116,000 cm−1 M−1. R6G solutions of lower concentration were prepared by serial dilutions. Loading of the sample solution (10 pM unless otherwise indicated) to the PDMS wells was accomplished by dispensing an aliquot of the sample solution onto a glass slide which was then covered by a PDMS sheet that was printed with micron-sized wells. After pressing on the PDMS sheet, any gas bubbles and excess solution were removed and the PDMS sheet bound to the glass slide tightly via Van der Waals interactions. Fluorescence correlation spectroscopy (FCS) and photobleaching measurements were then performed with the laser focused in individual wells. When the PDMS wells, each of which has a volume of approximately 30 fL, were filled with a 10 pM R6G solution, most of them contain either one or zero dye molecule. For those containing one R6G molecule, the effective dye concentration was approximately 50 pM.
2.4. Preparation of Giant Unilamellar Vesicles
Giant unilamellar vesicles (GUVs) were prepared by the standard method of electroswelling using a custom-made closed perfusion chamber and indium-tin-oxide (ITO) coated slides (Delta-Technologies, Stillwater, MN) as electrodes. Briefly, a 1 μmol/mL 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) lipid solution was prepared in chloroform. 100 mL of this lipid solution was then deposited onto an ITO slide. After chloroform evaporation, the electroswelling chamber was assembled from two lipid-coated ITO slides separated by a rubber spacer and filled with 25 pM R6G solution in 100 mM sucrose buffer. A voltage of 1.2 V/mm at a frequency of 5 Hz was applied to the system for 2 hours while incubating the sample at 60 °C. Before FCS measurement, the stock GUV solution was diluted 4 times with 100 mM sucrose buffer. 10 μL of the diluted GUV solution was then added to a custom-made, closed chamber where individual GUVs were allowed to settle for at least 1 hour on the bottom of the glass cover slide.
2.5. FCS and Photobleaching Measurements
The confocal setup used for FCS measurements has been described previously in detail.2 Excitation of R6G was accomplished by the 514 nm line of an Ar+ ion laser and the resulting fluorescence was split and detected by two avalanche photodiodes (Perkin Elmer, NJ). Cross-correlation of the fluorescence signals from the two detectors was accomplished by a Flex 03-LQ-01 correlator card (Correlator.com, NJ). Photobleaching experiments were carried out with a laser power of either 160 μW or 1.6 mW (measured at the input of the microscope).
