Abstract
Objectives
HIV infection is associated with dyslipidaemia and increased risk of cardiovascular disease. The effects of HIV infection and antiretroviral treatment on surrogate markers of atherosclerosis, and lipoprotein metabolism were evaluated in a 12 month prospective study.
Methods and Results
Treatment-naive HIV patients were recruited into one of three groups: untreated HIV infection not likely to require initiation of antiretroviral therapy (ART) for at least 12 months; initiating treatment with non nucleoside reverse transcriptase inhibitor-containing ART regimen and initiating treatment with protease inhibitor-containing ART regimen. The patients underwent assessment of carotid intima-media thickness (cIMT), pulse wave velocity (PWV), brachial flow-mediated dilation (FMD) and variables of plasma lipoprotein metabolism at baseline and 12 months. The findings were compared with published values for age and sex matched HIV-negative healthy subjects in a cross-sectional fashion. cIMT and FMD were lower while PWV was higher in HIV-patients compared with HIV-negative individuals; none of the markers changed significantly during 12 months follow up. HIV patients had hypoalphalipoproteinemia and elevated plasma levels of lecithin:cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein. The only significant changes in lipid-related variables were elevation of total cholesterol and triglycerides in patients treated with PI-containing regimen and elevation of plasma LCAT levels in patients treated with NNRTI-containing regimen. The ability of whole and apoB-depleted plasma to effect cholesterol efflux was not impaired in all three groups.
Conclusions
This study did not find evidence for rapid progression of subclinical atherosclerosis and deterioration of dyslipidaemia in HIV patients within 1 year.
Keywords: HIV, atherosclerosis, lipoproteins, cholesterol metabolism
1. Introduction
Current treatment for HIV infection has dramatically reduced mortality, however, co-morbidities that are not directly related to immunodeficiency are now increasingly recognized as a consequence of HIV infection. One such co-morbidity is an increased risk of cardiovascular disease. The current view is that HIV infection and/or its treatment are associated with elevated risk of development of atherosclerosis and consequently with increased prevalence of acute and chronic cardiovascular events [1, 2]. The evidence for this conclusion, however, is not unequivocal.
Many early antiretroviral regimens included protease inhibitors (PI) that caused dyslipidaemia [3] and most likely had other pro-atherogenic effects not directly related to dyslipidaemia [4, 5]. Enhanced development of atherosclerosis due to treatment with PI-containing regimens has been documented [6]. Current ART regimens are less likely to cause elevation of low density lipoprotein cholesterol (LDL-C) [7]. Another element of dyslipidaemia, low levels of high density lipoprotein cholesterol (HDL-C), persists in both treated and untreated patients [8]. We [9, 10] and others [11] suggested that HIV infection itself, in addition to antiretroviral regimens, may contribute to dyslipidaemia and elevated risk of atherosclerosis. Clinical evidence to support such a hypothesis is conflicting. Several studies have demonstrated an increased risk of atherosclerosis in HIV patients [12–17], but were cross-sectional or retrospective in design and did not assess the progression of atherosclerosis. Two short-term prospective studies demonstrated a faster increase of cIMT in HIV patients compared to HIV-negative individuals [18, 19], but both studies included subjects on PI-containing regimens. Other studies demonstrated no impact of HIV infection or non-PI treatment regimens on surrogate markers of atherosclerosis when adjusted for conventional risk factors [20–22] including three prospective studies [23–25]. The only outcome-based trial, SMART, did not produce a clear conclusion [26, 27]. On the one hand, interruption of antiretroviral therapy was associated with increased cardiovascular mortality pointing to the contributory role of HIV infection in the development of atherosclerosis. On the other hand, that study produced no evidence for association between HIV viral load and increased CVD risk [26, 27].
In this study we assessed progression of atherosclerosis over 12 months in treatment-naive HIV-infected patients using three surrogate measures of atherosclerosis, cIMT, PVW and FMD. We compared patients who remained untreated with patients who commenced treatment with non-nucleoside reverse transcriptase inhibitor (NNRTI)- or PI- containing regimens, and with an HIV-negative cohort. We also assessed changes in lipid variables and HDL functionality and related the progression of atherosclerosis to changes in HIV disease status and lipid variables.
2. Methods
2.1. Patients
HIV patients were recruited through the Infectious Disease Clinic at The Alfred Hospital. All patients were male reflecting the HIV patient population attending the Alfred Hospital (90% male). Patients taking lipid-lowering medications (including fish oil preparations) and those with history of Familial Hypercholesterolemia and BMI greater than 27 were excluded from participation. Smokers were not excluded, but patients abstained from smoking or taking caffeine within 4 h of the study procedures. All patients gave informed consent to the study which was approved by the Alfred Hospital Human Research and Ethics Committee (#54/05). Data for the HIV-negative subjects were obtained from our previously published studies [8, 28, 29].
