The majority of critically ill infants and children supported on mechanical ventilation receive some form of sedative therapy; most often various combinations of opioids and benzodiazepines1,2. Although there are clear benefits in using sedation in critically ill pediatric patients3 sedative use may also be associated with iatrogenic injury4-6. Clinical trials exploring optimal sedation management in mechanically ventilated pediatric patients are urgently needed to improve short and long-term outcomes in this vulnerable patient population.
Fundamental to any clinical trial is patient safety. During the study design phase, anticipated adverse events (AEs), specific to the patient population and the intervention studied, are operationally defined and event rates estimated based on best available evidence. Operational definitions for each AE should be clear and reproducible across multiple clinical sites. Expected AEs are also incorporated into the study’s risk-benefit profile and informed consent process. Adverse events are continuously monitored by the investigative team and Data and Safety and Monitoring Board (DSMB). If an AE rate exceeds that expected then the study’s risk-benefit burden is reevaluated.
Here, we describe the process used to identify and operationalize sedation-related AEs for the RESTORE clinical trial. RESTORE (Randomized Evaluation of Sedation Titration fOr Respiratory FailurE; U01 HL086622 and U01 HL086649) is a multicenter clinical trial evaluating the impact of a nurse-implemented goal-directed sedation protocol on clinical outcomes in pediatric patients with acute respiratory failure. Our objective was to develop concise operational definitions that defined, and provided best-available event rates, of sedation-related AEs in the pediatric population.
Methods
We conducted a multiphase systematic review of the literature. Sedation-related AEs were first identified and operationally defined. Next, each of the operational definitions and event rates were estimated specific to the pediatric intensive care unit (PICU) population. Adverse events were defined as “any untoward or unfavorable medical occurrence, including any abnormal sign or symptom, temporally associated with the use of ICU sedation”7.
Search Strategy
In Phase One we searched the OVID-MEDLINE and CINAHL databases from 1998 – 2008. Search terms included “sedation”, “intensive care unit” and “critical care”. We limited our search to English language data-based articles, involving Human subjects across the age spectrum from neonatal to adult. We included randomized clinical trials, prospective observational, or pre/post implementation study designs but excluded quality improvement projects, case reports, surveys, pharmacokinetics studies, and studies evaluating a single agent. We limited our search to include adverse events related to sedation management and not those related to a specific sedative or analgesic agents. Reference lists of retrieved articles were also reviewed. For each publication, we abstracted into data tables: methods, study population, results, reported operational definitions and event rates for each AE. Data pooling was not possible because of study heterogeneity. Data saturation was achieved when no further unique AE or rates of occurrence were identified.
Phase Two replicated our previous search strategy to search terms that included all AEs identified in Phase One, limited to data-based articles in the pediatric population. Unpublished data from the RESTORE pilot study (R21 HD045020)8 and available event rate data from participating sites where added. Pediatric-specific definitions and event rates were then added to the data table.
Data Extraction and Synthesis
The RESTORE Core Investigator team that included 2 adult and 3 pediatric intensivists, 3 PICU nurses and 1 PICU pharmacist reviewed the data table and, by consensus, made a recommendation that operationally defined each AE with an expected rate of occurrence. Excluded from further review at this stage were several phenomena uncommon in pediatrics (myocardial infarction, delirium), not specific to sedative use (multisystem organ failure), related to administration techniques (hypotension), unit-based standards of care (urinary tract infection, restraint use) or evaluated after ICU discharge (post-traumatic stress). Of note, delirium has not been fully explicated in the pediatric population, valid and reliable pediatric-specific assessment instruments have only recently been published for verbal cognitive-capable pediatric patients.
The RESTORE Steering Committee, that included 22 voting pediatric intensivist or advanced practice nurses representing each participating PICU, then reviewed the data tables and approved the final terms and rates with 100% agreement. These were then reviewed and approved by the RESTORE DSMB (see Table One). A replicated systematic review in January 2012 demonstrated no new AEs that would impact our findings. Institutional review board was not obtained for this review.
Table 1.