3. RESULTS AND DISCUSSION
3.1. Photobleaching Rate of R6G in GUVs
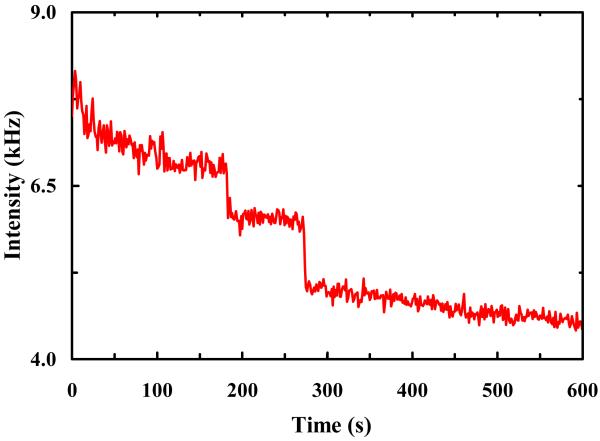
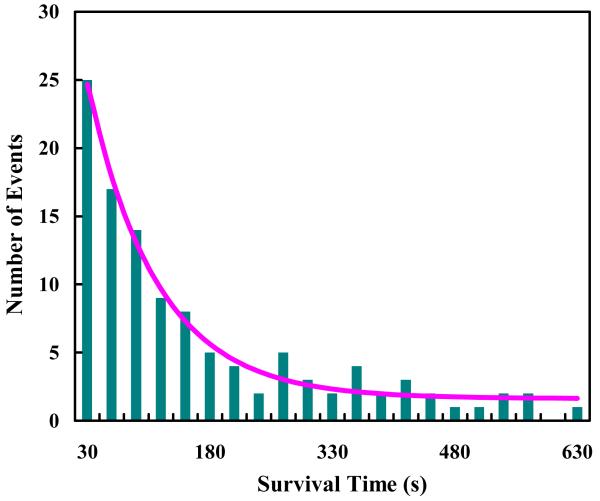
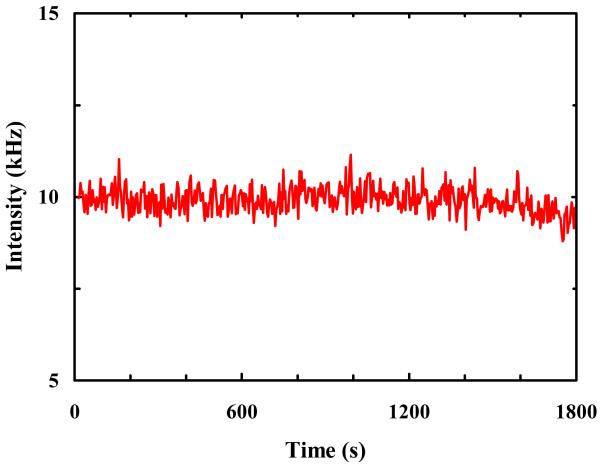
To establish a baseline for the photobleaching rate of R6G under normal solution conditions, we first conducted photobleaching measurements on R6G molecules encapsulated in individual immobilized POPC GUVs of ~5 μm in diameter. Haran and coworkers24,25 have shown that immobilized lipid vesicles offer a convenient avenue for confinement and observation of single-molecules. To avoid any potential surface effect, in the current case the laser focus was brought to the center of the respective GUVs. As indicated (Figure 1), the fluorescence intensity time trace shows stepwise decreases, characteristic of photobleaching of individual R6G molecules. Thus, a large number of such measurements allowed us to determine the distribution of survival times of individual R6G molecules and hence an estimate of the photobleaching rate of R6G (under the current experimental conditions). As shown (Figure 2), the resulting survival time distribution is dominated by a single-exponential component (~95%) with a time constant of 85.6 ± 10.0 s. This value is comparable to those measured for similar fluorescent molecules,26-28 suggesting that the role of GUVs in the present case is to provide a confined environment for the R6G molecules, and that the confinement thus generated has no apparent effect on the photostability of the encapsulated fluorescent molecules.
Figure 1.
A representative fluorescence intensity time trace of R6G molecules encapsulated in POPC GUVs.
Figure 2.
Photobleaching statistics of R6G molecules encapsulated in POPC GUVs. The smooth line is the best fit of these data to the following equation: S(t) = A·exp(−t/τ)+B, with A = 32.7, τ = 85.6 s, and B = 1.6.
3.2. Confinement of R6G Molecules in Micron-Sized PDMS Wells
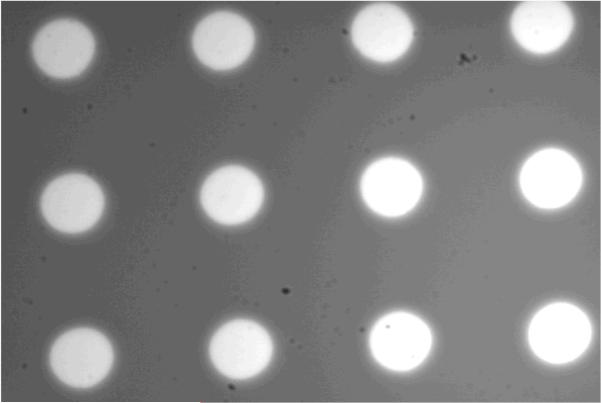
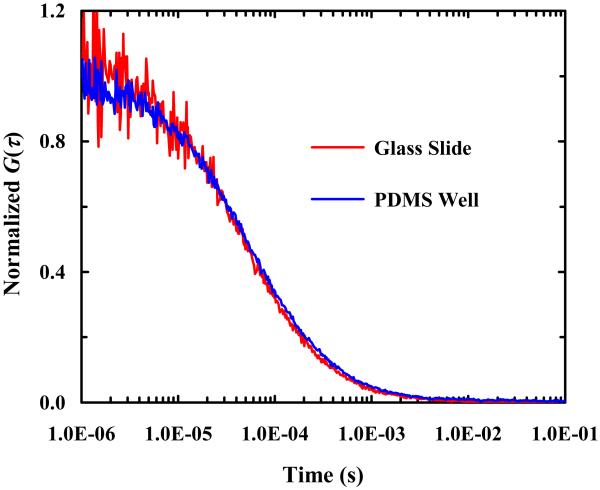
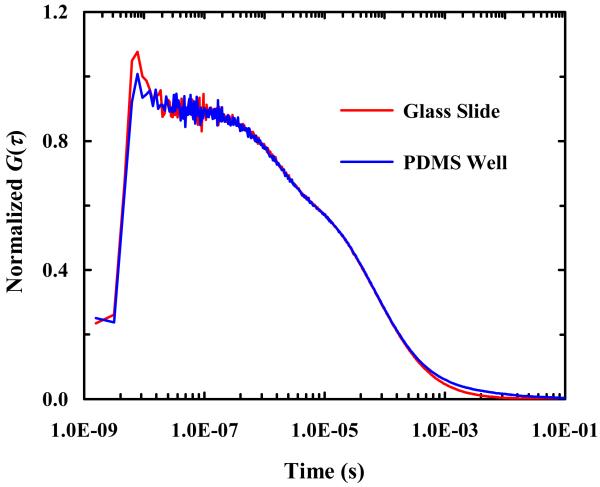
As shown (Figure 3), a wide-field image of arrays of micron-sized PDMS wells filled with a dilute R6G solution confirms that fluorescent molecules can be easily loaded into such wells. Further fluorescence recovery after photobleaching (FRAP) measurements performed on individual wells using a strong excitation laser intensity (data not shown) indicated that the encapsulated dye molecules cannot diffuse through the PDMS walls separating different wells, validating the confinement role of such PDMS wells. To