Following an 8 h fast, ten millilitres of blood was collected into EDTA tubes and plasma was obtained by low speed centrifugation, aliquoted and frozen at −80°C. ApoB-depleted plasma was obtained after precipitation of apoB-containing lipoproteins as described previously [30].
2.2. Surrogate markers of atherosclerosis
The right carotid artery was assessed for carotid wall intima-media thickness (cIMT) by ultrasound using Philips iE33 with 11 MHz Linear transducer as described previously [29]. The cIMT was defined as the distance between lumen-intima interface and media-adventitia interface and was measured from the 2D high resolution digital images obtained using an automated border-detection algorithm (Philips QLAB). cIMT was determined in areas identified as without plaque by 6 duplicate consecutive measurements made 1–3 cm proximal to the carotid bulb. The presence of plaques was also documented.
Pulse wave velocity (PWV) was measured as described previously [28]. PWV was calculated by the difference in time between the two waves obtained from tenometers divided by the distance between carotid and femoral arteries minus the distance between carotid and manubrium sternum.
Brachial flow-mediated dilation (FMD) was measured as described previously [29]. A continuous ultrasound image of the carotid artery in longitudinal cross-section was recorded at baseline, 60 seconds and at 30 second intervals thereafter post induced-ischemia for 4 minutes. The minimum arterial diameter in diastole was measured at all time points in triplicates to maintain reproducibility. FMD was expressed as the highest percentage change in artery dilation when compared to baseline.
All measurements of surrogate markers of atherosclerosis were performed by the same operator both at baseline and after 12 months. Coefficients of variation for repeated measures at a short time interval were 5.7%, 3.2%, and 10.0% for IMT, PWV and FMD, respectively.
2.3. Plasma analysis
Total cholesterol (TC), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C) and triacylglycerol (TG) levels were measured using a colorimetric microassays (Wako, Japan). Lecithin :cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein (CETP) concentrations in the human plasma were measured by ELISA (Sekisui Medical Co., Japan). Apolipoprotein A–I (apoA-I) and apolipoprotein B (apoB) levels in plasma were measured using a COBAS Integra 400 Plus blood analyzer. The CD4+ T cell count and the percentage of CD4+ T cells were determined by flow cytometric analysis of cells stained with a range of fluorescently labelled antibodies against lymphoid markers. The plasma HIV viral load was measured by commercial RT-PCR assay (Amplicor Roche).
2.4. Ex vivo Cholesterol Efflux Assay
Cholesterol efflux was measured as described previously [30]. Briefly, differentiated THP-1 cells were incubated in serum-containing medium supplemented with [3H]cholesterol (75 kBq/mL) for 48 h and simultaneously activated with the LXR agonist TO-901317 (final concentration 4 µmol/L). Cells were then incubated for 24 h in serum-free medium in the presence of TO-901317. Plasma or apoB-depleted plasma was then added to the final concentration of 1%. Cells were incubated for 2 h after which aliquots of medium and cells were counted. The efflux was calculated as a proportion of radioactivity that moved from medium to cells (after subtracting efflux to medium without acceptors).
2.5. Statistical Analysis
Means ± SD are shown. Paired t-test was used to assess the difference between baseline and 12 months values and unpaired test was used to assess differences between variables in HIV patients and controls. ANCOVA was used to assess the differences between the groups adjusted for baseline values at study entry. Pearson correlations (unadjusted) were applied to test associations between continuous variables. The sample size was sufficient for 80% power with differences exceeding 0.075 mm in cIMT, 3% in FMD and 1.2 m/s in PWV. The study also had 80% power to detect changes in plasma lipids and lipoproteins exceeding 15–20%, except in more variable LDL-C and apoB (35% change) and TG (60% change), and 0.3% change of cholesterol efflux.
3. Results
3.1. Patients
The number of patients in each treatment group and their anthropometric data are shown in Table 1. The first group consisted of treatment-naive patients who remained untreated for the duration of the study (12 month). The second group consisted of treatment-naive patients who initiated treatment with an NNRTI-based regimen at study baseline and remained on this treatment for the duration of the study. The third group consisted of treatment-naive patients who initiated treatment with a PI-containing regimen at study baseline and remained on this treatment for the duration of the study. Fewer patients were recruited into the PI-containing ART regimen group due to a decline in PI use as an initial regimen. The median (IQR) duration of HIV infection prior to recruitment into the study was 340 (117, 948) days. Table 1 includes the mean and standard deviation of known HIV infection. There was no significant difference in duration of HIV infection between the 3 groups. The three groups were similar for all variables, except for CD4 cell count and CD4 cell percentage, which are criteria for initiation of antiretroviral treatment.