RESTORE Specified Events Operational Definitions and Event Rates
|
Inadequate sedation management: Agitation defined by an SBS > 0 (or “assumed agitation present” in patients receiving neuromuscular blockade) for 2 consecutive hours not related to a planned extubation attempt. Event Rate: < 10% of patients |
|
Inadequate pain management: Pain score > 4 (or “assumed pain present” in patients receiving neuromuscular blockade) for 2 consecutive hours not related to a planned extubation attempt. Event Rate: < 20% of patients |
|
Clinically significant iatrogenic withdrawal: In patients weaning from ≥ 5 days of continuous infusion or round-the-clock narcotics, any patient receiving rescue therapy (defined as an opioid or benzodiazepine bolus or an increase in opioid or benzodiazepine infusion) to manage an increase in WAT-1 (59) symptoms after the start of weaning and not for treatment of new pain or sedation needs. Available evidence identifies iatrogenic withdrawal as a WAT-1 Score of ≥ 3. Event Rate: <75% of patients |
|
Unplanned endotracheal tube (ETT) extubation: Unplanned extubation. Event Rate < 3.0 per 100 ventilator days |
|
Post-extubation stridor with chest-wall retractions at rest: Stridor (defined as a high-pitched or harsh inspiratory noise) with chest-wall retractions after ETT extubation. Event Rate: < 30% |
|
Extubation failure: Reintubation within 24 hours. Less than 10% of patients electively extubated should require reintubation. Event Rate: < 10% of patients |
|
Unplanned removal of any invasive tube: Unplanned removal of any invasive tube (e.g., arterial access, central venous access, peripheral venous access, nasogastric drainage tube, bladder catheter, chest tube, “other” tube). Denominator data are required to determine accurate rates per 100 days. Event Rate: Unknown |
|
Ventilator-associated pneumonia VAP: Pneumonia occurring ≥48 hours after the initiation of mechanical ventilation (104) VAP rate as the number of VAP per 1000 ventilator days (92). VAP rates should be zero. All cases of suspected VAP are to be adjudicated by the local infectious disease officer using a standardized process (105) and National Healthcare Safety Network definition (106) Event rate: < 3.2 per 1,000 ventilator days |
|
Catheter-associated bloodstream infection CA-BSI: Defined using The National Nosocomial Infections Surveillance System (NNIS) (100) as the number of CA-BSI per 1,000 days of IV sedative use. CA-BSI rates should be zero. All cases of suspected CA-BSI are to be adjudicated by the local infectious disease officer using National Healthcare Safety Network definition (106) Event Rate: < 4 per 1,000 central line days |
|
Stage 2+ pressure ulcers: Stage II (or worse) partial thickness loss of skin layers involving epidermis and possibly penetrating into but not through dermis. May present as blistering with erythema and/or induration; wound base moist and pink; painful; free of necrotic tissue (107). Assign attribution using the Braden Q scale (102). Event Rate: <30% |
|
New tracheostomy: Track all new tracheostomy as a tracer for extreme airway trauma secondary to agitation. |
Results
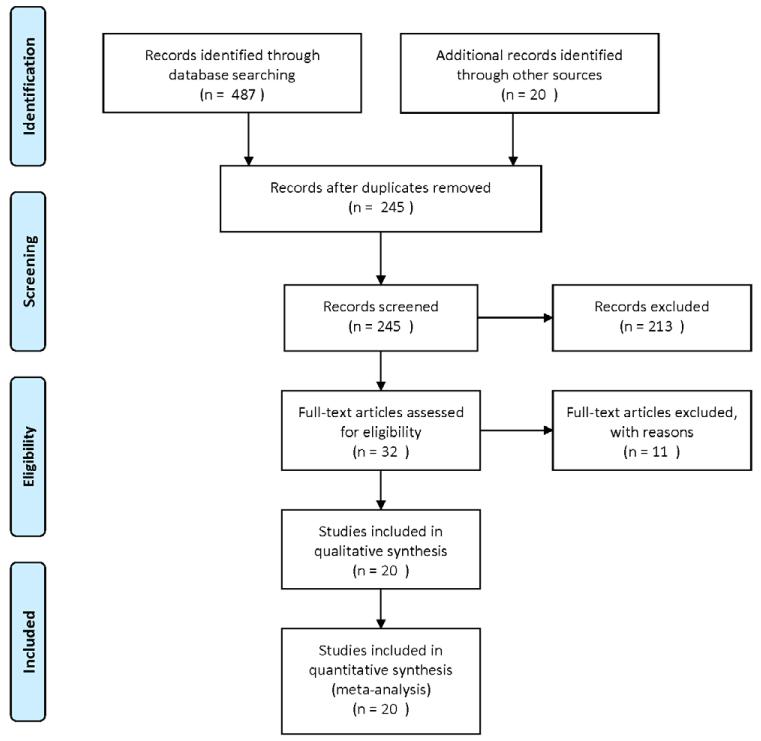
In Phase One we screened 245 papers (see Figure 1). Of these, 32 articles were retrieved for more detailed evaluation and 20 met Phase One inclusion/exclusion criteria9-28_ENREF_9_ENREF_9. As anticipated, none included pediatric patients. 9 were reported as RCTs, 8 prospective before/after, and 3 prospective, observational. The majority of studies were conducted in medical ICUs (N=12). Most studies evaluated the effect of guideline directed sedation management on patient outcomes (N=10).
Figure One.
Phase One Flow Diagram
In Phase Two we included 62 papers to refine our definitions and determine the rates of each AE in the PICU population. A summary of the sedation-related AEs is provided below. Anticipated rates for these events are presented in Table One. .
Inadequate sedation management
Achieving adequate and effective sedation levels, and controlling agitation, was evaluated by most studies in Phase One of this review. In pediatric patients, Fonsmark et al29. described the efficacy of sedation as” the quality of sedation assessed by the nursing staff; specifically, the patient is asleep, tolerating mechanical ventilation and able to show a slight response to nursing procedures”. The COMFORT scale 30was designed to assess distress in ventilated children but distress was operationalized to include the constructs of both pain and agitation. From a clinical perspective, separate valid and reliable pain and agitation assessment tools would allow more targeted therapeutic management.31In 2006, Curley et al 32 developed then validated State Behavioral Scale (SBS) for use in ventilated pediatric patients aged 6 wks to 6 yrs. The SBS range is −3 unresponsive to +2 agitated.
In the RESTORE trial, inadequate sedation management was defined as an SBS32 of +1 (restless and difficult to calm) or a SBS of +2 (agitated) for 2 consecutive hours, not related to a planned extubation attempt.
Inadequate pain management
While a number of studies reported daily or total administration of sedatives and analgesics 6,17,19,20,23,25,26,33-35 few systematically evaluated the effect of the sedation/analgesia regimen on a patient’s pain level. A variety of instruments were used to assess pain. The incidence of pain varied in adult studies ranging from 6-42%21,25.