verify that the diffusion of the encapsulated molecules of interest has not been altered due to such confinement, we performed FCS measurements on R6G molecules in bulk solution and in BSA-coated PDMS wells. As shown (Figure 4), the FCS curves obtained in both cases are practically identical, indicating that the diffusion of the encapsulated R6G molecules is not hindered by confinement and that the confinement is unlikely to change the physical properties of the encapsulated molecules. Taken together, these results demonstrated the feasibility of using micron-sized PDMS wells to confine a small number of fluorescent molecules for single-molecule studies.
Figure 3.
A fluorescence image of the PDMS wells. The unequal image brightness is caused by uneven laser illumination.
Figure 4.
Comparison of FCS traces obtained in a BSA-coated PDMS well and on a BSA-covered glass slide, as indicated. In both cases, the concentration of the R6G solution used was 1 nM.
3.3. Photobleaching Rate of R6G in PDMS Wells
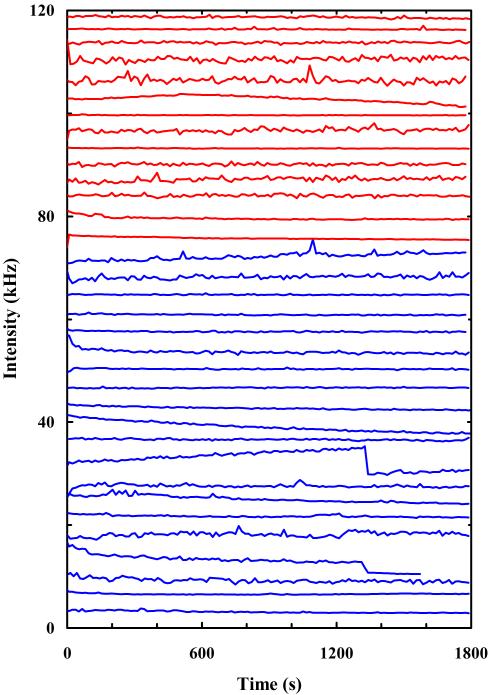
Initially, we did not anticipate that R6G molecules confined in micron-sized PDMS wells would show different photobleaching behaviors in comparison to those encapsulated in GUVs. Thus, it came as a surprise when the initial single-molecule photobleaching experiment suggested that the PDMS wells may enhance the photostability of the encapsulated R6G molecules. As shown (Figure 5), the fluorescence intensity time trace obtained with a BSA-coated PDMS well filled with a 10 pM R6G solution indicates that the confined dye molecule(s) is remarkably resistant to photobleaching, even at high laser powers. To further confirm that this result is not an anomaly, we conducted a series of single molecule photobleaching measurements using a large number of PDMS wells coated with either BSA or PEG. As indicated (Figure 6), among the 34 fluorescence intensity time traces obtained, only 2 showed a photobleaching event after a long period of time (>1200 s). Furthermore, it is worth pointing out that the autofluorescence of PDMS contributes little, if any, to the observed fluorescence signal because (1) the laser focus was tuned 2-3 μm away from the PDMS surface, minimizing any potential autofluorescence, and (2) the autocorrelation of the fluorescence signals obtained in the PDMS wells shows characteristic diffusion of R6G (e.g., Figure 4). Thus, taken together these data clearly demonstrate that both BSA- and PEG-coated PDMS wells are able to significantly enhance the photostability of R6G.