Table 1.
Anthropometric and disease related variables
| Variable | Group 1 (treatment naive) | Group 2 (treatment with NNRTI) | Group 3 (treatment with PI) | |||
|---|---|---|---|---|---|---|
| Baseline | 12 month | Baseline | 12 month | Baseline | 12 month | |
| Number | 19 | 18 | 18 | 15 | 9 | 8 |
| Age (y) | 40.6±10.2 | 41.6±10.2 | 39.6±8.9 | 40.6±8.9 | 40.3±8.4 | 41.3±8.4 |
| BMI (kg/m2) | 24.6±1.5 | 23.5±2.5 | 23.0±2.8 | 22.1±2.5 | 21.4±1.9 | 25.6±2.4 |
| Duration of HIV infection (days)¶ |
1089±1961 | 1187±1805 | 707±1398 | |||
| CD4 cell count | 443±158 | 450±157 | 258 ±109* | 412±103 | 245±77* | 416±100 |
| CD4 % | 25.8±8.2 | 24.6±5.1 | 14.5±5.2** | 24.3±8.5 | 15.2±8.1* | 21.1±8.8 |
| Viral load (x103 ml−1) | 47±27 | 13±11 | 51±17 | <0.05 | 62±30 | <0.05 |
| Systolic BP (mm Hg) | 121.7±12.7 | 115.7±9.1 | 112.6±10.8 | 117.8±8.3 | 125.8±5.8 | 134.0±31.5 |
| Diastolic BP (mm Hg) | 72.5±7.9 | 71.4±9.1 | 73.2±7.6 | 73.0±8.7 | 74.0±8.0 | 86.5±6.8 |
Means ±SD are shown;
p<0.01 versus treatment- naïve;
p<0.0001 versus treatment-naïve
Duration of HIV infection defined as time between first positive HIV test and study baseline assessment.
Surrogate markers of atherosclerosis and plasma lipid variables assessed in HIV-infected patients were cross-sectionally compared to corresponding values for HIV-negative subjects from our previously published studies. These studies used the methodologies identical to those used in this study and HIV-negative healthy subjects in these studies matched patients in the current study with respect to age, sex and BMI [8, 28, 29].
3.2. Surrogate markers of atherosclerosis
To assess progression of atherosclerosis in patients with HIV, three surrogate markers were used, cIMT, PWV and FMD. There was no difference between baseline and 12-month measurements of all three surrogate markers in untreated patients (group 1) (Table 2). There was a slight increase in cIMT and FMD in group 2 (treatment with NNRTI) and group 3 (treatment with PI) after 12 months follow up; the difference however did not reach statistical significance. There was also no statistically significant difference between baseline and 12 month observations when the data for all three HIV groups were combined (0.53±0.10 versus 0.56±0.10; 7.45±0.93 versus 8.00±1.43; 6.50±4.06 versus 7.84±3.72 for cIMT, PWV and FMD respectively, p>0.05 for all comparisons, n=41). However, ANCOVA analysis demonstrated that slope of changes in FMD values was steeper in Group 3 compared to groups 1 and 2 indicating a possible positive effect of treatment with PI in subjects with vascular dysfunction.
Table 2.
Surrogate measures of atherosclerosis
| Variable | Group 1 (treatment naive) | Group 2 (treatment with NNRTI) |
Group 3 (treatment with PI) | Published values for HIV-negative healthy subjects¶ |
|||
|---|---|---|---|---|---|---|---|
| Baseline | 12 month | Baseline | 12 month | Baseline | 12 month | ||
| cIMT (mm) | 0.56±0.11 | 0.55±0.08 | 0.52±0.08 | 0.55±0.11 | 0.49±0.04 | 0.52±0.03 | 0.64±0.1*1 |
| FMD (%) | 8.2±4.4 | 8.6±4.2 | 4.7±3.1 | 5.6±3.1 | 5.9±3.0 | 8.5±3.2§ | 13.1±4.8*1 |
| PWV (m/s) | 7.3±1.0 | 7.2±0.7 | 7.7±0.7 | 7.6±0.9 | 7.1±0.4 | 7.9±1.1 | 6.3±0.2*2 |
IMT, a structural marker of atherosclerosis, was statistically significantly lower (p<0.01) in HIV patients compared to published values for matched HIV-negative healthy subjects indicating less atherosclerosis. However, PWV was statistically significantly higher (p<0.01), while FMD was statistically significantly lower (p<0.01) indicating a vascular dysfunction, which is a risk factor for atherosclerosis, in HIV patients. Thus, two out of three surrogate markers indicate vascular dysfunction in HIV patients, however, we did not find evidence for rapid progression of atherosclerosis in HIV-infected subjects, at least within the limited period of follow up.