No pediatric study defined or reported the incidence of inadequate pain management in mechanically ventilated children. Pain scores of 4 on a 0-10 scale equate to a moderate level of pain infrequently experienced in nonsurgical patients supported on mechanical ventilation. Pain assessment tools were based on a 10-point scale. The validated pain assessment tool depended upon the age and verbal capacity of the subject: infant to 7 years and nonverbal: FLACC (Facial expression, Leg movement, Activity, Cry, and Consolability) 36,37; 3+ years of age and verbal: Wong-Baker FACES 38; 5+ yrs of age and verbal: Numeric Rating Scale; and ≥ 8 yrs and nonverbal: Individualized Numeric Rating Scale (INRS)39. The RESTORE’s pilot study8 showed the highest median daily pain score on a 0-10 scale to be 1 (IQR: 1-4).
In the RESTORE trial, inadequate pain management was defined as a pain score > 4 on a 0-10 age appropriate pain scale for 2 consecutive hours, not related to a planned extubation attempt..
Clinically significant iatrogenic withdrawal
Iatrogenic withdrawal syndrome related to opioid and or benzodiazepine use has not been thoroughly described in critically ill adult patients. In contrast, iatrogenic withdrawal syndrome has been reported to occur in up to 57% of PICU patients40. Iatrogenic withdrawal is influenced by the length and total exposure to opioids and benzodiazepines, increasing to over 50% after 5 days of continuous infusion or around-the-clock administration40,41 and by the rate of weaning29,40,42.
Several assessment instruments have been used to describe iatrogenic withdrawal syndrome29,42-49. The Finnegan Neonatal Abstinence Score50 (NAS) has been used in pediatric patients40,43,51,52 defining withdrawal as a NAS of ≥ 8 of 39 for three consecutive scores obtained every 2 hours51,52 or a NAS of >12 of 3943 or agitation requiring opioid rescue52. Franck et al. developed the pediatric-specific Withdrawal Assessment Tool 1 (WAT-1)53,54. WAT-1 score of > 3 of 12 is associated with iatrogenic withdrawal.
In the RESTORE trial, clinically significant iatrogenic withdrawal was defined any patient receiving rescue therapy to manage an increase in WAT-1 score.
Unplanned endotracheal tube (ETT) extubation
Numerous randomized controlled12,13,16,20,24,28,55,56 ENREF 36 and comparative observational studies 9,18,57-60 report unplanned extubation as a sedation-related AE in adult patients. Unplanned/self extubation rates in these studies ranged from 1-6 per 100 airway days.
ENREF 53Factors associated with unplanned extubation in pediatrics include the presence of agitation61-64 ENREF 55 ENREF 58, high patient/nurse and patient acuity/nurse ratios 64,65, younger age 63,66,67, medical vs. surgical patients 63, sedation not administered in the two hours before event 62, lack of two-point or more restraints 62, and performance of a patient procedure at the bedside62,63. Patients with a longer length of mechanical ventilation and children in the weaning phase were at a higher risk of unplanned extubation63. Unplanned extubation rates in pediatrics range from 0.2 to 1.7 per 100 airway days 62-70. Overall, 14-22% of pediatric patients who self-extubate require re-intubation 63,71.
Post-extubation stridor with chest-wall retractions at rest
No sedation articles in adults discussed this AE. However, excessive movement of the ETT within the airway is thought to precipitate airway trauma in the agitated pediatric patient. The reported pediatric incidence of post-extubation stridor requiring treatment ranges from 15% to 41%72-74. The frequency of pediatric reintubation for stridor ranges from 2% to 52%72-77. Other variables related to stridor included the number of racemic epinephrine treatments 73,76-80, use of a helium-oxygen gas mixture73, and stridor requiring some form of intervention74,77. Two RCTs evaluating the use of dexamethasone to prevent post-extubation stridor reported reintubation in 11%73 and 25%74 of patients with stridor.
The RESTORE trial defined stridor (high-pitched or harsh inspiratory noise) with chest-wall retractions at rest after ETT extubation to be phenomena of concern.
Extubation failure
In adults, extubation failure was defined as reintubation occurring within 24-48 hours of extubation16,18,81. The criteria for reintubation was not reported or left to the discretion of the treating physician. There were significant differences in reintubation rates across study groups10,13,18. Subjects treated with continuous IV sedation were more likely to require reintubation10. Subjects managed with a nurse-implemented sedation protocol were less likely than the control group to require reintubation 18. Weaning parameters were also found to be poor predictors of successful extubation82,83.
Reintubation rates in electively extubated pediatric patients ranged from 4-14% 67,71,84-86. Patients who failed extubation were typically younger 67,85,86, experiencing a longer PICU length of stay and length of mechanical ventilation 67,76,84-86, were receiving inotropic support with a low PaO2 87 , or were chronically ill with a respiratory or neurologic condition77.
Unplanned removal of any invasive tube
Six prospective adult observational studies reported the frequency of unplanned removal of any invasive tube/catheter 16,21,24,58,60,88. Devices removed included vascular catheters, bladder catheters, and gastric tubes. Studies implementing systematic pain and sedation assessments21, daily sedation interruption24 and protocolized sedation16 noted low rates of device removal. Similar pediatric data were not identified. Unplanned removal of any invasive tube in RESTORE trial was defined as removal of the device per 100 device days.