Figure 5.
A representative fluorescence intensity time trace of single R6G molecules confined in BSA-coated PDMS wells.
Figure 6.
Fluorescence intensity time traces of single R6G molecules confined in either BSA-coated (blue) or PEG-coated (red) PDMS wells. For ease of comparison, the original data have been offset. The variation in fluctuations among individual traces is resulting from different collection times (thus different bin time).
The molecular mechanism underlying photobleaching of fluorescent dyes is not well understood and may depend on the chemical and physical properties of the molecules of interest. For example, it has been suggested that photobleaching may involve triplet state formation. Indeed, many studies have shown that it is possible to alleviate the probability of photobleaching by decreasing the yield of the triplet state through the use of one or a cocktail of anti-photobleaching agents,16,29-31 or by employing a depletion laser pulse to enhance the yield of ground state recovery.32 To test whether the R6G molecules confined in micron-sized PDMS wells show different photophysical properties compared to those in bulk solution, we conducted high-resolution FCS measurements to determine the rate and yield of triplet state formation of R6G with and without the confinement of PDMS wells. As shown (Figure 7), the results clearly indicate, within our experimental uncertainties, that the confinement has not altered the rate of triplet state formation, suggesting that the aforementioned ‘protection’ effect of PDMS wells is not due to triplet state quenching. More specifically, these FCS curves (in the range of 100 ns – 1 s) can be well fit by the following equation,2
| (1), |
where the first term is related to diffusion, whereas the second term describes triplet state dynamics, with T and τtriplet representing the yield of triplet state formation and the lifetime of the triplet state, respectively. Fitting the FCS curves in Figure 7 yielded the following values: T = 0.28 ± 0.01 and τtriplet = 1.75 ± 0.05 μs for R6G diffusing in solution on BSA-covered glass slides and T = 0.30 ± 0.01 and τtriplet = 1.77 ± 0.05 μs for R6G diffusing in BSA-coated PDMS wells. In addition, this component (i.e., T) shows an increase with increasing laser power, consistent with our assignment to triplet state formation.
Figure 7.
Compassion of high resolution FCS curves obtained in a BSA-coated PDMS well and on a BSA-covered glass slide, as indicated. In both cases, the concentration of the R6G solution used to prepare the respective samples was 1 nM.
Another photobleaching mechanism that has been suggested involves formation of radical states via photoionization.4,33,34 Depending on the redox potential of the fluorophores and the environment, such non-fluorescent radical states can have a very long lifetime.35 Therefore, scavenging of radicals using antioxidants such as n-propyl gallate (nPG) and ascorbic acid (AA) has been shown as an effective way to improve the photostability of fluorescent dyes.7 Interestingly, most commonly used triplet state quenchers can also be used as radical scavengers, such as mercaptoethylamine (MEA),36 β-mercaptoethanol (2-BME), and Trolox.37 In fact, a series of reducing and oxidizing systems (ROXS) have been designed to alleviate photobleaching by recycling the fluorophore rapidly from the triplet state through a radical state to the ground state.7,38 According to this strategy, the triplet state is rapidly depopulated by reacting with a reducing agent such as Trolox.37 As a result, a radical anion is formed. The lifetime of this radical anion strongly depends on the availability for a second, reverse electron transfer reaction to return the fluorophore to the ground state. The rate of this process can be massively enhanced by adding an oxidizing agent. Based on these previous studies, we tentatively attribute the observed protective effect of BSA- and PEG-coated PDMS wells to their abilities to react with radical ions. For BSA-coated wells, this idea is consistent with the notion that BSA is an effective antioxidant and therefore can scavenge various radicals.39,40 Similarly, it is well known that oxygen plasma treatment generates a variety of reactive oxygen species41,42 such as superoxide anion, peroxide, hydrogen peroxide and hydroxyl radical that have the ability to react with other radicals. Thus, we hypothesize that the radical scavenging ability of PEG-coated PDMS wells arises from the reactive oxygen species generated in the oxygen plasma treatment step in the PEG coating process (see Experimental Section).