3.3. Lipids and lipoproteins
Levels of total and LDL cholesterol and apoB rose slightly during 12 months of observation in all three groups of HIV patients, but only elevation of total cholesterol in group 3 (treatment with PI) reached statistical significance (Table 3). ANCOVA analysis demonstrated that slope of changes in LDL was different between all three groups indicating possible negative effect of treatment on subjects with lower LDL-C levels. Plasma triglyceride levels did not change over 12 months in patients in groups 1 and 2, but rose significantly in group 3. Plasma total cholesterol, LDL cholesterol, apoB and triglyceride levels in group 1 (untreated) and group 2 (treatment with NNRTI) were similar to published values for matched HIV-negative healthy subjects. In group 3 (treated with PI) levels of total cholesterol and triglycerides after 12 month were significantly higher compared to published values for matched HIV-negative healthy subjects. Thus, levels of apoB-containing lipoproteins were not affected by HIV infection, but treatment with PI-containing regimen caused elevation of level of triglyceride-rich lipoproteins over 12 month period.
Table 3.
Lipids and Lipoproteins
| Variable | Group 1 (treatment naive) | Group 2 (treatment with NNRTI) |
Group 3 (treatment with PI) | Published values for HIV-negative healthy subjects (n=33)¶ |
|||
|---|---|---|---|---|---|---|---|
| Baseline | 12 month | Baseline | 12 month | Baseline | 12 month | ||
| Total cholesterol (mmol/l) | 4.5±0.9 | 4.8±1.0 | 4.4±0.9 | 5.3±1.1 | 4.4±0.9 | 6.1±1.6* | 4.9±0.8 |
| LDL cholesterol (mmol/l) | 2.9±0.8 | 3.2±0.9 | 2.8±0.8 | 3.4±0.8§ | 2.9±0.7 | 3.9±1.3§ | 2.9±0.9 |
| ApoB (g/l) | 0.7±0.2 | 0.8±0.2# | 0.8±0.2 | 0.9±0.2 | 0.7±0.3 | 0.9±0.3 | 0.7±0.3 |
| Triglycerides (mmol/l) | 1.3±0.6 | 1.4±0.6# | 1.8±1.0 | 1 8±1 4# | 1.7±1.0 | 2.5±0.5* | 1.3±0.8 |
| HDL cholesterol (mmol/l) | 1.0±0.3 | 1.0 ±0.3† | 0.8±0.3 | 1.0±0.3 | 0.8±0.2 | 0.9±0.2 | 1.4±0.4 |
| ApoA-I (g/l) | 1.3±0.3 | 1.3±0.2#† | 1.2±0.2 | 1.4±0.2 | 1.1±0.1 | 1.4±0.1 | 1.3±0.2 |
| HDL-C/ApoA-I | 0.8±0.1 | 0.8±0.1† | 0.7±0.1 | 0.7±0.1# | 0.7±0.1 | 0.6±0.1 | 1.1±0.4 |
| LCAT (µg/ml) | 8.5±1.9 | 8.3±1.9# | 7.7±2.2 | 9.4±2.4* | 6.0±0.9 | 7.7±1.9 | 4.3±1.6 |
| CETP (µg/ml) | 2.4±0.5 | 2.4±0.5 | 2.2±0.6 | 2.4±0.3 | 2.7±0.4 | 2.6±0.6 | 2.0±0.9 |
Previously published in [8]. Means ± SD are shown;
p<0.05 (versus baseline);
p<0.02 (for slope versus two other groups);
p<0. 05 (for elevation versus group 2);
p<0. 05 (for elevation versus group 3).
HDL cholesterol level did not change in the three HIV groups during 12 months, but was significantly lower than published values for matched HIV-negative healthy subjects (Table 3). Plasma apoA-I levels did not change in group 1 (untreated), but were slightly elevated in groups 2 (treatment with NNRTI) and 3 (treatment with PI), the difference, however, was not statistically significant. ANCOVA analysis demonstrated that elevations of changes in apoA-I and HDL-C were higher in untreated than in one or both treated groups indicating possible positive effect of treatment on subjects with lower HDL levels. Plasma apoA-I levels in all groups of HIV patients were similar to published values for matched HIV-negative healthy subjects. Ratio of HDL-C/apoA-I was similar in all three groups during 12 months observation period, but was lower compared to published values for matched HIV-negative healthy subjects, indicating that HDL particles in HIV patients may be cholesterol-poor.