Ventilator Associated Pneumonia (VAP)
Five adult studies reported an association between sedation practices and VAP rates 9,18,21,56,89. The incidence of VAP has been reported to be significantly lower in nurse-implemented sedation protocol vs. control groups21,18. RESTORE adopted the National Nosocomial Infections Surveillance System (NNIS)90,91 definition of VAP
Catheter Associated Bloodstream Infection (CA-BSI)
CA-BSI rates may be affected by the use of continuous versus intermittent sedation and/or the need to enter a central line to administer rescue sedation doses. One adult study reported a significantly lower rate of CA-BSI in patients managed with a sedation protocol21. RESTORE adopted the NNIS91,92 ENREF 79 definition of CA-BSI.
Stage two pressure ulcers
Sedated patients cannot communicate nor respond to pressure-related discomfort and thus are at high risk for pressure ulcers. Adult patients managed with a sedation algorithm experienced fewer pressure ulcers9. Using the National Pressure Ulcer Advisory Panel guidelines93, Curley et al94 ENREF 83 reported that 27% of critically ill pediatric patients developed pressure ulcers. Predictors of pressure ulcers included mechanical ventilation and lower Braden Q95 ENREF 97scores. RESTORE used the National Pressure Ulcer Advisory Panel guidelines93 and defined Stage II93 pressure ulcer (or worse) as reportable.
New tracheotomy
Tracheotomy practices differ between adult and pediatric critically ill patients. There is conflicting evidence on the rate of new tracheotomy in adult patients managed with a sedation protocol13,14,27,56 ENREF 27. There were no pediatric studies reporting the rate of new tracheotomy in sedated critically ill patients. We elected to follow all new tracheotomies as a tracer for extreme airway trauma secondary to agitation.
Discussion
We conducted this systematic literature review to operationally define, summarize and present a sedation-related AE monitoring plan for the RESTORE multi-institutional clinical trial. AE definitions and estimated event rates were derived through a multilayered consensus process using best available evidence. While clear and concise operational definitions and estimated rates of specified AE are paramount to the safe conduct of clinical trials, these data may also be useful to those evaluating patient safety initiatives.
Because of the paucity of pediatric data, pediatric clinicians frequently use data that are available from adult care. When relevant anatomical or maturational differences exist between adult and pediatric patients we based our definitions strictly on pediatric literature. For example, whereas extubation failure is universal, post-extubation stridor is unique in the pediatric population because of anatomical differences.
Our recommendations must be interpreted in light of several important limitations. The etiology of sedation related AE appear multi-factorial, and each may be associated with different risk or protective factors. Sedation practices are rapidly evolving and many papers now pre-date the trend towards a more awake ICU patient population. Not all tools are validated to the entire pediatric age spectrum; specifically the SBS was validated in 6 wk to 6 yr olds. While AE rates seem to vary with the use of a sedation protocol, there is significant heterogeneity among studies and different operational definitions of AE made comparisons across studies difficult. Careful consideration of the complex interaction between, age, development, sedation level and exposure, use of protocols and organizational factors will be fundamental to understanding the factors that alter patient risk. The proposed definitions and event rates represent a consensus evidence based opinion. Finally, although our search methods were quite comprehensive, we may have overlooked a relevant paper that should have been included in our review.
Conclusion
In this article, we propose operational definitions and recommendations for reporting sedation-related AE in critically ill ventilated pediatric patients. While this work provides the necessary foundation for the safe conduct of sedation clinical trials, we anticipate continued dialogue on this topic. These standard definitions may help us communicate, share results and advance the field.
Acknowledgements
The authors thank members of the RESTORE Steering Committee for their thoughtful review of this work and the RESTORE Investigative Team: Geoffrey L. Allen, MD, Children’s Mercy Hospital; Jeffrey Alten, MD, Children’s Hospital of Alabama; Judith A. Ascenzi, RN, MSN, Johns Hopkins Hospital; Frederick E. Barr, MD, Monroe Carell Jr. Children’s Hospital at Vanderbilt; Scot Bateman, MD, UMASS Memorial Medical Center; Pamela Brown, RN, PhD, CCRN, Doerbecher Children’s Hospital; Ira M Cheifetz, MD, Duke University Medical Center; Brenda Dodson, PharmD, Children’s Hospital Boston; Kimberly Eiden, RN, MS, Advocate Hope Children’s Hospital; Edward Vincent S. Faustino, MD, Yale-New Haven Children’s Hospital; Lori Fineman, RN, MS, UCSF Children’s Hospital; Heidi Flori, MD, Children’s Hospital & Research Center Oakland; Linda S. Frank, RN, PhD. FRCPCH, FAAN, UCSF School of Nursing; Rainer G. Gedeit, MD, Children’s Hospital of Wisconsin; Andrea Harabin, MD, National Institutes of Health; Larissa Hutchins, MSN, RN, CCRN, CCNS, The Children’s Hospital of Philadelphia; Dean Jarvis, BSN, MBA, Dartmouth Hitchcock Medical Center; Aileen Kirby, MD, Doernbecher Children’s Hospital; Nikoleta S. Kolovos, MD, St. Louis Children’s Hospital; Ruth Lebet, RN, MSN, CCNS, CPNP, Alfred I. DuPont Hospital for Children; Michael A. Matthay, MD, University of California at San Francisco; Joanne Natale, MD, PhD, UC Davis Children’s Hospital; Shari Simone, RN, MSN, CPNP-AC, FCCM, University of Maryland’s Children’s Hospital; Lauren Sorce, MSN, CCRN, CPNP-AC/PC, FCCM, Children’s Memorial Hospital; Philip F. Thurst, RN, St. Louis Children’s Hospital; Deborah Updegraff, MSN, RN, PNP, CNS, Lucile Packard Children’s Hospital at Stanford; R. Scott Watson, MD, MPH, Children’s Hospital of Pittsburgh; David Wypij, PhD, Children’s Hospital Boston
This work was supported by the National Institute of Health (NIH); National Heart, Lung, and Blood Institute (NHLBI); National Institute of Nursing Research (NINR): [Grant HL086622 and HL086649].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest.