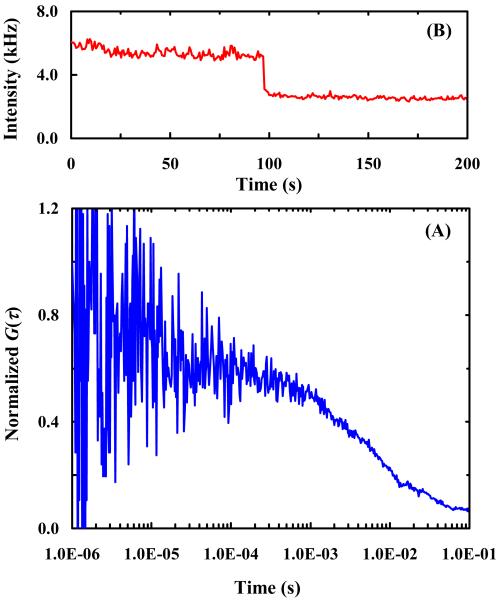
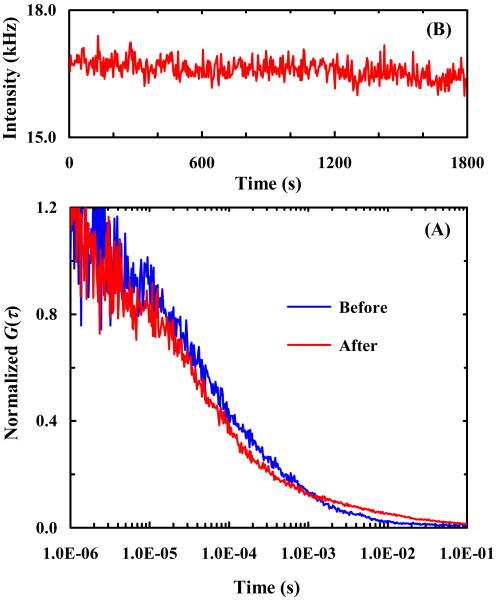
To test this hypothesis, we further conducted photobleaching experiments using PDMS wells with and without oxygen plasma treatment. Oxygen plasma treatment has been shown to effectively reduce the hydrophobicity of PDMS surface for a relatively long period of time (6 hours)43,44 and thus the surface stickiness of the molecules of interest. As expected (Figure 8), in untreated PDMS wells the encapsulated R6G molecules interact strongly with the PDMS surfaces, as judged by the poor quality of the correlation signal and also the lengthened correlation time (Figure 8A), and are quickly photobleached (Figure 8B). On the other hand, R6G molecules confined in PDMS wells that have been freshly treated by oxygen plasma cleaning, as shown (Figure 9), show drastic improvements in both the diffusivity and photostability of the encapsulated fluorescent molecules. These results thus corroborate our hypothesis that reactivity with radicals plays an important role in the protective effect provided by PEG- and BSA-coated PDMS wells. Currently, we are investigating whether the present method is effective in preventing photobleaching of other commonly used fluorescent dyes in single-molecules studies.45-48
Figure 8.
A representative FCS curve (A) and a fluorescence intensity time trace (B) of single R6G molecules in untreated PDMS wells.
Figure 9.
(A) Comparison of FCS curves of single R6G molecules in freshly oxygen plasma treated PDMS wells. As indicated, these data were obtained before and after 30 minutes of continuous laser (1.6 mW) illumination of the sample; and (B) the fluorescence intensity time trace obtained after the 30 minute photobleaching event.
4. CONCLUSIONS
In summary, we demonstrate a simple method to effectively prolong the survival time of R6G dye molecules before photobleaching occurs. Because R6G shares structural similarities with many commonly used fluorescent dye reporters, we believe that this method is not limited to R6G and can be used more broadly. Specifically, we show that micron-sized, BSA- or PEG-coated PDMS wells can enhance the photostability of one or a few R6G molecules. We hypothesize that the enhanced resistance to photobleaching in the present case arises from the ability of such PDMS wells to quench the electronic state(s) leading to photoreactions. This hypothesis is consistent with several previous studies suggesting that many fluorescent dyes, including R6G, undergo photobleaching via formation of radical ions and, thus, reactions that can effectively help such radical states return to the fluorephore’s electronic ground states will lead to an enhancement in the photostability of the fluorescent molecules. Furthermore, we believe that the current method could have many potential biophysical applications wherein both a long observation time and a confined environment are needed, for example, in the study of the structure and dynamics of individual amyloid peptide oligomers via FCS or FRET.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the National Institutes of Health (GM-065978) and the Nano/Bio Interface Center (NBIC) at the University of Pennsylvania. We also wish to thank Professor Haim H. Bau and Dr. Joseph M. Grogan for providing us with the silicon wafer used to prepare the PDMS wells.