Plasma levels of LCAT did not change over 12 months in group 1 (untreated), but elevated in groups 2 and 3, the difference reached statistical significance in group 2 (treatment with NNRTI) (Table 3). Plasma levels of LCAT were substantially higher in HIV patients compared with published values for matched HIV-negative healthy subjects (p<0.001). Plasma levels of CETP did not change in all three groups of HIV patients over 12 months of observation (Table 3) and were higher than published values for matched HIV-negative healthy subjects (p<0.05).
Thus, two elements of HDL metabolism, LCAT and CETP, were altered in HIV patients, but the magnitude of this effect did not change significantly during the 12 month study.
3.4. Cholesterol efflux
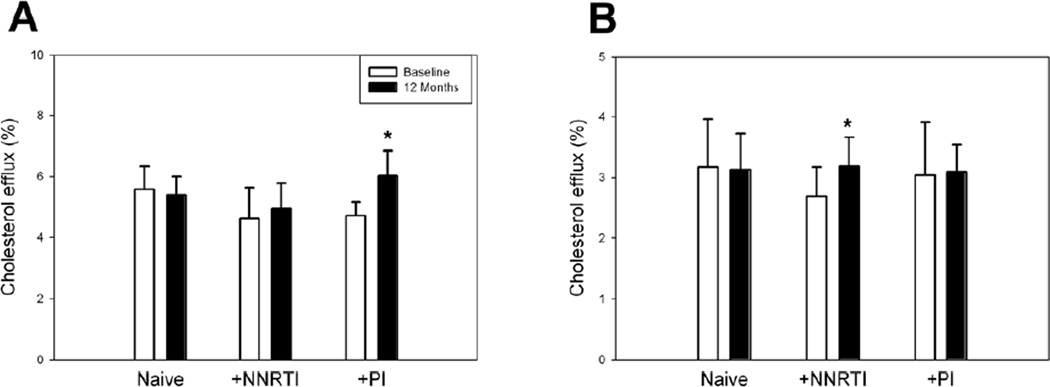
The capacity of plasma to effect cholesterol efflux in groups 1 and 2 did not change during 12 months of observation (Fig 1 A). Cholesterol efflux to plasma of patients in group 3 (treated with PI) has increased after 12 months. This increase could be a result of an increased functionality of HDL particles or of increased abundance of HDL particles. To distinguish between these two possibilities we normalized cholesterol efflux to the concentration of apoA-I, which is proportional to the abundance of HDL particles. After this normalization the difference in cholesterol efflux disappeared (data not shown) indicating that it was more likely due to an increased abundance of HDL particles than to changes in functionality of HDL particles. When apoB-depleted plasma was used as an acceptor in the cholesterol efflux assay, there was no difference in efflux between baseline and 12 months time points for groups 1 and 3, but the efflux in group 2 was elevated after 12 month of observation (Fig. 1 B). Again, when normalized to plasma apoA-I content, the differences disappeared. Thus, HIV infection did not impair the functional capacity of plasma to effect cholesterol efflux during the 12 month observation period.
Figure 1. Cholesterol efflux to whole plasma or apoB-depleted plasma of HIV patients.
THP-1 cells were differentiated, labeled with [3H] cholesterol and activated with TO-901317 (4 µ mol/L) as described in Methods. Cells were then incubated for 2 h with whole plasma (1%) (A) or apoB-depleted plasma (1%) (B). Cholesterol efflux is expressed as proportion of labeled cholesterol moved from cells to medium. *p<0.01 versus baseline of the same group; this difference disappeared when cholesterol efflux was normalized to plasma apoA-I content.
3.5. Analysis of associations
To gain an insight into possible relationships between severity of HIV infection, development of atherosclerosis and lipoprotein metabolism, we considered associations between the variables measured in this study (Table 4). To gain statistical power, we combined data from all three groups of HIV infected patients and used baseline values for analysis of correlations. Many of the associations, however, were valid for the individual groups of patients as also shown in Table 4. cIMT correlated positively with total cholesterol, LDL-C, TG and HIV viral load. Perhaps surprisingly, there was a positive correlation between cIMT and cholesterol efflux (to both whole and apoB-depleted plasma). Multivariate analysis, however, showed that the only independent variable associated with changes in cIMT was plasma levels of TG (beta coefficient 0.102, p<0.001). CD4+% correlated positively with HDL-C, apoA-I, LDL-C and cholesterol efflux; cholesterol efflux also correlated with CD4+ cell count. This is consistent with our previous findings [8] and points to an association between HIV replication and levels and function of HDL. Cholesterol efflux to whole plasma correlated with TC, HDL-C, LDL-C, TG, apoA-I, apoB and CD4+ cell count. Efflux to apoB-depleted plasma showed the same associations except for the associations with apoB-containing lipoproteins. Plasma LCAT concentration correlated positively with total and LDL cholesterol, apoB, apoA-I and cholesterol efflux to both whole and apoB-depleted plasma.