Contributor Information
Mary Jo C. Grant, Pediatric Critical Care Nurse Practitioner, Primary Children’s Medical Center, Maryjo.Grant@imail.org.
Michele C. Balas, Community-Based Health Nursing Department, University of Nebraska Medical Center, College of Nursing, MBalas@unmc.edu.
Martha A.Q. Curley, Ellen and Robert Kapito Professor in Nursing Science, School of Nursing, Anesthesia and Critical Care Medicine, University of Pennsylvania, Curley@nursing.upenn.edu.
References
- 1.Rhoney DH, Murry KR. National survey on the use of sedatives and neuromuscular blocking agents in the pediatric intensive care unit. Pediatr Crit Care Med. 2002;3:129–133. doi: 10.1097/00130478-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Twite MD, Rashid A, Zuk J, et al. Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med. 2004;5:521–532. doi: 10.1097/01.PCC.0000144710.13710.2E. [DOI] [PubMed] [Google Scholar]
- 3.Marx CM, Rosenberg DI, Ambuel B, et al. Pediatric intensive care sedation: survey of fellowship training programs. Pediatrics. 1993;91:369–378. [PubMed] [Google Scholar]
- 4.Barrientos-Vega R, Mar Sanchez-Soria M, Morales-Garcia C, et al. Prolonged sedation of critically ill patients with midazolam or propofol: impact on weaning and costs. Crit Care Med. 1997;25:33–40. doi: 10.1097/00003246-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kress JP, Pohlman AS, Hall JB. Sedation and analgesia in the intensive care unit. Am J Respir Crit Care Med. 2002;166:1024–0128. doi: 10.1164/rccm.200204-270CC. [DOI] [PubMed] [Google Scholar]
- 6.Randolph AG, Wypij D, Venkataraman ST, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288:2561–1568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 7.Office for Human Research Protections . Guidance on Reviewing and Reporting Unanticipated Problems Involving Risks to Subjects or Others and Adverse Events: U.S. Department of Health and Human Services. [Google Scholar]
- 8.Curley MAQ, Amling J, Gedeit RG, et al. Sedation management in pediatric patients supported on mechanical ventilation. Pediatr Crit Care Med. 2007;8:A 106. [Google Scholar]
- 9.De Jonghe B, Bastuji-Garin S, Fangio P, et al. Sedation algorithm in critically ill patients without acute brain injury. Crit Care Med. 2005;33:120–127. doi: 10.1097/01.ccm.0000150268.04228.68. [DOI] [PubMed] [Google Scholar]
- 10.Kolleff MH, Levy NT, Ahrens TS, et al. The use of continuous I.V. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 11.Arias-Rivera S, Sanchez-Sanchez Mdel M, Santos-Diaz R, et al. Effect of a nursing-implemented sedation protocol on weaning outcome. Crit Care Med. 2008;36:2054–2060. doi: 10.1097/CCM.0b013e31817bfd60. [DOI] [PubMed] [Google Scholar]
- 12.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Crit Care Med. 2008;36:1444–1450. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 13.de Wit M, Gennings C, Jenvey W, et al. Randomized trial comparing daily interruption of sedation and nursing-implemented sedation algorithm in medical intensive care unit patients. Critical Care. 2008;12:R70. doi: 10.1186/cc6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huey-Ling L, Chun-Che S, Jen-Jen T, et al. Comparison of the effect of protocol-directed sedation with propofol vs. midazolam by nurses in intensive care: efficacy, haemodynamic stability and patient satisfaction. Journal of Clinical Nursing. 2008;17:1510–1517. doi: 10.1111/j.1365-2702.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- 15.Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Medicine. 2008;36:427–433. doi: 10.1097/01.CCM.0000300275.63811.B3. [DOI] [PubMed] [Google Scholar]
- 16.Mehta S, Burry L, Martinez-Motta JC, et al. A randomized trial of daily awakening in critically ill patients managed with a sedation protocol: a pilot trial. Crit Care Medicine. 2008;36:2092–2099. doi: 10.1097/CCM.0b013e31817bff85. [DOI] [PubMed] [Google Scholar]
- 17.Kress JP, Vinayak AG, Levitt J, et al. Daily sedative interruption in mechanically ventilated patients at risk for coronary artery disease. Crit Care Med. 2007;35:365–371. doi: 10.1097/01.CCM.0000254334.46406.B3. [DOI] [PubMed] [Google Scholar]
- 18.Quenot J, Ladoire S, Devoucoux F, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Medicine. 2007;35:2031–2036. doi: 10.1097/01.ccm.0000282733.83089.4d. [DOI] [PubMed] [Google Scholar]
- 19.Adam C, Rosser D, Manji M. Impact of introducing a sedation management guideline in intensive care. Anaesthesia. 2006;61:260–263. doi: 10.1111/j.1365-2044.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- 20.Carson SS, Kress JP, Rodgers JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34:1326–1332. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 21.Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34:1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 22.Richman PS, Baram D, Varela M, et al. Sedation during mechanical ventilation: a trial of benzodiazepine and opiate in combination. Crit Care Med. 2006;34:1395–1401. doi: 10.1097/01.CCM.0000215454.50964.F8. [DOI] [PubMed] [Google Scholar]