REFERENCES
- (1).Sherman E, Itkin A, Kuttner YY, Rhoades E, Amir D, Haas H, Haran G. Using Fluorescence Correlation Spectroscopy to Study Conformational Changes in Denatured Proteins. Biophys. J. 2008;94:4819–4827. doi: 10.1529/biophysj.107.120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Guo L, Chowdhury P, Glasscock JM, Gai F. Denaturant-induced Expansion and Compaction of a Multi-domain Protein: IgG. J. Mol. Biol. 2008;384:1029–1036. doi: 10.1016/j.jmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wennmalm S, Edman L, Rigler R. Conformational Fluctuations in Single DNA Molecules. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10641–10646. doi: 10.1073/pnas.94.20.10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ha T, Tinnefeld P. Photophysics of Fluorescent Probes for Single-Molecule Biophysics and Super-Resolution Imaging. Annu. Rev. Phys. Chem. 2012;63:595–617. doi: 10.1146/annurev-physchem-032210-103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Joo C, Balci H, Ishitsuka Y, Buranachai C, Ha T. Advances in Single-Molecule Fluorescence Methods for Molecular Biology. Annu. Rev. Biochem. 2008;77:51–76. doi: 10.1146/annurev.biochem.77.070606.101543. [DOI] [PubMed] [Google Scholar]
- (6).Altman RB, Zheng Q, Zhou Z, Terry DS, Warren JD, Blanchard SC. Enhanced Photostability of Cyanine Fluorophores Across the Visible Spectrum. Nat. Methods. 2012;9:428–429. doi: 10.1038/nmeth.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Aitken CE, Marshall RA, Puglisi JD. An Oxygen Scavenging System for Improvement of Dye Stability in Single-Molecule Fluorescence Experiments. Biophys. J. 2008;94:1826–1835. doi: 10.1529/biophysj.107.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Donnert G, Eggeling C, Hell SW. Major Signal Increase in Fluorescence Microscopy through Dark-state Relaxation. Nat. Methods. 2007;4:81–86. doi: 10.1038/nmeth986. [DOI] [PubMed] [Google Scholar]
- (9).Bernas T, Zarebski M, Dobrucki JW, Cook PR. Minimizing Photobleaching During Confocal Microscopy of Fluorescent Probes Bound to Chromatin: Role of Anoxia and Photon Flux. J. Microsc. 2004;215:281–296. doi: 10.1111/j.0022-2720.2004.01377.x. [DOI] [PubMed] [Google Scholar]
- (10).Harada Y, Sakurada K, Aoki T, Thomas DD, Yanagida T. Mechanochemical Coupling in Actomyosin Energy Transduction Studied by in vitro Movement Assay. J. Mol. Biol. 1990;216:49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- (11).Mei E, Tang JY, Vanderkooi JM, Hochstrasser RM. Motions of Single Molecules and Proteins in Trehalose Glass. J. Am. Chem. Soc. 2003;125:2730–2735. doi: 10.1021/ja021197t. [DOI] [PubMed] [Google Scholar]
- (12).Widengren J, Rigler R. Mechanisms of Photobleaching Investigated by Fluorescence Correlation Spectroscopy. Bioimaging. 1996;4:149–157. [Google Scholar]
- (13).Zondervan R, Kulzer F, Kol’chenko MA, Orrit M. Photobleaching of Rhodamine 6G in Poly(vinyl alcohol) at the Ensemble and Single-Molecule Levels. J. Phys. Chem. A. 2004;108:1657–1665. [Google Scholar]
- (14).English DS, Furube A, Barbara PF. Single-molecule Spectroscopy in Oxygen-Depleted Polymer Films. Chem. Phys. Lett. 2000;324:15–19. [Google Scholar]
- (15).van Dijk MA, Kapitein LC, van Mameren J, Schmidt CF, Peterman EJG. Combining Optical Trapping and Single-molecule Fluorescence Spectroscopy: Enhanced Photobleaching of Fluorophores. J. Phys. Chem. B. 2004;108:6479–6484. doi: 10.1021/jp049805+. [DOI] [PubMed] [Google Scholar]