Table 4.
Analysis of correlations
| Variable | cIMT | CD4% | Efflux (%) | Efflux to ΔapoB (%) | LCAT(µg/ml) | Viral load |
|---|---|---|---|---|---|---|
| Total Cholesterol (mmol/l) | 0.36* | 0.51**#† | 0.8**† | |||
| HDL-C (mmol/l) | 0.34* | 0.31* | 0.39**# | |||
| LDL-C (mmol/l) | 0.49** | 0.30* | 0.42**† | 0.59*† | ||
| TG (mmol/l) | 0.6***†‡ | 0.42** | 0.49***† | |||
| ApoA-I (g/l) | 0.36* | 0.42** | 0.47** | 0.65*† | ||
| apoB (g/l) | 0. 37**#† | 0.31**#† | 0.72**† | |||
| CD4 | 0.31* | 0.34* | ||||
| Efflux (%) | 0.47** | 0.34* | 0.64*† | |||
| Efflux to Δ apoB (%) | 0.39* | 0.59*† | ||||
| Viral load | 0.36*† |
Means ± SD are shown; n=46;
p<0.05;
p<0.01;
p<0.001 (for combined group);
p<0.05 for group 1;
p<0.05 for group 2;
p<0.05 for group 3.
4. Discussion
In this study we investigated the progression of atherosclerosis and dyslipidaemia in patients with HIV disease followed for 1 year. Progression of atherosclerosis was assessed by three surrogate markers of atherosclerosis, cIMT, PVW and FMD. We compared three groups of initially treatment naive patients: those that continued without anti-retroviral therapy, patients that commenced treatment with NNRTI-containing regimen, and a small group of patients who started treatment with PI-containing regimen; the latter group was included mainly as a reference as the effects of PIs on dyslipidaemia and progression of atherosclerosis have been previously documented [3]. It is important to recognize that while treatment has started at the beginning of the observation period, the duration of the HIV infection was unknown. In a cross-sectional arm of the study, we compared surrogate markers of atherosclerosis and lipid variables in HIV infected patients with previously published values for sex-, age- and BMI-matched, medication-free healthy HIV-negative subjects.
The main outcomes of the analysis of the progression of atherosclerosis are as follows. First, we did not observe a progression of atherosclerosis in the untreated group and the group of patients treated with NNRTI over one year of observation. There was some progression of atherosclerosis in the group treated with PI-containing regimen, and although changes in surrogate markers of atherosclerosis did not reach statistical significance, this was almost certainly due to low number of patients in this group. Second, values of two out of three surrogate markers of atherosclerosis, specifically markers of vascular function, in HIV patients when compared to published values for matched HIV-negative healthy subjects were consistent with vascular dysfunction and possibly more atherosclerosis in HIV patients. The findings of the prospective arm of the study are consistent with three other available prospective studies investigating the impact of HIV infection on the development of atherosclerosis in a comparable cohort without treatment with PI-containing regimens [23–25]. Consistent with our study, two of these studies found that although progression of atherosclerosis was slow, it was associated not only with traditional risk factors, such as lipoprotein levels, but also with HIV-specific factors, such as CD4+ cell count, implying that there was a contribution of HIV infection to the progression of atherosclerosis [24, 25]. Specific contribution of HIV infection independent of the effects on lipid metabolism was also supported by a recent cross-sectional study [31]. The findings of the cross-sectional arm of the study are consistent with a number of reports showing increased atherosclerosis in HIV patients compared to HIV-negative subjects [12–16]. Limitations of this study were a small number of patients, short duration of the prospective arm of the study, and use of previously obtained values for the HIV-negative subjects in the cross-sectional arm of the study. Further, a connection between surrogate markers of atherosclerosis and clinical outcomes is not unequivocal. Recent meta-analysis demonstrated no association between the rate of changes in IMT and cardiovascular events, although a static IMT value was predictive [32].
Furthermore, atherosclerosis in HIV patients may be characterized by more unstable plaques rather than increased number of plaques, a difference not necessarily reflected in changes of surrogate markers. However, recognizing these limitations, our findings do not support a hypothesis that HIV disease is associated with rapid development of atherosclerosis. This is consistent with a number of other studies [20–22]. It is important to recognize that severity of HIV (viral load and/or CD4+ cell count) in patients in this study was controlled either naturally in the untreated group or by treatment, therefore conclusions cannot be extended to severe HIV infection.