- 23.Duane TM, Riblet JL, Golay D, et al. Protocol-driven ventilator management in a trauma intensive care unit population. Arch Surg. 2002;137:1223–1227. doi: 10.1001/archsurg.137.11.1223. [DOI] [PubMed] [Google Scholar]
- 24.Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 25.MacLaren R, Plamondon JM, Ramsay KB, et al. A prospective evaluation of empiric versus protocol-based sedation and analgesia. Pharmacotherapy. 2000;20:662–672. doi: 10.1592/phco.20.7.662.35172. [DOI] [PubMed] [Google Scholar]
- 26.Mascia MF, Koch M, Medicis JJ. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Medicine. 2000;28:2300–2306. doi: 10.1097/00003246-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 29.Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit Care Med. 1999;27:196–199. doi: 10.1097/00003246-199901000-00052. [DOI] [PubMed] [Google Scholar]
- 30.Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol. 1992;17:95–109. doi: 10.1093/jpepsy/17.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Wansbrough SR, White PF. Sedation scales: measures of calmness or somnolence? Anesthesia & Analgesia. 1993;76:219–221. doi: 10.1213/00000539-199302000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Curley MA, Harris SK, Fraser KA, et al. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7:107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartman ME, McCrory DC, Schulman SR. Efficacy of sedation regimens to facilitate mechanical ventilation in the pediatric intensive care unit: a systematic review. Pediatr Crit Care Med. 2009;10:246–255. doi: 10.1097/PCC.0b013e31819a3bb9. [DOI] [PubMed] [Google Scholar]
- 34.Hooper MH, Girard TD. Sedation and weaning from mechanical ventilation: linking spontaneous awakening trials and spontaneous breathing trials to improve patient outcomes. Crit Care Clin. 2009;25:515–525. doi: 10.1016/j.ccc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Kolleff MH, Shapiro SD, Silver P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–574. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Merkel S, Voepel-Lewis T, Tait AR, et al. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297. [PubMed] [Google Scholar]
- 37.Manworren RC, Hynan LS. Clinical validation of FLACC: Preverbal pain scale. Pediatr Nurs. 2003;29:140–146. [PubMed] [Google Scholar]
- 38.Wong DL, Hockenberry-Eaton M, Wilson D, et al. Whaley and Wong’s Nursing Care of Infants and Children. Mosby; St. Louis, MO: 1999. [Google Scholar]
- 39.Solodiuk J, Curley MAQ. Pain assessment in nonverbal children with severe cognitive impairments: The Individualized Numberic Rating Scale (INRS) J Pediatr Nurs. 2003;18:295–299. doi: 10.1016/s0882-5963(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 40.Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med. 1994;22:763–767. doi: 10.1097/00003246-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Arnold JH, Truog RD, Orav EJ, et al. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology. 1990;73:1136–1140. doi: 10.1097/00000542-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Franck L, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive Crit Care Nurs. 2004;20:344–351. doi: 10.1016/j.iccn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Tobias J. Tolerance, withdrawal, and phsical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28:2122–2132. doi: 10.1097/00003246-200006000-00079. [DOI] [PubMed] [Google Scholar]
- 44.Lugo RA, MacLaren R, Cash J, et al. Enteral methadone to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU. Pharmacotherapy. 2001;21:1566–1573. doi: 10.1592/phco.21.20.1566.34471. [DOI] [PubMed] [Google Scholar]
- 45.Siddappa R, Fletcher JE, Heard AMB, et al. Methadone dosage for prevention of opioid withdrawal in children. Paediatric Anaesthesia. 2003;13:805–810. doi: 10.1046/j.1460-9592.2003.01153.x. [DOI] [PubMed] [Google Scholar]
- 46.Robertson RC, Darsey E, Fortenberry JD, et al. Evaluation of an opiate-weaning protocol using methadone in pediatric intensive care unit patients. Pediatr Crit Care Med. 2000;1:119–123. doi: 10.1097/00130478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins IA, Playfor SD, Bevan C, et al. Current United Kingdom sedation practice in pediatric intensive care. Peadiatr Anaesth. 2007;17:675–683. doi: 10.1111/j.1460-9592.2006.02180.x. [DOI] [PubMed] [Google Scholar]
- 48.Bowens CD, Thompson JA, Thompson MT, et al. A trial of methadone tapering schedules in pediatric intensive care unit patients exposed to prolonged sedative infusions. Pediatr Crit Care Med. 2011;2:504–511. doi: 10.1097/PCC.0b013e3181fe38f5. [DOI] [PubMed] [Google Scholar]
- 49.Ista E, van Dijk M, de Hoog M, et al. Construction of the Sophia Observation Withdrawal Symptoms-Scale (SOS) for critically ill children. Intensive Care Med. 2009;35:1075–1081. doi: 10.1007/s00134-009-1487-3. [DOI] [PubMed] [Google Scholar]