- (16).Zheng Q, Jockusch S, Zhou Z, Altman RB, Warren JD, Turro NJ, Blanchard SC. On the Mechanisms of Cyanine Fluorophore Photostabilization. J. Phys. Chem. Lett. 2012;3:2200–2203. doi: 10.1021/jz300670p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Vogelsang J. A Reducing and Oxidizing System Minimizes Photobleaching and Blinking of Fluorescent Dyes. Angew. Chem. Int. Ed. 2008;47:5465–5469. doi: 10.1002/anie.200801518. [DOI] [PubMed] [Google Scholar]
- (18).Lemke E. Microfluidic Device for Single-Molecule Experiments with Enhanced Photostability. J. Am. Chem. Soc. 2009;131:13610–13612. doi: 10.1021/ja9027023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rondelez Y, Tresset G, Tabata KV, Arata H, Fujita H, Takeuchi S, Noji H. Microfabricated Arrays of Femtoliter Chambers Allow Single Molecule Enzymology. Nat. Biotechnol. 2005;23:361–365. doi: 10.1038/nbt1072. [DOI] [PubMed] [Google Scholar]
- (20).Iino R. Single-Molecule Assay of Biological Reaction in Femtoliter Chamber Array. Jpn. J. Appl. Phys. 2009;48 08JA04. [Google Scholar]
- (21).Yeh HC, Puleo CM, Lim TC, Ho YP, Giza PE, Huang RC, Wang TH. A Microfluidic-FCS Platform for Investigation on the Dissociation of Sp1-DNA Complex by Doxorubicin. Nucleic Acids Res. 2006;34:e144. doi: 10.1093/nar/gkl787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Jung S-Y, Liu Y, Collier CP. Fast Mixing and Reaction Initiation Control of Single-Enzyme Kinetics in Confined Volumes. Langmuir. 2008;24:4439–4442. doi: 10.1021/la800053e. [DOI] [PubMed] [Google Scholar]
- (23).MacDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- (24).Boukobza E, Sonnenfeld A, Haran G. Immobilization in Surface-Tethered Lipid Vesicles as a New Tool for Single Biomolecule Spectroscopy. J. Phys. Chem. B. 2001;105:12165, 12170. [Google Scholar]
- (25).Piwonski HM, Goomanovsky M, Bensimon D, Horovitz A, Haran G. Allosteric Inhibition of Individual Enzyme Molecules Trapped in Lipid Vesicles. Proc. Natl. Acad. Sci. USA. 2012;109:1437–1443. doi: 10.1073/pnas.1116670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Yeow EK, Melnikov SM, Bell TD, De Schryver FC, Hofkens J. Characterizing the Fluorescence Intermittency and Photobleaching Kinetics of Dye Molecules Immobilized on a Glass Surface. J. Phys. Chem. A. 2006;9:1726–1734. doi: 10.1021/jp055496r. [DOI] [PubMed] [Google Scholar]
- (27).Lee S, Lee J, Hohng J. Single-Molecule Three-Color FRET with Both Negligible Spectral Overlap and Long Observation Time. PLoS ONE. 2010;5:e12270. doi: 10.1371/journal.pone.0012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Yao G, Fang X, Yokota H, Yanagida T, Tan W. Monitoring Molecular Beacon DNA Probe Hybridization at the Single-Molecule Level. Chemistry. 2003;9:5686–5692. doi: 10.1002/chem.200304977. [DOI] [PubMed] [Google Scholar]
- (29).Song L, Varma CA, Verhoeven JW, Tanke HJ. Influence of the Triplet Excited State on the Photobleaching Kinetics of Fluorescein in Microscopy. Biophys. J. 1996;70:2959–2968. doi: 10.1016/S0006-3495(96)79866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rasnik I, McKinney SA, Ha T. Nonblinking and Long-lasting Single-molecule Fluorescence Imaging. Nat. Methods. 2006;3:891–893. doi: 10.1038/nmeth934. [DOI] [PubMed] [Google Scholar]
- (31).Dave R, Terry DS, Munro JB, Blanchard SC. Mitigating Unwanted Photophysical Processes for Improved Single-Molecule Fluorescence Imaging. Biophys. J. 2009;96:2371–2381. doi: 10.1016/j.bpj.2008.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Mondal PP. Minimizing Photobleaching in Fluorescence Microscopy by Depleting Triplet States. Appl. Phys. Lett. 2007;92:013902. [Google Scholar]