Analysis of the effect of HIV infection on lipids and lipoprotein metabolism largely confirmed the conclusions of our previous study [8]: modest hypoalphalipoproteinemia, accompanied with elevation of plasma levels of LCAT and CETP. Raise in total cholesterol and triglycerides as a result of treatment with PI is a well established phenomenon and the only other variable related to lipoprotein metabolism that changed over 12 month observation period was plasma concentration of LCAT in patients treated with NNRTI-containing regimen. This observation may explain HDL-raising effects of some NNRTI [33], in our study NNRTI also caused small elevation of HDL as well as small elevation of the capacity of apoB-depleted plasma to effect cholesterol efflux. Analysis of associations confirmed conclusions from our previous study [8] about possible effect of HIV disease on dyslipidaemia, but did not show any associations of surrogate markers of atherosclerosis and markers of severity of HIV disease independent of lipid variables. Interestingly, there was a strong correlation between plasma TG levels and viral load on one hand and cIMT on the other pointing to the possible role of TG-rich lipoproteins in pathogenesis of atherosclerosis in HIV-infected patients. Thus, although HIV patients had dyslipidaemia characteristic for this disease, there was no deterioration of dyslipidaemia in a 12 month period.
Overall this study demonstrates that although there are indications that HIV infection may be associated with vascular dysfunction and altered two elements of HDL metabolism, LCAT and CETP, these changes are stable, at least in the short term and in a cohort of relatively young subjects. Longer studies are required to compare the rate of progression of atherosclerosis and dynamics of dyslipidaemia in HIV patients.
Highlights.
We analysed cIMT, FMD, PWV and lipids in HIV patients in a prospective study
We compared treatment-naive, treated and HIV-negative subjects
We found more atherosclerosis and impairment of HDL metabolism in HIV patients
We found little progression of atherosclerosis and of dyslipidemia over 1 year
Acknowledgements
This study would not be possible without the help of the Clinical Research Trial coordinators, especially Ms. Sally Newall and Ms. Jo Maher. This study was supported by grants from the National Health and Medical Research Council of Australia (#317811) and NIH (#R01HL101274) and in part by the Victorian Government’s OIS Program. DS and AD are fellows of the National Health and Medical Research Council of Australia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Luscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Glesby MJ. Coronary heart disease in HIV-infected patients. Current HIV/AIDS reports. 2005;2:68–73. doi: 10.1007/s11904-005-0021-7. [DOI] [PubMed] [Google Scholar]
- 3.Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, Marcovina SM, Glauser MP, Nicod P, Darioli R, Mooser V. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 4.Dressman J, Kincer J, Matveev SV, Guo L, Greenberg RN, Guerin T, Meade D, Li X-A, Zhu W, Uittenbogaard A, Wilson ME, Smart EJ. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J. Clin. Invest. 2003;111:389–397. doi: 10.1172/JCI16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H, Pandak WM, Jr., Lyall V, Natarajan R, Hylemon PB. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol. 2005;68:690–700. doi: 10.1124/mol.105.012898. [DOI] [PubMed] [Google Scholar]
- 6.Maggi P, Perilli F, Lillo A, Gargiulo M, Ferraro S, Grisorio B, Ferrara S, Carito V, Bellacosa C, Pastore G, Chirianni A, Regina G. Rapid progression of carotid lesions in HAART-treated HIV-1 patients. Atherosclerosis. 2007;192:407–412. doi: 10.1016/j.atherosclerosis.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Murphy RL, Berzins B, Zala C, Fichtenbaum C, Dube MP, Guaraldi G, Torriani F, Belsey E, Mitchell C, Stein JH. Change to atazanavir/ritonavir treatment improves lipids but not endothelial function in patients on stable antiretroviral therapy. Aids. 2010;24:885–890. doi: 10.1097/QAD.0b013e3283352ed5. [DOI] [PubMed] [Google Scholar]
- 8.Rose H, Hoy J, Woolley I, Tchoua U, Bukrinsky M, Dart A, Sviridov D. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199:79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukrinsky M, Sviridov D. HIV and cardiovascular disease: contribution of HIV-infected macrophages to development of atherosclerosis. PLoS Med. 2007;4:e43. doi: 10.1371/journal.pmed.0040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV, Bukrinsky M, Sviridov D. Human Immunodeficiency Virus Impairs Reverse Cholesterol Transport from Macrophages. PLoS Biology. 2006;4:e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliviero U, Bonadies G, Apuzzi V, Foggia M, Bosso G, Nappa S, Valvano A, Leonardi E, Borgia G, Castello G, Napoli R, Sacca L. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis. 2009;204:586–589. doi: 10.1016/j.atherosclerosis.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Varriale P, Saravi G, Hernandez E, Carbon F. Acute myocardial infarction in patients infected with human immunodeficiency virus. Am Heart J. 2004;147:55–59. doi: 10.1016/j.ahj.