- 50.Finnegan LP, Connaughton JFJ, Kron RE, et al. Neonatal abstinence syndrome: Assessment and management. Addict Dis. 1975;2:141–158. [PubMed] [Google Scholar]
- 51.Meyer MM, Berens RJ. Efficacy of an enteral 10-day methadone wean to prevent opioid withdrawal in fentanyl-tolerant pediatric intensive care unit patients. Pediatr Crit Care Med. 2001;2:329–333. doi: 10.1097/00130478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Berens RJ, Meyer MT, Mikhailov TA, et al. A prospective evaluation of opioid weaning in opioid-dependent pediatric critical care patients. Anesthesia & Analgesia. 2006;102:1045–1050. doi: 10.1213/01.ane.0000202395.94542.3e. [DOI] [PubMed] [Google Scholar]
- 53.Franck LS, Harris SK, Soetenga DJ, et al. The Withdrawal Assessment Tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med. 2008;9:573–580. doi: 10.1097/PCC.0b013e31818c8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franck LS, Scoppettuolo LA, Wypij D, et al. Validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain. 2012;153:142–148. doi: 10.1016/j.pain.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 56.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomized trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 57.Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008;36:427–433. doi: 10.1097/01.CCM.0000300275.63811.B3. [DOI] [PubMed] [Google Scholar]
- 58.Mion LC, Minnick AF, Leipzig R, et al. Patient-initiated device removal in intensive care units: a national prevalence study. Critical Care Medicine. 2007;35:2714–2720. doi: 10.1097/01.ccm.0000291651.12767.52. [DOI] [PubMed] [Google Scholar]
- 59.Woods JC, Mion LC, Connor JT, et al. Severe agitation among ventilated medical intensive care unit patients: frequency, characteristics and outcomes. Intensive Care Medicine. 2004;30:1066–1072. doi: 10.1007/s00134-004-2193-9. [DOI] [PubMed] [Google Scholar]
- 60.Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest. 2005;128:2749–2757. doi: 10.1378/chest.128.4.2749. [DOI] [PubMed] [Google Scholar]
- 61.da Silva P, de Carvalho W. Unplanned extubation in pediatric critically ill patients: a systematic review and best practice recommendations. Pediatr Crit Care Med. 2010;11:2287–2942. doi: 10.1097/PCC.0b013e3181b80951. [DOI] [PubMed] [Google Scholar]
- 62.Little LA, Koenig JC, Jr., Newth CJ. Factors affecting accidental extubations in neonatal and pediatric intensive care patients. Critical Care Medicine. 1990;18:163–165. doi: 10.1097/00003246-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Sadowski R, Dechert RE, Bandy KP, et al. Continuous quality improvement: reducing unplanned extubations in a pediatric intensive care unit. Pediatrics. 2004;114:628–632. doi: 10.1542/peds.2003-0735-L. [DOI] [PubMed] [Google Scholar]
- 64.Marcin JP, Rutan E, Rapetti PM, et al. Nurse staffing and unplanned extubation in the pediatric intensive care unit. Pediatr Crit Care Med. 2005;6:254–257. doi: 10.1097/01.PCC.0000160593.75409.6B. [DOI] [PubMed] [Google Scholar]
- 65.Ream RS, Mackey K, Leet T, et al. Association of nursing workload and unplanned extubations in a pediatric intensive care unit. Pediatr Crit Care Med. 2007;8:366–371. doi: 10.1097/01.PCC.0000269379.40748.AF. [DOI] [PubMed] [Google Scholar]
- 66.da Silva PS, Aguiar VE, Neto HM. Unplanned extubation in a pediatric intensive care unit: Impact of a quality improvement programme. Anaesthesia. 2008;63:1209–1216. doi: 10.1111/j.1365-2044.2008.05628.x. [DOI] [PubMed] [Google Scholar]
- 67.Kurachek SC, Newth CJ, Quasney MW, et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Medicine. 2003;31:2657–2664. doi: 10.1097/01.CCM.0000094228.90557.85. [DOI] [PubMed] [Google Scholar]
- 68.Popernack ML, Thomas NJ, Lucking SE. Decreasing unplanned extubations: utilization of the Penn State Children’s Hospital Sedation Algorithm. Pediatr Crit Care Med. 2004;5:58–62. doi: 10.1097/01.CCM.0000105305.95815.91. [DOI] [PubMed] [Google Scholar]
- 69.Rivera R, Tibballs J. Complications of endotracheal intubation and mechanical ventilation in infants and children. Crit Care Medicine. 1992;20:193–199. doi: 10.1097/00003246-199202000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Fineman L, LaBrecque M, Shih M, et al. Prone positioning can be safely performed in critically ill infants and children. Pediatr Crit Care Med. 2006;7:413–422. doi: 10.1097/01.PCC.0000235263.86365.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farias JA, Alia I, Retta A, et al. An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Med. 2002;28:752–757. doi: 10.1007/s00134-002-1306-6. [DOI] [PubMed] [Google Scholar]
- 72.Markovitz BP, Randolph AG, Khemani RG. Corticosteroids for the prevention and treatment of post-extubation stridor in neonates, children and adults. Cochrane Database Syst Rev. 2008:CD001000. doi: 10.1002/14651858.CD001000.pub2. [DOI] [PubMed] [Google Scholar]