- (33).Eggeling C, Volkmer A, Seidel CA. Molecular Photobleaching Kinetics of Rhodamine 6G by One- and Two-Photon Induced Confocal Fluorescence Microscopy. Chemphyschem. 2005;6:791–804. doi: 10.1002/cphc.200400509. [DOI] [PubMed] [Google Scholar]
- (34).Zondervan R, Kulzer F, Orlinskii SB, Orrit M. Photoblinking of Rhodamine 6G in Poly(vinyl alcohol): Radical Dark State Formed through the Triplet. J. Phys. Chem. A. 2003;107:6770–6776. [Google Scholar]
- (35).Vogelsang J, Cordes T, Forthmann C, Steinhauer C, Tinnefeld P. Controlling the Fluorescence of Ordinary Oxazine Dyes for Single-molecule Switching and Superresolution Microscopy. Proc. Natl. Acad. Sci. USA. 2009;106:8107–8112. doi: 10.1073/pnas.0811875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Widengren J, Chmyrov A, Eggeling C, Löfdahl PA, Seidel CA. Strategies to Improve Photostabilities in Ultrasensitive Fluorescence Spectroscopy. J. Phys. Chem. A. 2007;111:429–440. doi: 10.1021/jp0646325. [DOI] [PubMed] [Google Scholar]
- (37).Cordes T, Vogelsang J, Tinnefeld P. On the Mechanism of Trolox as Antiblinking and Antibleaching Reagent. J. Am. Chem. Soc. 2009;131:5018–5019. doi: 10.1021/ja809117z. [DOI] [PubMed] [Google Scholar]
- (38).Vogelsang J, Kasper R, Steinhauer C, Person B, Heilemann M, Sauer M, Tinnefeld P. A Reducing and Oxidizing System Minimizes Photobleaching and Blinking of Fluorescent Dyes. Angew. Chem. Int. Ed. 2008;47:5465–5469. doi: 10.1002/anie.200801518. [DOI] [PubMed] [Google Scholar]
- (39).Davies KJ, Lin SW, Pacifici RE. Protein Damage and Degradation by Oxygen Radicals. J. Biol. Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- (40).Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The Antioxidant Properties of Serum Albumin. FEBS Lett. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- (41).Bodas D, Khan-Malek C. Hydrophilization and Hydrophobic Recovery of PDMS by Oxygen Plasma and Chemical Treatment—an SEM Investigation. Sensors and Actuators B. 2007;123:368–373. [Google Scholar]
- (42).Wong I, Ho C-M. Surface Molecular Property Modifications for Poly(dimethylsiloxane) (PDMS) Based Microfluidic Devices. Microfluid Nanofluidics. 2009;7:291–306. doi: 10.1007/s10404-009-0443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Tan SH, Nguyen N-T, Chua YC, Kang TG. Oxygen Plasma Treatment for Reducing Hydrophobicity of a Sealed Polydimethylsiloxane Microchannel. Biomicrofluidics. 2010;4:032204. doi: 10.1063/1.3466882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).de Menezes Atayde C, Doi I. Highly Stable Hydrophilic Surfaces of PDMS Thin Layer Obtained by UV Radiation and Oxygen Plasma Treatments. Phys. Status Solidi C. 2010;7:189–192. [Google Scholar]
- (45).Ly CT, Altuntop ME, Wang Y. Single-Molecule Study of Viomycin’s Inhibition Mechanism on Ribosome Translocation. Biochemistry. 2010;49:9732–9738. doi: 10.1021/bi101029g. [DOI] [PubMed] [Google Scholar]
- (46).Campos LA, Liu J, Wang X, Ramanathan R, English DS, Munoz V. A Photoprotection Strategy for Microsecond-resolution Single-molecule Fluorescence Spectroscopy. Nat. Methods. 2011;8:143–146. doi: 10.1038/nmeth.1553. [DOI] [PubMed] [Google Scholar]
- (47).Elbaum-Garfinkle S, Rhoades E. Identification of an Aggregation-Prone Structure of Tau. J. Am. Chem. Soc. 2012;134:16607–16613. doi: 10.1021/ja305206m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Soranno A, Buchli B, Nettels D, Cheng RR, Müller-Späth S, Pfeil SH, Hoffmann A, Lipman EA, Makarov DE, Schuler B. Quantifying Internal Friction in Unfolded and Intrinsically Disordered Proteins with Single-molecule Spectroscopy. Proc. Natl. Acad. Sci. USA. 2012;109:17800–17806. doi: 10.1073/pnas.1117368109. [DOI] [PMC free article] [PubMed] [Google Scholar]