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 13.van Wijk JP, de Koning EJ, Cabezas MC, Joven J, op’t Roodt J, Rabelink TJ, Hoepelman AM. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47:1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M, von Kegler S, Ruhkamp D, Steinmetz H, Sitzer M. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Hsue PY, Ordovas K, Lee T, Reddy G, Gotway M, Schnell A, Ho JE, Selby V, Madden E, Martin JN, Deeks SG, Ganz P, Waters DD. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am. J. Cardiol. 2012;109:742–747. doi: 10.1016/j.amjcard.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, Tien PC, Shlipak MG, Sidney S, Polak JF, O’Leary D, Bacchetti P, Kronmal RA. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. Aids. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden Cardiac Death in Patients With Human Immunodeficiency Virus Infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of Atherosclerosis as Assessed by Carotid Intima-Media Thickness in Patients With HIV Infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 19.Mangili A, Polak JF, Skinner SC, Gerrior J, Sheehan H, Harrington A, Wanke CA. HIV infection and progression of carotid and coronary atherosclerosis: the CARE study. J Acquir Immune Defic Syndr. 2011;58:148–153. doi: 10.1097/QAI.0b013e31822d4993. [DOI] [PubMed] [Google Scholar]
- 20.de Saint Martin L, Vandhuick O, Guillo P, Bellein V, Bressollette L, Roudaut N, Amaral A, Pasquier E. Premature atherosclerosis in HIV positive patients and cumulated time of exposure to antiretroviral therapy (SHIVA study) Atherosclerosis. 2006;185:361–367. doi: 10.1016/j.atherosclerosis.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 21.Currier JS, Kendall MA, Zackin R, Henry WK, Alston-Smith B, Torriani FJ, Schouten J, Mickelberg K, Li Y, Hodis HN. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. Aids. 2005;19:927–933. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross AC, Storer N, O'Riordan MA, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J. 2010;29:634–638. doi: 10.1097/inf.0b013e3181d770c4. [DOI] [PubMed] [Google Scholar]
- 23.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, Li Y, Hodis HN. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. Aids. 2007;21:1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 24.Mercie P, Thiebaut R, Aurillac-Lavignolle V, Pellegrin JL, Yvorra-Vives MC, Cipriano C, Neau D, Morlat P, Ragnaud JM, Dupon M, Bonnet F, Lawson-Ayayi S, Malvy D, Roudaut R, Dabis F Groupe d'Epidemiologie Clinique du Sida en, A. Carotid intima-media thickness is slightly increased over time in HIV-1-infected patients. HIV Med. 2005;6:380–387. doi: 10.1111/j.1468-1293.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Guaraldi G, Zona S, Orlando G, Carli F, Ligabue G, Fiocchi F, Rossi R, Modena MG, Raggi P. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. The international journal of cardiovascular imaging. 2012;28:935–941. doi: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]
- 26.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, Williams I, Drummond F, Duprez D, Belloso WH, Goebel FD, Grund B, Hatzakis A, Vera J, Lundgren JD. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 27.Lampe FC, Duprez DA, Kuller LH, Tracy R, Otvos J, Stroes E, Cooper DA, Hoy J, Paton NI, Friis-Moller N, Neuhaus J, Liappis AP, Phillips AN. Changes in lipids and lipoprotein particle concentrations after interruption of antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54:275–284. doi: 10.1097/qai.0b013e3181d32158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:773–779. doi: 10.1016/s0735-1097(02)02009-0. [DOI] [PubMed] [Google Scholar]
- 29.Liang YL, Teede H, Kotsopoulos D, Shiel L, Cameron JD, Dart AM, McGrath BP. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci (Lond) 1998;95:669–679. doi: 10.1042/cs0950669. [DOI] [PubMed] [Google Scholar]
- 30.Hoang A, Drew BG, Low H, Remaley AT, Nestel P, Kingwell BA, Sviridov D. Mechanism of cholesterol efflux in humans after infusion of reconstituted high-density lipoprotein. Eur. Heart J. 2012;33:657–665. doi: 10.1093/eurheartj/ehr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrinello CM, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, Anastos K, Tien PC, Xue X, Lazar J, Benning L, Tracy RP, Kaplan RC. Association of subclinical atherosclerosis with lipid levels amongst antiretroviral-treated and untreated HIV-infected women in the Women's Interagency HIV study. Atherosclerosis. 2012;225:408–411. doi: 10.1016/j.atherosclerosis.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, Dasmahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG on behalf of the P-I. M. T. S. G. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012 doi: 10.1016/S0140-6736(12)60441-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tohyama J, Billheimer JT, Fuki IV, Rothblat GH, Rader DJ, Millar JS. Effects of nevirapine and efavirenz on HDL cholesterol levels and reverse cholesterol transport in mice. Atherosclerosis. 2009;204:418–423. doi: 10.1016/j.atherosclerosis.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]