- 73.Anene O, Meert KL, Uy H, et al. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med. 1996;24:1666–1669. doi: 10.1097/00003246-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 74.Tellez DW, Galvis AG, Storgion SA, et al. Dexamethasone in the prevention of postextubation stridor in children. J Pediatr. 1991;118:289–294. doi: 10.1016/s0022-3476(05)80505-0. [DOI] [PubMed] [Google Scholar]
- 75.Harel Y, vardi A, Quigley R, et al. Extubation failure dure to post-extubation stridor is better correlated with neurologic impairment than with upper airway lesions in critically ill pediatric patients. International Journal of Pediatric Otorhinolaryngology. 1997;39:147–158. doi: 10.1016/s0165-5876(97)01488-2. [DOI] [PubMed] [Google Scholar]
- 76.Wratney AT, Benjamin DJ, Slonim A, et al. The endotracheal tube air leak test does not predict extubation outcome in critically ill pediatric patients. Pediatr Crit Care Med. 2008;9:490–496. doi: 10.1097/PCC.0b013e3181849901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss M, Dullenkoph A, Fischer JE, et al. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth. 2009;103:867–873. doi: 10.1093/bja/aep290. [DOI] [PubMed] [Google Scholar]
- 78.Deakers TW, Reynolds G, Stretton M, et al. Cuffed endotracheal tubes in pediatric intensive care. J Pediatr. 1994;125:57–62. doi: 10.1016/s0022-3476(94)70121-0. [DOI] [PubMed] [Google Scholar]
- 79.Newth CJl, Rachman B, Patel N, et al. The use of cuffed versus uncuffed endotracheal tubes in pediatric intensive care. Journal of Pediatrics. 2004;144:333–337. doi: 10.1016/j.jpeds.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 80.Harel Y, Vardi A, Quigley R, et al. Extubation failure due to post-extubation stridor is better correlated with neurologic impairment than with upper airway lesions in critically ill pediatric patients. Inter J of Ped Otorhinolaryngology. 1997;39:147–158. doi: 10.1016/s0165-5876(97)01488-2. [DOI] [PubMed] [Google Scholar]
- 81.Chevron V, Menard JF, Richard JC, et al. Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med. 1998;26:1049–1053. doi: 10.1097/00003246-199806000-00026. [DOI] [PubMed] [Google Scholar]
- 82.Farias JA, Alia I, Retta A, et al. An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Medicine. 2002;28:752–757. doi: 10.1007/s00134-002-1306-6. [DOI] [PubMed] [Google Scholar]
- 83.Farias JA, Monteverde E. We need to predict extubation failure. J Pediatr (Rio J) 2006;82:322–324. doi: 10.2223/JPED.1539. [DOI] [PubMed] [Google Scholar]
- 84.Baisch SD, Wheeler WB, Kurachek SC, et al. Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med. 2005;6:312–318. doi: 10.1097/01.PCC.0000161119.05076.91. [DOI] [PubMed] [Google Scholar]
- 85.Fontela PS, Piva JP, Garcia PC, et al. Risk factors for extubation failure in mechanically ventilated pediatric patients. Pediatr Crit Care Med. 2005;6:166–170. doi: 10.1097/01.PCC.0000154922.65189.48. [DOI] [PubMed] [Google Scholar]
- 86.Edmunds S, Weiss I, Harrison R. Extubation failure in a large pediatric ICU population. Chest. 2001;119:897–900. doi: 10.1378/chest.119.3.897. [DOI] [PubMed] [Google Scholar]
- 87.Harkel AD, van der Vorst MMJ, Hazekamp MG, et al. High mortality rate after extubation failure after pediatric cardiac surgery. Pediatric Cardiology. 2005;26:756–761. doi: 10.1007/s00246-005-0906-7. [DOI] [PubMed] [Google Scholar]
- 88.Carrion MI, Ayuso D, Marcos M, et al. Accidental removal of endotracheal and nasogastric tubes and intravascular catheters. Crit Care Med. 2000;28:63–66. doi: 10.1097/00003246-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 89.Schweickert WD, Gelbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Medicine. 2004;32:1272–1276. doi: 10.1097/01.ccm.0000127263.54807.79. [DOI] [PubMed] [Google Scholar]
- 90.Langley JM, Bradley JS. Defining pneumonia in critically ill infants and children. Pediatr Crit Care Med. 2005;9:S9–13. doi: 10.1097/01.PCC.0000161932.73262.D7. [DOI] [PubMed] [Google Scholar]
- 91.Centers for Disease Control and Prevention . National Healthcare Safety Network Manual. [Accessed January 5, 2011]. [Google Scholar]
- 92.Edwards JR, Peterson KD, Andrus ML, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007;35:290–301. doi: 10.1016/j.ajic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 93.Black J, Baharestani M, Cuddigan J, et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Dermatology Nursing. 2007;19:343–349. [PubMed] [Google Scholar]
- 94.Curley MAQ, Quigley SM, Lin M. Pressure ulcers in pediatric intensive care: incidence and associated factors. Pediatr Crit Care Med. 2003;4:284–290. doi: 10.1097/01.PCC.0000075559.55920.36. [DOI] [PubMed] [Google Scholar]
- 95.Curley MAQ, Razmus IS, Roberts KE, et al. Predicting pressure ulcer risk in pediatric patients: the Braden Q Scale. Nursing Research. 2003;52:22–33. doi: 10.1097/00006199-200301000-00004. [DOI] [PubMed] [Google Scholar]